Abstract
Background
Right ventricular (RV) late gadolinium enhancement (LGE) occurs due to surgical scarring and RV remodeling, and has been shown to be associated with clinical outcomes in Tetralogy of Fallot (TOF). However, it is not known if cardiovascular magnetic resonance (CMR) LGE extent progresses over time, and therefore, it is not known if serial reassessment of LGE is necessary. We determined the rate of progression in the extent of RV LGE on serial CMR examinations in repaired TOF.
Methods
Retrospective review of 127 patients after TOF repair (49% male, median age at first CMR 18.9 years (Interquartile range (IQR) 13.3,27.0) who had at least two CMRs (median follow-up duration of 4.0 years (IQR 2.1,5.9)) was performed. 84/127 patients had no interventions between serial CMRs (Group 1) while 43/127 patients had transcatheter or surgical intervention between CMRs (Group 2). The extent of RV LGE was assessed using 2 methods: a semiquantitative RV LGE score and a quantitative RV LGE extent expressed as % of RV mass. Mixed effects linear regression modeling to estimate changes in LGE over time.
Results
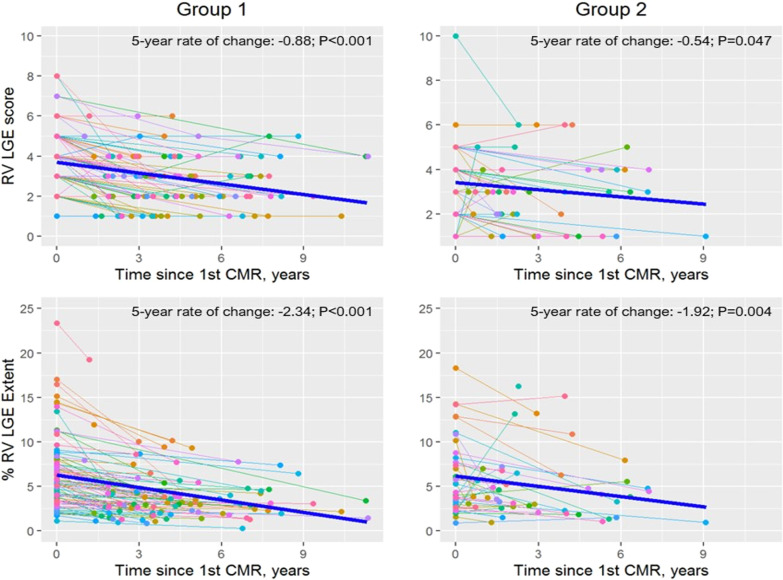
RV LGE was present in all patients on the first CMR. % RV LGE extent and LGE score did not increase over time in either patient group. The mean 5 year rates of change were small and negative for both % RV LGE extent [− 2.3 (95% CI − 2.9, − 1.8, p < 0.001) in Group 1, and − 1.9 (95% CI − 3.2, − 0.7, p = 0.004) in Group 2], and RV LGE score [− 0.9 (95% CI − 1.1, − 0.6, p < 0.001) in Group 1, and − 0.5 (95% CI − 1.1, − 0.0, p = 0.047) in Group 2].
Conclusions
In this serial CMR evaluation of children and adults with repaired TOF, no significant progression in the extent of RV LGE was seen on intermediate term follow-up. Given recent concerns regarding the safety of gadolinium-based contrast agents, frequent assessment of LGE may not be necessary in follow-up.
Keywords: Tetralogy of Fallot, Late gadolinium enhancement, Cardiovascular magnetic resonance, Longitudinal study
Background
Despite advances in the surgical, transcatheter, and medical management of Tetralogy of Fallot (TOF), late morbidity and mortality remain unacceptably high, related to chronic pulmonary regurgitation, ventricular dysfunction, and ventricular or atrial arrhythmias [1–5]. Right ventricular (RV) late gadolinium enhancement (LGE) on cardiovascular magnetic resonance (CMR) study can occur due to surgical scarring or RV remodeling and has been associated with ventricular dysfunction, arrhythmia, and serum biomarkers of heart failure [6–15]. Although the prognostic significance of LGE in TOF has been previously documented, data regarding LGE progression over time are sparse [16]. Understanding the rate of progression in RV LGE is important in informing the appropriate frequency of gadolinium administration to assess LGE on follow-up CMRs. This is particularly important in light of recent reports of long-term gadolinium deposits in the brain and other organs [17, 18]. The purpose of this study was to examine serial CMR data to determine the rate of progression in RV LGE after TOF repair.
Methods
Subjects
A retrospective review of all patients with repaired TOF who had undergone 2 or more CMR examinations with LGE imaging between January 2005 and November 2019 was performed. Patients with pulmonary atresia and major aortopulmonary collateral arteries and those with significant imaging artifacts were excluded. Demographic and clinical data were abstracted from electronic medical records. The hospital’s Committee on Clinical Investigation waived the requirement for informed consent.
CMR
All CMR examinations were performed using a whole-body 1.5 T CMR scanner (Achieva, Philips Healthcare, Best, The Netherlands) using standard imaging techniques recommended by the Society for Cardiovascular Magnetic Resonance [19]. Ventricular volumes and ejection fraction, and pulmonary regurgitation fraction were calculated using standard techniques as previously described by our laboratory [20, 21]. LGE imaging was performed 10–15 min after intravenous administration of a gadolinium-based contrast agent (0.15–0.2 mmol/kg of gadopentate dimeglumine or gadobutrol) using a 2-D phase-sensitive inversion-recovery prepared, fast-gradient echo sequence in ventricular long- and short-axis planes using the following imaging parameters: slice thickness 6–8 mm, slice gap 0–2 mm, reconstructed in-plane spatial resolution between 1.4 × 1.4 mm and 1.7 × 1.7 mm. The short-axis imaging planes were prescribed to cover both ventricles from base to apex. Image analyses were performed using commercially available software (cvi42, v 5.10.1, Circle Cardiovascular Imaging, Calgary, Alberta, Canada).
LGE assessment
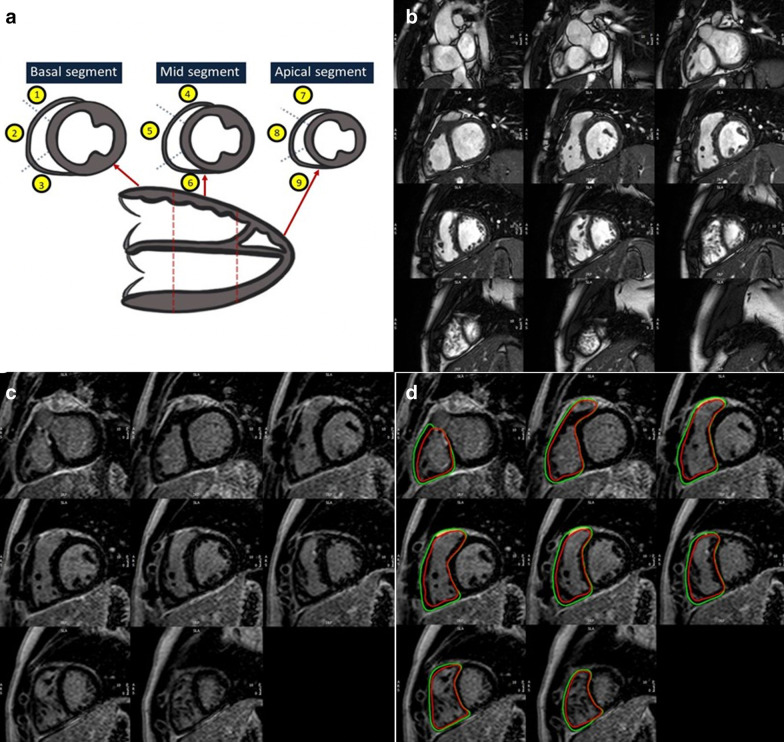
The extent of RV LGE was determined using 2 techniques (Fig. 1): a semiquantitative RV LGE score, and quantitative RV LGE extent expressed as % of RV mass as follows:
Semiquantitative RV LGE score: The score is a modification of a previously described technique [8]. The RV was divided into 9 segments as previously described [7]. Briefly, the RV was first divided into 3 longitudinal regions—basal, midventricular, and apical. Each longitudinal segment was then divided into 3 parts—superior, anterior, and inferior, yielding a total of 9 segments. For each RV segment, LGE extent was graded by a single-blinded observer using a semiquantitative scale as follows: 0 = No LGE; 1 = LGE involving < 1/3 of the segment; 2 = LGE involving 1/3 to 2/3 of the segment; 3 = LGE involving > 2/3 of the segment (Fig. 1). The maximum possible RV LGE score for each subject was 27. Septal insertion point LGE was excluded from RV LGE for this analysis.
Quantitative RV LGE Extent (%): RV endocardial and epicardial contours were drawn manually on the stack of short-axis LGE images, avoiding epicardial fat. Areas of LGE were identified visually and then a semi-automated thresholding technique was applied until the visually identified areas of LGE were marked as areas of positive LGE (Fig. 1). Percentage RV LGE extent was calculated as a sum of the RV mass exhibiting positive LGE, expressed as a percentage of total RV mass. Septal insertion point LGE was excluded from RV LGE for this analysis.
Fig. 1.
RV LGE Assessment. a RV LGE score: RV divided into 9 segments. Each segment graded as follows: 0—no LGE; 1—LGE involving < 1/3 of segment; 2—LGE involving 1/3—2/3 of segment; 3—LGE involving 2/3 of segment (maximum possible score 27) b Example of cine ventricular short axis in diastolic phase c Example of LGE imaging in the ventricular short axis. d % RV LGE Extent: Areas of LGE highlighted using semi- automated manual thresholding. % RV LGE Extent = (RV LGE mass/ Total RV mass) × 100
Statistical analysis
Comparisons among independent groups were performed using Student’s t-test, analysis of variance, Wilcoxon rank-sum test or Kruskal–Wallis test, or a Fisher exact test, as appropriate. Spearman correlation was used to estimate the magnitude of association between LGE score and % LGE extent. To evaluate whether change scores in CMR outcomes differed from zero, a one-sample t-test and Wilcoxon signed-rank test were used. Mixed effects linear regression (fixed time, random subject) was used to evaluate the rate of change in RV LGE over time. For this study, assuming a standard deviation of 2.0 for the change in RV LGE score between 2 CMRs and a 2-sided 0.05-level test, with 40 subjects in each of 2 independent groups, there is 80% power to detect a mean group difference of 1.0 between the mean RV LGE change scores. Intra-rater and inter-rater reliability were assessed using the intra-class correlation coefficient (ICC). A p-value of less than 0.05 was considered significant. Analyses were performed using SAS (version 9.4, SAS Institute, Inc., Cary, North Carolina, USA) and R (version 3.5.1, R Foundation for Statistical Computing, Vienna, Austria).
Results
Subjects
Patient characteristics are summarized in Table 1. A total of 269 CMR examinations were analyzed on 127 subjects with a median follow-up duration of 4.0 years (IQR 2.1,5.9) between initial and latest CMR examinations. Among the study cohort, 112 subjects (88%) had 2 CMR examinations, and 15 (12%) had 3 CMR examinations available for analysis. The study sample consisted of children and adults, a majority of whom had a transannular patch during their initial surgical repair, which was performed most commonly during the first year of life. 84/127 subjects had no interventions between serial CMRs (Group 1), while 43/127 subjects had transcatheter or surgical intervention between CMRs (Group 2). The types of interventions performed in Group 2 are summarized in Table 1.
Table 1.
Patient Characteristics on Initial CMR
| Group 1 (n = 84) | Group 2 (n = 43) | p-value | |
|---|---|---|---|
| Male | 41 (48.8%) | 26 (60.5%) | 0.261 |
| Age at repair (years) | 0.7 (0.3, 5.0) | 0.5 (0.3, 2.9) | 0.507 |
| Type of repair | 0.184 | ||
| Valve sparing | 15 (18.9%) | 2 (4.7%) | |
| Transannular patch | 52 (61.9%) | 33 (76.7%) | |
| RV-to-PA conduit | 7 (8.3%) | 3 (7.0%) | |
| Unknown | 10 (11.9%) | 5 (11.6%) | |
| Time since repair (years) | 18.9 (13.3, 27.0) | 14.8 (12.7, 27.8) | 0.179 |
| Age at first CMR, years | 20.4 (13.2, 34.2) | 15.6 (13.0, 28.7) | 0.164 |
| Age at last CMR, years | 25.6 (17.8, 39.6) | 19.9 (12.6, 31.8) | 0.067 |
| Type of intervention between CMRs | N/A | ||
| Surgical pulmonary valve replacement | N/A | 37 (86%) | |
| Transcatheter pulmonary valve replacement | N/A | 6 (14%) |
Variables are expressed as either frequency (%) or median (interquartile range). Group 1—patients with no interventions between serial CMRs; Group 2—patients with transcatheter or surgical interventions between CMRs. CMR cardiovascular magnetic resonance; RV-to-PA right ventricle to pulmonary artery. N/A not applicable
RV LGE presence, location, and extent
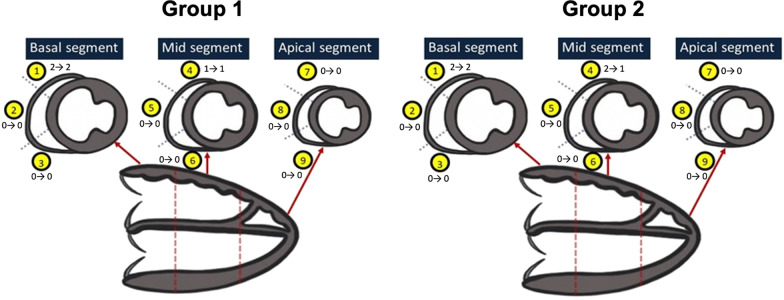
RV LGE was present in all patients on the first CMR examination in both groups. The location of LGE was a basal superior segment in 127 subjects (100%), at the mid superior segment in 82 (65%), and at the mid anterior segment in 16 subjects (13%) (Fig. 3). There was good correlation between RV LGE score and % RV LGE extent in both groups (r = 0.63 in Group 1, r = 0.80 in Group 2; p < 0.001 for both). Intra-observer reliability in measuring % RV LGE extent was good (ICC 0.83) in a subset of 40 CMR examinations that were reanalyzed at a later date by the same blinded observer (KS). Inter-observer reliability was modest (ICC 0.6), assessed in a subset of 20 CMR examinations by a blinded second observer (AP).
Fig. 3.
Longitudinal Changes in Segmental RV LGE Score. Group 1—patients with no interventions between serial CMRs; Group 2—patients with transcatheter or surgical interventions between CMRs. Median baseline and follow-up RV LGE scores for each segment are annotated next to each segment
Serial changes in RV LGE
Serial changes in RV LGE score and % RV LGE extent are summarized in Tables 2 and 3 and in Figs. 2 and 3. There was no increase in RV LGE over time, with a small decline in both LGE parameters over time in both patient groups. Mean 5 yeaar changes in both parameters from linear mixed-effects models are summarized in Table 3. As seen in Fig. 2, RV LGE score and % RV LGE extent increased over follow-up in a small minority of patients, and remained stationary or declined in most patients.
Table 2.
Serial Changes in RV LGE and Functional Parameters
| Initial CMR | Latest CMR | p-value | |
|---|---|---|---|
| Group 1 (n = 84) | |||
| RVEDVI (ml/m2) | 134.5 (114.0, 150.0) | 133.9 (117.1, 152.1) | 0.013 |
| RVESVI (ml/ m2) | 65.7 (55.1, 81.3) | 72.0 (60.8, 84.9) | < 0.001 |
| RVEF (%) | 49.3 (45.8, 54.8) | 47.6 (41.4, 51.5) | < 0.001 |
| PR fraction (%) | 29.0 (7.0, 40.0) | 33.0 (9.0, 43.0) | 0.002 |
| RV LGE score | 4 (3, 5) | 3 (2,4) | < 0.001 |
| RV LGE extent (%) | 5.5 (3.4, 8.0) | 3.1 (2.0, 4.6) | < 0.001 |
| RV Mass/BSA, g/m2 | 16.6 (14.0, 19.4) | 16.6 (14.7, 19.0) | 0.652 |
| RV Mass:Volume, g/ml | 0.12 (0.10, 0.16) | 0.12 (0.10, 0.15) | 0.318 |
| Group 2 (n = 43) | |||
| RVEDVI (ml/m2) | 175.7 (141.6, 200.4) | 118.7 (102.2, 144.3) | < 0.001 |
| RVESVI (ml/ m2) | 85.6 (67.8, 106.1) | 63.8 (51.5, 82.8) | < 0.001 |
| RVEF (%) | 50.1 (42.7, 55.0) | 46.0 (40.7, 50.5) | 0.013 |
| PR fraction (%) | 46.0 (30.0, 55.0) | 5.0 (0.0, 13.0) | < 0.001 |
| RV LGE score | 4 (2, 5) | 3 (2, 4) | 0.030 |
| RV LGE extent (%) | 4.4 (2.7, 7.7) | 3.9 (2.6, 6.3) | 0.009 |
| RV Mass/BSA, g/m2 | 19.1 (14.9, 22.5) | 14.7 (12.5, 16.8) | < 0.001 |
| RV Mass:Volume, g/ml | 0.11 (0.09, 0.12) | 0.12 (0.09, 0.14) | 0.018 |
Variables are expressed as median (interquartile range). Group 1—patients with no interventions between serial CMRs; Group 2—patients with transcatheter or surgical interventions between CMRs. Median time between initial and latest CMR was 4.3 years (IQR 2.9, 6.6) for Group 1, and 2.7 years (IQR 1.6 to 5.3) for Group 2). BSA body surface area, CMR cardiovascular magnetic resonance, EDVI end-diastolic volume index, ESVI end-systolic volume index, EF ejection fraction, PR pulmonary regurgitation, LGE late gadolinium enhancement, RV right ventricle
Table 3.
Mixed effects model of longitudinal changes
| Group 1 (n = 84) | Group 2 (n = 43) | |||
|---|---|---|---|---|
| Variables | Mean 5 year change (95% CI) | p-value | Mean 5 year change (95% CI) | p-value |
| RV LGE score | − 0.88 (− 1.12, − 0.64) | < 0.001 | − 0.54 (− 1.07, − 0.01) | 0.047 |
| % RV LGE extent | − 2.34 (− 2.91, − 1.76) | < 0.001 | − 1.92 (− 3.18, − 0.67) | 0.004 |
| RVEDVI (ml/m2) | 1.63 (− 2.37, 5.63) | 0.421 | − 42.2 (− 60.0, − 24.4) | < 0.001 |
| RVESVI (ml/m2) | 4.07 (1.15, 6.98) | 0.007 | − 19.1 (− 28.9, − 9.27) | < 0.001 |
| RVEF (%) | − 2.81 (− 3.92, − 1.71) | < 0.001 | − 3.11 (− 5.97, − 0.26) | 0.033 |
| PR fraction (%) | 1.36 (− 0.04, 2.76) | 0.057 | − 24.1 (− 33.4, − 14.9) | < 0.001 |
| RV Mass/BSA, g/m2 | − 0.28 (− 1.03, 0.48) | 0.470 | − 3.46 (− 5.53, − 1.39) | 0.002 |
| RV Mass:Volume, g/ml | − 0.004 (− 0.01, 0.03) | 0.243 | 0.01 (− 0.006, 0.03) | 0.212 |
p-values < 0.05 denote that the slope of longitudinal change differs significantly from zero. Group 1—patients with no interventions between serial CMRs; Group 2—patients with transcatheter or surgical interventions between CMRs. BSA body surface area, CMR cardiovascular magnetic resonance, EDVI end-diastolic volume index, ESVI end-systolic volume index, EF ejection fraction, PR pulmonary regurgitation, LGE late gadolinium enhancement, RV right ventricle
Fig. 2.
Longitudinal Changes in RV LGE Score and % RV LGE Extent. Group 1—patients with no interventions between serial CMRs; Group 2—patients with transcatheter or surgical interventions between CMRs. The thick blue line represents the estimated slope of change over time determined using linear mixed-effects modeling
Comparison between groups
RV LGE score and % RV LGE extent showed a similar serial trend of gradual decline over serial CMRs in both Groups 1 and 2, with similar 5 yeaar rates of decline (Table 3). As seen in Fig. 1, in Group 1, no patient showed a serial increase in % RV LGE extent, while a small minority showed a slight increase in the RV LGE score. In Group 2, although the overall trend was similar to that in Group 1, a somewhat larger number of patients showed an increase in % RV LGE extent and RV LGE score. In this group, the only factor associated with an interval increase in both % RV LGE extent and LGE score (n = 6) was older age at repair (p = 0.009, supplemental Table 1).
Factors associated with decline in RV LGE
We explored the hypothesis that a serial increase in RV end-diastolic volume indexed to body surface area (RVEDVI) with a corresponding increase in RV mass may lead to a serial decline in RV LGE score or % RV LGE extent. However, we found no significant association between the rate of change in either parameter of RV LGE, and the rate of change in RVEDVI.
Discussion
In this serial CMR study of children and adults with repaired TOF, although RV LGE was present in all subjects on the initial CMR, the extent of RV LGE analyzed using 2 measures (LGE score and % LGE extent) did not significantly increase during follow-up, rather showing a slight decline over serial examinations.
RV LGE—patterns and serial changes
The patterns of RV LGE in our cohort were similar to those previously reported, with the most common site for LGE being the RV outflow tract. [6] Several prior studies have identified the presence and extent of RV LGE as a risk factor for adverse long-term outcomes in TOF patients [2, 12, 13, 22]. However, the serial rate of change in the RV LGE extent has not been previously studied. Ylitalo et al. [23]. found in a cross-sectional study that the extent of RV LGE is higher in patients with a longer duration of follow-up and they hypothesized that LGE extent increased over time. However, the results of our longitudinal study suggest a lack of significant progress in RV LGE extent during a median follow-up period of 4 years. Further, we found no correlation between the extent of RV LGE and changes in RV volumes during follow-up. It is possible that the finding of a higher LGE extent in patients with a longer duration of follow-up in prior studies may reflect a higher extent of RV surgical scarring, possibly related to an older surgical era. The strengths of the current analysis are its longitudinal design and the quantitative assessment of RV LGE using 2 methods by a single observer.
Comparison between groups
In the present analysis, we evaluated 2 groups of patients—those free of intervention between serial CMRs and those who had surgical or transcatheter intervention between serial CMRs. We analyzed these groups separately, since surgical manipulation of the outflow tract, as well as artifact caused by implanted valves could potentially confound the serial assessment of LGE extent. The findings of a similar lack of progression in RV LGE in both groups suggests that RV LGE in TOF patient possibly represents fixed post-surgical scarring that is not significantly modulated by long term volume loading due to pulmonary regurgitation, or by intervening procedures, at least in the intermediate term (median follow-up duration was 4 years).
LGE assessment
There are inherent difficulties in quantifying RV LGE due to the thin free wall. We used a previously described method for calculation a semiquantitative RV LGE score, in addition to a new method to quantify RV LGE extent [8]. In using the second method for quantifying RV LGE extent, we avoided techniques that automatically designate areas of LGE using a pre-defined standard deviation cutoff relative to normal myocardium, as this can be fraught with error in the thin-walled RV. Instead, we manually identified areas of RV LGE and then quantified the extent of RV LGE in these areas. We found good correlation in the extent of RV LGE calculated using both methods and longitudinal trends in RV LGE were also similar using both techniques. We found good inter-rater and modest inter-observer reliability in quantifying % RV LGE extent. Good inter-rater reliability in assessing the RV LGE score has been previously reported [8].
Clinical implications
Our results have important clinical implications. Since we did not observe a significant increase in RV LGE over a median follow-up duration of 4 years, this suggest that RV LGE does not progress rapidly. Therefore, frequent serial assessment of LGE may not be necessary, thereby avoiding the frequent administration of gadolinium-based contrast agents in these patients, in light of several reports of long-term retention of gadolinium in patients who receive multiple doses of contrast [17, 18]. It should be noted that our findings apply to patients in a more recent surgical era.
Limitations
Several limitations of the current work are worth considering. First, there are known limitations related to its retrospective single-center design. However, these are likely offset by the use of serial longitudinal data using a consistent imaging protocol and analysis by a single observer. Second, while we used serial CMRs, the median duration of follow-up was 4 years and therefore we are not able to exclude progression in RV LGE over longer periods of time. Studies with larger numbers of patients with a longer duration of follow-up may help answer this question more definitively. Third, due to its retrospective design, we did not have high resolution 3-D LGE imaging or imaging for diffuse fibrosis available for serial assessment, as these techniques have only become available more recently. It is possible that follow-up studies using these more sensitive techniques may detect progression in RV fibrosis. Finally, we did not have sufficient numbers of subjects with LV LGE to analyze longitudinal changes in LV LGE over time.
Conclusions
In children and adults with repaired TOF, the RV LGE extent does not increase significantly based upon serial CMRs during a median follow-up of 4 years. Given recent concerns regarding long-term gadolinium retention, our data do not support the routine frequent serial assessment of LGE in TOF patients.
Acknowledgements
None
Abbreviations
- CMR
Cardiovascular magnetic resonance
- ICC
Intraclass correlation coefficient
- IQR
Interquartile range
- LGE
Late gadolinium enhancement
- PA
Pulmonary artery
- PR
Pulmonary regurgitation
- RV
Right ventricle/right ventricular
- RVEDVI
Right ventricular end-diastolic volume index
- RVEF
Right ventricular ejection fraction
- RVESVI
Right ventricular end-systolic volume index
- TOF
Tetralogy of Fallot
Authors' contributions
KS designed the study, analyzed the CMR images and drafted and revised the manuscript; ML performed statistical analyses; LS helped with study design, and supervised the statistical analyses; TG provided guidance during study design and data analysis and helped revise the manuscript; AP was overall in-charge of the study, designed the study, supervised CMR image analysis, and revised the manuscript. All authors read and approved the final manuscript.
Funding
Dr. Saengsin’s research fellowship was funded by the Chiang Mai University Hospital, Chiang Mai, Thailand.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available, as this was not approved by the hospital’s Committee on Clinical Investigation for this study.
Declarations
Ethics approval and consent to participate
The Department of Cardiology’s Scientific Review Committee approved the study, and the Hospital’s Committee on Clinical Investigation waived the requirement for informed consent.
Consent for publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stirrat J, Rajchl M, Bergin L, Patton DJ, Peters T, White JA. High-resolution 3-dimensional late gadolinium enhancement scar imaging in surgically corrected Tetralogy of Fallot: clinical feasibility of volumetric quantification and visualization. J Cardiovasc Magn Reson. 2014;16:76. doi: 10.1186/s12968-014-0076-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obeyesekere MN, Mechulan A, White JA, Bergin L, Khairy P, Gula LJ. Right ventricular outflow tract ventricular tachycardia ablation post-Rastelli repair. Europace. 2011;13:1050–1052. doi: 10.1093/europace/eur111. [DOI] [PubMed] [Google Scholar]
- 3.Khairy P, Landzberg MJ, Gatzoulis MA, Lucron H, Lambert J, Marcon F, Alexander ME, Walsh EP. Value of programmed ventricular stimulation after Tetralogy of Fallot repair: a multicenter study. Circulation. 2004;109:1994–2000. doi: 10.1161/01.CIR.0000126495.11040.BD. [DOI] [PubMed] [Google Scholar]
- 4.Geva T, Mulder B, Gauvreau K, Babu-Narayan SV, Wald RM, Hickey K, Powell AJ, Gatzoulis MA, Valente AM. Preoperative predictors of death and sustained ventricular tachycardia after pulmonary valve replacement in patients with repaired Tetralogy of Fallot enrolled in the INDICATOR Cohort. Circulation. 2018;138:2106–2115. doi: 10.1161/CIRCULATIONAHA.118.034740. [DOI] [PubMed] [Google Scholar]
- 5.Valente AM, Gauvreau K, Assenza GE, Babu-Narayan SV, Schreier J, Gatzoulis MA, Groenink M, Inuzuka R, Kilner PJ, Koyak Z, Landzberg MJ, Mulder B, Powell AJ, Wald R, Geva T. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired Tetralogy of Fallot enrolled in the INDICATOR cohort. Heart. 2014;100:247–253. doi: 10.1136/heartjnl-2013-304958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Secchi F, Lastella G, Monti CB, Barbaro U, Capra D, Zanardo M, Sardanelli F. Late gadolinium enhancement in patients with Tetralogy of Fallot: a systematic review. Eur J Radiol. 2021;136:109521. doi: 10.1016/j.ejrad.2021.109521. [DOI] [PubMed] [Google Scholar]
- 7.Wald RM, Haber I, Wald R, Valente AM, Powell AJ, Geva T. Effects of regional dysfunction and late gadolinium enhancement on global right ventricular function and exercise capacity in patients with repaired tetralogy of fallot. Circulation. 2009;119:1370–1377. doi: 10.1161/CIRCULATIONAHA.108.816546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babu-Narayan SV, Kilner PJ, Li W, Moon JC, Goktekin O, Davlouros PA, Khan M, Ho SY, Pennell DJ, Gatzoulis MA. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired Tetralogy of fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006;113:405–413. doi: 10.1161/CIRCULATIONAHA.105.548727. [DOI] [PubMed] [Google Scholar]
- 9.Haggerty CM, Suever JD, Pulenthiran A, Mejia-Spiegeler A, Wehner GJ, Jing L, Charnigo RJ, Fornwalt BK, Fogel MA. Association between left ventricular mechanics and diffuse myocardial fibrosis in patients with repaired Tetralogy of Fallot: a cross-sectional study. J Cardiovasc Magn Reson. 2017;19:100. doi: 10.1186/s12968-017-0410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oosterhof T, Mulder BJ, Vliegen HW, de Roos A. Corrected Tetralogy of Fallot: delayed enhancement in right ventricular outflow tract. Radiology. 2005;237:868–871. doi: 10.1148/radiol.2373041324. [DOI] [PubMed] [Google Scholar]
- 11.Hunold P, Jakob H, Erbel R, Barkhausen J, Heilmaier C. Accuracy of myocardial viability imaging by cardiac MRI and PET depending on left ventricular function. World J Cardiol. 2018;10:110–118. doi: 10.4330/wjc.v10.i9.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghonim S, Ernst S, Keegan J, Giannakidis A, Spadotto V, Voges I, Smith GC, Boutsikou M, Montanaro C, Wong T, Ho SY, McCarthy KP, Shore DF, Dimopoulos K, Uebing A, Swan L, Li W, Pennell DJ, Gatzoulis MA, Babu-Narayan SV. Three-dimensional late gadolinium enhancement cardiovascular magnetic resonance predicts inducibility of ventricular tachycardia in adults with repaired Tetralogy of Fallot. Circ Arrhythm Electrophysiol. 2020;13:e008321. doi: 10.1161/CIRCEP.119.008321. [DOI] [PubMed] [Google Scholar]
- 13.Cochet H, Iriart X, Allain-Nicolai A, Camaioni C, Sridi S, Nivet H, Fournier E, Dinet ML, Jalal Z, Laurent F, Montaudon M, Thambo JB. Focal scar and diffuse myocardial fibrosis are independent imaging markers in repaired Tetralogy of Fallot. Eur Heart J Cardiovasc Imaging. 2019;20:990–1003. doi: 10.1093/ehjci/jez068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prakash A, Powell AJ, Krishnamurthy R, Geva T. Magnetic resonance imaging evaluation of myocardial perfusion and viability in congenital and acquired pediatric heart disease. Am J Cardiol. 2004;93:657–661. doi: 10.1016/j.amjcard.2003.11.045. [DOI] [PubMed] [Google Scholar]
- 15.Preim U, Sommer P, Hoffmann J, Kehrmann J, Lehmkuhl L, Daehnert I, Gutberlet M, Grothoff M. Delayed enhancement imaging in a contemporary patient cohort following correction of Tetralogy of Fallot. Cardiol Young. 2015;25:1268–1275. doi: 10.1017/S1047951114002248. [DOI] [PubMed] [Google Scholar]
- 16.Karur GR, Mawad W, Grosse-Wortmann L. Progressive right ventricular outflow tract fibrosis after repair of Tetralogy of Fallot. Cardiol Young. 2020;30:1366–1367. doi: 10.1017/S1047951120002164. [DOI] [PubMed] [Google Scholar]
- 17.Radbruch A. Are some agents less likely to deposit gadolinium in the brain? Magn Reson Imaging. 2016;34:1351–1354. doi: 10.1016/j.mri.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Runge VM. Critical questions regarding gadolinium deposition in the brain and body after injections of the gadolinium-based contrast agents, safety, and clinical recommendations in consideration of the EMA's pharmacovigilance and risk assessment committee recommendation for suspension of the marketing authorizations for 4 linear agents. Invest Radiol. 2017;52:317–323. doi: 10.1097/RLI.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 19.Fratz S, Chung T, Greil GF, Samyn MM, Taylor AM, Valsangiacomo Buechel ER, Yoo SJ, Powell AJ. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson. 2013;15:51. doi: 10.1186/1532-429X-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blalock SE, Banka P, Geva T, Powell AJ, Zhou J, Prakash A. Interstudy variability in cardiac magnetic resonance imaging measurements of ventricular volume, mass, and ejection fraction in repaired Tetralogy of Fallot: a prospective observational study. J Magn Reson Imaging. 2013;38:829–835. doi: 10.1002/jmri.24050. [DOI] [PubMed] [Google Scholar]
- 21.Geva T. Repaired Tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson. 2011;13:9. doi: 10.1186/1532-429X-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuypers JA, Menting ME, Konings EE, Opic P, Utens EM, Helbing WA, Witsenburg M, van den Bosch AE, Ouhlous M, van Domburg RT, Rizopoulos D, Meijboom FJ, Boersma E, Bogers AJ, Roos-Hesselink JW. Unnatural history of Tetralogy of Fallot: prospective follow-up of 40 years after surgical correction. Circulation. 2014;130:1944–1953. doi: 10.1161/CIRCULATIONAHA.114.009454. [DOI] [PubMed] [Google Scholar]
- 23.Ylitalo P, Pitkänen OM, Lauerma K, Holmström M, Rahkonen O, Heikinheimo M, Sairanen H, Jokinen E. Late gadolinium enhancement (LGE) progresses with right ventricle volume in children after repair of Tetralogy of Fallot. Int J Cardiol Heart Vessel. 2014;3:15–20. doi: 10.1016/j.ijchv.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available, as this was not approved by the hospital’s Committee on Clinical Investigation for this study.