Abstract
Background
COVID-19 outcomes and risk factors, including comorbidities and medication regimens, in people living with diabetes (PLWD) are poorly defined for low- and middle-income countries.
Methods
The Provincial Health Data Centre (Western Cape, South Africa) is a health information exchange collating patient-level routine health data for approximately 4 million public sector health care seekers. Data from COVID-19 patients diagnosed between March and July 2020, including PLWD, were analysed to describe risk factors, including dispensed diabetes medications and comorbidities, and their association with COVID-19 outcomes in this population.
Findings
There were 64,476 COVID-19 patients diagnosed. Of 9305 PLWD, 44.9% were hospitalised, 4.0% admitted to ICU, 0.6% received ventilation and 15.4% died. In contrast, proportions of COVID-19 patients without diabetes were: 12.2% hospitalised, 1.0% admitted, 0.1% ventilated and 4.6% died. PLWD were significantly more likely to be admitted (OR:3.73, 95 %CI: 3.53, 3.94) and to die (OR:3.01, 95 %CI: 2.76,3.28). Significant hospitalised risk factors included HIV infection, chronic kidney disease, current TB, male sex and increasing age. Significant risk factors for mortality were CKD, male sex, HIV infection, previous TB and increasing age. Pre-infection use of insulin was associated with a significant increased risk for hospitalisation (OR:1·39, 95 %CI:1·24,1·57) and mortality (OR1·49, 95 %CI:1·27; 1·74) and metformin was associated with a reduced risk for hospitalisation (OR:0·62,95 %CI:0·55, 0·71) and mortality (OR 0·77, 95 %CI:0·64; 0·92).
Interpretation
Using routine health data from this large virtual cohort, we have described the association of infectious and noncommunicable comorbidities as well as pre-infection diabetes medications with COVID-19 outcomes in PLWD in the Western Cape, South Africa.
Funding
This research was funded in part, by the Wellcome Trust 203135/Z/16/Z, through support of NT. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. The Wellcome Centre for Infectious Diseases Research in Africa is supported by core funding from the Wellcome Trust [203135/Z/16/Z]. NT receives funding from the CIDRI-Africa Wellcome Trust grant (203135/Z/16/Z), and NT and TT receive funding from the NIH H3ABioNET award (U24HG006941). NT receives funding from the UKRI/MRC (MC_PC_MR/T037733/1).
Keywords: COVID-19, Diabetes, Sub-Saharan Africa
1. Introduction
Infection with SARS-CoV-2 has caused a global pandemic that has not spared any country. At the time of writing, 107 million people have been infected and 2·4 million people have died from Coronavirus Disease in 2019 (COVID-19) [1].
It has now been well established that people living with diabetes (PLWD) are at increased risk of more severe infection with SARS-CoV-2 [2]. Observational data of COVID-19 from countries which were at the forefront of this pandemic have reported greater morbidity and mortality in PLWD than those without diabetes, when studied in well-defined populations from the UK [3], [4], [5], [6], [7]. Publications from low-middle income countries of Mexico, Brazil and South Africa have similarly confirmed this risk [8], [9], [10].
Determinants of COVID-19 mortality risk among people with diabetes have been explored in a few studies from the UK, Ireland and France [3], [4], [5], [11]. Age, sex, duration of diabetes, body mass index (BMI), black and South Asian ethnicity, lower socio-economic status, poorer glycaemic control, and pre-existing cardiovascular disease were reported to increase risk.
In this study, we used linked-routine health data at the end of the first wave of COVID-19, collated from a variety of electronic platforms for adults attending public sector health facilities in the Western Cape Province, South Africa, to identify whether diabetes is associated with greater morbidity and mortality from COVID-19. By using routinely collected data, we aimed to determine whether there were any predictors for more severe COVID-19, among these patients.
2. Methods
2.1. Study design
In this cohort study, we used data from the first wave of the pandemic in the Western Cape Province, from 04 March 2020 (when the first case was identified) to 15 July 2020, when infection rates had dropped.
2.2. Selection of study population
The Provincial Health Data Centre (PHDC) is a health information exchange, housed by the Western Cape Department of Health that collates and links routine health data from a variety of electronic platforms used across the Western Cape Province [12]. These include demographic data from facilities, dispensing data for medications, laboratory data, and data from a variety of disease-specific and service delivery data systems. The data are updated daily and linked to a de-duplicated patient master index (PMI), which represents approximately 5.25 million that rely solely on the public sector for health care.
The study population was identified from the Western Cape Population, as represented in the PHDC. Inclusion criteria were: (1) having attended at least one Government Health Facility in the Western Cape, South Africa, in the period 1 January 2010 to 31 December 2019, used as a proxy for patients accessing public sector health care and (2) a laboratory confirmation of SARS-CoV-2 infection up and until the 15 July 2020. A COVID-19 diagnosis was inferred from PHDC records, using evidence of a positive SARS-CoV-2 polymerase chain reaction (PCR) laboratory result. Records without patient COVID-19 outcome (deceased or recovered) by October 2020, were excluded.
Data descriptors All retrospective, routine health data accessed in PHDC for public health sector patients with a COVID-19 outcome, were analysed using R version 3·6·1 (2019-07-05). Descriptive statistical methods assessed population demographics as of 31 July 2020. ‘Age’ was the age at COVID-19 diagnosis in years, ‘sex’ was the gender recorded in PHDC records (male, female), ‘pregnant’ indicated pregnancy status at COVID-19 diagnosis. ‘Hospital_admission’ referred to hospital admission contemporaneous with COVID-19, ‘admitted_to_ICU’ represents admission to an intensive care unit (ICU) due to COVID-19 and ‘ventilated’ means a ventilator was required, as part of COVID-19 patient care. ‘New_diabetes’ is diabetes diagnosed subsequent to the COVID-19 diagnosis, ascertained from the date of first evidence of diabetes using PHDC records.
Comorbidity data were provided for six comorbidities: Human Immunodeficiency Virus (HIV), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD) or asthma, hypertension, diabetes mellitus (DM) and tuberculosis (TB). TB was further stratified into ‘TB-current’, a TB episode ongoing at time of COVID-19 diagnosis; and ‘TB-previously’, a TB episode occurring prior to COVID-19 diagnosis. Comorbidity episodes are inferred from a combination of facility visits, laboratory results and medications, are not clinician-validated for individual patients, and may have some margin of error. Data were not available for cardiovascular disease (CVD) episodes in this group. In brief: diabetes status was inferred in people who ever had an HBA1c of ≥ 6·5%, a 2-hr glucose of ≥ 11·1mmol/l after an oral glucose tolerance test, had been dispensed oral hypoglycaemic agents used exclusively for the management of diabetes or insulin, or had been assigned a diabetes ICD-10 diagnostic code [12]. For CKD, TB and HIV, laboratory results, specialist facility visits, and dispensed medications were used to define the nature of the condition. For hypertension and COPD or asthma, only dispensed medication was used to define the condition, without identifying patients with specific diagnoses who were untreated. Currently, CVD algorithms are under development and were not available for this study.
Pharmacy data from PHDC described the dispensing date for medicines. The association of medication on hospitalisation and mortality in PLWD diagnosed with COVID-19 was analysed from a subset of patients collecting medication from healthcare facilities linked to the electronic pharmacy records. Patient counts estimated the proportions of patients on various drugs in the year preceding COVID-19 diagnosis and post diagnosis, recognising that not all issued pharmacy drugs are always captured in the PHDC records. The medications selected and grouped were as follows: oral diabetes drugs (metformin, glimepiride), insulin (actrapid, Protaphane®, Actraphane®, Humulin N®, Humlin R®, Humulin 30/70®, insulin lispro, insulin aspart, insulin glargine, insulin detemir), statin (simvastatin, atorvastatin), angiotensin converting enzyme (ACE) inhibitor (enalapril), angiotensin receptor blocker (ARB) (losartan), steroids (dexamethasone, prednisone), hydrochlorothiazide (HCTZ) and anti-retroviral therapy (ART). These drugs were selected specific classes reflecting the formulary for the Western Cape Department of Health.
Laboratory data for all patients admitted to hospital for COVID-19 were analysed. The first available blood results in the period 2 days before and up to 5 days after the hospital admission for COVID-19, were considered as “admission investigations”.
2.3. Outcomes
We assessed the cumulative incidence of hospital admissions and deaths in PLWD diagnosed with COVID-19. For COVID-19 cases, the PHDC collates deaths data from hospital records, forensic pathology services, the National Institute for Communicable disease (NICD) notifications and death certification records.
Statistical analysis Summary statistics were generated for the whole population and stratified by different sub-groups in the population. For continuous data, median and interquartile range were calculated and for grouped data, percentages were calculated. Multivariate logistic regression was used to estimate the effect on two outcomes, hospital admission and mortality and included all available co-variates.
2.4. Research and ethics
The study was approved by the University of Cape Town Faculty of Health Sciences Ethics Review Board (HREC Ref: 286/2020). As this study comprised anonymized and perturbed data, a waiver was granted for informed consent.
2.5. Data sharing statement
The Western Cape Government Health approved the use of these data for research. Approval has not been provided for secondary use or onward sharing, the authors can be contacted for advice on applying for the use of these data.
2.6. Role of the funding source
There was no funding source available for this study.
3. Results
3.1. Patient characteristics
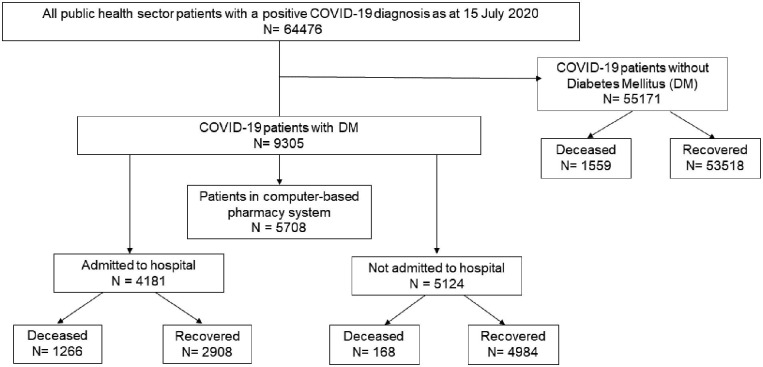
Selection of the study population is described in Fig. 1 . Of approximately 4·0 million active patients aged ≥ 20 years in the PHDC database, 64 476 were diagnosed with COVID-19 by 15 July 2020, of whom 2993 (4·6%) died (Table 1 ).
Fig. 1.
Flow chart showing the selection of the study population from the PHDC routine health data.
Table 1.
Characteristics of the Western Cape public health sector patients with COVID-19. The results have been grouped by Diabetes status (No Diabetes or Diabetes).
|
All |
No Diabetes |
Diabetes |
|
|---|---|---|---|
| N = 64476 | N = 55171 | N = 9305 | |
| Sex: | |||
| Female | 39,752 (61.7%) | 34,107 (61.8%) | 5645 (60.7%) |
| Male | 24,669 (38.3%) | 21,012 (38.1%) | 3657 (39.3%) |
| Age (years) | 40.0 [30.0;52.0] | 37.0 [29.0;49.0] | 55.0 [46.0;63.0] |
| Age distribution: | |||
| 0–18 | 2654 (4.1%) | 2635 (4.8%) | 19 (0.2%) |
| 18–39 | 29,379 (45.6%) | 28,135 (51.0%) | 1244 (13.4%) |
| 40–49 | 13,098 (20.3%) | 11,246 (20.4%) | 1852 (19.9%) |
| 50–59 | 10,613 (16.5%) | 7727 (14.0%) | 2886 (31.0%) |
| 60–69 | 5323 (8.3%) | 3260 (5.9%) | 2063 (22.2%) |
| 70–79 | 2340 (3.6%) | 1390 (2.5%) | 950 (10.2%) |
| >=80 | 1069 (1.7%) | 778 (1.4%) | 291 (3.1%) |
| Outcome: | |||
| Active | 11 (<0.1%) | 10 (<0.1%) | 1 (<0.1%) |
| Died | 2993 (4.6%) | 1559 (2.8%) | 1434 (15.4%) |
| Recovered | 61,374 (95.3%) | 53,518 (97.2%) | 7856 (84.6%) |
| HIV | 7933 (12.3%) | 7022 (12.7%) | 911 (9.8%) |
| TB current | 791 (1.2%) | 679 (1.2%) | 112 (1.2%) |
| TB previously | 3945 (6.1%) | 3302 (6.0%) | 643 (6.9%) |
| COPD or Asthma | 4202 (6.5%) | 2981 (5.4%) | 1221 (13.1%) |
| Hypertension | 12,623 (19.6%) | 7462 (13.5%) | 5161 (55.5%) |
| CKD | 1448 (2.2%) | 596 (1.1%) | 852 (9.2%) |
| Pregnant | 958 (1.5%) | 873 (1.6%) | 85 (0.9%) |
| Hospital admission | 10,887 (16.9%) | 6706 (12.2%) | 4181 (44.9%) |
| ICU admission | 917 (1.4%) | 544 (1.0%) | 373 (4.0%) |
| Ventilated | 130 (0.2%) | 78 (0.1%) | 52 (0.6%) |
| New diabetes | 1053 (11.3%) | 0 (0.0%) | 1053 (11.3%) |
HIV, Human immunodeficiency virus; TB, Tuberculosis; COPD, Chronic obstructive pulmonary disease; CKD, chronic kidney disease; ICU, intensive care unit.
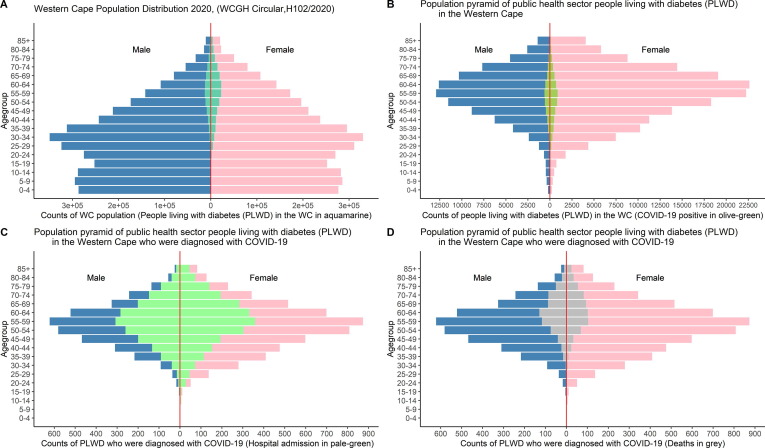
The COVID-19 patient population: Population pyramids illustrate the youthful distribution of the Western Cape population, with 80% of the population under 50 years of age (Fig. 2 A). For COVID-19 patients, a greater proportion (61·7%, n = 39 752) were women. Most patients (45·6%, n = 29 379) were 18 to 39 years old, with 70% of patients ≤ 50 years old. In this group, hypertension (19·6%, n = 12 623), diabetes (14·4%, n = 9305) and HIV infection (12·3%, n = 7933) were most prevalent co-morbidities. There were 16·9% (n = 10 887) hospitalised, 1·4% (n = 917) required intensive care, and 0·2% (n = 130) needed ventilation (Table 1).
Fig. 2.
Population pyramids showing the distribution of people living with diabetes (PLWD) in the Western Cape (WC) population (A), PLWD with COVID-19 (B), PLWD with COVID-19 who got admitted into hospital for COVID-19 (C) and PLWD who died from COVID-19 (D).
The diabetes/COVID-19 patient population: In 9305 PLWD with COVID-19, 11·3% (n = 1053) were newly diagnosed with DM during their COVID-19 episode. The 45–69-year-old age group had the most COVID-19 cases, with 66·5% of patients ≥ 50 years old and most patients in the 50–59 years category (31·0%; n = 2886). More women (60·7%, n = 5645) were diagnosed across all age groups (Fig. 2B). Hospital admissions appeared similar across genders, with the largest proportion of admissions for the 45–69-year age group (Fig. 2C). The distribution of COVID-19 deaths was similar, but men aged 55–69 years had the highest mortality (Fig. 2D). Compared to those without diabetes, a larger proportion of PLWD with COVID-19 were hospitalised (44·9%, c.f. 12·2%), admitted to ICU (4·0% c.f. 1·0%), and required ventilation (0·6% c.f. 0·1%) (Table 1).
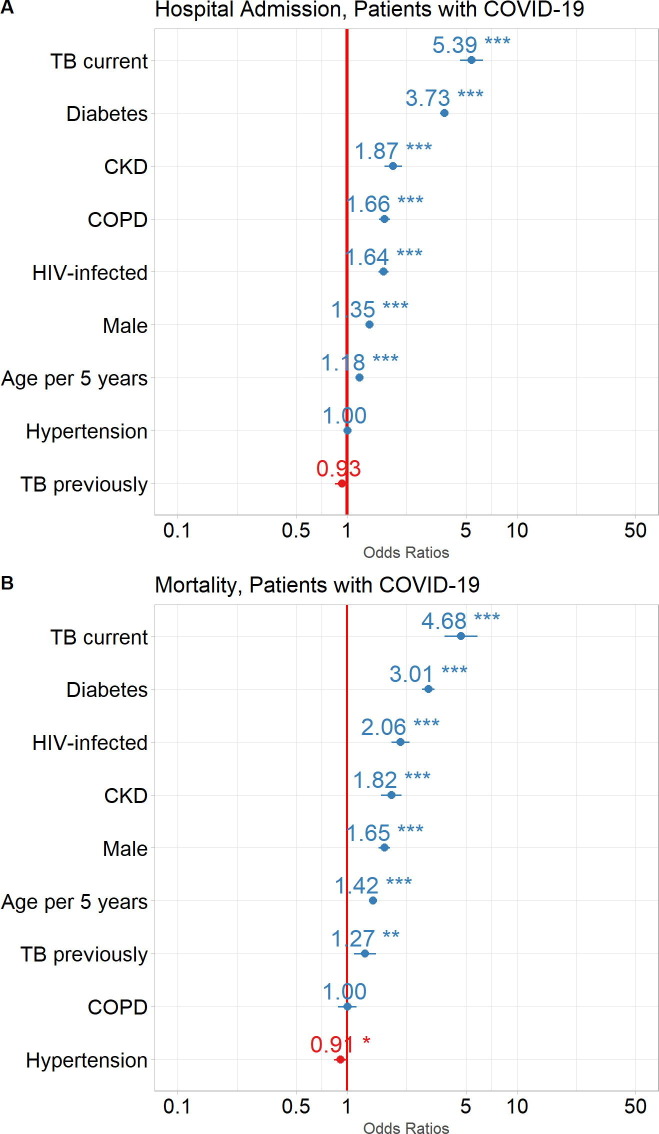
Risk factors for admission and mortality in patients with COVID-19: Logistic regression assessed the association of patient comorbidities and demographics with hospital admissions and mortality. For the total population of COVID-19 patients, current TB (OR:5·39, 95% CI: 4·61, 6·29), DM (OR:3·73, 95% CI: 3·53, 3·94), CKD (OR:1·87, 95% CI: 1·65, 2·10), COPD (OR:1·66, 95% CI: 1·54, 1·79), HIV infection (OR:1·64, 95% CI: 1·53, 1·75), male sex (OR:1·35, 95% CI: 1·29, 1·41), age per 5-year intervals (OR:1·18, 95% CI: 1·17, 1·19) were all associated with an increased risk for admission to hospital. Treated hypertension and previous TB were not associated with an increased risk for admission (Fig. 3 A).
Fig. 3.
Impact of comorbidities and demographics on COVID-19 patient outcomes. Odds Ratios (circles) with 95% Confidence Intervals (horizontal lines) are shown for COVID-19 patient outcomes: A. Admission to hospital, and B. Mortality (death). ***p<0.0001, **p<0.001, *p<0.01. TB, tuberculosis; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus.
Current TB (OR:4·68, 95% CI: 3·74, 5·82), DM (OR:3·01, 95% CI: 2·76, 3·28), HIV infection (OR:2·06, 95% CI: 1·82, 2·32), CKD (OR:1·82, 95% CI: 1·58, 2·09), male sex (OR:1·65, 95% CI: 1·52, 1·79), age per 5-year intervals (OR:1·42, 95% CI: 1·40, 1·44) and previous TB (OR:1·27, 95% CI: 1·10, 1·47) were associated with an increased risk for mortality. Treated hypertension (OR:0·91, 95% CI: 0·83, 0·99) was however, associated with a reduced mortality and COPD appeared to have a neutral effect on mortality (Fig. 3B).
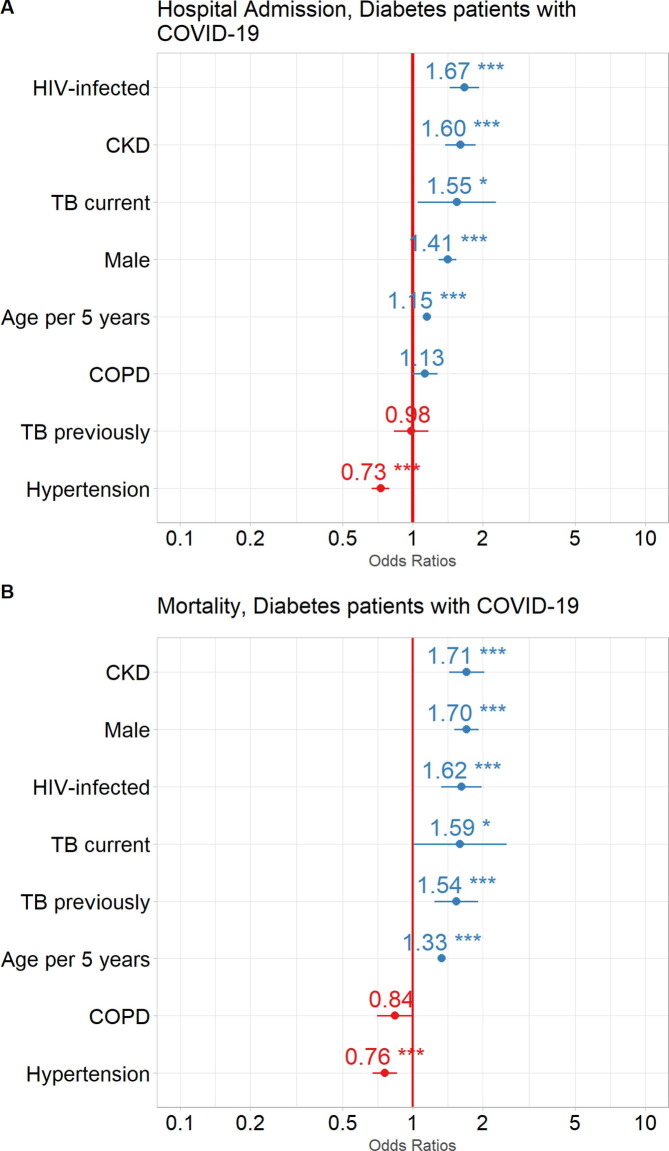
Risk factors for hospitalisation and mortality in PLWD diagnosed with COVID-19: For PLWD who had COVID-19, HIV infection (OR:1·67, 95% CI: 1·44, 1·93), CKD (OR:1·60, 95% CI: 1·39, 1·87), current TB (OR:1·55, 95% CI: 1·05, 2·29), male sex (OR:1·41, 95% CI: 1·29, 1·54) and age per 5-year intervals (OR:1·15, 95% CI: 1·13, 1·17), were associated with an increased risk for hospitalisation. Treated hypertension (OR:0·73, 95% CI: 0·67, 0·79) was associated with a reduced risk for hospitalisation. Previous TB was not associated with hospitalisation (Fig. 4 A).
Fig. 4.
Impact of comorbidities and demographics on outcomes in COVID-19 patients with DM. Odds Ratios (circles) with 95% Confidence Intervals (horizontal lines) are shown for COVID-19 patient outcomes: A. Admission to hospital, and B. Mortality (death). ***p<0.0001, **p<0.001, *p<0.01. HIV, human immunodeficiency virus; CKD, chronic kidney disease; TB, tuberculosis; COPD, chronic obstructive pulmonary disease.
CKD (OR:1·71, 95% CI: 1·44, 2·02), male sex (OR:1·70, 95% CI: 1·51, 1·92), HIV infection (OR:1·62, 95% CI: 1·32, 1·98), current TB (OR:1·59, 95% CI: 0·99, 2·50), previous TB (OR:1·54, 95% CI: 1·23, 1·90) and age per 5-year intervals (OR:1·33, 95% CI: 1·30, 1·37) were associated with an increased risk of mortality. Treated hypertension (OR:0·76, 95% CI: 0·67, 0·86) was also associated with a reduced risk for mortality and COPD was not associated with mortality (Fig. 4B).
The effect of medication on hospitalisation and mortality in PLWD: Dispensing records for the preceding six months were available for 61·4% (n = 5708) of PLWD who were diagnosed with COVID-19 and who accessed their healthcare from a facility with a computer-based pharmacy system. Of these, 928 (16·3%) died and 4780 (83·7%) recovered (Supplementary Table 1).
When comparing PLWD who were diagnosed with COVID-19 and who accessed their healthcare from a facility with a computer-based pharmacy system to those PLWD who accessed their healthcare from a facility with no computer-based pharmacy system it was noted that they were older [57·0 (48·0; 65·0) vs 52·0 (42·0; 61·0)] years old; 72·3% >50 years old vs 57·2% >50 years old] and were more likely to have co-morbidities such as HIV infection, current TB, asthma/COPD, hypertension and CKD but had a similar outcome (Supplementary Table 2). Furthermore, in this cohort of patients accessing their healthcare from a facility with a computer-based pharmacy system there were similar risk factors associated with hospitalisation and mortality when compared to the whole group (Fig. 5 A and 5B)
Fig. 5.
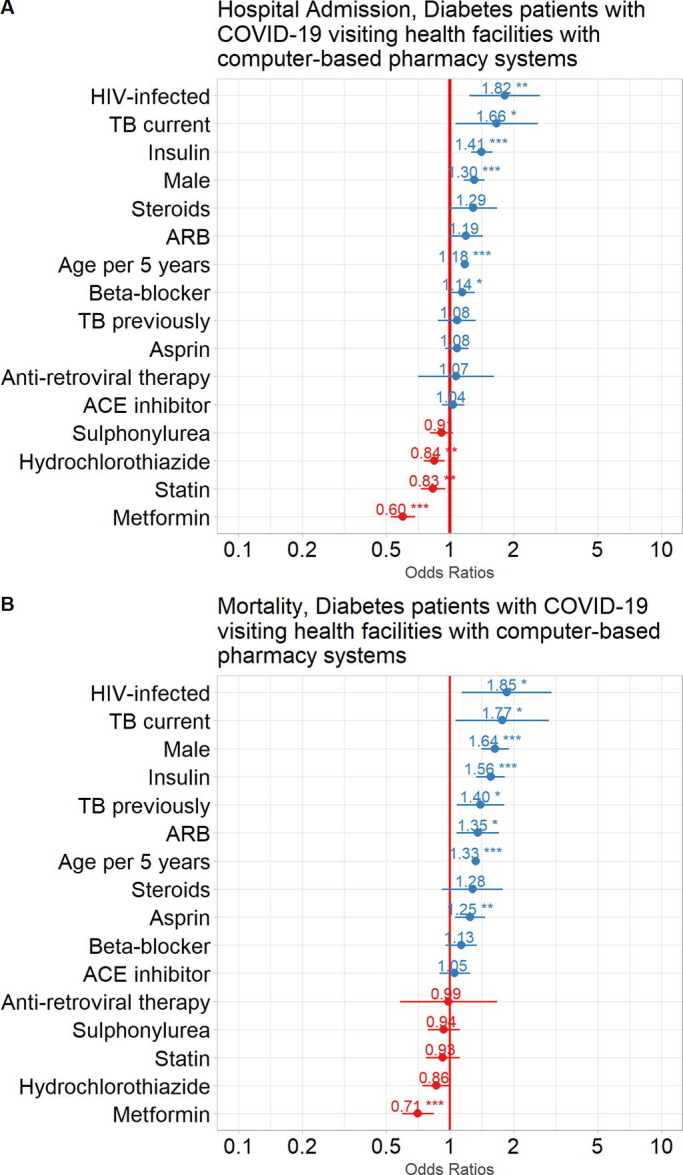
Impact of common comorbidities, medications dispensed in the 6 months prior to COVID-19 diagnosis and demographic factors to the outcomes of COVID-19 patients with DM. Odds Ratios (circles) with 95% Confidence Intervals (horizontal lines) are shown for COVID-19 patient outcomes: A. Admission to hospital, and B. Mortality (death). ***p<0.0001, **p<0.001, *p<0.01. HIV, human immunodeficiency virus; TB, tuberculosis; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ARB, angiotensin receptor blocker; ACE, angiotensin converting enzyme.
Use of insulin (OR:1·39, 95% CI: 1·24,1·57), was associated with an increased risk for hospitalisation whereas use of hydrochlorothiazide (OR:0·87, 95% CI: 0·77,0·97), a statin (OR:0·83, 95% CI: 0·72, 0·94) and metformin (OR:0·62, 95% CI: 0·55,0·71) were associated with a reduced risk for hospitalisation. Use of steroids, ARB, beta-blocker ART, aspirin, ACE-I, or a sulphonylurea were not associated with hospitalisation (Fig. 5A).
Use of insulin (OR1·49, 95% CI: 1·27; 1·74), ARB (OR 1·34, 95% CI: 1·06; 1·70) and aspirin (OR1·24, 95% CI: 1·05; 1·46) were associated with an increased mortality whereas use of metformin (OR 0·77, 95% CI: 0·64; 0·92) was associated with a reduction in mortality. The use of steroids, beta-blocker, ACE-I, ART, sulphonylurea, statin and hydrochlorothiazide were not associated with mortality (Fig. 5B).
Risk factors for mortality in PLWD hospitalised with COVID-19 Detailed laboratory investigations were available for 3664 PLWD who were hospitalised, shown in detail in Supplementary Table 3.
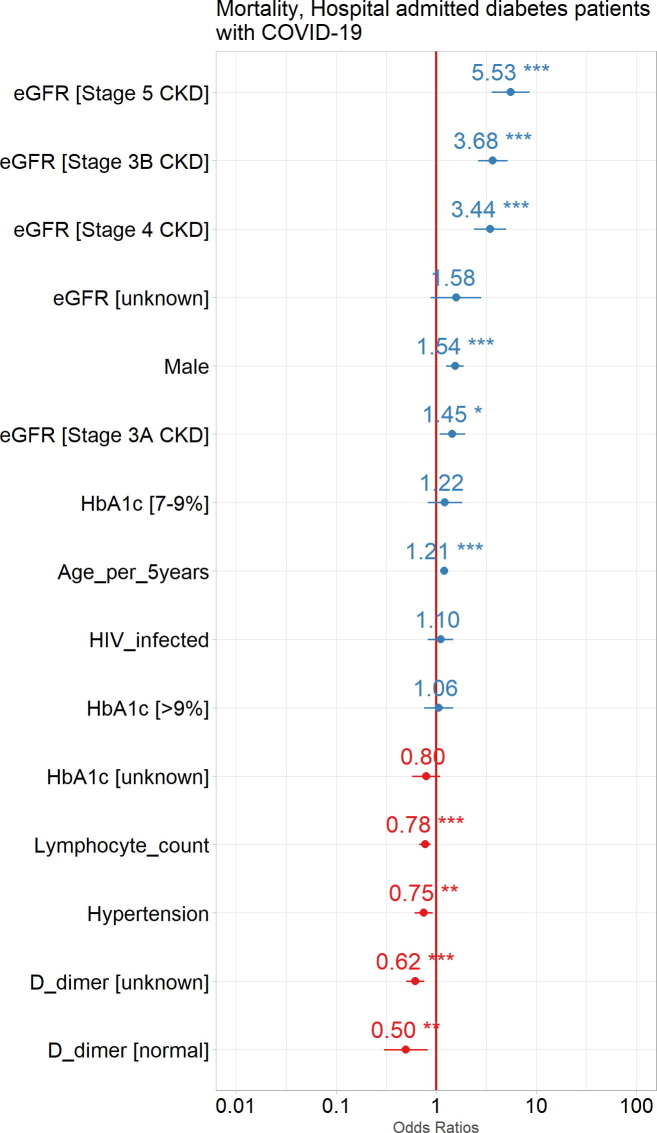
All stages of CKD were associated with an increased mortality [stage 5 (OR:5·53, 95% CI: 3·60, 8·60), stage 4 (OR:3·44, 95% CI: 2·39, 4·98), stage 3B (OR:3·68, 95% CI: 2·63, 5·16), stage 3A (OR:1·45, 95% CI: 1·08, 1·93)]. Other factors associated with an increased mortality were male sex (OR:1·54, 95% CI: 1·26, 1·89) and age per 5-year intervals (OR:1·21, 95% CI: 1·15, 1·26) (Fig. 6 ).
Fig. 6.
Impact of common comorbidities and laboratory results at admission, as well as demographic factors to mortality in hospital admitted diabetes patients with COVID-19. Odds Ratios (circles) with 95% Confidence Intervals (horizontal lines) are shown for COVID-19 patient outcomes. ***p<0.0001, **p<0.001, *p<0.01. eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus.
Treated hypertension (OR:0·77, 95% CI: 0·61, 0·92), a normal lymphocyte count (OR:0·76, 95% CI: 0·68, 0·88) and an unknown (OR:0·62, 95% CI: 0·50, 0·76) or normal (OR:0·50, 95% CI: 0·30, 0·80) d-dimer were associated with a reduced risk for mortality. No level of HbA1c was associated with mortality (Fig. 6).
4. Discussion
To our knowledge, this is the largest study of patients with COVID-19 from Africa and the Southern Hemisphere, which describes the association of diabetes with severe COVID-19 (hospitalisation and mortality), adjusting for key confounding factors. The key findings were that increasing age, male sex, diabetes, current tuberculosis, HIV infection and chronic kidney disease were significantly associated with admission to hospital and mortality. In PLWD, HIV-infection, chronic kidney disease, current tuberculosis, male sex and increasing age were significantly associated with admission to hospital and mortality. Use of metformin was associated with a reduced risk of hospitalisation and mortality in PLWD.
Our results show an increased risk of hospital admission, ICU admission and death in people diagnosed with COVID-19 who have diabetes, with half of all COVID-19 deaths occurring in people with diabetes. After adjusting for various variables such as age, sex and co-morbid disease, including HIV status, PLWD had almost four times the risk for hospitalisation and three times the risk of death relative to people without diabetes. This larger study also confirms earlier findings from an analysis performed before 1 June 2020, of COVID-19 death in the general population, being associated with age, male sex, chronic kidney disease, and in people with active tuberculosis and HIV [9].
This high risk for hospitalisation and death for PLWD is well recognised in other series. Metanalyses of studies including small numbers of patients with COVID-19 have reported a mortality risk of between 1·9-3·5 for patients with diabetes [13] and the largest cohort study of PLWD in primary care reported a risk for mortality of 2·03 for patients with T2DM [4].
In this cohort mortality was high with 15·4% of PLWD and COVID-19 having died, 3·3% of those not hospitalised and 30·3% of those admitted to hospital. Early data from Wuhan reported a mortality rate of 20% for hospitalised patients with COVID-19 and diabetes [14]. This mortality rate for hospitalised patients is similar to a retrospective cohort analysis of 1126 patients with diabetes hospitalised with COVID-19 at a large academic medical centre in New York City, where the mortality rate was 33·1%, but in patients with a mean age almost 10 years older than in this cohort [15]. Updated results from the French CORONADO Study of 2796 PLWD (mean age 69·7) who were hospitalised reported a mortality rate of 11·2% within 7 days, which continued to increase to 20·6% by 28 days [16]. We can only speculate about factors that account for such a high mortality in our setting, even in relatively young patients, which includes social determinants such as poor access to care, general poor standards of chronic care for diabetes such as glycaemic control and management of complications, higher threshold for admission and reduced access to ICU beds once admitted [17]. The high background of infectious diseases in the population may also contribute to mortality.
Amongst PLWD we corroborated well-described associations for death including age, male sex, and CKD [3], [13]. In this population, 9·8% were infected with HIV and 1·2% had active tuberculosis, with concurrent HIV and tuberculosis exhibiting more severe disease, as evidenced by higher hospitalisation and death rates. PLWD with treated hypertension had a reduced risk of mortality in our dataset, acknowledging that the hypertension episode is inferred from prescribed medications, and the impact of untreated hypertension on outcomes could not be assessed. Although hypertension has frequently been found to be associated with poor outcomes of COVID-19 in other studies, we could not find an association and even described a small but significant reduction in risk of poor outcomes in our cohort. McGurnaghan et al (2021), also failed to demonstrate an association between HPT or HPT treatment and worse outcomes in PLWD in Scotland [5]. In our dataset, a hypertension episode is defined only on the basis of patients receiving hydrochlorothiazide dispensed as an antihypertensive and blood pressure observational data are not available for analysis, meaning that undiagnosed/untreated hypertension is not documented in the current dataset. Given that Berry et al (2017), described that 48·7% of South Africans with hypertension are undiagnosed [18], it is plausible that patients who are receiving medication for hypertension have better outcomes than the many patients in the cohort who are likely to have undiagnosed and/or untreated hypertension, and the population of patients that are diagnosed with hypertension and dispensed medication may be different from those in other series.
In the subset of PLWD for whom full prescription data were available for the 6 months preceding the diagnosis of COVID-19, use of ARB, insulin and aspirin had a moderate independent association with mortality, yet metformin was protective.
Episode data for cardiovascular disease, as a risk factor were not available for this cohort, but dispensing records for aspirin, statin, betablocker, ACE and ARB were all associated with worse outcomes in the cohort. We postulate that these medications may be a surrogate marker for cardiovascular disease, potentially explaining the higher risk of poor outcomes in patients receiving these medications. An early report from Wuhan, China, also suggested that metformin was associated with a decreased mortality in hospitalized COVID-19 patients [19]. Moreover, metformin was found to be associated with reduced risk of early death in the French CORONADO study [20] and with decreased mortality in women with COVID-19, based on a United Health data analysis [21]. Three metanalyses have [22], [23], [24] corroborated these findings. It is possible that metformin use, may reflect PLWD with less advanced disease and fewer complications, such as chronic kidney disease in which it is contraindicated or possibly, patients who are more adherent to prescribed therapy. A recent paper using propensity scoring to adjust for such confounders failed to show an association of metformin use on susceptibility or outcome [25]. Although we show, as have others, that use of insulin is associated with worse outcomes, this most likely simply reflects a group of PLWD of longer disease duration and thus more vascular and other complications.
We did not demonstrate an association with HbA1c results and poorer outcomes in PLWD. Similarly, the Italian CORONADO Study and the population-based study from Scotland also failed to demonstrate an association between HbA1c and mortality [5], [26]. However, there are studies that have shown an association between poor outcome and worse glycaemic control. In some of these population studies PLWD were compared to a ‘non-diabetic’’ population [9], but in other studies where increments in HbA1c levels were compared exclusively in PLWD an association with poorer outcomes was shown [3]. Our data should, however, be considered with caution, as only a small proportion (31·5%) of PLWD had an HbA1c analysed in the preceding year. The reasons for this could be due to poor linkage to care, and also may reflect missing data due to patients opting for either public or private health care depending on their changing financial and employment circumstances. Private healthcare data in our system are not available, limiting analysis of this subset.
Strengths of the study include the large study size, with near complete ascertainment of outcome data, laboratory confirmed SARS-CoV-2 diagnosis in all COVID-19 cases, the inclusion of hospitalised and non-hospitalised cases and deaths, as well as modelling the independent association of diabetes with death. Patients newly diagnosed with diabetes during testing or admission, based on a diagnostic HbA1c were also included. Limitations include that the classification cascade used to identify somebody as having diabetes using the routine health data in the PHDC is still in the process of formal validation, but, if anything, may provide an underestimate of actual diabetes prevalence. Also, given the predominantly administrative nature of data capture, important comorbidities, lack of data on other potential risk factors including socio-economic status, lack of observational clinical data such as blood pressure, smoking and body mass index (BMI) limit the included comorbidities. In addition, data relating to some biochemical variables may be incomplete due to potential public/private health sector patient mobility, for example HbA1c data. Admission criteria for ICU and data capturing in the various facilities in the Western Cape is variable and possibly incomplete. The missing data for some comorbidities for example, cardiovascular disease, along with the absence of other potential confounders may result in large residual confounding in the associations described.
In conclusion, there is convincing evidence from many large population-based studies that PLWD are at greater risk of severe COVID-19 disease (hospital and ICU admissions) and of death than those without diabetes. This study adds to the body of evidence from a low and middle-income country, where the burden of DM affects younger people, compared to high income countries where older people are predominantly affected. We show that the population with diabetes is at particularly high risk, possibly due to poorer access to optimal care for diabetes. We also show that concurrent HIV infection and DM are associated with more severe disease and that metformin use, in particular, is associated with a reduced mortality. These findings are of major public health importance, which raise the question of how to ameliorate the high risk burden of PLWD in COVID-19. It is incumbent upon us as healthcare providers to offer education and close monitoring of risk in PLWD. It is too premature to recommend widespread use of metformin. Additional interventions may include home oxygen saturation monitoring, ensuring adequate glycaemic control, early identification of deterioration in symptoms with rapid access to hospital admission and consideration for pre-emptive admissions to hospital for those PLWD who are deemed to be at very high risk of severe COVID-19.
Funding
This research was funded in part, by the Wellcome Trust 203135/Z/16/Z, through support of NT. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. The Wellcome Centre for Infectious Diseases Research in Africa is supported by core funding from the Wellcome Trust [203135/Z/16/Z]. NT receives funding from the CIDRI-Africa Wellcome Trust grant (203135/Z/16/Z), and NT and TT receive funding from the NIH H3ABioNET award (U24HG006941). NT receives funding from the UKRI/MRC (MC_PC_MR/T037733/1).
Data Sharing
These data were provided for analysis by the Western Cape Department of Health, Provincial Health Data Centre. Whilst the data are anonymised, they are highly granular health data linked to individual health care clients in the Province and no informed consent has been given for research use of these routine health data. For this reason the Western Cape Department of Health only grants primary use permission for the anonymised data. Applications for each new use case are reviewed by the Western Cape Department of Health, Contact: Dr M. Moodley, Director: HIA, Western Cape Department of Health, South Africa. Email: Melvin.moodley@westerncape.gov.za, dataset reference ID:DAVE_2020_07.
Research in context
Evidence before this study
Several large studies have reported on the association of increased severity of COVID-19 with diabetes, but most large studies are from higher income countries. No large study with a focus on diabetes from Africa has been reported. The population in low and middle income countries with diabetes differ from those in large published series especially in respect of age – a major risk factor for severity of COVID-19, but also in terms of baseline diabetes and risk factor control. Whether diabetes poses the same risk in these younger populations is unclear, but important for future mitigation strategy planning.
Added value of this study
In this large study of patients confirmed with COVID-19 and with a median age of 40 years, typical of patients with diabetes in low or middle income countries, we show that people living with diabetes have an increased risk for morbidity and mortality from COVID-19 and that pre-infection use of metformin may mitigate this risk.
Implications of all the available evidence
People living with diabetes in low- and middle-income countries, where median age is lower than high income countries, are at high risk of severe COVID-19 and death. This population might warrant special designed protection measures and should be prioritized for vaccination. The potential protective effect of metformin requires further study.
Declaration of Competing Interest
JAD has been a speaker and on advisory board for Novartis, Novo Nordisk, Sanofi, Lilly, Servier, MSD, Aspen, Abbott, AstraZeneca, MSD and Boehringer Ingelheim; ILR, WT, and PJR have been a speaker for Sanofi and Aspen. AC has been a speaker for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Medtronic, Merck, Novo Nordisk, Sanofi and on an advisory board for Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, Novo Nordisk, Sanofi.
All other authors declare no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2021.108925.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.worldOmeter. COVID-19 Coronavirus Pandemic. 2021. https://www.worldometers.info/coronavirus/ (accessed 06 April 2021).
- 2.International Diabetes Federation COVID-19: Perspectives from people with diabetes. Diabetes Res Clin Pract. 2020;163:108201. doi: 10.1016/j.diabres.2020.108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holman N., Knighton P., Kar P., et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barron E., Bakhai C., Kar P., et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGurnaghan S.J., Weir A., Bishop J., et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9(2):82–93. doi: 10.1016/S2213-8587(20)30405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of covid-19. N Engl J Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bello-Chavolla O.Y., Bahena-López J.P., Antonio-Villa N.E., et al. Predicting mortality due to SARS-CoV-2: A mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab. 2020;105:2752–2761. doi: 10.1210/clinem/dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulle A., Davies M.A., Hussey H., et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martins-Filho P.R., de Souza A., Araujo A., Pereira L.X., et al. Factors associated with mortality among hospitalized patients with COVID-19: A retrospective cohort study. Am J Trop Med Hyg. 2021;104(1):103–105. doi: 10.4269/ajtmh.20-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cariou B., Hadjadj S., Wargny M., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020:1–16. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulle A., Heekes A., Tiffin N., et al. Data centre profile: the provincial health data centre of the Western Cape Province, South Africa. Int J Popul Data Sci. 2019;4(2):1143. doi: 10.23889/ijpds.v4i2.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Q., Zhang X., Jiang F., et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: A two-center, retrospective study. Diabetes Care. 2020;43:1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal S., Schechter C., Southern W., Crandall J.P., Tomer Y. Preadmission diabetes-specific risk factors for mortality in hospitalized patients with diabetes and coronavirus disease 2019. Diabetes Care. 2020;43(10):2339–2344. doi: 10.2337/dc20-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wargny M., Potier L., Gourdy P., et al. Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia. 2021;64(4):778–794. doi: 10.1007/s00125-020-05351-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govender I., Ehrlich R., Van Vuuren U., et al. Clinical audit of diabetes management can improve the quality of care in a resource-limited primary care setting. Int J Qual Health Care. 2012;24(6):612–618. doi: 10.1093/intqhc/mzs063. [DOI] [PubMed] [Google Scholar]
- 18.Berry K.M., Parker W.A., McHiza Z.J., et al. Quantifying unmet need for hypertension care in South Africa through a care cascade: evidence from the SANHANES, 2011–2012. BMJ Glob Health. 2017;2(3) doi: 10.1136/bmjgh-2017-000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo P., Qiu L., Liu Y., et al. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg. 2020;103(1):69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lalau J.D., Al-Salameh A., Hadjadj S., et al. Metformin use is associated with a reduced risk of mortality in patients with diabetes hospitalised for COVID-19. Diabetes Metab. 2021;47(5):101216. doi: 10.1016/j.diabet.2020.101216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bramante C.T., Ingraham N.E., Murray T.A., et al. Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis. Lancet Healthy Longev. 2021;2(1):e34–e41. doi: 10.1016/S2666-7568(20)30033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukito A.A., Pranata R., Henrina J., Lim M.A., Lawrensia S., Suastika K. The effect of metformin consumption on mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(6):2177–2183. doi: 10.1016/j.dsx.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kow C.S., Hasan S.S. Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: A meta-analysis. J Med Virol. 2021;93:695–697. doi: 10.1002/jmv.26498. [DOI] [PubMed] [Google Scholar]
- 24.Hariyanto T.I., Kurniawan A. Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection. Obes Med. 2020;19 doi: 10.1016/j.obmed.2020.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J., Cooper J.M., Gokhale K., et al. Association of metformin with susceptibility to COVID-19 in people with Type 2 diabetes. J Clin Endocrinol Metab. 2021;106(5):1255–1268. doi: 10.1210/clinem/dgab067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cariou B., Pichelin M., Goronflot T., et al. Phenotypic characteristics and prognosis of newly diagnosed diabetes in hospitalized patients with COVID-19: results from the CORONADO study. Diabetes Res Clin Pract. 2021;108695 doi: 10.1016/j.diabres.2021.108695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.