Abstract
1. Individual differences in cytochrome P450 (CYP) enzymes contribute to responses to drugs and environmental chemicals. The expression of CYPs are influenced by sex, age and ethnicity. Human CYP studies are often conducted with human liver microsomes and liver cells to evaluate chemical induction and drug interactions. However, the basal or constitutive expression of CYP transcripts and enzyme activities in the intact liver are also important in our understanding of individual variation in CYPs.
2. This study utilized 100 human liver samples to profile the constitutive expression of CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4, and 4A11 enzyme activity and transcript levels. The mRNA expression of the CYPs and xenobiotic receptors AhR, CAR and PXR was examined via qPCR.
3. Results showed that there was greater inter-individual variation in mRNA expression than in enzyme activities, except for CYP2C19. Females had higher CYP3A4 activity than males. Children had lower CYP4A14 activity, while elderly had lower P450 oxidoreductase activity. Compared to Caucasians, Hispanics had higher CYP2C8 activity and higher CYP2B6, CYP2C9 and CYP2C19 mRNA expression, whereas African Americans had lower CYP2D6 mRNA expression.
4. These results add to our understanding of individual variations in xenobiotic metabolism and toxicology.
Keywords: Cytochrome P450 activity, CYP mRNA expression, Individual variation, Sex, Age, Ethnicity
Introduction
Human hepatic cytochrome P450 enzymes vary markedly in abundance, activity, and expression among age, sex, and ethnicity, which affects individual susceptibility to drugs and toxicants (Guengerich 2008; Sadler and others 2016). Functional human P450 isozyme variations are implicated with drug toxicities such as S-acenocoumarol, phenytoin and warfarin (CYP2C9), metoclopramide, codeine, mirtazapin and tramadol (CYP2D6), methadone and efavirenz (CYP2B6), clopidogrel and desipramine (CYP2C19), and tacrolimus (CYP3A5) (Johansson and Ingelman-Sundberg 2011).
Differences in cytochrome P450 enzymes also affect individual susceptibility to diseases. For example, people who exhibit low enzyme activities of CYP2D6 and CYP2C19 are at increased risk of developing adult acute leukemia (Roddam and others 2000), high levels of CYP2A6 activity are positively associated with pancreatic cancer in a Midwestern US population (Kadlubar and others 2009), and polymorphic variations in CYP1A2 and CYP1B1 are associated with colorectal cancer susceptibility (Bethke and others 2007). In an ethnic Han population in China, CYP1A1 Val/Val genotype and CYP2E1 C1/C1 genotype correlate with an increased the risk for prostate cancer (Yang and others 2006).
The importance of CYP enzymes in drug metabolism is not solely dependent on their relative abundance in the liver. CYP3A4/5 is the major human P450 for drug metabolism, accounting for approximately 20–30% of total P450 in the liver, followed by CYP2E1 (15–25%), CYP1A2 (10–25%), CYP2C9 (10–20%), CYP2D6 (1.5–5%), CYP2B6 (1–5%), and CYP2C19 (1–4%) (Achour and others 2014; Chen and others 2019; Gao and others 2016; Gröer and others 2014; Michaels and Wang 2014; Zhang and others 2016). For example, CYP2E1 (15–25%) plays a key role in the metabolic activation of acetaminophen and a large number of toxicants and carcinogens resulting in hepatotoxicity (Chen and others 2019; Gonzalez 2007). CYP2B6 accounts for a minor portion (1–4%) of total hepatic CYP content, but contributes to the hepatic metabolism of 2–10% of drugs (Wang and Tompkins 2008). Thus, all CYP enzymes regardless of their relative expression are potentially important and are dependent on the substrates under investigation.
Human CYP studies are often conducted with human liver microsomes, liver cell lines or isolated hepatocytes (Gómez-Lechón and others 2007; Wang and others 2020). Human primary hepatocyte cultures have become the “gold standard” for evaluating hepatic metabolism and toxicity of drugs and other xenobiotics in vitro (Lecluyse and Alexandre 2010), as the chemical induction and drug-drug interactions can be easily evaluated in these models. However, the basal or constitutive expression of CYP enzyme activities and transcript levels in intact livers are equally important in our understanding of individual variation in CYP-mediated drug metabolism and drug interactions (Boobis and others 2009; Parkinson and others 2004). To better understand the CYPs in human livers, the present study profiled the mRNA levels of the 9 major CYP genes and their encoded enzyme activities in 100 human liver donor samples to determine inter-individual variations in each CYP, and the effects of sex, age, and ethnicity.
Materials and methods
Liver samples and basal P450 enzyme activities
Human liver samples (n = 100) were purchased from Xenotech LCC (Lenexa, KS, USA), and the sample demographics are shown in Table 1 including gender (39% are females), age (0–79 years), and ethnic origin (10% are Hispanics, 10% African Americans, 77% Caucasians and 3% Asian Americans). This study received Institutional Review Board exemption status by the University of Kansas Medical Center Human Subjects Committee because the specimens were obtained commercially, and donors were not identified to the authors.
Table 1.
Sample demographics
| Sex | Female | n = 39 |
| Male | n = 61 | |
| Age | Children (0–18) | n = 11 |
| Adult (19–59) | n = 64 | |
| Elderly (60 and over) | n = 25 | |
| Race | African American | n = 10 |
| Asian American | n = 3 | |
| Hispanic | n = 10 | |
| Caucasian | n = 77 | |
Cytochrome P450 isoenzyme activity assays
Liver microsomes were prepared to determine total P450 content and P450 isoenzyme activities as described previously (Parkinson and others 2004) using probe substrates listed in Table 2. CYP1A2 was determined by 7-ethoxyresoruifn O-dealkylation, CYP2B6 by S-mephenytoin N-demethylation, CYP2C8 by paclitaxel 6α-hydroxylation, CYP2C9 by diclofenac 4’-hydroxylation, CYP2C19 by S-mephenytoin 4’-hydroxylation, CYP2D6 by dextromethorphan O-demethylation, CYP2E1 by chlorzoxazone 6-hydroxylation, CYP3A4/5 by testosterone 6β-hydroxylation, and CYP4A9/11 by lauric acid 12’-hydroxylation. The total cytochrome P450 content was expressed as pmol/mg protein, the activity of cytochrome P450 oxidoreductase was expressed as nmol/mg protein, and the rates for all 9 cytochrome P450 isozymes were expressed as pmol/mg protein/min.
Table 2.
Cytochrome P450 activities in human liver microsomes
| Enzyme | Activity | Minimum | Maximum | Variation (fold) | Mean ± SD |
|---|---|---|---|---|---|
| CYP1A2 | 7-Ethoxyresoruifn O-dealkylation | 2.2 | 258 | 117 | 51.0 ± 45.4 |
| CYP2B6 | S-Mephenytoin N-demethylation | 10.1 | 1290 | 127 | 107 ±160 |
| CYP2C8 | Paclitaxel 6α-hydroxylation | 11.1 | 2040 | 184 | 346±417 |
| CYP2C9 | Diclofenac 4’-hydroxylation | 127 | 5870 | 46 | 1806 ± 863 |
| CYP2C19 | S-Mephenytoin 4’-hydroxylation | 2.4 | 895 | 373 | 99.4 ± 142 |
| CYP2D6 | Dextromethorphan O -demethylation | 18 | 1160 | 64 | 306 ±192 |
| CYP2E1 | Chlorzoxazone 6-hydroxylation | 201 | 11500 | 57 | 2098 ±1530 |
| CYP3A4/5 | Testosterone 6β-hydroxylation | 113 | 20300 | 180 | 3387 ± 3710 |
| CYP4A9/11 | Lauric acid 12’-hydroxylation | 297 | 4350 | 15 | 1685 ± 831 |
| Total P450 | Total cytochrome P450 content (pmol/mg) | 99 | 1737 | 17.5 | 346 ± 200 |
| CPR | Cytochrome P450 oxidoreductase (nmol/mg/iin) | 82 | 343 | 4.2 | 177 ± 53.8 |
QuantiGene multiplex suspension array
Total RNA was isolated from each liver sample using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA quality was determined by the 260/280 absorbance ratio (>1.8) and by the formaldehyde-agarose gel electrophoresis for visualization of 18S and 28S rRNA bands. Individual bead-based oligonucleotide probe sets specific for each human gene examined were developed by Panomics, Inc (Fremont, CA, USA) using glyceraldehyde 3-phosphate dehydrogenase (G3PDH) mRNA expression as the internal control for each sample. Samples were analyzed using a Bio-Plex System array reader with Luminex 100 xMAP technology (multi-analyte profiling beads). Briefly, 3 μg of total RNA were incubated overnight at 53°C with X-MAP beads containing oligonucleotide capture probes, label extenders, and blockers. The beads and bound target RNA were washed and subsequently incubated with streptavidin-conjugated R-phycoerythrin (Affymetrix/Panomics, Santa Clara CA). Fluorescence was analyzed using a Bio-Plex reader and analyzed with Bio-Plex Data Manager Software (BioRad, Hercules, CA). All data were standardized to the internal control G3PDH and expressed as the ratio of relative light units of target gene mRNA normalized to G3PDH mRNA.
Real-time RT-PCR analysis.
Total RNA was purified with RNeasy columns (Qiagen, Valencia, CA), and reverse transcribed with Multiscript reverse transcriptase using High Capacity RT kits from Applied Biosystems (Applied Biosystems, Foster City, CA). Primers were designed with Primer3 software (version 4), and are listed in Supplementary Table 1. The Power SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA) was used for real-time RT-PCR analysis. Differences in gene expression between groups were calculated using cycle threshold (Ct) values, which were normalized with G3PDH and RPL13A (averaged) and expressed as relative to the housekeeping gene levels.
Statistical analysis
Data was calculated as mean and SD. The SPSS 17 software (IBM Corporation Armonk, NY) was used for statistical analysis. For comparisons between two groups, Student’s t test was used. Sex effects were determined using males as the baseline; age effects were determined relative to adults; and ethnic effects were determined relative to Caucasians. The black filled bars in figures indicate the male, adult, and Caucasian data for comparisons. The significant level was set at p < 0.05 in all cases.
Results
Basal cytochrome P450 activities
The total cytochrome P450 contents, cytochrome P450 oxidoreductase and basal cytochrome P450 enzyme activities from 100 normal human liver microsomes are shown in Table 2. Among the 9 human CYP isoenzymes, the highest was CYP3A4/5 activity towards testosterone 6β-hydroxylation (3387 ± 3710 pmol/mg protein/min), and the lowest was CYP1A2 activity towards 7-ethoxyresorufin O-dealkylation (51.0 ± 45.4 pmol/mg protein/min). CYP2E1 activity towards chlorzoxazone 6-hydroxylation, CYP2C9 towards diclofenac 4′-hydroxylation, and the CYP4A9/11 activity towards lauric acid 12′-hydroxylation were approximately 2000 pmol/mg/min, while CYP2C8 activity towards paclitaxel 6α-hydroxylation, CYP2D6 towards dextromethorphan O-demethylation, CYP2B6 towards S-mephenytoin N-demethylation, and CYP2C19 towards S-mephenytoin 4′-hydroxylation were between 100–300 pmol/mg/min. The biggest variation in P450 activity was seen with CYP2C19 (370-fold), and the least variable was CYP4A9/11 (15-fold). Total P450 content (346 ± 200 pmol/mg) and the activity of cytochrome p450 oxidoreductase (177 ± 54 pmol/mg/min) were within the normal range (Parkinson and others 2004).
Basal cytochrome P450 transcript levels
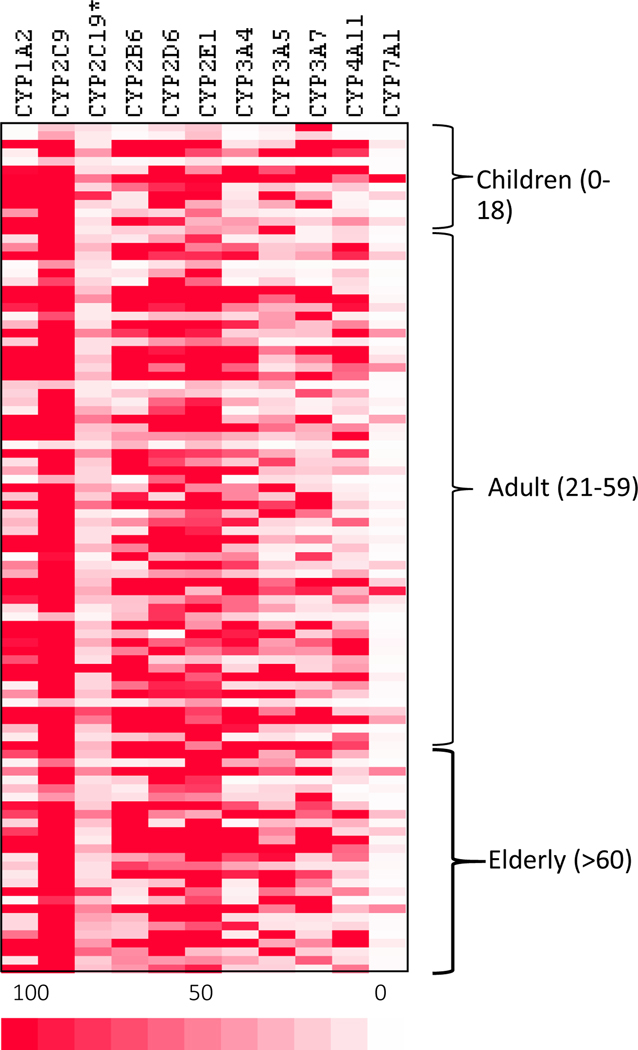
Figure 1 shows the heatmap of 11 CYP relative transcript levels of the 9 CYP mRNA levels via QuantiGene multiplex suspension array. The CYP with the lowest expression was CYP2C19, and CYP with the highest expression was CYP2C9. The data was sorted by age: Children (0–18), Adult (19–59), and Elderly (60 and over). No apparent difference in basal expression patterns among the three age groups was visually apparent.
Figure 1.
Heatmap of the expression of 11 CYP genes in normal human livers. Total RNA was extracted from 100 human livers and subjected to QuantiGene multiplex suspension array. The deeper the red color the higher expression.
The basal mRNA expression of 8 CYPs and 3 xenobiotic receptors were further examined via real-time RT-qPCR. The expression was normalized with the housekeeping genes (G3PDH and RPL13A) and is shown in Table 3. Among the 8 human CYP genes, the highest was CYP2E1 (1260% of housekeeping genes), followed by CYP3A4 (522%) and CYP2C9 (330%); the lower transcript levels were for CAR (9.99%), followed by AhR (17.2%) and PXR (32.5% of housekeeping gene). The variation in CYP mRNAs levels is more than CYP enzyme activities (Table 2), with many CYPs having over a hundred-fold variation. The transcription factor AhR (12-fold) and PXR (33-fold) had lower variations than the CYPs. The largest variation in transcript levels was seen with CYP1A2 (1000-fold), whereas CYP1A2 activity was only 112-fold variable.
Table 3.
mRNA Expression of cytochrome P450 and corresponding nuclear receptor
| Gene | Minimum | Maximum | Variation (fold) | Mean ± SD |
|---|---|---|---|---|
| AhR | 4.27 | 51.3 | 12 | 17.2 ± 8.8 |
| C/P1A2 | 0.91 | 895 | 1000 | 98.2 ± 137 |
| CAR | 0.09 | 40.3 | 438 | 9.99 ±8.6 |
| CYP2B6 | 0.27 | 1270 | 474 | 183 ±271 |
| CYP2C9 | 8.01 | 1204 | 158 | 330 ±291 |
| CYP2C19 | 6.34 | 847 | 134 | 132 ±161 |
| CYP2D6 | 4.34 | 505 | 116 | 91.2 ± 76.9 |
| CYP2E1 | 5.88 | 4770 | 811 | 1260 ±838 |
| PXR | 2.55 | 83.1 | 32.5 | 31.7± 16.2 |
| CYP3A4 | 5.81 | 3330 | 573 | 522 ±691 |
| CYP4A14 | 0.96 | 626 | 650 | 78.7 ±97.6 |
Sex-, age-, and race-dependent differences in constitutive cytochrome P450 activity levels
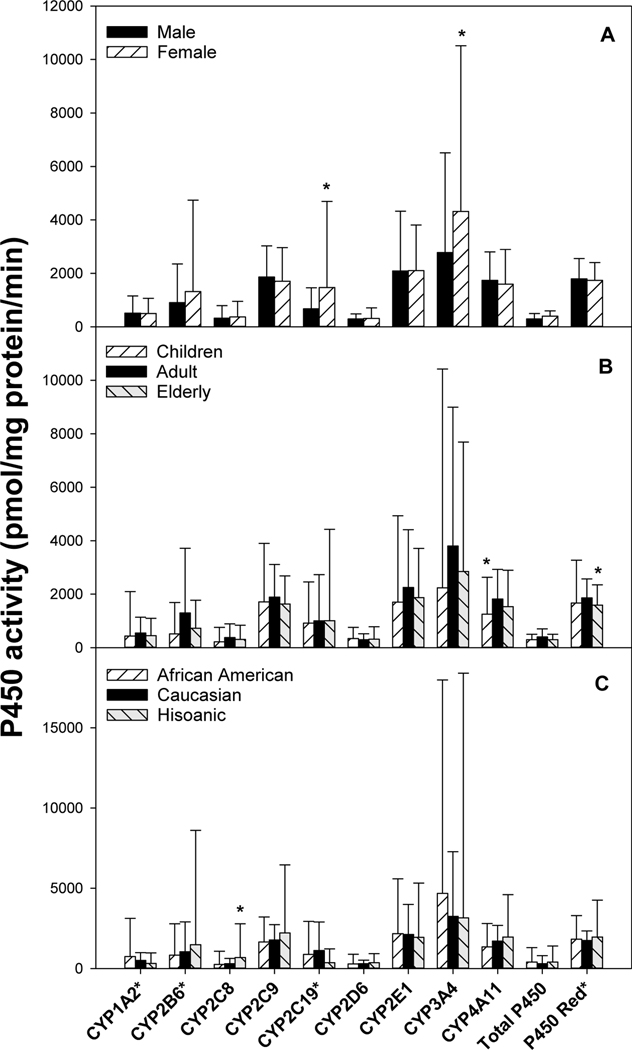
Sex differences in P450 enzyme activities are shown in Fig. 2A. Females had higher CYP2C19 (147 vs 67.7 pmol/mg protein/min) and CYP3A4 activities (4310 vs 2780). Age differences in P450 enzyme activities are shown in Fig. 2B. Compared to adults (19–59y), children (0–18y) had lower CYP4A14 activity (1700 vs 2250), while elderly (>60y) had lower P450 oxidoreductase activity (159 vs 186). Race differences in P450 isozyme activities are shown in Fig. 2C. Compared to Caucasians (n= 77), Hispanic (n = 10) had higher CYP2C8 activities (698 vs 303%).
Figure 2.
Sex-, age-, and race-dependent differences in P450 isozyme activities. Liver microsomes were extracted from 100 normal human livers, and P450 isozyme activities were examined by specific substrates outlined in Table 2. Values for CYP1A2*, CYP2B6*, and CYP2C9* were multiplied10-fold to be better visualized. Data are mean ± SD. *Significantly different from male (A), adults (B), and Caucasian (C), respectively, p < 0.05.
Sex-, age-, and race-dependent differences in constitutive cytochrome P450 mRNA levels
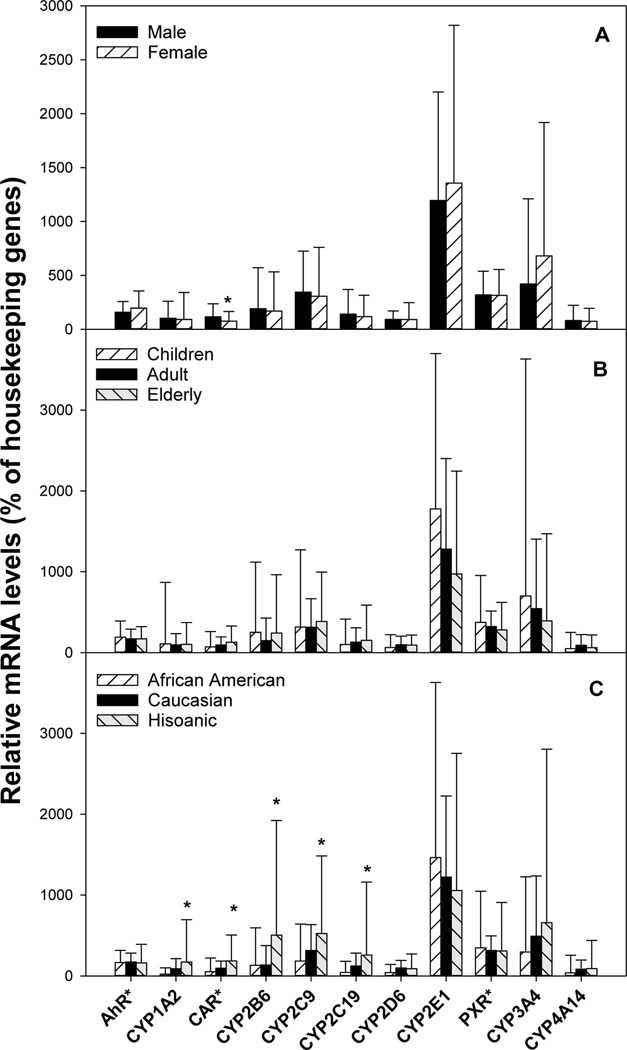
Sex differences in mRNA of CYPs are shown in Fig. 3A. Females (n = 39) had lower CAR mRNA (7.5 vs 11.6%). Females also tended to have higher CYP3A4 mRNA than males (n =61) (681 vs 421% of housekeeping genes) but was not statistically significant (P=0.06). Age differences in CYP mRNAs are shown in Fig. 3B. Compared to adults, no significant differences were evident between children and adults. Children tended to have slightly higher CYP2E1 mRNA, but the difference was not statistically significant (1780 vs 1280, P=0.08). Race/ethnicity differences in CYP mRNAs are shown in Fig. 3C. Compared to Caucasians, African Americans (n = 10) had lower CYP2D6 mRNA (41 vs 98%). Hispanics had higher CYP1A2 mRNA (172 vs 90%), CAR mRNA (19 vs 10%), CYP2B6 mRNA (504 vs 136%), CYP2C9 mRNA (524 vs 316%) and CYP2C19 mRNA (258 vs 124%).
Figure 3.
Sex-, age-, and race-dependent differences in CYPs mRNA expression. Total liver RNA was isolated and purified from 100 normal human livers, and the expression of specific CYP was probed with specific primers listed in Supplementary Table 1. Values for AhR*, CAR*, and PXR* were multiplied10-fold to be better visualized. Data are mean ± SD. *Significantly different from male (A), adults (B), and Caucasian (C), respectively, p < 0.05.
Discussion
This study examined constitutive P450 activity and mRNA levels of 100 normal human livers including both sexes (female n = 39, male n = 61) and three major ethnic groups (African Americans n = 10, Hispanics n =10, and Caucasians n = 77), and three age groups (children n =11, adults n = 64, and elderly n =25). There were marked individual variations in CYP enzyme expression and activities. Females had higher CYP3A4 activity and mRNA levels; children had lower CYP4A activity, while the elderly had lower P450 oxidoreductase activity. Compared to Caucasians, Hispanics had higher CYP2C8 activity, and relative higher expression of CYP2B6, CYP2C9 and CYP2C19 mRNA. The information obtained on constitutive expression of human CYP adds to our understanding of individual variations in xenobiotic metabolism and toxicology.
Individual variation in drug clearance is often responsible for efficacy and toxicity of drugs. In the present study, variations in the total P450 (17.5-fold) and cytochrome P450 reductase activities (4.2 fold) were relatively small, whereas variations in CYP isozymes such as CYP3A4, CYP2D6, and CYP1A2 were over a hundred fold. In general, variations at the transcript levels were even larger than that at the activity level. For example, CYP1A2 showed a 1000-fold variation in mRNA but only a 117-fold variation in enzyme activity; CYP2C9 had a 158-fold variation in mRNA but only a 46-fold variation in activity; CYP3A4 had a 573-fold variation in mRNA but a 180-fold variation in activity, similar to that observed in previous publications (Parkinson and others 2004). Indeed there exist differences between P450 enzyme activity and relative mRNA levels (Zhang and others 2016). These variations in P450 could be caused by sex, age and genetic factors, which are known to be responsible for individual differences in drug metabolism and toxicity (Croom and others 2010; Johansson and Ingelman-Sundberg 2011; Sadler and others 2016).
Sex/gender differences in CYP enzymes are a common phenomenon in rodents (Waxman and Holloway 2009). Our Lab recently analyzed 78 mouse CYP genes, and sex-dependent expression was observed with 27 CYPs, and 22 CYPs were higher in females including Cyp2a4, Cyp2b9, Cyp2c37, Cyp3a41 and Cyp4a14 (Renaud and others 2011). Human CYP3A4/5 is the most important P450 in human liver because it metabolizes the most drugs, and is considered to be female-predominant (Waxman and Holloway 2009). Cytochrome P450 3A activity can be assessed by midazolam disposition; women exhibited 16% higher weight-corrected midazolam oral clearance than men (Hu and Zhao 2010). In the present study, the enzyme activity and mRNA of CYP3A4 as well as CYP2E1 tended to be higher in females. There is also evidence for females having higher activity of CYP2A6 and CYP2B6, but no differences in CPY2C9 and CYP2D6 activity (Anderson 2008). In the present study, no major difference in CYP1A2, 2B6, 2C9, 2C19, 2D6, and 4A11 was evident. Sexually dimorphic regulation and induction of P450s could be mediated in part by the constitutive androstane receptor (CAR) (Hernandez and others 2009). Apparently, the dramatic sex-differences in CYP mRNA and enzyme activities seen in experimental animals (Renaud and others 2011) are not observed in humans.
Murine liver cytochrome P450s showed three distinct patterns of gene expression during development: (1) higher in perinatal period but undetectable by 30 days of age (Cyp3a16, 3a41b in male), (2) rapidly increased after birth and reached maximal expression levels by day 5 (Cyp2e1, 3a11, and 4a10), and (3) low until days 10 to 15, and then markedly increased at day 20 to a high and stable level (Cyp1a2, 2a4, 2b10, 2c29, 2d22, 2f2, 3a13, and 3a25)(Hart and others 2009). The ontogeny of human CYPs has also been reported (Hines 2007); CYP2D6 showed a developmental pattern from high in gestation days to maturation (Stevens and others 2008). In the present study (mainly adult livers, only a few livers were from children of 0–7 years old), and no apparent age-differences in CYP expression were observed. In the literature, age and sex did not influence the basal expression of CYP3A activity towards midazolam, but sex-differences exist during the induction by rifampin (Gorski and others 2003). In nonsmokers, no significant age-differences in CYP1A2 activity towards caffeine was observed (Simon and others 2001). The present study did not reveal significant age-differences in CYP enzymes at both mRNA levels and enzyme activity levels, which fortify the conclusion that human CYPs do not exhibit marked age-differences in basal levels (Parkinson and others 2004), in sharp contrast to those seen in rodents (Hart and others 2009).
Ethnic differences in P450 expression contribute to differences in drug metabolism. In rodents, strain-differences in P450s often occur. In a large-scale study on 165 genetic variants, comprising 27 drug-metabolizing enzyme and transporter genes from 2188 participants across 3 major ethnic populations (Caucasians, Africans, and East Asians), three distinct populations were observed using standard multi-dimensional scaling calculations (Man and others 2010). Enhanced CAR transcriptome expression and its target gene CYP2B6 in Hispanic livers over Caucasian livers has been reported (Finkelstein and others 2006). However, the small sample size of this study (5 Hispanic females vs 13 White females) limit the generalization of this study in regards to Hispanics. In other published studies, ethnic differences were not clear. For example, among the genetic variation of 11 CYPs from Caucasians, African Americans, and Asian Americans, the largest genetic viability was the CYP2 family, but there was little difference among the ethnic groups (Solus and others 2004). In a study of pharmacokinetics of CYP2B6-mediated metabolism of bupropion between Caucasians and Chinese subjects, CYP2B6 activity was similar among the ethnic groups (Loboz and others 2006). Similarly, plasma concentration-time profiles of chlorzoxazone and its 6-hydroxy metabolite (attributed to CYP2E1 and CYP3A metabolism) were not different between Mexicans and European Americans (Poland and others 2002). P450 activities in the livers of ethnic Chinese also showed some differences (Gao and others 2016; Wang and others 2020; Zhang and others 2016). In the present studies, no apparent differences in P450 activities among African Americans, Hispanics, and Caucasians were observed except for higher CYP2C8 activities in Hispanics (Fig. 2). However, for the mRNA levels, Hispanics had higher CYP1A2, 2B6, 2C9, 2C19, and CAR levels than Caucasians (Fig. 3). Genetic variants in CYPs should be taken into consideration in future studies.
Conclusions
This study compared human CYP isozyme activities and mRNA expression in 100 human liver samples, and the data showed large variations in P450 enzymes among individual samples, with even greater variations seen at transcript levels. However, when compared between sex, age and ethnic groups, no dramatic differences in CYP enzyme expression were evident. The constitutive expressions of P450 genes are important for our understanding of individual susceptibility to xenobiotics and for better risk assessments.
Supplementary Material
Acknowledgements
We would like to thank Drs. Andrew Parkinson, David B. Buckley, and Ronnie L. Yeager for their contributions to this work.
Funding
This study was supported by NIH Research Grant ES-013714, ES-09716, ES-009649, DK-081461; RR-021940 and NIH Training Grant ES-07079. We thank Drs. Mike Hughes and Janice Lee for critical in-house review of the manuscript.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors
The information in this document has been subjected to review by the Center for Computational Toxicology and Exposure and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References:
- Achour B, Russell MR, Barber J, Rostami-Hodjegan A. (2014). Simultaneous quantification of the abundance of several cytochrome P450 and uridine 5’-diphospho-glucuronosyltransferase enzymes in human liver microsomes using multiplexed targeted proteomics. Drug Metab Dispos, 42, 500–10. [DOI] [PubMed] [Google Scholar]
- Anderson GD. (2008). Gender differences in pharmacological response. Int Rev Neurobiol, 83, 1–10. [DOI] [PubMed] [Google Scholar]
- Bethke L, Webb E, Sellick G, Rudd M, Penegar S, Withey L, Qureshi M, Houlston R. (2007). Polymorphisms in the cytochrome P450 genes CYP1A2, CYP1B1, CYP3A4, CYP3A5, CYP11A1, CYP17A1, CYP19A1 and colorectal cancer risk. BMC Cancer, 7, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boobis A, Watelet JB, Whomsley R, Benedetti MS, Demoly P, Tipton K. (2009). Drug interactions. Drug Metab Rev, 41, 486–527. [DOI] [PubMed] [Google Scholar]
- Chen J, Jiang S, Wang J, Renukuntla J, Sirimulla S, Chen J. (2019). A comprehensive review of cytochrome P450 2E1 for xenobiotic metabolism. Drug Metab Rev, 51, 178–95. [DOI] [PubMed] [Google Scholar]
- Croom EL, Wallace AD, Hodgson E. (2010). Human variation in CYP-specific chlorpyrifos metabolism. Toxicology, 276, 184–91. [DOI] [PubMed] [Google Scholar]
- Finkelstein D, Lamba V, Assem M, Rengelshausen J, Yasuda K, Strom S, Schuetz E. (2006). ADME transcriptome in Hispanic versus White donor livers: evidence of a globally enhanced NR1I3 (CAR, constitutive androstane receptor) gene signature in Hispanics. Xenobiotica, 36, 989–1012. [DOI] [PubMed] [Google Scholar]
- Gao N, Tian X, Fang Y, Zhou J, Zhang H, Wen Q, Jia L, Gao J, Sun B, Wei J and others. (2016). Gene polymorphisms and contents of cytochrome P450s have only limited effects on metabolic activities in human liver microsomes. Eur J Pharm Sci, 92, 86–97. [DOI] [PubMed] [Google Scholar]
- Gómez-Lechón MJ, Castell JV, Donato MT. (2007). Hepatocytes--the choice to investigate drug metabolism and toxicity in man: in vitro variability as a reflection of in vivo. Chem Biol Interact, 168, 30–50. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ. (2007). The 2006 Bernard B. Brodie Award Lecture. Cyp2e1. Drug Metab Dispos, 35, 1–8. [DOI] [PubMed] [Google Scholar]
- Gorski JC, Vannaprasaht S, Hamman MA, Ambrosius WT, Bruce MA, Haehner-Daniels B, Hall SD. (2003). The effect of age, sex, and rifampin administration on intestinal and hepatic cytochrome P450 3A activity. Clin Pharmacol Ther, 74, 275–87. [DOI] [PubMed] [Google Scholar]
- Gröer C, Busch D, Patrzyk M, Beyer K, Busemann A, Heidecke CD, Drozdzik M, Siegmund W, Oswald S. (2014). Absolute protein quantification of clinically relevant cytochrome P450 enzymes and UDP-glucuronosyltransferases by mass spectrometry-based targeted proteomics. J Pharm Biomed Anal, 100, 393–401. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. (2008). Cytochrome p450 and chemical toxicology. Chem Res Toxicol, 21, 70–83. [DOI] [PubMed] [Google Scholar]
- Hart SN, Cui Y, Klaassen CD, Zhong XB. (2009). Three patterns of cytochrome P450 gene expression during liver maturation in mice. Drug Metab Dispos, 37, 116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JP, Mota LC, Huang W, Moore DD, Baldwin WS. (2009). Sexually dimorphic regulation and induction of P450s by the constitutive androstane receptor (CAR). Toxicology, 256, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines RN. (2007). Ontogeny of human hepatic cytochromes P450. J Biochem Mol Toxicol, 21, 169–75. [DOI] [PubMed] [Google Scholar]
- Hu ZY, Zhao YS. (2010). Sex-dependent differences in cytochrome P450 3A activity as assessed by midazolam disposition in humans: a meta-analysis. Drug Metab Dispos, 38, 817–23. [DOI] [PubMed] [Google Scholar]
- Johansson I, Ingelman-Sundberg M. (2011). Genetic polymorphism and toxicology--with emphasis on cytochrome p450. Toxicol Sci, 120, 1–13. [DOI] [PubMed] [Google Scholar]
- Kadlubar S, Anderson JP, Sweeney C, Gross MD, Lang NP, Kadlubar FF, Anderson KE. (2009). Phenotypic CYP2A6 variation and the risk of pancreatic cancer. Jop, 10, 263–70. [PMC free article] [PubMed] [Google Scholar]
- Lecluyse EL, Alexandre E. (2010). Isolation and culture of primary hepatocytes from resected human liver tissue. Methods Mol Biol, 640, 57–82. [DOI] [PubMed] [Google Scholar]
- Loboz KK, Gross AS, Williams KM, Liauw WS, Day RO, Blievernicht JK, Zanger UM, McLachlan AJ. (2006). Cytochrome P450 2B6 activity as measured by bupropion hydroxylation: effect of induction by rifampin and ethnicity. Clin Pharmacol Ther, 80, 75–84. [DOI] [PubMed] [Google Scholar]
- Man M, Farmen M, Dumaual C, Teng CH, Moser B, Irie S, Noh GJ, Njau R, Close S, Wise S and others. (2010). Genetic variation in metabolizing enzyme and transporter genes: comprehensive assessment in 3 major East Asian subpopulations with comparison to Caucasians and Africans. J Clin Pharmacol, 50, 929–40. [DOI] [PubMed] [Google Scholar]
- Michaels S, Wang MZ. (2014). The revised human liver cytochrome P450 “Pie”: absolute protein quantification of CYP4F and CYP3A enzymes using targeted quantitative proteomics. Drug Metab Dispos, 42, 1241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson A, Mudra DR, Johnson C, Dwyer A, Carroll KM. (2004). The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicol Appl Pharmacol, 199, 193–209. [DOI] [PubMed] [Google Scholar]
- Poland RA, Lin KM, Nuccio C, Wilkinson GR. (2002). Cytochrome P450 2E1 and 3A activities do not differ between Mexicans and European Americans. Clin Pharmacol Ther, 72, 288–93. [DOI] [PubMed] [Google Scholar]
- Renaud HJ, Cui JY, Khan M, Klaassen CD. (2011). Tissue distribution and gender-divergent expression of 78 cytochrome P450 mRNAs in mice. Toxicol Sci, 124, 261–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddam PL, Rollinson S, Kane E, Roman E, Moorman A, Cartwright R, Morgan GJ. (2000). Poor metabolizers at the cytochrome P450 2D6 and 2C19 loci are at increased risk of developing adult acute leukaemia. Pharmacogenetics, 10, 605–15. [DOI] [PubMed] [Google Scholar]
- Sadler NC, Nandhikonda P, Webb-Robertson BJ, Ansong C, Anderson LN, Smith JN, Corley RA, Wright AT. (2016). Hepatic Cytochrome P450 Activity, Abundance, and Expression Throughout Human Development. Drug Metab Dispos, 44, 984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon T, Becquemont L, Hamon B, Nouyrigat E, Chodjania Y, Poirier JM, Funck-Brentano C, Jaillon P. (2001). Variability of cytochrome P450 1A2 activity over time in young and elderly healthy volunteers. Br J Clin Pharmacol, 52, 601–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solus JF, Arietta BJ, Harris JR, Sexton DP, Steward JQ, McMunn C, Ihrie P, Mehall JM, Edwards TL, Dawson EP. (2004). Genetic variation in eleven phase I drug metabolism genes in an ethnically diverse population. Pharmacogenomics, 5, 895–931. [DOI] [PubMed] [Google Scholar]
- Stevens JC, Marsh SA, Zaya MJ, Regina KJ, Divakaran K, Le M, Hines RN. (2008). Developmental changes in human liver CYP2D6 expression. Drug Metab Dispos, 36, 1587–93. [DOI] [PubMed] [Google Scholar]
- Wang H, Tompkins LM. (2008). CYP2B6: new insights into a historically overlooked cytochrome P450 isozyme. Curr Drug Metab, 9, 598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, He B, Shi J, Li Q, Zhu HJ. (2020). Comparative Proteomics Analysis of Human Liver Microsomes and S9 Fractions. Drug Metab Dispos, 48, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, Holloway MG. (2009). Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol, 76, 215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Qian LX, Wu HF, Xu ZQ, Sui YG, Wang XR, Zhang W. (2006). Genetic polymorphisms in the cytochrome P450 1A1 and 2E1 genes, smoking, drinking and prostate cancer susceptibility: a case-control study in a Han nationality population in Southern China. Int J Urol, 13, 773–80. [DOI] [PubMed] [Google Scholar]
- Zhang HF, Wang HH, Gao N, Wei JY, Tian X, Zhao Y, Fang Y, Zhou J, Wen Q, Gao J and others. (2016). Physiological Content and Intrinsic Activities of 10 Cytochrome P450 Isoforms in Human Normal Liver Microsomes. J Pharmacol Exp Ther, 358, 83–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.