Abstract
There is a growing interest in the development of portable, cost-effective, and easy-to-use biosensors for the rapid detection of diseases caused by infectious viruses: COVID-19 pandemic has highlighted the central role of diagnostics in response to global outbreaks. Among all the existing technologies, screen-printed electrodes (SPEs) represent a valuable technology for the detection of various viral pathogens. During the last five years, various nanomaterials have been utilized to modify SPEs to achieve convincing effects on the analytical performances of portable SPE-based diagnostics. Herein we would like to provide the readers a comprehensive investigation about the recent combination of SPEs and various nanomaterials for detecting viral pathogens. Manufacturing methods and features advances are critically discussed in the context of early-stage detection of diseases caused by HIV-1, HBV, HCV, Zika, Dengue, and Sars-CoV-2. A detailed table is reported to easily guide readers toward the “right” choice depending on the virus of interest.
Keywords: Screen-printed electrodes (SPEs), Nanomaterials, Immunoassays, Viruses, SARS CoV-2
1. Introduction
Today the world is more interconnected than ever before, and as a result of the tremendous increase in global travel, there are more chances of spreading contagious pathogens. Among those, viruses play a fundamental role as the causative agents of various diseases. The infectious pathogens like the Influenza virus, Middle East Respiratory Syndrome Coronavirus (MERS-CoV), henipaviruses (Hendra and Nipah), Ebola, Zika, Dengue, West Nile virus, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have caused severe global disease outbreaks [1,2]. Therefore, the development of reliable methods for the early-stage detection of emerging viruses is a vital requirement of time. The recent pandemic of SARS-CoV-2 has made it obvious that portable, rapid, and highly sensitive diagnostic devices are the critical need of the day. The early-stage detection of infected patients plays a huge role in reducing the spread of infectious diseases [3,4]. Viruses are usually identified using various laboratory-based assays, including immunological assays, Polymerase Chain Reaction (PCR), plaque assays, and transmission electron microscopy [[5], [6], [7]]. These methods are laboratory-bound and require specialized personnel and sophisticated equipment. In addition to these practical limitations, nanometer size and an extremely small level of the viruses in human bodily samples further exacerbate their monitoring. Afterward, alternative analytical approaches such as colorimetric [[8], [9], [10]], chemiluminescent [11], fluorescence-based [12,13], electrochemical [14,15] have been utilized for viral quantification. Among these, the electrochemical ones are characterized by a unique advantage regarding real samples: electrochemical-based methods are not affected by the color/turbidity of biological matrices [16,17], i.e., blood, serum, saliva. Among the diverse technologies around the world of electrochemical methods, screen-printed electrodes (SPEs) are without any doubt the platforms that highlight the portability and easiness of electroanalysis.
SPEs can be manufactured on various substrates such as plastic, paper, tattoo, etc., and they are characterized by high versatility in selecting the size, geometry, dimensionality, and customization methods [[18], [19], [20], [21], [22]]. The use of screen-printed electrodes (SPEs) could be of the highest interest to develop new methods and research.
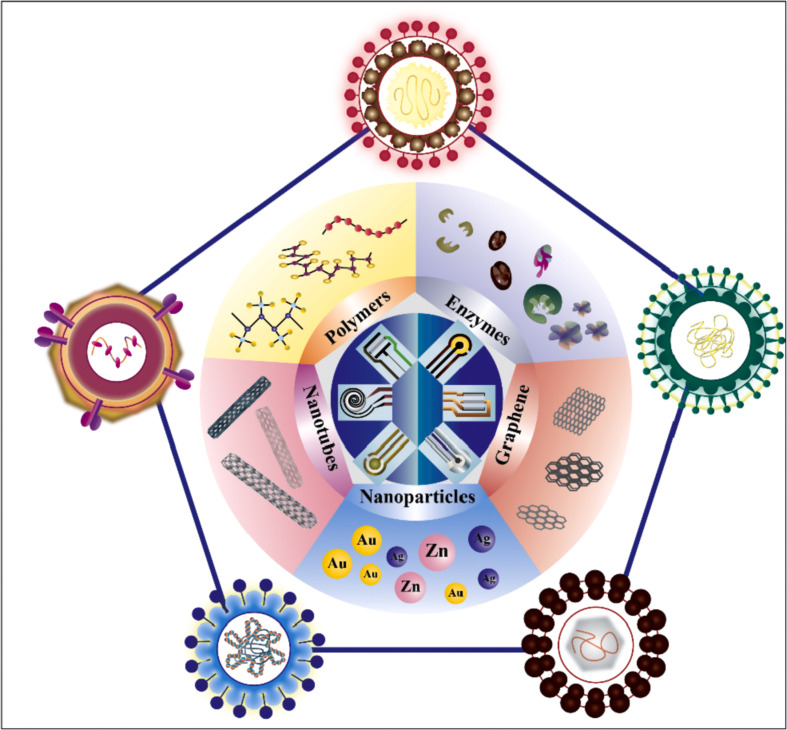
The advancement in material science highlights the fundamental role of novel nanomaterials in enhancing specificity, stability, and sensitivity of portable diagnostics: metal nanoparticles, magnetic nanoparticles, carbonaceous-based nanomaterials, conductive polymers are only some of the enhancers that are generally exploited [[23], [24], [25], [26], [27], [28], [29], [30], [31], [32]]. Thus, this paper aims to give a critical overview of the role of various nanomaterials, the manufacture methods for screen-printed electrodes, and the strategies for the forthcoming sustainable detection of Human Immunodeficiency Virus (HIV), Hepatitis B and C Viruses, Zika Virus, Dengue Virus and Sars-CoV-2 [[33], [34], [35], [36], [37]], Fig. 1 .
Fig. 1.
Illustration of virus structures and their detection using the screen-printed electrodes. HIV-1 [38], Hepatitis C virus [39], Dengue virus [40], Zika Virus [41], Hepatitis B virus [42] and Sars-CoV-2 [43].
2. Tips on SPEs manufacture and customization
The main feature of screen printing is the easiness of fabrication and application. This manufacturing method does not require cleanroom facilities and can be easily performed using low-cost materials, even with hand-made settings. The screen printing process requires conductive inks, a mesh screen with electrode patterns, a squeegee, and an oven [44,45]. The patterns can be of various geometrical shapes, and they can be designed using common drawing softwares such as AutoCAD, Adobe Illustrator, PowerPoint. Successively masks are obtained using that initial design, and they can be manufactured on plastics, fibers, or stainless-steel platforms. The conductive inks are successively spread with a squeegee onto various substrates like plastic, paper, fabric, tattoo, etc. The analytical features of SPEs (sensitivity, specificity, stability) can be enhanced through the use of different customization approaches, i.e., drop-casting, screen-printing, inkjet printing, langmuir-blodgett, spray, etc. [[46], [47], [48]]. Depending on the nature of the modifier and the final application of the devices, the right approach can be chosen: for instance, working with porous paper-based device that exploits paper-based flow needs to have whole-modified ink and screen-printing is suggested, while surface modification by drop casting/inkjet printing is enough if a classic drop-based configuration is used, perhaps on rigid substrates like plastic and office paper [[49], [50], [51]]. Metallic nanoparticles (Ag, Pt, Au, etc.), carbonaceous nanomaterials (carbon black, graphene, carbon nanotubes, etc.), Reduced Graphene Oxide (RGO) nanocomposite in situ decorated with gold nanoparticles, Au nanorods-functionalized nanostructured TiO2 transparent electrodes, conductive polymers (polypyrrole, polyaniline polythiophene, etc.) have demonstrated to enhance the features of SPEs in terms of cost-effectiveness, biocompatibility, conductivity, mechanical resistance and micro-environment stabilizers [[52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65]].
A printed electrochemical sensor consists of layers of conductive as well as dielectric pastes printed on an inert substrate [66]. Pastes are mainly consisting of nanoparticles colloidal suspension, containing additive, solvent, and binders. To obtain sustainable electrochemical devices, greener materials like polylactic acid, silk protein, and biochar have also been utilized. The next sections will be dedicated to the comprehension of the role and the effectiveness of nanomaterials when coupled to SPEs.
3. SPEs for detection of viruses
3.1. Human Immunodeficiency Virus (HIV)
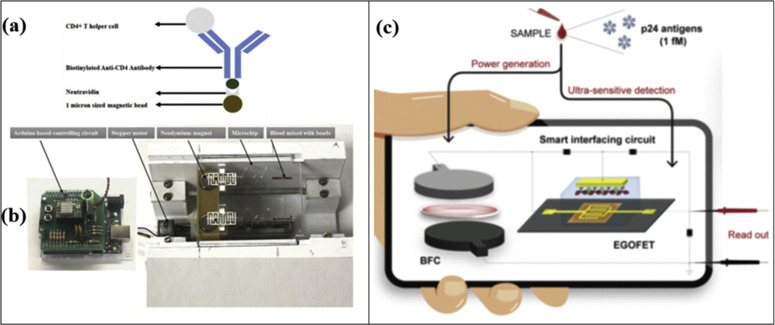
In 1981, the Human Immunodeficiency Virus (HIV) type I was discovered by Luc Montagnier's team at the Pasteur Institute in Paris. Later in 1984, Robert Gallo's team at the National Cancer Institute in Bethesda, Maryland, established that HIV-1 is the etiological agent of Acquired Immunodeficiency Disease (AIDS). HIV-1 is currently affecting an estimated 38 million people worldwide [67,68]. Berta et al. detected the presence of the HIV-1 virus in the Peripheral Mononuclear Cell (PMC) and bone marrow of 22 out of 45 randomly selected patients with AIDS [69]. The effective management of this disease is dependent on early-stage detection, rapid antiretroviral therapy (ART) initiation, and regular monitoring of HIV-1 viral load [70]. HIV-1 viral infections are routinely diagnosed with anti-HIV1 antibody-based tests. Molecular biology-based techniques can be utilized to quantify the HIV-1 virus with higher sensitivity and accuracy. But nucleic acid-based tests are quite time time-consuming and labor-intensive. Highly Active Antiretroviral Therapy (HAART) has successfully reduced the mortality associated with HIV-1/AIDS and kept the viral load under control. Nowadays, the major dilemma is that HIV-1 viral infections are highly prevalent in those resource-limited regions where healthcare facilities are not sufficient. Hence, it is of the utmost importance to develop cost-effective, simple, and easy-to-use devices that can help early-stage HIV-1 detection. Several researchers have demonstrated the detection of HIV-1 viruses using screen-printed electrodes. A plastic microchip containing screen-printed electrodes was utilized for the viral load quantification purpose [34]. The silver-vinyl ink was mixed with silicon adhesive in a ratio of 1:5 (w/w) to print the microelectrodes. A printed flexible plastic microchip, through capacitance spectroscopy of bioagent lysate, has been adopted for detecting and quantifying multiple Human Immunodeficiency Virus (HIV) subtypes (A, B, C, D, E, G and panel (circulating recombinant forms, CRF01_AE and CRF02_AG)). HIV-1 particles were captured by biotinylated polyclonal anti-gp120 antibodies anchored to streptavidin-coated magnetic beads. Successively, glycerol was used to remove residual high electrically conductive background, and a solution containing 1% Triton x-100 has been used to release the charged molecules: the release provokes a change of the electrical properties of the solution, that has been used to detect the viral lysate samples at the silver SPEs. The conductive silver ink-based SPEs printed on flexible plastic material provided a good platform to quantify HIV-1 subtypes A, B, C, D, E, G and panel, respectively, down to 103,103,102,102, 102, 103 and 104 viral load spiked plasma samples. The experimental setup has been characterized by a cost lower than $2 with a 1-h total assay time. The preliminary results on spiked samples have demonstrated that capacitance spectroscopy allowed a more sensitive method than impedance spectroscopy [33]. In another effort to detect the HIV-1 virus, capsid protein p24 has been revealed in untreated human serum samples [71]. The platform consists of a single-walled carbon nanotube functionalized with screen-printed electrodes. SWCNT-SPCEs are the key to the detection process due to their superior properties of carbon nanotubes, including efficient immobilization of bioreceptors, enhancement of biochemical active area, and significant improvement in the electronic transfer process. The protein p24 was conjugated on the surface of SWCNT-SPCEs modified with chitosan/glutaraldehyde (CS/GA). The detection results were very promising, and a linear detection range of 10 pM to 1 nM was achieved, with a detection limit equal to 2 pM in spiked human serum. Another interesting target for HIV monitoring is CD4 cells. Their quantification provides information about the overall health of the immune system. In this regard, a flow-free automatic immunoassay was developed to quantify CD4+ T cells [52]. CD4+ T cells were isolated from whole blood using antibody-coated magnetic beads. SPEs fabricated on the base layer of the composite microfluidic chip were used to perform the electrical impedance spectroscopy. SPEs consisting of 20% (w/w) graphene-modified silver conductive paste and were printed on PMMA that provided an ample platform for CD4+ T cells quantification. Fig. 2 (a,b) presents the process of making a screen for the fabrication of interdigital microelectrodes and the final interdigitated electrodes printed using this developed screen. This assay successfully quantified CD4+ T cells from whole blood down to 25 cells/μL. This test is completed within 5 min time frame, and setup provided a fully automated assay for the quantification of CD4+ T cells. Antibody-tagged magnetic beads were utilized to isolate CD4+ T cells from the whole blood sample, and impedance spectroscopy was performed to quantify the CD4 cells (on lysate). Another route is represented by serological assays that are capable of detecting the presence of HIV-1 antibodies in human bodily samples. HIV-1 antibodies are detectable after 1–3 months of initial infection [72]. SPEs can also be utilized for the detection of the anti-human HIV1 antibody. In one such effort, an immunosensor has been realized for the detection of HIV-1 antibodies in serum [73]. The biofunctionalization process of the working electrode was carried out with 3-amino-propyldimethylethoxysilane (APDES) and glutaraldehyde (GA). The GA bound with –NH2 groups of APDES and provided aldehyde groups for the immobilization of proteins. Then HIV p24 core antigen was immobilized to the surface of the electrode. This method was utilized for the accurate detection and quantification of HIV-1 antibodies in mouse serum at a limit of detection (LOD) of 300 pg/mL. The developed platform consists of an array of paper-based immunosensors and a handheld multi-channel potentiostat. The distinct advantage of this platform is the ability to rapidly conduct enzyme-linked immunosorbent assays simultaneously on 8 samples in only 20 min. Another example has been represented by the use of electrolyte-gated field-effect transistor (EGOFET) [74]. The gate of the transistor was functionalized with the anti-HIV-1 p24 antibody. The process consisted of the attachment of a self-assembled chemical monolayer (SAM) to the surface of the gold electrode and subsequent immobilization of the antibodies. Screen printing was utilized to print anode and cathode electrodes on a polyethylene terephthalate (PET) substrate, and the anode and cathode were separated by a glass-fibre-paper disk that includes the analyte to be tested, as shown in Fig. 2b. The bio-recognition takes place at this gate electrode after the addition of HIV-1 p24 protein. This sensor is powered by a paper-based biofuel cell (BFC). Carboxyl-functionalized multi-walled-carbon-nanotubes dispersed in isopropanol was deposited on these carbon-based anode electrodes. A final layer was deposited with a cross-linker ethylene glycol diglycidyl ether in ultrapure water (10% v/v) with naphthoquinone-linear poly(ethylenimine) (NQ-LPEI) polymer solution (67% v/v in deionized water) and FAD-GDH (30 mg/mL in deionized water) mixture. The developed setup was able to detect down to 1 fM HIV-1 p24 antigen (the range of p24 antigen in acute infection is 50 aM to 15 fM). It should be noted that the presence of multi-walled in printed transistor can be utilized for HIV-1 detection at the acute infection stage, although the viral load is at a peak at this stage (106–108 virus copies per mL) [[75], [76], [77], [78]]. Individuals in acute HIV-1 infection state are unaware of their disease status, are highly infectious due to higher viral load, and, as a result, can significantly contribute to the further spread of HIV-1 [79].
Fig. 2.
(a,b) The actual picture of developed disposable microchip containing screen-printed electrodes for CD4+ T cell quantification (Reprinted from Ref. [52]. Copyright 2019, Elsevier). (c) Illustration of the self-powered platform consisting of EGOFET and paper-based BFC for the quantification of HIV-1 p24 protein (Reprinted from Ref. [74]. Copyright 2020, Elsevier).
In another work, a screen-printed platform was utilized for the detection of double-stranded DNA sequences of HIV in serum samples. Electrodes were fabricated on different types of paper-based substrates (Copy and filter papers) [80]. Paper-based substrates allows to obtain numerous advantages like cost-effectiveness, ease of availability, and environment-friendly nature [81]. Carbon-based electrodes were coupled with gold nanoparticles (AuNPs) dispersion to immobilize the DNA probes. AuNPs were purposely chosen for this application due to their good electrical properties and higher surface-to-volume ratio—the AuNPs increase the charge transfer kinetics occurring at the surface of the electrode and allowed to covalently immobile recognition probes for DNA. These platforms were used for ssDNA and dsDNA detection. The developed platform was successfully utilized to detect single and double-stranded sequences with a detection limit of 3 nM and 7 nM, respectively. The performance of the developed setup was also tested in undiluted serum samples, and it provided similar results compared to the measurements performed in a buffer solution. The method provided the direct quantification of the dsDNA and ssDNA sequences and eliminated the need for the benchtop PCR instrument.
3.2. Hepatitis
3.2.1. Hepatitis C virus (HCV)
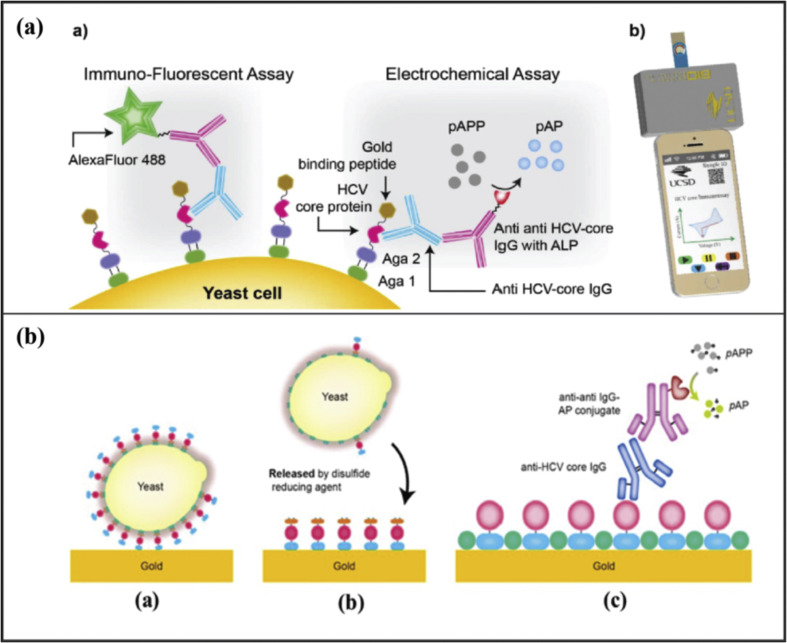
Hepatitis C virus (HCV) causes various chronic liver diseases such as hepatocellular carcinoma, cirrhosis, and end-stage liver disease [82]. HCV is a single-stranded positive-sense RNA virus with a relatively small size (40–80) nm [83]. HCV has six known genotypes and multiple subtypes. Genotype 1 is the most prevalent worldwide [84]. It is estimated that there are approximately 71 million people worldwide are infected with HCV [85]. HCV can be diagnosed with various different methods. Enzyme-Linked Immunosorbent Assay (ELISA) and Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR) are the two most common detection methods of HCV. Here, we summarize the screen-printing methods utilized for the detection of HCV. Spencer et al. have used SPEs to detect HCV antibodies [86]. The anti-HCV antibodies were detected with the help of yeast bio-bricks. Yeast cells were genetically nano-engineered to display HCV core antigen protein linked to the gold-binding peptide. The screen-printed electrodes sensor was made up of high-temperature curing ink. These genetically engineered yeast cells were used to functionalize the surface of gold-coated SPEs demonstrated in Fig. 3 (a). This method has provided a LOD of 2 nM. This antigen-based antibody capture method offers a cost-effective solution for POC diagnostics. In another research, an electrochemical sensor was developed for the detection of core antigen of HCV [87]. Gr-IL-Fu nanocomposites were utilized to modify the screen-printed carbon electrodes, and then rhodium nanoparticles (RhNPs) were deposited on the surface of the modified electrode. The addition of RhNPs thin-film facilitates the immobilization of the primary antibody. Initially, the HCV core protein was loaded on the surface of this electrode. TiO2 nanoparticles doped with Celestine Blue were used as a label to identify the captured HCV. CB/Nafion@TiO2 composite nanoparticles were utilized to quantify the captured HCV antigen. The utilization of Nafion@TiO2 composite nanoparticles enhances the electrochemical output signal down to a LOD of 25 fg/mL. Alternatively, Au-SPEs were utilized for the detection of HCV [88]. These electrodes, screen-printed with high-temperature inks, are suitable candidates for analyte sensing from micro volumes (up to 50 μL) samples in decentralized settings. The surface was nanostructured by CV cycles in sulfuric acid. HCV-core gold-binding-peptide fusion protein was used to bind primary anti-HCV antibodies, and a detection limit of 32 nM was achieved (Fig. 3b).
Fig. 3.
(a) Genetically engineered yeast cells based electrochemical immunoassay for detection of HCV (Reprinted from Ref. [86]. Copyright 2016, Elsevier). (b) The illustration of GBP yeast fusion proteins immobilization to the surface of a gold electrode (Reprinted from Ref. [88]. Copyright 2018, Elsevier).
3.2.2. Hepatitis B virus (HBV)
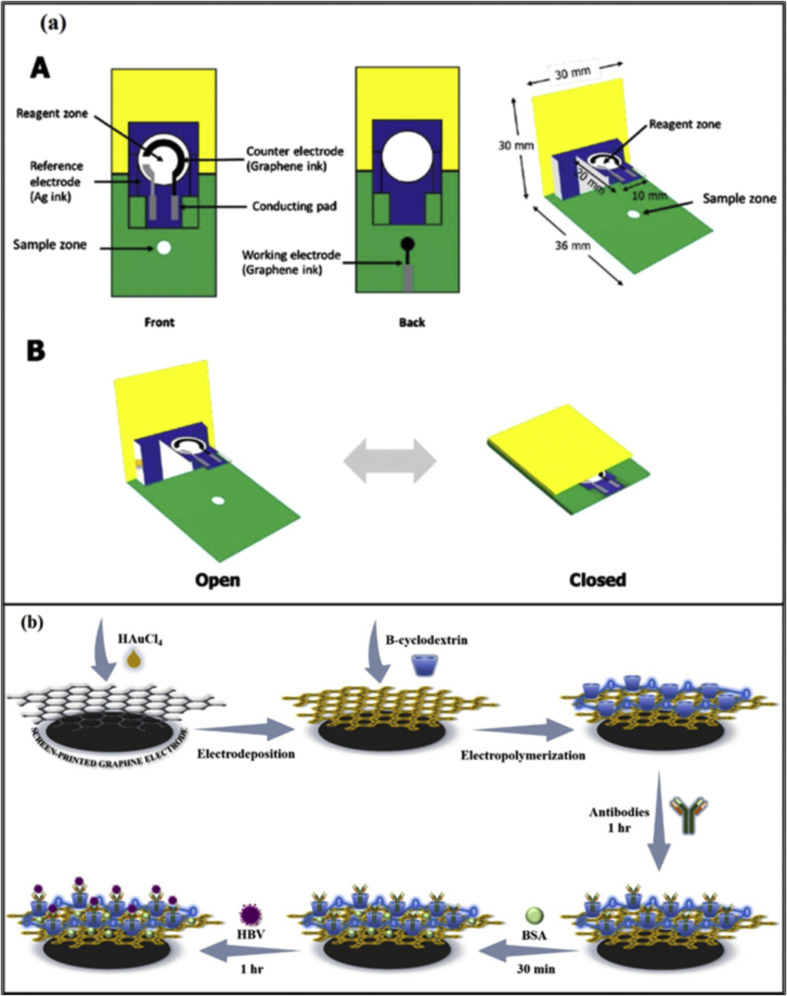
Hepatitis B virus (HBV) is causing a global chronic viral disease. It is a double-stranded DNA virus that belongs to the Hepadnaviridae family [39]. As per estimates of the Center for Disease Control and Prevention (CDC), there are 257 million people worldwide infected with HBV [85]. Immunoassays and polymerase chain reaction (PCR) are reported to detect HBV [89]. Various SPE-based examples have been reported in the literature. For instance, a paper-based electrochemical sensor was developed for HBV-DNA detection [85]. The device was in the shape of a pop-up micropad. These graphene-based electrodes were fabricated on the front of the device, and the working electrode was printed on the back of the pop-up device, as shown in figure Fig. 4 (a). Their developed device had a separate sample zone and the reagent zone. The device's design enabled the establishment of a fluidic path, electrical connectivity and reaction time controlled by folding the pop-up. The developed device reached a limit of detection of 1.45 pM and worked in the liner range from 0.05 to 100 nM. In another research, HBV-DNA was detected with the use of AgNPs in the electrochemical experimental setting [90]. AgNPs were modified with the recognition DNA probe. The utilization of AgNPs provided an amplification factor of approximately 250k-fold. In addition, 2.8 micron-sized magnetic microbeads (MμBs) have been involved as mobile solid phase supports for the capture probes. These magnetic microbeads were concentrated at the detection electrode using a magnet, and this arrangement enhanced the amplification by approximately 25-times. The assay did not involve any antibody or enzyme and consists of a single-step sample incubation. The two steps amplifications enabled picomolar detection of HBV-DNA target. This paper-based electrochemical sensor achieved a LOD of 85 pM. A simple and user-friendly biosensor was developed exploiting the use of a smartphone to establish a near field communication (NFC) enabled platform [91]. The entire setup was consisting of an NFC antenna, potentiostat circuit, and connections to an electrode. The screen-printing method was used for the printing of electrodes on Polyvinyl chloride (PVC) substrate using graphene ink and a nano-layer of gold was electrodeposited on top of the printed platform to host the β-cyclodextrin (β-CD) that was utilized to immobilize highly sensitive antibodies, as demonstrated in Fig. 4b. The immunosensor was used to successfully detect HBsAg in serum samples in the range 10–200 μg/mL, with a LOD of 0.17 μg/mL.
Fig. 4.
(a) A step-by-step illustration of the pop-up PAD DNA sensor (Reprinted from Ref. [85]. Copyright 2020, Elsevier). (b) Screen-printed electrodes on PVC substrate immunosensor to detect HBV (Reprinted from Ref. [91]. Copyright 2021, Elsevier).
3.3. Zika Virus (ZIKV)
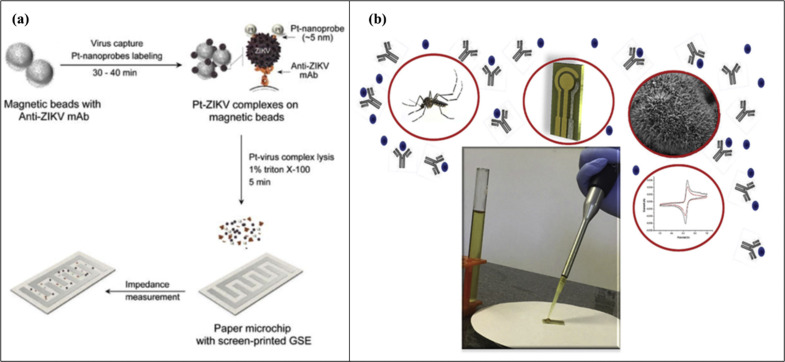
Zika virus (ZIKV) is a flavivirus that spreads through the bite of an Aedes species mosquito. Zika virus can cause Guillain–Barré Syndrome, microcephaly, and other birth defects [92]. This mosquito-borne pathogen is usually detected using RT-PCR and ELISA in a laboratory setting. Several biosensors have been fabricated for the rapid detection of ZIKV. Mohamed et al. have developed a paper-based microchip with printed electrodes for the quantification of the viral lysate [37]. The biosensor was fabricated using a screen-printing process, and 4-finger-like interdigitated electrodes were printed on paper using graphene-modified silver nanocomposite in a 4:1 ratio (graphene: silver), to promote good conductivity and measurement stability. The whole detection process is showed in Fig. 5 a. The viral particles of interest were isolated from the biological samples with the help of Anti-ZIKV monoclonal antibodies coated magnetic beads and tagged with platinum nanoparticles (PtNPs). In addition, PtNPs were incorporated to enhance the electrical signal. ZIKV-PtNPs complexes were lysed using a detergent solution, and the limit of detection was approximately 100 viruses per microliter in the complex sample matrix, i.e., serum. Electrochemical nucleic acid biosensing, also known as genosensing utilizes sequence-specific probes as a biorecognition element, and as a result of nucleic acid hybridization, electrochemical transduction produces a measurable signal [93,94]. Another strategy focused on genosensor that was developed to detect ZIKV in saliva, urine, and serum samples [95]. A biotinylated capture probe was anchored to streptavidin-coated magnetic particles. The target was prehybridized with the digoxigenin (Dig)-coated signal probe and further hybridized to the capture probe-coated magnetic particles. The addition of an anti-Dig monoclonal antibody tagged with the HRP resulted in recognition of a signal probe. The beads were magnetically attracted to the surface of Au-SPE and as a result of this process, genosensor was successfully used to detect ZIKV and discriminate it from the dengue (DENV) and chikungunya (CHIKV) viruses. The LOD was 0.7 and 3 pmol/L for two regions R1 and R2, respectively. However, the effectiveness of nanomaterials modification can help to achieve a lower detection limit [95]. In addition, the use of zinc oxide nanorods (ZnO NRs) also provided a suitable surface for attaching anti-ZIKV NS1 antibody [96]. Fig. 5(b) ZnO nanorods, that were grown onto Au-SPE, allowed to attach ZIKV-NS1 antibody via cystamine (Cys) and glutaraldehyde (Glut). Electrochemical measurements were performed using cyclic voltammetry, and spiked urine containing the ZIKV was successfully analyzed in the linear range comprised between 0.1 and 100 ng/mL and a LOD of approximately 1 pg/mL. An elegant approach involving electrochemical impedance spectroscopy has also been reported with application in serum and saliva samples [97]. In this case, a SPE has been nano-engineered with a portion of the recombinant structural proteins domain III of the envelope protein (EDIII). This architecture was characterized by high selectivity, and the biosensor obtained with the ZIKV EDIII was capable of detecting 53 fg/mL mAb.
Fig. 5.
(a) Illustration of the nanoparticle enhanced method for the detection of ZIKV using a paper chip containing screen-printing electrodes consisting of graphene-silver nanocomposite ink (Reprinted from Ref. [37]. Copyright 2018, Royal Society of Chemistry). (b) The illustration of immunosensor based on ZnO nanostructures conjugated with ZIKV-NS1 antibody to detect ZIKV (Reprinted from Ref. [96]. Copyright 2019, Elsevier).
3.4. Dengue virus
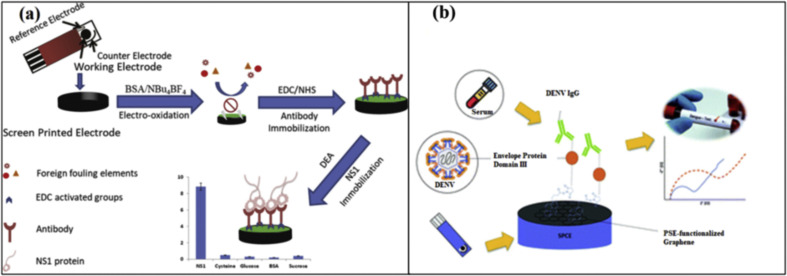
Dengue virus is a single positive-stranded RNA virus that belongs to the Flavivirus family. It is transmitted by infected Aedes female mosquitoes and causes dengue hemorrhagic fever (DHF) and dengue shock syndrome [98]. Even if RT-PCR and ELISA are normally utilized for the detection of DENV, the use of SPE-based biosensors has been demonstrated for the early-stage detection of DENV. The adoption of a nanocomposite formed with silicon nanowires (SiNWs) and AuNPs has been reported in the literature [99]. The major role of SiNWs/AuNPs was to increase the conductivity of SPGE, and it also provided a good site for DNA probe immobilization and hybridization. A thiolated ssDNA probe was immobilized on the surface SiNWs/AuNPs modified SPE. The developed biosensor was tested with various concentrations of complementary DNA, and it was able to detect DENV-DNA oligomer in the linear range of 100 nM-10 pM with a detection limit of 1.63 pM. In another work, to avoid interferents species, an SPE has been engineered by electrografting a protein layer, with the results of obtaining a conductive and anti-fouling surface [100]. A non-structural 1(NS1) monoclonal antibody was attached to the grafted surface, as indicated in Fig. 6 a and DENV protein was linearly detected up to 200 ng/mL, with a limit of detection of 0.3 ng/mL. In another research, an immunosensor was developed for the detection of DENV IgG antibodies [101]. In this case, graphene was used to provide a better electron transfer and a high surface to volume ratio to SPE. The envelope glycoprotein domain III (cEDIII) was immobilized on the nanostructured SPE, and the immunosensor allowed to determine the dengue antibodies in mouse serum samples with a detection limit of 22.5 ng/mL (Fig. 6b).
Fig. 6.
(a) An illustration of screen-printed carbon electrode-based immunosensor for the detection of dengue virus (Reprinted from Ref. [100]. Copyright 2018, Elsevier). (b) Graphene modified screen-printed electrodes based immunosensor to detect DENV IgG (Reprinted from Ref. [101]. Copyright 2021, Royal Society of Chemistry).
3.5. SARS-CoV-2
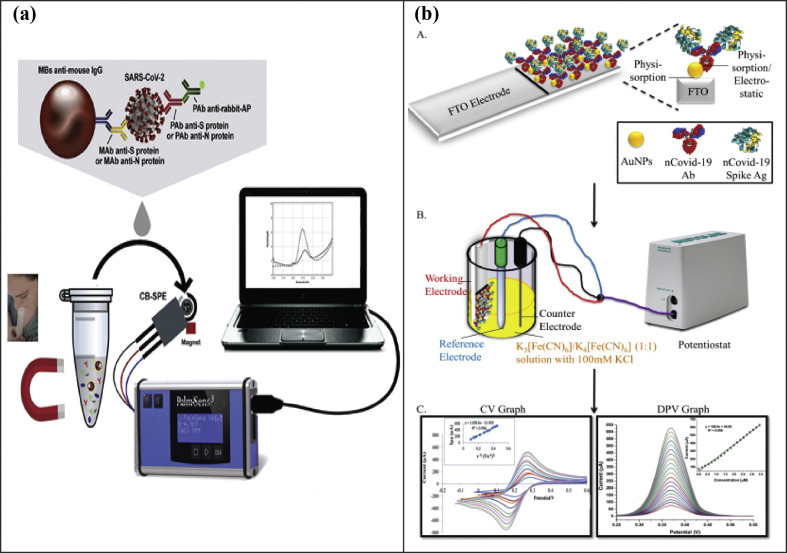
SARS-CoV-2 has recently caused a global pandemic in the form of coronavirus disease 2019 (COVID-19). Due to its wide infectivity, SARS-CoV-2 has exacerbated the outbreak's management [102]. It is of utmost importance to quickly identify the infected patients and isolate them to reduce the further spread of the virus. Sars-CoV-2 is a single-stranded RNA virus. It has four structural proteins: envelope protein (E), spike surface glycoprotein (S), matrix protein (M), and nucleocapsid protein (N) [103]. Various diagnostic assays have been developed to detect SARS-CoV-2. Some of these assays are classified as direct tests that detect the presence of viral RNA, while other indirect tests measure the antibodies against the virus [104]. Various biosensors using SPEs have also been adopted to detect SARS-CoV-2 [56]. Recently, a biosensor was developed to rapidly detect SARS-CoV-2 coronavirus [102]. The immunoassay was introduced for the detection of Nucleocapsid (N) protein or Spike (S) protein utilizing magnetic beads and carbon black-based screen-printed electrodes. The magnetic beads act as a support of the immunological chain. As a result of the high surface-to-volume ratio of these magnetic beads, it is possible to immobilize a higher amount of primary antibody resulting in an increase of sensitivity. The antibodies for S and N proteins were conjugated to magnetic beads. The binding of the Sars-CoV-2 was evaluated by utilizing a secondary antibody labeled with Alkaline Phosphatase as the indicator enzyme. The enzymatic by-product 1-naphtol was detected with screen-printed electrodes. These SPEs were modified with carbon black that demonstrates enhanced electrochemical sensitivity if compared to the bare counterpart. The whole process of SARS-CoV-2 detection is shown in Fig. 7 a. The LOD for this assay was 8 ng/mL and 19 ng/mL in unprocessed saliva samples, respectively, for N and S protein. The cultured virus samples and clinical saliva samples were also successfully detected with the developed immunoassay. In another effort, a biosensing device was developed for the detection of nCovid-19 spike antigen in human saliva samples [105]. The biosensor was developed by attaching nCovid-19 monoclonal antibody on screen-printed carbon electrodes. Gold nanoparticles were utilized as the signal amplifiers due to their biocompatibility, stability, and higher conductivity. The Ab-Ag interactions provide a change in the electrical signal that can be measured using the transducer, as shown in Fig. 7b. This device has been demonstrated to detect nCovid-19 antigen down to 10 fM in standard buffer and a detection limit of 90 fM when tested with spiked saliva samples. The developed device termed “eCovSens” has allowed response within 10–30 s. Similarly, ZnO nanowires (ZnO-NWs), in combination with paper-based microfluidics, have been used to develop an immunoassays for the serological testing of antibody markers (IgA, IgM, and IgG) [106]. After the electrodes were screen-printed, ZnO-NWs were grown on top of the sensing surface. This biosensor was utilized for the detection of specific antibody, i.e., CR3022 antibody, in human serum. It was demonstrated that the paper-based ZnO-NW biosensor allowed detection in spiked human serum samples at different concentrations in the range of ng/mL and μg/mL in less than 30 min.
Fig. 7.
(a) Illustration of the magnetic beads-based assay for the rapid detection of SARS-CoV-2 (Reprinted from Ref. [102]. Copyright 2021, Elsevier). (b) AuNP based immunoassay on FTO electrode for detecting SARS-Cov-2 (Reprinted from Ref. [106]).
In the following Table 1 , a comprehensive guide that summarizes the various strategies for detecting viral pathogens at SPEs is reported. Each example is associated with experimental features.
Table 1.
Summary of various methods of viral pathogens detection.
| Target | Platform | Sensing element | LOD | Time | Matrix | Ref. |
|---|---|---|---|---|---|---|
| HIV | ||||||
| HIV-1 viral particles | Printed flexible plastic microfluidic chip | Biotinylated polyclonal anti-HIV1 gp120 antibodies attached to streptavidin-coated magnetic microbeads. | 100 copies/mL | 60 min | Plasma | [34] |
| CD4+ T cells | Point-of-care (POC) CD4 enumeration platform. | Biotinylated monoclonal anti-CD4 antibody-coated magnetic beads | 25 cells per μL | 5 min | Blood | [52] |
| HIV-Antibodies | Microfluidic paper-based | HIV p24 protein | 300 pg/mL | 20 min | Serum | [73] |
| HIV-1 p24 protein | Electrolyte-gated field-effect transistor (EGOFET) | anti-HIV1 p24 antibody | Approximately 1 fM range of proteins. | – | Blood | [74] |
| HIV-DNA | Paper-based strip | Triplex forming oligonucleotides | 3 nM and 7 nM respectively for ssDNA and dsDNA. | – | Serum | [80] |
| HIV p24 capsid protein | CNT-SPE | Monoclonal anti-HIV-1 p24 IgG1 | 2 pM | . | Serum | [71] |
| HCV | ||||||
| anti-HCV core IgG | Au-SPE | HCV-core antigen | 32 nM | Approximately a few minutes time frame. | Serum | [88] |
| HCV core antigen | Nafion@TiO2-SPE | Primary antibody | 25 fg/mL | – | Serum | [87] |
| HCV antibodies | Smartphone-based | HCV core antigen linked to a gold binding peptide (GBP) | 12.3 pM | – | PBS | [86] |
| HBV | ||||||
| Hepatitis B virus target DNA extracted from plasmid constructs. | Paper-based | Pyrrolidinyl PNA | 1.45 pM | – | Culture supernatants | [85] |
| Hepatitis B Virus DNA | Paper-based | Magnetic microbeads, DNA probe, AgNPs | 85 pM | – | PBS buffer | [90] |
| Hepatitis B surface antigen (HBsAg) | Immunosensor | Primary antibody | 0.17 μg/mL | – | Serum | [91] |
| SARS-CoV-2 | ||||||
| SARS-CoV-2 particles | CB-SPE immunosensor | Anti-S/N antibodies on magnetic beads | 19 ng/mL and 8 ng/mL in unprocessed saliva samples for S and N proteins, respectively | 30 min | Saliva | [102] |
| nCovid-19 spike antigen (nCovid-19Ag) | Fluorine doped tin oxide electrode + AuNPs | nCovid-19 monoclonal antibody | 90 fM | 10–30 s | Saliva | [106] |
| Zika Virus | ||||||
| Zika Virus particles. | Paper-based microchip | Magnetic beads conjugated with anti-ZIKV monoclonal antibody | 100 viral particles per microliter. | Assay time is not specified. | Urine, plasma, semen | [37] |
| Zika Virus NS1 antigen | ZnO nanostructures | Anti-ZIKV NS1 antibody. | 1 pg/mL | Assay time is not mentioned. | Urine | [96] |
| ZIKV-specific antibodies | Screen-printed electrodes modified with carbon-nanotubes containing ZIKV-derived proteins. | ZIKV non-structural protein 1(NS1) and domain III of the envelope protein (EDIII) as biorecognition element. | LOD is not specified. | Assay time is not specified. | Serum, saliva | [97] |
| Dengue Virus | ||||||
| Dengue virus NS1 | Impedimetric immunosensor consisting of BSA-SPCE modified with antibody. | anti-NS1 monoclonal antibody | 0.30 ng/mL | Assay time is not specified. | Serum | [100] |
| Dengue Virus DNA Oligomer | Au-SPE | SiNWs/AuNPs | 1.63 pM | Assay time is not mentioned. | TE buffer | [99] |
| Dengue virus antibodies | Graphene-SPE | Envelope glycoprotein domain III (EDIII) antigen. | 22.5 ng/mL | Assay time is not specified. | Serum | [101] |
4. Role of nanomaterials for improving SPEs’ performance
Nanomaterials are primarily used as labels or carriers that remarkably enhances the detection capacity of SPEs. Among various classes of nanomaterials, nanoparticles are the most widely used nanomaterials for the modification of SPEs. The application of metal nanoparticles is a standard practice employed for the enhancement of SPEs. In this aspect, AuNPs are extensively reported to provide an anchoring platform due to many features that make them the favorite choice for a wide range of applications. To list a few, flexible size, shape, fabrication capacity offers extensive applications in the development of low-cost, portable electrochemical sensors. AuNPs, due to their easy fabrication, high conductivity, biocompatibility, and electrochemical activity, they have been utilized to detect HCV [88], and in another work a dispersion of AuNPs has been exploited both to immobilize the DNA probes and for the efficient detection of HIV-related DNA [80]. AuNPs combined with silicon nanowires were utilized to detect the Dengue virus [99].
Besides, magnetic particles like Iron NPs are mainly utilized as a carrier due to excellent stability and their capability in the separation/accumulation of probe/target conjugates [90], as antibody-coated magnetic beads for HIV detection [52] and coupled to streptavidin for HIV and Zika virus detection [34,95]. Also, Platinum NPs have been capable of providing remarkable enhancement in signal transduction anti-ZIKV antibodies detection [37]. Rhodium NPs along with TiO2 have played a significant role in HCV detection due to their high biocompatibility towards the recognition probe [87]. Apart from nanoparticles, SPEs modified with carbon-based nanomaterials and their derivatives have drastically revolutionized diagnostics. Owing to the high surface area, immobilization capacity, excellent electrical conductivity, solvent dispersibility, rapid reaction kinetics, and eco-friendly nature, several studies have reported carbon-based electrodes for effective detection of viruses [85,97,100,101]. Graphene is often reported with other nanoparticles such as AgNPs to improve catalytic activity (with respect to bulk Ag electrodes) when peroxide-based assay is involved. In another study, carbonaceous porous structure allowed ZnO-NWs to be synthesized and providing a stable support for immobilizing probe for protein-SARS-CoV-2 detection [105]. ZnO nanorod were also employed as an efficient platform for detecting Zika antibodies, even without the use of carbon-based enhancers [96]. Polymers, on the other hand, also stand significant in diagnostics; likewise, carbon nanotubes are typically utilized thanks to their increase of analytical performances demonstrated by SWCNT-SPCEs modified with chitosan/glutaraldehyde (CS/GA) [71]. Carbon black, even if less famous than graphene and carbon nanotubes, has displayed a great sensitivity enhancement in detecting both S and N-protein of SARS-CoV-2 [102]. SPEs have been also modified with biomolecules such as portion of proteins and nucleic acids for identifying causative agents of various diseases, as for the case of genetically engineered yeast cells that have been able to detect HCV antigen protein [86]. Nanoengineering SPEs has provided multiple portable platforms for effective viruses’ detection with the use of low volume of samples. It should be noted that, even if their presence has been able to improve selectivity and stability, the majority of the studies have been demonstrated onto spiked samples. Even if the advantages related to the use of nanomaterials are obvious, the further development of SPE for novel strategies in virus sensing should be coupled to other technologies like microfluidics and chemometrics tools [[107], [108], [109], [110], [111]], with the aim in reducing complexity of in real-worlds scenarios.
5. Conclusion
The present paper reports a comprehensive overview of the principal strategies that have been developed for viral disease diagnostic by employing screen-printed technologies. The role of nanomaterials and magnetic particles has been highlighted for the enhancement of portable electrochemical methods. As the study case, nanomaterial-modified SPEs applied to HIV, HBV, HCV, Zika virus, Dengue virus, and SARS-CoV-2 detection have been considered. The adoption of nanomaterials as customizers for SPEs implementation represents a valuable tool for obtaining low-cost, user-friendly, and eco-designed diagnostics. In our opinion, the examples reported within the field of SPEs represents a good starting point for next-generation sensing and biosensing; however, to make these methods suitable for the real environment, they should be coupled to complementary technologies such as microfluidics, chemometrics, and artificial intelligence, with the aim of reducing background signals and interferents from non-processed samples, i.e., blood.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
S.C. acknowledges MIUR Grant “Dipartimento di Eccellenza 2018–2022” to Department of Pharmacy of University of Naples “Federico II”. Authors acknowledge Julian Ramirez for proofreading the manuscript.
References
- 1.Thomson E.C., Smith J.A., Klenerman P. The natural history of early hepatitis C virus evolution; lessons from a global outbreak in human immunodeficiency virus-1-infected individuals. J. Gen. Virol. 2011;92:2227. doi: 10.1099/vir.0.033910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das P., Choudhuri T. Decoding the global outbreak of COVID-19: the nature is behind the scene. Virus Dis. 2020:1–7. doi: 10.1007/s13337-020-00605-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yousefi H., Mahmud A., Chang D., Das J., Gomis S., Chen J.B., et al. Detection of SARS-CoV-2 viral particles using direct, reagent-free electrochemical sensing. J. Am. Chem. Soc. 2021;143:1722–1727. doi: 10.1021/jacs.0c10810. [DOI] [PubMed] [Google Scholar]
- 4.Weiss C., Carriere M., Fusco L., Capua I., Regla-Nava J.A., Pasquali M., et al. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano. 2020;14:6383–6406. doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- 5.Patolsky F., Zheng G., Hayden O., Lakadamyali M., Zhuang X., Lieber C.M. Electrical detection of single viruses. Proc. Natl. Acad. Sci. Unit. States Am. 2004;101:14017–14022. doi: 10.1073/pnas.0406159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang J., Tahir A., Wang H., Chang J. Applications of nanotechnology in virus detection, tracking, and infection mechanisms. Wiley Interdiscipl. Rev.: Nanomed. Nanobiotechnol. 2021 doi: 10.1002/wnan.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roingeard P., Raynal P.I., Eymieux S., Blanchard E. Virus detection by transmission electron microscopy: still useful for diagnosis and a plus for biosafety. Rev. Med. Virol. 2019;29 doi: 10.1002/rmv.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi Y., Hwang J.H., Lee S.Y. Recent trends in nanomaterials-based colorimetric detection of pathogenic bacteria and viruses. Small Methods. 2018;2:1700351. doi: 10.1002/smtd.201700351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morbioli G.G., Mazzu-Nascimento T., Stockton A.M., Carrilho E. Technical aspects and challenges of colorimetric detection with microfluidic paper-based analytical devices (μPADs)-A review. Anal. Chim. Acta. 2017;970:1–22. doi: 10.1016/j.aca.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 10.Coarsey C., Coleman B., Kabir M.A., Sher M., Asghar W. Development of a flow-free magnetic actuation platform for an automated microfluidic ELISA. RSC Adv. 2019;9:8159–8168. doi: 10.1039/c8ra07607c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali Z., Wang J., Tang Y., Liu B., He N., Li Z. Simultaneous detection of multiple viruses based on chemiluminescence and magnetic separation. Biomater. Sci. 2017;5:57–66. doi: 10.1039/c6bm00527f. [DOI] [PubMed] [Google Scholar]
- 12.Gong X., Cai J., Zhang B., Zhao Q., Piao J., Peng W., et al. A review of fluorescent signal-based lateral flow immunochromatographic strips. J. Mater. Chem. B. 2017;5:5079–5091. doi: 10.1039/c7tb01049d. [DOI] [PubMed] [Google Scholar]
- 13.Rong Z., Wang Q., Sun N., Jia X., Wang K., Xiao R., et al. Smartphone-based fluorescent lateral flow immunoassay platform for highly sensitive point-of-care detection of Zika virus nonstructural protein 1. Anal. Chim. Acta. 2019;1055:140–147. doi: 10.1016/j.aca.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z., Cong Y., Huang Y., Du X. Nanomaterials-based electrochemical immunosensors. Micromachines. 2019;10:397. doi: 10.3390/mi10060397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piro B., Reisberg S. Recent advances in electrochemical immunosensors. Sensors. 2017;17:794. doi: 10.3390/s17040794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clifford A., Das J., Yousefi H., Mahmud A., Chen J.B., Kelley S.O. Strategies for biomolecular analysis and continuous physiological monitoring. J. Am. Chem. Soc. 2021;143:5281–5294. doi: 10.1021/jacs.0c13138. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed M.U., Hossain M.M., Safavieh M., Wong Y.L., Rahman I.A., Zourob M., et al. Toward the development of smart and low cost point-of-care biosensors based on screen printed electrodes. Crit. Rev. Biotechnol. 2016;36:495–505. doi: 10.3109/07388551.2014.992387. [DOI] [PubMed] [Google Scholar]
- 18.Mincu N.-B., Lazar V., Stan D., Mihailescu C.M., Iosub R., Mateescu A.L. Screen-printed electrodes (SPE) for in vitro diagnostic purpose. Diagnostics. 2020;10:517. doi: 10.3390/diagnostics10080517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yáñez-Sedeño P., Campuzano S., Pingarrón J.M. Screen-printed electrodes: promising paper and wearable transducers for (Bio)Sensing. Biosensors. 2020;10:76. doi: 10.3390/bios10070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J., Jeerapan I., Imani S., Cho T.N., Bandodkar A., Cinti S., et al. Noninvasive alcohol monitoring using a wearable tattoo-based iontophoretic-biosensing system. ACS Sens. 2016;1:1011–1019. [Google Scholar]
- 21.Hallam P.M., Kampouris D.K., Kadara R.O., Banks C.E. Graphite screen printed electrodes for the electrochemical sensing of chromium (VI) Analyst. 2010;135:1947–1952. doi: 10.1039/c0an00228c. [DOI] [PubMed] [Google Scholar]
- 22.Arduini F., Cinti S., Scognamiglio V., Moscone D. vol. 77. 2017. (Paper-based Electrochemical Devices in Biomedical Field: Recent Advances and Perspectives). ISSN 0166-526X. [Google Scholar]
- 23.Cinti S. Polymeric materials for printed-based electroanalytical (Bio) applications. Chemosensors. 2017;5:31. [Google Scholar]
- 24.Pirzada M., Altintas Z. Nanomaterials for healthcare biosensing applications. Sensors. 2019;19:5311. doi: 10.3390/s19235311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Draz M.S., Shafiee H. Applications of gold nanoparticles in virus detection. Theranostics. 2018;8:1985. doi: 10.7150/thno.23856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Z., Cui H., Song W., Ru X., Zhou W., Yu X. A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. BioRxiv. 2020:1–18. doi: 10.1101/2020.02.22.961268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakhalian D., Shultz R.B., Miles C.E., Kohn J. Opportunities for biomaterials to address the challenges of COVID-19. J. Biomed. Mater. Res. 2020;108:1974–1990. doi: 10.1002/jbm.a.37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehtesabi H. Application of carbon nanomaterials in human virus detection. J. Sci.: Adv. Mater. Dev. 2020;5(4):436–450. ISSN 2468-2179. [Google Scholar]
- 29.Power A.C., Gorey B., Chandra S., Chapman J. Carbon nanomaterials and their application to electrochemical sensors: a review. Nanotechnol. Rev. 2018;7:19–41. [Google Scholar]
- 30.Crapnell R.D., Hudson A., Foster C.W., Eersels K., Grinsven B.v., Cleij T.J., et al. Recent advances in electrosynthesized molecularly imprinted polymer sensing platforms for bioanalyte detection. Sensors. 2019;19:1204. doi: 10.3390/s19051204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkash O., Yean C.Y., Shueb R.H. Screen printed carbon electrode based electrochemical immunosensor for the detection of dengue NS1 antigen. Diagnostics. 2014;4:165–180. doi: 10.3390/diagnostics4040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez-Paredes G., González-García M.B., Costa-García A. Genosensor for SARS virus detection based on gold nanostructured screen-printed carbon electrodes. Electroanalysis: Int. J. Devoted Fund. Pract. Aspects Electroanalysis. 2009;21:379–385. [Google Scholar]
- 33.Valizadeh A., Sohrabi N., Badrzadeh F. Electrochemical detection of HIV-1 by nanomaterials. Artificial Cells Nanomed. Biotechnol. 2017;45:1467–1477. doi: 10.1080/21691401.2017.1282494. [DOI] [PubMed] [Google Scholar]
- 34.Shafiee H., Kanakasabapathy M.K., Juillard F., Keser M., Sadasivam M., Yuksekkaya M., et al. Printed flexible plastic microchip for viral load measurement through quantitative detection of viruses in plasma and saliva. Sci. Rep. 2015;5:1–9. doi: 10.1038/srep09919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uliana C.V., Riccardi C.S., Yamanaka H. Diagnostic tests for hepatitis C: recent trends in electrochemical immunosensor and genosensor analysis. World J. Gastroenterol.: WJG. 2014;20:15476. doi: 10.3748/wjg.v20.i42.15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei S., Xiao H., Cao L., Chen Z. A label-free immunosensor based on graphene oxide/Fe3O4/Prussian blue nanocomposites for the electrochemical determination of HBsAg. Biosensors. 2020;10:24. doi: 10.3390/bios10030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Draz M.S., Venkataramani M., Lakshminarayanan H., Saygili E., Moazeni M., Vasan A., et al. Nanoparticle-enhanced electrical detection of Zika virus on paper microchips. Nanoscale. 2018;10:11841–11849. doi: 10.1039/c8nr01646a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dawany N. 2010. Large-scale Integration of Microarray Data: Investigating the Pathologies of Cancer and Infectious Diseases. [Google Scholar]
- 39.Wang Q., Michailidis E., Yu Y., Wang Z., Hurley A.M., Oren D.A., et al. Public broadly neutralizing antibodies against hepatitis B virus in individuals with elite serologic activity. bioRxiv. 2020:1–36. doi: 10.1101/2020.03.04.976159. [DOI] [Google Scholar]
- 40.Oviya S., Kaviya S., Udhaya S. Dengue fever: causes, complications, and vaccine strategies–A review. GSC Biol. Pharmaceut. Sci. 2019;6 [Google Scholar]
- 41.Rombi F., Bayliss R., Tuplin A., Yeoh S. The journey of Zika to the developing brain. Mol. Biol. Rep. 2020;47:3097–3115. doi: 10.1007/s11033-020-05349-y. [DOI] [PubMed] [Google Scholar]
- 42.Hepatitis B Virus – Part 6 – Hepatitis B Virus (Hepatotropic virus), HBV Complete Picture. https://www.labpedia.net/lecture-on-hepatitis-b-virus/ Available:
- 43.Parvez M.K., Jagirdar R.M., Purty R.S., Venkata S.K., Agrawal V., Kumar J., et al. COVID-19 pandemic: understanding the emergence, pathogenesis and containment. World Acad. Sci. J. 2020;2:1. [Google Scholar]
- 44.Cinti S., Mazzaracchio V., Cacciotti I., Moscone D., Arduini F. Carbon black-modified electrodes screen-printed onto paper towel, waxed paper and parafilm M®. Sensors. 2017;17:2267. doi: 10.3390/s17102267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J., Campbell A.S., de Ávila B.E.-F., Wang J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019;37:389–406. doi: 10.1038/s41587-019-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cinti S., Arduini F., Moscone D., Palleschi G., Killard A.J. Development of a hydrogen peroxide sensor based on screen-printed electrodes modified with inkjet-printed Prussian blue nanoparticles. Sensors. 2014;14:14222–14234. doi: 10.3390/s140814222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guimarães J.A., Ferraz H.C., Alves T.L.M. Langmuir–Blodgett films of cholesterol oxidase and S-layer proteins onto screen-printed electrodes. Appl. Surf. Sci. 2014;298:68–74. [Google Scholar]
- 48.Ping J., Wang Y., Fan K., Wu J., Ying Y. Direct electrochemical reduction of graphene oxide on ionic liquid doped screen-printed electrode and its electrochemical biosensing application. Biosens. Bioelectron. 2011;28:204–209. doi: 10.1016/j.bios.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 49.Cinti S. Novel paper-based electroanalytical tools for food surveillance. Anal. Bioanal. Chem. 2019;411:4303–4311. doi: 10.1007/s00216-019-01640-5. [DOI] [PubMed] [Google Scholar]
- 50.Tomei M.R., Cinti S., Interino N., Manovella V., Moscone D., Arduini F. Based electroanalytical strip for user-friendly blood glutathione detection. Sensor. Actuator. B Chem. 2019;294:291–297. [Google Scholar]
- 51.Moccia M., Caratelli V., Cinti S., Pede B., Avitabile C., Saviano M., et al. Based electrochemical peptide nucleic acid (PNA) biosensor for detection of miRNA-492: a pancreatic ductal adenocarcinoma biomarker. Biosens. Bioelectron. 2020;165:112371. doi: 10.1016/j.bios.2020.112371. [DOI] [PubMed] [Google Scholar]
- 52.Sher M., Asghar W. Development of a multiplex fully automated assay for rapid quantification of CD4+ T cells from whole blood. Biosens. Bioelectron. 2019;142:111490. doi: 10.1016/j.bios.2019.111490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J. Nanomaterial-based electrochemical biosensors. Analyst. 2005;130:421–426. doi: 10.1039/b414248a. [DOI] [PubMed] [Google Scholar]
- 54.Chen A., Chatterjee S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013;42:5425–5438. doi: 10.1039/c3cs35518g. [DOI] [PubMed] [Google Scholar]
- 55.Cinti S., Arduini F. Graphene-based screen-printed electrochemical (bio) sensors and their applications: efforts and criticisms. Biosens. Bioelectron. 2017;89:107–122. doi: 10.1016/j.bios.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Arduini F., Cinti S., Scognamiglio V., Moscone D., Palleschi G. How cutting-edge technologies impact the design of electrochemical (bio) sensors for environmental analysis. A review. Anal. Chim. Acta. 2017;959:15–42. doi: 10.1016/j.aca.2016.12.035. [DOI] [PubMed] [Google Scholar]
- 57.Su W.-Y., Cheng S.-H. Electrocatalysis and sensitive determination of cysteine at poly (3, 4-ethylenedioxythiophene)-modified screen-printed electrodes. Electrochem. Commun. 2008;10:899–902. [Google Scholar]
- 58.Hyun W.J., Lim S., Ahn B.Y., Lewis J.A., Frisbie C.D., Francis L.F. Screen printing of highly loaded silver inks on plastic substrates using silicon stencils. ACS Appl. Mater. Interf. 2015;7:12619–12624. doi: 10.1021/acsami.5b02487. [DOI] [PubMed] [Google Scholar]
- 59.Metters J.P., Kadara R.O., Banks C.E. New directions in screen printed electroanalytical sensors: an overview of recent developments. Analyst. 2011;136:1067–1076. doi: 10.1039/c0an00894j. [DOI] [PubMed] [Google Scholar]
- 60.Miserere S., Ledru S., Ruillé N., Griveau S., Boujtita M., Bedioui F. Biocompatible carbon-based screen-printed electrodes for the electrochemical detection of nitric oxide. Electrochem. Commun. 2006;8:238–244. [Google Scholar]
- 61.Cumba L.R., Camisasca A., Giordani S., Forster R.J. Electrochemical properties of screen-printed carbon nano-onion electrodes. Molecules. 2020;25:3884. doi: 10.3390/molecules25173884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bettazzi F., Ingrosso C., Sfragano P.S., Pifferi V., Falciola L., Curri M.L., et al. Gold nanoparticles modified graphene platforms for highly sensitive electrochemical detection of vitamin C in infant food and formulae. Food Chem. 2021;344:128692. doi: 10.1016/j.foodchem.2020.128692. [DOI] [PubMed] [Google Scholar]
- 63.Ingrosso C., Corricelli M., Disha A., Fanizza E., Bianco G., Depalo N., et al. Solvent dispersible nanocomposite based on Reduced Graphene Oxide and in-situ decorated gold nanoparticles. Carbon. 2019;152:777–787. [Google Scholar]
- 64.Ingrosso C., Corricelli M., Bettazzi F., Konstantinidou E., Bianco G.V., Depalo N., et al. Au nanoparticle in situ decorated RGO nanocomposites for highly sensitive electrochemical genosensors. J. Mater. Chem. B. 2019;7:768–777. doi: 10.1039/c8tb02514b. [DOI] [PubMed] [Google Scholar]
- 65.Bettazzi F., Laschi S., Voccia D., Gellini C., Pietraperzia G., Falciola L., et al. Ascorbic acid-sensitized Au nanorods-functionalized nanostructured TiO2 transparent electrodes for photoelectrochemical genosensing. Electrochim. Acta. 2018;276:389–398. [Google Scholar]
- 66.Sfragano P.S., Laschi S., Palchetti I. Sustainable printed electrochemical platforms for greener analytics. Front. Chem. 2020;8:644. doi: 10.3389/fchem.2020.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sher M., Coleman B., Caputi M., Asghar W. Development of a point-of-care assay for HIV-1 viral load using higher refractive index antibody-coated microbeads. Sensors. 2021;21:1819. doi: 10.3390/s21051819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.90-90-90: Treatment for all. Available online: https://www.unaids.org/en/resources/909090 (accessed on 27 June 2021).
- 69.Levy J.A., Hoffman A.D., Kramer S.M., Landis J.A., Shimabukuro J.M., Oshiro L.S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984;225:840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- 70.Manoto S.L., Lugongolo M., Govender U., Mthunzi-Kufa P. Point of care diagnostics for HIV in resource limited settings: an overview. Medicina. 2018;54(3) doi: 10.3390/medicina54010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giannetto M., Costantini M., Mattarozzi M., Careri M. Innovative gold-free carbon nanotube/chitosan-based competitive immunosensor for determination of HIV-related p24 capsid protein in serum. RSC Adv. 2017;7:39970–39976. [Google Scholar]
- 72.Rogers M.F., Fowler M.G., Lindegren M.L. Revised recommendations for HIV screening of pregnant women. MMWR Recomm. Rep. (Morb. Mortal. Wkly. Rep.) 2001:59–85. [PubMed] [Google Scholar]
- 73.Zhao C., Liu X. A portable paper-based microfluidic platform for multiplexed electrochemical detection of human immunodeficiency virus and hepatitis C virus antibodies in serum. Biomicrofluidics. 2016;10 doi: 10.1063/1.4945311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sailapu S.K., Macchia E., Merino-Jimenez I., Esquivel J.P., Sarcina L., Scamarcio G., et al. Standalone operation of an EGOFET for ultra-sensitive detection of HIV. Biosens. Bioelectron. 2020;156:112103. doi: 10.1016/j.bios.2020.112103. [DOI] [PubMed] [Google Scholar]
- 75.Rouet F., Ekouevi D.K., Chaix M.-L., Burgard M., Inwoley A., Tony T.D.A., et al. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a West African resource-limited setting. J. Clin. Microbiol. 2005;43:2709. doi: 10.1128/JCM.43.6.2709-2717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pope M., Haase A.T. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat. Med. 2003;9:847–852. doi: 10.1038/nm0703-847. [DOI] [PubMed] [Google Scholar]
- 77.Cohen M.S., Shaw G.M., McMichael A.J., Haynes B.F. Acute HIV-1 infection. N. Engl. J. Med. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Daar E.S., Moudgil T., Meyer R.D., Ho D.D. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N. Engl. J. Med. 1991;324:961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- 79.Shafiee H., Jahangir M., Inci F., Wang S., Willenbrecht R.B., Giguel F.F., et al. Acute on-chip hiv detection through label-free electrical sensing of viral nano-lysate. Small. 2013;9:2553–2563. doi: 10.1002/smll.201202195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cinti S., Proietti E., Casotto F., Moscone D., Arduini F. Based strips for the electrochemical detection of single and double stranded DNA. Anal. Chem. 2018;90:13680–13686. doi: 10.1021/acs.analchem.8b04052. [DOI] [PubMed] [Google Scholar]
- 81.Sher M., Zhuang R., Demirci U., Asghar W. Based analytical devices for clinical diagnosis: recent advances in the fabrication techniques and sensing mechanisms. Expert Rev. Mol. Diagn. 2017;17:351–366. doi: 10.1080/14737159.2017.1285228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Griffin J., Singh A.K., Senapati D., Lee E., Gaylor K., Jones-Boone J., et al. Sequence-specific HCV RNA quantification using the size-dependent nonlinear optical properties of gold nanoparticles. Small. 2009;5:839–845. doi: 10.1002/smll.200801334. [DOI] [PubMed] [Google Scholar]
- 83.Bukh J. The history of hepatitis C virus (HCV): basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control. J. Hepatol. 2016;65:S2–S21. doi: 10.1016/j.jhep.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 84.Barr H. Detection challenges in clinical diagnostics. R. Soc. Chem. 2013;2 [Google Scholar]
- 85.Srisomwat C., Teengam P., Chuaypen N., Tangkijvanich P., Vilaivan T., Chailapakul O. Pop-up paper electrochemical device for label-free hepatitis B virus DNA detection. Sensor. Actuator. B Chem. 2020;316:128077. [Google Scholar]
- 86.Aronoff-Spencer E., Venkatesh A., Sun A., Brickner H., Looney D., Hall D.A. Detection of Hepatitis C core antibody by dual-affinity yeast chimera and smartphone-based electrochemical sensing. Biosens. Bioelectron. 2016;86:690–696. doi: 10.1016/j.bios.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 87.Valipour A., Roushani M. TiO 2 nanoparticles doped with Celestine Blue as a label in a sandwich immunoassay for the hepatitis C virus core antigen using a screen printed electrode. Microchimica Acta. 2017;184:2015–2022. [Google Scholar]
- 88.Venkatesh A., Brickner H., Looney D., Hall D., Aronoff-Spencer E. Clinical detection of Hepatitis C viral infection by yeast-secreted HCV-core: gold-binding-peptide. Biosens. Bioelectron. 2018;119:230–236. doi: 10.1016/j.bios.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 89.Giamblanco N., Conoci S., Russo D., Marletta G. Single-step label-free hepatitis B virus detection by a piezoelectric biosensor. RSC Adv. 2015;5:38152–38158. [Google Scholar]
- 90.Li X., Scida K., Crooks R.M. Detection of hepatitis B virus DNA with a paper electrochemical sensor. Anal. Chem. 2015;87:9009–9015. doi: 10.1021/acs.analchem.5b02210. [DOI] [PubMed] [Google Scholar]
- 91.Teengam P., Siangproh W., Tontisirin S., Jiraseree-amornkun A., Chuaypen N., Tangkijvanich P., et al. NFC-enabling smartphone-based portable amperometric immunosensor for hepatitis B virus detection. Sensor. Actuator. B Chem. 2021;326:128825. [Google Scholar]
- 92.Kabir M.A., Zilouchian H., Sher M., Asghar W. Development of a flow-free automated colorimetric detection assay integrated with smartphone for Zika NS1. Diagnostics. 2020;10:42. doi: 10.3390/diagnostics10010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Campuzano S., Yáñez-Sedeño P., Pingarrón J.M. Electrochemical genosensing of circulating biomarkers. Sensors. 2017;17:866. doi: 10.3390/s17040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Labuda J., Brett A.M.O., Evtugyn G., Fojta M., Mascini M., Ozsoz M., et al. Electrochemical nucleic acid-based biosensors: concepts, terms, and methodology (IUPAC Technical Report) Pure Appl. Chem. 2010;82:1161–1187. [Google Scholar]
- 95.Alzate D., Cajigas S., Robledo S., Muskus C., Orozco J. Genosensors for differential detection of Zika virus. Talanta. 2020;210:120648. doi: 10.1016/j.talanta.2019.120648. [DOI] [PubMed] [Google Scholar]
- 96.Faria A.M., Mazon T. Early diagnosis of Zika infection using a ZnO nanostructures-based rapid electrochemical biosensor. Talanta. 2019;203:153–160. doi: 10.1016/j.talanta.2019.04.080. [DOI] [PubMed] [Google Scholar]
- 97.Cabral-Miranda G., Cardoso A.R., Ferreira L.C., Sales M.G.F., Bachmann M.F. Biosensor-based selective detection of Zika virus specific antibodies in infected individuals. Biosens. Bioelectron. 2018;113:101–107. doi: 10.1016/j.bios.2018.04.058. [DOI] [PubMed] [Google Scholar]
- 98.Dhal A., Kalyani T., Ghorai S., Sahu N.K., Jana S.K. Recent development of electrochemical immunosensor for the diagnosis of dengue virus NSI protein: a review. Sensors Int. 2020:100030. [Google Scholar]
- 99.Abd Rashid J.I., Yusof N.A., Abdullah J., Hashim U., Hajian R. A novel disposable biosensor based on SiNWs/AuNPs modified-screen printed electrode for dengue virus DNA oligomer detection. IEEE Sensor. J. 2015;15:4420–4427. [Google Scholar]
- 100.Nawaz M.H., Hayat A., Catanante G., Latif U., Marty J.L. Development of a portable and disposable NS1 based electrochemical immunosensor for early diagnosis of dengue virus. Anal. Chim. Acta. 2018;1026:1–7. doi: 10.1016/j.aca.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 101.Siew Q.Y., Tan S.H., Pang E.L., Loh H.-S., Tan M.T. A graphene-based dengue immunosensor using plant-derived envelope glycoprotein domain III (EDIII) as the novel probe antigen. Analyst. 2021;146:2009–2018. doi: 10.1039/d0an02219e. [DOI] [PubMed] [Google Scholar]
- 102.Fabiani L., Saroglia M., Galatà G., De Santis R., Fillo S., Luca V., et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: a reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021;171:112686. doi: 10.1016/j.bios.2020.112686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2021;54(2):159–163. doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Giri B., Pandey S., Shrestha R., Pokharel K., Ligler F.S., Neupane B.B. Review of analytical performance of COVID-19 detection methods. Anal. Bioanal. Chem. 2020:1–14. doi: 10.1007/s00216-020-02889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li X., Qin Z., Fu H., Li T., Peng R., Li Z., et al. Enhancing the performance of paper-based electrochemical impedance spectroscopy nanobiosensors: an experimental approach. Biosens. Bioelectron. 2021;177:112672. doi: 10.1016/j.bios.2020.112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mahari S., Roberts A., Shahdeo D., Gandhi S. eCovSens-ultrasensitive novel in-house built printed circuit board based electrochemical device for rapid detection of nCovid-19 antigen, a spike protein domain 1 of SARS-CoV-2. BioRxiv. 2020:1–20. doi: 10.1101/2020.04.24.059204. [DOI] [Google Scholar]
- 107.Kaushik A.K., Dhau J.S., Gohel H., Mishra Y.K., Kateb B., Kim N.-Y., et al. Electrochemical SARS-CoV-2 sensing at point-of-care and artificial intelligence for intelligent COVID-19 management. ACS Appl. Bio Mater. 2020;3:7306–7325. doi: 10.1021/acsabm.0c01004. [DOI] [PubMed] [Google Scholar]
- 108.Tortorella S., Cinti S. ACS Publications; 2021. How Can Chemometrics Support the Development of Point of Need Devices? [DOI] [PubMed] [Google Scholar]
- 109.Paulovich F.V., De Oliveira M.C.F., Oliveira O.N., Jr. A future with ubiquitous sensing and intelligent systems. ACS Sens. 2018;3:1433–1438. doi: 10.1021/acssensors.8b00276. [DOI] [PubMed] [Google Scholar]
- 110.Cui F., Yue Y., Zhang Y., Zhang Z., Zhou H.S. Advancing biosensors with machine learning. ACS Sens. 2020;5:3346–3364. doi: 10.1021/acssensors.0c01424. [DOI] [PubMed] [Google Scholar]
- 111.Fennell R.D., Sher M., Asghar W. Design, development, and performance comparison of wide field lensless and lens-based optical systems for point-of-care biological applications. Opt Laser. Eng. 2021;137:106326. doi: 10.1016/j.optlaseng.2020.106326. [DOI] [PMC free article] [PubMed] [Google Scholar]