Abstract
Targeted cognitive training (TCT) has been reported to improve verbal learning deficits in patients with schizophrenia (SZ). Despite positive findings, it is not clear whether demographic factors and clinical characteristics contribute to the success of TCT on an individual basis. Medication-associated anticholinergic burden has been shown to impact TCT-associated verbal learning gains in SZ outpatients, but the role of anticholinergic medication burden on TCT gains in treatment refractory SZ patients has not been described. In this study, SZ patients mandated to a locked residential rehabilitation center were randomized to treatment as usual (TAU; n = 22) or a course of TAU augmented with TCT (n = 24). Anticholinergic medication burden was calculated from medication data at baseline and follow-up using the Anticholinergic Cognitive Burden (ACB) Scale. MATRICS Consensus Cognitive Battery Verbal Learning domain scores were used as the primary outcome variable. The TAU and TCT groups were matched in ACB at baseline and follow-up. While baseline ACB was not associated with verbal learning in either group, increases in ACB over the course of the study were significantly associated with deterioration of verbal learning in the TAU group (r = −0.51, p = 0.02). This was not seen in subjects randomized to TCT (r = −0.13, p = 0.62). Our results suggest that TCT may blunt anticholinergic medication burden associated reduction in verbal learning in severely disabled SZ inpatients.
Keywords: Targeted cognitive training, Schizophrenia, Anticholinergic medication, Verbal learning, Cognitive impairment
1. Introduction
Several randomized controlled clinical trials have demonstrated that computerized auditory-based targeted cognitive training (TCT) interventions enhance verbal learning in schizophrenia (SZ) (Biagianti et al., 2017a; Biagianti et al., 2016; Biagianti et al., 2017b; Dale et al., 2016; Fisher et al., 2016; Fisher et al., 2009; Fisher et al., 2010; Fisher et al., 2015; Nahum et al., 2014; Ramsay et al., 2018; Schlosser et al., 2017; Subramaniam et al., 2012; Subramaniam et al., 2014; Vinogradov et al., 2012). TCT consists of a suite of auditory-based exercises usually delivered in 20–40 1 h sessions, and is thought to improve verbal learning by enhancing the fidelity of low-level auditory information processing in a “bottom up” manner (Adcock et al., 2009). As impaired verbal learning is a robust predictor of psychosocial disability and currently approved medications for SZ do not meaningfully target verbal learning deficits, TCT is regarded as a promising intervention for SZ patients (Rajji et al., 2014; Revell et al., 2015). Despite these encouraging results, not all SZ patients benefit from this time and resource intensive treatment. In fact, up to 40% of SZ patients fail to show meaningful cognitive gains after a full course of TCT, even with up to 100 h of training (Biagianti et al., 2016; Fisher et al., 2010; Thomas et al., 2018). Identifying patient characteristics that affect therapeutic response thus remains an important next-step for more widespread implementation of TCT in real-world clinical settings.
Previous studies have demonstrated that anticholinergic medication burden both contributes to cognitive decline and may blunt the effectiveness of TCT in SZ (Minzenberg et al., 2004; Vinogradov et al., 2009). In one of the few studies assessing anticholinergic burden on TCT performance, Vinogradov and colleagues found that serum anticholinergic burden in stable SZ outpatients was negatively associated with TCT gains over the course of treatment (Vinogradov et al., 2009). Chronic exposure to anticholinergic medications is common, and is linked to cognitive impairment not only in SZ, but in other illnesses such as Alzheimer’s disease (Brebion et al., 2004; Drimer et al., 2004; Gray et al., 2015; McGurk et al., 2004; Pristed et al., 2017; Strauss et al., 1990; Su et al., 2017; Veselinovic et al., 2015; Xiang et al., 2013; Xiang et al., 2011). Indeed, cholinergic signaling from the basal forebrain throughout the cerebral cortex is critical for a number of different cognitive processes, including verbal learning (Demeter and Sarter, 2013; Duzel et al., 2010; Peter et al., 2016; Wallace and Bertrand, 2013). These cholinergic projections are thought to be active in brain neuroplasticity and detection of salient stimuli in bottom-up sensory processing, both of which are thought to underlie TCT-associated gains in SZ (Froemke et al., 2007; Kilgard and Merzenich, 1998; Kuo et al., 2007; Leanza et al., 1996; McGaughy et al., 2002; Risbrough et al., 2002; Sarter et al., 2005).
The aim of the present study was to assess the role of anticholinergic load on TCT treatment effects in a cohort of SZ patients who were mandated to long-term, inpatient care at a non-academic community treatment center. Studying this patient population allows a unique opportunity to assess how TCT can be implemented in real-world settings, especially in patients who typically are more severely disabled, have a longer illness duration, and may be exposed to a greater number of psychotropic medications with potential for anticholinergic properties. More broadly, we chose to study such an impaired SZ population as this presented an opportunity to test a bias in the field that more severely impaired patients may not be able to benefit from TCT (Thomas et al., 2018).
In the present study, the Anticholinergic Cognitive Burden Scale (ACB) was used to calculate the overall anticholinergic medication burden from medication records. The ACB has been widely used in multiple contexts to determine the longitudinal cognitive impact of anticholinergic medications (Cai et al., 2013; Campbell et al., 2010; Campbell et al., 2016; Fox et al., 2011; Kolanowski et al., 2009). Recent analyses by Tsoutsoulas and colleagues have found even modest exposure to anticholinergics as calculated by the ACB is associated with cognitive impairment in SZ patients (Tsoutsoulas et al., 2017). We have previously reported that patients randomized to a ~30 h course of TCT in this cohort significantly improved verbal learning relative to a matched treatment as usual (TAU) group (Thomas et al., 2018). Based on Vinogradov et al., 2009, and the above supporting literature, we investigated the impact of anticholinergic medication burden on cognitive gains produced by TCT with the following hypotheses in mind: 1) greater anticholinergic medication burden would be associated with poorer verbal learning across all SZ subjects, and 2) higher levels of anticholinergic burden would be associated with attenuated TCT-associated gains in verbal learning gains as previously reported.
2. Materials and methods
2.1. Participants
Participants, study design, and intervention (summarized below) are described in Thomas et al., 2018. Below, we provide a brief overview of the participants, design and intervention previously reported. Subjects with chronic psychosis (N = 46) who were court mandated to reside in a non-academic residential treatment program were enrolled in this study. All participants were under conservatorship (i.e., public guardianship) by San Diego or Los Angeles counties, or by a private party, due to being gravely disabled due to mental illness—unable to provide food, water, and/or shelter due to the severity of their symptoms. This community-based residential program serves a transitional role, bridging acute crisis and independent living, with patients undergoing phased community reintegration. Patients typically stay approximately 6 months before being able to discharge to a lower level of care (i.e., a board and care).
2.2. Design
Subjects were randomized to treatment as usual (TAU, n = 22) or treatment as usual with TCT (TCT, n = 24). Randomization was stratified by sex, age and ethnicity. Subjects had to meet formal diagnostic criteria for schizophrenia or schizoaffective disorder based on an abbreviated Structured Clinical Interview for DSM-IV-TR (First et al., 2002). Subjects were initially considered for eligibility by their treatment team who were blinded to the study and after subjects indicated interest, written informed consent was obtained with subsequent written approvals ultimately granted from public guardians/conservators before initiating any study activities. The Institutional Review Board of University of California, San Diego approved all experimental procedures (IRB#130874). As reported previously (Thomas et al., 2018), 8 participants randomized to the TCT group and 2 assigned to TAU group did not complete the study; however, the completion rate did not significantly differ between groups.
2.3. Intervention
Participants randomized to the TAU group received their usual care and study assessments only. Participants randomized to TAU with TCT additionally completed 1 h of training per day, 3 to 5 days a week for up to 40 h. TCT was administered using laptops with headphones with exercises from the BrainHQ suite from Posit Science Corporation (Posit Science, 2016). Exercises applied an n-up/m-down algorithm to participant responses to estimate threshold, allowing participants to be continuously challenged at approximately 80% criterion accuracy throughout training.
2.4. Outcome measures
Age and gender corrected T-scores from the MATRICS Consensus Cognitive Battery (MCCB; (Nuechterlein et al., 2008) verbal learning scale was used as the primary cognitive outcome variable. Medication data was collected for all subjects from electronic medication records. The Anticholinergic Cognitive Burden scale (ACB) was used to quantify anticholinergic medication burden. The ACB is a validated expert-based list of medications with significant anticholinergic properties (Cai et al., 2013; Campbell et al., 2010; Campbell et al., 2016; Fox et al., 2011; Kolanowski et al., 2009). All medications were assigned a number on a 4-point Likert-type scale based on the ACB ranging from 0 for no anticholinergic activity to 3 for definite anticholinergic activity. ACB was calculated for each subject both at baseline as well as the end of treatment by summing the ratings from all medications administered on the day of assessment. For further analysis of what medication classes contributed to the overall ACB score, we separated medications into antipsychotics, non-antipsychotic psychotropics, and non-psychotropic medications, which are shown in Supplemental Table 1.
2.5. Analyses
Inferential tests of group differences were based on t-tests and ANOVA. Relationships between ACB and MCCB verbal learning were examined using Pearson correlation coefficients. For all statistical comparisons, an alpha of 0.05 was used for determining significance.
3. Results
Demographic characteristics, clinical symptoms, CPZs, days to follow-up assessment and training hours are reported in Table 1 (see also, Thomas et al., 2018). TAU and TCT groups did not differ on any of the baseline demographic variables (age, gender, race, education, illness duration). TAU and TCT groups also had similar levels of positive and negative symptoms as assessed by SAPS and SANS, similar antipsychotic load, and similar baseline cognitive function. TCT produced significant improvement on MCCB verbal learning t-scores with a moderately large effect size (TAU, baseline = 33.09 ± 6.09; TAU, follow-up = 33.75 ± 3.99; TCT, baseline = 32.75 ± 6.19; TCT, follow-up = 37.62 ± 6.18; b = 4.13, SE = 1.86, df = 33.22, t = 2.21, d = 0.65).
Table 1.
Demographic and clinical characteristics. Means +/− standard deviations are given where applicable. TAU = treatment as usual group. TCT = targeted cognitive training. SAPS = Scale for the Assessment of Positive Symptoms. SANS = Scale for the Assessment of Negative Symptoms.
| TAU | TCT | p | |
|---|---|---|---|
| Sample size | 22 | 24 | 0.75 |
| Age | 35.73 (13.00) | 34.54 (12.13) | 0.55 |
| Gender: Males | 9 (41% | 13 (54%) | 0.61 |
| Hispanic | 6 (27%) | 4 (17%) | |
| Race | |||
| African American | 3 (14%) | 5 (21%) | 0.51 |
| Asian | 2 (9%) | 1 (4%) | |
| Caucasian | 12 (55%) | 13 (54%) | |
| More than one race | 5 (23%) | 3 (12%) | |
| Native American | 0 (0%) | 2 (8%) | |
| Education | 11.95 (2.17) | 11.71 (1.99) | 0.69 |
| Illness duration | 15.23 (12.78) | 16.12 (13.67) | 0.82 |
| Chlorpromazine equivalents | 982.534 (758.10) | 1329.42 (972.78) | 0.82 |
| SAPS | 5.36 (5.02) | 6.46 (4.26) | 0.62 |
| SANS | 13.09 (3.41) | 12.96 (4.19) | 0.22 |
| Days to follow-up | 99.30 (24.26) | 89.44 (19.79) | 0.2 |
| Hours of training | 27.97 (10.20) | ||
Both TAU and TCT groups had similar ACB ratings at baseline (TAU = 5.50 ± 2.98; TCT = 5.06 ± 2.83) and follow up (TAU = 6.0 ± 3.28; TCT = 4.56 ± 2.22) with no significant group (F = 1.09, p = 0.34), time (F = 0.00, p = 1.0) or group x time interaction (F = 1.87, p = 0.180). As shown in Fig. 1, in both TAU and TCT groups, the major contribution to the overall anticholinergic burden was due to antipsychotic medications and non-antipsychotic psychotropic medications.
Fig. 1.
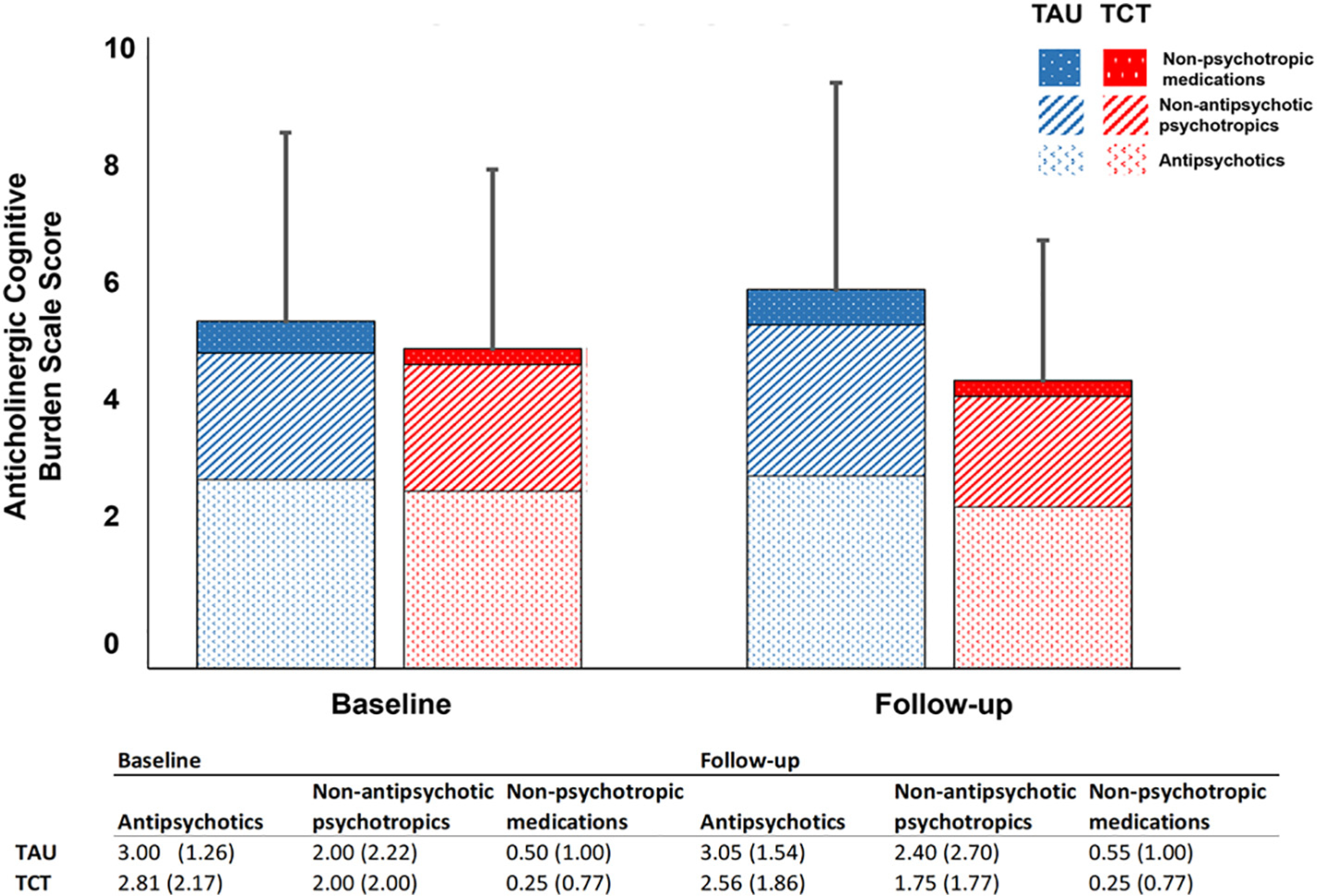
Anticholinergic burden in TAU and TCT groups. Bar graph represents sum total of ACB scores from antipsychotics, non-antipsychotic psychotropics and non-psychotropic medications in both groups. Table reports mean (standard deviation).
In contrast to expectations, baseline ACB scores were not significantly correlated with baseline verbal learning (r = − 0.10, p = 0.57) at the group level prior to randomization. ACB scores at follow up were also not significantly correlated with verbal learning (TAU, r = −0.28, p = 0.24; TCT, r = 0.11, p = 0.67). Changes in ACB over the course of the study, however, were negatively correlated with changes in verbal learning t-scores in the TAU group such that an increase in ACB over the course of the study was associated with reduced verbal learning (TAU, r = −0.51, p = 0.02; Fig. 2). This relationship was not seen in the TCT group (r = −0.13, p = 0.62).
Fig. 2.
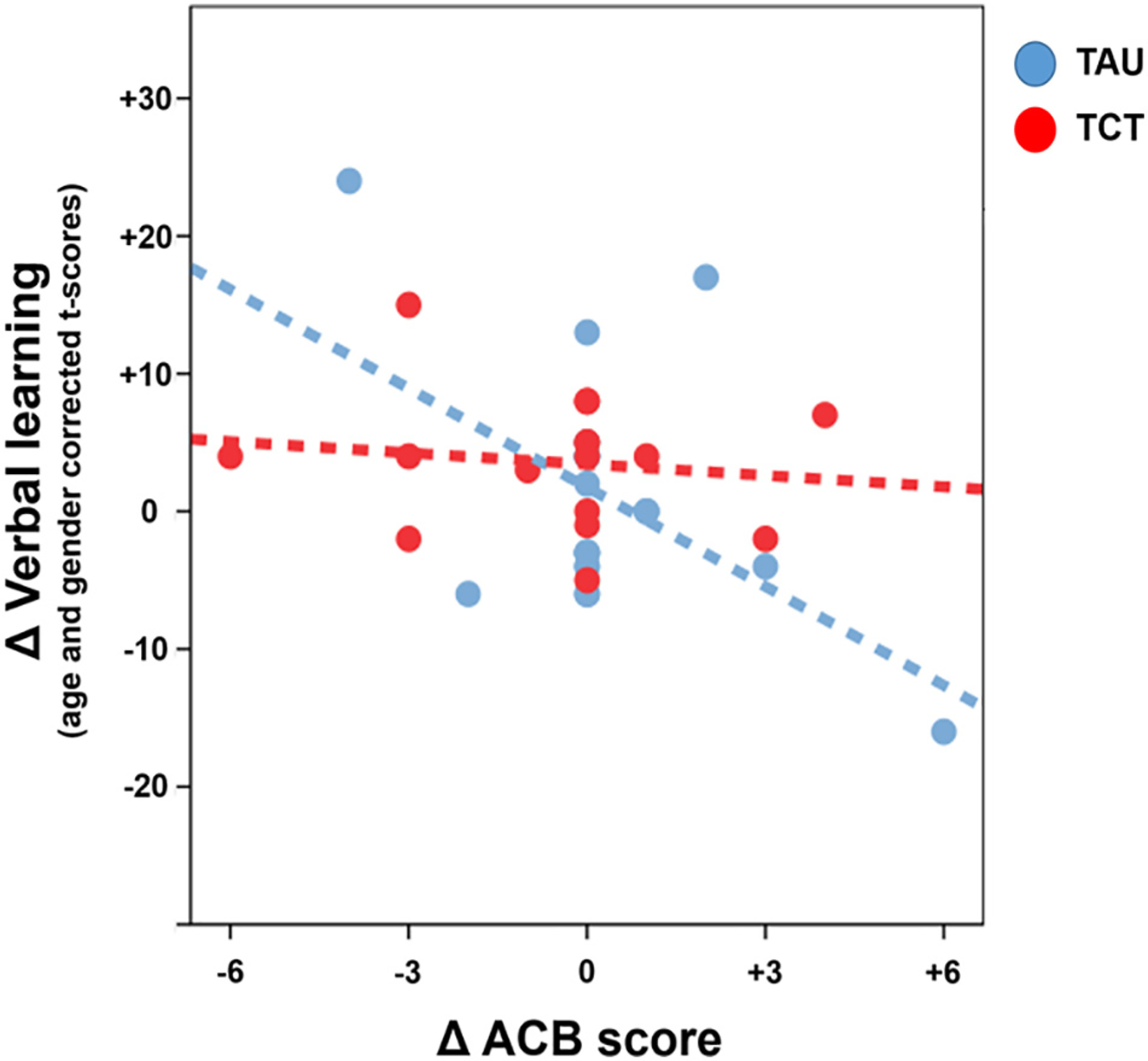
Relationship between change in verbal learning and ACB score in SZ patients undergoing targeted cognitive training. Change in anticholinergic burden between baseline and end of study was correlated negatively with gains in verbal learning in SZ patients in the treatment as usual group (TAU), but not in those who received targeted cognitive training (TCT). An increase in ACB score between baseline and end of study was associated with worsening verbal learning scores in the TAU but not in the TCT group.
We further investigated the relationship between antipsychotic load, verbal learning and ACB score. We did not find significant correlations between verbal learning and antipsychotic load (r = −0.11, p = 0.50) at baseline. At the end of the study, verbal learning and antipsychotic load were not correlated in either TAU (r = −0.003, p = 0.99) or TCT (r = −0.12, p = 0.65). Change in antipsychotic load did not correlate with change in verbal learning in either TAU (r = −0.19, p = 0.47) or TCT (r = 0.053, p = 0.85). Partial correlation analyses between ACB score and change in verbal learning adjusting for antipsychotic load was not significant in subjects at baseline (r = −0.042, p = 0.82) nor at follow up in either TAU (r = −0.48, p = 0.06) or TCT (r = 0.93, p = 0.74) groups.
4. Discussion
The impact of anticholinergic medication burden on treatment effects of TCT was examined in a cohort of treatment-refractory SZ patients mandated to locked inpatient care. We hypothesized that higher baseline anticholinergic medication burden, as measured by ACB, would be associated with poorer baseline cognition and that higher anticholinergic burden would be associated with reduced TCT gains. In contrast to our expectations, anticholinergic medication burden was not significantly associated with verbal learning at baseline or follow up, but increases in ACB over the course of the study were significantly associated with a decline in verbal learning in the TAU, but not TCT group.
High anticholinergic burden has been repeatedly linked with cognitive impairment, increasing risk for dementing illnesses in non-SZ populations, and has also been recently linked to cognitive deficits in SZ patients in a pattern which resembles early Alzheimer’s disease (Eum et al., 2017; O’Reilly et al., 2016; Tsoutsoulas et al., 2017). Since neurocognitive impairment in SZ can be present in both prodromal and early illness patients, and strongly predicts functional outcomes, additional cognitive impairment imparted by a high anticholinergic load could further jeopardize the potential success of pro-cognitive interventions (Kremen et al., 1994; Revheim et al., 2014; Thomas et al., 2017; Wallace and Bertrand, 2013; Welsh et al., 2018; Zheng et al., 2018). Consistent with the extant literature described above, we found that subjects in the TAU group who experienced an increase in ACB over the course of our study had reduction in their verbal learning t-scores. Solutions to blunt such cognitive impairment in older adults have largely been to either de-prescribe medications with high anticholinergic burden when possible, or transition to alternate medications with fewer anticholinergic properties for the same indication. However, in the case of chronic psychotic illnesses like schizophrenia, a large portion of the anticholinergic burden is related to antipsychotics and other psychotropics which are necessary to avoid disability stemming from psychosis. De-prescribing antipsychotics carries substantial risk of decompensation, and, often SZ patients may not benefit from changing to alternative antipsychotics and psychotropics due to the complexities of treatment response from such medications even if they are mechanistically similar. Other methods to reduce the cognitive burden of anticholinergics include cholinergic system-based cognitive enhancers (i.e., acetylcholinesterase inhibitors) or other strategies (i.e., nicotine supplementation via nicotine patches), but these are currently not approved for patients with chronic psychoses for this purpose, have had inconsistent effectiveness in previous studies assessing their pro-cognitive benefits in SZ (Erickson et al., 2005), and have the potential for significant side effects or unwanted reactions. In this context, using TCT to potentially blunt high anticholinergic medication burden-associated cognitive impairment could present a novel therapeutic approach to improve functional outcomes in SZ. Intriguingly, the present data implies that participation in TCT may protect against cognitive decline associated with very high anticholinergic load.
These results differ somewhat from those previously reported by Vinogradov and colleagues who reported a negative correlation between baseline serum anticholinergic activity as measured by radioimmunoassay and TCT performance in a group of SZ patients (Vinogradov et al., 2009). This discrepancy may be due to differences in the patient characteristics and assessment methods for anticholinergic burden. First, it is noteworthy that the present study enrolled severely disabled SZ inpatients with refractory illness while Vinogradov and colleagues reported on chronically ill, but stable outpatients. Indeed, all patients in our cohort met the legal criteria of grave disability and were unable to maintain independently in the community to the point of requiring guardianship/permanent conservatorship and inpatient residential level of care.
Second, the average ACB load in our study participants was substantially higher than studies of other SZ populations. It is possible that our failure to detect relationships between baseline cognition and anticholinergic medication burden as reported by Vinogradov and Fisher may be due to a floor effect on cognition induced by a high anticholinergic medication burden in our cohort of disabled patients (Fisher et al., 2009; Vinogradov et al., 2009). Indeed, mean ACB scores in chronically stable community dwelling SZ outpatients have been described to be between 2 and 3 (Tsoutsoulas et al., 2017). In forensic inpatient settings the average ACB score in patients with chronic psychosis has been reported to be between 4 and 5 (O’Reilly et al., 2016). Given that our participant group was mandated to locked-inpatient care—although not in a forensic setting—the ACB values we observed are not surprising. It is interesting to note, however, that an ACB score of 1.5 is a sensitive cutoff for predicting cognitive impairment in older patients with SZ, and large longitudinal studies in non-SZ older adults have found that being exposed to drugs with strong anticholinergic properties (i.e., an ACB of 2 or above) is associated with a greater decline in cognition over time (Campbell et al., 2018; Campbell et al., 2016; Tsoutsoulas et al., 2017). Similarly, the antipsychotic load as measured by chlorpromazine equivalents in our study approximates those described in patients requiring a state-hospital-level of care, double what is seen in most studies of chronically stable SZ outpatients (Diaz and De Leon, 2002), including previous studies of TCT in SZ (Fisher et al., 2009; Vinogradov et al., 2009).
Third, perhaps consequently, while both Vinogradov et al. and our study enrolled SZ patients with similar levels of verbal learning impairment (~ 2 standard deviations below the population mean), the patients in the current report had a greater global cognitive impairment by nearly an additional standard deviation. It is possible that in such a severely-impaired population that anticholinergic effects may affect TCT performance differently than those with lower levels of baseline cognitive deficit. However, it should be noted that it is not clear whether such differences in baseline cognition reflect inherent impairment in our subjects or other contextual factors (e.g., Thomas et al., 2017).
Notably, Vinogradov and colleagues utilized a blood-based measure of anticholinergic activity, while the present study relied upon a well-validated clinical scale. While both serum-based and scale-based studies have repeatedly demonstrated that high anticholinergic load is associated with cognitive impairment in SZ as well as other illnesses, both types of assessments have strengths and limitations (Mayer et al., 2016). Serum-based measures of anticholinergic load are unbiased and can account for anticholinergic activity that might be otherwise missed using drug scales. This is particularly relevant for those medications which have limited data or are newly approved or are not typically thought to have anticholinergic effects—such methods can also take into account individual differences in basal cholinergic tone in subjects. However, peripheral assessment of anticholinergic activity may not accurately represent concentrations of brain levels of anticholinergic medications, and serum anticholinergic activity has been shown to be affected by acute stressors and endogenous hormones (Hachisu et al., 2015; Plaschke et al., 2010; Sulon et al., 1978; Todorova et al., 2001). In contrast, drug scales have the benefit of being empirically derived from longitudinal data on cognitive outcomes and are low-cost and easy to administer in real-world settings where additional blood draws might not be readily feasible. Unfortunately, however, such scales may assign differing anticholinergic risk to the same drug, generally fail to account for dose effects, and presume that anticholinergic effects can be summed in a linear fashion potentially ignoring complex pharmacology (Naples et al., 2015; Welsh et al., 2018). To the extent that anticholinergic load—regardless of how it is measured—impacts potential TCT gains, and that TCT could inform and facilitate a future comprehensive neurorehabilitation strategy in SZ, it is likely that scales like ACB could be useful.
The results of this study should be interpreted with other limitations in mind. Due to the severity of functional impairment in our subjects, the present findings may not generalize to less severely impaired SZ patients. While the efficacy of TCT in SZ outpatients has already been established in carefully controlled trials, the current trial was designed to determine the feasibility and effectiveness of delivering TCT in this more severely impaired cohort of patients who were mandated to longer-term inpatient treatment. We were not able to follow up with patients beyond the end of the course of TCT—thus, we are not able to describe whether anticholinergic burden may affect functional outcomes in a durable way. More broadly, despite the encouraging overall findings of beneficial effects of TCT on verbal learning, additional studies are required to determine which individual patient characteristics or biomarkers predict individual patient response to this and other treatments (Hochberger et al., 2018). Since to date, demographic, clinical, or other cognitive features at baseline fail to predict benefit to TCT or other pro-cognitive therapeutics, and in light of the results presented, the role of anticholinergic burden in the effectiveness of TCT warrants further study.
Supplementary Material
Acknowledgements
The authors wish to thank George B. Handran and the Sidney R. Baer, Jr. Foundation, USA for their generous support of this research. We also wish to thank all of the participants and non-author support staff that made this study possible, including the following key personnel: Sean Pianka, Sonia Rackelmann, and Alexandra L. Shiluk.
Role of funding source
Funding sources had no role in the design of this study, its execution, analyses, interpretation of data, writing of the manuscript or decision to submit results.
Footnotes
Conflict of interest
Dr. Light reports having been a consultant to Astellas, Boehringer-Ingelheim, Dart Neuroscience, Heptares, Lundbeck, Merck, NeuroSig, Neuroverse, and Takeda.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.schres.2019.01.016.
References
- Adcock RA, Dale C, Fisher M, Aldebot S, Genevsky A, Simpson GV, Nagarajan S, Vinogradov S, 2009. When top-down meets bottom-up: auditory training enhances verbal memory in schizophrenia. Schizophr. Bull. 35 (6), 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagianti B, Fisher M, Neilands TB, Loewy R, Vinogradov S, 2016. Engagement with the auditory processing system during targeted auditory cognitive training mediates changes in cognitive outcomes in individuals with schizophrenia. Neuropsychology 30 (8), 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagianti B, Fisher M, Howard L, Rowlands A, Vinogradov S, Woolley J, 2017a. Feasibility and preliminary efficacy of remotely delivering cognitive training to people with schizophrenia using tablets. Schizophr. Res. Cogn. 10, 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagianti B, Roach BJ, Fisher M, Loewy R, Ford JM, Vinogradov S, Mathalon DH, 2017b. Trait aspects of auditory mismatch negativity predict response to auditory training in individuals with early illness schizophrenia. Neuropsychiatric Electrophysiology. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebion G, Bressan RA, Amador X, Malaspina D, Gorman JM, 2004. Medications and verbal memory impairment in schizophrenia: the role of anticholinergic drugs. Psychol. Med. 34 (2), 369–374. [DOI] [PubMed] [Google Scholar]
- Cai X, Campbell N, Khan B, Callahan C, Boustani M, 2013. Long-term anticholinergic use and the aging brain. Alzheimers Dement. 9 (4), 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell NL, Boustani MA, Lane KA, Gao S, Hendrie H, Khan BA, Murrell JR, Unverzagt FW, Hake A, Smith-Gamble V, Hall K, 2010. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology 75 (2), 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell NL, Perkins AJ, Bradt P, Perk S, Wielage RC, Boustani MA, Ng DB, 2016. Association of Anticholinergic Burden with cognitive impairment and health care utilization among a diverse ambulatory older adult population. Pharmacotherapy 36 (11), 1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell NL, Lane KA, Gao S, Boustani MA, Unverzagt F, 2018. Anticholinergics influence transition from normal cognition to mild cognitive impairment in older adults in primary care. Pharmacotherapy 38 (5), 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale CL, Brown EG, Fisher M, Herman AB, Dowling AF, Hinkley LB, Subramaniam K, Nagarajan SS, Vinogradov S, 2016. Auditory cortical plasticity drives training-induced cognitive changes in schizophrenia. Schizophr. Bull. 42 (1), 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter E, Sarter M, 2013. Leveraging the cortical cholinergic system to enhance attention. Neuropharmacology 64, 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz FJ, De Leon J, 2002. Excessive antipsychotic dosing in 2 U.S. State hospitals. J. Clin. Psychiatry 63 (11), 998–1003. [DOI] [PubMed] [Google Scholar]
- Drimer T, Shahal B, Barak Y, 2004. Effects of discontinuation of long-term anticholinergic treatment in elderly schizophrenia patients. Int. Clin. Psychopharmacol. 19 (1), 27–29. [DOI] [PubMed] [Google Scholar]
- Duzel S, Munte TF, Lindenberger U, Bunzeck N, Schutze H, Heinze HJ, Duzel E, 2010. Basal forebrain integrity and cognitive memory profile in healthy aging. Brain Res. 1308, 124–136. [DOI] [PubMed] [Google Scholar]
- Erickson SK, Schwarzkopf SB, Palumbo D, Badgley-Fleeman J, Smirnow AM, Light GA, 2005. Efficacy and tolerability of low-dose donepezil in schizophrenia. Clin. Neuropharmacol. 28 (4), 179–184. [DOI] [PubMed] [Google Scholar]
- Eum S, Hill SK, Rubin LH, Carnahan RM, Reilly JL, Ivleva EI, Keedy SK, Tamminga CA, Pearlson GD, Clementz BA, Gershon ES, Keshavan MS, Keefe RSE, Sweeney JA, Bishop JR, 2017. Cognitive burden of anticholinergic medications in psychotic disorders. Schizophr. Res. 190, 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). Biometrics Research New York State Psychiatric Institute, New York. [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S, 2009. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am. J. Psychiatry 166 (7), 805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S, 2010. Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophr. Bull. 36 (4), 869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Loewy R, Carter C, Lee A, Ragland JD, Niendam T, Schlosser D, Pham L, Miskovich T, Vinogradov S, 2015. Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia. Schizophr. Bull. 41 (1), 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Herman A, Stephens DB, Vinogradov S, 2016. Neuroscience-informed computer-assisted cognitive training in schizophrenia. Ann. N. Y. Acad. Sci. 1366 (1), 90–114. [DOI] [PubMed] [Google Scholar]
- Fox C, Richardson K, Maidment ID, Savva GM, Matthews FE, Smithard D, Coulton S, Katona C, Boustani MA, Brayne C, 2011. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J. Am. Geriatr. Soc. 59 (8), 1477–1483. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE, 2007. A synaptic memory trace for cortical receptive field plasticity. Nature 450 (7168), 425–429. [DOI] [PubMed] [Google Scholar]
- Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, Yu O, Crane PK, Larson EB, 2015. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern. Med. 175 (3), 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachisu M, Konishi K, Hosoi M, Tani M, Tomioka H, Kitajima Y, Inamoto A, Hirata A, Koganemaru T, Tomita A, Akashi N, Hori K, 2015. Serum anticholinergic activity as an index of anticholinergic activity load in Alzheimer’s disease. Neurodegener. Dis. 15 (3), 134–139. [DOI] [PubMed] [Google Scholar]
- Hochberger WC, Joshi YB, Thomas ML, Zhang W, Bismark AW, Treichler EBH, Tarasenko M, Nungaray J, Sprock J, Cardoso L, Swerdlow N, Light GA, 2018. Neurophysiologic measures of target engagement predict response to auditory-based cognitive training in treatment refractory schizophrenia. Neuropsychopharmacology 44 (3), 606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM, 1998. Cortical map reorganization enabled by nucleus basalis activity. Science (New York, N.Y.) 279 (5357), 1714–1718. [DOI] [PubMed] [Google Scholar]
- Kolanowski A, Fick DM, Campbell J, Litaker M, Boustani M, 2009. A preliminary study of anticholinergic burden and relationship to a quality of life indicator, engagement in activities, in nursing home residents with dementia. J. Am. Med. Dir. Assoc. 10 (4), 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Seidman LJ, Pepple JR, Lyons MJ, Tsuang MT, Faraone SV, 1994. Neuropsychological risk indicators for schizophrenia: a review of family studies. Schizophr. Bull. 20 (1), 103–119. [DOI] [PubMed] [Google Scholar]
- Kuo MF, Grosch J, Fregni F, Paulus W, Nitsche MA, 2007. Focusing effect of acetylcholine on neuroplasticity in the human motor cortex. J. Neurosci. 27 (52), 14442–14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanza G, Muir J, Nilsson OG, Wiley RG, Dunnett SB, Bjorklund A, 1996. Selective immunolesioning of the basal forebrain cholinergic system disrupts short-term memory in rats. Eur. J. Neurosci. 8 (7), 1535–1544. [DOI] [PubMed] [Google Scholar]
- Mayer T, Kopitz J, Plaschke K, Weiss J, Seidling HM, Haefeli WE, 2016. Limitations of the anticholinergic activity assay and assay-based anticholinergic drug scales. Am. J. Geriatr. Psychiatry 24 (12), 1182–1188. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW, 2002. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a five-choice serial reaction time task. J. Neurosci. 22 (5), 1905–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk SR, Green MF, Wirshing WC, Wirshing DA, Marder SR, Mintz J, Kern R, 2004. Antipsychotic and anticholinergic effects on two types of spatial memory in schizophrenia. Schizophr. Res. 68 (2–3), 225–233. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Poole JH, Benton C, Vinogradov S, 2004. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am. J. Psychiatry 161 (1), 116–124. [DOI] [PubMed] [Google Scholar]
- Nahum M, Fisher M, Loewy R, Poelke G, Ventura J, Nuechterlein KH, Hooker CI, Green MF, Merzenich M, Vinogradov S, 2014. A novel, online social cognitive training program for young adults with schizophrenia: a pilot study. Schizophr. Res. Cogn. 1 (1), e11–e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naples JG, Marcum ZA, Perera S, Gray SL, Newman AB, Simonsick EM, Yaffe K, Shorr RI, Hanlon JT, Health A, Body Composition S, 2015. Concordance between anticholinergic burden scales. J. Am. Geriatr. Soc. 63 (10), 2120–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR, 2008. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am. J. Psychiatry 165 (2), 203–213. [DOI] [PubMed] [Google Scholar]
- O’Reilly K, O’Connell P, Donohoe G, Coyle C, O’Sullivan D, Azvee Z, Maddock C, Sharma K, Sadi H, McMahon M, Kennedy HG, 2016. Anticholinergic burden in schizophrenia and ability to benefit from psychosocial treatment programmes: a 3-year prospective cohort study. Psychol. Med. 46 (15), 3199–3211. [DOI] [PubMed] [Google Scholar]
- Peter J, Lahr J, Minkova L, Lauer E, Grothe MJ, Teipel S, Kostering L, Kaller CP, Heimbach B, Hull M, Normann C, Nissen C, Reis J, Kloppel S, 2016. Contribution of the cholinergic system to verbal memory performance in mild cognitive impairment. J. Alzheimers Dis. 53 (3), 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaschke K, Kopitz J, Mattern J, Martin E, Teschendorf P, 2010. Increased cortisol levels and anticholinergic activity in cognitively unimpaired patients. J. Neuropsychiatr. Clin. Neurosci. 22 (4), 433–441. [DOI] [PubMed] [Google Scholar]
- Posit Science, 2016. Brain Training that Works.
- Pristed SG, Correll CU, Nielsen J, 2017. Frequency and correlates of anticholinergic use among patients with schizophrenia in Denmark: a nation-wide pharmacoepidemiological study. Psychiatry Res. 255, 198–203. [DOI] [PubMed] [Google Scholar]
- Rajji TK, Miranda D, Mulsant BH, 2014. Cognition, function, and disability in patients with schizophrenia: a review of longitudinal studies. Can. J. Psychiatry 59 (1), 13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay IS, Fryer S, Boos A, Roach BJ, Fisher M, Loewy R, Vinogradov S, Mathalon DH, 2018. Response to targeted cognitive training correlates with change in thalamic volume in a randomized trial for early schizophrenia. Neuropsychopharmacology 43 (3), 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell ER, Neill JC, Harte M, Khan Z, Drake RJ, 2015. A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophr. Res. 168 (1–2), 213–222. [DOI] [PubMed] [Google Scholar]
- Revheim N, Corcoran CM, Dias E, Hellmann E, Martinez A, Butler PD, Lehrfeld JM, DiCostanzo J, Albert J, Javitt DC, 2014. Reading deficits in schizophrenia and individuals at high clinical risk: relationship to sensory function, course of illness, and psychosocial outcome. Am. J. Psychiatry 171 (9), 949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough V, Bontempi B, Menzaghi F, 2002. Selective immunolesioning of the basal forebrain cholinergic neurons in rats: effect on attention using the 5-choice serial reaction time task. Psychopharmacology 164 (1), 71–81. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B, 2005. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res. Brain Res. Rev. 48 (1), 98–111. [DOI] [PubMed] [Google Scholar]
- Schlosser DA, Campellone TR, Truong B, Anguera JA, Vergani S, Vinogradov S, Arean P, 2017. The feasibility, acceptability, and outcomes of PRIME-D: a novel mobile intervention treatment for depression. Depress. Anxiety 34 (6), 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss ME, Reynolds KS, Jayaram G, Tune LE, 1990. Effects of anticholinergic medication on memory in schizophrenia. Schizophr. Res. 3 (2), 127–129. [DOI] [PubMed] [Google Scholar]
- Su YA, Yan F, Li Q, Xiang YT, Shu L, Yu X, Ning YP, Zhang KR, Li T, Mei QY, Li KQ, Si TM, 2017. Anticholinergic use trends in 14,013 patients with schizophrenia from three national surveys on the use of psychotropic medications in China (2002−2012). Psychiatry Res. 257, 132–136. [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S, 2012. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron 73 (4), 842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Garrett C, Chung C, Fisher M, Nagarajan S, Vinogradov S, 2014. Intensive cognitive training in schizophrenia enhances working memory and associated prefrontal cortical efficiency in a manner that drives long-term functional gains. NeuroImage 99, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulon J, Sparano F, Sciarra F, Giaquinto G, Genard P, 1978. 24 hour profile of 18-hydroxy-11-deoxycorticosterone in normal supine man: relationship with cortisol and aldosterone. Clin. Endocrinol. 8 (5), 367–372. [DOI] [PubMed] [Google Scholar]
- Thomas ML, Green MF, Hellemann G, Sugar CA, Tarasenko M, Calkins ME, Greenwood TA, Gur RE, Gur RC, Lazzeroni LC, Nuechterlein KH, Radant AD, Seidman LJ, Shiluk AL, Siever LJ, Silverman JM, Sprock J, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL, Light GA, 2017. Modeling deficits from early auditory information processing to psychosocial functioning in schizophrenia. JAMA Psychiat. 74 (1), 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ML, Bismark AW, Joshi YB, Tarasenko M, Treichler EBH, Hochberger WC, Zhang W, Nungaray J, Sprock J, Cardoso L, Tiernan K, Attarha M, Braff DL, Vinogradov S, Swerdlow N, Light GA, 2018. Targeted cognitive training improves auditory and verbal outcomes among treatment refractory schizophrenia patients mandated to residential care. Schizophr. Res. 202, 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorova A, Vonderheid-Guth B, Dimpfel W, 2001. Effects of tolterodine, trospium chloride, and oxybutynin on the central nervous system. J. Clin. Pharmacol. 41 (6), 636–644. [DOI] [PubMed] [Google Scholar]
- Tsoutsoulas C, Mulsant BH, Kumar S, Ghazala Z, Voineskos AN, Menon M, Pollock BG, Rajji TK, 2017. Anticholinergic Burden and Cognition in Older Patients With Schizophrenia. J. Clin. Psychiatry 78 (9), e1284–e1290. [DOI] [PubMed] [Google Scholar]
- Veselinovic T, Vernaleken I, Janouschek H, Kellermann T, Paulzen M, Cumming P, Grunder G, 2015. Effects of anticholinergic challenge on psychopathology and cognition in drug-free patients with schizophrenia and healthy volunteers. Psychopharmacology 232 (9), 1607–1617. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Warm H, Holland C, Kirshner MA, Pollock BG, 2009. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am. J. Psychiatry 166 (9), 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, de Villers-Sidani E, 2012. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology 37(1), 43–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TL, Bertrand D, 2013. Importance of the nicotinic acetylcholine receptor system in the prefrontal cortex. Biochem. Pharmacol. 85 (12), 1713–1720. [DOI] [PubMed] [Google Scholar]
- Welsh TJ, van der Wardt V, Ojo G, Gordon AL, Gladman JRF, 2018. Anticholinergic drug burden tools/scales and adverse outcomes in different clinical settings: a systematic review of reviews. Drugs Aging in press. [DOI] [PubMed] [Google Scholar]
- Xiang YT, Wang CY, Si TM, Lee EH, He YL, Ungvari GS, Chiu HF, Yang SY, Chong MY, Tan CH, Kua EH, Fujii S, Sim K, Yong KH, Trivedi JK, Chung EK, Udomratn P, Chee KY, Sartorius N, Shinfuku N, 2011. Use of anticholinergic drugs in patients with schizophrenia in Asia from 2001 to 2009. Pharmacopsychiatry 44 (3), 114–118. [DOI] [PubMed] [Google Scholar]
- Xiang YT, Dickerson F, Kreyenbuhl J, Ungvari GS, Wang CY, Si TM, Lee EH, Chiu HF, Lai KY, He YL, Yang SY, Chong MY, Tan CH, Kua EH, Fujii S, Sim K, Yong MK, Trivedi JK, Chung EK, Udomratn P, Chee KY, Sartorius N, Shinfuku N, 2013. Common use of anticholinergic medications in older patients with schizophrenia: findings of the research on Asian psychotropic prescription pattern (REAP) study, 2001–2009. Int. J. Geriatr. Psychiatry 28 (3), 305–311. [DOI] [PubMed] [Google Scholar]
- Zheng W, Zhang QE, Cai DB, Ng CH, Ungvari GS, Ning YP, Xiang YT, 2018. Neurocognitive dysfunction in subjects at clinical high risk for psychosis: a meta-analysis. J. Psychiatr. Res. 103, 38–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


