Abstract
Background
Since information on the pathology of COVID-19 from sub-Saharan Africa (SSA) remains scarce, the objective of our study was to define the gross pathology and histological features of COVID-19. We report data from 29 whole-body autopsies of COVID-19 deaths occurring in hospitals in Lusaka, Zambia - the first large autopsy case series from Africa.
Methods
We performed a descriptive post-mortem examination study of inpatient COVID-19 related deaths at two hospitals in Lusaka, Zambia. Whole-body autopsies were conducted according to Standard Operating Procedures. Gross and histopathological examinations of all organs were performed. Patient demographics, history, co-morbidities, autopsy gross and microscopic findings, and cause(s) of death were recorded and analyzed using STATA version 14. Variables were grouped and presented as frequencies and percentages.
Findings
Autopsies were performed on 29 decedents (mean age = 44 ± 15.8years; age range = 19-82; 17/29 [58.8%] males). 22/29 [75.9%] cases were <55 years of age. A spectrum of pathological manifestations of COVID-19 were seen in all organs. The commonest causes of death were pulmonary thromboembolism (13/29, 45%), Diffuse Alveolar Damage (9/29, 31%), and COVID-19 pneumonia (7/29, 25%). 22/29 (76%) had co-morbidities. Common co-morbidities included HIV (8/29, 28%), Hypertension (6/29, 20%) Tuberculosis (3/29, 10%), Diabetes (3/29, 10%).
Conclusions
A spectrum of gross anatomical and histopathological findings are seen in COVID-19 deaths in hospitalized decedents. These appear broadly similar to those reported from China, Europe and USA. Differences include a younger age group, and co-morbidities of HIV and TB co-infection which require further investigation.
Keywords: SARS-CoV-2, COVID-19, Zambia, Africa, Autopsy, hospital deaths, pathology, post-mortem
Introduction
The SARS-CoV-2 pandemic has caused 171,292,827 confirmed COVID-19 cases with 3,687,589 deaths globally as of June 4th, 2021. Of these 3,530,845 cases occurred in sub-Saharan Africa (SSA) with 131,630 deaths (WHO Coronavirus dashboard, 2021; Africa CDC - Coronavirus Disease 2019 (COVID-19), 2021). Zambia reported its first COVID-19 case in March 2020 and has since recorded 96, 563 cases with 1,284 deaths as of June 4th, 202 (World Health Organisation, 2021). Eighteen months after the first COVID-19 cases were reported from Wuhan, China, several major knowledge gaps on COVID-19 pathology and pathogenesis remain, particularly in SSA. The case-fatality ratio (CFR) for COVID-19 in Africa appears lower than the Case Fatality Rate in western countries, indicating that COVID-19 may manifest less severe disease and that the pathogenesis of COVID-19 in SSA may be different (Lawal, 2021, Bamgboye et al., 2020).
An area of continuing neglect and missed opportunities for COVID-19 research in SSA is that of accurately defining the pathological features of COVID-19 and ascertaining the underlying cause(s) of death from COVID-19 in hospitals (Bamgboye et al., 2020, Mawalla, 2020, Mucheleng’anga and Himwaze, 2020). Whilst several reports of whole body autopsy case series and organ specific pathology have been published from China, Europe and USA (Maiese et al., 2020), there is little information on post-mortem examination and the pathology of COVID-19 in SSA. There remains scanty information on the actual causes of death and pathogenesis of COVID-19 from the African continent. There are only two individual case reports of whole body autopsies and lung biopsy studies of four South African ICU patients obtained after death (Mulale et al., 2021, Khaba et al., 2020, Bruce-Brand et al., 2020). Several reports have emphasized the need for rekindling and revamping autopsy and post-mortem studies in SSA (Mawalla, 2020, Mucheleng’anga and Himwaze, 2020, Maiese et al., 2020, Mulale et al., 2021, Khaba et al., 2020, Bruce-Brand et al., 2020, Mudenda et al., 2020), to determine the underlying pathology and causes of COVID-19 deaths occurring in hospital inpatients.
Whole-body autopsies in general, and on confirmed or suspected SARS-CoV-2 cases in Africa have not been forthcoming due to scarcity of pathologists, inadequate infrastructure, and difficulties in obtaining consent for autopsies (Mawalla, 2020, Mucheleng’anga and Himwaze, 2020, Khaba et al., 2020, Rampatige and Gilks, 2015). In addition, performing autopsies in environments where infectious pathogens such as SARS-CoV-2 are suspected or confirmed, requires optimal biosafety facilities (World Health Organization, 2020, Baj et al., 2021, Liang et al., 2020), which are unavailable in most African countries.
The objective of our case series study was to present the histopathological findings following whole-body autopsies in COVID-19 inpatients. We present post-mortem examination data from 29 whole-body autopsies of COVID-19 deaths occurring in hospitals, in Lusaka, Zambia - the first large autopsy case series study from Africa.
Methods
Study design and sites
We conducted a retrospective descriptive study of reports of 29 whole body autopsies we had perfomed of COVID-19 inpatient deaths whose proximate cause of death was SARS-CoV-2 infection (positive SARS-CoV-2 reverse transcriptase-polymerase chain reaction (RT-PCR). The hospital deaths occurred between March 2020 and March 2021 in the COVID-19 isolation wards at the University Teaching Hospital (UTH) and Levy Mwanawasa University Teaching Hospital (LMUTH), Lusaka, Zambia. Selection for autopsy was based according to request by physician
Ethics permission
Informed consent from the next-of-kin was obtained to conduct the autopsy and publish the findings. The Office of State Forensic Pathology (OSFP) granted permission to access postmortem data.
Pre-autopsy procedures
All staff involved underwent safety training procedures. Clinical case notes were reviewed prior to autopsy. The two autopsy pathologists had rehearsed on how they were to work together, classify and define the autopsy findings. Placement and handling of equipment was also planned. The manner in which the pathologists’ assistant would operate was also discussed and practiced.
The mortuary and autopsy rooms used to perform the autopsy are had a working refrigeration system and good lighting and water reticulation system, but have no negative pressure ventilation. The bodies were well preserved as the refrigeration was in working condition. The bodies were placed in the mortuary two to three hours after death. The timing of autopsy was between 24 to 72 hours after death. The body was washed using chlorine before autopsy.
Autopsy procedures
Whole body autopsies were performed in accordance with the Practice Manual for Medicolegal Death Investigations. The practice manual for medicolegal death investigation is used in Zambia as a local guideline for conducting forensic postmortem examinations. It covers processes to follow for autopsies on bodies infected with category three and four infectious organisms including SARS-CoV-2. In all cases, we followed universal precautions using personal protective equipment (PPE) (hair caps, eye protection, goggles), long sleeved, non-waterproof gown, covered by a water-proof apron. Double pair of standard disposable surgical latex gloves were used. Cut resistant gloves were also used. We used guidance in place with regards to dressing and undressing of PPE when dealing with infected bodies as in the practice manual for medicolegal death investigations.
Tissues sampled and histological examination
Representative samples were obtained from the various organs (Brain, Lungs, Heart, Liver, Spleen, Kidneys) and other tissues as required and submitted in standard tissue cassettes and fixed in 10% neutral buffered formalin for 72 hours. The samples were processed, embedded in paraffin, sectioned, mounted onto glass slides, and stained using hematoxylin and eosin (H&E) staining according to the Standard Operating Procedure Manual at the UTH histopathology Laboratory. All slides were examined together by the two forensic and anatomical pathologists.
The cause of death was formulated within the context of the history, clinical findings, circumstances of death, autopsy findings, and ancillary studies.
Data collection and analyses
Data on the decedents’ demographics, history, circumstances, autopsy findings, and opinion of the cause of death was entered in excel and analyzed using STATA version 14. The variables were grouped and presented as frequencies and percentages.
Results
Decedent demographics
Demographics of the autopsied cases are given in Table 1 .
Table 1.
Demographic characteristics of COVID-19 hospital decedents autopsied.
| Characteristic/Variable | Hospital Decedents autopsied |
|---|---|
| Gender | N (%) |
| Male | 17 (58.8) |
| Female | 12 (41.4) |
| Total No. decedents autopsied | 29 |
| Mean Age (years) | 44 |
| Age range | 19-82 |
| Age group | |
| 15-25 | 2 (6.9) |
| 26-35 | 5 (17.2) |
| 36-45 | 9 (31.0) |
| 46-55 | 6 (20.7) |
| 56-65 | 4 (13.8) |
| 66-75 | 2 (6.9) |
| 76-85 | 1 (3.5) |
Autopsies were performed on 29 decedents (mean age = 44 ± 15.8years; age range = 19-82; 17/29 [58.8%] males).
Symptoms and Co-morbidities
The symptoms and comorbidities of the 29 decedents are given in Table 2 . Common symptoms observed were; difficulty breathing (n = 24/29, 83%), cough (n = 9/29, 31%), fever (n = 11/29, 38%), headache (n = 6/29, 21%), general body weakness (n = 4/29, 14%).
Table 2.
Symptoms and Comorbidities of 29 decedents autopsied.
| SYMPTOM(S) AT ADMISSION | Number (%) |
|---|---|
| Difficulty breathing | 24(82.8) |
| Cough | 9(31.0) |
| Fever | 11(37.9) |
| Headache | 6(20.7) |
| General Body Weakness | 4(13.8) |
| Vomiting | 2(6.9) |
| Diarrhea | 2(6.9) |
| Oral Thrush | 1(100) |
| Abdominal Pain | 1(3.4) |
| CO-MORBIDITIES AT ADMISSION | |
| HIV positive | 8(27.6) |
| Hypertension | 6(20.7) |
| Tuberculosis | 3(10.3) |
| Diabetes | 3(10.3) |
| Pregnancy | 2(6.9) |
| Cerebral Vascular Accident | 1(3.4) |
| Malaria | 1(3.4) |
| Leprosy | 1(3.4) |
| Atherosclerotic heart disease | 1(3.4) |
| Paraquat toxicity | 1(3.4) |
| Sickle Cell Anaemia | 1(3.4) |
Overall, 22/29 (76%) decedents had comorbidities. Co-morbidities were present in 22/29 (76%) of decedents. Common co-morbities included HIV (8/29, 27%), Hypertension (6/29, 21%) Tuberculosis (3/29, 10%), Diabetes (3/29, 10%).
Autopsy findings
The main gross pathology and microscopic autopsy findings, and cause(s) of death, are given in Table 3 .
Table 3.
Main autopsy findings and causes of death.
| Autopsy findings | Number (%) |
|---|---|
| Diffuse Alveolar Damage | 12/29 (41.4) |
| Emboli | 10/29 (34.5) |
| Saddle Emboli | 6/29 (20.7) |
| Shower Emboli | 4/29 (13.8) |
| Pneumonia | 9/29 (31) |
| Granular kidneys | 5/29 (17.2) |
| Deep Venous Thrombosis | 3/29 (10.3) |
| Disseminated Thrombi | 4/29 (13.8) |
| Tuberculosis | 1/29 (3.4) |
| Anaemia | 1/29 (3.4) |
| Kaposi Sarcoma | 1/29 (3.4) |
| Colorectal adenocarcinoma | 1/29 (3.4) |
| Main Autopsy Causes of Death | |
| Pulmonary Thromboembolism | 13/29 (44.8) |
| Diffuse Alveolar Damage | 9/29 (31.0) |
| Pneumonia | 7/29 (24.2) |
The commonest causes of death were pulmonary thromboembolism (13/29, 45%), Diffuse Alveolar Damage (DAD) (9/29, 31%), and COVID-19 pneumonia (7/29, 25%).
Gross pathology and Microscopic Findings
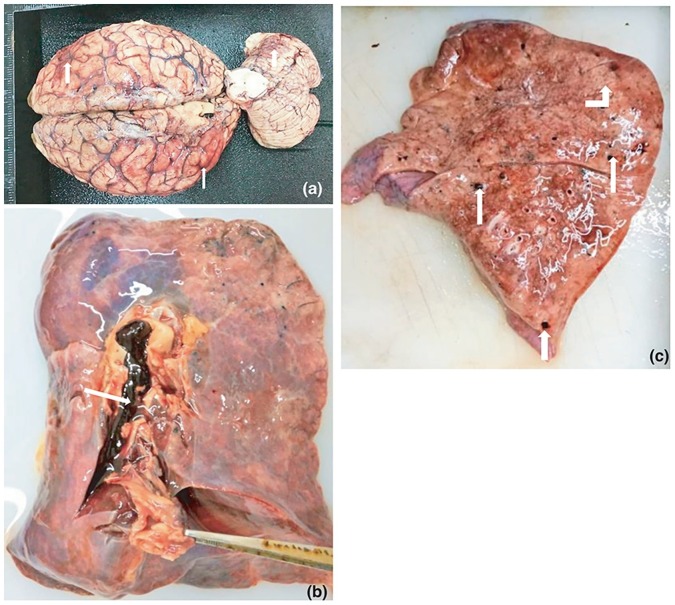
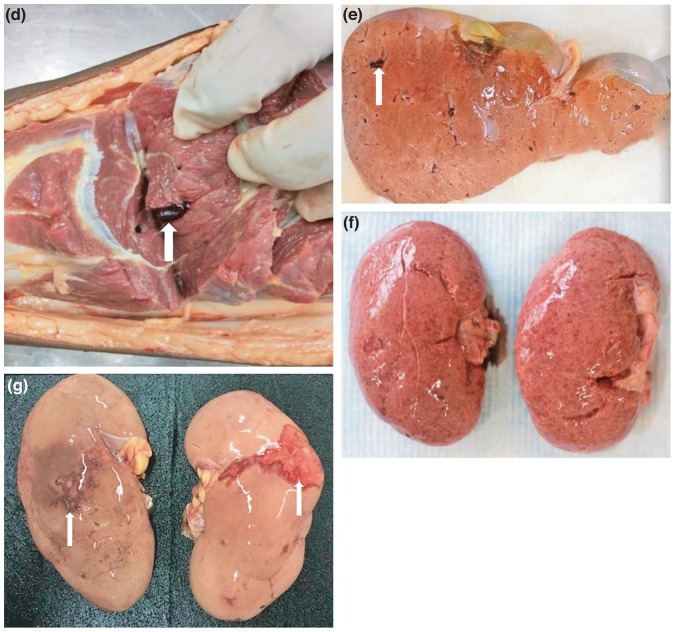
A selection of images representative gross and microscopic autopsy findings are given. Figure 1 (Figures 1A to G) show gross pathology findings.
Figure 1.
A: Brain: Gross appearance showing hyperaemia (white arrow heads).
B: Lung: Pulmonary thrombus in the pulmonary artery (white arrow head).
C: Lung: Shower thrombo-emboli and grey hepatisation (white arrow head and bent white arrow up).
D: Leg: Deep venous thrombosis (white arrow head).
E: Liver: Thromboemboli in the liver in a case with disseminated thrombosis (white arrow head).
F: Kidney: Granular surfaces of the kidneys in a case with hypertension.
G: Kidney: Infarction due to disseminated thrombosis (white arrow heads).
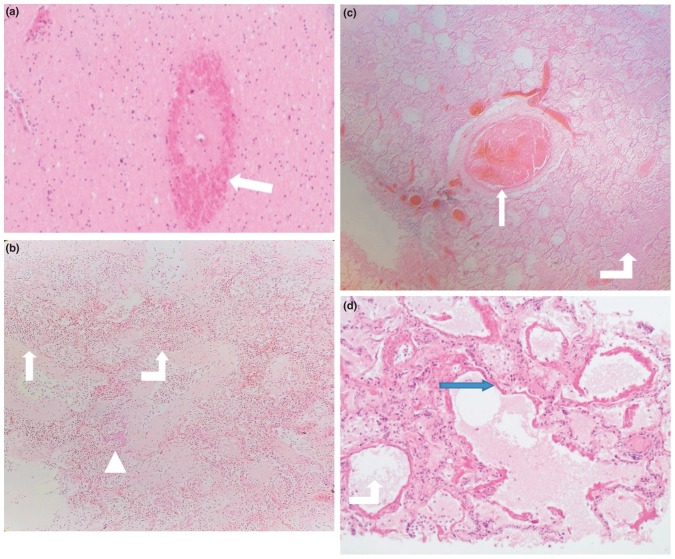
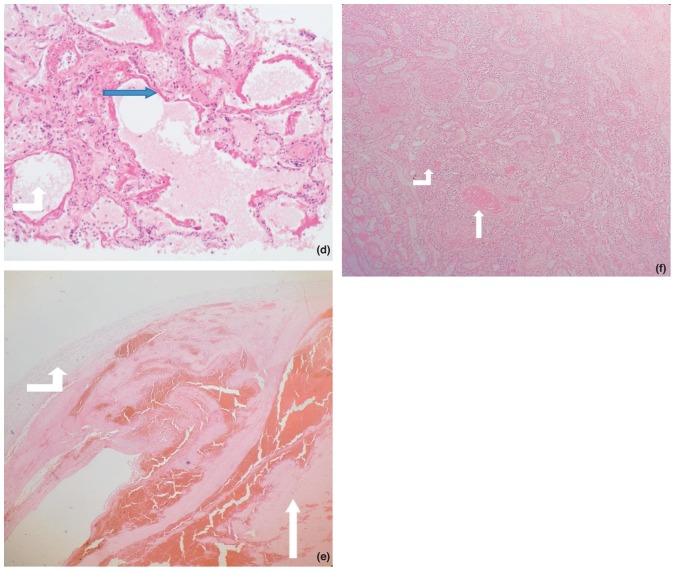
Figure 2 (Figures 2A-F) shows microscopic histopathological findings.
Figure 2.
A: Brain: Micrograph (X 40) of the brain parenchyma showing hemorrhage (white arrow head), around a vessel.
B: Micrograph (X 20) of lung showing acute (white arrow head) and organising pneumonia (bent white arrow up), and squamous metaplasia (triangle).
C: Micrograph (X 20) of lung showing a thrombus (white arrow head) and organising pneumonia (bent white arrow up).
D: Micrograph (X 40) of lung tissue showing diffuse alveolar damage within the lung. The hyaline membranes (blue arrow head) are observed to line the alveolar walls. The alveolar space is indicated by the bent white arrow up.
E: Micrograph (X 40) of a vessel (bent white arrow up) showing a thrombus (white arrow head).
F: Micrograph (X 20) of an infarcted kidney showing disseminated thrombus (white arrow head) and neutrophils in the parenchyma in a case (bent white arrow up).
Overall individual decedent characteristics and autopsy findings
Table 4 depicts demographic, clinical and autopsy characteristics of each of the 29 decedents that were autopsied.
Table 4.
Summary: demographic, clinical and autopsy characteristics of each individual decedent autopsied.
| Case # | Sex/Age | Symptoms | Duration of illness | Comorbidities | Gross pathology and microscopic findings | Cause(s) of death |
|---|---|---|---|---|---|---|
| 1 | M/38 | Cough, Oral thrush, Diarrhea, Fever, Difficulty in breathing | 7 days | Leprosy Pneumonia |
Heavy lungs (>1000 g each), consolidation of lungs and neutrophils in alveolar spaces and DAD | Pulmonary thromboembolism due to COVID-19 |
| 2 | M/37 | Cough Difficulty in breathing |
6 days | HIV | Consolidation of lungs and neutrophils in alveolar spaces | Pneumonia due to COVID-19 |
| 3 | M/40 | Difficulty in breathing Headache, Fever |
14 days | None | Heavy lungs (>1000 g each), and DAD | Diffuse Alveolar Damage due to COVID-19 |
| 4 | M/29 | Difficulty in breathing | 5 days | Disseminated Kaposi Sarcoma HIV/AIDS | Heavy lungs (>1000 g each), Kaposi sarcoma lesions in the esophagus, stomach, bowel, lungs, skin and DAD | Diffuse Alveolar Damage due to COVID-19 |
| 5 | F/40 | General body weakness Vomiting, Fever | 7 days | HIV Malaria |
Consolidation of lungs and neutrophils in alveolar spaces | Pneumonia due to COVID-19 |
| 6 | M/62 | Cough Difficulty in breathing, Fever |
4 days | Diabetes, Hypertension | Heavy lungs (>1000 g each), shower thromboemboli, thromboemboli in the heart, granular kidneys, and DAD | Pulmonary thromboembolism due to COVID-19 |
| 7 | F/47 | Cough Difficulty in breathing, Fever |
5 days | HIV | Consolidation of lungs and organising pneumonia | Pneumonia due to COVID-19 |
| 8 | M/55 | Cough, Fever | 120 days | HIV Disseminated Tuberculosis |
Heavy lungs (>1000 g each) and DAD | Diffuse Alveolar Damage due to COVID-19 |
| 9 | 33/F | Difficulty in breathing, cough, Pregnant on claxane | 5 days | None | Heavy lungs (>1000 g each), shower thromboemboli, iliac artery thromboembolic, thromboemboli in the heart, and DAD | Pulmonary thromboembolism due to COVID-19 |
| 10 | 38/M | Cough Difficulty in breathing, Fever |
14 days | Colorectal adenocarcinoma HIV |
Heavy lungs (>1000 g each), colorectal adenocarcinoma, and DAD | Diffuse Alveolar Damage due to COVID-19 |
| 11 | 37/F | Cough Difficulty in breathing Diarrhea and on claxane |
5 days | Hypertension | Heavy lungs (>1000 g each), shower thromboemboli, iliac artery thromboembolic, thromboemboli in the heart, and DAD | Pulmonary thromboembolism due to COVID-19 |
| 12 | 48/F | Difficulty in breathing, headache | 8 days | Hypertensive heart disease HIV |
Consolidation of lungs, granular kidneys, large heart (700 g) and neutrophils in alveolar spaces, glomerular sclerosis | Pneumonia due to COVID-19 |
| 13 | M/62 | Difficulty in breathing | 3 days | Hypertension, Diabetes |
Consolidation of lungs, granular kidneys and neutrophils in alveolar spaces | Pneumonia due to COVID-19 |
| 14 | 32/M | General body pains, vomiting | 14 days | Diabetes | Heavy lungs (>1000 g each) and DAD | Diffuse Alveolar Damage due to COVID-19 |
| 15 | 27/F | Difficulty in breathing in pregnancy | 3 days | Sickle cell anemia | Heavy lungs (>1000 g each), and DAD. No sickle cells identified | Diffuse Alveolar Damage due to COVID-19 |
| 16 | 42/F | Difficulty in breathing Palpitations |
7 days | Pulmonary tuberculosis | Consolidation of lungs, and neutrophils in alveolar spaces | Pneumonia due to COVID-19 |
| 17 | 47/F | Difficulty in breathing | 5 days | None | Saddle emboli, heavy lungs (>1000 g each), deep venous thrombosis, and DAD | Pulmonary thromboembolism due to Deep Venous Thrombosis due to COVID-19 |
| 18 | 65/M | Difficulty in breathing | None | Cerebral Vascular Accident | Saddle emboli. | Pulmonary thromboembolism due to COVID-19 |
| 19 | 58/M | Difficulty in breathing, Headache, Fever on claxane | 5 days | Hypertension Obese |
Heavy lungs (>1000 g each), shower emboli, deep venous thrombosis, myocarditis, kidney and brain infarcts and DAD | Pulmonary thromboembolism due to Deep Venous Thrombosis due to COVID-19 |
| 20 | 19/F | Difficulty in breathing and Fever | 3 days | Paraquat toxicity treated for 14 days | Heavy hemorrghic lungs (>1000 g each), and DAD | Diffuse Alveolar Damage due to COVID-19 |
| 21 | 82/M | Difficulty in breathing | 1 day | Diabetes, Atherosclerotic coronary artery heart disease | Heavy lungs (>1000 g each) and DAD | Diffuse Alveolar Damage due to COVID-19 |
| 22 | 66/M | Difficulty in breathing | 14 days | Hypertension | Heavy lungs (>1000 g each), granular kidneys, and DAD | Diffuse Alveolar Damage due to COVID-19 |
| 23 | 70/F | Cough Difficulty in breathing on claxane. Death on operation table |
6 days | Hypertension | Heavy lungs (>1000 g each), deep venous thrombosis, thrombosed mesenteric vessels, saddle emboli, and DAD | Pulmonary thromboembolism due to COVID-19 |
| 24 | 33/M | Difficulty in Breathing, Fever | 6 days | HIV | Saddle emboli, heavy lungs (>1000 g each), consolidated lungs, and neutrophils in alveolar spaces and DAD | Pulmonary thromboembolism due to COVID-19 |
| 25 | 36/M | Difficulty in in breathing, Abdominal pains | 14 days | Abdominal TB | Multiples abdominal caseating nodules, saddle thromboembolism | Pulmonary thromboembolism due to COVID-19 |
| 26 | 20/F | Headache, general body weakness | 3 days | None | Heavy lungs (>1000 g each), shower emboli, and DAD | Pulmonary thromboembolism due to COVID-19 |
| 27 | 43/M | Difficulty in in breathing | 1 day | None | Disseminated thrombi in lungs, liver, iliac vessels | Pulmonary thromboembolism due to COVID-19 |
| 28 | 50/M | General body weakness, headache | 1 day | None | Heavy lungs (>1000 g each), bilateral shower emboli, and DAD | Pulmonary thromboembolism due to COVID-19 |
| 29 | 47/F | Difficulty in in breathing, fever, headache | 6 days | None | Shower emboli, heavy lungs (>1000 g each), organising pneumonia and DAD | Pulmonary thromboembolism due to COVID-19 |
Abbreviations: DAD = Diffuse Alveolar Damage.
Discussion
To our knowledge, this is the first large COVID-19 whole-body autopsy series of hospital inpatient deaths to be reported from sub-Saharan Africa (SSA) to date. Autopsy studies and data on the pathology of COVID-19 from SSA is scanty and has been restricted only to examination of tissue samples obtained through minimally invasive post-mortem examination of 4 cases in South Africa (Bruce-Brand et al., 2020) and two autopsy reports, one of a COVID-19 and HIV co-infected person (Khaba et al., 2020) and another of an infant co-infected with SARS-CoV-2 and Mycobacterium tuberculosis (Mulale et al., 2021). Our study provides data from 29 autopsies of hospital COVID-19 deaths. Our descriptions of symptoms ante-mortem, comorbidities, the extent of disease, the cause(s) of death, fill an important knowledge gap on COVID-19 pathology in Africa.
There were more males than females in our autopsied cases. Published reports suggest that males may be more susceptible to SARS-CoV-2 infection due to their higher plasma Acetylcholinesterase Enzyme 2 (ACE2) levels, less effective antiviral immune defenses than women (Gadi et al., 2020). Understanding COVID-19 the gender differences in Africa will require larger cohort studies and include biopsychosocial and immunological investigations (Griffith et al., 2020).
The age of our study decedents ranged from 19-82 years, with a mean age of 44. Notably, 22/29 (76.0%) were below the age of 55 years. This is at variance with reported autopsy studies from Europe, China and North America, where the deaths were commonly observed in those above 65 years old (Edler et al., 2020, Falasca et al., 2020, Bryce et al., 2021, Bian and The COVID-19 Pathology Team, 2020). The comparatively younger age may be attributed to Zambia's age structure, where people above the age of 55 are less than 6% of the entire population (Index Mundi, 2021). It could also due to HIV being more prevalent in people less than 55 years (Himwaze et al., 2020). HIV may also play a role since it is considered a risk factor for acquiring SARS-CoV-2 infection and is associated with a higher risk of mortality from COVID-19 (Ssentongo et al., 2021).
As reported from autopsy studies from outside Africa (Edler et al., 2020, Falasca et al., 2020, Bryce et al., 2021, Bian and The COVID-19 Pathology Team, 2020), difficulty in breathing, cough, fever, and headache were the most common symptoms. This may reflect admission criteria, which selected patients with moderate and severe respiratory symptoms (COVID CONTINENT HUB, 2021, Grant et al., 2020) and assessment of temperatures during admission (Bian and The COVID-19 Pathology Team, 2020).
HIV infection and hypertension co-morbidities were common among our study group and these comorbidities are important compared to the observations made in other autopsy studies from outside Africa (Maiese et al., 2020, Edler et al., 2020, Falasca et al., 2020, Bryce et al., 2021, Bian and The COVID-19 Pathology Team, 2020).
The range of macroscopic and microscopic pathological features of COVID-19 in hospital deaths in Lusaka, Zambia, greatly resemble those seen in autopsy case series from China, Europe, and USA (Maiese et al., 2020, Edler et al., 2020, Falasca et al., 2020, Bryce et al., 2021, Bian and The COVID-19 Pathology Team, 2020). Diffuse Alveolar Damage (DAD), pulmonary thromboembolism, and pneumonia were the most prevalent autopsy findings, respectively.
Pulmonary thromboembolism due to SARS-CoV-2 was the most common cause of death in our series. It is now known that thrombosis in decedents with SARS-CoV-2 infection is part of the microangiopathic changes observed in COVID-19 (Maiese et al., 2020). We observed that four patients on anticoagulant therapy (enoxaparin) had died of pulmonary thromboembolism with disseminated thrombosis. Further studies are required to establish the reason why coagulopathy occurs despite anticoagulant preventive therapy. Thromboembolic disease is not uncommon in Zambia at autopsy, however the occurrence of deep vein thrombosis, pulmonary embolism and microthrombi in smaller pulmonary vessels should alert the physician to the possibility of COVID-19 and perform a SARS-CoV-PCR test to confirm of rule out COVID-19.
Nonetheless, It is important to emphasize that deep vein thrombosis and pulmonary embolism, and microthrombi in the small pulmonary vessels should alert clinicians that they may be pathognomonic signs of COVID-19, especially in SSA.
DAD due to SARS-CoV-2 was the second most common cause of death. SARS-CoV-2 enters the epithelial lining cells of the respiratory tract using the ACE2 enzyme as a viral receptor leading to DAD and eventually death (Mohanty et al., 2020). Pneumonia due to COVID-19 was the third most common cause of death. Patients admitted to the hospital were empirically treated with ceftriaxone and azithromycin (COVID-19 rapid guideline, 2020) to cover the possibility of bacterial pneumonia. Pneumonia due to SARS-CoV-2 was a common finding and thus empiric use of antibiotics may not be appropriate in these cases and needs further study and discussion.
Autopsies provide the most accurate data about cause of death, but remain operationally difficult and expensive. The World Health Organisation (WHO) recommended that autopsy rooms must be adequately ventilated (World Health Organization, 2020). Maximal infection prevention should be provided using full-body suits with air-purifying respirators (Baj et al., 2021, Liang et al., 2020). Autopsies were conducted in a mortuary with inadequate ventilation, and 2 cases were conducted in a well-ventilated mortuary. Although there is no evidence of transmission of SARS-CoV-2 from decedents, we washed the bodies with chlorine and used standard personal protective equipment during the autopsy (World Health Organization, 2020, Baj et al., 2021, Liang et al., 2020). The autopsy team members were tested for COVID-19 every two months (or when they became symptomatic) by reverse transcriptase-polymerase chain reaction (RT-PCR) on nasopharyngeal swabs. None of our pathologists or the assistants have tested positive for SARS-CoV-2 indicating that optimal infection control practice reduces risk of acquiring SARS-CoV-2 during autopsies.
There are several limitations to our study which are inherent to all autopsy studies, including selection bias and the relatively small numbers. However, our study illustrates that autopsies during the COVID-19 era are possible to conduct on SARS-CoV-2 infected subjects in Zambia. There is a need to revive clinical and research autopsies througout sub-Saharan African countries (Mawalla, 2020, Mucheleng’anga and Himwaze, 2020, Bruce-Brand et al., 2020, Mudenda et al., 2020, Rampatige and Gilks, 2015, Barth et al., 2020, Yi et al., 2020) to add to the provisional information provided by our study. Further autopsies with accurate data are required to conjure a more comprehensive picture of the pathogenesis and impact of COVID-19 in SSA.
Our autopsy study serves as a pivotal starting point for conversations around future autopsy studies in SSA and worldwide, to further understand factors which influence expression of clinical spectrum of disease and the impact on other co-morbidities such as HIV, tuberculosis, malaria, hypertension, and diabetes. The COVID-19 pandemic serves as a reminder to authorities responsible for health care and policy formulation to invest more into autopsy pathology services (Yi et al., 2020). Furthermore, with new SARS-CoV-2 variants in circulation and decedents dying after re-infection, sequencing data will need to be aligned to autopsy studies to better understand the full scope of COVID-19 in Zambia and the rest of Africa. Further investments are required to improve the accuracy of diagnosis, recording and reporting of all causes of death and to ascertain the generic validity of our findings, An international COVID-19 postmortem platform is required for gathering uniform autopsy and postmortem data, and for enabling comparisons between countries.
Conflicts of interest
All authors declare no conflict of interest.
Authors contributions
Conceptualization: Luchenga Adam Mucheleng’anga, Cordelia Maria Himwaze and Alimuddin Zumla.
Methodology: Luchenga Adam Mucheleng’anga and Cordelia Maria Himwaze.
Software and formal analysis: Amos Hamukale.
Validation: Viktor Telendiy, Fred Maate, Mupeta Songwe, Lunda Shibemba, Chitalu Chanda, Duncan Chanda, Peter Julius, Chibamba Mumba, Clemence Marimo, Amos Hamukale, Llyod Mulenga, and Alimuddin Zumla.
Data curation : Luchenga Adam Mucheleng’anga, Cordelia Maria Himwaze, Viktor Telendiy
Writing: First and final drafts: Cordelia Maria Himwaze, Alimuddin Zumla and Luchenga Adam Mucheleng’anga. Review and editing: All authors contributed to writing of the manuscript.
Visualization: Luchenga Adam Mucheleng’anga, Cordelia Maria Himwaze, Amos Hamukale, Peter Julius and Alimuddin Zumla.
Supervision: Alimuddin Zumla and Luchenga Adam Mucheleng’anga.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
We are indebted to Dr. Chitalu Chanda, who campaigned for the idea of all deaths in the isolation wards at UTH to be autopsied and counseled the next-of-kin about the importance of autopsies in these deaths. We thank our brave pathologist’s assistants, Mr. Mwanza Rabson and Mr. Chanda Brain, without whom the autopsies would have been impossible to conduct. Our gratitude goes to our hard-working biomedical scientists Ms. Lomeo Chifunda, Ms. Alice Chibondwe, and Mr. Chrispin Chiyabi. We also thank Dr. Suwilanji Sivile and Dr. Francis Mupeta for supporting autopsy work on hospital Inpatient COVID-19 Deaths.
Sir Prof Alimuddin Zumla, is a co-Principal Investigator of the (PANDORA-ID-NET), the Pan-African Network For Rapid Research, Response, Relief and Preparedness for Infectious Disease Epidemics, supported by the EDCTP. He is in receipt of a UK National Institutes of Health Research, Senior Investigator Award and is a Mahathir Foundation Science Award laureate.
References
- Africa CDC - Coronavirus Disease 2019 (COVID-19). Latest updates on the COVID-19 crisis from Africa CDC. https://africacdc.org/COVID-19/ -accessed June 4th, 2021.
- Baj J., Ciesielka M., Buszewicz G., Maciejewski R., Budzyńska B., Listos P., Teresiński G. COVID-19 in the autopsy room–requirements, safety, recommendations and pathological findings. Forensic Sci Med Pathol. 2021;17:101–113. doi: 10.1007/s12024-020-00341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamgboye E.L., Omiye J.A., Afolaranmi O.J. COVID-19 Pandemic: Is Africa Different? J Natl Med Assoc. 2020 doi: 10.1016/j.jnma.2020.10.001. S0027-9684(20)30345-X.pmid:33153755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth R.F., Xu X., Buja L.M. A Call to Action: The Need for Autopsies to Determine the Full Extent of Organ Involvement Associated With COVID-19. Chest. 2020;158(July (1)):43–44. doi: 10.1016/j.chest.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Xiu-Wu, The COVID-19 Pathology Team Autopsy of COVID-19 patients in China. National Science Review. 2020;7(9):1414–1418. doi: 10.1093/nsr/nwaa123. -Accessed on 10th April, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Brand C., Allwood B.W., Koegelenberg C.F.N., Lalla U., Louw E., Diacon A.H. Postmortem lung biopsies from four patients with COVID-19 at a tertiary hospital in Cape Town, South Africa. S Afr Med J. 2020;110(October (12)):1195–1200. doi: 10.7196/SAMJ.2020.v110i12.15290. PMID: 33403965. [DOI] [PubMed] [Google Scholar]
- Bryce C., Grimes Z., Pujadas E. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol. 2021;(April):1–12. doi: 10.1038/s41379-021-00793-y. Epub ahead of print. PMID: 33795830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID CONTINENT HUB, Interim Clinical Guidance for Management of Patients with Coronavirus Disease 2019 (COVID-19). https://www.c19hub.io/interim-clinical-guidance-for-management-of-patients-with-coronavirus-disease-2019-COVID-19/. -Accessed on 24/03/2021.
- National Institute for Health and Care Excellence (UK); London: 2020. COVID-19 rapid guideline: antibiotics for pneumonia in adults in hospital. (NICE Guideline, No. 173.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK566162/ -Accessed April 10th, 2021. [PubMed] [Google Scholar]
- Edler C., Schröder A.S., Aepfelbacher M. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134(July (4)):1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falasca L., Nardacci R., Colombo D. Postmortem Findings in Italian Patients With COVID-19: A Descriptive Full Autopsy Study of Cases With and Without Comorbidities. J Infect Dis. 2020;222(11):1807–1815. doi: 10.1093/infdis/jiaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadi N., Wu S.C., Spihlman A.P. What’s Sex Got to Do With COVID-19? Gender-Based Differences in the Host Immune Response to Coronaviruses. Frontiers in Immunology. 2020;11:2147. doi: 10.3389/fimmu.2020.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M.C., Geoghegan L., Arbyn M. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0234765. PMID: 32574165; PMCID: PMC7310678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith D.M., Sharma G., Holliday C.S. 2020. Men and COVID-19: A Biopsychosocial Approach to Understanding Sex Differences in Mortality and Recommendations for Practice and Policy Interventions.https://www.cdc.gov/pcd/issues/2020/20_0247.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himwaze C., Mucheleng’anga L., Siyumbwa S. Prevalence of Human Immunodeficiency Virus, Hepatitis B, and Hepatitis C Viral Infections among Forensic Autopsy Cases at the University Teaching Hospital in Lusaka, Zambia. Forensic Science International: Reports. 2020;2 doi: 10.1016/j.fsir.2020.100133. https://www.sciencedirect.com/science/article/pii/S2665910720300827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Index Mundi, Zambia Age structure, https://www.indexmundi.com/zambia/age_structure.html. Accessed on 14/03/2021.
- Khaba M.C., Ngale T.C., Madala N. COVID-19 in an HIV-infected patient. Lessons learned from an autopsy case. Int J Infect Dis. 2020;101:243–246. doi: 10.1016/j.ijid.2020.09.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal Y. Africa’s low COVID-19 mortality rate: A paradox? Int J Infect Dis. 2021;102:118–122. doi: 10.1016/j.ijid.2020.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T., Cai H., Chen Y. 2020. Handbook of COVID-19 prevention and treatment: The First Affiliated Hospital, Zhejiang University School of Medicine, complied according to clinical experience.https://books.google.pl/books?id=p8dzzQEACAAJ [Google Scholar]
- Maiese A., Manetti A.C., La Russa R. Autopsy findings in COVID-19-related deaths: a literature review. Forensic Sci Med Pathol. 2020;(October):1–18. doi: 10.1007/s12024-020-00310-8. Epub ahead of print. PMID: 33026628; PMCID: PMC7538370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawalla W.F. The contribution of autopsy in COVID-19 pandemic: Missed opportunity in Sub-Saharan Africa. Forensic Science International: Reports. 2020;2 doi: 10.1016/j.fsir.2020.100136. https://www.sciencedirect.com/science/article/pii/S2665910720300852 ISSN 2665-9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty S.K., Satapathy A., Naidu M.M. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19) - anatomic pathology perspective on current knowledge. Diagn Pathol. 2020;15(August (1)):103. doi: 10.1186/s13000-020-01017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucheleng’anga L., Himwaze C. The role of forensic pathology in the COVID-19 pandemic in Zambia. Forensic Science International: Reports. 2020;2 doi: 10.1016/j.fsir.2020.100147. https://www.sciencedirect.com/science/article/pii/S2665910720300979 ISSN 2665-9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudenda V., Malyangu E., Sayed S. Addressing the shortage of pathologists in Africa: Creation of a MMed Programme in Pathology in Zambia. Afr J Lab Med. 2020;9(1):974. doi: 10.4102/ajlm.v9i1.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulale U.K., Kashamba T., Strysko J., Kyokunda L.T. Fatal SARS-CoV-2 and Mycobacterium tuberculosis coinfection in an infant: insights from Botswana. BMJ Case Rep. 2021;14(April (4)) doi: 10.1136/bcr-2020-239701. PMID: 33883111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampatige R., Gilks C.F. Autopsies and better data on causes of death in Africa. Lancet Infect Dis. 2015;15(May (5)):492–494. doi: 10.1016/S1473-3099(15)70096-4. Epub 2015 Mar 10. PMID: 25765218. [DOI] [PubMed] [Google Scholar]
- Ssentongo P., Heilbrunn E.S., Ssentongo A.E. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Sci Rep. 2021;11(1):6283. doi: 10.1038/s41598-021-85359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Coronavirus dashboard 2021. https://covid19.who.int/ -accessed June 4th, 2021.
- World Health Organisation, Zambia, https://www.who.int/countries/zmb/ -accessed June 4th, 2021.
- World Health Organization . 2020. Infection prevention and control for the safe management of a dead body in the context of COVID-19.https://apps.who.int/iris/bitstream/handle/10665/331538/WHO-COVID-19-lPC_DBMgmt-2020.1-eng.pdf [Google Scholar]
- Yi E.S., Cecchini M.J., Bois M.C. Pathologists in pursuit of the COVID-19 culprit. Lancet Infect Dis. 2020;20(October (10)):1102–1103. doi: 10.1016/S1473-3099(20)30449-7. Epub 2020 Jun 8. PMID: 32526191; PMCID: PMC7279719. [DOI] [PMC free article] [PubMed] [Google Scholar]