Abstract
Introduction
Mexican Americans remain severely underrepresented in Alzheimer's disease (AD) research. The Health & Aging Brain among Latino Elders (HABLE) study was created to fill important gaps in the existing literature.
Methods
Community‐dwelling Mexican Americans and non‐Hispanic White adults and elders (age 50 and above) were recruited. All participants underwent comprehensive assessments including an interview, functional exam, clinical labs, informant interview, neuropsychological testing, and 3T magnetic resonance imaging (MRI) of the brain. Amyloid and tau positron emission tomography (PET) scans were added at visit 2. Blood samples were stored in the Biorepository.
Results
Data was examined from n = 1705 participants. Significant group differences were found in medical, demographic, and sociocultural factors. Cerebral amyloid and neurodegeneration imaging markers were significantly different between Mexican Americans and non‐Hispanic Whites.
Discussion
The current data provide strong support for continued investigations that examine the risk factors for and biomarkers of AD among diverse populations.
Keywords: Alzheimer's disease, amyloid, biomarkers, diversity, Hispanic, Mexican American, mild cognitive impairment, neurodegeneration
1. INTRODUCTION
The percentage of Hispanics 65 and older in the United States will triple by the year 2050.1 Along with this population growth, when compared to other racial/ethnic groups, Hispanic Americans are expected to experience the largest increase in Alzheimer's disease (AD) and AD‐related dementias (ADRD) by 2060.2 AD is the eighth leading cause of death among Hispanics in the United States, although research on AD among Hispanics in the United States remains limited. Approximately 65% of Hispanics in the United States are of Mexican origin 3 ; however, few studies have explicitly examined mild cognitive impairment (MCI) and AD among Mexican Americans. 4 , 5 The extant literature suggests significant differences in MCI and AD among Mexican Americans as compared to non‐Hispanic Whites with regard to age at onset, 6 genetic risks, 4 , 5 medical co‐morbidities, 4 , 5 and biological profiles. 7 , 8 , 9
The Health & Aging Brain among Latino Elders (HABLE) study was initiated in September of 2017 under award R01AG054073 with the goals of (1) investigating factors underlying health disparities in MCI and AD among Mexican Americans (eg, younger age at onset) and (2) examining differential pathways to MCI and AD among Mexican Americans (ie, metabolic, inflammatory, depressive) as compared to non‐Hispanic Whites. The HABLE study is intended to examine long‐term factors associated with incident MCI and AD from mid to late life; therefore, the age for inclusion was set at 50.
The 2018 AT(N) framework 10 provided the field with a biological system for studying AD with the explicit goal of advancing novel clinical trials; however, there remains very little research on amyloid (A), tau (T) or neurodegeneration (N) among diverse populations. 11 In fact, the publication itself calls for examination among community‐based, diverse populations. 10 Currently, the sequence, trajectories, timing, and even clinical impact of cerebral amyloid, tau, or neurodegeneration biomarkers among Mexican Americans is unknown. On the other hand, not only do traditional risk factors, proteomic profiles, and apolipoprotein E (APOE) ε4 genotype vary by racial/ethnic group, but data also point to racial/ethnic variability in core AD pathological markers in cerebrospinal fluid (CSF) 12 , 13 , 14 and autopsy. 15 , 16 , 17 , 18 In August 2020, the HABLE‐AT(N) grant was funded under award number R01AG058533 to examine the hypothesis that the presence, sequence, progression, incidence, and cognitive impact of amyloid, tau, and neurodegenerative biomarkers will be different among Mexican Americans as compared to non‐Hispanic Whites. Together grants R01AG054073 and R01AG058533 provide the structure for a large‐scale multi‐ethnic examination of the AT(N) framework.
The goal of this article is to provide an overview of the HABLE methods for the field. HABLE data, images, and biofluid samples are now publicly available. 19
2. METHODS
The HABLE protocol takes place over multiple appointments completed within a 4‐month timeframe at the Institute for Translational Research (ITR) 20 at the University of North Texas Health Science Center, Fort Worth, Texas. The protocol components are listed in Table 1 . Figure 1 provides a timeline for the HABLE study. Components of the HABLE protocol overlap with other large‐scale studies to facility cross‐study comparisons. For example, several cognitive tests and sociocultural questions overlap with Study of Latinos – Investigation of Neurocognitive Aging (SOL‐INCA) to examine cross‐ethnic factors that contribute to cognitive aging among Hispanic populations. Cognitive testing, magnetic resonance imaging (MRI) and positron emission tomography (PET) overlap with the Alzheimer's Disease Neuroimaging Initiative (ADNI) to enable examinations of differences between clinic‐based and community‐based cohorts as well as the impact of race/ethnicity on AT(N) biomarkers. Imaging and cognitive testing overlap exist with the Longitudinal Early‐Onset Alzheimer's Disease Study (LEADS) to examine factors associated with early onset cognitive loss across populations. Sociocultural questionnaires overlap with the Washington Heights‐Hamilton Heights‐Inwood Community Aging Project (WHICAP) study to foster cross‐study comparisons of diverse cohorts and the impact of sociocultural factors on cognitive aging and biomarkers of AD. These and other cross‐study methodological overlap was intended to allow investigators to combine and analyze data from the HABLE study with other large‐scale clinic‐ and community‐based studies to rapidly advance our understanding of health disparities in AD. All aspects of the study protocol can be conducted in Spanish or English.
TABLE 1.
HABLE Assessments
| Procedure | Baseline Visit* | Visit 2* 24‐30mo | Visit 3* 48‐60mo |
|---|---|---|---|
| Interview | X | X | X |
| Functional Exam | X | X | X |
| Cognitive Battery | X | X | X |
| Physical Exam | X | X | X |
| Informant Interview | X | X | X |
| Blood Draw | X | X | X |
| Proteomics | X | X | X |
| Exosome assays | X | X | |
| MRI | X | X | X |
| Amyloid PET | X | X | |
| Tau PET | X | X | |
| Consensus Classification | X | X | X |
Waves of the HABLE protocol are scheduled for ≈24 month follow‐up timepoints. Due to coronavirus diseases 2019 (COVID‐19), completion of ongoing Wave 1 as well as Wave 2 assessments was longer.
FIGURE 1.
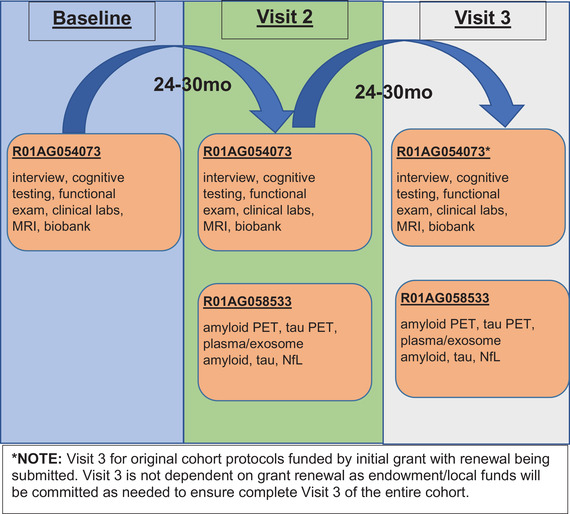
HABLE Study Timeline
2.1. Recruitment
A full description of the HABLE recruitment methods is being published elsewhere. The HABLE study enrolls study participants (ie, Mexican American and non‐Hispanic White) using our previously published community‐based participatory research (CBPR) approach, 21 , 22 , 23 which we and others have demonstrated as a successful method for engaging minority populations in research. 23 , 24 , 25 Our work demonstrated that the community‐based approach yields a representative sample of the larger community. 23 The HABLE study replaces for attrition to ensure that the cohort continues with adequate sample size over time. The team began building community ties and the foundation for the HABLE in 2012. All recruitment efforts target both the Mexican American and non‐Hispanic White communities. Recruitment efforts are managed by the Outreach Core and include a wide range of presentations at events, health fairs, businesses, senior citizen centers, bingo events, churches, and others. The team has provided hundreds of community presentations and educational events since study inception. Participants can provide contact information at community‐based events to research staff as well as contact our team directly based on materials provided at outreach events to express interest in joining the study. Recruitment methods also include newspaper, television, and radio advertisements as well as social media campaigns. Participants regularly refer others into the study (ie, snowball recruitment). A significant component of the HABLE approach is the “give back” to the community. Specifically, any procedures that are used clinically (eg, clinical labs, neuropsychological testing, MRI clinical reads, PET clinical reads) can be provided back to the participant and/or a health care provider of his/her choice. The participant is provided the capacity to make such decisions in the informed consent process. Birthday cards, holiday cards, thank you cards, sympathy cards, and HABLE‐based reports are provided back to the participants.
RESEARCH IN CONTEXT
Systematic Review: The authors used traditional (e.g., PubMed) sources. Mexican Americans are a segment of the U.S. Hispanic population but remain significantly underrepresented in Alzheimer's disease (AD) research. The Health & Aging Brain among Latino Elders (HABLE) study was created to study a broad range of factors contributing to health disparities in mild cognitive impairment (MCI) and AD among Mexican Americans.
Interpretation: Our findings demonstrate that the risk factor for and biomarkers of MCI and AD are different among Mexican Americans as compared to non‐Hispanic Whites.
Future Directions: With the new addition of an African American cohort, the HABLE study will examine the AT(N) framework within a health disparities context among the three largest racial/ethnic groups in the United States. The long‐term goal of this project is to establish population‐guided precision medicine interventions for AD.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria: (1) self‐reported ethnicity of Mexican American or non‐Hispanic White, (2) willingness to provide blood samples, (3) capable of undergoing neuroimaging studies, (4) age 50 and older, and (5) fluent in English or Spanish. Exclusion criteria: (1) type 1 diabetes (2) presence of active infection, (3) current/recent (12 months) cancer (other than skin cancer), (4) current severe mental illness that could impact cognition (except depression), (5) recent (12 months) traumatic brain injury with loss of consciousness, (6) current/recent alcohol/substance abuse, (7) active severe medical condition that could impact cognition (eg, end‐stage renal disease, chronic heart failure, chronic obstructive pulmonary disease), and (8) current diagnosis of dementia other than AD.
2.3. Interview and Medical/Functional Exam
A custom electronic data capture (EDC) system was generated. The HABLE interview includes, but is not limited to, questions regarding medical diagnoses (personal/family, current and past), medical coverage, medications, physical activity, residential, educational and occupational history, household annual income, acculturation, health care access, primary and secondary language, chronic stress, social support, and social engagement. Affective measures include the Geriatric Depression Scale 26 and the Penn State Worry Questionnaire. 27 Possible substance abuse is collected using the Alcohol Use Disorder Inventory (AUDIT). 28 The functional exam includes the Timed Up and Go (TUG) 29 and the Short Physical Performance Battery (SPPB). 30 Anthropomorphic measures (ie, height, weight, abdominal circumference, blood pressure readings) are collected during the interview. Structured questions are collected regarding subjective cognitive complaints. Participants go to Quest Laboratories for collection of fasting bloods and clinical labs, which includes a complete blood count (CBC) with differential, comprehensive metabolic panel, lipid panel, hemoglobin A1c (HbA1c), liver panel, thyroid stimulating hormone, thyroxine (T4), and vitamin B12/folate.
2.4. Informant Interview
All participants provide an informant who is familiar with the participant to answer questions regarding daily functioning. A standardized assessment is administered for the Clinical Dementia Rating (CDR) 31 scale and the physician's estimate of duration (PED). 32
2.5. Cognitive Assessment
The cognitive battery includes tests to assess global cognition, attention/executive functioning, memory, language, and premorbid intelligence (see Table 2 ). Also indicated are the tests that overlap with ADNI, SOL/INCA, and LEADS. Based on our recently published methods, 33 , 34 HABLE normative ranges were calculated stratified by education (0‐7 years, 8‐12 years, and 13+), primary language (English or Spanish), and age (median split; < = 65 and > = 66), which are used to assign cognitive diagnoses.
TABLE 2.
Neuropsychological test battery
| Cognitive Domain | Test |
|---|---|
| Global cognition | Clinical Dementia Rating Scale 48 , Mini‐Mental State Examination 49 |
| Attention/executive functioning | WMS‐III Digit Span 50 , Trail Making Test 51 , Digit Symbol Substitution 52 |
| Memory | Spanish‐English Verbal Learning Test 53 , Wechsler Memory Scale‐III Logical Memory I and II* 50 |
| Language | Animal naming 52 and FAS 52 |
| Premorbid IQ | American National Adult Reading Test 52 or Word Accentuation Test 54 |
NOTE: Bolded tests overlap with ADNI and underlined tests overlap with SOL/INCA. Also note that item‐level data is entered for most neuropsychological testing. *ADNI uses LM Story A only. Italicized tests overlap with LEADS and crosswalk conversions can compare WMS‐III LM scores and Digit Span to LEADS scores.
2.6. Imaging
All HABLE neuroimaging scans are stored, managed, and processed by the University of Southern California (UCS) Laboratory of Neuroimaging (LONI). 35 The HABLE MRI protocol is based on that of ADNI3 using a 3T Siemens Magnetom SKYRA whole‐body scanner. The following scans sequences were captured: T1‐weighted whole brain volumetric spoiled Magnetization‐Prepared Rapid Gradient (MPRAGE), whole‐brain volumetric fluid‐attenuated inversion recovery (FLAIR), susceptibility‐weighted imaging (SWI), diffusion tensor MRI (DTI), 3D arterial spin labeling (3DPASL), resting‐state functional (rsfMRI), and high‐resolution (0.4 × 0.4 mm x 2 mm) T2‐weighted hippocampal high resolution (HHR) scans. FreeSurfer was used to segment the T1‐weighted volume scans by tissue and cortical and subcortical region, to derive whole hippocampal volume and mean cortical thickness in regions of interest (ROIs) across the brain, and to estimate intracranial volume. To segment the HHR scans into subregions in and near the hippocampus, we used Automatic Segmentation of Hippocampal Subfields (ASHS) software. DTI spatial resolution (voxel size) is 1.72 mm x 1.72 mm x 2.5 mm with 64 gradient directions (b = 1000 s/mm2). The Statistical Parametric Mapping Lesion Segmentation Toolbox, Lesion Growth Algorithm 36 was used to quantify white matter hyperintensity volume from T1 and FLAIR images. Supplemental Table 1 provides a comparison of HABLE and ADNI3 MRI protocols. PET Amyloid (Neuraceq; aka florbetaben). Beginning with visit 2, all study subjects undergo PET amyloid imaging using Siemens Biograph Vision 450 whole‐body PET/CT scanner following the ADNI3 protocol for Neuraceq scans. Briefly, participants are injected with an 8.1 mCi (±10%) bolus of Neuraceq. A 4‐frame by 5‐min each (20 min total) dynamic emission acquisition is started 90 min post injection following the acquisition of a low‐dose CT scan used for attenuation correction. The emission images are processed by iterative reconstruction: 4 iterations and 16 subsets. FreeSurfer‐defined regions (frontal, anterior/posterior cingulate, lateral parietal, lateral temporal cortex) were used to derive a summary cortical ROI. Normalization to whole cerebellum reference region was conducted to obtain global standardized update value ratios (SUVRs). An SUVR of 1.08 was used to define positivity. PET Tau Scan ( 18 F‐PI‐2620). Beginning at Visit 2, all HABLE participants undergo PET tau scans using 18F‐PI‐2620 (PI‐2620). All participants are injected with a 10.0 mCi (±10%) bolus of PI‐2620 administered with a total volume of up to 10 mL. A 4 6‐frame by 5 min each dynamic emission acquisition is started 45‐75 minutes post injection and immediately after a CT attenuation scan. The PET images are reconstructed in a 440 reconstruction matrix, zoom = 2, results in 0.825 mm pixels (440 if the full resolution size, like 336 for the TruePoint, 400 for the mCT, and upper 300 s for Horizon). 3D‐OSEM+ToF 8 iterations (5 subsets), pass filter. Match CT slice location OFF, intrinsic slice thickness (1.64557 mm), and 159 slices. Images are reconstructed immediately after the 30‐min emission scan.
2.7. Blood Collection and Processing Procedures
Fasting blood samples are collected and processed per the international guidelines. 37
2.8. Proteomic Assays
All assay preparation is completed using a custom automated StarPlus system from Hamilton Robotics. Serum samples are assayed via a multi‐plex biomarker assay platform using electrochemiluminescence (ECL) per our previously published protocols. 7 , 38 , 39 , 40 The markers assayed include: insulin, pancreatic polypeptide (PPY), peptide YY(PYY), intercellular adhesion molecule 1 (sICAM‐1), vascular cell adhesion molecule 1 (sVCAM‐1), c‐reactive protein (CRP), tumor necrosis factor alpha (TNFα), interleukin (IL)‐6, IL10, GLP1, glucagon, and fatty acid binding protein3 (FABP3). Plasma samples are assayed for amyloid beta 40 (Aβ40) and 42 (Aβ42), tau (total), and neurofilament light (NfL) using the ultra‐sensitive Simoa (single molecule array) technology platform (Quanterix.com).
2.9. Exosome Processing
Plasma neuronal‐derived exosomes (NDEs) are assayed per our previously published protocols. 41 Detailed protocols will be available from the Omics Core. L1CAM‐positive NDE cargo proteins will be quantified using Quanterix Simoa assay for Aβ40, Aβ42, tau, and NfL. Evidence for enrichment of exosomes from neural sources in plasma has been demonstrated previously. 41 , 42
2.10. Cognitive Diagnosis and Consensus Review
Research cognitive diagnoses were assigned based on self‐report and informant report of daily function, expert clinician assignment of CDR scores (using daily function and cognitive information), and neuropsychological testing results. Imaging biomarker data were not used for cognitive assignment. Normal cognition: no complaints of cognitive change (self or other), clinical dementia rating scale sum of boxes score = 0, cognitive test scores above MCI cut scores (below). Of note, participants with isolated cognitive test scores ≤ 1.5 SD below adjusted z‐scores, who had no cognitive or functional complaints were assigned as normal cognition. Participants with subjective cognitive concerns (SCCs) and normal cognition, were assigned as normal cognition and SCC. MCI: complaint of cognitive change (self or other), CDR sum of boxes score of 0.5‐2.0 based on previously published criteria, 43 , 44 and performance at or below 1.5 SD below z‐score adjusted norms on at least one cognitive test. Dementia (in alignment with clinical AD diagnoses 45 ): CDR sum of boxes score > = 2.5 based on prior work 43 , 44 and cognitive test score at or below 2 SD below the mean on two or more tests. To assign these criteria in a consistent and near real‐time manner, the diagnostic system was automated within the EDC system. Medical research diagnoses were assigned by a licensed clinician (MD, DO, or NP) based on clinical labs, medical history, objective measures, and current medications. Assignment of the AT(N) framework research‐based diagnostic criteria is done algorithmically.
3. RESULTS
3.1. Participants and Preliminary Data
As of June 2020, there were a total of n = 1786 participants enrolled in HABLE with data entry and consensus completed on n = 1705, which were included in the current analyses. Due to coronavirus disease 2019 (COVID‐19), recruitment was halted in April 2020. Study procedures began again in July 2020, with Visit 1 assessments ongoing until n = 2000 participants have been enrolled.
3.2. Demographics
Demographic characteristics of the cohort (total and split by ethnicity) are presented in Table 3 . The Mexican American cohort was significantly younger (p < 0.001), had fewer years of education (p < 0.001), had a lower annual household income (p < 0.001), and had a higher body mass index (BMI) (p < 0.001) than the non‐Hispanic White cohort. The Mexican American cohort was less likely to own their residence (p < 0.001), less likely to have insurance (p < 0.001) and less likely to have a primary care provider (p < 0.001). The Mexican American cohort was more likely to have a consensus diagnosis of hypertension (p = 0.002) and more likely to have a diagnosis of type 2 diabetes (p < 0.001). There was no significant difference in dyslipidemia or depression prevalence.
TABLE 3.
HABLE Characteristics
| Total Cohort N = 1705 | Mexican American N = 890 | Non‐Hispanic White N = 813 | |
|---|---|---|---|
| Age | 66.47 (8.75) | 63.88 (7.97) | 69.29 (8.69)*** |
| Gender (% female) | 61% | 67% | 54%*** |
| Education | 12.34 (4.82) | 9.46 (4.59) | 15.48 (2.57)*** |
| BMI | 29.95 | 30.82 | 28.99*** |
| Diabetes (% yes) | 25% | 36% | 13%*** |
| Dyslipidemia (% yes) | 62% | 64% | 60% |
| Hypertension (% yes) | 60% | 63% | 56%** |
| Depression (% yes) | 32% | 33% | 30% |
| Annual Income | $59,155.02 ($70,108.71) | $35,735.10 ($47,821.37) | $84,651.21*** ($80,889.99) |
| Current Residence | |||
| Own | 75% | 71% | 79%*** |
| Rent | 19% | 20% | 18% |
| Live Rent Free/Other | 6% | 9% | 3% |
| Insurance (% no) | 14% | 24% | 4%*** |
| Have Primary Care Provider (% no) | 13% | 21% | 4%*** |
| Control | 79% | 76% | 83% |
| MCI | 14% | 17% | 11%*** |
| Dementia | 7% | 7% | 6% |
p < 0.001 significance after controlling for covariates.
p < 0.01 significance after controlling for covariates.
p < 0.05 after controlling for covariates.
3.3. Diagnostic Classification
Table 3 provides the prevalence rates of MCI and dementia in the HABLE cohort by ethnicity. Mexican Americans were more likely to be classified as MCI (17%) compared to non‐Hispanic Whites (11%; p < 0.01). There was no significant difference in prevalence of dementia between Mexican Americans (7%) and non‐Hispanic Whites (6%). Mexican American MCI cases (mean age = 64.23, SD = 8.11) were younger than non‐Hispanic White MCI cases (mean age = 71.07, SD = 9.94) (p < 0.001).
3.4. Cognitive Testing
Raw neuropsychological test scores for the cohort (total and split by ethnicity) are provided in Table 4 . Analysis of covariance (ANCOVA) models were conducted using age, gender, education, and primary language as covariates to determine the impact of ethnicity. Ethnicity remained a significant predictor of the following domains: Memory (Wechsler Memory Scale (WMS)‐III Logical Memory (LM) 1, Fethnicity[1,1693] = 35.51, p < 0.001; WMS‐III LM2, Fethnicity[1,1693] = 20.30, p < 0.001; SEVLT Trials 1‐5 Total, Fethnicity[1,1694] = 12.93, p < 0.001), Attention (WMS‐III Digit Span, Fethnicity[1,1676] = 72.42, p < 0.001), and Language (FAS, Fethnicity[1,1695] = 15.59, p < 0.001).
TABLE 4.
HABLE Characteristics – Cognitive Testing
| Total Cohort N = 1705 | Mexican American N = 890 | Non‐Hispanic White N = 813 | |
|---|---|---|---|
| Mini Mental State Exam (MMSE) | 27 (3.32) | 26.05 (3.75) | 28.75 (1.96) |
| Trails A | 44.72 (28.05) | 52.01 (32.61) | 36.77 (19.12) |
| Trails B | 121.69 (79.75) | 151.41 (88.70) | 91.94 (55.49) |
| WMS‐III Digit Span | 13.68 (4.27) | 11.42 (3.52) | 16.13 (3.63)*** |
| Digit Symbol Substitution Test (DSST) | 39.78 (13.65) | 34.70 (13.43) | 45.32 (11.60) |
| Verbal Fluency (FAS) | 31.85 (12.25) | 27.13 (10.99) | 37.00 (11.44)*** |
| Category Naming (Animals) | 17.47 (5.16) | 16.28 (4.83) | 18.77 (5.21) |
| WMS‐LM 1 | 35.16 (11.99) | 30.71 (10.60) | 39.98 (11.54)*** |
| WMS‐LM 2 | 21.24 (8.95) | 18.50 (8.07) | 24.20 (8.92)*** |
| SEVLT Trials 1‐5 | 30.69 (9.08) | 28.90 (8.29) | 32.68 (9.49)*** |
| SEVLT 30 min Delay | 7.60 (3.45) | 6.97 (3.31) | 8.29 (3.47) |
| AMNART (errors) | 16.09 (9.86) | 23.92 (9.69) N = 318 | 13.05 (8.09) |
| WAT (correct) | 14.38 (6.37) | 14.38 (6.37) | N/A |
p < 0.001 significance after controlling for covariates of age, gender, education and language.
3.5. Blood Biomarkers
Table 5 provides values for a range of serum and plasma‐based biomarkers for the cohort (total and split by ethnicity). ANCOVA models were conducted using age, sex, education, and primary language as covariates to determine the impact of ethnicity. After we controlled for covariates, Mexican Americans had significantly lower plasma Aβ40 levels (Fethnicity[1,1624] = 12.55, p < 0.001), significantly higher plasma total tau (Fethnicity[1,1624] = 15.80, p < 0.001), significantly higher plasma insulin (Fethnicity[1,1297] = 31.71, p < 0.001), and significantly higher plasma glucagon (Fethnicity[1,1250] = 12.70, p < 0.001) levels.
TABLE 5.
HABLE Characteristics – Blood Biomarker Biomarkers
| Total Cohort N = 1705 | Mexican American N = 890 | Non‐Hispanic White N = 813 | |
|---|---|---|---|
| Plasma AT(N) Markers | |||
| Plasma A | |||
| Aβ40 | 252.55 (67.69) | 239.25 (67.31) | 267.30 (65.06)*** |
| Aβ42 | 12.06 (3.31) | 11.86 (3.41) | 12.26 (3.18) |
| Plasma T (total tau) | 2.47 (0.96) | 2.57 (0.98) | 2.35 (0.92)*** |
| Plasma N (NfL) | 18.97 (11.36) | 17.31 (11.47) | 20.81 (10.98) |
| Metabolic Endophenotype | |||
| PPY | 634.01 (377.71) | 606.95 (386.84) | 660.91 (366.43) |
| PYY | 44.26 (29.77) | 41.81 (27.90) | 46.63 (31.34) |
| GLP1 | 1.32 (1.64) | 1.44 (1.83) | 1.20 (1.14) |
| Insulin | 295.65 (268.99) | 344.47 (301.70) | 247.07 (221.93)*** |
| Glucagon | 64.30 (44.83) | 66.93 (46.08) | 61.43 (43.00)*** |
| FABP3 | 5032.94 (2267.02) | 4619.31 (2259.08) | 5487.06 (2187.72) |
| Inflammatory Endophenotype | |||
| IL6 | 1.32 (2.20) | 1.42 (2.27) | 1.21 (2.12) |
| CRP | 42875365.58 (67985315.03) | 44341610.06 (69216737.06) | 41288343.28 (66691123.43) |
| TNFα | 3.16 (1.03) | 3.18 (1.04) | 3.14 (1.00) |
NOTE: All blood‐based biomarkers were capped at 4 SD above the mean.
p < 0.001 significance after controlling for covariates.
3.6. Neuroimaging Biomarkers
MRI: In order to measure “neurodegeneration” from the AT(N) framework (ie, N), the “metaROI” for N was calculated per Jack. 46 In addition, total brain volume and hippocampal thickness were examined. Mean metaROI for N was significantly different among Mexican Americans (2.73, SD = 0.14) as compared to non‐Hispanic Whites (2.71, SD = 0.15)(F[1,1309] = 8.79, p = 0.003) after controlling for age. After controlling for age and intracranial volume (ICV), whole brain volume was significantly higher among non‐Hispanic Whites (1055224.30, SD = 108753.63) than Mexican Americans (993876.80, SD = 99515.28) (F[1,1524] = 51.73, p < 0.001). Whole brain volume minus the ventricles, CSF, and choroid plexus was also significantly higher among non‐Hispanic Whites (1015871.81, SD = 105928.23) as compared to Mexican Americans (9664447.54; SD = 97253.15) (F[1,1524] = 42.49, p < 0.001) after controlling for age and ICV. Mexican Americans had significantly greater left hippocampal thickness (3810.31, SD = 493.62) as compared to non‐Hispanic Whites (3755.55, SD = 545.17) (F[1,1334] = 18.36, p < 0.001) after covarying for age. Mexican Americans had significantly greater right hippocampal thickness (3939.10, SD = 506.87) as compared to non‐Hispanic Whites (3864.63, SD = 572.95) (F[1,1467] = 13.31, p < 0.001) after covarying for age. Amyloid PET: A total of 60 participants underwent amyloid PET and had data available for preliminary analyses. Clinical reads were conducted on amyloid PET scans, which in certain circumstances (based on IRB protocols) could be returned to the participant's health care provider. A total of n = 52 scans had both clinical and SUVR‐based positivity data available in the database. Amyloid positivity among clinical reads was 27% positive among the Mexican American cohort and 48% among the non‐Hispanic White cohort. Using an SUVR cut score of 1.08, 15% of the Mexican American cohort were classified as positive, whereas 27% of the non‐Hispanic White cohort were classified as amyloid positive. SUVR‐based and clinical reads of positivity were significantly different (p < 0.001). The clinical reads classified 9 of 11 SUVR‐based positive cases as positive (82% agreement). However, the clinical reads classified nine SUVR‐negative scans as positive. A global SUVR score of 0.99 picked up n = 4 additional clinical read positive cases, with n = 3 clinical read positive scans having global SUVR scores less than 0.99. Clinical reads of positivity among Mexican Americans were as follows: normal control 30% positivity, MCI 8% positivity, and AD 45% positivity. Non‐Hispanic White positivity rates were as follows: normal control 44%, MCI 33%, and AD 67%.
4. SUMMARY AND CONCLUSIONS
To date, there have been few comprehensive studies of biomarkers associated with MCI and AD among Mexican Americans. The HABLE study was designed to fill this critical gap in the scientific knowledge. By conducting baseline and longitudinal assessments among both Mexican Americans and non‐Hispanic Whites, the HABLE study will provide the field with an unprecedented amount of data to understand if the biology of AD among diverse populations within the context of sociocultural, behavioral, and environmental factors per the NIA Health Disparities Research Framework. 47
As with prior work, there are significant differences between Mexican American and non‐Hispanic White adults and elders regarding medical and social factors. Mexican Americans had higher rates of diabetes and hypertension as well as higher BMIs as compared to non‐Hispanic Whites. Mexican Americans in HABLE were also less likely to own their own residence, have a primary care provider, or have medical insurance, and had significantly lower household income levels. Mexican Americans also obtained fewer average years of formal education. These findings are also important when considering putative factors (risk and/or causal) associated with MCI and AD among Mexican Americans. Mexican Americans were classified as having MCI at significantly younger ages, which is consistent with our prior work. 5 Mexican Americans were more likely to be classified as MCI as compared to non‐Hispanic Whites, whereas no differences in dementia prevalence were observed. The HABLE team examined ADNI‐criteria for MCI, which resulted in a 30% MCI rate in this cohort, which was considered over‐pathologizing. Therefore, the more traditional ≤1.5 SD cut‐score was implemented. In addition, normative references were created based on prior work 33 ; however, the team has ongoing studies to examine multiple methods for normative consideration that may impact prevalence rates of diagnostic categories across ethnic groups.
The current findings demonstrate a link between ethnicity and biological markers thought to be associated with MCI and AD. Mexican Americans had higher levels of glucagon‐like peptide‐1 (GLP‐1), insulin, and glucagon. The same held for glucose and HbA1c (data not shown). Mexican Americans also had significantly higher levels of plasma Aβ40 and total tau. With regards to imaging, Mexican Americans had lower levels of amyloid positivity and significant differences were observed in multiple MRI‐based measures of neurodegeneration.
By the year 20451 the United States will become largely “non‐White,” with 14% of the U.S. population being African American and 25% being Latino. 1 In addition, by the year 2060, the U.S. population age 65 and older will grow by more among the African American and Hispanic communities as compared to the non‐Hispanic White community. 13 African Americans currently have highest the prevalence of AD and ADRD, whereas Hispanics will experience the greatest increase in ADRDs 2 by 2060. Based on these data, the HABLE study (now entitled the Health & Aging Brain Study – Health Disparities, HABS‐HD) has expanded to add 1000 African Americans and now includes the three largest racial/ethnic groups in the United States (75% of the population). The overall goal of HABS‐HD is to examine the biomarkers of AD within a health disparities framework. All HABS‐HD data are available to the global scientific community to foster a more advanced understanding of the biological, social, cultural, and environmental factors associated with MCI and AD.
DECLARATIONS OF INTEREST
none
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
Research reported here was supported by the National Institute on Aging of the National Institutes of Health under Award Numbers R01AG054073 and R01AG058533. This work was also supported in part by NIH/National Institute of Biomedical Imaging and Bioengineering award P41‐EB015992. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The research team also thanks the local Fort Worth community and participants of the HABLE study.
O'Bryant SE, Johnson LA, Barber RC, et al. The Health & Aging Brain among Latino Elders (HABLE) study methods and participant characteristics. Alzheimer's Dement. 2021;13:e12202. 10.1002/dad2.12202
REFERENCES
- 1. Jacobsen Linda A, et al. America's Aging Population. Popul Bull. 2011;66(1). https://www.prb.org/americas‐aging‐population/. [Google Scholar]
- 2. Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer's disease and related dementias in the United States (2015‐2060) in adults aged ≥65 years. Alzheimer's Dement. 2019. Published online.15(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. US_Census_Bureau. American Fact Finder. Published 2004. http://www.census.gov/
- 4. O'Bryant SE, Johnson L, Balldin V, et al. Characterization of Mexican Americans with mild cognitive impairment and Alzheimer's disease. J Alzheimer's Dis. 2013. Published online.33(2):373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Bryant SE, Johnson L, Reisch J, et al. Risk factors for mild cognitive impairment among Mexican Americans. Alzheimer's Dement. 2013. Published online.9(6):622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson LA, Gamboa A, Vintimilla R, et al. A depressive endophenotype for predicting cognitive decline among mexican American adults and elders. J Alzheimer's Dis. 2016. Published online.54(1):201–206. [DOI] [PubMed] [Google Scholar]
- 7. O'Bryant SE, Ferman TJ, Zhang F, et al. A proteomic signature for dementia with Lewy bodies. Alzheimer's Dement Diagnosis, Assess Dis Monit. 2019. Published online.11: 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Bryant SE, Xiao G, Edwards M, et al. Biomarkers of Alzheimer's disease among Mexican Americans. J Alzheimer's Dis. 2013. Published online.34(4):841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pathak GA, Silzer TK, Sun J, et al. Genome‐Wide Methylation of Mild Cognitive Impairment in Mexican Americans Highlights Genes Involved in Synaptic Transport, Alzheimer's Disease‐Precursor Phenotypes, and Metabolic Morbidities. J Alzheimer's Dis. 2019. Published online.72(3):733–749. [DOI] [PubMed] [Google Scholar]
- 10. Jack CR Jr, DA Bennett, K Blennow, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on ethnic and racial disparities in Alzheimer's disease and related dementias: update and areas of immediate need. Alzheimer's Dement. 2019;15(2):292‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Howell JC, Watts KD, Parker MW, et al. Race modifies the relationship between cognition and Alzheimer's disease cerebrospinal fluid biomarkers. Alzheimer's Res Ther. 2017. Published online.9(1):88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weuve J, Korrick SA, Weisskopf MA, et al. Cumulative exposure to lead in relation to cognitive function in older women. Environ Health Perspect. 2009;117(4):574‐580. http://www.scopus.com/inward/record.url?eid=2‐s2.0‐64049092153&partnerID=40&md5=37427a3441407721ebfe253d93c0793f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morris JC, Schindler SE, McCue LM, et al. Assessment of Racial Disparities in Biomarkers for Alzheimer Disease. JAMA Neurol. 2019. Published online.76(3):264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Bryant SE, Lista S, Rissman RA, et al. Comparing biological markers of Alzheimer's disease across blood fraction and platforms: comparing apples to oranges. Alzheimer's Dement Diagnosis, Assess Dis Monit. 2015. Published online.3: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graff‐Radford NR, Besser LM, Crook JE, Kukull WA, Dickson DW. Neuropathologic differences by race from the National Alzheimer's Coordinating Center. Alzheimer's Dement. 2016. Published online.12(6):669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322‐2326. [DOI] [PubMed] [Google Scholar]
- 18. Barnes LL, Leurgans S, Aggarwal NT, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. Published online 2015. 2015;85(6):528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Institute for Translational Research. Accessed December 20, 2020. https://apps.unthsc.edu/itr/studies/hable
- 20. Institute for Translational Research . Accessed March 23, 2021. https://apps.unthsc.edu/itr/
- 21. Johnson LA, Gamboa A, Vintimilla R, et al. Comorbid Depression and Diabetes as a Risk for Mild Cognitive Impairment and Alzheimer's Disease in Elderly Mexican Americans. J Alzheimer's Dis. 2015. Published online.47(1):129–136. [DOI] [PubMed] [Google Scholar]
- 22. Szerlip HM, Edwards ML, Williams BJ, Johnson LA, Vintimilla RM, O'Bryant SE. Association between cognitive impairment and chronic kidney disease in Mexican Americans. J Am Geriatr Soc. 2015. Published online.63(10):2023–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Bryant SE, Edwards M, Menon C V, Gong G, Barber R. Long‐term low‐level arsenic exposure is associated with poorer neuropsychological functioning: a project FRONTIER study. Int J Environ Res Public Health. 2011. Published online.8(3):861–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O'Fallon A. LR & D. Commitment of the National Institute of Environmental Health Sciences to Community‐Based Participatory Research for Rural Health. Environ Health Perspect. 2001;109:469‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ravenell JE, Ogedegbe G. Community programs for hypertension: a means of identification and intervention in the highest‐risk population. Hypertension in High Risk African Americans: Current Concepts, Evidence‐Based Therapeutics and Future Considerations. In: Ferdinand K. (eds) Hypertension in High Risk African Americans. Clinical Hypertension and Vascular Diseases. Humana Press, NY., 2015. [Google Scholar]
- 26. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37‐49. [DOI] [PubMed] [Google Scholar]
- 27. Meyer TJ, Miller ML MR and BT. Development and validation of the penn state worry questionnaire. Behav Res Ther. 1990;28:487‐495. [DOI] [PubMed] [Google Scholar]
- 28. Alcohol Use Disorders Identification Test (AUDIT). Accessed December 14, 2020. https://auditscreen.org/
- 29. (No Title). Accessed December 14, 2020. https://www.cdc.gov/steadi/pdf/TUG_Test‐print.pdf
- 30. Short Physical Performance Battery (SPPB) | National Institute on Aging. Accessed December 14, 2020. https://www.nia.nih.gov/research/resource/short‐physical‐performance‐battery‐sppb
- 31. Morris JC, Ernesto C, Schafer K, et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer's Disease Cooperative Study experience. Neurology. 1997;48(6):1508‐1510. [DOI] [PubMed] [Google Scholar]
- 32. Doody RS, Dunn JK, Huang E, Azher S, Kataki M. A method for estimating duration of illness in alzheimer's disease. Dement Geriatr Cogn Disord. 2004;17(1‐2):1‐4. http://www.scopus.com/inward/record.url?eid=2-s2.0-0344629372&partnerID=40&md5=b8e6bb8e68822f63a52ff192360f3b3a. [DOI] [PubMed] [Google Scholar]
- 33. O'Bryant SE, Edwards M, Johnson L, Hall J, Gamboa A, O'jile J. Texas Mexican American adult normative studies: normative data for commonly used clinical neuropsychological measures for English‐ and Spanish‐speakers. Dev Neuropsychol. 2018. Published online.43(1):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hall JR, Balldin VH, Gamboa A, Edwards ML, Johnson LA, O'Bryant SE. Texas Mexican American adult normative studies: normative data for the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). Dev Neuropsychol. 2018;43(1):27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laboratory of Neuro Imaging. Accessed March 23, 2021. http://www.loni.usc.edu/research/projects
- 36. Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR‐hyperintense white‐matter lesions in Multiple Sclerosis. Neuroimage. 2012;59(4):3774‐3783. [DOI] [PubMed] [Google Scholar]
- 37. O'Bryant SE, Gupta V, Henriksen K, et al. Guidelines for the standardization of preanalytic variables for blood‐based biomarker studies in Alzheimer's disease research. Alzheimer's Dement. 2015. Published online.11(5):549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Bryant SE, Edwards M, Zhang F, et al. Potential two‐step proteomic signature for Parkinson's disease: pilot analysis in the Harvard Biomarkers Study. Alzheimer's Dement Diagnosis, Assess Dis Monit. 2019. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. O'Bryant SE, Edwards M, Johnson L, et al. A blood screening test for Alzheimer's disease. Alzheimer's Dement Diagnosis, Assess Dis Monit. 2016. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Bryant SE, Xiao G, Zhang F, et al. Validation of a serum screen for alzheimer's disease across assay platforms, species, and tissues. J Alzheimer's Dis. 2014. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Winston CN, Goetzl EJ, Akers JC, et al. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimer's Dement Diagnosis, Assess Dis Monit. 2016. Published online.3: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goetzl EJ, Boxer A, Schwartz JB, et al. Low neural exosomal levels of cellular survival factors in Alzheimer's disease. Ann Clin Transl Neurol. Published online 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Waring O'Bryant, SC Cullum, CM Hall, et al. Staging dementia using Clinical Dementia Rating Scale sum of boxes scores. Arch Neurol. 2008;65(8):1091‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. O'Bryant SE, Lacritz LH, Hall J, et al. Validation of the new interpretive guidelines for the clinical dementia rating scale sum of boxes score in the National Alzheimer's Coordinating Center database. Arch Neurol. 2010;67(6):746‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McKhann Drockman D, Folstein M, et al. D. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group. Neurology. 1984;34:939‐944. [DOI] [PubMed] [Google Scholar]
- 46. Jack CR, Wiste HJ, Therneau TM, et al. Associations of Amyloid, Tau, and Neurodegeneration Biomarker Profiles with Rates of Memory Decline among Individuals Without Dementia. JAMA ‐ J Am Med Assoc. 2019;321(23):2316‐2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hill CV, Pérez‐Stable EJ, Anderson NA, Bernard MA. The national institute on aging health disparities research framework. Ethn Dis. 2015;25(3):245‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatrics. 1997;1:173‐176. [DOI] [PubMed] [Google Scholar]
- 49. Folstein MF, Folstein SE, McHugh PR. Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 50. Wechsler D. Weschler Adult Intelligence Scale ‐ III. The Psychological Corporation; 1997. [Google Scholar]
- 51. Strauss Sherman EMS, Spreen OE. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed.. Oxford University Press; 2006. [Google Scholar]
- 52. Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th Ed. Oxford University Press; 2004. [Google Scholar]
- 53. González HM, Mungas D, Haan MN. A verbal learning and memory test for English‐ and Spanish‐speaking older Mexican‐American adults. Clin Neuropsychol. 2002. Published online. [DOI] [PubMed] [Google Scholar]
- 54. Sierra Sanjurjo N, Montañes P, Sierra Matamoros FA, Burin D. Estimating Intelligence in Spanish: regression Equations with the Word Accentuation Test and Demographic Variables in Latin America. Appl Neuropsychol. 2015. Published online. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


