Abstract
Objective
The aim of this in vitro study was to investigate the expression of SARS-CoV-2 entry and processing genes in human gingival fibroblasts (HGnF) following treatment with Porphyromonas gingivalis-derived lipopolysaccharide (PgLPS) or inflammatory cytokines/mediators.
Design
We assessed the expression of SARS-CoV-2 entry and processing genes; angiotensin-converting enzyme 2 (ACE2), cellular serine proteases transmembrane serine protease 2 (TMPRSS2), Furin, and basigin (BSG) in HGnF by real-time PCR. To further asses the contribution of PgLPS and inflammatory cytokines/mediators to proliferation and SARS-CoV-2 entry and processing gene expression, HGnF were treated with PgLPS, IL1β, TNFα, and PGE2.
Results
The expression for ACE2 in HGnF was significantly elevated after PgLPS or IL1β, TNFα, PGE2 treatment. The expression of TMPRSS2 was increased by PgLPS, IL1β, or PGE2 while BSG was elevated by PgLPS and IL1β. The expression of BSG and FURIN decreased after TNFα treatment.
Conclusion
SARS-CoV-2 entry and processing genes are expressed in human gingival fibroblasts and their expressions are altered by PgLPS, IL1β, TNFα and PGE2 treatment.
Keywords: COVID-19, Angiotensin-converting enzyme 2, Transmembrane serine protease 2, Basigin, Furin
1. Introduction
Since the first discovery of pneumonia of unknown cause in December 2019, later designated coronavirus disease 2019 (COVID-19) (Huang et al., 2020), World Health Organization reported more than 174 million confirmed cases of COVID-19 including more than 3.7 million death worldwide as of June 9, 2021 (https://covid19.who.int/). COVID-19 is caused by transmission of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, previously known as 2019-nCoV) (Zhu et al., 2020). Presence of SARS-CoV-2 is detected in different types of clinical specimens such as nasopharyngeal swabs, faces, blood (Wang, Xu et al., 2020), saliva (Fernandes et al., 2020; Huang et al., 2021; To et al., 2020) and gingival crevicular fluid (Gupta et al., 2021). In addition, COVID-19 can affect multiple systems such as lungs, airways, gastrointestinal tract, kidney, liver, heart (Chen et al., 2020; Huang et al., 2020; Wang, Hu et al., 2020), and oral regions (Corchuelo & Ulloa, 2020; Halboub et al., 2020; Halepas et al., 2020; La Rosa et al., 2021; Mortazavi et al., 2020). In addition, patients with COVID-19 exhibited wide range of symptoms, ranging from mild symptoms to severe illness. According to data from China, nearly 80 % of people with Covid-19 had mild or moderate symptoms while 20 % of the patients had severe disease with mortality rate of 6% (Huang et al., 2020; Xu, Li et al., 2020). Older age (65 years old and older) is identified as one of the highest risk factors for developing severe symptoms of COVID-19 (Wu et al., 2020). In addition, sex, patients with chronic lung disease, moderate to severe asthma, severe obesity, diabetes, chronic kidney disease, and liver disease are also at high risk for severe COVID-19 symptoms (Garibaldi et al., 2020; Wu et al., 2020; Xu, Li et al., 2020).
Recent research on SARS-CoV-2 has identified a list of key entry and processing genes used by the virus to infect host cells. SARS-CoV-2 enters host cells by binding spike (S) protein embedded in the viral lipid envelope to human host cell receptor, angiotensin-converting enzyme 2 (ACE2) (Hoffmann et al., 2020). For viral entry via ACE2, it is thought that the SARS-CoV-2 S protein is primed, and ACE2 is cleaved by the cellular serine proteases transmembrane serine protease 2 (TMPRSS2) (Hoffmann et al., 2020). Furin cleaves viral enveloping proteins, providing another putative priming step for the S protein of SARS-COV-2 (Coutard et al., 2020). In addition, basigin (BSG, also known as cluster of differentiation 147; CD147, or extracellular matrix metalloproteinase inducer; EMMPRIN) is reported as an alternative receptor by which SARS-CoV-2 may enter host cells (Ulrich & Pillat, 2020; Wang, Chen et al., 2020). For viral entry via BSG, less is known about specific receptor and viral processing partners for SARS-CoV-2 (Ahmetaj-Shala et al., 2020).
Periodontal diseases are highly prevalent inflammatory diseases affecting the periodontal tissue initiated by microbial pathogens in oral biofilm (Pihlstrom et al., 2005). Although periodontopathic bacteria (i.e. Porphyromonas gingivalis) are essential for the initiation and progression of the disease, periodontal tissue damage and formation of periodontal pockets are primarily mediated by inflammatory cytokines and mediators (e.g. interleukin 1β; IL1β, tumor necrosis factor α; TNFα, prostaglandin E2; PGE2) (Cekici et al., 2014). In addition, it is known that periodontal disease is highly prevalent among older adults (65 years old and older) (Eke et al., 2016) and affect systemic conditions such as cardiovascular disease, diabetes mellitus, and adverse pregnancy outcomes (Beck et al., 2019). Moreover, several studies have reported the existence of a bidirectional link between periodontal diseases and systemic disease (Kim & Amar, 2006). Since the prevalence of both COVID-19 and periodontal disease are high in older adults and the fact that periodontal health is linked to systemic disease, there might be a link between COVID-19 and periodontal disease. Indeed, Kara et al. (Kara et al., 2020) recently emphasized possible relationship between periodontal diseases severity and COVID-19 infections.
Progression of periodontal disease leads to ulcerated periodontal pocket epithelium with an exposed connective tissue (Takata & Donath, 1988). Gingival fibroblasts are the major constituents of the periodontal connective tissue. Although few studies reported expression profiles of ACE2 and TMPRSS2 in oral mucosa (Hamming et al., 2004; Huang et al., 2021; Sakaguchi et al., 2020; Xu, Zhong et al., 2020; Zhong et al., 2020), expression of SARS-CoV-2 entry and processing genes in gingival fibroblasts have not been studied in detail. In addition, effect of Porphyromonas gingivalis-derived lipopolysaccharide or inflammatory cytokines and inflammatory mediators on the expression of these genes is not well understood. Identifying and studying cell types that can be infected by SARS-CoV-2 via expression of SARS-CoV-2 entry and processing genes could inform our understanding of COVID-19 heterogeneity in disease outcomes. For this, the current study utilized human gingival fibroblasts to determine the expression level of SARS-CoV-2 entry and processing genes upon treatment with Porphyromonas gingivalis-derived lipopolysaccharide or inflammatory cytokines/mediators, in vitro.
2. Materials & methods
2.1. Cell culture
Primary human gingival fibroblasts (HGnF), isolated from human gingiva, were purchased from ScienCell Research Laboratories and were cultured in alpha-minimum essential medium (αMEM; Sigma-Aldrich) containing 10 % fetal bovine serum (FBS, Sigma-Aldrich), 100 U/mL penicillin and 100 U/mL streptomycin (FUJIFILM Wako Pure Chemical) as described previously (Noguchi et al., 2002). During culturing, cells were incubated in a humidified atmosphere of 5% CO2 and 95 % air at 37 °C. The cells between the third and six passages were used in this study.
For experiments, HGnF were seeded at a density of 1 × 104 cells/cm2 in multiwell culture plates. After confluence, cells were serum-starved in αMEM containing 0.5 % FBS to minimize the effect of serum components. After 24 h of starvation, the cells were treated either with lipopolysaccharide from the gram-negative bacteria Porphyromonas gingivalis (PgLPS, InvivoGen), recombinant human interleukin 1 beta protein (IL1β, Sigma-Aldrich), recombinant human tumor necrosis factor alpha protein (TNFα, R&D Systems), or prostaglandin E2 (PGE2, FUJIFILM Wako Pure Chemical), in the concentrations indicated. After further 24 h of incubation, cells were either processed for cell proliferation assay or collected and stored at −80 °C until further analysis.
2.2. Cell proliferation assay
Cells were seeded into 96-well plates. Cell Counting Kit-8 (CCK-8, Dojindo) was used to investigate cell proliferation after 24 h of treatment. In brief, after 24 h treatment, a ready-to-use WST-8 solution was added, and incubation continued for 2 h at 37 °C. The WST-8 formazan complex was quantitatively measured at a wavelength of 450 nm using a microplate reader according to the manufacturer's protocol.
2.3. Isolation of total RNA and cDNA synthesis
Total RNA was extracted from the cells with TRIzol reagent (Invitrogen), according to the manufacturer’s instructions. Reverse-transcription was performed to generate cDNA by the ReverTra Ace qPCR RT Master Mix with gDNA Remover kit (Toyobo). cDNAs were used for the PCR template as described below.
2.4. Quantification of mRNAs by real-time polymerase chain reaction (real-time PCR)
Quantitative gene-expression analyses were carried out using real-time PCR by means of the Thunderbird SYBR qPCR mix (Toyobo) and the Real-time PCR System 7300 (Applied Biosystems) as described previously (Furue et al., 2017). Genes studied in this investigation were angiotensin-converting enzyme 2 (ACE2), transmembrane serin protease 2 (TMPRSS2), basigin (BSG, also known as CD147 or EMMPRIN), and furin (FURIN).
All data were normalized to 18 s ribosomal RNA (18S) expression. Information on the primer sets is listed in Table 1 .
Table 1.
Primer sequences used in this study for real-time PCR.
| Gene symbol | Primer sequence | Product size (bp) |
|---|---|---|
| Accession No. | ||
| ACE2 | forward: 5′-GGGATCAGAGATCGGAAGAAGAAA-3′ | 124 |
| reverse: 5′-AGGAGGTCTGAACATCATCAGTG-3′ | NM_001371415 | |
| TMPRSS2 | forward: 5′-AATCGGTGTGTTCGCCTCTAC-3′ | 106 |
| reverse: 5′-CGTAGTTCTCGTTCCAGTCGT-3′ | NM_001135099 | |
| BSG | forward: 5′-GCAGCGGGCAGCACC-3′ | 61 |
| reverse: 5′-CCACCTGCCTCAGGAAGAGTT-3′ | AB085790 | |
| FURIN | forward: 5′-CCTGGTTGCTATGGGTGGTAG-3′ | 187 |
| reverse: 5′-AAGTGGTAATAGTCCCCGAAGA-3′ | NM_002569 | |
| 18S | forward: 5′-GTAACCCGTTGAACCCCATT-3′ | 151 |
| reverse: 5′-CCATCCAATCGGTAGTAGCG-3′ | M10098 |
ACE2, angiotensin-converting enzyme 2; TMPRSS2, cellular serine proteases transmembrane serine protease 2, BSG; basigin, 18S; 18 s ribosomal RNA.
2.5. Statistical analysis
For cell proliferation assay and real-time PCR, values are presented as mean ± SD of at least three replicates. All experiments were repeated twice and similar results were obtained. The results from one representative experiment are shown. The statistical significance of differences between groups was analyzed using one-way ANOVA and Bonferroni’s multiple comparisons test (GraphPad Prism 9, Version 9.1.0). Values of P < 0.05 were considered to be statistically significant.
3. Results
3.1. Effect of PgLPS, IL1β, TNFα, and PGE2 on HGnF cell proliferation
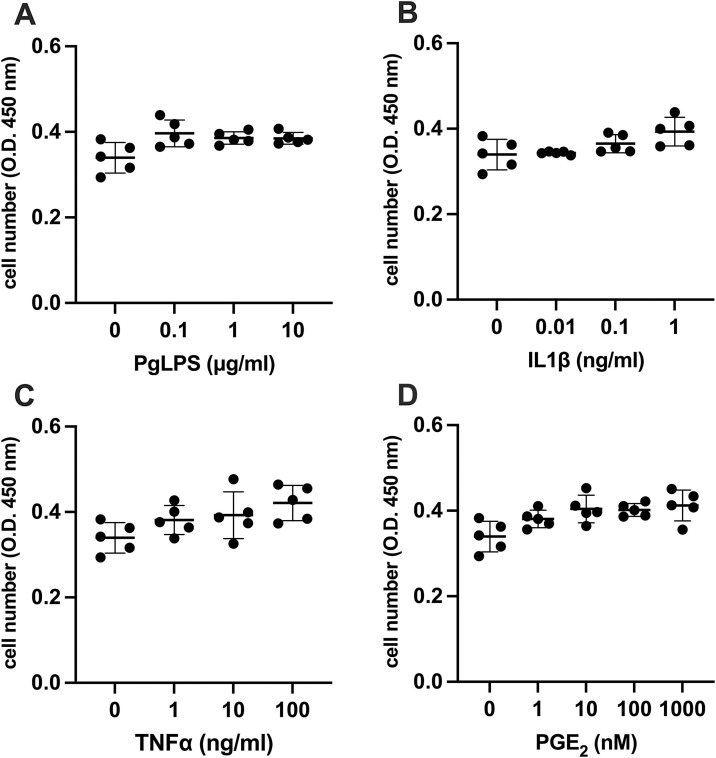
To investigate the effect of PgLPS, IL1β, TNFα and PGE2 on SARS-CoV-2 entry gene expression, cell proliferation assay was performed to investigate the cytotoxicity of PgLPS, IL1β, TNFα, and PGE2 on HGnF. Our results indicated that PgLPS, IL1β, TNFα, and PGE2 treated with different concentrations for 24 h did not inhibit the proliferation of the HGnF (Fig. 1 ). These data suggest that 0.1−10 μg/mL of PgLPS, 0.01−1 ng/mL of IL1β, 1−100 ng/mL of TNFα, or 1−1000 nM of PGE2 did not have significant cytotoxicity against the growth of HGnF.
Fig. 1.
Effect of PgLPS, IL1β, TNFα and PGE2 on HGnF cell proliferation. Cell numbers were analyzed after treatment with (A) PgLPS (0, 0.1, 1, 10 μg/mL), (B) IL1β (0, 0.01, 0.1, 1 ng/mL), (C) TNFα (0, 1, 10, 100 ng/mL) or (D) PGE2 (0, 1, 10, 100, 1000 nM) for 24 h. Data are presented as dot plots. Values are shown as mean ± SD. n = 5. O.D., optical density.
3.2. Effect of PgLPS, IL1β, TNFα, and PGE2 on expression of ACE2
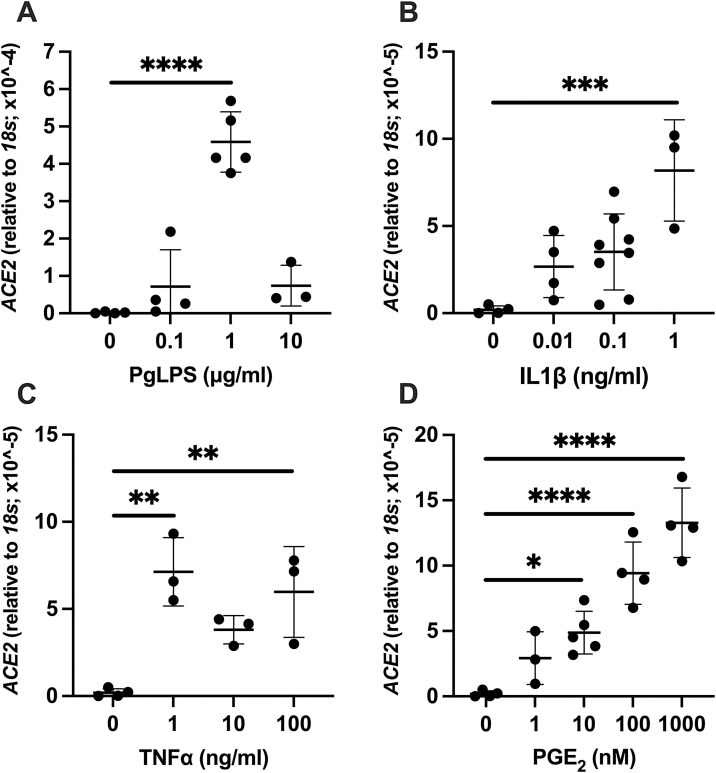
Elevated expression of ACE2 was observed by PgLPS, IL1β, TNFα, and PGE2 treatment. For PgLPS, significantly higher expression of ACE2 was observed at 1 μg/mL (P < 0.0001, Fig. 2 A). At 10 μg/mL of PgLPS, the levels of gene expression were similar to the control group. The level of ACE2 after PgLPS stimulation at 1 μg/mL was 244-fold higher compared to control. For IL1β, ACE2 gene expression level was significant higher at 1 ng/mL (P = 0.0006, Fig. 2B) which was 44-fold higher than control. With TNFα treatment, biphasic significant increase in ACE2 expression was observed at 1 ng/mL (P = 0.0017) and 100 ng/mL (P = 0.0059) compared to control (Fig. 2C). Increased expression of ACE2 was observed by 10−1000 nM PGE2 stimulation (Fig. 2D). Significantly higher level of ACE2 was observed at concentration as low as 10 nM of PGE2 (P = 0.0277) and dose dependent increase was noted at 100 nM (P < 0.0001) and 1000 nM (P < 0.0001) concentration.
Fig. 2.
Effect of PgLPS, IL1β, TNFα, and PGE2 on expression of ACE2. Expression level of ACE2 mRNA was analyzed after treatment with (A) PgLPS (0, 0.1, 1, 10 μg/mL), (B) IL1β (0, 0.01, 0.1, 1 ng/mL), (C) TNFα (0, 1, 10, 100 ng/mL) or (D) PGE2 (0, 1, 10, 100, 1000 nM) for 24 h. Data are presented as dot plots. Values are shown as mean ± SD. n = 3-8. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
3.3. Effect of PgLPS, IL1β, TNFα, and PGE2 on expression of TMPRSS2
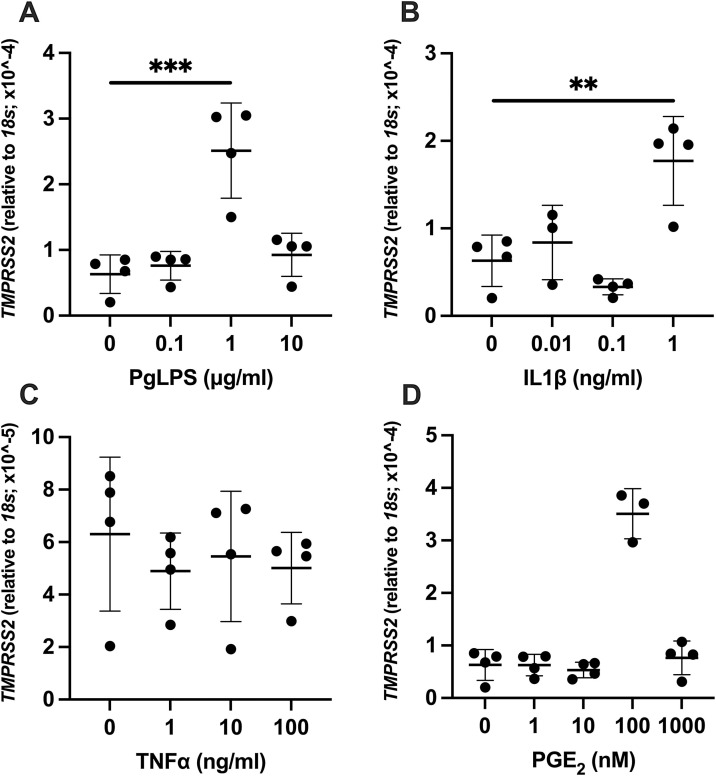
Higher mRNA level for TMPRSS2 was observed with PgLPS, IL1β, and PGE2 treatment in HGnF. PgLPS treatment resulted in significantly higher expression of TMPRSS2 at 1 μg/mL (P < 0.0003, Fig. 3 A). With IL1β at the dose of 1 ng/mL, significantly higher expression of TMPRSS2 was noted (P = 0.0054, Fig. 3B). TNFα did not affect expression level of TMPRSS2 at all doses (1−100 ng/mL) utilized in the current study (Fig. 3C). Treatment of HGnF with PGE2 concentration at 100 nM showed significantly higher level of TMPRSS2 expression compared to control (P < 0.0001, Fig. 3D).
Fig. 3.
Effect of PgLPS, IL1β, TNFα, and PGE2 on expression of TMPRSS2. Expression level of TMPRSS2 mRNA was analyzed after treatment with (A) PgLPS (0, 0.1, 1, 10 μg/mL), (B) IL1β (0, 0.01, 0.1, 1 ng/mL), (C) TNFα (0, 1, 10, 100 ng/mL) or (D) PGE2 (0, 1, 10, 100, 1000 nM) for 24 h. Data are presented as dot plots. Values are shown as mean ± SD. n = 3-4. **P < 0.01, ***P < 0.001, ****P < 0.0001.
3.4. Effect of PgLPS, IL1β, TNFα, and PGE2 on expression of BSG
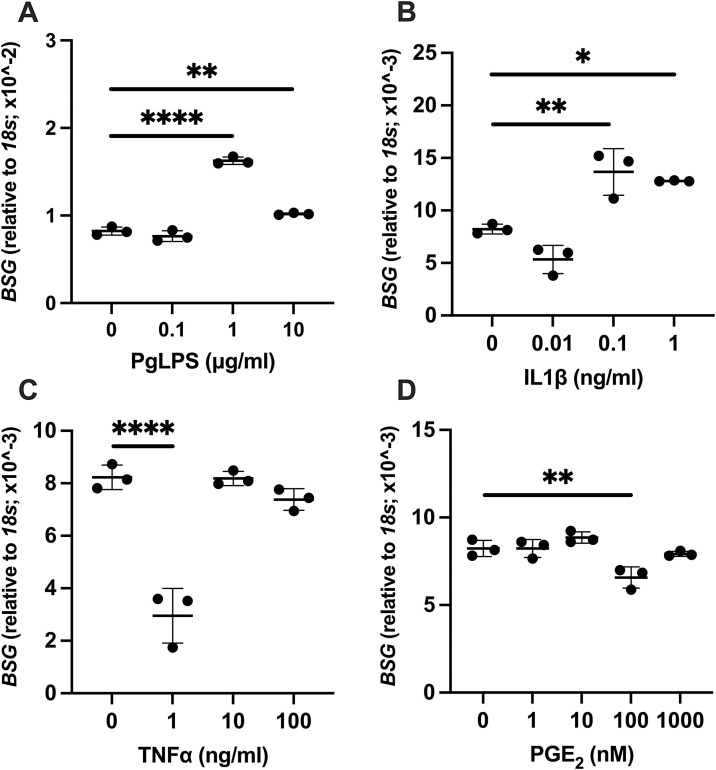
Significantly elevated expression of BSG by PgLPS and IL1β, while decreased expression by TNFα and PGE2 was observed with some concentrations. Compared to control, significantly elevated levels of BSG expression were noted at the dose of 1 μg/mL (P < 0.0001) and 10 μg/mL (P = 0.0036) of PgLPS (Fig. 4 A). Administration of IL1β increased the expression of BSG at 0.1 ng/mL (P = 0.0059) and 1 ng/mL (P = 0.0166) (Fig. 4B). For TNFα or PGE2 administration, significant decrease of BSG was observed by TNFα at 1 ng/mL (P < 0.0001, Fig. 4C) as well as PGE2 at 100 nM (P = 0.0097, Fig. 4D) while the expression remained at the level of control for the other concentration studied.
Fig. 4.
Effect of PgLPS, IL1β, TNFα, and PGE2 on expression of BSG. Expression level of BSG mRNA was analyzed after treatment with (A) PgLPS (0, 0.1, 1, 10 μg/mL), (B) IL1β (0, 0.01, 0.1, 1 ng/mL), (C) TNFα (0, 1, 10, 100 ng/mL) or (D) PGE2 (0, 1, 10, 100, 1000 nM) for 24 h. Data are presented as dot plots. Values are shown as mean ± SD. n = 3. *P < 0.05, **P < 0.01, ****P < 0.0001.
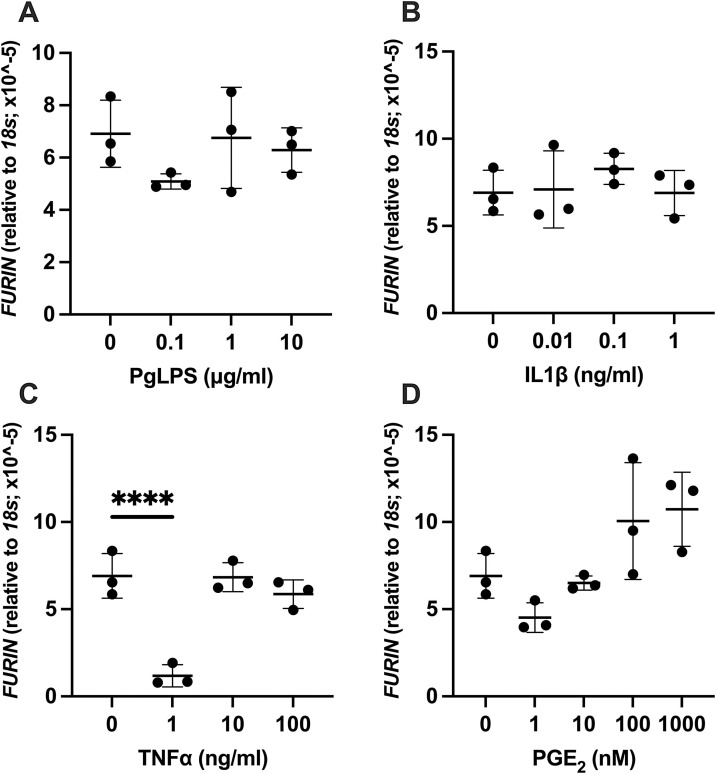
3.5. Effect of PgLPS, IL1β, TNFα, and PGE2 on expression of FURIN
Administration of PgLPS, IL1β or PGE2 did not alter the expression level of FURIN in HGnF (Fig. 5 A, B, and D). By TNFα administration, significant decrease of FURIN was observed by TNFα at 1 ng/mL (P < 0.0001, Fig. 5C) while the expression level remained same to the level of control for 10 ng/mL and 100 ng/mL group.
Fig. 5.
Effect of PgLPS, IL1β, TNFα, and PGE2 on expression of FURIN. Expression level of FURIN mRNA was analyzed after treatment with (A) PgLPS (0, 0.1, 1, 10 μg/mL), (B) IL1β (0, 0.01, 0.1, 1 ng/mL), (C) TNFα (0, 1, 10, 100 ng/mL) or (D) PGE2 (0, 1, 10, 100, 1000 nM) for 24 h. Data are presented as dot plots. Values are shown as mean ± SD. n = 3. ****P < 0.0001.
4. Discussion
The results of the present study revealed that SARS-CoV-2 entry and processing genes are expressed in gingival fibroblasts and these expressions were altered by PgLPS, IL1β, TNFα, and PGE2 treatment. HGnF were used in this study, since these cells are the major constituents of the periodontal connective tissue and not an established cell line that may harbor possible by-products of transformation process.
The increased expression level of ACE2 was observed after treatment with PgLPS or inflammatory cytokines/mediators; IL1β, TNFα, and PGE2, while elevated expression of TMPRSS2, and BSG was evident by PgLPS and not all but some of the inflammatory cytokines/mediators in HGnF. Decreased expression level of TMPRSS2, BSG and FURIN was noted after treatment with some of the cytokines.
Expression of ACE2 as well as BSG in HGnF has been reported previously (Santos et al., 2015). ACE2 is a cell surface receptor and peptidase that cleaves angiotensin II and other peptide hormones (Xu, Zhong et al., 2020). ACE2 is the known binding partner of the SARS coronavirus S protein and recent study reveal ACE2 to be the receptor for the entry of SARS-CoV-2 (Walls et al., 2020). Although, several studies have shown that ACE2 was highly expressed in respiratory epithelium, kidney, cardiovascular system and testis, it has also been reported that ACE2 was expressed in oral tissues based on RNA sequencing data based in public databases (Hoffmann et al., 2020). Xu et al. utilized single-cell sequencing data to further prove the expression of ACE2 receptor in tongue, buccal mucosa and gingiva (Xu, Zhong et al., 2020). More recently, mRNA expression of ACE2 was reported in epithelial cells of the glands and oral mucosa by in situ hybridization (Huang et al., 2021). Our findings on the expression of ACE2 gene using real-time PCR is in line with these reports. Furthermore, expression of ACE2 has been reported to be affected by aging, sex hormones, smoking, and diet (Li et al., 2020). In this report, we have identified significant increase in ACE2 expression by PgLPS and inflammatory cytokines/mediator (IL1β, TNFα, and PGE2) treatment which suggests periodontal disease may affect local expression of ACE2 in periodontal tissues. This finding follows report from a previous study which characterized the local renin-angiotensin system in human and rat periodontal tissues between healthy and periodontally affected tissue (Santos et al., 2015). ACE2 expression in HGnF was detected by PgLPS as well as E. coli-derived LPS while its expression was not detected in the control group (without LPS treatment) (Santos et al., 2015). In addition, modulation of ACE2 as well as other renin-angiotensin-system by cytokines such as IL1β has been reported in osteosarcoma cells (Ender et al., 2014). Due to the fact that ACE2 is the known binding partner of the SARS-CoV-2, the expression of ACE2 in HGnF may suggest gingiva/periodontal tissue as a potential infection routes of SARS-CoV-2 and contracting gum disease as a risk factor for SARS-CoV-2 infection or progression of COVID-19.
BSG is a highly glycosylated and plasma membrane-bound glycoprotein that belongs to the immunoglobulin superfamily (Gabison et al., 2005; Muramatsu, 2016). The expression of BSG is considered to be responsible for the induction of fibroblasts to produce or secrete matrix metallopeptidases (Foda et al., 2001) and play a critical role in development, tissue repair, rheumatoid arthritis, cardiovascular diseases, and inflammation (Gabison et al., 2005). An elevated expression of BSG has been found in the gingival tissue (Dong et al., 2009; Wang et al., 2014) and gingival crevicular fluid (Emingil et al., 2006) collected from chronic periodontitis patients and it has been indicated that BSG regulate the collagenolytic balance in favor of the expression and activation of matrix metallopeptidases in periodontal disease (Wang et al., 2014). Lai et al. reported expression of BSG in HGnF and its effect on the enhancement of matrix metallopeptidase expression stimulated by monocytes (Lai et al., 2020). Recently, BSG is implicated as a possible alternative receptor for the SARS-CoV-2 S protein (Wang, Chen et al., 2020). In our study, BSG expression was detected by real-time PCR and significant increase was noted by PgLPS and IL1β treatment while significant decrease was noted by TNFα at 1 ng/mL, and PGE2 at 100 nM. To the best of our knowledge, altered expression of BSG has not been previously reported in response to PgLPS, inflammatory cytokines, and PGE2 in HGnF. Our results indicate that in addition to its role in periodontal disease to destruct periodontal connective tissue, BSG expression in HGnF may act as a potential infection routes of SARS-CoV-2.
TMPRSS2 is a cell surface protease known to cleave both ACE2 and the S protein of coronaviruses (Heurich et al., 2014; Hoffmann et al., 2020). Its cleavage of ACE2 is considered to promote viral uptake (Heurich et al., 2014), while cleavage of S primes the viral particle for membrane fusion into the host cell (Hoffmann et al., 2020). FURIN is another protease known to cleave inactive precursor proteins into their biologically active product (Thomas, 2002). Notably, it functions in cleaving viral envelope proteins including those of HIV, influenza, dengue virus, ebolavirus, and some coronaviruses (Izaguirre, 2019). While a FURIN-specific cleavage site has not been found in the SARS coronavirus, a site has been discovered in the protein sequence of the SARS-CoV-2 spike protein (Coutard et al., 2020). So far, compared to ACE2 and BSG, expression of TMPRSS2 as well as FURIN has not been studied in HGnF. However, a search of the GEO Profiles database (Barrett et al., 2013; Edgar et al., 2002) revealed that not only ACE2 and BSG but also TMPRSS2 and FURIN are expressed in HGnF (Agis et al., 2014; Kuk et al., 2015). Our findings detecting the expression of TMPRSS2 and FURIN by real-time PCR are in agreement with these reports. In addition, high level of TMPRSS2 was noted by PgLPS, IL1β, and PGE2 treatment while only a significantly decrease by TNFα treatment was observed of FURIN expression. Interestingly, among the genes studied, expression of FURIN was either not affected or decreased by inflammatory cytokines and PGE2. These results suggest FURIN might be modulated in different manner compared to ACE2, TMPRSS2, and BSG. Further study is required to elucidate the regulation of FURIN in HGnF.
The pathophysiological mechanisms underlying COVID-19 disease severity and progression remain unclear. Several cohort studies have observed markedly elevated levels of circulating proinflammatory cytokines (cytokine storm), significantly correlating to disease severity and mortality. Due to the fact that periodontal disease is an inflammatory disease and elevated levels of inflammatory cytokines are detected in locally inflamed gingival tissue and in the systemic circulation, Sahni and Gupta (Sahni & Gupta, 2020) emphasized possible association between periodontitis and COVID-19 related adverse outcomes. Our current results may provide another piece of information to support association between periodontitis and COVID-19 by showing the expression of SARS-CoV-2 entry and processing genes in HGnF and its regulation by PgLPS, IL1β, TNFα, and PGE2. As they stated, understanding of this association underscores the importance of keeping periodontal disease under check and the value of maintaining meticulous oral hygiene in the COVID-19 era and also points towards the possibility of the presence of periodontal disease as predisposing towards COVID-19-related adverse outcomes. More recently Marouf et al. (Marouf et al., 2021) reported an association between periodontitis and severity of COVID-19 infection in a case-control study. In their report, periodontitis was associated with higher risk of ICU admission, need for assisted ventilation and death of COVID‐19 patients, and with significantly higher blood levels of white blood cells, D‐dimer and C-reactive protein linked to worse disease outcomes (Marouf et al., 2021). Infection of SARS-CoV-2 to periodontal tissues may cause oral manifestation. Indeed, recent review by Santos (Amorim dos Santos et al., 2021) on oral symptoms in COVID-19 patients reported taste alterations as the most prevalent oral manifestation but also a low certainty of evidence of oral mucosal lesions, including gingiva. Patel and Wooley (Patel & Woolley, 2020) presented a case of a patient with necrotizing periodontal disease and suspected COVID‐19. Taken together, our results suggest that, with reference to the expression of SARS-CoV-2 entry and processing genes in HGnF, periodontal tissue could potentially be infected by SARS-CoV-2 and presence of periodontal diseases may affect clinical outcomes of COVID-19.
Since the purpose of the current investigation was to determine whether periodontal pathogen-derived lipopolysaccharide or inflammatory cytokines/mediator could affect the expression level of SARS-CoV-2 entry and processing factors in HGnF by means of gene expression, the exact roles of these genes in HGnF were not investigated. In addition, we do not know whether the increased expression of SARS-CoV-2 entry and processing genes relates to disease severity of COVID-19. However, to date, research on COVID-19/ SARS-CoV-2 in dental field is mainly focused on prevention of the disease. Further research aimed toward elucidating the association between periodontal disease and COVID-19 are strongly warranted.
5. Conclusion
In conclusion, SARS-CoV-2 entry and processing genes are expressed in human gingival fibroblasts and their expressions are altered by PgLPS, IL1β, TNFα and PGE2 treatment. Therefore, periodontal tissue could potentially be infected by SARS-CoV-2. In addition, the results may indicate that presence of periodontal diseases as a predisposition to the negative consequences associated with COVID-19.
Authors’ contributions
Kotaro Sena (Conceptualization; Methodology; Data curation; Investigation; Supervision; Writing original draft), Kirara Furue (Conceptualization; Methodology; Writing original draft), Fumiaki Setoguchi (Investigation; Writing original draft), Kazuyuki Noguchi (Supervision; Writing original draft).
Funding
This study was supported by JSPS KAKENHI Grants Numbers JP18K09639 and JP18K17100. The funder had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Agis H., Collins A., Taut A.D., Jin Q., Kruger L., Görlach C., Giannobile W.v. Cell population kinetics of collagen scaffolds in Ex Vivo oral wound repair. PloS One. 2014;9(11) doi: 10.1371/journal.pone.0112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmetaj-Shala B., Vaja R., Atanur S.S., George P.M., Kirkby N.S., Mitchell J.A. Cardiorenal tissues express SARS-CoV-2 entry genes and basigin (BSG/CD147) increases with age in endothelial cells. JACC: Basic to Translational Science. 2020;5(11) doi: 10.1016/j.jacbts.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim dos Santos J., Normando A.G.C., Carvalho da Silva R.L., Acevedo A.C., de Luca Canto G., Sugaya N.…Guerra E.N.S. Oral manifestations in patients with COVID-19: A living systematic review. Journal of Dental Research. 2021;100(2):141–154. doi: 10.1177/0022034520957289. [DOI] [PubMed] [Google Scholar]
- Barrett T., Wilhite S.E., Ledoux P., Evangelista C., Kim I.F., Tomashevsky M.…Soboleva A. NCBI GEO: Archive for functional genomics data sets - Update. Nucleic Acids Research. 2013;41(D1) doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J.D., Papapanou P.N., Philips K.H., Offenbacher S. Periodontal medicine: 100 years of progress. Journal of Dental Research. 2019;98(10):1053–1062. doi: 10.1177/0022034519846113. [DOI] [PubMed] [Google Scholar]
- Cekici A., Kantarci A., Hasturk H., van Dyke T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000. 2014;64(1) doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y.…Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corchuelo J., Ulloa F.C. Oral manifestations in a patient with a history of asymptomatic COVID-19: Case report. International Journal of Infectious Diseases. 2020;100:154–157. doi: 10.1016/j.ijid.2020.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Research. 2020;176 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Xiang J., Li C., Cao Z., Huang Z. Increased expression of extracellular matrix metalloproteinase inducer is associated with matrix metalloproteinase-1 and -2 in gingival tissues from patients with periodontitis. Journal of Periodontal Research. 2009;44(1):125–132. doi: 10.1111/j.1600-0765.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- Edgar R., Domrachev M., Lash A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Research. 2002;30(1):207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke P.I., Wei L., Borgnakke W.S., Thornton-Evans G., Zhang X., Lu H.…Genco R.J. Periodontitis prevalence in adults ≥ 65 years of age, in the USA. Periodontology 2000. 2016;72(1) doi: 10.1111/prd.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emingil G., Tervahartiala T., Mãntylã P., Määttä M., Sorsa T., Atilla G. Gingival crevicular fluid matrix metalloproteinase (MMP)-7, extracellular MMP inducer, and tissue inhibitor of MMP-1 levels in periodontal disease. The Journal of Periodontology. 2006;77(12):2040–2050. doi: 10.1902/jop.2006.060144. [DOI] [PubMed] [Google Scholar]
- Ender S.A., Dallmer A., Lässig F., Lendeckel U., Wolke C. Expression and function of the ACE2/angiotensin(1–7)/Mas axis in osteosarcoma cell lines U-2 OS and MNNG-HOS. Molecular Medicine Reports. 2014;10(2) doi: 10.3892/mmr.2014.2266. [DOI] [PubMed] [Google Scholar]
- Fernandes L.L., Pacheco V.B., Borges L., Athwal H.K., de Paula Eduardo F., Bezinelli L.…Heller D. Saliva in the diagnosis of COVID-19: A review and new research directions. Journal of Dental Research. 2020;99(13):1435–1443. doi: 10.1177/0022034520960070. [DOI] [PubMed] [Google Scholar]
- Foda H.D., Rollo E.E., Drews M., Conner C., Appelt K., Shalinsky D.R., Zucker S. Ventilator-induced lung injury upregulates and activates gelatinases and EMMPRIN: Attenuation by the synthetic matrix metalloproteinase inhibitor, prinomastat (AG3340) American Journal of Respiratory Cell and Molecular Biology. 2001;25(6):717–724. doi: 10.1165/ajrcmb.25.6.4558f. [DOI] [PubMed] [Google Scholar]
- Furue K., Sena K., Sakoda K., Nakamura T., Noguchi K. Involvement of the phosphoinositide 3-kinase/Akt signaling pathway in bone morphogenetic protein 9-stimulated osteogenic differentiation and stromal cell-derived factor 1 production in human periodontal ligament fibroblasts. European Journal of Oral Sciences. 2017;125(2):119–126. doi: 10.1111/eos.12336. [DOI] [PubMed] [Google Scholar]
- Gabison E.E., Hoang-Xuan T., Mauviel A., Menashi S. EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie. 2005;87(3–4 SPEC. ISS):361–368. doi: 10.1016/j.biochi.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Garibaldi B.T., Fiksel J., Muschelli J., Robinson M.L., Rouhizadeh M., Perin J.…Gupta A. Patient trajectories among persons hospitalized for COVID-19. Annals of Internal Medicine. 2020 doi: 10.7326/M20-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Mohindra R., Chauhan P.K., Singla V., Goyal K., Sahni V.…Singh M.P. SARS-CoV-2 detection in gingival crevicular fluid. Journal of Dental Research. 2021;100(2):187–193. doi: 10.1177/0022034520970536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halboub E., Al-Maweri S.A., Alanazi R.H., Qaid N.M., Abdulrab S. Orofacial manifestations of COVID-19: A brief review of the published literature. Brazilian Oral Research. 2020;34 doi: 10.1590/1807-3107bor-2020.vol34.0124. [DOI] [PubMed] [Google Scholar]
- Halepas S., Lee K.C., Myers A., Yoon R.K., Chung W., Peters S.M. Oral manifestations of COVID-19 related multi-system inflammatory syndrome in children: A review of 47 pediatric patients. Journal of the American Dental Association. 2020;152(3):202–208. doi: 10.1016/j.adaj.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pohlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. Journal of Virology. 2014;88(2):1293–1307. doi: 10.1128/jvi.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S.…Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.052. 271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M.…Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223) doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N., Pérez P., Kato T., Mikami Y., Okuda K., Gilmore R.C.…Byrd K.M. SARS-CoV-2 infection of the oral cavity and saliva. Nature Medicine. 2021;27(5):892–903. doi: 10.1038/s41591-021-01296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaguirre G. The proteolytic regulation of virus cell entry by furin and other proprotein convertases. Viruses. 2019;11(9) doi: 10.3390/v11090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara C., Çelen K., Dede F.Ö., Gökmenoğlu C., Kara N.B. Is periodontal disease a risk factor for developing severe Covid-19 infection? The potential role of Galectin-3. Experimental Biology and Medicine. 2020;245(16) doi: 10.1177/1535370220953771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Amar S. Periodontal disease and systemic conditions: A bidirectional relationship. Odontology. 2006;94(1):10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuk H., Hutchenreuther J., Murphy-Marshman H., Carter D., Leask A. 5z-7-oxozeanol inhibits the effects of TGFβ1 on human gingival fibroblasts. PloS One. 2015;10(4) doi: 10.1371/journal.pone.0123689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G.R.M., Libra M., De Pasquale R., Ferlito S., Pedullà E. Association of viral infections with oral cavity lesions: Role of SARS-CoV-2 infection. Frontiers of Medicine. 2021;7 doi: 10.3389/fmed.2020.571214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai T.M., Kuo P.J., Lin C.Y., Chin Y.T., Lin H.L., Chiu H.C.…Fu E. CD147 self-regulates matrix metalloproteinase-2 release in gingival fibroblasts after coculturing with U937 monocytic cells. The Journal of Periodontology. 2020;91(5):651–660. doi: 10.1002/JPER.19-0278. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhou W., Yang L., You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacological Research. 2020;157 doi: 10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marouf N., Cai W., Said K.N., Daas H., Diab H., Chinta V.R.…Tamimi F. Association between periodontitis and severity of COVID‐19 infection: A case–control study. Journal of Clinical Periodontology. 2021 doi: 10.1111/jcpe.13435. jcpe.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi H., Rezaeifar K., Nasrabadi N. Oral manifestations of coronavirus disease-19: A mini-review. Journal of Medical Sciences. 2020;8(T1):286–289. doi: 10.3889/oamjms.2020.4999. [DOI] [Google Scholar]
- Muramatsu T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. Journal of Biochemistry. 2016;159(5):481–490. doi: 10.1093/jb/mvv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K., Shitashige M., Endo H., Kondo H., Ishikawa I. Binary regulation of interleukin (IL)-6 production by EP1 and EP2/EP4 subtypes of PGE2 receptors in IL-1beta-stimulated human gingival fibroblasts. Journal of Periodontal Research. 2002;37(1):29–36. doi: 10.1034/j.1600-0765.2002.00641.x. [DOI] [PubMed] [Google Scholar]
- Patel J., Woolley J. Necrotizing periodontal disease: Oral manifestation of COVID-19. Oral Diseases. 2020;27(Suppl. 3) doi: 10.1111/odi.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- Sahni V., Gupta S. COVID-19 & periodontitis: The cytokine connection. Medical Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi W., Kubota N., Shimizu T., Saruta J., Fuchida S., Kawata A.…Tsukinoki K. Existence of SARS-CoV-2 entry molecules in the oral cavity. International Journal of Molecular Sciences. 2020;21(17):1–16. doi: 10.3390/ijms21176000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos C.F., Morandini A.C., Dionísio T.J., Faria F.A., Lima M.C., Figueiredo C.M.…Greene A.S. Functional local renin-angiotensin system in human and rat periodontal tissue. PloS One. 2015;10(8) doi: 10.1371/journal.pone.0134601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata T., Donath K. The mechanism of pocket formation. The Journal of Periodontology. 1988;59(4):215–221. doi: 10.1902/jop.1988.59.4.215. [DOI] [PubMed] [Google Scholar]
- Thomas G. Furin at the cutting edge: From protein traffic to embryogenesis and disease. Nature Reviews Molecular Cell Biology. 2002;3(10):753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K.W., Tsang O.T.Y., Yip C.C.Y., Chan K.H., Wu T.C., Chan J.M.C.…Yuen K.Y. Consistent detection of 2019 novel coronavirus in saliva. Clinical Infectious Diseases. 2020;71(15):841–843. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich H., Pillat M.M. CD147 as a target for COVID-19 treatment: Suggested effects of azithromycin and stem cell engagement. Stem Cell Reviews and Reports. 2020;16(3):434–440. doi: 10.1007/s12015-020-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.058. 281-292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yang D., Li C., Shang S., Xiang J. Expression of extracellular matrix metalloproteinase inducer glycosylation and caveolin-1 in healthy and inflamed human gingiva. Journal of Periodontal Research. 2014;49(2):197–204. doi: 10.1111/jre.12095. [DOI] [PubMed] [Google Scholar]
- Wang K., Chen W., Zhang Z., Deng Y., Lian J.Q., Du P.…Chen Z.N. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduction and Targeted Therapy. 2020;5(1):1–10. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J.…Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S.…Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Medicine. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Li S., Tian S., Li H., Kong L. Full spectrum of COVID-19 severity still being depicted. Lancet. 2020;395(10228):947–948. doi: 10.1016/S0140-6736(20)30308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X.…Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. International Journal of Oral Science. 2020;12(1) doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M., Lin B., Pathak J.L., Gao H., Young A.J., Wang X.…Wang L. ACE2 and furin expressions in oral epithelial cells possibly facilitate COVID-19 infection via respiratory and fecal–oral routes. Frontiers of Medicine. 2020;7 doi: 10.3389/fmed.2020.580796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J.…Tan W. A novel coronavirus from patients with pneumonia in China, 2019. The New England Journal of Medicine. 2020;382(8):727–733. doi: 10.1056/nejmoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]