Abstract
Background and Aims:
A large proportion of statin eligible candidates have a baseline absence of coronary artery calcium (CAC) and low 10-year atherosclerotic cardiovascular disease (ASCVD) risk. We sought to determine the proportion of statin eligible individuals who had long-term healthy arterial aging (persistent CAC=0) and their examine 15-year ASCVD outcomes.
Methods:
We included 561 statin eligible candidates from the Multi-Ethnic Study of Atherosclerosis who were not on statin therapy with CAC=0 at Visit 1 (2000–02) and underwent a subsequent CAC scan at Visit 5 (2010–11). Adjusted Cox proportional hazards regression assessed the association between persistent CAC=0 and ASCVD events over 15.9 years.
Results:
Participants were on average 61.7 years old, 50% were women, and 43% maintained long-term CAC=0. Individuals with an LDL-C ≥190 mg/dL (54%) and those with an ASCVD risk ≥20% (33%) had the highest and lowest proportion of persistent CAC=0, respectively. There were 57 ASCVD events and 15-year ASCVD event rates were low for individuals with and without healthy arterial aging (4.3 versus 8.6 per 1,000 persons-years), but the 10-year number needed to treat to prevent one ASCVD event differed by more than two-fold (117 versus 54). In multivariable modeling, persistent CAC=0 conferred a 51% lower risk of ASCVD compared to those with incident CAC (HR=0.49, 95% CI: 0.27–0.90, p=0.02).
Conclusions:
More than 40% of statin eligible individuals with baseline CAC=0 had long-term healthy arterial aging. Statin eligible candidates with persistent CAC=0 had a very low 15-year ASCVD risk, suggesting that statin therapy may be of limited benefit among this group of individuals.
Keywords: coronary artery calcium, aging, prevention, cardiovascular diseases, multidetector computed tomography, statins
Introduction
An estimated 44.2 million (42%) of U.S. adults between 40 to 75 years of age are eligible for statin pharmacotherapy(1) for the primary prevention of atherosclerotic cardiovascular disease (ASCVD), which is the leading cause of morbidity and mortality in the nation(2). Assessment of ASCVD risk is the first step towards creating a personalized approach for primary prevention treatment strategies. While increasing age, type 2 diabetes, hyperlipidemia, hypertension, and tobacco use are associated with an increased ASCVD risk, these traditional factors incompletely describe an individual’s ASCVD risk and there is significant heterogeneity among individuals with such risk factors(3–6). For example, up to half of middle-aged individuals with familial hypercholesterolemia and approximately 40% of middle-aged adults with metabolic syndrome or diabetes do not have coronary artery calcium (CAC)(4) upon cross-sectional assessment(5).
Measurement of CAC is a non-invasive imaging approach that improves ASCVD risk stratification beyond traditional risk factors and is recommended by the 2018 AHA/ACC Management of Cholesterol Guideline(7), 2019 ACC/AHA Prevention of Cardiovascular Disease Guidelines(8), and European Society of Cardiology Guidelines for the Management of Dyslipidaemias(9) when there is uncertainty regarding ASCVD risk. The absence of CAC (CAC=0)(10) is associated with a low 10-year ASCVD risk and can “de-risk” individuals aged 45–75 years old who are classified as moderate-high risk by traditional ASCVD risk factor-based risk scores such as the Pooled Cohort Equations (PCE)(8). Approximately 40% of statin eligible individuals between 40–75 years of age have CAC=0 with an associated low 10-year ASCVD event rate, but little is known with regards to how many of these individuals maintain long-term healthy arterial aging and whether their ASCVD event rate remains low beyond 10-years follow-up(11,12). Furthermore, statin therapy is recommended for persons with type 2 diabetes or a low-density lipoprotein (LDL-C) ≥190 mg/dL regardless of their estimated ASCVD and accordingly CAC scoring is not recommended. However, previous studies have demonstrated that between 37–41% of these individuals do not have any CAC, suggesting significant heterogeneity in ASCVD risk (3,13). Compared to a non-CAC based risk estimation approach, a decision making process on the initiation of statin pharmacotherapy that includes CAC has been shown to provide higher cost-effectiveness and quality-adjusted life expectancy for individuals who have an intermediate 10-year ASCVD risk, while also reducing daily pill burden and potential significant side effects, such as myalgias (14,15).
Therefore, we sought to determine 1) the proportion of statin eligible candidates who maintained persistent CAC=0 over 10-years follow-up (healthy arterial aging), 2) whether the proportion of individuals with healthy arterial aging varies by statin eligibility subtype, and 3) whether there is a significant difference in very long-term ASCVD risk among individuals with and without long-term healthy arterial aging. A better understanding of healthy arterial aging and its associated long-term ASCVD risk among statin eligible individuals may help to develop a more individualized approach for primary prevention treatment strategies.
Methods
Study Population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study of non-institutionalized men and women, aged 45–84 years old, who were free of clinical ASCVD upon study entry. Details regarding the study design and methodology of MESA have been previously described elsewhere(16). Participants eligible for this analysis had CAC=0 at Visit 1 and attended Visit 5 at which time randomization to a repeat CAC scan occurred (Supplemental Figure 1). Individuals ≥75 years of age (n=51) were excluded because this demographic group is not included in the current ASCVD guidelines(8). Participants already on statin therapy at baseline (n=202) and those who were not among one of the four statin benefit groups were also excluded (n=1,036).
Statin eligibility was defined according to the 2018 Management of Cholesterol(7) and 2019 ACC/AHA Primary Prevention Guidelines(8) as a 1) 10-year ASCVD risk ≥ 20%, 2) 10-year ASCVD risk between 7.5% to 20%, 3) type 2 diabetes mellitus, or 4) a low-density lipoprotein cholesterol (LDL-C) ≥190 mg/dL. In this study, type 2 diabetes mellitus was defined by a fasting blood glucose ≥126 mg/dL or use of a blood glucose lowering medication, as hemoglobin A1c measurements were not obtained at MESA Visit 1 (16–18). All participants provided written informed consent, and protocols were approved by site-specific Institutional Review Boards at MESA-participating institutions.
Measurement of coronary and thoracic calcification
The entire MESA cohort underwent a CAC scan at Visit 1 (2000–2002). Of these participants, half of those who attended Visit 5 (2010–2012) were randomized to undergo a repeat CAC scan without regard to their participant characteristics, ASCVD risk factor profile, or occurrence of an ASCVD event between Visits 1 and 5. Calcium scores were calculated using the Agatston method and a calcium phantom was used as a means to standardize results among MESA field centers(19,20). Electron beam computed tomography was used at the Chicago, Los Angeles, and New York field centers, while multidetector computed tomography was used at the Baltimore, Forsyth County, and St. Paul field centers to acquire CAC scans. The Kappa statistics for inter- and intra-observer agreement for the electron beam and multidetector computed tomography scanners were 0.93 and 0.90, respectively, indicative of robust rescan agreement(10,21).
Ascertainment of ASCVD Outcomes
MESA participants and/or family members of participants were contacted by study staff via telephone to ascertain hospital admissions, outpatient ASCVD diagnoses, and deaths every nine to twelve months. The telephone interview response rate was 92% and medical information on nearly all inpatient events (98%) and outpatient ASCVD diagnoses (95%) was obtained(22). Events were adjudicated independently by two separate MESA physicians on the morbidity and mortality review committee. Disagreements were resolved by the full review committee. Incident ASCVD events were defined by definite or probable myocardial infarction, resuscitated cardiac arrest, fatal coronary heart disease (CHD), fatal and non-fatal stroke, and other atherosclerotic or cardiovascular death. CHD events were defined as angina, myocardial infarction, resuscitated cardiac arrest, or fatal CHD. A further description(16) of the adjudication protocol and categorization of events is available on the MESA website (www.mesa-nhlbi.org).
General Clinical Examination and ASCVD Measures
Demographic and clinical information, including sex, race, education status, income, smoking status, and medication utilization history, were collected using standardized survey methods13. Height and weight were measured in duplicate using a standardized protocol and were averaged to calculate body mass index. Blood pressure was measured in triplicate on the right arm after participants rested in the seated position for five minutes, and the average of the second and third readings was used in analyses. Fasting blood glucose was measured using the Vitros 950 analyzer (Johnson & Johnson, Rochester, NY)14. Fasting lipid values were measured using the cholesterol oxidase method (Roche Diagnostics)14 and low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation15.
Statistical Analysis
Differences between categorical variables were evaluated through the Chi-square test. Differences in normally and non-normally distributed continuous variables were assessed through the Student’s t-test and Wilcoxon signed-rank test, respectively.
For the main analyses, we compared participants with and without the long-term absence of CAC (CAC=0) and those with incident CAC, ASCVD, and CHD events were expressed as absolute numbers and proportions for the overall study sample and according to CAC category. The total number of events was divided by person-years to calculate ASCVD and CHD event rates (per 1,000-year follow-up). Kaplan-Meier survival curves were computed for ASCVD and CHD events among individuals with long-term absence of CAC and those with incident CAC. Differences in survival between long-term absence of CAC and incident CAC groups were assessed through the log-rank test.
Multivariable Cox proportional hazards regression was used to estimate the hazard of ASCVD and CHD events associated with long-term absence of CAC. The proportional hazards assumption was satisfied and was tested by assessing the significance of time-dependent independent variables concurrently. The association of long-term absence of CAC with ASCVD and CHD events was assessed in two models: model 1, adjusting for age, race, sex, and model 2, adjusting for age, race, sex, educational attainment, income, body mass index, total cholesterol, high-density lipoprotein-cholesterol, fasting blood glucose, systolic blood pressure, diastolic blood pressure, cigarette smoking, and blood pressure- and glucose-lowering medications.
A number needed to treat (NNT) analysis for statin LDL-C lowering was conducted for ASCVD and CHD using the Altman-Anderson method(23). A primary prevention hazard ratio associated with a 30% relative event reduction for every 1 mmol/L lower LDL-C was used for the NNT analysis(24). We calculated the NNT using a follow-up time of 10.9 years, rather than the complete MESA follow up time of 15.9 years, because 10-years is the risk estimate timeframe recommended by the ACC/AHA guidelines. In addition, this timeframe is most easily interpretable from a clinical standpoint and also more directly comparable to previously published reports(11). Among participants with persistent CAC=0, there were only four CHD events over 15.9 years follow-up and only 1 CHD event within the first 10.9 years follow-up (at 4.6 years follow-up). Given the relatively small sample size and very small number of CHD events in this group, we used a follow-up time of 12 years for this group, which incorporated one additional CHD event (at follow-up time 11.5 years), in order to provide a more conservative an estimate of the 10-year NNT.
We conducted three a priori sensitivity analyses. First, we calculated event rates and assessed the association of CAC with ASCVD and CHD events using a three-level ordinal independent variable, differentiating individuals with CAC=0, CAC 1–10, and those with CAC >10 at Visit 5. Next, we excluded individuals who reported a prescription for statin therapy (n=175) at MESA Visit 5. Lastly, we computed ASCVD event rates for participants whom met study inclusion criteria for statin eligibility, but whom were not randomized to a repeat CAC scan at Visit 5 (n=508). Statistical analyses were performed using SAS 9.3 (Cary, NC). All hypothesis tests were two-sided used an alpha threshold of 0.05.
Results
Participants were on average 61.7 years old, 51% were women, and 41% were African American. Individuals with long-term CAC=0 were an average of 2 years younger and had a lower fasting blood glucose versus participants with incident CAC (Table 1). The estimated 10-year ASCVD risk was also lower among those with long-term persistent CAC=0 compared to those who developed incident CAC, although both groups had an intermediate 10-year risk (mean 12.0% versus 13.8%, p=0.003). There were no significant differences in lipids, systolic blood pressure, antihypertensive medication use, diabetes, or smoking status between the two CAC groups. At follow-up, the average age of participants with persistent CAC=0 was 69.9 years and the average age of participants with incident CAC was 72.0 years.
Table 1.
Characteristics of 561 Statin Eligible MESA Participants
| Variable | All (n=561) | Persistent CAC=0 (n=244) | Incident CAC (n=317) | P-ValueA |
|---|---|---|---|---|
| Sociodemographic | ||||
| Age, mean (SD), years | 61.7 (7.9) | 60.5 (8.2) | 62.5 (7.5) | 0.003 |
| Female | 285 (50.8) | 131 (53.6) | 163 (51.4) | 0.23 |
| Race | 0.08 | |||
| Caucasian | 146 (26.0) | 50 (20.5) | 96 (30.3) | |
| Chinese | 48 (8.6) | 23 (9.4) | 25 (7.9) | |
| African American | 231 (41.2) | 108 (44.3) | 123 (38.8) | |
| Hispanic | 136 (24.2) | 63 (25.8) | 73 (23.0) | |
| Post-High School Education | 348 (62.0) | 144 (59.0) | 204 (64.4) | 0.20 |
| Income (≥ $40,000) | 247 (44.0) | 97 (39.8) | 150 (47.3) | 0.07 |
| Imaging | ||||
| CAC Score at Visit 5, median (IQR), AU | 4.1 (39.7) | 0.0 (0.0) | 29.7 (58.5) | <0.001 |
| ASCVD Risk Factors | ||||
| Ten-Year ASCVD Risk | 13.1 (0.1) | 12.0 (0.1) | 13.8 (0.1) | 0.003 |
| Total Cholesterol, mean (SD), mg/dL | 200.5 (40.3) | 198.5 (43.3) | 201.9 (38.0) | 0.33 |
| HDL Cholesterol, mean (SD), mg/dL | 49.6 (14.5) | 50.0 (14.5) | 49.3 (14.6) | 0.58 |
| Body Mass Index, mean (SD), kg/m2 | 29.1 (5.2) | 29.2 (5.0) | 29.0 (5.3) | 0.63 |
| Fasting Blood Glucose, mean (SD), mg/dL | 102.6 (36.3) | 98.6 (27.7) | 105.6 (41.4) | 0.02 |
| Type 2 Diabetes Mellitus | 95 (16.9) | 37 (15.2) | 58 (18.3) | 0.33 |
| Glucose-Lowering Medication | 66 (11.8) | 28 (11.5) | 38 (12.0) | 0.85 |
| Systolic Blood Pressure, mean (SD), mmHg | 132.6 (20.0) | 132.2 (19.1) | 132.8 (20.7) | 0.71 |
| Hypertension | 338 (60.3) | 136 (55.7) | 202 (63.7) | 0.06 |
| Anti-Hypertensive Medication | 237 (42.3) | 95 (38.9) | 142 (44.8) | 0.16 |
| Never Smokers | 282 (50.6) | 116 (48.3) | 166 (52.4) | 0.35 |
comparison between persistent CAC=0 and incident CAC.
AU=Agatston units; ASCVD=atherosclerotic cardiovascular disease; CAC=coronary artery calcium; mmHg=millimeters of mercury
The overall proportion of individuals with long-term absence of CAC was 43.5% (Figure 1). However, more than one-half of all persons (54.2%) with an LDL-C ≥ 190 mg/dL maintained persistent CAC=0, while 33% of participants with an ASCVD risk ≥20% maintained persistent CAC=0.
Figure 1.
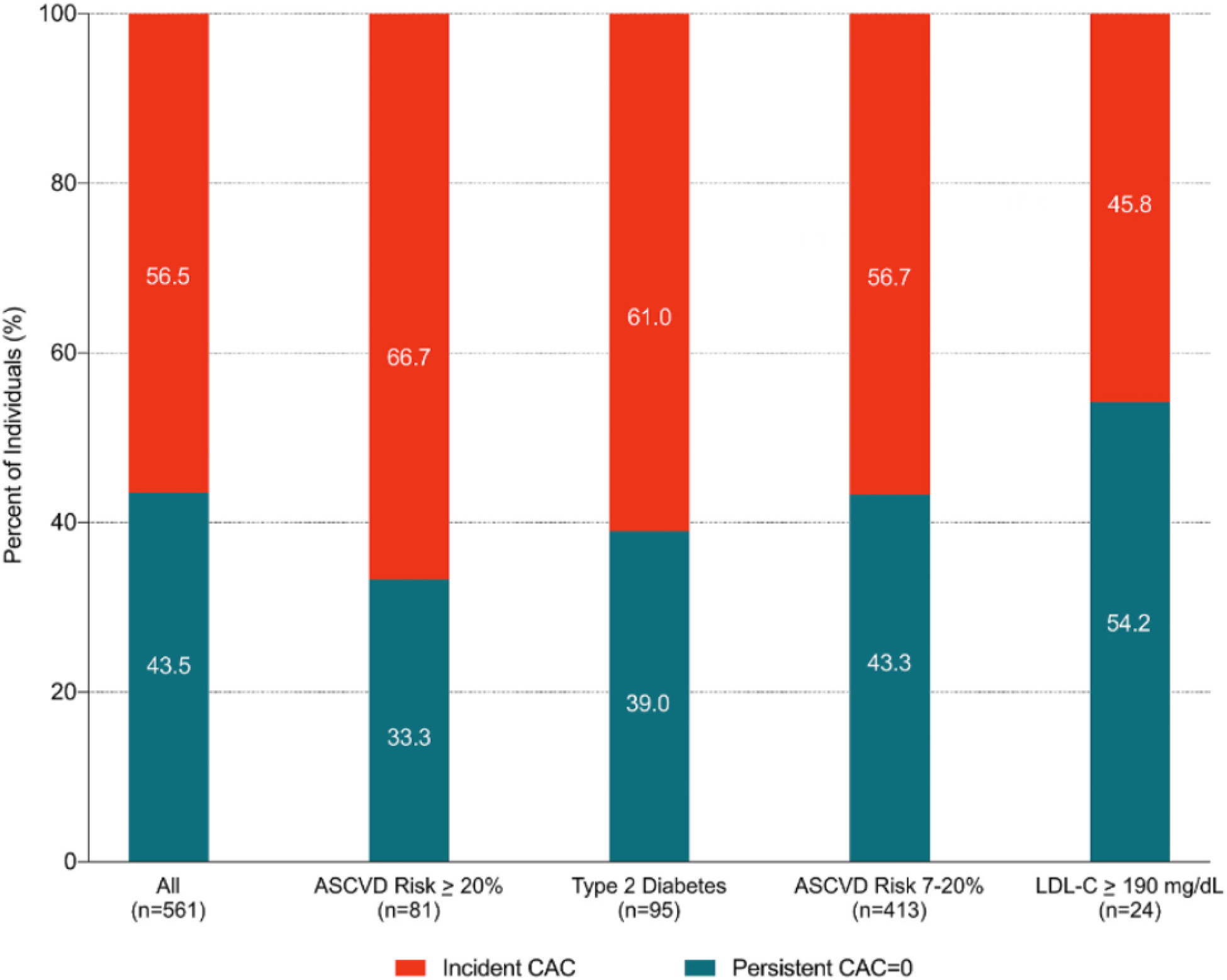
Proportion of participants with persistent CAC = 0 versus incident CAC, stratified by statin eligibility group Heterogeneity in the proportion of persistent CAC = 0 among statin eligible groups.
Overall, there were 57 (10.2%) ASCVD events, and 28 (5.0%) were attributable to CHD (Table 2). Over a median 15.9 years follow-up, individuals with long-term CAC=0 had an ASCVD event rate of 4.3 per 1,000 person-years and a CHD event rate of 1.1 per 1,000 person-years, compared to an ASCVD and CHD event rate of 8.6 and 5.0 per 1,000 person-years for those with incident CAC, respectively. Among individuals with persistent CAC=0, 75% of the ASCVD events were non-CHD, while among individuals with incident CAC 41% of ASCVD events were non-CHD. The 10-year NNT to prevent one ASCVD event was 117 for persons with long-term absence of CAC and 54 for persons with incident CAC. There was a larger discordance in the 10-year NNT for CHD prevention between individuals with long-term absence of CAC (398) and incident CAC (106).
Table 2.
Absolute Event Rates and Estimated 10-Year Number Needed to Treat for ASCVD and CHD According to CAC Status
| Outcome | Persistent CAC=0 (N=244) | Incident CAC (N=317) | |
|---|---|---|---|
| All ASCVD | N (%) | 16 (6.6) | 41 (12.9) |
| Event rate (95% CI)A | 4.3 (2.2, 6.4) | 8.6 (6.0, 11.2) | |
| 10-year NNT | 117 | 54 | |
| Coronary Heart Disease | N (%) | 4 (1.6) | 24 (2.5) |
| Event rate (95% CI)A | 1.1 (0.0, 2.1) | 5.0 (3.0, 6.9) | |
| 10-year NNT | 398 | 106 | |
Events per 1,000 person-years
NNT calculations assume a 30% relative risk reduction of events on statin therapy.
ASCVD=atherosclerotic cardiovascular disease. CAC=coronary artery calcification. NNT=number needed to treat
Kaplan Meier survival curves showed divergence in ASCVD and CHD events early on for participants with and without healthy arterial aging, with a more significant divergence occurring after 10 years of follow-up (Figures 2a & 2b). In adjusted Cox proportional hazard models, individuals with long-term CAC=0 had a 51% lower risk for an ASCVD event compared to those with incident CAC (HR=0.49, 95% CI: 0.27, 0.90; p=0.02) over a median follow up time of 15.9 years. The association between long-term absence of CAC and CHD was even stronger with an adjusted hazard ratio of 0.21 (95% CI: 0.07, 0.62; p=0.005).
Figure 2a.
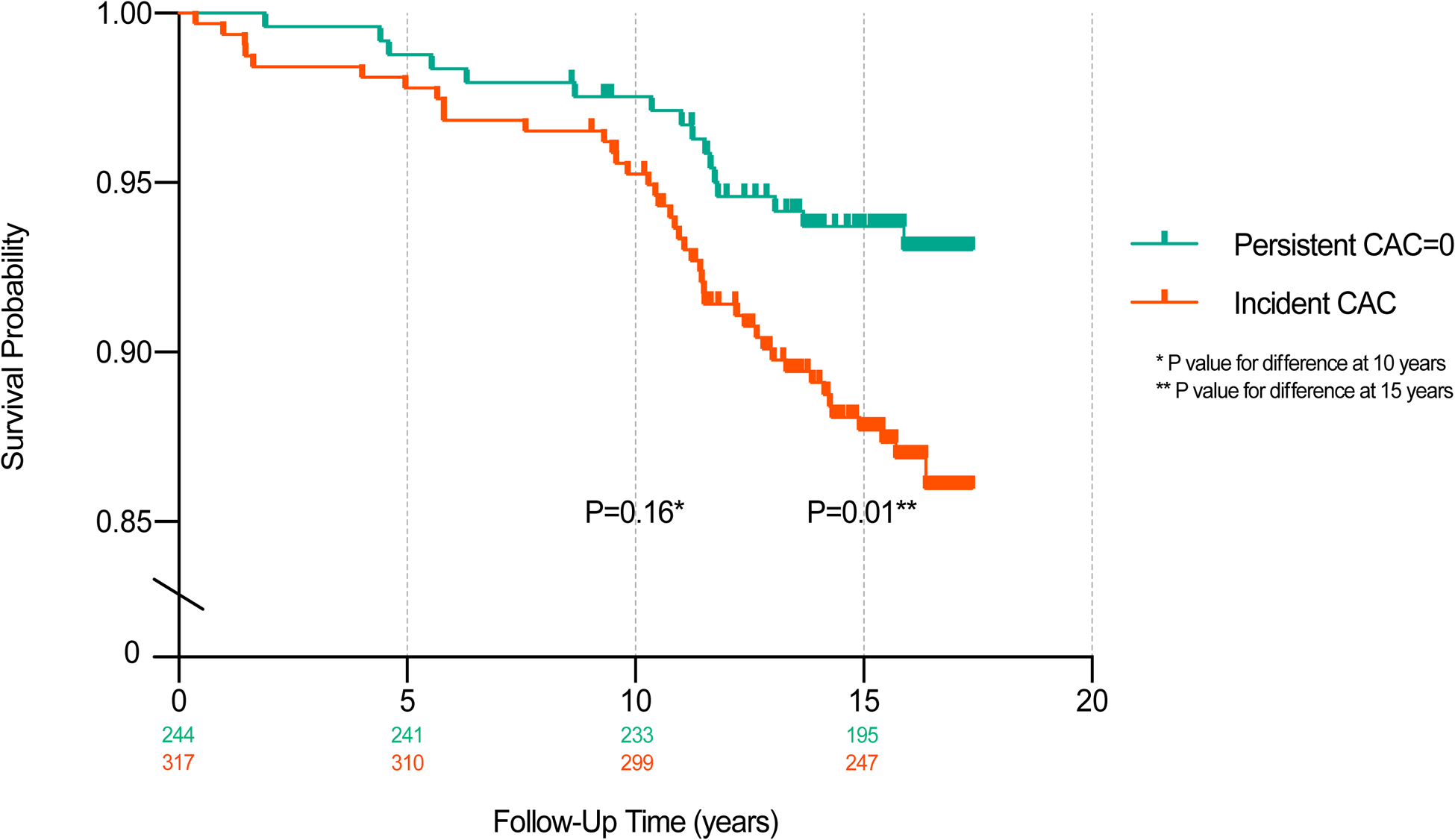
Kaplan Meier Estimate of ASCVD Event Probability According to Incident vs. Long-Term Absence of CAC
Figure 2b.

Kaplan Meier Estimate of CHD Event Probability According to Incident vs. Long-Term Absence of CAC
Individuals with a CAC score between 1–10 at Visit 5 (23% of participants with incident CAC) had higher event rates compared to those with long-term CAC=0 (6.3 versus 4.3 per 1000 person-years) and lower event rates compared to those with CAC > 10 (6.3 versus 9.3 per 1000 person-years) (Supplemental Table 1). Compared to individuals with long-term CAC=0, a significant trend was observed for the association of increasing CAC with ASCVD and CHD, although the adjusted hazards were only significant for individuals with incident CAC >10 (ASCVD HR=2.16, 95% CI: 1.15, 4.07; CHD HR=5.43, 95% CI: 1.79, 16.47) (Supplemental Table 2).
After excluding individuals prescribed statin therapy at follow-up, we observed similar ASCVD event rates and adjusted hazards for participants with and without the long-term absence of CAC (3.8 versus 8.1 per 1,000 person-years) (Supplemental Tables 3 & 4). The ASCVD event rates among statin eligible participants who were prescribed statin therapy at follow-up were modestly higher for individuals with persistent CAC=0 (5.5 versus 3.8 events per 1,000 person-years) and incident CAC (9.7 versus 8.1 events per 1,000 person-years) compared to the ASCVD event rate among statin eligible participants who remained off of statins. Correspondingly, participants who were on statin therapy at Visit 5 also had a higher total cholesterol (214.0 mg/dL versus 194.5 mg/dL, p<0.001) and fasting blood glucose (108.5 mg/dL versus 100 mg/dL, p=0.02) compared to those who remained off of therapy. Compared to the main sample, ASCVD incidence rates after including statin eligible participants who were >75 years of age and had CAC=0 at baseline (n=38) were similar for participants with persistent CAC=0 (4.6 versus 4.3 events per 1,000 person-years) and incident CAC (8.0 versus 8.6 events per 1,000 person-years).
Among all statin eligible candidates with baseline CAC=0, those who underwent follow-up CAC scans were younger, had a lower prevalence of diabetes, and had a lower ASCVD event rate compared to statin eligible participants with baseline CAC=0 who were not randomized to a follow-up scan (6.7 versus 11.8 per 1,000 person-years) (Supplemental Tables 5 and 6). However, the number of participants and events in all subgroup analyses were small and accordingly the results should be cautiously interpreted.
Discussion
Among an ethnically diverse cohort, we found that a substantial proportion of statin eligible participants with baseline CAC=0 had long-term healthy arterial aging, although there was significant variability between statin benefit groups that ranged between 33% for participants with an ASCVD risk ≥20% to 54% of participants with LDL-C ≥190 mg/dL. Overall, individuals with baseline CAC=0 had low 15-year ASCVD and CHD event rates. However, persons with the persistent absence of CAC had event rates that were even lower than individuals with incident CAC and lower than even the 10-year ASCVD risk threshold for guideline recommended primary prevention treatment. Accordingly, our results demonstrate that approximately twice as many individuals with the long-term absence of CAC would need to be treated with statin therapy to prevent one ASCVD event compared to individuals with incident CAC.
The persistent CAC=0 and incident CAC groups had a relatively similar distribution of risk factors, although the incident CAC group had a higher estimated 10-year ASCVD risk (13.8% versus 12.0%), likely due principally to their slightly higher mean age(25). However, this observation also demonstrates that traditional risk factors incompletely describe a person’s ASCVD profile and is further strengthened by our findings that show a particularly low event rate among statin eligible candidates with persistent CAC=0.
Our study builds on previous research that has demonstrated significant heterogeneity in ASCVD risk among statin eligible candidates and that approximately 40% of statin eligible candidates cross-sectionally have CAC=0(11,26). By leveraging two measurements of CAC 10-years apart, we were able to show that a large proportion of statin eligible participants with baseline CAC=0 have not only long-term healthy arterial aging over 10-years, but also very low 15-year ASCVD event rates. Defining this phenotype in the clinical setting may facilitate improved allocation of primary prevention treatment strategies particularly among statin eligible individuals with type 2 diabetes, intermediate ASCVD risk, or an LDL ≥190, as between 39–54% of these participants had long-term healthy arterial aging, but may be less useful among individuals with a high ASCVD risk in whom only 33% had long-term healthy arterial aging. Such improved accuracy in ASCVD risk assessment may aid in the allocation of primary prevention resources on both an individual and population-health level and also further enhance shared decision-making between providers and patients.
In our study, the event rates were low for persons with and without a long-term absence of CAC, but all participants in our study sample had a baseline absence of CAC, which is consistently associated with a low 10-year ASCVD event rate, across a spectrum of ASCVD risk, traditional risk factor burden, and among groups that are traditionally considered at an increased ASCVD risk(3–6). In general, optimal timing or age at which a CAC scan is performed would minimize the need for a follow-up scan. However, among statin eligible participants at MESA Visit 1, 44% had CAC=0 and without performing a CAC scan these participants would otherwise be misclassified as moderate or high ASCVD risk based on traditional ASCVD risk factors.
Broadly speaking, statin eligible individuals with the baseline absence of CAC on CT imaging have reported ASCVD and CHD event rates between 5–6 and 2–3 event per 1,000 person-years respectively over a 10-year follow-up(3,11,26). In this study, we found that individuals with the long-term absence of CAC had 15-year event rates that were even lower at 4.3 and 1.1, respectively, demonstrating that maintenance of the healthy arterial aging phenotype is an even stronger protective factor, which can be considered for refining an individual’s ASCVD risk over time. Long-term CAC=0 was particularly protective for CHD compared to ASCVD (79% versus 51% risk reduction) after considering traditional risk factors, and it is important to note that 12/16 (75%) of ASCVD events among persons with persistent CAC=0 in the current study were non-CHD, of which consisted of stroke (11/12) and congestive heart failure (1/12). Furthermore, we found that the NNT for individuals with persistent CAC=0 was 117, approximately double the estimated NNT for statin eligible participants in MESA with a single measure of CAC=0(11).
The proportion of participants who maintained long-term absence of CAC over one decade varied according to statin eligibility group, providing further evidence that that the optimal timing of obtaining a repeat CAC scan may differ based on baseline ASCVD risk factors(27). While the warranty period of CAC=0 for future ASCVD events has generally been considered to be five years(28–30), our findings support the concept that conversion from CAC=0 to CAC >1 is heterogenous, even among statin benefit candidates. For example, more than half of all individuals with very-high LDL-C (≥190 mg/dL) maintained persistent CAC=0 over one decade, while a considerably lower prevalence of healthy arterial aging was observed among those with diabetes (39%) and those with a very-high 10-year risk of ASCVD (33%). Among those with healthy arterial aging, subgroups with very-high LDL-C or diabetes also had a younger average age (57 and 58 years) and a larger proportion who were prescribed statin therapy at follow-up (42% and 45%) compared to subgroups with an intermediate or high ASCVD risk who had an average age of 61 and 69 years and 31% precent prescribed statin therapy at follow-up. Nevertheless, long-term CAC=0 was a significant protective factor for ASCVD and CHD events, even after accounting for age and excluding those who were prescribed statin therapy at follow-up. The clinical translation of these findings suggests that individuals with diabetes or an LDL-C ≥190 who have CAC=0 may benefit from a repeat CAC scan within approximately 5–10 years to re-evaluate if they still have CAC=0 (31). Moving forward, future studies with larger sample sizes will be required to strengthen these thought-provoking findings and further phenotype statin eligible candidates to better predict which persons are most likely to have healthy arterial aging.
Recent studies have noted that the average time for conversion from CAC=0 to CAC >1 is between 3–7 years among middle-aged adults, with older individuals, men, those with type 2 diabetes, and those with high 10-year PCE risk progressing the most rapidly (27,31). Thus, repeat CAC scoring should be performed earlier for patients with a higher traditional ASCVD risk factor burden, such as those in the statin eligibility groups. Furthermore, while traditional risk factors may help predict incident CAC, our findings underline a notable discordance between an atherogenic environment (e.g. high LDL-C, diabetes) and the burden of subclinical atherosclerosis among presumably high-risk individuals. Future mechanistic studies that incorporate genetic, metabolomics, and clinical data are needed to more fully understand the biological processes and predictors of healthy arterial aging among both low- and high-risk individuals.
Our study had several strengths, among which included a 15-year follow up for ASCVD events and long time period between CAC scans. Likewise, women (50%) and African Americans (41%) comprised a large percentage of our sample, enhancing the generalizability our findings. Findings from our study should also be evaluated with certain limitations in mind. First, while all participants at baseline were not on statin-therapy, changes in primary prevention management throughout the duration of follow-up may have influenced our measured association between persistent CAC=0 and ASCVD events. However, after excluding participants who started statin medication after Visit 1 (n=175) we observed event rates, hazard ratios, and significance values that were consistent between the two analytical approaches.
Our sample size was also relatively small in comparison to the broader MESA cohort in large part because only half of MESA participants were randomized to receive a CAC scan at Visit 5. For example, while our results regarding persistent CAC=0 among those with an LDL-C ≥190 are provocative, there were only 24 individuals in this group. Compared to the main study sample, there was a higher ASCVD event rate in participants with CAC=0 who were not randomized to a repeat CAC scan and those who did not attend Visit 5, but these participants were also older and had a greater ASCVD risk-factor burden, consistent with individuals who were more likely to develop incident CAC and have higher ASCVD event rates. Of note, a majority of participants (67%) who had baseline CAC=0 and were not randomized to a repeat CAC scan at Visit 5 did not attend Visit 5 altogether and thus could not have been eligible to receive repeat imaging. Accordingly, the proportion of individuals with long-term CAC=0 may be somewhat lower than the 43% we observed in this study. Lastly, we were unable to directly compare event rates between individual statin benefit groups due to a low frequency of events within each subgroup.
Overall, almost half of statin eligible individuals with baseline CAC=0 had long-term healthy arterial aging, which varied considerably based on statin benefit subgroup. In particular, ≥50% of individuals with LDL-C ≥190 mg/dL had long-term healthy arterial aging, although there were only a small number of participants (n=24) with LDL-C ≥ 190 mg/dL. Statin eligible individuals with long-term healthy arterial aging had very low 15-year ASCVD event rates that were below the 10-year ASCVD risk thresholds for primary prevention therapy, which suggests a limited utility of statin therapy for reducing ASCVD risk among these individuals. Evolving ASCVD risk assessment to include baseline and repeat CAC scoring more broadly across risk subgroups may improve the allocation of primary prevention therapies.
Supplementary Material
Table 3.
ASCVD and CHD Outcomes According to CAC Status Among Statin-Eligible Participants
| Outcome | Hazard Ratio (95% CI) | P-value |
|---|---|---|
| All ASCVD | ||
| Model 1 | 0.50 (0.28, 0.89) | 0.02 |
| Model 2 | 0.49 (0.27, 0.90) | 0.02 |
| Coronary Heart Disease | ||
| Model 1 | 0.20 (0.07, 0.59) | 0.003 |
| Model 2 | 0.21 (0.07, 0.62) | 0.005 |
Model 1: adjusted for age, sex, race.
Model 2: adjusted for age, sex, race, educational attainment, income, body mass index, total cholesterol, HDL-C, fasting blood glucose, systolic blood pressure, diastolic blood pressure, cigarette smoking, blood pressure-lowering medication, and glucose-lowering medication.
ASCVD-atherosclerotic cardiovascular disease CAC=coronary artery calcium.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This publication was developed under the Science to Achieve Results (STAR) research assistance agreements, No. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the U.S Environmental Protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication.
Financial Support
This research was supported by R01 HL071739 and MESA was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1 TR 001079, and UL1-RR-025005 from National Center for Research Resources.
Abbreviations:
- ASCVD
atherosclerotic cardiovascular disease
- CAC
coronary artery calcification
- CHD
coronary heart disease
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein
- MESA
Multi-Ethnic Study of Atherosclerosis
Footnotes
Declaration of Competing Interest
The authors declare that they do not have any conflicts of interest to disclose.
References
- 1.Pencina MJ, Navar-Boggan AM, D’Agostino RB, Williams K, Neely B, Sniderman AD, et al. Application of New Cholesterol Guidelines to a Population-Based Sample. N Engl J Med. 2014; [DOI] [PubMed] [Google Scholar]
- 2.Murray CJL, Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, et al. The state of US health, 1990–2016: Burden of diseases, injuries, and risk factors among US states. JAMA - J Am Med Assoc. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandesara PB, Mehta A, O’Neal WT, Kelli HM, Sathiyakumar V, Martin SS, et al. Clinical significance of zero coronary artery calcium in individuals with LDL cholesterol ≥190 mg/dL: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2020; [DOI] [PubMed] [Google Scholar]
- 4.Miname MH, Bittencourt MS, Moraes SR, Alves RIM, Silva PRS, Jannes CE, et al. Coronary Artery Calcium and Cardiovascular Events in Patients With Familial Hypercholesterolemia Receiving Standard Lipid-Lowering Therapy. JACC Cardiovasc Imaging. 2019; [DOI] [PubMed] [Google Scholar]
- 5.Malik S, Zhao Y, Budoff M, Nasir K, Blumenthal RS, Bertoni AG, et al. Coronary Artery Calcium Score for Long-term Risk Classification in Individuals With Type 2 Diabetes and Metabolic Syndrome From the Multi-Ethnic Study of Atherosclerosis. JAMA Cardiol [Internet]. 2017. December 1 [cited 2019 Apr 10];2(12):1332–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29117273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEvoy JW, Martin SS, Dardari ZA, Miedema MD, Sandfort V, Yeboah J, et al. Coronary Artery Calcium to Guide a Personalized Risk-Based Approach to Initiation and Intensification of Antihypertensive Therapy. Circulation. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sc S, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285–350.30423393 [Google Scholar]
- 8.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J Am Coll Cardiol. 2019;74(10):e177–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. European Heart Journal. 2020. [DOI] [PubMed] [Google Scholar]
- 10.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. N Engl J Med [Internet]. 2008. March 27 [cited 2019 Apr 9];358(13):1336–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18367736 [DOI] [PubMed] [Google Scholar]
- 11.Nasir K, Bittencourt MS, Blaha MJ, Blankstein R, Agatson AS, Rivera JJ, et al. Implications of Coronary Artery Calcium Testing among Statin Candidates According to American College of Cardiology/American Heart Association Cholesterol Management Guidelines MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015. October 13;66(15):1657–68. [DOI] [PubMed] [Google Scholar]
- 12.Whelton SP, Silverman MG, McEvoy JW, Budoff MJ, Blankstein R, Eng J, et al. Predictors of Long-Term Healthy Arterial Aging. JACC Cardiovasc Imaging. 2015. December;8(12):1393–400. [DOI] [PubMed] [Google Scholar]
- 13.Razavi AC, Wong N, Budoff M, Bazzano LA, Kelly TN, He J, et al. Predicting Long-Term Absence of Coronary Artery Calcium in Metabolic Syndrome and Diabetes. JACC Cardiovasc Imaging. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts ET, Horne A, Martin SS, Blaha MJ, Blankstein R, Budoff MJ, et al. Cost-effectiveness of coronary artery calcium testing for coronary heart and cardiovascular disease risk prediction to guide statin allocation: The Multi-Ethnic Study of Atherosclerosis (MESA). PLoS One. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spahillari A, Zhu J, Ferket BS, Hunink MGM, Carr JJ, Terry JG, et al. Cost-effectiveness of Contemporary Statin Use Guidelines with or without Coronary Artery Calcium Assessment in African American Individuals. JAMA Cardiol. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am J Epidemiol. 2002. November 1;156(9):871–81. [DOI] [PubMed] [Google Scholar]
- 17.Levin G, Kestenbaum B, Ida Chen Y-D, Jacobs DR, Psaty BM, Rotter JI, et al. Glucose, Insulin, and Incident Hypertension in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol [Internet]. 2010. November 15 [cited 2020 Feb 1];172(10):1144–54. Available from: https://academic.oup.com/aje/article-lookup/doi/10.1093/aje/kwq266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972. June;18(6):499–502. [PubMed] [Google Scholar]
- 19.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: Results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2006;113(1):30–7. [DOI] [PubMed] [Google Scholar]
- 20.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990; [DOI] [PubMed] [Google Scholar]
- 21.Budoff MJ, McClelland RL, Chung H, Wong ND, Carr JJ, Gray MMN, et al. Reproducibility of coronary artery calcified plaque with cardiac 64-MDCT: The multi-ethnic study of atherosclerosis. Am J Roentgenol. 2009; [DOI] [PubMed] [Google Scholar]
- 22.Budoff MJ, Young R, Burke G, Carr JJ, Detrano RC, Folsom AR, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. Br Med J. 1999; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Angelantonio E, Sarwar N, Perry P. Emerging risk factors collaboration: Major Lipids, Apolipoproteins, and Risk of Vascular Disease. JAMA-J Am Med Assoc. 2009; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miedema MD, Dardari ZA, Kianoush S, Virani SS, Yeboah J, Knickelbine T, et al. Statin eligibility, coronary artery calcium, and subsequent cardiovascular events according to the 2016 United States Preventive Services Task Force (USPSTF) statin guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7(12):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dzaye O, Dardari ZA, Cainzos-Achirica M, Blankstein R, Szklo M, Budoff MJ, et al. Incidence of New Coronary Calcification: Time to Conversion From CAC = 0. Journal of the American College of Cardiology. 2020. [DOI] [PubMed] [Google Scholar]
- 28.Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010; [DOI] [PubMed] [Google Scholar]
- 29.Min JK, Lin FY, Gidseg DS, Weinsaft JW, Berman DS, Shaw LJ, et al. Determinants of Coronary Calcium Conversion Among Patients With a Normal Coronary Calcium Scan. What Is the “Warranty Period” for Remaining Normal? J Am Coll Cardiol. 2010; [DOI] [PubMed] [Google Scholar]
- 30.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, et al. Absence of Coronary Artery Calcification and All-Cause Mortality. JACC Cardiovasc Imaging. 2009. June;2(6):692–700. [DOI] [PubMed] [Google Scholar]
- 31.Dzaye Omar, Dardari Zeina A., Cainzos-Achirica Miguel, Blankstein Ron, Dzaye ArthuOmar, Dardari Zeina A., Cainzos-Achirica Miguel, Blankstein Ron, Agatston Arthur S., Duebgen Matthias, Yeboah Joseph, Szklo Moyses, Budoff Matthew J., Lima Joao A.C., Roger and MJ B. Warranty Period of a Calcium Score of Zero: Comprehensive Analysis From the Multiethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


