Abstract
Inhalation toxicity testing, which provides the basis for hazard labeling and risk management of chemicals with potential exposure to the respiratory tract, has traditionally been conducted using animals. Significant research efforts have been directed at the development of mechanistically based, non-animal testing approaches that hold promise to provide human-relevant data and an enhanced understanding of toxicity mechanisms. A September 2016 workshop, “Alternative Approaches for Acute Inhalation Toxicity Testing to Address Global Regulatory and Non-Regulatory Data Requirements”, explored current testing requirements and ongoing efforts to achieve global regulatory acceptance for non-animal testing approaches. The importance of using integrated approaches that combine existing data with in vitro and/or computational approaches to generate new data was discussed. Approaches were also proposed to develop a strategy for identifying and overcoming obstacles to replacing animal tests. Attendees noted the importance of dosimetry considerations and of understanding mechanisms of acute toxicity, which could be facilitated by the development of adverse outcome pathways. Recommendations were made to (1) develop a database of existing acute inhalation toxicity data; (2) prepare a state-of-the-science review of dosimetry determinants, mechanisms of toxicity, and existing approaches to assess acute inhalation toxicity; (3) identify and optimize in silico models; and (4) develop a decision tree/testing strategy, considering physicochemical properties and dosimetry, and conduct proof-of-concept testing. Working groups have been established to implement these recommendations.
Keywords: Acute inhalation toxicity, Alternative approaches, Dosimetry, In vitro, In silico, Globally harmonized system (GHS) additivity, formula
1. Introduction
Inhalation is a major route of human exposures for substances such as particles, fibers, nanomaterials, gases, and volatile organic chemicals. As the respiratory tract serves as both target tissue and portal of entry (POE) from the external environment to the systemic circulation for these substances, it is important to characterize the hazards they may present. Historically, acute inhalation toxicity tests expose rodents in whole-body or nose-only systems for 24 h or less to identify substances that could cause toxicity after a short-term exposure. Several critical differences between the rodent and human respiratory tract have the potential to affect the precision with which the rodent test predicts the human dose and response. These differences include (1) respiratory physiology (e.g., breathing mode and ventilation rates; metabolic rates), (2) anatomy (e.g., airway architecture and branching pattern; cell types and composition within the regions of the respiratory tract), and (3) biochemistry (e.g., composition and capacity of biotransformation enzymes) (Prytherch and BéruBé, 2014a; Parent, 2015). To explore approaches with the potential for a more accurate prediction of human response, recent research has focused on the development and application of human-relevant in vitro and in silico methods that can be used in testing approaches that facilitate a mechanistic understanding of the toxic effects of inhaled materials (Loizou et al., 2008; Fröhlich and Salar-Behzadi, 2014).
Recommendations from a 2015 workshop, “Alternative Approaches for Identifying Acute Systemic Toxicity: Moving from Research to Regulatory Testing,” highlighted a need to identify and further develop approaches that could reduce or replace animal use for acute inhalation toxicity testing (Hamm et al., 2017). To address this need, an international group of experts convened at a workshop on September 22–23, 2016 to discuss progress and challenges associated with the development, validation, and implementation of alternatives. The product of this workshop was a defined strategy for further development and implementation of approaches to acute inhalation toxicity testing that would reduce or replace animal use for both regulatory and non-regulatory purposes. In advance of the workshop, a six-part webinar series was organized to review the state-of-the-science of non-animal approaches to acute inhalation toxicity testing (Table 1).
Table 1.
List of webinar and workshop presentations. Presentation slides and webinar recordings can be found online at www.piscltd.org.uk/acute-inhalation-toxicity.
| Webinars | ||
|---|---|---|
| Speaker | Affiliation | Title |
| Ian Indans | Health and Safety Executive, UK | Acute Inhalation Toxicity Testing: The 3Rs, Current Needs and Future Prospects. |
| Jon Hotchkiss | The Dow Chemical Company | A Primer on Acute Inhalation Toxicity Testing: Where do Alternative Methods Fit? |
| Marianna Gaca | British American Tobacco | Acute Inhalation Toxicity: In Vitro and Ex Vivo Systems. |
| Annie Jarabek | U.S. EPA, National Center for Environmental Assessment, ORD | What a Difference the Dose Makes: Dosimetry Approaches to Aid Experimental Design, Evidence Integration, and Inferences for Risk Assessment. |
| Grace Patlewicz | U.S. EPA, National Center for Computational Toxicology, ORD | State-of-the-Science and Practical Application of In Silico Methods. |
| Daniel Wilson | The Dow Chemical Company | In Silico Approaches for Acute Inhalation Toxicity. |
| Marco Corvaro | Dow AgroSciences | GHS Additivity Approach to Classify Mixtures based on Ingredient Toxicity. A Case Example: Agrochemical Formulations. |
| Mathieu Vinken | Free University of Brussels | Adverse Outcome Pathways as Tools to Assess Chemical-Induced Toxicity. |
| Barbara Buckley | U.S. EPA, National Center for Environmental Assessment, ORD | A Conceptual Model for Assessing Criteria Air Pollutants in a Multipollutant Context: A Modified Adverse Outcome Pathway Approach. |
| Kelly BéruBé | School of Biosciences, Cardiff University | The NHBE and Metabo-Lung™ Models: Normal and Metabolizing In Vitro Alternatives for Lung Research. |
| Dongeun Huh | University of Pennsylvania | Microengineered Physiological Bio-mimicry: Human Organs-on-Chips. |
| Workshop presentations | ||
| Jon Hotchkiss | The Dow Chemical Company | The Case for an Integrated Approach to Acute Inhalation Toxicity Testing and Assessment |
| Dan Wilson | The Dow Chemical Company | An Alternative Framework for Acute toxicity using Mechanistic In Silico and In Vitro Approaches |
| Paul Hinderliter | Syngenta | An Alternative Approach for Evaluating the Human Health Risk from Exposure to an Irritant Aerosol |
| Michael Bartels | ToxMetrics.com, LLC | Toxicokinetics in Risk Assessment: Evaluation of In Silico Approaches |
| Miyoung Yoon | ScitoVation, LLC | Assessing Bioavailability and Systemic Delivery of Inhaled Compounds: Current Status and Future Directions |
| Anna Lowit | U.S. EPA Office of Pesticide Programs | U.S. EPA OPP Regulatory Perspective on Acute Inhalation Toxicity Testing |
| Iris Camacho | U.S. EPA Office of Pollution Prevention and Toxics | U.S. EPA OPPT Regulatory Perspective on Acute Inhalation Toxicity Testing |
| Grace Patlewicz | U.S. EPA, National Center for Computational Toxicology, ORD | ICCVAM’s Vision and Strategy for Acute Toxicity Testing |
This paper aims to summarize the presentations and discussions that took place during the webinars and workshop. Specifically, it describes (1) the current regulatory and non-regulatory needs for acute inhalation toxicity data; (2) the information obtained from acute animal inhalation tests that is currently used by regulators to define hazard, in order to determine what information is needed from alternative approaches; (3) the mechanistic determinants of dosimetry and toxic effects as these will have a large bearing on the development and application of alternative approaches; (4) what alternative approaches are currently used by companies for in-house decisions and/or accepted for regulatory purposes; (5) the data gaps or other issues that are precluding the uptake and acceptance of alternative approaches; and (6) the final recommendations agreed on at the workshop.
2. Regulatory and non-regulatory needs for acute inhalation toxicity data
Acute inhalation toxicity data are used for both regulatory and non-regulatory purposes. For regulatory purposes, data are used in hazard identification as part of product or substance registration; classification and labeling; determining handling and shipping requirements; and providing information for safety data sheets. Non-regulatory purposes for which data may be used include development of risk assessments to determine short-term occupational exposure levels and emergency response values to inform first responders in cases of an unexpected release or accident. Other applications for which data may be used include product stewardship; filling data gaps by read-across (i.e., applying data from one substance(s) to predict the same property or effect for a ‘similar’ substance); assessing the impact of alterations in the safety profile due to product reformulation; and determining when development of products should progress or be halted due to toxicity concerns.
In a global economy, achieving international harmonization is a significant challenge. Many regulatory authorities require acute inhalation toxicity data (Fig. 1). While there are differences in specific testing requirements across regional regulatory authorities, the general principles of the acute inhalation toxicity testing guidelines are discussed below.
Fig. 1.
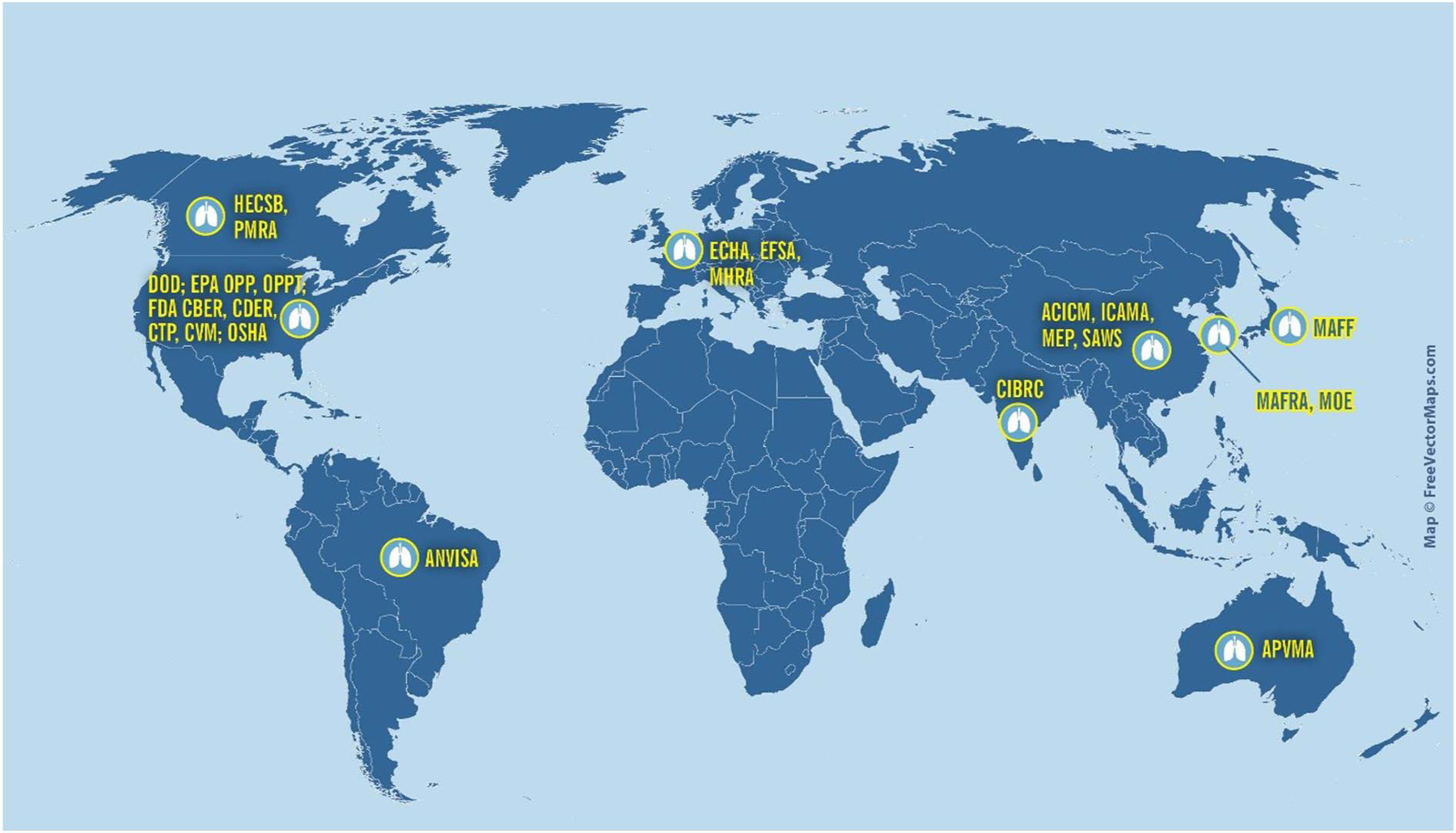
Agencies that require or use acute inhalation data. Abbreviations:
ACICM = Chinese Association of International Chemical Manufacturers;
ANVISA = Brazilian Agência Nacional de Vigilância Sanitária (The National Health Surveillance Agency);
APVMA = Australian Pesticides and Veterinary Medicines Authority;
CIBRC = Indian Central Insecticide Board & Registration Committee,
DOD = Department of Defense;
ECHA = European Chemicals Agency;
EFSA = European Food Safety Authority;
EPA OPP = United States Environmental Protection Agency Office of Pesticide Programs;
EPA OPPT = United States Environmental Protection Agency Office of Pollution Prevention and Toxics;
FDA CBER = United States Food and Drug Administration Center for Biologics Evaluation and Research;
FDA CDER = United States Food and Drug Administration Center for Drug Evaluation and Research;
FDA CTP = United States Food and Drug Administration Center for Tobacco Products;
FDA CVM = United States Food and Drug Administration Center for Veterinary Medicine;
HECSB = Canadian Healthy Environments and Consumer Safety Branch;
ICAMA = Chinese Institute for the Control of Agrochemicals, Ministry of Agriculture;
MAFF = Japanese Ministry of Agriculture, Forestry and Fisheries;
MAFRA = South Korean Ministry of Agriculture, Food and Rural Affairs;
MEP = Chinese Ministry of Environmental Protection;
MHRA = United Kingdom Medicines & Healthcare Products Regulatory Agency;
MOE = South Korean Ministry of Environment;
OSHA – United States Occupational Safety and Health Administration;
PMRA = Canadian Pest Management Regulatory Agency;
SAWS = Chinese State Administration of Work Safety.
2.1. Regulatory test guidelines
Data on the potential effects from airway exposures may be required if inhalation is likely, taking into account the various potential uses of the substance, the vapor pressure, and the potential aerodynamic size and distribution of materials that are liquids or solids under standard conditions (Doiron, 2007).
Test guidelines (TGs) adopted by the Organization for Economic Co-operation and Development (OECD) (Table 2) may be used to fulfill specific requirements for acute inhalation data when an animal study is requested by a regulatory agency. OECD TG 403 (OECD, 2009a) recommends ways to minimize animal usage, including consideration of any information on the test substance use scenario or expected human exposure, as well as existing toxicological data (i.e., in vivo human or animal, in vitro, or in silico) on the test substance or structurally similar substances. For example, one approach to reducing regulatory-required animal use when the test article is known or expected to be virtually non-toxic would be to conduct a limit test, in which a single dose group is exposed to the limit concentration (or maximum attainable concentration if a limit concentration cannot be attained), generally for 4 h. OECD TG 436 (OECD, 2009b) uses fewer animals than OECD TG 403; it follows a stepwise procedure of 4 h exposure to fixed concentrations, with the outcome of the previous step determining the subsequent step. Lethality is used as the main endpoint for both OECD TG 436 and 403. A third test guideline for acute inhalation toxicity, OECD TG 433 (OECD, 2017) is a refinement alternative that follows the fixed concentration approach used in OECD TG 436 but replaces lethality as an endpoint with the observation of evident clinical signs of toxicity. Evident toxicity is defined as clear signs of toxicity that indicate that exposure to the next highest concentration will cause severe toxicity or death in most animals (e.g., irregular respiration or tremors) (Sewell et al., 2015). Ultimately, the choice of test guideline is driven by the regulatory requirement for the intended use of the inhaled material.
Table 2.
Comparison of select features of OECD acute inhalation TGs.
| OECD TG 403: acute inhalation toxicity | OECD TG 436: acute inhalation toxicity - acute toxic class method | OECD TG 433: acute inhalation toxicity - fixed concentration procedure | |
|---|---|---|---|
| Use | Used for quantitative risk assessments where a concentration-response relationship is required, as well as for classification and labeling purposes | Used mainly for classification and labeling purposes | Used mainly for classification and labeling purposes |
| Number of animals (generally rats) |
Traditional protocol1: Limit test (single concentration tested): 10 animals/limit concentration (5 M/5 F); total 10 animals |
Main study: 6 animals (3 animals per sex, 1 or more concentrations) |
Main study: 5 animals (5 animals, 1 or more concentrations) |
|
Main Study (at least 3 concentrations tested): 10 animals/concentration (5 M/5 F); total at least 30 animals |
|||
|
Concentration × time protocol (C × t): 8–10 animals/concentration (1 M/1 F at 4–5 exposure durations); 4–5 exposure concentrations; total 40–50 animals |
|||
| Limit test concentration | 5000 ppm (gas) | 5000 ppm (gas) | 5000 ppm (gas) |
| 20 mg/L (vapor) | 20 mg/L (vapor) | 20 mg/L (vapor) | |
| 5 mg/L (aerosol) | 5 mg/L (aerosol) | 5 mg/L (aerosol) | |
| Exposure duration | 4 h (other durations permitted with justification) | 4 h (other durations permitted with justification) | 4 h (other durations permitted with justification) |
| Endpoint | Lethality | Lethality | Evident toxicity |
| Lethality estimate2 | Point estimate | Range estimate | Range estimate |
| Relevance to the 3Rs (replace, reduce, and refine animal use) | Careful selection of initial exposure concentration can minimize animal use | Decreased group size and fixed concentration testing result in reduction in animal use compared to OECD TG 403 | Reduction and refinement to OECD TG 403. Based on evident toxicity. |
OECD TG 403 includes two study types: (1) a traditional protocol and (2) a concentration × time (C × t) protocol. The traditional protocol provides concentration-response data used to derive a LC50 and establish the exposure-response slope. A C × t study can be used as an alternative to the traditional protocol when there is a regulatory requirement to test over multiple time durations (e.g., emergency response planning).
A point estimate is a single lethality estimate (e.g., LC50 = 5 mg/L) calculated from the study results. A range estimate is a range of lethality estimates (e.g., 0.5 mg/L < LC50 ≤ 2 mg/L) determined based on stepwise exposures to fixed concentrations.
2.2. Regulatory efforts to advance alternative approaches for acute inhalation toxicity
The ICCVAM Authorization Act of 2000 (42 U.S.C. 285l-3) established the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM). ICCVAM is comprised of 16 federal regulatory and research agencies that require, use, generate, or disseminate toxicological and safety testing information (NTP, 2017). Within ICCVAM, the Environmental Protection Agency (EPA) and the Department of Defense (DoD) sponsor an acute toxicity working group with additional members from the Consumer Product Safety Commission, the Department of Transportation, the Occupational Safety and Health Administration (OSHA), and the National Institute of Environmental Health Sciences (NIEHS). The working group also includes liaison representatives from the European Union Reference Laboratory for alternatives to animal testing and the Korean Center for the Validation of Alternative Methods. The aim of the ICCVAM working group is to evaluate existing in vivo, in silico, and in vitro tests for acute systemic toxicity and to contribute to an ICCVAM strategic roadmap1 on using in vitro and in silico approaches to reduce or replace current in vivo acute systemic toxicity tests.
The ICCVAM working group’s current activities are being informed in part by a recent National Research Council publication, “Application of Modern Toxicology Approaches for Predicting Acute Toxicity for Chemical Defense,” which was sponsored by DoD (National Research Council, 2015). The report reflects DoD’s interest in advancing a mechanistic testing framework that first considers properties of the test substance to inform the need for additional evaluation using in silico or in vitro methods.
In addition to the DoD interests, EPA is actively pursuing implementation of alternatives to animal testing. The EPA Office of Pesticide Programs (OPP) has publicly committed to significantly reduce the number of animals used in the agrochemical registration process (US EPA, 2016a). To accomplish this goal, EPA OPP’s strategy includes considering acceptance of a broader suite of in silico approaches and in vitro assays, waiving study requirements under certain circumstances (e.g., when a data endpoint is not relevant to the chemical), and supporting an improved understanding of toxicity mechanisms to inform data needs and allow for the development of non-animal tests that better predict how exposures are related to adverse effects. EPA OPP is working in partnership with other governmental entities, industry, and non-governmental organizations to achieve the mutual goal of more efficient human-predictive testing that does not use animals.
EPA OPP has released several guidance documents to aid in reducing animal use including “Guiding Principles for Data Requirements” (US EPA, 2013) and “Process for Establishing and Implementing Alternative Approaches to Traditional In Vivo Acute Toxicity Studies” (US EPA, 2016c). Subsequent to an EPA OPP 2012 guidance (US EPA, 2012b), the OECD released a “Guidance Document for Waiving or Bridging Acute Toxicity Tests” (OECD, 2016a) (discussed further in Section 6.1).
EPA OPP has also initiated a voluntary pilot program where registrants may submit calculations of toxicity for agrochemical formulations using the United Nations Globally Harmonized System of Classification and Labeling of Chemicals (GHS) additivity formula along with currently required in vivo acute oral and inhalation test data (US EPA, 2016b). The GHS additivity formula can be used to classify a mixture (such as an agrochemical formulation) based on acute toxicity values and concentrations of its ingredients, without requiring any additional testing of the final formulation (United Nations, 2015). The goal of the pilot program is to evaluate the ability of the GHS additivity formula to predict the acute toxicity categories for oral and inhalation routes (i.e., compare the in vivo and calculated LD50/LC50 values for each ingredient, and compare the EPA and GHS category based on the in vivo test and the calculated value). If the analysis shows the approaches to be comparable, EPA OPP will take steps towards waiving the in vivo test requirement for these substances, as appropriate.
EPA OPP is also working with the NTP Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM) to develop a database of curated acute toxicity data from agrochemical products, including acute inhalation toxicity data. NICEATM provides scientific and operational support for ICCVAM technical evaluations and related activities. The resulting database will be used to assess the variability within and across studies, to develop (Q)SAR and/or read-across approaches, and to compare with the results from alternative approaches, such as the GHS additivity formula or in vitro studies.
3. Inhalation dosimetry
Accurate dosimetry characterization requires determining the amount, rate, distribution, and form of a substance delivered to the target tissue of interest (Kuempel et al., 2015). Anatomical and physiological differences in the various species used in inhalation toxicological studies can result in different doses delivered both to analogous respiratory tract regions and systemically to other tissues. Therefore, an understanding of the comparative dosimetry of inhaled compounds in humans versus animals is critical both for translation of exposure-response relationships found in existing in vivo data and to inform the design of novel in vitro and in silico approaches to assess acute inhalation toxicity. Consideration of the concepts discussed in this section will be used in the development of a decision strategy to guide case studies.
3.1. Factors controlling comparative disposition of inhaled agents
The adverse toxic effects that are considered in a risk assessment are more related to the quantitative pattern of deposition within the respiratory tract rather than to the exposure concentration (Brain and Mensah, 1983). The deposition pattern of an inhaled substance determines not only the initial dose in the portal of entry (POE) but also the specific pathways by which inhaled material is cleared and redistributed. This section briefly discusses the two major factors impacting comparative inhalation dosimetry: (1) respiratory anatomy and physiology and (2) the physicochemical characteristics of the inhaled toxicant. Although these factors are discussed as distinct entities, their influence on the disposition of an inhaled agent are dynamic and interactive, with the relative contribution different in each species and respiratory tract region. Thus, an accurate description of the disposition of inhaled substances requires integration best afforded by the models described in the next section. Further, these factors are influenced by exposure concentration, duration, and frequency, all of which are discussed in more detail elsewhere (Fiserova-Bergovera, 1983a; Fiserova-Bergovera, 1983b; International Commission on Radiological Protection (ICRP), 1994; US EPA, 1994; McClellan and Henderson, 1995; Miller, 1995; Phalen et al., 1995; Gardner et al., 1999; Harkema, 1999; Gardner, 2005; Harkema et al., 2006; Morris and Shusterman, 2010; Parent, 2015).
Disposition of inhaled agents encompasses the processes of initial deposition and absorption, distribution, metabolism, and elimination (ADME). The phrase “initial deposition” is used when referring to gases and particles because contact with the respiratory tract surface precedes absorption or uptake. Clearance mechanisms are defined herein to include processes such as dissolution, phagocytosis by macrophages, transport to the gastrointestinal tract via the mucociliary escalator or nasal outflow, translocation via the lymphatics, absorption into the blood, and metabolic transformation. Disposition varies across species and among the respiratory tract regions; for example, interspecies variations in cell type, morphology, number, distribution, and functional capacities contribute to variations in clearance of initially deposited doses (Bogdanffy and Jarabek, 1995). Retained dose in a given region is defined as deposition minus clearance; in other words, the actual amount of particles or gas found in the respiratory tract at any time is determined by the relative rates of deposition and clearance. The efficiencies of the deposition mechanisms are different in each respiratory tract region and species. The defense mechanisms and clearance rates for each of these regions are also different and ideally require quantitation to arrive at an accurate description of the dose in each species.
3.1.1. Respiratory tract anatomy and physiology
The respiratory tract in humans and animals can be divided into three major regions on the basis of structure, size, and function: (1) the extrathoracic region that extends from just posterior to the external nares to just anterior to the trachea; (2) the tracheobronchial region, defined as the trachea to the terminal bronchioles where proximal mucociliary transport begins; and (3) the pulmonary region, including the terminal bronchioles and alveolar sacs. The thoracic region is defined as the tracheobronchial and pulmonary regions combined. The anatomic structures included in each of these respiratory tract regions are depicted in Fig. 2 and listed in Table 3A.
Fig. 2.
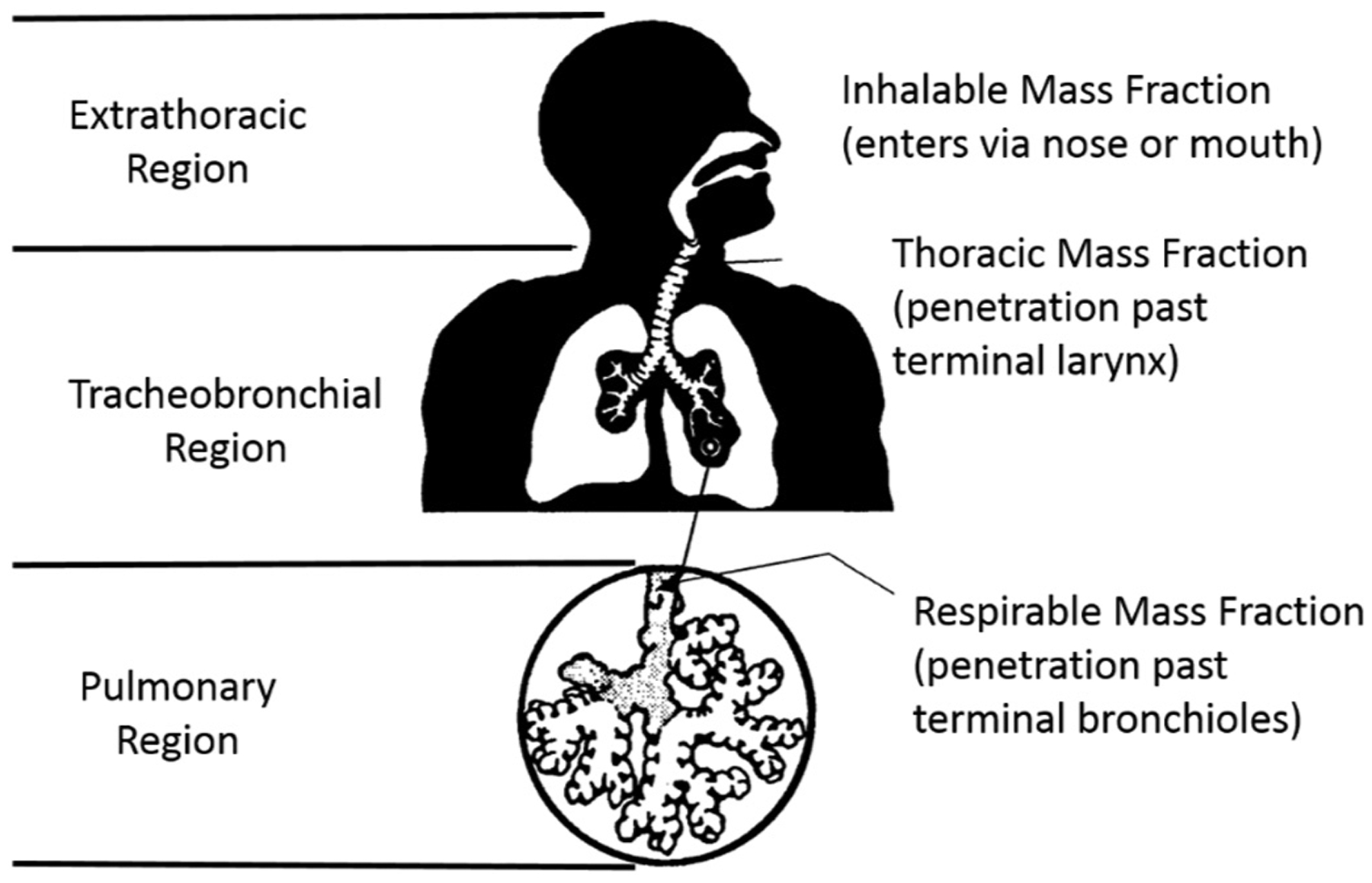
Three respiratory tract regions, with definitions of mass fractions for particle inhalation and exposure sampling (US EPA, 1994).
Table 3A.
Respiratory tract regions. Adapted from (US EPA, 1994).
| Anatomic structure | Other terminology | |
|---|---|---|
| Region | ||
| Extrathoracic (ET) | Nose | Head airways region |
| Mouth | Nasopharynx (NP) | |
| Nasopharynx | Upper respiratory tract (URT) | |
| Oropharynx | ||
| Laryngopharynx | ||
| Larynx | ||
| Tracheobronchial (TB) | Trachea | Conducting airways |
| Bronchi | ||
| Bronchioles (to terminal bronchioles) | ||
| Pulmonary (PU) | Respiratory bronchioles | Gas exchange region |
| Alveolar ducts | Alveolar region | |
| Alveolar sacs | ||
| Alveoli |
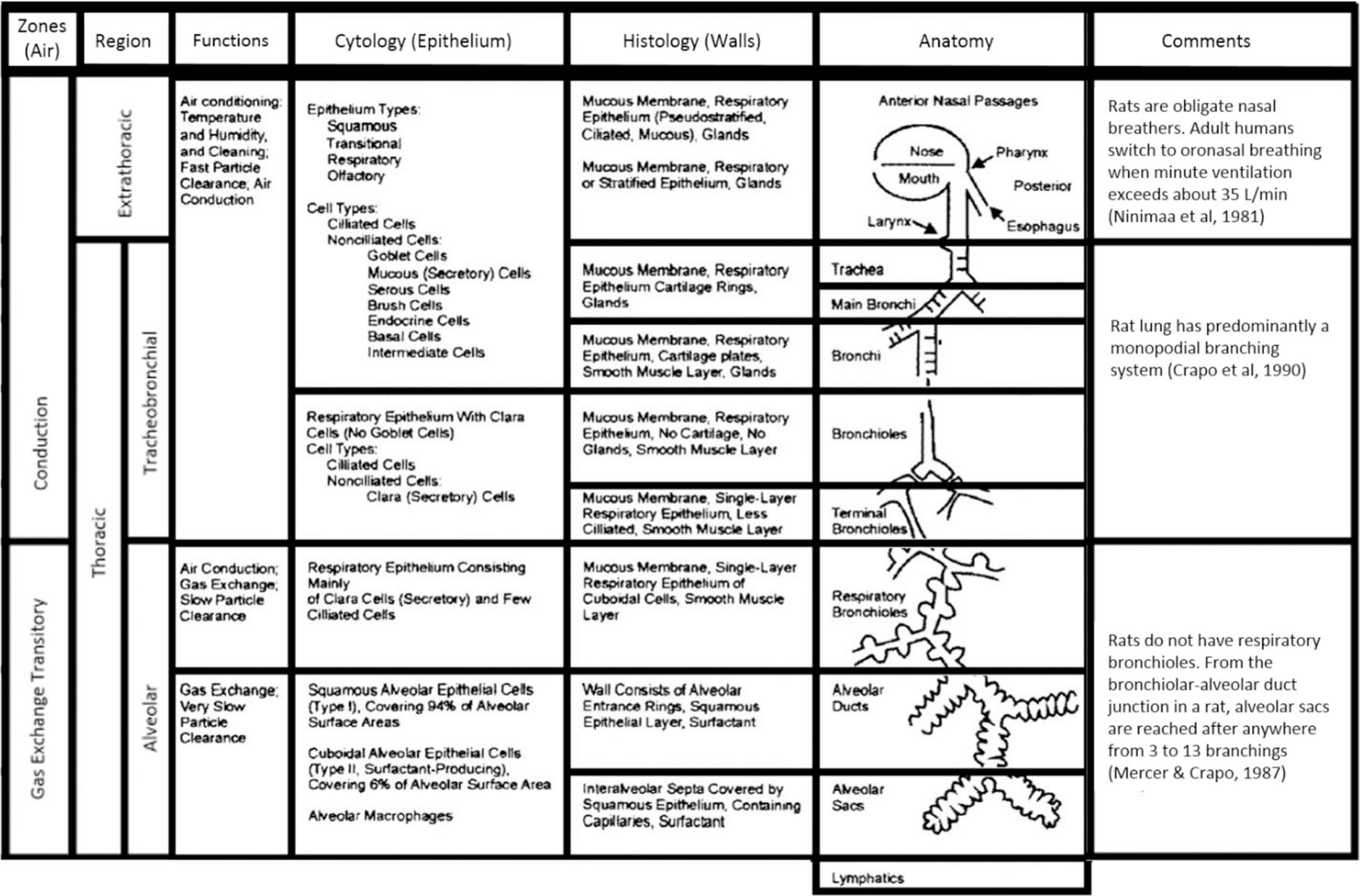
The respiratory tract of humans and animals differ in anatomy and physiology in several quantitative and qualitative ways (Table 3B). These variations affect airflow pattern in the respective respiratory tract architecture, which affects in turn the deposition of the given inhaled agent as well as its clearance and retention. Human and animal respiratory tracts exhibit differences in, for example, gross anatomy, types and location of nasal epithelia, and the distribution of mucous secretory products. Interspecies differences in structure of the upper respiratory tract and resultant differences in airflow dynamics (Fig. 3) and lesion distribution have been shown to result in quantitative differences relevant to dose-response analysis and interspecies extrapolation (Kimbell et al., 1997; Kimbell et al., 2001a; Kimbell et al., 2001b; Overton et al., 2001). There are also clear differences in the anatomy and geometry of airways in the lower respiratory tract that influence deposition and uptake of inhaled substances (International Commission on Radiological Protection (ICRP), 1994; US EPA, 1994; Dahl, 1995). An obvious difference between rodents and humans is the branching pattern and angle of the bronchi and bronchioles in the lower respiratory tract. As shown in Fig. 3, the branching pattern in rodents is asymmetric or monopodial, which results in a relatively unimpeded flow, whereas the branching pattern in humans is symmetric that results in an airflow pattern more susceptible to deposition at its bifurcation points. Airway dimensions such as length and diameter, tissue volumes, cell types and their distribution, and mucous composition also differ across species (Parent, 2015). Differences in cell types for the pulmonary region are less dramatic than in other regions, with significant homogeneity in populations of epithelial, endothelial, interstitial, and macrophage cells as well as in the percentage of the alveolar surface area covered by Type I and Type II cells (Crapo et al., 1983; Parent, 2015). Consideration of these parameters can be used to guide the development of in vitro test systems, and to extrapolate results from in vivo tests and to target human exposure scenarios.
Table 3B.
Key aspects of the structure and function of the respiratory tract of rats and humans. Adapted from (International Commission on Radiological Protection (ICRP), 1994; Miller, 1999).
|
Fig. 3.
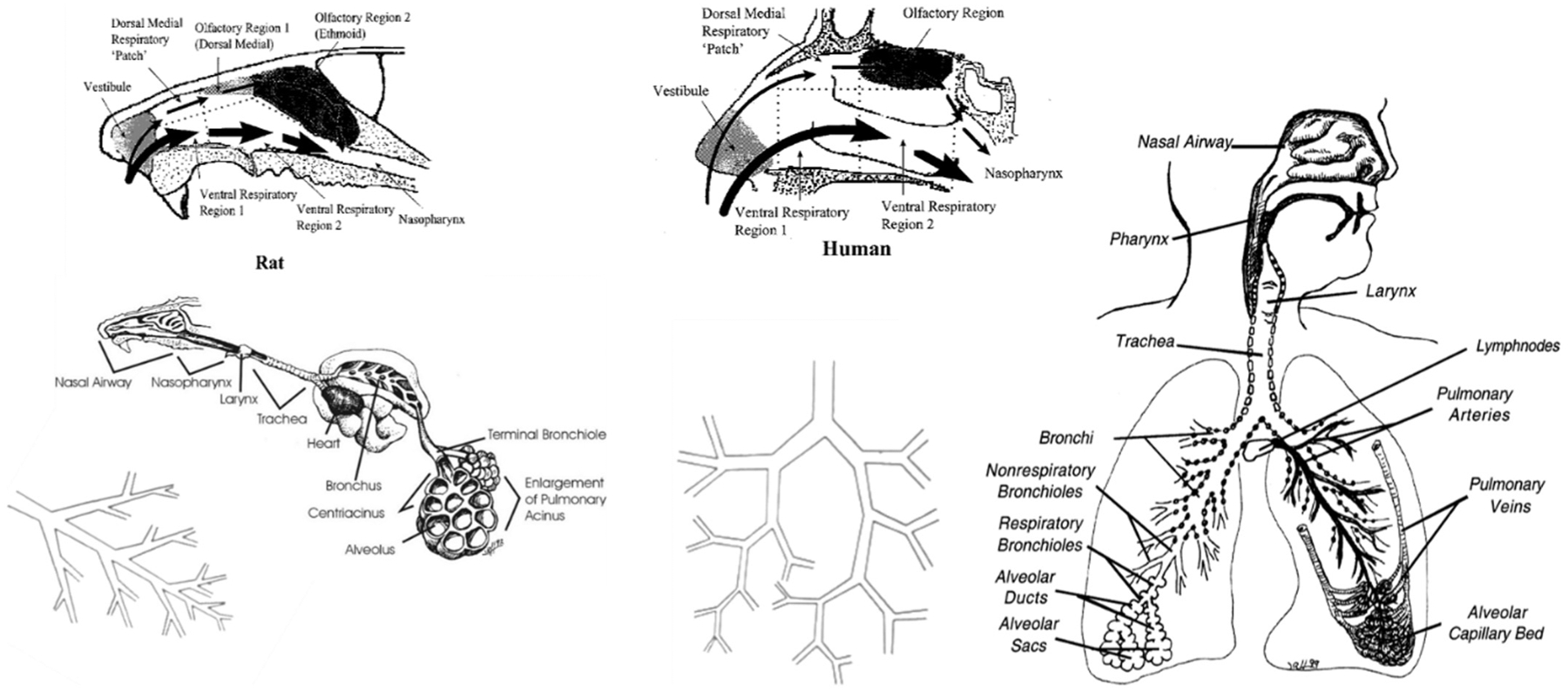
Comparative respiratory tract anatomy and airflow in the upper respiratory tract. Illustrations courtesy of Dr. Jack R. Harkema, Professor of Comparative Pathology, Michigan State University.
One of the most obvious and significant physiological differences between rodents and humans is breathing mode. Humans are oronasal breathers while rodents are obligate nose breathers. This difference has important ramifications for particle and gas deposition in the URT of humans as compared to rodents. For example, there is less filtering of particles and gases in oral breathing compared to nasal breathing, resulting in a greater delivery of material to the peripheral airways. Oral breathing increases with exertion (Niinimaa et al., 1981; International Commission on Radiological Protection (ICRP), 1994; US EPA, 1994), thus the ventilation rate and activity pattern associated with various exposure scenarios (e.g., occupational versus resting) is an important factor when constructing internal dose metrics for response analysis. Differences in ventilation rates affect the tidal volume and ventilation-to-perfusion ratios across species, and cardiac outputs also vary; all interact with the anatomical differences described above to result in dramatic differences in deposition and uptake across species. Another factor that differs among species is the chemotactic attraction of macrophages involved in clearance. Biochemical mechanisms of airway activation, detoxification, and response are other factors affecting internal dose, which need to be characterized to target development of in vitro test systems. Metabolic capabilities of critical enzymes are different across species (Csanady et al., 1992; Bogdanffy and Jarabek, 1995; Bond and Medinsky, 1995; Dahl, 1995; Fisher, 1995; Bogdanffy and Keller, 1999; Sarangapani et al., 2002a); for example, cytochrome P450 activities for a variety of substrates are metabolized less efficiently in microsomes from human nasal mucosa than in microsome preparations from rodents, whereas phase II enzymes such as epoxide hydrolase and glutathione S-transferase appear to be less active in rodents. Carboxylesterase activity is particularly prominent in the nasal tissues of rodents. In the lower respiratory tract, cytochrome P450 and glutathione S-transferase activities are lower in humans than in most species, and considerably lower in humans than in mice.
3.1.2. Physicochemical properties
The physicochemical properties of an inhaled agent will influence the initial deposition and subsequent disposition within the respiratory tract, distribution to other tissues, and ultimately the toxic effect. Two general categories for inhalation dosimetry can be made: particles (including fibers and nanomaterials) and gases; within gases there are three major categories for dosimetry model selection (1, 2, and 3). Consideration of physicochemical properties according to these categories will be essential to the design of test systems and the evaluation and extrapolation of the effects of a given inhalation exposure. A number of anatomical and physiological factors that affect deposition and uptake of particles and gases in the respiratory tract, which are diagramed in Fig. 4 and further described in Sections 3.1.2.1 and 3.1.2.2.
Fig. 4.
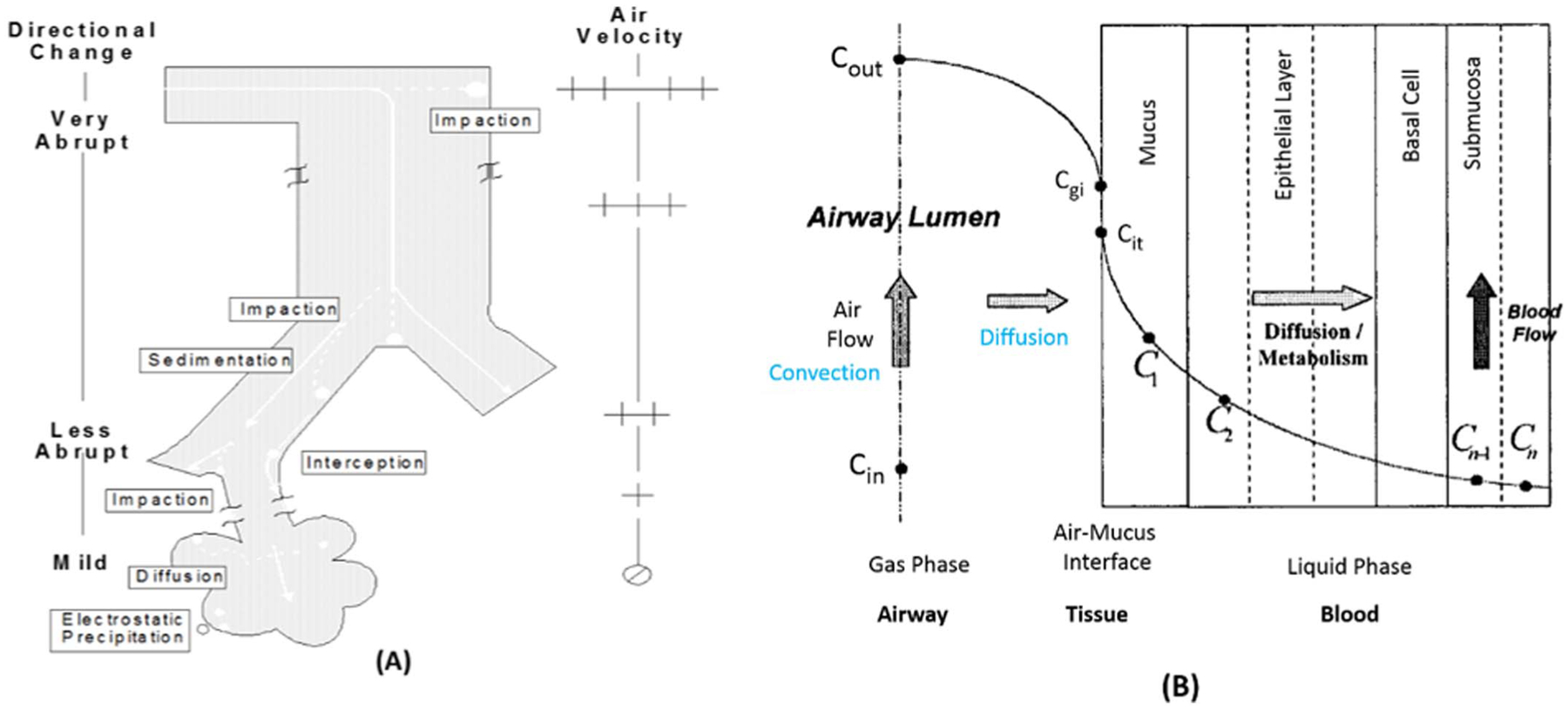
Selected anatomical or physiological parameters and mechanisms of inhaled particle deposition (a) and gas uptake (b) in the respiratory tract. Particle clearance is not illustrated. Directional change and air velocity refer to airflow direction and rate, respectively, as it travels through the respiratory tract. As shown in (a), airflow in the extrathoracic region is distinguished by abrupt directional changes and high velocity; deposition here is principally through impaction. As the airways bifurcate, airway volume increases with increasing cross-sectional area thereby decreasing air velocity and allowing more gradual directional airflow changes such that sedimentation can also occur. (b) provides a schematic of the uptake and metabolism of an inhaled chemical from the air phase into the tissue sub-compartments. Cin is the concentration of inspired gas entering an airway region and Cout is the concentration exiting the same airway by convection if no reactions occur. Cgi is the gas concentration at the interface of the airway and epithelial lining fluid or mucus layer, and due to molecular diffusivity Cit is the concentration at the epithelium interface. C1 and C2 illustrate concentrations in mucus and epithelial layers, and Cn-1 and Cn are concentrations in the submucosa and blood due to perfusion. Adapted from (US EPA, 1994; Bogdanffy et al., 1999).
3.1.2.1. Particles.
Particle, fibre, and nanomaterial dosimetry has been evaluated in a number of reviews (Schlesinger, 1995; Snipes, 1995; Miller, 1999; Asgharian et al., 2005; Bernstein, 2005; Jarabek et al., 2005; Oberdorster et al., 2005; Warheit, 2005; Teeguarden et al., 2007; Morris et al., 2010). Factors affecting deposition of particles in the respiratory tract include mechanisms of impaction, sedimentation, interception, diffusion, and electrostatic precipitation (Fig. 4a) (US EPA, 1994; Jarabek et al., 2005). The density, size, and distribution of particles influence their aerodynamic behavior in the respiratory tract according to these mechanisms (International Commission on Radiological Protection (ICRP), 1994; US EPA, 1994; McClellan and Henderson, 1995). The mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD) of a particle are used to characterize these properties in dosimetry modeling. Mass median aerodynamic diameter may be influenced by the hygroscopic nature of some particles and can be used to refine applied dosimetric adjustments. For fibers and nanomaterials, shape is also a critical determinant, which can be described by aspect ratios or bivariate distributions of length and width, coil length, and coil diameter (US EPA, 1994). Subsequent clearance of a deposited dose is dependent on both the initial site of deposition and the solubility or biodurability of the particle. Time since deposition is another important consideration.
3.1.2.2. Gases.
Dosimetry of gases has also been considered in a number of reviews (Bond and Medinsky, 1995; Dahl, 1995; Fisher, 1995; Miller and Kimbell, 1995; Bogdanffy and Keller, 1999; Kimbell and Miller, 1999; Medinsky et al., 1999; Sarangapani et al., 2002a; Morris et al., 2010; Morris, 2012). Uptake of a gas requires that it move from the gas phase in the airway lumen to the tissue phase (i.e., the surface-liquid lining layer, the tissue layers of the respiratory tract, the capillary endothelium or the blood. The major mechanisms of gas transport in the respiratory tract involve convection, diffusion, absorption, dissolution, and chemical reaction (Fig. 4b). Convection is comprised of advection (i.e., horizontal movement of a mass of air relative to the airway wall) and eddy dispersion (i.e., air mixing by turbulence) so that individual fluid elements transport the gas and generate a flux to the epithelial lining fluid and tissue. Molecular diffusion is superimposed at all times on convection due to local concentration gradients. Absorption removes gases from the lumen and affects concentration gradients. Chemical reactions in the respiratory tract tissue can increase absorption by acting as a sink to drive the concentration gradient. Systemic metabolism can also drive the concentration gradient for soluble gases that are removed from the respiratory tract primarily by perfusion. Thus, gas dosimetry is dictated by its mass transfer coefficient consisting of a gas-phase component and tissue-phase component (US EPA, 1994; McClellan and Henderson, 1995; Hanna et al., 2001; US EPA, 2012a; Kuempel et al., 2015).
3.2. Dosimetry modeling
Recent advances in the availability of mechanistic data and mathematical models that describe the behavior of inhaled particles and gases in the respiratory tract discussed above can serve as the basis for approaches that integrate critical determinants of ADME into testing strategies and risk assessment. “Dosimetry modeling” is a comprehensive term encompassing model structures that describe the inhaled disposition of non-volatile and irritant gases, particles, and fibers, including physiologically based pharmacokinetic (PBPK) models.
Selection of the dosimetry model to use in a particular risk assessment depends on the purpose of the predictions (e.g., screening versus full characterization), the physicochemical properties of the inhaled agent, the degree of understanding of ADME and the mechanism of toxicity, and the level of detail and specificity of the available data. A general equation for a dosimetric adjustment factor (DAF) is shown below (US EPA, 1994; Jarabek, 1995a):
where:
POD = the point of departure (i.e., threshold for activity) of a given endpoint or key event relevant to the adverse outcome pathway;
HEC = human equivalent concentration, constructed using parameters such as ventilation rate for age and exertion level relevant for the human exposure scenario under consideration;
ADJ = if needed, adjustment for animal exposure level (e.g., 6 h/day, 7 day/week);
DAFr = is either the regional deposited dose ratio or the regional gas dose ratio in the respiratory tract region associated with the observed toxicity, r, in the study being extrapolated.
The DAF approach can be used to adjust for interspecies differences or in vitro to in vivo extrapolation (IVIVE), and a similar strategy can be used to adjust to different target human exposure scenarios such as occupational exposures by adjusting the terms used to construct the DAF ratio (Kuempel et al., 2015).
A two-tiered hierarchy of model structures that can be used as the DAF is shown in Table 4. Analysis of more mature and sophisticated models can be used to construct reduced forms that are conceptually consistent with the more detailed model structures (Jarabek, 1995a). In the first tier, the default structure relies on categorical descriptions of key processes and parameters; in the second tier, the incorporation and integration of the critical mechanistic determinants allows more elucidation of the exposure-dose-response continuum and may represent a range of model structures preferred to the default. Depending on the knowledge of model parameters and fidelity to the biological system, a comprehensive model structure can be constructed that provides a more accurate characterization of the pathogenic process.
Table 4.
Hierarchy of model structures for dosimetry and extrapolation (US EPA, 1994).
| Hierarchy of model structures for dosimetry and extrapolation |
|---|
| “Optimal” or preferred model structure |
|
| Default model structure |
|
For particle dosimetry, the freely available Multiple-Path Particle Dosimetry (MPPD) computational model (Applied Research Associates Inc., 2017) is widely used, including by EPA and the National Institute for Occupational Safety and Health (NIOSH), which is working to expand the model to describe nanoparticles. EPA also supports a semi-empiric model, the regional deposited dose ratio model, for animal species not yet available in MPPD (US EPA, 1994). The same mechanisms responsible for inhaled particle deposition in the respiratory tract are operative in in vitro systems. The in vitro sedimentation, diffusion, and dosimetry (ISDD) model was developed to describe in vitro kinetic mechanisms of particles to better describe the “cellular dose,” or dose delivered to the cell (Hinderliter et al., 2010). These models can be used to target test concentrations to be used in in vitro systems, and provide context for inferences and support integration across in vitro and in vivo tests.
Numerous dosimetry models are also available to describe gas uptake and disposition in the respiratory tract. Since inhaled gases can cover such a large range of potential physicochemical properties, the EPA gas category scheme describes three different model structures that can be used to arrive at dose estimates (Table 5; (US EPA, 1994; Hanna et al., 2001). The scheme considers properties such as water solubility and reactivity, which includes the propensity for dissociation of the parent gas in tissue (e.g., hydrolysis) and its ability to react either spontaneously or via enzymatic reactions in the respiratory tract (US EPA, 1994). The goal of the EPA gas category scheme is to guide approaches to dosimetry adjustment that are commensurate with the available data on physicochemical properties, the nature and location of the toxicity, and the level of detail regarding the mechanism (US EPA, 1994; Jarabek, 1995a; US EPA, 2012a). However, it should be recognized that the gas category scheme represents a way to select specific model components from a continuum and that the same comprehensive model structure could be applied to all categories (Kuempel et al., 2015). The scheme does not apply to stable gases that exert effects by reversible physical interactions of gas molecules with biomolecules (e.g., displacement asphyxiants).
Table 5.
Gas category scheme and characteristics (US EPA, 1994; Hanna et al., 2001; Kuempel et al., 2015).
| Gas category | Water solubility | Reactivity and tissue uptake | Examples |
|---|---|---|---|
| 1 | Higha |
|
Chlorine, formaldehyde, hydrogen fluoride, vinyl acetate |
| 2 | Moderate |
|
Ozone, sulfur dioxide, xylene, propanol |
| 3 | Insoluble |
|
Chloroform, styrene, trichloroethelene |
Gases with either of these characteristics are included in Category 1.
In the case of Category 1 gases, description of dose delivered to the tissue in a given region must account for the scrubbing of the gas out of the convective airstream as it travels through the nose (proximal) to pulmonary (distal) airways. Scrubbing is caused by uptake into the tissue, for example, by dissolution or reactions such as metabolism in the tissue. If the reaction rate is fast, then the gas-phase component dictates the overall mass transfer and would thus be the basis of default modeling algorithms (US EPA, 1994; US EPA, 2012a). Thus, for Category 1 gases, computational fluid dynamic (CFD) models (Kimbell et al., 2001a; Kimbell et al., 2001b; Corley et al., 2015) or single-path mass transfer models (Hanna et al., 2001; Overton, 2001) more accurately describe the species-specific anatomical influences on airflow delivery (mass transfer) and PBPK models then employed to describe tissue reaction kinetics. In fact, hybrid CFD-PBPK model structures are used to best capture behavior of gas uptake and disposition because they consider the combined influences of airway architecture and tissue metabolism (Frederick et al., 2002; Schroeter et al., 2006; Schroeter et al., 2010; Asgharian et al., 2011).
Such hybrid models represent a more comprehensive structure suitable for modeling of Category 2 gases, as well as those that are intermediate in reactivity and water solubility. Category 2 gases may have local effects in the POE, accumulate in the blood to cause systemic effects, or may deliver the toxicant back to airway tissues from the endothelial side of the respiratory/circulatory tissues where they may react with respiratory tract or be exhaled (Jarabek, 1995a; Kuempel et al., 2015).
Gases in Category 3, such as volatile organic solvents, have limited reactivity in the respiratory epithelium and are generally insoluble in water. These gases are not scrubbed out in the upper respiratory tract or conducting airways but instead readily penetrate to the pulmonary region where they are available to be absorbed into the systemic circulation. Toxic effects from these gases typically occur in systemic target tissues, although some metabolism in airways can lead to POE effects (Kuempel et al., 2015). The underlying model structure for gases in Category 3 is a ventilation:perfusion model in which the blood:gas partition coefficient is used to modulate the rate of transfer from the pulmonary region to the blood. For these gases, the human-to-animal ratio of blood:air partition coefficients is used as a default for the regional gas-dose ratio (Kuempel et al., 2015).
Typically, PBPK models used to describe systemic distribution of Category 3 gases represent the lung as a single homogenous tissue compartment in equilibrium with arterial blood for simulating inhalation exposure. These simple models do not reflect respiratory tract dosimetry as target tissue. In addition, they do not capture air-phase delivery of the inhaled substance to the target cells in the respiratory tract; species differences in the metabolic constants for formation and clearance of metabolites by club cells, the primary detoxifying cells that also produce secretory proteins to protect the bronchiolar epithelium; or regional club cell density. In contrast, PBPK models with regional compartments in the respiratory tract can improve the estimation of dosimetry at the target site after inhalation exposure, capturing both POE and systemic dosimetry (Sarangapani et al., 2002b; Campbell et al., 2014; Campbell et al., 2015).
Selection of the appropriate dose metric (i.e., the measure of the dose) is another important consideration for the design of in vitro test systems and interpretation of results. The best dose metrics are those that are most closely associated with the mechanism determining the adverse response in the target tissue. Calculation of a DAF for interspecies extrapolation for particles typically involves construction of dose metrics based on fractional deposition of mass normalized by factors related to the mechanism (Jarabek et al., 2005). PBPK models to characterize tissue reactions and systemic delivery can be used in combination with particle models like the MPPD as an approach for development of a DAF based on tissue concentrations (Ramoju et al., 2017).
The selection of a relevant dose metric also depends on whether a disease or adverse outcome is better described by an acute or chronic pathogenesis process. The challenge is to select a dose metric that is mechanistically associated with or experimentally closely correlated to the biological response (Jarabek, 1995b). Internal dose may be accurately described by particle deposition alone if the particles exert their primary action on the epithelial surface tissues (Dahl, 1990). When different types of particles are compared, inhaled dose may be more appropriately expressed as particle volume, particle surface area, or number of particles rather than mass, depending on the toxic effect being evaluated (Oberdorster et al., 1994). For gases, consideration of the mechanism and available data are also used to motivate selection of the dose metric. Descriptions of internal dose can be based on the parent compound or metabolite in various tissues. Dose metrics must take into account the frequency, duration, and magnitude of the exposure as well as the toxicity mechanism to be characterized (Jarabek, 1995b; Kuempel et al., 2015). For example, a given toxicity may be described by the peak concentration or the area under the blood or tissue concentration and time (AUC).
The cellular dose-response is modified by fractional deposition, local metabolism, and sensitivity of cell populations in specific regions of the respiratory tract. The major challenge in investigating respiratory tract metabolism is the non-homogenous distribution of metabolic enzymes in the respiratory tract. Metabolic enzymes are differentially expressed in various regions of the respiratory tract. For example, CYP450 metabolism only occurs in certain pulmonary epithelial cells, such as Type I, Type II, and club cells, with club cells being responsible for most of the metabolic activity (Plopper et al., 1980; Plopper et al., 1992). In addition, there are species differences in respiratory tract metabolism. For example, in mice, club cells are found throughout the respiratory tract, but they are only found in the transitional airway of rats and humans immediately before the alveolar regions of the lung (Plopper et al., 1992; Mercer et al., 1994).
Dosimetry modeling approaches are used during product development to aid in toxicology study designs based on predicted systemic exposures, as well as to understand how differences in toxicity between exposure routes, life stages, genders, and/or species correlate with differences in internal dose. However, to facilitate adoption of dosimetry modeling tools to support non-animal testing, there is a need to standardize modeling approaches with common tools and a large user base. In developing a suite of standardized toxicokinetic models, a number of modeling criteria are important to consider.
The models should be predictive of internal target site exposure (i.e., either in the respiratory tract or to systemic target tissues) after acute, episodic, or steady-state exposures.
The models should incorporate critical (quantitative) structure-activity relationships ((Q)SARs) for key ADME parameters, including absorption rates and amounts, metabolic clearance and metabolite structure prediction, plasma protein binding, and disposition in both the respiratory tract and systemic tissues.
The suite of models should be flexible so that the components are tractable across different structures and can be adapted for use with either compartmental PK or more complex physiologically based structures (i.e., dosimetry or PBPK models) depending on the needs of the application, and provide predictions for either parent compound only or parent and metabolite(s).
The models should provide outputs in a user-friendly format and be easy to communicate with regulatory agencies.
To increase use of dosimetry/PBPK modeling in support of alternatives to animal testing for safety evaluations of chemicals and also to improve efficiency by accepting more high throughput testing results, novel in vitro and in silico approaches will be required to provide the necessary ADME parameters for dosimetry/PBPK models. Numerous in silico tools exist for prediction of one or more parameters involved in PBPK modeling of oral exposures and systemic toxicity (i.e., solubility, absorption, metabolism, or tissue distribution) (Bessems et al., 2014); however, these tools were primarily developed for use with pharmaceuticals. In order for these tools to support non-animal approaches to acute inhalation toxicity testing, additional work will be needed to develop in vitro test systems that address the physicochemical properties across the range of inhaled substances, including nanomaterials, fibers, and gases (e.g., volatile organics and reactive gases). A cross-cutting need is defining metabolic rate terms for the various cell types and capacities for various enzymes in the different respiratory tract regions (e.g., carboxylesterases). Key to the use of predictive data from in silico expert systems is an understanding of the relevant adverse outcome pathways and associated mechanistic key events that result in acute inhalation toxicity, either in the POE or in systemic tissues. In follow-up to the workshop, a decision strategy is under development that will assess the likelihood of adverse outcomes in either the POE or systemic delivery, based on physicochemical properties and guided by the gas category scheme described above. Approaches to describe and extrapolate different dose metrics for target site exposures, at a minimum in the respiratory tract versus systemic delivery, will be developed and explored.
3.3. In vitro to in vivo extrapolation
In silico high-throughput PBPK models that incorporate IVIVE are becoming more widely used to prioritize chemicals for testing in large safety evaluation programs (Wambaugh et al., 2015; Wetmore et al., 2015). IVIVE can build on current understanding of biological and physicochemical mechanisms and thereby aid in the comparison and translation of results across exposure conditions, between species, and across exposure or use scenarios. IVIVE may also facilitate comparisons of regional to local estimates of different doses (either to the respiratory tract or systemic distribution), provide insight on mechanism, and refine risk assessment predictions when developing alternatives for acute inhalation testing.
Models for IVIVE that are developed to estimate inhalation exposures should use as inputs cellular exposure concentrations corresponding to the appropriate dose metric in the target respiratory tract region under in vivo conditions (e.g., for comparison to animal studies) and incorporate parameters based on likely human exposure to predict estimates that characterize target exposure scenarios. A challenge for the application of IVIVE to inhalation is that current IVIVE practices and approaches are largely based on the experience with hepatic metabolism of select pharmaceuticals after oral dosing, for which specific dosimetry within a tissue, such as the respiratory tract as the POE, is not an issue (Basketter et al., 2012; Yoon et al., 2012). Therefore, developing in vitro respiratory tract models that can represent target dosimetry, cellular components, and toxicity mechanisms in specific regions of the respiratory tract in vivo and which characterize in vitro dosimetry of the test material will be critical to developing a strategy for IVIVE for inhalation testing alternative methods.
Ultimately, user-friendly IVIVE programs that do not require computational expertise will be needed for routine regulatory application. Open source tools are being developed that allow modelers to incorporate computational workflows for IVIVE. For example, an open source “httk” package is available in R, a software platform for statistical computing and graphics (https://www.r-project.org/), that provides a set of tools for IVIVE using high throughput screening data (e.g., ToxCast) to estimate real-world exposures. Resources being developed by EPA (e.g., the EPA Chemistry Dashboard available at https://comptox.epa.gov/dashboard) and NICEATM (Integrated Chemical Environment) will include QSAR model predictions for hepatic clearance and protein binding that can be applied for IVIVE.
4. Mechanisms of toxicity and adverse outcome pathways
An understanding of mechanisms that lead to toxicity can help in devising relevant non-animal testing approaches, and it must be kept in mind that these mechanisms are highly dependent on the physicochemical properties of the inhaled agent. Basal cytotoxicity assays address many of these mechanisms, which include reactivity, nonspecific lipid membrane disruption, chelation, mitotic spindle poison by binding tubulin, disruption of energy production, vitamin interference, protein synthesis inhibition, and nucleotide synthesis inhibition (Vinken and Blaauboer, 2017). In vitro testing can be conducted using assays targeted to one of more of these mechanisms, for example, assessing mitochondrial membrane potential depolarization or dopamine receptor binding. In addition to basal cytotoxicity assays, tests may be used to assess more specific mechanisms, such as those acting through G-protein coupled receptors or Cys-loop ligand gated ion channels.
An adverse outcome pathway (AOP) is a framework used to organize data across a series of casually linked key events, beginning with a molecular initiating event and ending with an adverse outcome (Ankley et al., 2010; Villeneuve et al., 2014b; Villeneuve et al., 2014a). The molecular initiating event and adverse outcome are linked by key events, which are measurable and essential to the progression of one or more defined biological perturbations leading to the adverse outcome. AOPs can be built using existing in vivo, in vitro, in chemico, and in silico data from the published literature. AOPs can facilitate the study of potential effects in the respiratory tract subsequent to inhalation exposures. Construction of AOPs can also help identify research gaps and with design of non-animal testing strategies (Wittwehr et al., 2017). The confidence in an AOP determines its potential use, with more quantitative AOPs being useful for risk assessment and less developed AOPs useful for prioritization or hazard identification (Vinken, 2013; Becker et al., 2015; Patlewicz et al., 2015; OECD, 2016b). The general mechanisms listed above may be used as a starting point for the development of AOPs that characterize key events of pathogenesis for diseases and toxic effects in either the respiratory tract or systemic tissues following inhalation exposure. These AOPs can then be used to select in vitro assays that assess specific mechanisms of toxicity.
5. In vitro testing approaches
Numerous reviews on in vitro inhalation toxicity testing models have been published (BéruBé et al., 2009; BéruBé et al., 2010a; BéruBé et al., 2010b; Gordon et al., 2015; Wiemann et al., 2016). This review focuses on three specific examples presented during the 2016 webinar series: (1) in vitro cell cultures, (2) lung-on-a-chip models, and (3) ex vivo human precision cut lung slices.
5.1. In vitro cell culture
In vitro test systems can range in complexity from relatively simple submerged mono- or co- culture systems to co-culture systems incorporating human respiratory tract cells at the air-liquid interface (ALI). The overlying medium in submerged cell cultures can interfere with the maintenance of a normal epithelial phenotype, specific gene expression, and sedimentation and aggregation of particles, as well as being a diffusion barrier for gases (Aufderheide, 2005; Xie et al., 2012; Rach et al., 2014). Therefore, to study the effects of airborne substances in a more human-relevant manner, systems have been developed that allow direct exposure of the cells of the respiratory tract at the ALI. There are many types of ‘laboratory-based’ or commercially available in vitro ALI exposure systems, each of which has unique design features that may pose advantages or disadvantages for different exposure scenarios focusing on specific particle sizes, types, physicochemistries, and concentrations (Polk et al., 2016).
Many human cell-based in vitro systems were developed with the intention of best mimicking human respiratory biology. Co-culture models have been developed that incorporate embryonic stem cells, tumor-derived cell lines (e.g., NIH-H292 cells), immortalized cell lines (e.g., BEAS-2B cells), and primary cells (e.g., normal human bronchial epithelial cells). There are also three-dimensional organotypic models in which cells are used in physiologically-relevant ratios and arranged in a way that mimics certain regions of the human respiratory tract (Diabaté et al., 2008; Kirkpatrick et al., 2008; Lehmann et al., 2011; Prytherch et al., 2011; Kuehn et al., 2015). These tissues are generated using human-derived cells cultured at the ALI, allowing them to mimic the biology of the in vivo respiratory tract by modeling barrier function (e.g., trans-epithelial electrical resistance and functionality of tight junctions), mucous production, and cilia function, and can be used to study infection and acute or long-term toxicity (Mathis et al., 2013; Neilson et al., 2015; Essaidi-Laziosi et al., 2017). There are a number of three-dimensional organotypic respiratory tract models available that can be used to study inhalation toxicity (e.g., MucilAir™ or SmallAir™ [Epithelix Sàrl]; EpiAirway™ or EpiAlveolar™ [MatTek Corporation]; and Micro-Lung™ and Metabo-Lung™ [Cardiff University]), and each of these models have different properties which make them best suited for specific studies (Table 6). For example, these models all contain primary human cells, but vary in the specific cell types included (e.g., nasal, tracheal/bronchial epithelial cells, fibroblasts, goblet cells, or alveolar cells from healthy or diseased donors). The field of three-dimensional tissue model development is rapidly evolving with new and improved systems continuing to enter the market. In the webinar series, two examples (the Micro-Lung™ and Metabo-Lung™, Cardiff University) that are not yet commercially available, were used to illustrate the state-of-the-science. They are described in brief in the following section.
Table 6.
Characteristics relevant to regulatory applicability of the in vitro models presented in the webinar series. PCLS, precision-cut lung slices.
| MucilAir (Epithelix) | EpiAirway (MatTek) | Micro-Lung | Metabo-Lung | Lung-on-a-chip | PCLS | |
|---|---|---|---|---|---|---|
| Uses normal human cells | x | x | x | x | x | x |
| Can be maintained at the ALI | x | x | x | x | x | x |
| Mimics aspects of human lung morphology and physiology | x | x | x | x | x | x |
| Cilia beating | x | x | x | x | x | x |
| Production of mucous | x | x | x | x | x | x |
| Potential for metabolic activity | x | x | – | x | x | x |
| Suitable for long-term culture | x | x | x | x | x | x |
| Incorporates cyclic breathing and hemodynamic flow | – | – | – | – | x | – |
| Includes immune cells | x | x | – | – | x | x |
| Commercially available | x | x | – | – | – | – |
5.1.1. Three-dimensional in vitro lung models
Examples of three-dimensional in vitro lung models include the Micro-Lung™ and Metabo- Lung™, both developed at Cardiff University. The Micro-Lung model uses normal human bronchial epithelial (NHBE) cells, isolated from surgical patients and post-mortem donors (Prytherch et al., 2011; BéruBé, 2013; Prytherch and BéruBé, 2014b; Prytherch and BéruBé, 2015). After basal epithelial cells are removed from isolated NHBE cells, they differentiate into a mucociliary phenotype when cultured at the ALI. After exposure, the cells can be used to assess a wide range of histopathology, toxicogenomics, and proteomics endpoints. The apical wash (i.e., fluid collected from apical surface wash after dosing) can also be analyzed for proteins or inflammatory mediators, and the basal media can be studied for metabolic processes using high performance liquid chromatography (HPLC).
Such models can reproduce features of intact physiological anatomy, such as the formation of tight and adherens junctions, desmosomes, cilia, and microvilli (Prytherch, 2010). Prytherch (2010) has demonstrated the appropriate morphological organization of these features using light and electron microscopies. The mature tissue expresses cytokines, secretes mucin (i.e., mucous glycoproteins), and contains non-cancerous or transfected genes. Transepithelial electrical resistance can be measured to quantify the integrity of the epithelial barrier, with high resistance indicative of a healthy barrier function and vice versa. Transepithelial electrical resistance readings can be supported by conventional toxicological assays to assess changes in histology, cell secretions, viability, and cell death.
Prytherch (2010) investigated the irritation potential of a range of compounds using the methods described above with 77% concordance to in vivo irritancy (Prytherch, 2010). The NHBE model was co-cultured with human primary hepatocytes to create a metabolizing bronchial model (Metabo-Lung). Cells in the respiratory tract can biotransform compounds, either reducing their toxicity or contributing to the in situ activation of inhaled toxins and leading to adverse reactions. Cells grown in isolation do not permit in situ metabolism to take place, but primary human bronchial cells that are co-cultured at the ALI with primary human hepatocytes achieve metabolic activity comparable to that found in vivo (BéruBé, 2011a). NHBE cells co-cultured with donor-matched hepatocytes have been used to detect acetaminophen toxicity after 24-h exposure to phenacetin and dextromethorphan (BéruBé, 2011b; Prytherch and BéruBé, 2014b).
5.2. Lung-on-a-chip
Lung-on-a-chip systems provide an in vitro platform to model and predict physiological responses of human lung tissue to environmental materials. Typically, these in vitro models are created in microfabricated polymeric devices consisting of multiple layers of cell culture chambers to mimic three-dimensional micro-architecture of the tracheobronchial airways and alveoli (Huh et al., 2010; Huh et al., 2012; Esch et al., 2015). Lung-on-a-chip devices offer unique capabilities to recapitulate the dynamic microenvironment of native lung tissue produced by cyclic breathing and hemodynamic flow that play a crucial role in organ structure and function. Moreover, the ability of these systems to co-culture multiple cell types makes it possible to mimic complex tissue-tissue interactions and resultant integrated physiological responses at the organ level.
A breathing lung-on-a-chip model has been designed to mimic the alveolar-capillary unit of the human lung (Huh et al., 2010). This microdevice consists of two parallel cell culture chambers separated by a thin, flexible, porous membrane (Fig. 5). These elements are all fabricated from an optically transparent and gas permeable elastomer. The device architecture allows for co-culture of human alveolar epithelial cells and pulmonary microvascular endothelial cells on the opposite sides of the membrane to recreate the structural organization of the air-blood interface. By using computer-controlled cyclic vacuum suction applied to the hollow chambers adjacent to the cell culture channels, this system can also mimic physiological breathing motions and con-comitant tissue deformation. Importantly, this lung-on-a-chip system has been used to successfully model lung infection and nanoparticle transport across the in vitro alveolar-capillary barrier (Huh et al., 2010).
Fig. 5.
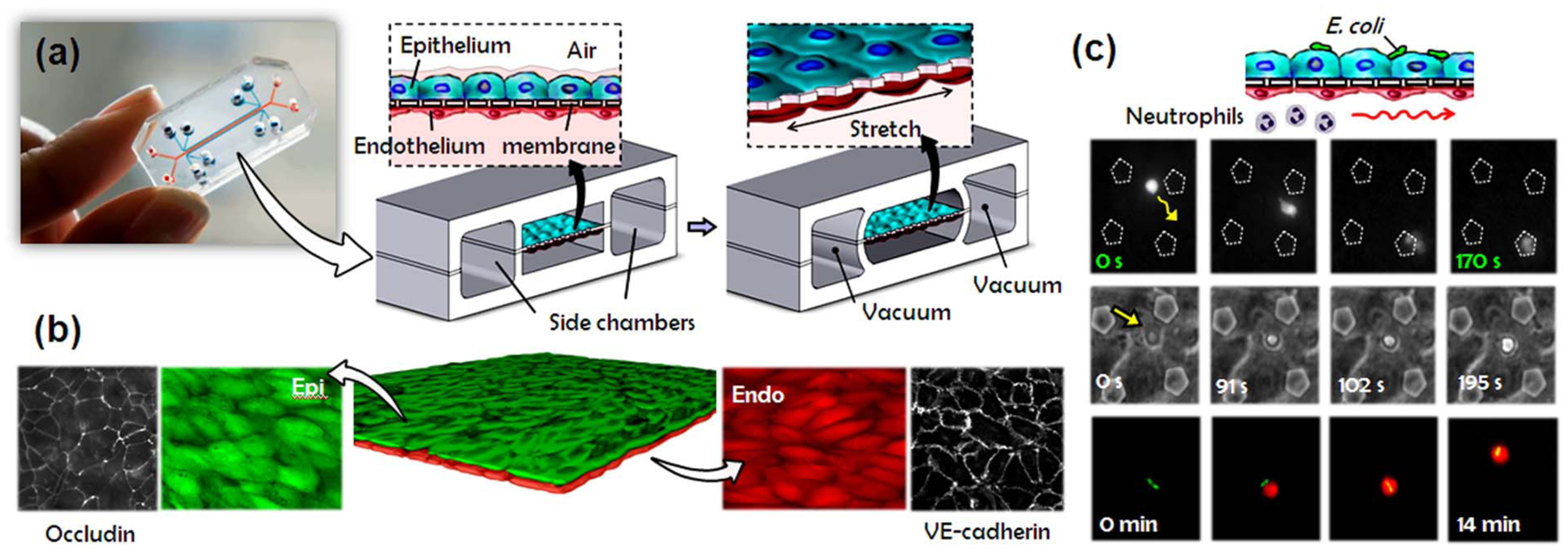
A human breathing lung-on-a-chip. (a) The microfabricated lung mimic device recreates physiological breathing movements by applying vacuum to the side chambers and causing mechanical stretching of the PDMS membrane forming the alveolar-capillary barrier. (b) Long-term microfluidic co-culture produces a tissue-tissue interface consisting of a single layer of the alveolar epithelium (Epi; green) closely apposed to a monolayer of the microvascular endothelium (Endo; red), both of which express intercellular junctional structures such as occludin or VE-cadherin. (c) Neutrophils flowing in the lower vascular channel adhere to the endothelium activated by E. coli in the alveolar chamber, transmigrate (top row), emigrate into the alveolar space (middle row), and engulf the bacteria (bottom row).
5.3. Ex vivo lung slices
Ex vivo precision-cut lung slices can be produced from a variety of species, including humans, and offer the advantage of maintaining the spatial orientation and structural microenvironment of all the cell types present in the lung, including those associated with the immune response (i.e., dendritic cells, macrophages, and mast cells) and intercellular communication (Fisher et al., 1994). Additionally, precision-cut lung slices may be maintained at the ALI for weeks or more and can be created from healthy or diseased human donor lungs. However, because there is no standardized production method, variations in thickness of the tissue slice are inevitable, which impacts comparative functionality. Studies using precision-cut lung slices have shown that high doses of cigarette smoke result in a substantial loss of alveolar epithelium (Lin et al., 2012).
5.4. In vitro testing conclusion
There are many in vitro tools that can be used to evaluate acute inhalation toxicity, and each tool should be evaluated for its potential use as a component of an integrated approach. Table 6 describes a number of currently available models along with their advantages and limitations.
Important considerations for selecting an in vitro model include cost, complexity, reproducibility, accessibility, endpoints modeled, and the amenability to high-throughput testing; the most appropriate in vitro system will depend on the specific data needs of the study and ultimately the accurate prediction of acute human toxicity.
While numerous systems that model specific regions of the respiratory tract exist, none is currently accepted by regulatory agencies as a standalone replacement for the animal test. Because substances may cause toxicity through differing mechanisms, a single in vitro approach is unlikely to provide sufficient coverage of the chemical universe. Regulatory use also requires consideration of the varying classification systems used in different jurisdictions, complicating the development of sufficiently predictive decision criteria.
Alternative methods have historically been evaluated based on direct comparison to the in vivo test method, despite the recognized inherent variability of in vivo methods and the interspecies uncertainty of predicting human responses based on an animal result. To maximize the utility of in vitro methods for regulatory decision-making, it will be critical to consider potential human exposure during the interpretation of in vitro results and how they relate to potential human effects. Application of IVIVE will be useful in this context.
Additional case study examples, such as those presented below, will be important in showing the regulatory utility of in vitro approaches. Regardless of the in vitro system used, an understanding of in vitro and in vivo dosimetry is critical. In addition, in vitro results may be improved using computer models that take into account bioavailability and metabolic clearance, although these models need to be further developed to reliably predict toxicity from inhalation exposure.
6. Non-testing approaches
Non-testing approaches use existing information to make predictions about the likelihood of a substance to cause toxicity without running tests to generate new data. Non-testing approaches for substances may include (Q)SARs, expert systems, grouping and read-across, and for mixtures, bridging principles and the theory of additivity (United Nations, 2015). These approaches, as shown in the following examples, can substantially reduce the number of animals used for acute inhalation toxicity assessment.
6.1. General opportunities for waivers
Non-testing approaches can be used to waive acute inhalation toxicity testing requirements (OECD, 2016a) based on:
Little or no risk of human inhalation exposure
The theory of additivity (the GHS mixtures equation)
Read-across from a substantially similar, well-characterized mixture or formulated product
Read-across using data from another route of entry, in a weight-of-evidence approach (i.e., using acute oral toxicity data to predict acute inhalation toxicity).
Severe local irritation and corrosivity
Low volatility
Inability to safely generate a toxic concentration (e.g., for a gas or vapor that is explosive or an asphyxiant at the concentrations needed for testing)
Non-inhalable aerosol particle size (if > 99% of particles are > 100 μm aerodynamic diameter and are resistant to mechanical size reduction by attrition)
The above criteria are included in OECD (2016a) and EPA OPP (US EPA, 2012b) guidance documents on waiving or bridging existing acute toxicity data from one chemical or product formulation to a similar substance instead of conducting additional tests. The European Union also provides for waiving acute inhalation tests based on a lack of exposure (Annex VIII, 8.5.2) and general adaptations noted under Annex XI of REACH; for example, use of existing data; weight-of-evidence; grouping and read-across; QSARs; in vitro approaches; or if testing is not technically possible (e.g., the vapor pressure is too low resulting in the inability to generate an atmosphere suitable for testing). For certain industry sectors (e.g., agrochemicals) in the European Union, acute inhalation testing is not a registration requirement unless specific conditions are met. For example, requirements for inhalation testing for agrochemical end-use products are triggered only if the physical state and properties of the product make it likely for the substance to be inhaled (European Commission, 2013).
6.2. Read-across using data from oral route of exposure
Data from one route of exposure can, in certain circumstances, be used to predict the toxicity of the same substance exposed by a different route. Use of acute oral toxicity data, while not definitively establishing toxicity specific to the airway epithelium, may provide a conservative means to categorize acute inhalation toxicity when used in a weight-of-evidence approach, particularly for the most toxic hazard categories. A review of in vivo oral versus inhalation data suggests that, for 30 agrochemical formulations, those chemicals classified as GHS Category I by the oral route are expected to be Category I via the inhalation route and those classified as Category II by the oral route are expected to be classified as Category I or II via the inhalation route (Wilson et al., unpublished data). Therefore, when evaluated using a weight-of-evidence approach with physicochemical properties and other information, agrochemicals that are GHS Category I or II by the oral route may be classified as GHS Category I for the inhalation route. Another study demonstrated that orally non-toxic substances are unlikely to be toxic via the inhalation route (Corvaro et al., 2016). More specifically, 96% (95 out of 98) of products with an oral LD50 > 2000 mg/kg (i.e., GHS not classified) were Not Classified for acute inhalation (LC50 equal to or higher than 5.0 mg/L air). In the remaining case of formulations with an oral LD50 > 2000 mg/kg, 18 out of 25 had LC50 equal or higher than 5.0 mg/L air. Further comparison of curated data from archived acute oral and inhalation toxicity studies across different product types is needed to show how or when oral data may be used in a regulatory context to waive inhalation testing.
6.3. (Q)SAR models for inhalation toxicity
QSAR models can be used to predict a biological effect based on the structure of a chemical. Only a handful of QSAR regression models have been developed for predicting inhalation toxicity. These models are typically only applicable to semi-volatile substances, with parameters such as vapor pressure and boiling point used as descriptors to predict the LC50 value. These QSAR models for inhalation toxicity assume that toxicity occurs by the non-specific mechanism of narcosis and that the LC50 data used as inputs for the models are from tests in which a steady-state concentration has been reached in the blood. An example of one of these QSAR models is the baseline model derived by Veith et al., which relates vapor pressure to the 4-h molar LogLC50 using data from inhalation studies conducted in rats and mice (Veith et al., 2009).
Veith and Wallace (2006a, 2006b) determined that vapor pressure is not a good predictor of LC50 for chemicals that are reactive as electrophiles, in that the inhalation toxicity of such chemicals is underestimated by the baseline model. They were able to establish a QSAR for electrophilic chemicals (such as acrylates) where reactivity was quantified by the RC50 value, the concentration of test compound that produced a 50% reaction of glutathione thiol groups in 120 min in a glutathione depletion assay (Schultz et al., 2005; Veith and Wallace, 2006a, 2006b).
In silico models, such as the OECD QSAR Toolbox, contain profilers, or rules based on structural alerts, that can be useful for creating chemical categories for acute inhalation toxicity. For example, profilers based on the mechanism for acute aquatic toxicity can provide evidence for potential inhalation toxicity by identifying substances acting as non-specific narcotics, electrophilic substances, or substances acting through a specific mechanism. The OECD QSAR Toolbox also contains experimental data on acute inhalation toxicity from ECHA Chem, the Rodent Inhalation Toxicity database, and the Toxicity Japan MHLW.
TOPKAT (or Toxicity Prediction from Komputer Assisted Technology) contains a model based on rat LC50 data with five submodels related to different chemical classes, including (1) single benzenes, (2) heteroaromatics and multiple benzenes, (3) alicyclics, and (4) acyclics with or without halogens (Accelrys Inc., 2004). This model is underpinned by in vivo data from rat studies with exposure times in the range of 0.5–14 h. To normalize the data to adjust for different durations of exposure, it was assumed that toxicity was proportional to duration, per Haber’s Law (Cn × t = k, where n = 1). This normalization ignores the possibility that the slope at the observed time may not be the unit slope (i.e., n does not equal 1), but the approach was a pragmatic one to ensure the broadest coverage. Data presented during the workshop indicated poor sensitivity of the TOPKAT model for GHS class 1–2 compounds when it was challenged using broad chemical categories, suggesting a need to optimize the model and/or better define its applicability domain.
Regardless of the model, there is a need for a better understanding of the mechanisms of acute inhalation toxicity in order to further develop and optimize in silico approaches to predict acute inhalation toxicity potential for a broad spectrum of chemicals.
7. Case studies
This section presents three examples of alternative approaches for predicting toxicity following inhalation exposure: (1) a mathematical approach to justify study waivers for agrochemical formulations; (2) a combined approach using computational dosimetry modeling in conjunction with an in vitro airway epithelium to evaluate an agrochemical formulation; and (3) an integrated in vitro approach to testing next generation tobacco products. These examples illustrate the utility of in silico and in vitro models to predict human toxicity and the potential of these models to reduce and replace animal use.
7.1. Using the GHS additivity approach for classification of agrochemical mixtures
An additivity approach to estimating the toxicity of a chemical mixture assumes that at high single doses, as is the case in an acute systemic toxicity test, toxicity of the components of the mixture is additive. This approach applies a mathematical model to predict the toxicity of a mixture based on the relative contribution (concentration and potency) of each component (Finney, 1952; Pozzani et al., 1959; Smyth et al., 1969). Accordingly, the potential toxicity of a formulation could be estimated and used to apply for study waivers for the formulation without any additional testing.
The GHS additivity formula is essentially a harmonic mean calculation:
where Ci = concentration of ingredient i (%w/w or v/v); i = the individual ingredient from l to n; n = the number of ingredients; and ATEi = acute toxicity estimate of ingredient i.
The acute toxicity estimate for classification of a substance in an inhaled mixture is derived using an LC50 where available; however, a classification category or LC50 range can also be used (United Nations, 2015). From a regulatory use perspective, the theory of additivity approach can be used as a stand-alone replacement for animal tests according to GHS (United Nations, 2015), in the EU Classification, Labeling, and Packaging (CLP) Regulation (European Union, 2008), the New Zealand and Australian regulations on agrochemical formulations (New Zealand Government, 2012; APVMA, 2015), and in global transport regulations (United Nations, 2011).
In this case study of a non-testing approach, 225 agrochemical formulations were retrospectively reviewed and the resulting GHS additivity calculations compared against existing in vivo data (Corvaro et al., 2016). This dataset included 123 acute inhalation studies (122 liquid/dust aerosol and one vapor) with the vast majority of the substances tested having low toxicity (e.g., > 90% were GHS Not Classified based on animal tests). The predicted classifications using the GHS additivity formula were 94.3% accurate for GHS/CLP (3.3% underestimated; 2.4% overestimated) and 96.7% accurate for EPA and the Brazilian National Health Surveillance Agency classifications (1.6% over and underestimated). While this data set included a broad range of different pesticide formulations including herbicides, insecticides, and fungicides, the low number of toxic substances included necessitates the retrospective analysis of additional products using this approach.
Considering this information, the authors proposed an approach to significantly reduce the testing conducted for agrochemical formulations (Corvaro et al., 2016). The approach first considers the need for inhalation data and potential to waive data requirements (OECD, 2016a). If a regulatory agency determines that quantitative hazard characterization is needed, the GHS additivity calculation can be performed to predict acute inhalation (and oral) toxicity. Concentrations of 1.0, 2.0 or 5.0 mg/L would result in human exposures of 5000, 10,000 and 25,000 mg, respectively. According to the classification proposed by Hoffmann and colleagues, 1000, 2000 and 5000 mg/m3 corresponds to the concentration of PM10 dust particle that would be expected, respectively, in a normal, strong, or severe dust storm (ISO, 1995; Hoffmann et al., 2008). As such, the authors propose that when testing is justified, a concentration of 1.0 mg/L air represents a worst-case scenario for agrochemical formulations, since those are tested in concentrated forms and diluted remarkably with water before the actual use in the field. Therefore, if the LC50 is > 1.0 mg/L air, the results may be considered as negative. If the LC50 is < 1.0 mg/L (which is expected in only 2.4% of cases), the weight-of-evidence and physicochemical characteristics should be evaluated to determine whether there is sufficient information to require additional personal protective equipment as opposed to requiring additional testing. Currently, this proposed approach can be used internally within a company (e.g., for formulation design), to meet certain regulatory requirements (e.g., for any EU-only products), and as a tool to predict a starting dose level when animal testing is required by a government agency.
7.2. Evaluating the human health risk following exposure to an irritant aerosol
By default, inhalation dosimetry typically assumes that all of the material in the air is available for inhalation exposure. For non-volatiles, however, material is suspended as an aerosol or particulate, and thus the dosimetry approach used to estimate internal exposure will be different from that used for a volatile gas. The inhalation risk of a non-volatile substance can be assessed using a source-to-outcome approach including the evaluation of the exposure and internal particle size distribution, dosimetry considerations, and in vitro test data.
Exposure-based risk assessment takes into account the expected human exposure level to a substance. Agrochemical exposure in agriculture workers can be monitored using wearable OSHA Versatile Sampler (OVS) tubes. OVS tube data is typically reported only as the total concentration without consideration of the particle size; however, studies have been conducted to compare the concentration determined with use of an OVS tube and standard methods to derive particle size distributions (using internationally accepted sampling conventions for the inhalable, thoracic, and respirable aerosol fractions) (Hewitt, 1995; Brown et al., 2013). Particle size varies with spray nozzle type, with fine sprays being more inhalable than coarse particles. Regardless of the nozzle type, the fraction of particles available for systemic exposure (as defined by thoracic or respirable fractions) is approximately 40% of the total air concentration levels. Thus, the OVS data can be considered as the external exposure, which can be used to calculate the particle amount deposited at the target site.
The particle size distribution in a typical rodent inhalation toxicity study does not accurately represent human exposure to aerosols (Table 7). Human versus rat deposition in the respiratory tract can be estimated using the MPPD model (Anjilvel and Asgharian, 1995; Asgharian and Anjilvel, 1998; Asgharian et al., 2001). The MPPD model calculates the deposited dose of monodisperse and polydisperse aerosols in the respiratory tracts of rats and humans. Within each airway, deposition is calculated using theoretically derived efficiencies for the mechanisms of diffusion, sedimentation, and impaction within the airways or airway bifurcation (Table 8). Inhalability and filtration of aerosols by the nose and mouth is also determined using empirical efficiency functions. Clearance can also be calculated in the MPPD model but is not used in this assessment as data are not available for validation. The lack of clearance is also health protective as it then over-estimates total exposure. For chemicals with known modes of action, dosimetry may be needed at more exact locations in the respiratory tract. CFD models can be used to simulate the deposition of a large number of individual particles at very fine resolutions in the respiratory tract (Corley et al., 2015). Additionally, pharmacokinetic models can be coupled to dosimetry models if enough data are available for quantification within tissues.
Table 7.
Exposure distributions for aerosol exposure mass in size-selected sampling of human use case and rat inhalation studies.
| Droplet size cutoff (mm) | Fraction of total mass below cutoff (%) |
|---|---|
| Fine/medium reference nozzle | |
| 2.5 | 0.77 |
| 10 | 1.03 |
| 30 | 5.5 |
| 100 | 13.8 |
| 150 | 22.4 |
| 200 | 37.3 |
| Rat inhalation study | |
| 0.37 | 16.5 |
| 0.90 | 22.8 |
| 1.6 | 28.4 |
| 3.7 | 40.4 |
| 5.6 | 70.6 |
| 8.0 | 94.1 |
Table 8.
Fraction of total mass deposited in respiratory tract regions of rats and humans. Human exposures are approximately an order of magnitude lower than rodents; conducting and alveolar ratios are many orders of magnitude different.
| Particle type | Exposure | Fraction of total mass | |||
|---|---|---|---|---|---|
| Head | Conducting | Alveolar | Total | ||
| Polydisperse MMAD(GSD) | Rat – 2.33 (4) μm | 0.331 | 0.01 | 0.035 | 0.376 |
| Human – 100 (1.5) μm | 0.035 | 2.3 × 10–6 | 3.1 × 10–10 | 0.035 | |
| Rat:Human Ratio | 9.5 | 10.7 | |||
The exposure and deposition calculations provide a concentration for comparison with toxicology data. In vitro testing can provide such endpoints for inhalation toxicity. For example, commercially-available three-dimensional in vitro reconstructed human tissue models of the upper or lower respiratory tract can be used to assess point-of-contact toxicity following inhalation exposure. Multiple markers of membrane/cell damage and functional competence, such as trans-epithelial electrical resistance and lactate dehydrogenase leakage, can be evaluated. An in vitro POE can be calculated from the in vitro tissue models by using MPPD or CFD to calculate either (1) the equivalent inhalation exposure conditions necessary to give the same surface concentrations in rats or (2) the equivalent inhalation exposure conditions for predicted human exposures.
7.3. In vitro assessment of tobacco and nicotine products
DNA damage and oxidative stress have emerged as important endpoints to consider when assessing the potential toxicity of next-generation nicotine products. Given this emphasis, an opportunity exists to apply a new approach to testing based on mechanistic, human-relevant in vitro assays that could provide necessary regulatory information without using animals. Haswell et al. used NHBE cells cultured at the ALI to assess the impact of tobacco smoke on cell function as a model of goblet cell hyperplasia (Haswell et al., 2010). Repeated exposures over 28 days to cigarette smoke total particulate matter increased the number of mucous-secreting cells as determined by examination of cell morphology by transmission electron microscopy and decreased transepithelial electrical resistance (Haswell et al., 2010). In another study, NCI-H292 human pulmonary epithelial cells were exposed to total cigarette smoke aerosol for 30 min at the ALI. An assessment of oxidative DNA damage using the comet assay showed that cigarette smoke increased DNA damage in a concentration-dependent manner (Thorne et al., 2016). In addition, ALI systems have been used to assess the cytotoxicity of e-cigarettes aerosols compared to reference cigarettes. These systems were used to demonstrate that e-cigarette aerosols were less cytotoxic than cigarette smoke in NCI-H292 cells using a neutral red uptake assay (Azzopardi et al., 2016). E-cigarette aerosols were demonstrated to be less mutagenic than the reference cigarette (Thorne et al., 2016) and caused less DNA damage (Thorne et al., 2017). Three-dimensional reconstructed human airway tissues have also been used to study cytotoxicity in vitro following exposure to e-cigarette aerosol (Neilson et al., 2015; Banerjee et al., 2017). Cytotoxicity assay results showed that while cigarette smoke reduced cell viability in a time-dependent manner, e-cigarette smoke did not (Neilson et al., 2015; Banerjee et al., 2017; Haswell et al., 2017). In addition, studies have demonstrated the metabolic competency of the tissues, in particular, MucilAir™ has been shown to express CYP1A1/1B1 and activity of CYP2A6/2A13 for several months in culture (Baxter et al., 2015). This case study demonstrates the utility of human cells or reconstructed tissue models grown at the ALI to assess various toxicity endpoints, and their use in an integrated approach could prove useful for replacing animal tests.
8. Workshop recommendations
The need for more high-throughput and human-relevant methods that don’t use animals has led to the development of multiple in vitro and in silico approaches to assess acute inhalation toxicity. When designing in vitro or in silico methods to assess acute inhalation toxicity, researchers must consider several factors, including (1) which region of the respiratory tract the test substance will reach and at what dose; (2) whether acute toxicity may include POE or systemic effects involving other organs or organ systems; (3) that mechanisms of exposure generation, respiratory tract delivery, and location of toxicity will vary with the physicochemical properties of the test materials; and (4) that toxicokinetics of inhaled versus orally administered test materials may differ because of POE effects and for systemic effects because there is no first-pass hepatic metabolism associated with test materials absorbed by the upper and lower respiratory tract.
There is growing acceptance of the use of predictive in silico and in vitro models for hazard assessment. In order to be accepted, the models must be robust, reproducible, accurate, and transparent in how the data are generated. Currently, the greatest impact on reducing animal use can be achieved by using the in silico and in vitro models early in the development cycle to flag materials of high hazard, or to identify existing data that are informative of the toxicity of the substance of interest. Although many regulatory agencies still require in vivo testing in a number of contexts, animal-free testing approaches can be incorporated into current testing strategies as a means to validate and optimize the non-animal tests. Additionally, data from non-animal tests can be incorporated into read-across models, further helping to build confidence in the predictive power of the non-animal models and allowing regulatory authorities to see how the methods can be used. Simplification and standardization of non-animal models will facilitate regulatory acceptance. Ultimately, there is a need for government, industry, academics, and non-governmental organizations to work together to advance the implementation and global regulatory acceptance of non-animal approaches.
The purposes of the September 2016 workshop were to bring experts together to discuss experiences using alternative approaches for acute inhalation toxicity testing and how to build upon those approaches to develop strategies that regulatory agencies will accept. Because acute inhalation toxicity data are used for a variety of regulatory and non-regulatory purposes, participants agreed that a key first step in designing any testing strategy is defining the question to be addressed and establishing the specific informational needs for decision-making. Participants also agreed that alternative approaches could be developed to identify POE risks to prioritize compounds that require further assessment or to justify waiving the inhalation toxicity testing requirement altogether.
Ultimately, the workshop discussions were refined into four recommendations, which are listed below. Working groups were established and tasked with follow-up actions to implement each of these recommendations.
Develop a database of existing acute systemic toxicity data. Central to implementing alternative approaches for acute inhalation toxicity testing will be consolidating existing databases and obtaining additional data that have not yet been shared publicly. This working group will catalog the many existing acute toxicity databases and determine the most efficient and user-friendly way to consolidate information relevant to acute inhalation toxicity assessment.
Prepare a state-of-the-science review on mechanisms and available in vitro/in silico models for acute inhalation toxicity. This working group will catalog the available alternative approaches—in vitro, in chemico, and in silico—and define their usefulness and limitations, including the specific applicability domain for each approach. Additionally, the group will detail the numerous mechanisms of action associated with acute inhalation toxicity, with careful consideration of the physicochemical properties of the inhaled agents and the impact of dosimetry, including metabolism and the potential for POE effects. Central to these efforts will be defining relevant adverse outcome pathways that can be used to inform the appropriate integrated testing approach. The resulting review article (in preparation) will discuss mechanisms of acute systemic toxicity in general, as well as focus on specific POE considerations and the influence of physicochemical properties.
Identify and optimize in silico approaches. This working group will identify in silico models that can be used to predict toxicity that may result from general acute toxicity mechanisms (e.g., reactivity) or mechanisms specific to the inhalation route and to predict whether exposure via the inhalation route is feasible based on a substance’s physicochemical properties. This work will directly inform the work of Working Groups 2 and 4.
Develop a decision tree/testing strategy and conduct an in silico and in vitro proof-of-concept study. This working group will design a testing approach to show the utility of in vitro and in silico methods in assessing acute inhalation toxicity. Building off the research of the other three working groups, this group will (1) develop a list of reference chemicals based on curated in vivo data (rat, human, and/or other species) that can be used to interrogate available alternative approaches; (2) develop a decision tree that will inform the need to conduct testing following in silico modeling; (3) select the relevant in vitro assays to include in an integrated approach based on mechanisms of acute inhalation toxicity in humans; and (4) optimize the in vitro and in silico assays and develop standardized protocols that can be used across laboratories. The resulting tiered testing strategy will use both in silico and in vitro models.
Coordination of the working groups and progress on established milestones and timelines is being coordinated by the workshop co-sponsors.
Acknowledgements
The authors thank Catherine Sprankle, ILS, for editing the manuscript. This workshop was co-sponsored by the PETA International Science Consortium Ltd. and NICEATM. This project was funded in part with federal funds from the NIEHS, NIH under Contract No. HHSN273201500010C to ILS in support of NICEATM.
Footnotes
Transparency document
The http://dx.doi.org/10.1016/j.tiv.2017.12.011 associated with this article can be found, in online version.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References
- Accelrys Inc, 2004. Topkat Version 6.2 User Guide. San Diego. [Google Scholar]
- Anjilvel S, Asgharian B, 1995. A multiple-path model of particle deposition in the rat lung. Fundam. Appl. Toxicol 28 (1), 41–50. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, et al. , 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem 29 (3), 730–741. [DOI] [PubMed] [Google Scholar]
- Applied Research Associates Inc, 2017. Multiple-path Particle Dosimetry Model (mppd v 3.04).
- APVMA, 2015. Registration of Agricultural Products, Data Guidelines, Toxicology (Part 3; Version 2) (From). http://apvma/cov/au/node/1036.
- Asgharian B, Anjilvel S, 1998. A multiple-path model of fiber deposition in the rat lung. Toxicol. Sci 44 (1), 80–86. [DOI] [PubMed] [Google Scholar]
- Asgharian B, Hofman W, et al. , 2001. Particle deposition in a multiple-path model of the human lung. Aerosol Sci. Technol 34, 332–339. [Google Scholar]
- Asgharian B, Hofmann W, et al. , 2005. Dosimetry of Particles in Humans: From Children to Adults. Taylor and Francis, Philadelphia PA. [Google Scholar]
- Asgharian B, Price OT, et al. , 2011. Derivation of mass transfer coefficients for transient uptake and tissue disposition of soluble and reactive vapors in lung airways. Ann. Biomed. Eng 39 (6), 1788–1804. [DOI] [PubMed] [Google Scholar]
- Aufderheide M, 2005. Direct exposure methods for testing native atmospheres. Exp. Toxicol. Pathol 57 (Suppl. 1), 213–226. [DOI] [PubMed] [Google Scholar]
- Azzopardi D, Patel K, et al. , 2016. Electronic cigarette aerosol induces significantly less cytotoxicity than tobacco smoke. Toxicol. Mech. Methods 26 (6), 477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Haswell LE, et al. , 2017. Differential gene expression using rna-seq profiling in a reconstituted airway epithelium exposed to conventional cigarette smoke or electronic cigarette aerosols. Applied In Vitro Toxicol. 3 (1), 84–98. [Google Scholar]
- Basketter DA, Clewell H, et al. , 2012. A roadmap for the development of alternative (non-animal) methods for systemic toxicity testing - t4 report*. ALTEX 29 (1), 3–91. [DOI] [PubMed] [Google Scholar]
- Baxter A, Thain S, et al. , 2015. Targeted omics analyses, and metabolic enzyme activity assays demonstrate maintenance of key mucociliary characteristics in long term cultures of reconstituted human airway epithelia. Toxicol. in Vitro 29 (5), 864–875. [DOI] [PubMed] [Google Scholar]
- Becker RA, Ankley GT, et al. , 2015. Increasing scientific confidence in adverse outcome pathways: application of tailored bradford-hill considerations for evaluating weight of evidence. Regul. Toxicol. Pharmacol 72 (3), 514–537. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, 2005. Fiber Toxicology. Taylor and Francis, Philadelphia PA. [Google Scholar]
- BéruBé K, 2011a. Alternatives for lung research: stuck between a rat and a hard place. Altern. Lab. Anim 39 (2), 121–130. [DOI] [PubMed] [Google Scholar]
- BéruBé K, 2011b. Trends in integrated testing strategies for in vitro to in vivo extrapolation in chemical and drug safety for inhalation toxicology. Biotechnol. Int 22, 10–14. [Google Scholar]
- BéruBé K, 2013. Medical waste tissues - breathing life back into respiratory research. Altern. Lab. Anim 41 (6), 429–434. [DOI] [PubMed] [Google Scholar]
- BéruBé K, Aufderheide M, et al. , 2009. In vitro models of inhalation toxicity and disease. The report of a frame workshop. Altern. Lab. Anim 37 (1), 89–141. [PubMed] [Google Scholar]
- BéruBé K, Pitt A, et al. , 2010a. Filter-well technology for advanced three-dimensional cell culture: perspectives for respiratory research. Altern. Lab. Anim 38 (Suppl. 1), 49–65. [DOI] [PubMed] [Google Scholar]
- BéruBé K, Prytherch Z, et al. , 2010b. Human primary bronchial lung cell constructs: the new respiratory models. Toxicology 278 (3), 311–318. [DOI] [PubMed] [Google Scholar]
- Bessems JG, Loizou G, et al. , 2014. Pbtk modelling platforms and parameter estimation tools to enable animal-free risk assessment: recommendations from a joint epaa—eurl ecvam adme workshop. Regul. Toxicol. Pharmacol 68 (1), 119–139. [DOI] [PubMed] [Google Scholar]
- Bogdanffy MS, Jarabek AM, 1995. Understanding mechanisms of inhaled toxicants: implications for replacing default factors with chemical-specific data. Toxicol. Lett 82–83, 919–932. [DOI] [PubMed] [Google Scholar]
- Bogdanffy MS, Keller DA, 1999. Metabolism of Xenobiotics by the Respiratory Tract. Taylor and Francis, Philadelphia PA. [Google Scholar]
- Bogdanffy MS, Sarangapani R, et al. , 1999. A biologically based risk assessment for vinyl acetate-induced cancer and noncancer inhalation toxicity. Toxicol. Sci 51 (1), 19–35. [DOI] [PubMed] [Google Scholar]
- Bond JA, Medinsky MA, 1995. Factors Modifying the Disposition of Inhaled Organic Compounds. Taylor and Francis, Washington DC. [Google Scholar]
- Brain J, Mensah G, 1983. Comparative toxicology of the respiratory tract. Am. Rev. Respir. Dis 128, S87–90. [DOI] [PubMed] [Google Scholar]
- Brown JS, Gordon T, et al. , 2013. Thoracic and respirable particle definitions for human health risk assessment. Part. Fibre Toxicol 10, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JL, Andersen ME, et al. , 2014. A hybrid CFD-PBPK model for naphthalene in rat and human with IVIVE for nasal tissue metabolism and cross-species dosimetry. Inhal. Toxicol 26 (6), 333–344. [DOI] [PubMed] [Google Scholar]
- Campbell J, Van Landingham C, et al. , 2015. A preliminary regional PBPK model of lung metabolism for improving species dependent descriptions of 1,3-butadiene and its metabolites. Chem. Biol. Interact 238, 102–110. [DOI] [PubMed] [Google Scholar]
- Corley RA, Kabilan S, et al. , 2015. Comparative risks of aldehyde constituents in cigarette smoke using transient computational fluid dynamics/physiologically based pharmacokinetic models of the rat and human respiratory tracts. Toxicol. Sci 146 (1), 65–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvaro M, Gehen S, et al. , 2016. Ghs additivity formula: a true replacement method for acute systemic toxicity testing of agrochemical formulations. Regul. Toxicol. Pharmacol 82, 99–110. [DOI] [PubMed] [Google Scholar]
- Crapo JD, Young SL, et al. , 1983. Morphometric characteristics of cells in the aveolar region of mammals. Am. Rev. Respir. Dis 128, S42. [DOI] [PubMed] [Google Scholar]
- Csanady GA, Guengerich FP, et al. , 1992. Comparisons of the biotransformation of 1,3-butadiene and its metabolite, butadiene monexpoxide, by hepatic and pulmonary tissues from humans, rats and mice. Carcinogenesis 13, 1143–1153. [DOI] [PubMed] [Google Scholar]
- Dahl AR, 1990. Dose concepts for inhaled vapors and gases. Toxicol. Appl. Pharmacol 103 (2), 185–197. [DOI] [PubMed] [Google Scholar]
- Dahl AR, 1995. Metabolic Characteristics of the Respiratory Tract. Taylor and Francis, Washington DC. [Google Scholar]
- Diabaté S, Muelhopt S, et al. , 2008. The response of a co-culture lung model to fine and ultrafine particles of incinerator fly ash at the air-liquid interface. Altern. Lab. Anim 36 (3), 285–298. [DOI] [PubMed] [Google Scholar]
- Doiron T, 2007. 20 degrees c-a short history of the standard reference temperature for industrial dimensional measurements. J. Res. Natl. Inst. Stand. Technol 112 (1), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch EW, Bahinski A, et al. , 2015. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov 14, 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essaidi-Laziosi M, Brito F, et al. , 2017. Propagation of respiratory viruses in human airway epithelia reveals persistent virus-specific signatures. J. Allergy Clin. Immunol 10.1016/j.jaci.2017.07.018. https://www.ncbi.nlm.nih.gov/pubmed/28797733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission, 2013. Commission Regulation (Eu) no 283/2013 of 1 March 2013 Setting out the Data Requirements for Active Substances, in Accordance with Regulation (ec) no 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market (From). http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:093:0001:0084:EN:PDF.
- European Union, 2008. European union (eu). In: Regulation (ec) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures. [Google Scholar]
- Finney DJ, 1952. Probit analysis. In: Hayes’ Principles and Methods of Toxicology, 6th edition. Cambridge University Press, London U.K. [Google Scholar]
- Fiserova-Bergovera V, 1983a. Fundamentals in modeling. FL, CRC Press Inc, Boca Raton. [Google Scholar]
- Fiserova-Bergovera V, 1983b. Physicochemical Properties and Biological Activity. CRC Press Inc, Boca Raton, FL. [Google Scholar]
- Fisher AR, 1995. Lung Biochemistry and Intermediary Metabolism for Concepts in Toxicology. Taylor and Francis, Washington DC. [Google Scholar]
- Fisher RL, Smith MS, et al. , 1994. The use of human lung slices in toxicology. Hum. Exp. Toxicol 13 (7), 466–471. [DOI] [PubMed] [Google Scholar]
- Frederick CB, Lomax LG, et al. , 2002. Use of a hybrid computational fluid dynamics and physiologically based inhalation model for interspecies dosimetry comparisons of ester vapors. Toxicol. Appl. Pharmacol 183 (1), 23–40. [DOI] [PubMed] [Google Scholar]
- Fröhlich E, Salar-Behzadi S, 2014. Toxicological assessment of inhaled nanoparticles: role of in vivo, ex vivo, in vitro, and in silico studies. Int. J. Mol. Sci 15, 4795–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DE, 2005. Toxicology of the Lung. Taylor and Francis, Philadelphia PA. [Google Scholar]
- Gardner DE, Crapo JA, et al. , 1999. Toxicology of the Lung. Taylor and Francis, Philadelphia PA. [Google Scholar]
- Gordon S, Daneshian M, et al. , 2015. Non-animal models of epithelial barriers (skin, intestine and lung) in research, industrial applications and regulatory toxicology. ALTEX 32 (4), 327–378. [DOI] [PubMed] [Google Scholar]
- Hamm J, Sullivan K, et al. , 2017. Alternative approaches for identifying acute systemic toxicity: moving from research to regulatory testing. Toxicol. in Vitro 41, 245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna LM, Lou SR, et al. , 2001. Mass transport analysis: inhalation rfc methods framework for interspecies dosimetric adjustment. Inhal. Toxicol 13 (5), 437–463. [DOI] [PubMed] [Google Scholar]
- Harkema JR, 1999. Comparative Structure, Function and Toxicity of the Nasal Airways: Predicting its Effect on Laboratory Animal Studies. Taylor and Francis, Washington DC. [Google Scholar]
- Harkema JR, Carey SA, et al. , 2006. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol. Pathol 34 (3), 252–269. [DOI] [PubMed] [Google Scholar]
- Haswell LE, Hewitt K, et al. , 2010. Cigarette smoke total particulate matter increases mucous secreting cell numbers in vitro: a potential model of goblet cell hyperplasia. Toxicol. in Vitro 24 (3), 981–987. [DOI] [PubMed] [Google Scholar]
- Haswell LE, Baxter A, et al. , 2017. Reduced biological effect of e-cigarette aerosol compared to cigarette smoke evaluated in vitro using normalized nicotine dose and rna-seq-based toxicogenomics. Sci. Rep 7 (1), 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt AJ, 1995. Spray Drift Task Force Atomization Droplet Size Spectra for Selected Active Ingredients. (Sdtf study number a92–004. Epa mrid no. 43766502). [Google Scholar]
- Hinderliter PM, Minard KR, et al. , 2010. Isdd: a computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studies. Part. Fibre Toxicol 7 (1), 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Funk R, et al. , 2008. The effects of grazing on wind driven carbon and nitrogen ratios in the grasslands of inner Mongolia. Catena 75 (2), 182–190. [Google Scholar]
- Huh D, Matthews BD, et al. , 2010. Reconstituting organ-level lung functions on a chip. Science 328 (5986), 1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D, Leslie DC, et al. , 2012. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci. Transl. Med 4 (159) (159ra147). [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Commission on Radiological Protection (ICRP), 1994. Human Respiratory Tract Model for Radiological Protection Annals of the ICRP. 24. pp. 1–3. [PubMed] [Google Scholar]
- ISO, 1995. Iso 7708:1995: Air Quality – Particle Size Fraction Definitions for Health-related Sampling. (Iso/tc 146/sc 2, stage 90.92). [Google Scholar]
- Jarabek AM, 1995a. The application of dosimetry models to identify key processes and parameters for default dose-response assessment approaches. Toxicol. Lett 79 (1–3), 171–184. [DOI] [PubMed] [Google Scholar]
- Jarabek AM, 1995b. Consideration of temporal toxicity challenges current default assumptions. Inhal. Toxicol 7, 927–946. [Google Scholar]
- Jarabek AM, Asgharian B, et al. , 2005. Dosimetric adjustments for interspecies extrapolation of inhaled poorly soluble particles (psp). Inhal. Toxicol 17 (7–8), 317–334. [DOI] [PubMed] [Google Scholar]
- Kimbell JS, Miller FJ, 1999. Regional Respiratory-tract Absorption of Inhaled Reactive Gases: A Modeling Approach. Taylor and Francis, Philadelphia PA. [Google Scholar]
- Kimbell JS, Gross EA, et al. , 1997. Correlation of regional formaldehyde flux predictions with the distribution of formaldehyde-induced squamous metaplasia in f344 rat nasal passages. Mutat. Res 380 (1–2), 143–154. [DOI] [PubMed] [Google Scholar]
- Kimbell JS, Overton JH, et al. , 2001a. Dosimetry modeling of inhaled formaldehyde: binning nasal flux predictions for quantitative risk assessment. Toxicol. Sci 64 (1), 111–121. [DOI] [PubMed] [Google Scholar]
- Kimbell JS, Subramaniam RP, et al. , 2001b. Dosimetry modeling of inhaled formaldehyde: comparisons of local flux predictions in the rat, monkey, and human nasal passages. Toxicol. Sci 64 (1), 100–110. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CJ, Pohl C, et al. , 2008. Co-culture model of the human bronchial epithelium: cytokine-dependent reduction of ciliary and barrier function. FASEB J. 22 (1_MeetingAbstracts) (1214–1211). [Google Scholar]
- Kuehn D, Majeed S, et al. , 2015. Impact assessment of repeated exposure of organotypic 3d bronchial and nasal tissue culture models to whole cigarette smoke. J. Vis. Exp 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuempel ED, Sweeney LM, et al. , 2015. Advances in inhalation dosimetry models and methods for occupational risk assessment and exposure limit derivation. J. Occup. Environ. Hyg 12 (Suppl. 1), S18–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann AD, Daum N, et al. , 2011. An in vitro triple cell co-culture model with primary cells mimicking the human alveolar epithelial barrier. Eur. J. Pharm. Biopharm 77 (3), 398–406. [DOI] [PubMed] [Google Scholar]
- Lin JC, Roy JP, et al. , 2012. An ex vivo approach to the differential parenchymal responses induced by cigarette whole smoke and its vapor phase. Toxicology 293 (1–3), 125–131. [DOI] [PubMed] [Google Scholar]
- Loizou G, Spendiff M, et al. , 2008. Development of good modelling practice for physiologically based pharmacokinetic models for use in risk assessment: the first steps. Regul. Toxicol. Pharmacol 50 (3), 400–411. [DOI] [PubMed] [Google Scholar]
- Mathis C, Poussin C, et al. , 2013. Human bronchial epithelial cells exposed in vitro to cigarette smoke at the air-liquid interface resemble bronchial epithelium from human smokers. Am. J. Phys. Lung Cell. Mol. Phys 304 (7), L489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan RO, Henderson RF, 1995. Concepts in Inhalation Toxicology. Taylor and Francis, Washington DC. [Google Scholar]
- Medinsky MA, Bond JA, et al. , 1999. Mechanisms and Models for Respiratory Tract Uptake of Volatile Organic Chemicals. Taylor and Francis, Philadelphia PA. [Google Scholar]
- Mercer RR, Russell ML, et al. , 1994. Alveolar septal structure in different species. J Appl Physiol (1985) 77 (3), 1060–1066. [DOI] [PubMed] [Google Scholar]
- Miller FJ, 1995. Nasal Toxicology and Dosimetry of Inhaled Xenobiotics. CRC Press, Washington DC. [Google Scholar]
- Miller FJ, 1999. Dosimetry of Particles in Laboratory Animals and Humans. Taylor and Francis, Philadelphia PA. [Google Scholar]
- Miller FJ, Kimbell JS, 1995. Regional Dosimetry of Inhaled Reactive Gases. Taylor and Francis, Washington DC. [Google Scholar]
- Morris JB, 2012. Biologically-based modeling insights in inhaled vapor absorption and dosimetry. Pharmacol. Ther 136 (3), 401–413. [DOI] [PubMed] [Google Scholar]
- Morris JB, Shusterman DJ, 2010. Toxicology of the Nose and Upper Airways. Informa Healthcare, New York. [Google Scholar]
- Morris JB, Asgharian B, et al. , 2010. Upper Airway Dosimetry of Gases, Vapors, and Particulate Matter in Rodents. Informa Healthcare, New York. [Google Scholar]
- National Research Council, 2015. Application of Modern Toxicology Approaches for Predicting Acute Toxicity for Chemical Defense (From). https://www.nap.edu/catalog/21775/application-of-modern-toxicology-approaches-for-predicting-acute-toxicity-for-chemical-defense. [PubMed]
- Neilson L, Mankus C, et al. , 2015. Development of an in vitro cytotoxicity model for aerosol exposure using 3d reconstructed human airway tissue; application for assessment of e-cigarette aerosol. Toxicol. in Vitro 29 (7), 1952–1962. [DOI] [PubMed] [Google Scholar]
- New Zealand Government, 2012. Thresholds and Classifications Under the Hazardous Substances and New Organisms Act 1996 (From). http://www.epa.govt.nz/Publications/ER-UG-03-2.pdf.
- Niinimaa V, Cole P, et al. , 1981. Oronasal distribution of respiratory airflow. Respir. Physiol 43 (1), 69–75. [DOI] [PubMed] [Google Scholar]
- NTP, 2017. Iccvam Member Agencies (From). https://ntp.niehs.nih.gov/pubhealth/evalatm/iccvam/iccvam-agencies/index.html.
- Oberdorster G, Ferin J, et al. , 1994. Correlation between particle size, in vivo particle persistence, and lung injury. Environ. Health Perspect 102 (Suppl. 5), 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G, Maynard A, et al. , 2005. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part. Fibre Toxicol 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD, 2009a. Test No. 403: Acute Inhalation Toxicity. OECD Guidelines for the Testing of Chemicals, Section 4 (From). http://www.oecd-ilibrary.org/environment/test-no-403-acute-inhalation-toxicity_9789264070608-en.
- OECD, 2009b. Test No. 436: Acute Inhalation Toxicity – Acute Toxic Class Method. OECD Guidelines for the Testing of Chemicals, Section 4 (From). http://www.oecd-ilibrary.org/environment/test-no-436-acute-inhalation-toxicity-acute-toxic-class-method_9789264076037-en.
- OECD, 2016a. No. 237: Guidance Document on Considerations for Waiving or Bridging of Mammalian Acute Toxicity Tests OECD Guidelines for the Testing of Chemicals, Section 4 (From). http://www.oecd.org/env/ehs/testing/mono%202016%2032.pdf.
- OECD, 2016b. Users’ Handbook Supplement to the Guidance Document for Developing and Assessing Aops (From). http://www.oecd-ilibrary.org/environment/users-handbook-supplement-to-the-guidance-document-for-developing-and-assessing-adverse-outcome-pathways_5jlv1m9d1g32-en.
- OECD, 2017. Test no. 433: acute inhalation toxicity - fixed concentration procedure. In: OECD Guidelines for the Testing of Chemicals, Section 4, (From). http://www.oecd-ilibrary.org/environment/test-no-433-acute-inhalation-toxicity-fixed-concentration-procedure_9789264284166-en.
- Overton JH, 2001. Dosimetry modeling of highly soluble reactive gases in the respiratory tract. Inhal. Toxicol 13 (5), 347–357. [DOI] [PubMed] [Google Scholar]
- Overton JH, Kimbell JS, et al. , 2001. Dosimetry modeling of inhaled formaldehyde: the human respiratory tract. Toxicol. Sci 64 (1), 122–134. [DOI] [PubMed] [Google Scholar]
- Parent RA, 2015. Comparative Biology of the Normal Lung. Academic Press, New York. [Google Scholar]
- Patlewicz G, Simon TW, et al. , 2015. Proposing a scientific confidence framework to help support the application of adverse outcome pathways for regulatory purposes. Regul. Toxicol. Pharmacol 71 (3), 463–477. [DOI] [PubMed] [Google Scholar]
- Phalen RF, Yeh H-C, et al. , 1995. Morphology of the Respiratory Tract. Taylor and Francis, Washington DC. [Google Scholar]
- Plopper CG, Hill LH, et al. , 1980. Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung. III. A study of man with comparison of 15 mammalian species. Exp. Lung Res 1 (2), 171–180. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Suverkropp C, et al. , 1992. Relationship of cytochrome p-450 activity to Clara cell cytotoxicity. I. Histopathologic comparison of the respiratory tract of mice, rats and hamsters after parenteral administration of naphthalene. J. Pharmacol. Exp. Ther 261 (1), 353–363. [PubMed] [Google Scholar]
- Polk WW, Sharma M, et al. , 2016. Aerosol generation and characterization of multi-walled carbon nanotubes exposed to cells cultured at the air-liquid interface. Part. Fibre Toxicol 13, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzani UC, Weil CS, et al. , 1959. The toxicological basis of the threshold limit values: 5. The experimental inhalation f vapor mixtures by rats, with notes upon the relationship between single dose inhalation and single dose oral data. Am. Ind. Hyg. Assoc. J 20, 364–369. [DOI] [PubMed] [Google Scholar]
- Prytherch Z, 2010. In Vitro NHBE Model of the Human Bronchial Epithelium for Toxicological Testing (PhD). Cardiff University. [Google Scholar]
- Prytherch Z, BéruBé K, 2014a. Advances in in-vitro 3d lung cell culture. In: Haycock J, Ahluwalia A, Wilkinson MJ (Eds.), Cellular In Vitro Testing: Methods and Protocols. Pan Stanford Publishing, Singapore. [Google Scholar]
- Prytherch ZC, BéruBé KA, 2014b. A normal and biotransforming model of the human bronchial epithelium for the toxicity testing of aerosols and solubilised substances. Altern. Lab. Anim 42 (6), 377–381. [DOI] [PubMed] [Google Scholar]
- Prytherch Z, BéruBé K, 2015. 3-Dimensional Cell and Tissue Culture Models of the Human Respiratory System: Approaches for In Vitro Safety Testing and Drug Discovery. Royal Society of Chemistry Publishing, Cambridge. [Google Scholar]
- Prytherch Z, Job C, et al. , 2011. Tissue-specific stem cell differentiation in an in vitro airway model. Macromol. Biosci 11 (11), 1467–1477. [DOI] [PubMed] [Google Scholar]
- Rach J, Budde J, et al. , 2014. Direct exposure at the air–liquid interface: evaluation of an in vitro approach for simulating inhalation of airborne substances. J. Appl. Toxicol 34 (5), 506–515. [DOI] [PubMed] [Google Scholar]
- Ramoju SP, Mattison DR, et al. , 2017. The application of PBPK models in estimating human brain tissue manganese concentrations. Neurotoxicology 58, 226–237. [DOI] [PubMed] [Google Scholar]
- Sarangapani R, Clewell HJ, et al. , 2002a. Comparing respiratory-tract and hepatic exposure-dose relationships for metabolized inhaled vapors: a pharmacokinetic analysis. Inhal. Toxicol 14 (8), 835–854. [DOI] [PubMed] [Google Scholar]
- Sarangapani R, Teeguarden JG, et al. , 2002b. Physiologically based pharmacokinetic modeling of styrene and styrene oxide respiratory-tract dosimetry in rodents and humans. Inhal. Toxicol 14 (8), 789–834. [DOI] [PubMed] [Google Scholar]
- Schlesinger RB, 1995. Deposition and Clearance of Inhaled Particles. Taylor and Francis, Washington DC. [Google Scholar]
- Schroeter JD, Kimbell JS, et al. , 2006. Incorporation of tissue reaction kinetics in a computational fluid dynamics model for nasal extraction of inhaled hydrogen sulfide in rats. Toxicol. Sci 90 (1), 198–207. [DOI] [PubMed] [Google Scholar]
- Schroeter JD, Garcia GJ, et al. , 2010. A computational fluid dynamics approach to assess interhuman variability in hydrogen sulfide nasal dosimetry. Inhal. Toxicol 22 (4), 277–286. [DOI] [PubMed] [Google Scholar]
- Schultz TW, Yarbrough JW, et al. , 2005. Structure-activity relationships for reactivity of carbonyl-containing compounds with glutathione. SAR QSAR Environ. Res 16 (4), 313–322. [DOI] [PubMed] [Google Scholar]
- Sewell F, Ragan I, et al. , 2015. A global initiative to refine acute inhalation studies through the use of ‘evident toxicity’ as an endpoint: towards adoption of the fixed concentration procedure. Regul. Toxicol. Pharmacol 73 (3), 770–779. [DOI] [PubMed] [Google Scholar]
- Smyth HJ, Weil CS, et al. , 1969. An exploration of joint toxic action: twenty-seven industrial chemicals intubated in rats of all possible pairs. Toxicol. Appl. Pharmacol 14, 340–347. [DOI] [PubMed] [Google Scholar]
- Snipes MB, 1995. Pulmonary Retention of Particles: Biokinetics and Effects of Exposure Concentration. Taylor and Francis, Washington DC. [Google Scholar]
- Teeguarden JG, Hinderliter PM, et al. , 2007. Particokinetics in vitro: dosimetry considerations for in vitro nanoparticle toxicity assessments. Toxicol. Sci 95 (2), 300–312. [DOI] [PubMed] [Google Scholar]
- Thorne D, Crooks I, et al. , 2016. The mutagenic assessment of an electronic-cigarette and reference cigarette smoke using the ames assay in strains ta98 and ta100. Mutat. Res 812, 29–38. [DOI] [PubMed] [Google Scholar]
- Thorne D, Larard S, et al. , 2017. The comparative in vitro assessment of e-cigarette and cigarette smoke aerosols using the gammaH2AX assay and applied dose measurements. Toxicol. Lett 265, 170–178. [DOI] [PubMed] [Google Scholar]
- United Nations, 2011. United nations (un). Model regulations. In: Recommendations on the Transport of Dangerous Goods, 17th revised edition. Vol i St/sg/ac.10/1/rev.17. (New York and Geneva: ). [Google Scholar]
- United Nations, 2015. Globally Harmonized System of Classification and Labelling of Chemicals, 6th revised edition. (From). www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev06/English/ST-SG-AC10-30-Rev6e.pdf. [Google Scholar]
- US EPA, 1994. Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry (600-8-90-066f) (From). https://www.epa.gov/risk/methods-derivation-inhalation-reference-concentrations-and-application-inhalation-dosimetry.
- US EPA, 2012a. Advances in Inhalation Gas Dosimetry for Derivation of a Reference Concentration (rfc) and Use in Risk Assessment (epa/600/r-12/044) (From). https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=244650.
- US EPA, 2012b. Guidance for Waiving or Bridging of Mammalian Acute Toxicity Tests for Pesticides and Pesticide Products (Acute Oral, Acute Dermal, Acute Inhalation, Primary Eye, Primary Dermal, and Dermal Sensitization). From. http://www.epa.gov/sites/production/files/documents/acute-data-waiver-guidance.pdf.
- US EPA, 2013. Guiding Principles for Data Requirements (From). http://www.epa.gov/sites/production/files/2015-04/documents/data-require-guide-principle.pdf.
- US EPA, 2016a. Letter to Stakeholders on EPA Office of Pesticide Programs’s Goal to Reduce Animal Testing From Jack E. Housenger, Director Office of Pesticide Programs (From). https://www.epa.gov/pesticides/new-epa-guidance-testing-pesticides-will-reduce-animal-testing.
- US EPA, 2016b. Mixtures Equation Pilot Program to Reduce Animal Testing (From). https://www.epa.gov/pesticide-registration/mixtures-equation-pilot-program-reduce-animal-testing.
- US EPA, 2016c. Process for Establishing and Implementing Alternative Approaches to Traditional In Vivo Acute Toxicity Studies for Fifra Regulatory Use (From). https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/process-establishing-implementing-alternative.
- Veith GD, Wallace KB, 2006a. Inhalation Toxicity in Fish and Rodents; Presented at the Mckim Conference; June 27–29, 2006; Duluth, Mn. 2006. [Google Scholar]
- Veith GD, Wallace KB, 2006b. Inhalation Toxicity in Fish and Rodents; Presented at the Mckim Conference; June 27–29, 2006; Duluth, Mn. 2006. [Google Scholar]
- Veith GD, Petkova EP, et al. , 2009. A baseline inhalation toxicity model for narcosis in mammals. SAR QSAR Environ. Res 20 (5–6), 567–578. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Crump D, et al. , 2014a. Adverse outcome pathway (AOP) development i: strategies and principles. Toxicol. Sci 142 (2), 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve DL, Crump D, et al. , 2014b. Adverse outcome pathway development II: best practices. Toxicol. Sci 142 (2), 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinken M, 2013. The adverse outcome pathway concept: a pragmatic tool in toxicology. Toxicology 312, 158–165. [DOI] [PubMed] [Google Scholar]
- Vinken M, Blaauboer BJ, 2017. In vitro testing of basal cytotoxicity: establishment of an adverse outcome pathway from chemical insult to cell death. Toxicol. in Vitro 39, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambaugh JF, Wetmore BA, et al. , 2015. Toxicokinetic triage for environmental chemicals. Toxicol. Sci 147 (1), 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warheit DE, 2005. Effects of engineered nanoscale particulates on the lung. In: DE G (Ed.), Toxicology of the Lung Taylor and Francis, Philadelphia PA. [Google Scholar]
- Wetmore BA, Wambaugh JF, et al. , 2015. Incorporating high-throughput exposure predictions with dosimetry-adjusted in vitro bioactivity to inform chemical toxicity testing. Toxicol. Sci 148 (1), 121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann M, Vennemann A, et al. , 2016. An in vitro alveolar macrophage assay for predicting the short-term inhalation toxicity of nanomaterials. J Nanobiotechnol. 14, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittwehr C, Aladjov H, et al. , 2017. How adverse outcome pathways can aid the development and use of computational prediction models for regulatory toxicology. Toxicol. Sci 155 (2), 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Williams NG, et al. , 2012. Aerosolized ZnO nanoparticles induce toxicity in alveolar type II epithelial cells at the air-liquid interface. Toxicol. Sci 125 (2), 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M, Campbell JL, et al. , 2012. Quantitative in vitro to in vivo extrapolation of cell-based toxicity assay results. Crit. Rev. Toxicol 42 (8), 633–652. [DOI] [PubMed] [Google Scholar]


