Abstract
Slow-wave sleep, defined by low frequency (<4 Hz) electrical brain activity, is a basic brain function affecting metabolite clearance and memory consolidation. The origin of low-frequency activity is related to cortical up and down states, but the underlying cellular mechanism of how low-frequency activities affect metabolite clearance and memory consolidation has remained elusive. We applied electrical stimulation with voltages comparable to in vivo sleep recordings over a range of frequencies to cultured glial astrocytes while monitored the trafficking of GFP-tagged intracellular vesicles using total internal reflection fluorescence microscopy (TIRFM). We found that during low frequency (2 Hz) electrical stimulation the mobility of intracellular vesicle increased more than 20%, but remained unchanged under intermediate (20 Hz) or higher (200 Hz) frequency stimulation. We demonstrated a frequency-dependent effect of electrical stimulation on the mobility of astrocytic intracellular vesicles. We suggest a novel mechanism of brain modulation that electrical signals in the lower range frequencies embedded in brainwaves modulate the functionality of astrocytes for brain homeostasis and memory consolidation. The finding suggests a physiological mechanism whereby endogenous low-frequency brain oscillations enhance astrocytic function that may underlie some of the benefits of slow-wave sleep and highlights possible medical device approach for treating neurological diseases.
Keywords: slow-wave sleep, astrocytes, electrical stimulation, intracellular vesicles
1. INTRODUCTION
Mounting evidence suggests that sleep is a basic brain function promoting memory-consolidation and brain metabolite clearance [1–5]. Sleep is commonly classified into rapid eye movement (REM) and non-rapid eye movement (NREM) stages that are characterized by the frequency and pattern of brain electrical activity, called the electroencephalogram (EEG). NREM sleep includes three stages; with the stage III being called slow-wave sleep (SWS) which is characterized by slow-wave activity (SWA). SWA occurs when cortical neurons oscillate at low-frequency (1 – 4 Hz) between a hyperpolarized down-state and neuronal silence and a depolarized up-state and active neuronal firing [6].
Several independent lines of evidence support the notion that the effect of sleep on metabolite clearance is associated with brain waves at certain frequencies ranges. First, the rate of β-amyloid (Aβ) clearance in mice is improved during sleep and this improvement is associated with an increase in the prevalence of slow waves in sleep [3]; Second, the Aβ/tau levels in the cerebrospinal fluid (CSF) are inversely correlated with SWA [7]; Third, slow waves are coupled to blood oxygen level-dependent signals and pulsatile CSF oscillations during NREM sleep [2], and the pulsatile CSF oscillations increase brain fluid mixing and diffusion in the fluid. Thus the SWS brain state shows improved clearance of brain waste products [8, 9] and is also important in memory consolidation [10]. On the other hand, low-frequency EEG oscillations in SWS reflect the underlying transitions between cortical up and down states [11. However, whether the endogenous slow-waves recorded with EEG have any direct mechanistic role in metabolite clearance and memory consolidation has remained elusive.
The glial astrocyte has been selected as a targeting subject to study the effect of brain waves on metabolic homeostasis and memory consolidation because, in the central nervous system (CNS) astrocytes are believed to play an important role on removing excess of toxic waste, recycling neurotransmitters, transporting major ions, modulating neuronal excitability, and promoting synaptic remodeling [12]. Cellular functions of astrocytes can be monitored by their activities of intracellular vesicles. Various types of intracellular vesicles serve a variety of functions [13], such as transporting proteins and other materials within a cell, carrying compounds (enzymes, hormones, and carbohydrates) to be secreted, and involving digestion and waste removal. Intracellular vesicles in astrocytes exhibited two types of mobility, directional or non-directional [14]. The directional movement appears almost rectilinear, whereas the non-directional movement moves in Brownian motion.
Here, we utilized total internal reflection fluorescence microscopy (TIRFM) to study the frequency-dependent effect on the mobility of astrocytic intracellular vesicles. We applied electrical stimulation within a range of frequency (2, 20, 200Hz) on cultured astrocytes expressing GFP-labelled membrane protein (VAMP3 or CD63), and found that the mobility of intracellular vesicles in both directional and non-directional movement increased more than 20% under 2 Hz electrical stimulation, but remained unchanged under higher frequencies (20 Hz or 200 Hz) of electrical stimulation. This finding indicates that only slow waves elevate the intracellular activity of astrocytes, suggesting a novel glial mechanism of electrical brain activity that the functionality of astrocytes in brain homeostasis and memory formation can be directly modulated by electrical signals embedded in endogenous low-frequency brainwaves during SWS.
2. MATERIALS AND METHODS
2.1. Cell cultures
Animal experiments complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Mayo Clinic Institutional Animal Care and Use Committee. Pregnant Lewis rats were purchased from Harlan Sprague Dawley (Indianapolis, IN, USA). Astrocyte cultures were established from the cerebral cortices of fetal rats (embryonic days 18-21) as previously described [15]. Astrocytes cultures were maintained in a growth medium composed of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS), 1 mM sodium pyruvate, 100 IU/mL of penicillin, 100 μg/mL of streptomycin, and 292 μg/mL of L-glutamine at 37°C in a 5% CO2-95% air incubator. Microglia depletion was achieved by adding 100 μg/mL of Clodrosome (Encapsula NanoSciences, Brentwood, TN, USA) to the growth medium for 12 h, then replacing with fresh medium for 48 h, and confirmed by Iba1 immunostaining (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan).
2.2. Cell transfection
Astrocytic cultures transfection was achieved using TransIT-293 (Mirus, Madison, WI, USA). Plasmid encoding EGFP-VAMP3 was a gift from Dr. Thierry Galli (Addgene plasmid # 42310) [16] and CD63-pEGFP C2 was a gift from Paul Luzio (Addgene plasmid # 62964). Plasmid DNA was prepared using HiSpeed Plasmid Maxi Kit (QIAGEN, Germantown, MD, USA). The purified DNA was incubated for 20 min at room temperature with TransIT-293 in Gibco™ Opti-MEM reduced-serum medium. The mixture (130 μL) was dispersed into a 35 mm poly-D-lysine coated glass-bottom dishes (P35GC-1.0-14C, MatTek, Ashland, MA, USA) of astrocytes in complete growth medium (2.5 mL). And then the astrocytes in the medium were incubated for 2-3 days at 37°C in a 5% CO2-95% air incubator. Cell density was 0.6 – 1.0 × 105 count/dish.
2.3. Electrical stimulation of astrocytes
Electrical stimulation was applied to astrocytes using a stimulus isolator (A365, World Precision Instruments, Sarasota, FL, USA) and a digitizer (Axon Digidata 1440A, Molecular Devices, San Jose, CA, USA). The bipolar current output from stimulus isolator A365 was converted to constant voltage output with a 1KΩ bridging resistor, and then connected to a perfusion insert designed for electric field stimulation on 35 mm culture dishes (RC-37FS, Warner Instruments, Holliston, MA, USA) that provides 5 mV/mm field strength in the open region of the insert, which is analogous to endogenous electric fields of 2.5–5.0 mV/mm that have been recorded within mammalian brain [17, 18]. Five minutes of electrical stimulation was applied for each experiment. We selected three frequencies, 2 Hz, 20 Hz, and 200 Hz, all have 0.1 ms pulse width. To ensure that final delivered electrical energy are at the same level for all three frequencies during 5 mins stimulation, we applied exact number of pulses by inserting longer gaps at higher frequencies to achieve 600 total pulses for each experiment.
2.4. Imaging acquisition and processing
Cells were observed by using an inverted microscope (Axio Observer Z1, ZEISS, Oberkochen, Germany) equipped with total internal reflection fluorescence (TIRF) illumination (see Figure S1) in supplement that is confined to the glass surface with an evanescent field in ~100 nm depth penetrating cells setting above the glass surface [19]. An excitation wavelength of 488 nm was provided by a 100 mW argon laser. Images were captured through a 100×/1.46 alpha Plan-Apochromat oil-immersion objective (ZEISS) and a CMOS camera with a pixel size of 6.5 μm × 6.5 μm (ORCA-flash4.0, HAMAMATSU, Hamamatsu City, Japan) driven by ZEN imaging software. TIRF imaging were recorded at 100 ms/frame with a 512 ×512-pixel field of view. The growth medium was replaced with phosphate-buffered saline (PBS) supplemented with 0.9 mM CaCl2 and 0.5 mM MgCl2 before imaging acquisition. All experiments were performed at 37°C. Dia Track software [20] was used to localize and track movements of all detected vesicles. Based on a directionality index (a ratio comparing the maximal displacement to the total track length 14), we categorized movements of vesicles into two groups: directional (index >= 0.5) and non-directional (index < 0.5). Custom Matlab codes were used to categorize and calculate the speed of vesicle movements. Tracks with less than 10 continuous frames were excluded from speed calculation.
All experiments were performed at 37 °C using the CO2 incubation system equipped with the Zeiss microscope. Temperature of solution inside the sample dish was also monitored at ±0.1°C accuracy (TC-324C, Warner Instruments, Holliston, MA, USA) during electrical stimulation. No change of the temperature was detected.
2.5. Statistics
Experiments with/without electrical stimulation were repeated 4-5 times for both EGFP-CD63 and EGFP-VAMP3 transfected astrocytes. Cells from one culture dish were used only for one experiment. All data were presented as mean ± standard deviation unless specified otherwise. Significance was tested using one-way ANOVA in Matlab (MathWorks, Natick, MA, USA).
3. RESULTS
To address the electrical frequency-dependent effect on the mobility of intracellular vesicles, we selected three different frequencies (2 Hz as the low, 20 Hz as the intermediate and 200 Hz as the high) for electrical stimulation, and compared resulting movements of vesicles to that obtained when no electrical stimulation was applied. Each experiment was taken following the same timeline: two minutes of pre-stimulation, five minutes stimulation, and then another five minutes of post-stimulation. TIRF samplings were all collected in one-minute duration. Two consecutive samplings were performed before the stimulation; during the 5-minute stimulation we collected three samplings separated by two one-minute gaps to reduce photo-bleaching; and then we recorded four consecutive samplings one minute after when electrical stimulation was complete. We observed directional and non-directional movements of intracellular vesicles in astrocytes, same as the previous report[14]. The mobility of non-directional vesicles can be described well as free Brownian diffusion and the tracks of directional vesicles appear almost rectilinear (Figure 1).
Figure 1.
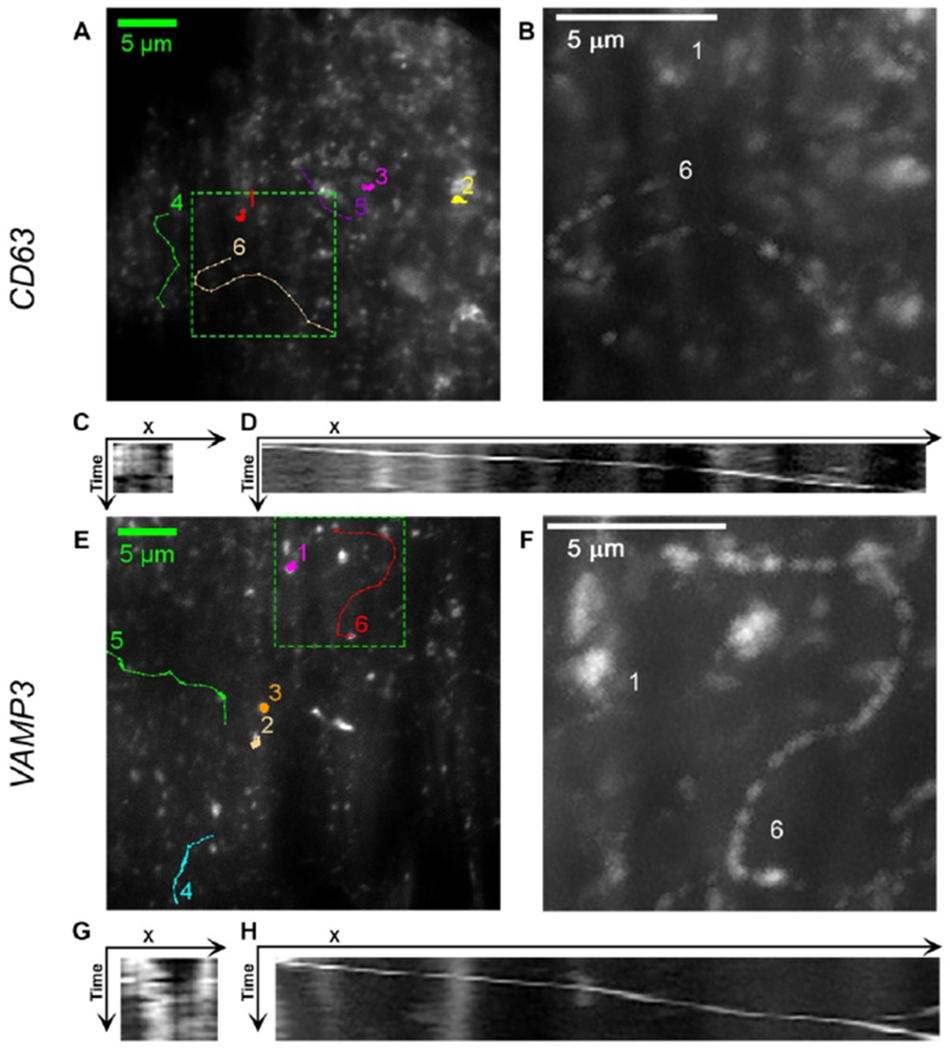
Images of EGFP-CD63 (A, B, C, D) and EGFP-VAMP3 (E, F, G, H) labeled vesicles. (A, E) Trajectories of EGFP-CD63 and EGFP-VAMP3 labeled vesicles. (B, F) Maxium intensity Z-projection of the tracks 1 and 6 in the region surrounded by dashed line rectangle in A) and E). (C, G) Kymograph of the movements of vesicle in track 1 in A) and E). (D, H) Kymograph of the movements of vesicle in track 6 in A) and E). Track 1, 2, and 3 are the trajectories of vesicles in non-directional movement. Track 4, 5, and 6 are the trajectories of vesicles in directional movement. Kymographs were generated using Multi Kymograph plugin in ImageJ.
Results are presented in normalized rates that compare every speed measurements to a common scale achieved by averaging two starting samplings of each experiment. For VAMP3 labeled vesicles, the average speeds of two starting samplings were 1.03 ± 0.06 μm/s (non-directional) and 1.27 ± 0.06 μm/s (directional). For CD63 labeled vesicles the average speeds of two starting samplings were 1.24 ± 0.06 μm/s (non-directional) and 1.29 ± 0.05 μm/s (directional), respectively. Normalized rates of average speeds are illustrated in Figure 2 (non-directional) and Figure 3 (directional).
Figure 2.
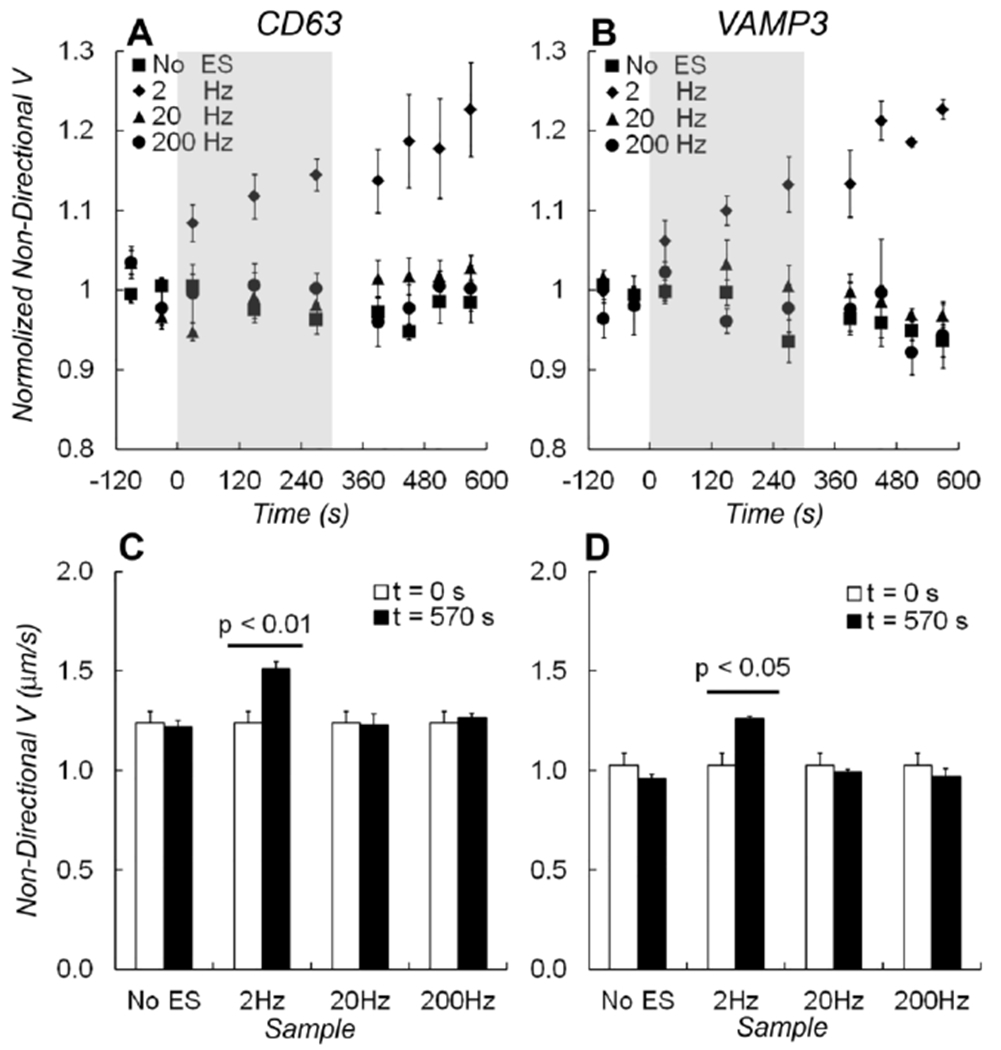
Average speeds of non-directional movement Vnon-d. The normalized speeds for non-directional movement of A) EGFP-CD63 and B) EGFP-VAMP3 labeled vesicles during the experiment timeline. Electrical stimulations were applied at 2 Hz (diamonds), 20 Hz (triangles), 200 Hz frequency (circles), or without stimulation (squares). The grey shades indicated the time when electrical stimulation was applied. C) A bar graph shows changes of Vnon-d measured before (at t = 0 s) and after (at t = 570 s) electrical stimulations from EGFP-CD63 labeled vesicles. D) A bar graph shows changes in Vnon-d measured from EGFP-VAMP3 labeled vesicles. One-way ANOVA test showed that the average speed of non-directional vesicles changed significantly after 2 Hz electrical stimulation (p < 0.01 in EGFP-CD63 data and p < 0.05 in EGFP-VAMP3 data). The changes in speed caused by electrical stimulation at 20 Hz and 200 Hz frequency, or without stimulations were insignificant.
Figure 3.
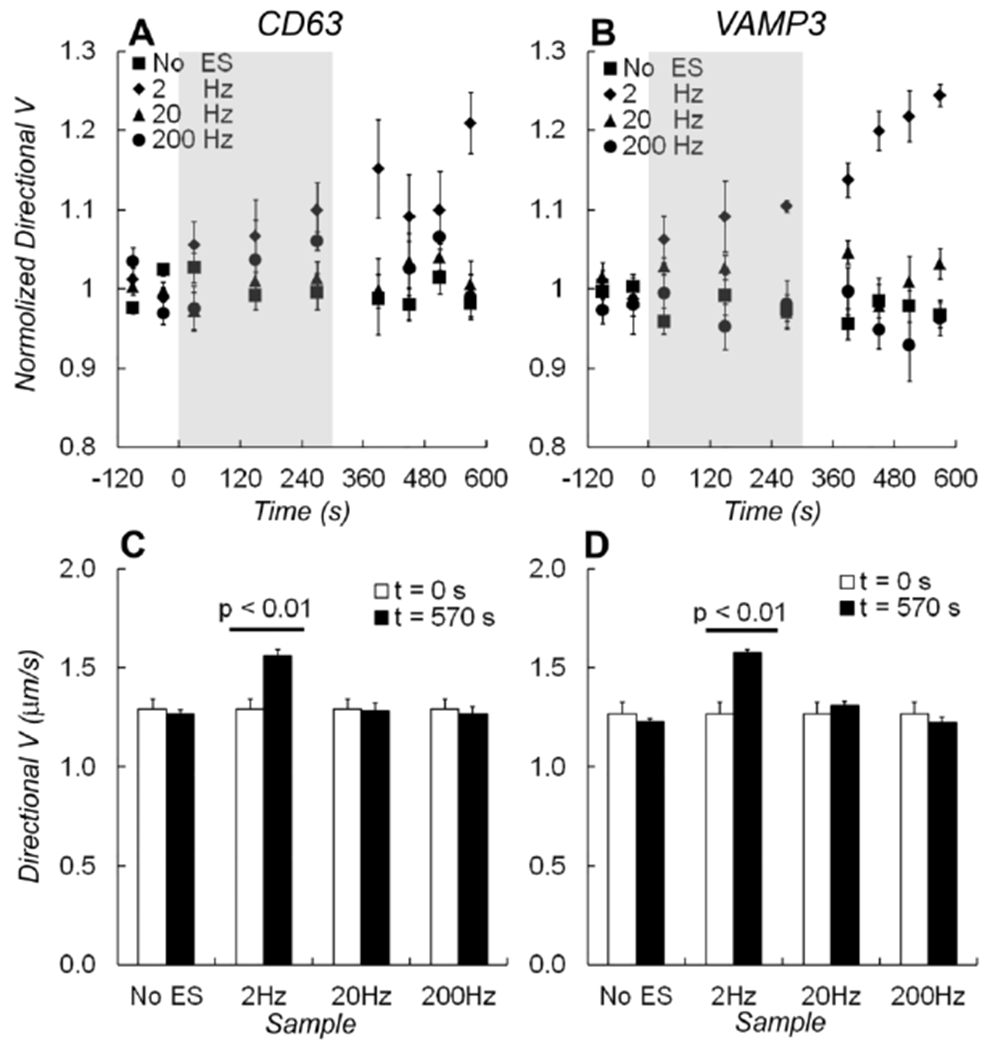
Average speeds of directional movement Vd. The normalized speeds for directional movement of A) EGFP-CD63 and B) EGFP-VAMP3 labeled vesicles during the experiment timeline. Electrical stimulations were applied at 2 Hz (diamonds), 20 Hz (triangles), 200 Hz frequency (circles), or without stimulation (squares). The grey shades indicated the time when electrical stimulation was applied. C) A bar graph shows changes of Vd measured before (at t = 0 s) and after (at t = 570 s) electrical stimulations from EGFP-CD63 labeled vesicles. D) A bar graph shows changes in Vd measured from EGFP-VAMP3 labeled vesicles. One-way ANOVA test showed that the average speed of non-directional vesicles changed significantly after 2 Hz electrical stimulation (p < 0.01). The changes in speed caused by electrical stimulation at 20 Hz and 200 Hz frequency, or without stimulations were insignificant.
For non-directional vesicles, the average speed (Vnon-d) increased immediately when 2 Hz electrical stimulation started. It kept increasing linearly during the stimulation course and 2 ~ 3 minutes later after the stimulation ended (Fig. 2A and 2B). With 2 Hz electrical stimulation, the total increase of Vnon-d was 23% for CD63 labeling vesicles (Fig. 2A) and 21% for VAMP3 labeling vesicles (Fig. 2B), respectively. To compare measurements made before and after electrical stimulation, one-way ANOVA test showed a significant increase (p = 0.007 in CD63 data, p = 0.024 in VAMP3 data. Analysis details are in the supplement table S1). However, without electrical stimulation or with electrical stimulations in 20 Hz or 200 Hz, changes in average speeds for both CD63- and VAMP3- labeling vesicles are insignificant (Fig. 2C, 2D). These results for non-directional vesicles indicated that only 2 Hz electrical stimulation caused a significant increase of Vnon-d for both CD63 and VAMP3 containing vesicles.
For directional vesicles, the average speed (Vd) was also significantly increased under 2 Hz electrical stimulation (Fig. 3A, 3B). Vd increased 20% for CD63 labeled vesicles and 22% for VAMP3 labeled vesicles, respectively (p = 0.004 in CD63 data, and p = 0.002 in VAMP3 data. Analysis details are in the supplement table S1). There was no significant change in Vd with 20 Hz or 200 Hz electrical stimulation (Fig. 3C, 3D).
To examine time-dependent changes of the speed caused by electrical stimulation, we compared average speed at each time point (1, 3, 5 minutes of electrical stimulation) with one-way ANOVA followed by a Tukey-Kramer post hoc comparison (Fig. 4). Our analysis displayed no significant changes at the 1st minute of electrical stimulation in all three stimulation frequencies (Fig. 4A1, 4B1, 4C1, 4D1); but displayed significant changes for non-directional vesicles at the 3rd minute (Fig. 4A2, 4B2, p = 0.013 in CD63 data, and p = 0.015 in VAMP3 data) and at 5th minute for both non-directional and directional vesicles (Fig. 4A3, 4B3, p = 5.1×10−5 in CD63 data, and p = 0.006 in VAMP3 data; Fig. 4C3, 4D3, p = 0.038 in CD63 data, and p = 0.006 in VAMP3 data) in 2 Hz electrical stimulation. Analysis details are also listed in supplement table S1.
Figure 4.
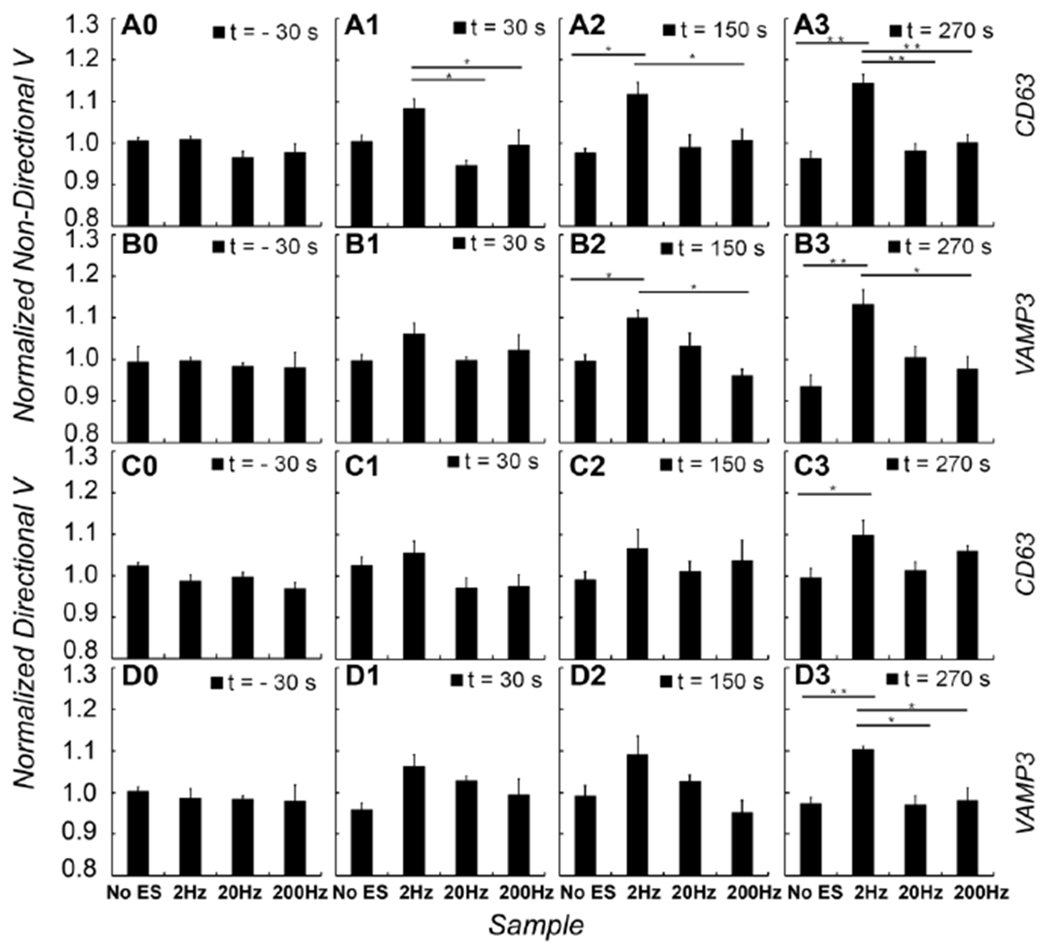
Relationships between the duration of electrical stimulation and changes in the speeds of vesicles. The effects of electrical stimulation on the speeds of vesicles were assessed at each time point. Bar graphs show normalized speeds of EGFP-CD63 (A0, A1, A2, A3) and EGFP-VAMP3 (B0, B1, B2, B3) labeled vesicles in non-directional movement, and normalized speeds of EGFP-CD63 (C0, C1, C2, C3) and EGFP-VAMP3 (D0, D1, D2, D3) labeled vesicles in directional movement. Four representative time points are corresponding to one minute before the electrical stimulation is applied (t= − 30 s), the 1st minute (t = 30 s), the 3rd minute (t = 150 s), and the 5th minute (t = 270 s) when electrical stimulation was applied, respectively. At each time point, normalized speeds of any pair of the four conditions (No ES, 2Hz, 20Hz and 200Hz ES) were compared with one-way ANOVA followed by a Tukey-Kramer post hoc comparison. *p < 0.05; **p < 0.01.
4. DISCUSSIONS
In this work, we investigated the frequency-dependent effect of externally applied electrical stimulation on the trafficking of astrocytic intracellular vesicles. Using EGFP tagged membrane proteins (CD63 and VAMP3), we found that the low frequency 2 Hz electrical stimulation increases the mobility for both directional and non-directional vesicles by more than 20%, but both the intermediate (20 Hz) and high (200 Hz) frequency electrical stimulation has no effect. We concluded that the intracellular activity of astrocytes can be elevated by electrical slow waves.
CD63 and VAMP3 reside in various types of intracellular vesicles and are involved in different processes of substance degradation and transportation. CD63 mainly resides in lysosomes and multivesicular bodies (MVBs), with a small pool on the cell surface [21]. CD63 containing vesicles in astrocytes are involved in the regulation of ATP release [22]. VAMP3 can be found in early endosomes, recycling endosomes, and intermediate transport vesicles [23]. VAMP3 containing vesicles in astrocytes participate in the recycling of plasma membrane glutamate transporters and in modulating the efficacy of glutamate uptake [24].
The increased mobility of intracellular vesicles caused by 2 Hz electrical stimulation is likely nonspecific to CD63 or VAMP3. Low-frequency oscillations in electrical fields may improve the mobility of vesicles carrying other molecules as well, including neurotransmitters, neurotransmitter transporters, neuromodulators, hormones and peptides, and the specific water channel of aquaporin-4 [25]. These substances secreted by astrocytes contribute to CNS homeostasis and development.
Astrocytes release their substances through several distinct pathways, including diffusion through plasma membrane channel, translocation by multiple transporters, and regulated exocytosis [13]. The mobility of intracellular vesicles is a limiting fact controlling the substance release [26]. Thus, increased mobility of intracellular vesicles leads to an enhanced release of astrocytic cargo and therefore affects CNS homeostasis and development.
Our finding that the low-frequency electrical stimulation elevates the intracellular activity in astrocytes is a possible mechanism by which the endogenous SWA supports cellular homeostasis. The brainwave frequencies vary in a wide range between sleep-wake cycles. The effect of sleep on metabolite clearance is stronger during the most restorative deep sleep stage with more low-frequency (< 4 Hz) EEG oscillations [3–5, 7].
Astrocytes are important to remove the excess of toxic waste in the CNS and maintain neurotransmitters homeostasis [12]. Glymphatic pathway [8, 9] enables exchange between the para-arterial space and the interstitial space and allows waste products to be removed away from the CNS. It is facilitated by the AQP4 water channels presented on the astrocytic endfeet. A recent study demonstrated that pulsatile CSF oscillations inflow to brain ventricles every 20 s during SWS [2], which facilitates the clearance of waste products during SWS. Also, the clearance rate of Aβ and the interstitial space volume in mouse brain increase during sleep associated with an increase in the prevalence of slow waves [3]. Our finding on the elevation of intracellular activity in astrocytes induced by low-frequency electrical stimulation may explain why this increase of waste clearance occurs in SWS.
The traffic deficiency of astrocytic intracellular vesicles is associated with many neurological diseases [27]. Using electrical stimulations to enhance the traffic of intracellular vesicles may prove to have therapeutic benefits.
The molecular mechanism for the frequency-dependent effect of electrical stimulation on the mobility of intracellular vesicles remains unclear. Cytoskeleton and motor proteins could be involved in the directional mobility, whereas the diffusion in local microenvironments of cytoplasm might affect the non-directional movement of vesicles [14, 28–30]. The rate of vesicle diffusion is determined by hydrodynamic interactions, vesicle collisions, cytoplasm viscosity, and other factors [31]. Interestingly, the vesicles mobility didn’t immediately fall back to the original value after 2 Hz stimulation completed. It suggests that the mobility increase caused by 2 Hz stimulation does not come from direct interaction between vesicles and electrical field. The mobility of vesicles can be changed by any factors that affect the function of cytoskeleton and motor proteins or the rate of vesicles diffusion. The 2 Hz stimulation triggered the change of some of those factors, and this change couldn’t be restored immediately after the stimulation completed. Further investigations are needed.
Besides the mobility of intracellular vesicles, the profiles of extracellular vesicles (EVs) size distribution, EV surface proteins, and EV-carrying microRNAs can also be affected by externally applied electrical stimulation with a frequency dependence [32].
This report, to the best of our knowledge, demonstrates for the first time that the mobility of intracellular vesicles in astrocytes can be increased only at a low-frequency electrical stimulation. This frequency-dependent effect of electrical fields on cellular functionalities likely applies to other cell types, such as neurons, microglial, or even cancer cells.
In summary, we reported our study on the trafficking of intracellular vesicles in astrocytes under externally applied electrical stimulation at different frequencies. We found that the mobility of intracellular vesicles for both directional and non-directional movements was elevated by more than 20% with low frequency (2 Hz) electrical stimulation, but remained unchanged with electrical stimulations at an intermediate (20 Hz) or higher frequency (200 Hz). This finding of the frequency-dependent effect on astrocytes provides a unique glial mechanism that may be important for both brain homeostasis and memory consolidation, and also have therapeutic implications of medical devices to provide electrical brain stimulation. It certainly extends our understanding of the basic biology about why deep sleep is the most restorative stage of sleep. Further investigations on the molecular mechanisms of how electrical fields modulate cellular functions are needed.
Supplementary Material
Highlights:
We applied electrical stimulation to cultured glial astrocytes while monitored the trafficking of GFP-tagged intracellular vesicles using TIRFM.
We found a frequency-dependent effect of electrical stimulation that low-frequency electrical stimulation elevates the mobility of astrocytic intracellular vesicles.
We suggest a novel mechanism of brain modulation that electrical signals in the lower range frequencies embedded in brainwaves modulate the functionality of astrocytes for brain homeostasis and memory formation.
ACKNOWLEDGMENTS
We thank Dr. Vanda A Lennon’s group who prepared rat astrocyte cultures.
Funding:
This work was supported by an NIH BRAIN initiative R01 grant to HLW and GAW (NS112144 from NINDS and NIMH) and the Minnesota Partnership for Biotechnology and Medical Genomics (MNP #17.16).
Footnotes
CONFLICT OF INTEREST
The authors HLW and GW have a pending patent (US2019-012375) on the application of electric fields for modulating astrocytic function.
DATA AVAILABILITY STATEMENT
The partial data set to support the findings of this study are available on Zenodo with the fingerprint ‘md5:fe22e942a527dc4884e15bcb71026cce’. A full data set is available from the corresponding author upon reasonable request.
REFERENCES
- 1.Diekelmann S, Born J, The memory function of sleep. Nat Rev Neurosci 11, 114–126 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Fultz NE et al. , Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 366, 628–631 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie L et al. , Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mander BA et al. , beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci 18, 1051–1057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holth JK et al. , The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363, 880–884 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steriade M, Timofeev I, Grenier F, Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol 85, 1969–1985 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Varga AW et al. , Reduced Slow-Wave Sleep Is Associated with High Cerebrospinal Fluid Abeta42 Levels in Cognitively Normal Elderly. Sleep 39, 2041–2048 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iliff JJ et al. , A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4, 147ra111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nedergaard M, Neuroscience. Garbage truck of the brain. Science 340, 1529–1530 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilckens KA, Ferrarelli F, Walker MP, Buysse DJ, Slow-Wave Activity Enhancement to Improve Cognition. Trends Neurosci 41, 470–482 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez-Vives MV, McCormick DA, Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3, 1027–1034 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Verkhratsky A, Nedergaard M, Physiology of Astroglia. Physiol Rev 98, 239–389 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verkhratsky A, Matteoli M, Parpura V, Mothet JP, Zorec R, Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. Embo J 35, 239–257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potokar M, Kreft M, Pangrsic T, Zorec R, Vesicle mobility studied in cultured astrocytes. Biochem Biophys Res Commun 329, 678–683 (2005). [DOI] [PubMed] [Google Scholar]
- 15.McCarthy KD, de Vellis J, Alpah-adrenergic receptor modulation of beta-adrenergic, adenosine and prostaglandin E1 increased adenosine 3[ISP CHK]′:5[ISP CHK]′-cyclic monophosphate levels in primary cultures of glia. J Cyclic Nucleotide Res 4, 15–26 (1978). [PubMed] [Google Scholar]
- 16.Galli T et al. , A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol Biol Cell 9, 1437–1448 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frohlich F, McCormick DA, Endogenous electric fields may guide neocortical network activity. Neuron 67, 129–143 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu C, Shivacharan RS, Zhang M, Durand DM, Can Neural Activity Propagate by Endogenous Electrical Field? J Neurosci 35, 15800–15811 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Axelrod D, Burghardt TP, Thompson NL, Total internal reflection fluorescence. Annu Rev Biophys Bioeng 13, 247–268 (1984). [DOI] [PubMed] [Google Scholar]
- 20.Vallotton P, Olivier S, Tri-track: free software for large-scale particle tracking. Microsc Microanal 19, 451–460 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Pols MS, Klumperman J, Trafficking and function of the tetraspanin CD63. Exp Cell Res 315, 1584–1592 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z et al. , Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol 9, 945–953 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Ropert N, Jalil A, Li D, Expression and cellular function of vSNARE proteins in brain astrocytes. Neuroscience 323, 76–83 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Li D et al. , Astrocyte VAMP3 vesicles undergo Ca2+ -independent cycling and modulate glutamate transporter trafficking. J Physiol 593, 2807–2832 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vardjan N, Verkhratsky A, Zorec R, Pathologic potential of astrocytic vesicle traffic: new targets to treat neurologic diseases? Cell Transplant 24, 599–612 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Burke NV et al. , Neuronal peptide release is limited by secretory granule mobility. Neuron 19, 1095–1102 (1997). [DOI] [PubMed] [Google Scholar]
- 27.Zorec R, Parpura V, Verkhratsky A, Astroglial vesicular network: evolutionary trends, physiology and pathophysiology. Acta Physiol (Oxf) 222, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potokar M et al. , Cytoskeleton and vesicle mobility in astrocytes. Traffic 8, 12–20 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Hehnly H, Stamnes M, Regulating cytoskeleton-based vesicle motility. Febs Lett 581, 2112–2118 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian T et al. , Dynamics of exosome internalization and trafficking. J Cell Physiol 228, 1487–1495 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Rothman JS, Kocsis L, Herzog E, Nusser Z, Silver RA, Physical determinants of vesicle mobility and supply at a central synapse. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Melvin R, Bemis LT, Worrell GA, Wang H-L, Programmable Modulation for Extracellular Vesicles. bioRxiv, 566448 (2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


