Abstract
Coronaviruses are named after the crown-like spike proteins on their surface. In the 21st century, three coronaviruses, namely severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), SARS-CoV, and Middle East respiratory syndrome related coronavirus (MERS-CoV), have emerged in the human population, presumably evolving from pathogens infecting other animals. Coronaviruses are enveloped viruses responsible for 15–30% of the atypical pneumonia cases in humans worldwide. The current coronavirus disease 2019 (COVID-19) pandemic is caused by the newest SARS virus, SARS-CoV-2, an enveloped, positive-sense, single-stranded RNA betacoronavirus of the family Coronaviridae. As of April 2021, the World Health Organization has reported more than 3 million deaths from COVID-19 and more than 140 million people have been infected with the virus, thereby making it the worst SARS pandemic of all time. Here, I review the current understanding of the molecular biology of coronaviruses and their host interactions, bringing together knowledge of the infection process to aid in the development of therapeutic drugs and/or vaccines against SARS-CoV-2. I also briefly overview the current situation of available treatments, vaccinations, and emerging strains.
Keywords: ACE2, coronavirus, spike protein, mechanism, vaccine pandemic
Introduction
Coronaviruses are characterized by and named for the crown-like spikes on the virion surface. There are four main subgroups of coronaviruses, alpha, beta, gamma, and delta. Alpha and beta subgroups are suggested to have originated from bats and mammals, whereas gamma and delta subgroups primarily infect birds. There are four common human coronaviruses, 229E (Alphacoronavirus), NL63 (Alphacoronavirus), OC43 (Betacoronavirus), and HKU1 (Betacoronavirus). NL63 and HKU1 were recently discovered after the severe acute respiratory syndrome (SARS) epidemic but are thought to have been circulating in the human population for some time.1 Coronaviruses are enveloped viruses responsible for 15–30% of atypical pneumonia in humans worldwide.2 In the 21st century, three coronaviruses, SARS coronavirus (SARS-CoV), Middle East respiratory syndrome related coronavirus (MERS-CoV), and SARS-CoV-2, have emerged in the human population, presumably after transmission from other animals.3 The intermediate host of SARS-CoV is the civet or raccoon and that of MERS-CoV is the camel,4 but the host of the current SARS-CoV-2 pandemic strain is yet to be proven, although it is believed to be closely related to BatCov RaTG13 (Table 1). Although the RaTG13 bat virus exhibits similarity to SARS-CoV-2 across the genome, coronaviruses in Malayan pangolins (Manis javanica) remain closest to SARS-CoV-2.5 In particular, all six key receptor-binding domain (RBD) residues are identical in the coronaviruses of M. javanica and SARS-CoV-2.6,7
Table 1.
Zoonotic Transmission of Coronaviruses
| Virus | Origin of Pathogen | Natural Reservoir Host | Virulent Coronavirus | Cell Receptor |
|---|---|---|---|---|
| SARS-CoV | Palm civets, raccoon, dogs, domestic cat, and ferret badgers | Bat | SARS | ACE269 |
| MERS-CoV | Dromedary camels | Bat | MERS | DPP470 |
| SARS-CoV-2 | Unknown | Unknown | COVID-19 | ACE27 |
Abbreviations: SARS-CoV, severe acute respiratory syndrome coronavirus; MERS-CoV, Middle East respiratory syndrome related coronavirus; COVID-19, coronavirus disease 2019; ACE2, angiotensin-converting enzyme 2; DPP4, dipeptidyl peptidase 4.
The fact that there have been three events of widespread zoonotic transmission within the past 20 years is concerning, and it suggests that additional emergence events are likely to occur in the future.8 During the first SARS pandemic in 2002, more than 8000 cases and 774 deaths were reported worldwide. However, fewer numbers of MERS cases have been reported – 2567 cases to date; however, 866 total deaths have resulted (from the WHO website: https://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html, viewed February 2021). The current virus, SARS-CoV-2, is the most pathogenic of the three coronaviruses, resulting in more than 3.1 million deaths from coronavirus disease 2019 (COVID-19) and more than 150 million people infected worldwide as of April 2021. Whereas the majority of COVID-19 cases are asymptomatic, symptomatic patients can experience mild to severe symptoms. Mild symptoms include fever, cough, headache, and fatigue, with mild or no pneumonia, and with severe clinical manifestations, acute respiratory syndrome, hypoxia, and dyspnea can develop. In critical cases, patients suffer from multi-organ dysfunction, respiratory failure, and shock. Disease fatality rates are higher in older people with chronic comorbidities such as diabetes, coronary artery disease, hypertension, chronic obstructive disease, and obesity. Despite the relatively high number of deaths, the rate of recovery from COVID-19 is substantial; the death rate varies regionally between 0.8% and 14.5% of the total population (mortality analyses, Johns Hopkins University of Medicine https://coronavirus.jhu.edu/data/mortality; for daily case report, please see the World Health Organization [WHO] coronavirus disease dashboard at https://covid19.who.int/). The actual number of presumed cases is thought to be much higher owing to the current testing limitations,9 and the number of cases is expected to continue to grow exponentially.10 The nomenclature of the new emerging coronavirus as SARS-CoV-2 has been found to be misleading owing to its differences from previous SARS-CoV in epidemiology, transmission, and unique genetic structure, although the two viruses share approximately 79% similarity in genome structure and cause severe acute respiratory syndrome. It was suggested that the virus be named nCov19 to resemble the disease announced by the WHO, COVID-19.11
Virus Genomic Structure
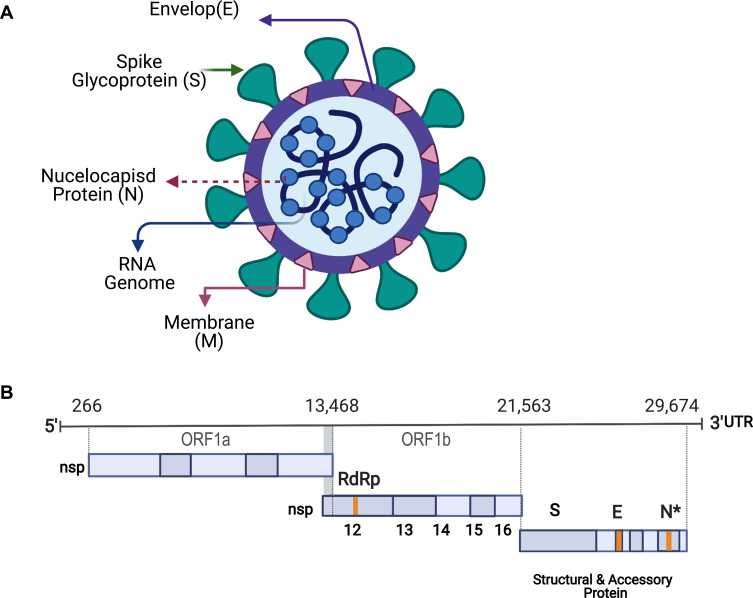
The current COVID-19 pandemic is caused by the novel coronavirus strain SARS-CoV-2, a positive-sense, single-stranded enveloped RNA belonging to the Coronaviridae family12 (Figure 1A). The SARS-CoV-2 genome is >30 kb in length and consists of 14 open reading frames (ORFs) encoding 27 proteins.13 The 5′ end of the SARS-CoV-2 genome consists of ORF1a/b encoding a polyprotein that is post-translationally cleaved into 16 non-structural proteins (nsp1–16), which form the replicase/transcriptase complex (RTC). This complex contains enzymes involved in the replication mechanism, including papain-like protease (nsp3), main protease (nsp5), nsp7-nsp8 primase complex, primary RNA-dependent RNA polymerase (RdRp; nsp12), helicase/triphosphatase (nsp13), exoribonuclease (nsp14), endonuclease (nsp15), and N7- and 2′-O-methyltransferases (nsp10/nsp16). The 3′ terminus of the viral genome contains ORFs encoding the four main structural proteins, spike (S), envelope (E), membrane (M), and nucleocapsid (N), as well as nine putative accessory factors (Figure 1B).14,15
Figure 1.
Schematic diagram of SARS-CoV-2: (A) SARS-CoV-2 is a positive-sense, single-stranded enveloped RNA.
Notes: The lipid envelope (E) is studded with several spike (S) glycoproteins giving the virus a crown-like appearance. The membrane (M) glycoprotein is the most abundant structural protein and spans the envelope. (B) The 5′ end of the SARS-CoV-2 genome consists of open reading frame (ORF)1a/b that encodes a polyprotein, which is post-translationally cleaved into 16 non-structural proteins (nsp1–16), including RNA-dependent RNA polymerase (RdRp). The 3′ terminus of the viral genome contains ORFs encoding the four main structural proteins, spike (S), envelope (E), membrane (M), and nucleocapsid (N), as well as nine putative accessory factors. Figure created with BioRender.com. Figure adapted from Wang N, Shang J, Jiang S, Du L. Subunit vaccines against emerging pathogenic human coronaviruses. Front Microbiol. 2020;11:298.30 Creative Commons Attribution License (CC-BY 4.0; https://creativecommons.org/licenses/by/4.0/legalcode).
The virus uses host machinery to produce its lipid envelope, which is studded with several S proteins giving the virus a crown-like appearance. The M glycoprotein, also called the matrix protein, is the most abundant structural protein, traversing the viral envelope and attaching the viral membrane to the nucleic acid within the virion. The N protein is important for the morphogenesis phase of the viral life cycle (Figure 1A).14
The S protein plays a critical role in promoting cell attachment and fusion of the virus to the host membrane; it is trimeric and contains two distinct domains, the RBD at the amino-terminus of the S1 subunit and the carboxy-terminus domain (CTD) of the S2 subunit, which is responsible for membrane fusion.16 SARS-CoV and SARS-CoV-2 share a highly conserved S protein RBD with 79.5% genome sequence identity.8 Cryo-electron microscopy images of SARS-CoV-2 showed sequence conservation of all related coronaviruses across the S2 subunit CTD.17,18 However, the S1 subunit RBD protein sequence contains unique regions, and this uniqueness tends to be common to coronaviruses in general. This is likely a result of intense evolutionary pressure due to constant interactions with the host immune system.19
SARS-CoV-2 antigenic evolution continues to be observed, particularly in the spike protein RBD. The first variant was reported and sampled in Italy in February of 2020, a D614G mutation in the S protein gene. Over time, this became the dominant variant displacing the original SARS-CoV-2 strain.20 Thereafter, additional variants have been emerging in different countries. The most widespread variant to date is B.1.1.7 or SARS-CoV-2 VOC 202012/01, a variant that emerged in the United Kingdom and was reported to the WHO in December 2020 (Table 2). Initial clinical studies showed that the 17-nucleotide mutation or deletion21 increases the transmissibility of the virus with little or no impact on severity, diagnosis, pathogenesis, reoccurrence, or response to vaccination.22 According to the WHO report on April 20, 2021, this variant has been reported in over 137 different countries. Another variant reported on December 18, 2020 emerged in South Africa, was named B.1.351 or 501Y.V2 after the mutation showed high similarity to the variant of the UK lineage (https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—20-april-2021). The latest variant, P.1, was identified first in January 2021 in Japan from travelers returning from Brazil.23 Various aspects of these three variants are summarized in Table 2.
Table 2.
Variants of SARS-CoV-2 Reported Worldwide20
| Variant | Country of First Case | Countries Reported in (no.) a | Transmissibility b | Virulence | Number of Mutation | Diagnostic Assay c | Mutation Type |
|---|---|---|---|---|---|---|---|
| B.1.1.7 | United Kingdom | 137 | 43% to 90%71 | NA | 18 | Work | H69/V70 del,Y144 del, N501Y, A570D, P681H, S106/G107/F108 del |
| B.1.351 | South Africa | 85 | NA | NA | 8 | Work | L242/A243/L244 del, K417N, E484K, N501Y, S106/G107/F108 del |
| P.1 | Brazil | 52 | 42%72 | NR | 21 | Work | K417T, E484K, N501Y, S106/G107/F108 del |
Notes: aBased on weekly report published on 20 April 2021 by WHO; data from reference;73 bTransmissibility is relative to that of the original SARS-CoV-2 strain reported in UK, USA, Ireland, Switzerland, and Denmark; cDiagnostic test capable of identifying those variants.
Abbreviations: NA, not available; NR, not reported.
Mechanism of Viral Cell Entry
Both SARS-CoV-2 and SARS-CoV bind to the angiotensin-converting enzyme 2 (ACE2) receptor protein to gain entry into the host cell, whereas MERS-CoV uses dipeptidyl peptidase 4 (DPP4) as a host receptor through the RBD (CTD) region.17 ACE2 is located on the membrane surface of host cells and after virus binding, proteolytic activity is critical for fusion of the S protein; its exposure facilitates fusion of the virus with the host membrane. Thereafter, host cell proteolytic cleavage of the S protein into S1 and S2 subunits proceeds via activity of the transmembrane protease serine 2 (TMPRSS2) and human airway trypsin-like protease (HAT).24 The S2 subunit is composed of a fusion peptide (FP) and heptad repeat domains (HR)1 and HR2. Upon insertion of the hydrophobic FP into the host membrane, the pre-hairpin coiled coils of HR1 and HR2 undergo irreversible conformational changes and interact to form a six-helix bundle. This brings the viral envelope and cell membrane close enough for host penetration.25
SARS-CoV-2 Distinguishing Criteria
Three factors have been identified as possible contributors to the virulence of SARS-CoV-2, and they might impact pathogenicity. They include the following: 1) differences in the S protein RBD, 2) differences in properties of accessory proteins, and 3) the addition of a polybasic cleavage site in the S protein. Interestingly, five of the six critical amino acids in the S protein RBD that are necessary for interaction of S with the ACE2 receptor are different between SARS-CoV and SARS-CoV-2.17 The SARS-CoV-2 S protein exhibits a stronger binding affinity to ACE2 than that of the SARS-CoV S protein, which could lead to the reportedly higher transmissibility and contagiousness of SARS-CoV-2 during the current pandemic.19,26 As indicated earlier, the genome sequences of SARS-CoV-2 and SARS-CoV share more similarities than differences. The differences are associated with the accessory proteins ORF33a, ORF8b, ORF6, and protein E,27 which are known to be involved in the NLRP3 inflammasome complex and innate immunity, perhaps countering the interferon response.28 The functions of many of the accessory proteins are not yet established. It would be worthwhile to identify whether the differences in these proteins contribute to the enhanced virulence and pathogenicity of SARS-CoV-2.8,17
The acquisition of a polybasic cleavage site (RRAR) at the junction of S1 and S2 allows for effective cleavage by furin and other proteases and is a determinant of viral infectivity and host range.7 In addition to this polybasic cleavage site, there are O-linked glycans and RBD mutations that appear to be specific to SARS-CoV-2; they were not previously seen in other B-lineage betacoronaviruses. Insertion of a polybasic site increases the transmissibility of pathogenic influenza viruses.7,29 Thus, it is important to elucidate whether the same is true for SARS-CoV-2.30
Virion Replication and Assembly
Coronavirus RNA transcription occurs in the host cytoplasm via the catalytic subunit RdRp. Viral transcription generates a large viral mRNA transcript containing two overlapped ORFs, 1a and 1ab, and the other subgenomic mRNAs (sgmRNAs). In general, all of the mRNAs encode polyproteins with few exceptions; however, only the most 5′-ORF of the smallest mRNA is translatable.31
All of the mRNAs possess a 5′-common leader sequence derived from the 5′-end of the viral genome. This short stretch of sequence represents a signal for the discontinuous transcription of sgmRNAs. The transcriptional regulatory sequences (TRSs) act as a “slow-down” or “stop” signal for the RTC.15 mRNA gene expression is copied to a direct base pair with the leader TRS strand and the 3′ end of the viral genome.32,33 This discontinuous transcription mechanism is quite complex and orchestrated by a replicase that includes a polymerase and several other proteins required for functional integration of the RNA polymerase, capping, and proofreading activities. The mechanism of discontinuous transcription likely facilitates the extraordinary recombination rates observed within coronaviruses, which are as high as ~25%.34 Most RNA viruses, and especially positive-sense RNA viruses, have vanishingly low levels of recombination. This unique feature of the large and complex genomic RNA of coronaviruses might have evolved to enable the maintenance of such large genomes.35
The SARS-CoV-2 replication complex is composed of nsp12, 10, and 14.36,37 The nsp12 RdRp is the major machinery of transcription/translation; the holoenzyme structure is constructed by two processivity factors forming nsp7/nsp8 complexes.37 The polymerase complex (nsp12/8/7) is associated with nsp14, which serves in both capping and as a source of exonuclease activity. The heterodimer structure of nsp14 is composed of a bifunctional enzyme of two different domains, a basic exoribonuclease (ExoN) activity domain involved in proofreading and a methyl transferase domain involved in the mRNA capping reaction. These two domains are separated by a flexible hinge region, which allows the protein to be positioned in different orientations for the mismatch repair mechanisms to occur.38 Silencing nsp14-ExoN induces a 21-fold increase in genome mutations in infected cells compared to that observed in wild-type viruses.39,40 Acquisition of such complex RdRp activity has allowed coronaviruses to evolve with a larger genome and to increase their fidelity rate.41 In addition, there are a variety of other viral processing proteins, undefined sets of cellular proteins, and activities involved in orchestrating this discontinuous transcription mechanism, which are not all biochemically understood.42
Viral-infected cells exhibit convoluted membranes and interconnected double-membrane vesicles where viral replication and transcription occur. These are derived from the endoplasmic reticulum (ER). The compartmentalization of different RNA parts of replication in different vesicles protects the viral genome from potential attack by antiviral mechanisms, exonucleases, or nucleases generally present in the host cytosol.43 It can also help concentrate the factors necessary to efficiently replicate and transcribe the viral genome. Integral membrane proteins that are part of the replication complex are thought to function in vesicle biogenesis, and the three replication components, nsp3, 4, and 6, are predicted to have transmembrane domains and to be indirectly involved in vesicle formation.44,45 Expressing nsp3 and nsp4 alone, outside of the context of infection, is sufficient to drive double-membrane vesicle formation.45 This occurs through an interaction between the luminal loops of nsp3 and nsp4 proteins that drive membrane curvature and vesicle formation.46 Components of the proteome are associated with these RTCs.
The co-expression of M protein with either E or N protein is sufficient to form membrane-enveloped vesicles and virus-like particles.14 Assembly is driven first by the association of N protein with the genomic RNA leading to the formation of helical nucleic acids. Mature coronavirus particles inserted into the ER and then bud into the ER-Golgi intermediate compartment. Assembly involves the interaction among components of the viral S, M, and E proteins and the interlinkage of proteins to the viral genome. After the accumulation of virions in large, smooth-walled vesicles, they are released from the host cells by exocytosis.
Immune Response
SARS pathogenesis has been shown to be related to delayed interferon (IFN)-1 signaling and subsequent immune toxicity.47,48 Immunopathology associated with these infection responses are thought to cause acute respiratory distress syndrome. Several nsps, accessory factors, and matrix nuclear proteins were identified as putative interferon antagonists. Early prevention of the antiviral interferon response results in high initial viral titers and the accumulation of less toxic inflammatory monocyte-macrophages (IMMs). This results in elevated lung cytokine/chemokine levels, vascular leakage, and impaired virus-specific T cell responses. Knockdown of the IFN-αβ receptor or IMM depletion prevents the development of SARS-CoV-associated pneumonia, which can be lethal.48 A key coronavirus pathogenicity factor, nsp1, restricts gene expression originating from the host cell via a multi-pronged approach. Nsp1 interacts directly with the 40S ribosomal subunit to block the translation of host RNAs. Nsp1 also mediates endonucleolytic cleavage of host RNAs leading to broad, accelerated degradation. Widespread RNA degradation is also an immune evasion tactic to delay the IFN response. Mice infected with a wild-type virus die approximately 6 days after infection, but when the nsp1 protein is mutated, survival improves.48
Beyond the nsp1 virulence factor, several other proteins contribute to virus infectivity, such as nsp7 and nsp8.49,50 These two proteins play a role in IFN degradation for both SARS-CoV and MERS-CoV. Additionally, most of the structural proteins, including the N protein, which were found to regulate the host-cell cycle by preventing the S phase, block or antagonize the immune response by preventing the virus from presenting on cells.51,52 Understanding the infective properties of these proteins is essential for the development of novel therapeutic targets.
The neutralizing antibodies produced by natural immunity mainly target the RBD at the amino-terminus of the S1 subunit. Convalescent plasma from recovered donors has been used as treatment or prophylaxis to prevent the disease.53 However, a clinical trial in Canada has stopped recruiting patients due to a lack of significance. Antibodies’ durability after infection varied between 3 to 7 months. Diminishing IgG and IgA antibodies after a natural infection is normal but further studies are required to assess the ability of both humoral and cellular immunity to remember the infection. Results from longitudinal cohort studies of neutralizing antibodies vary between approximately 77–89% protection, with an incidence of reinfection less than 1% in the 5–7 months following the initial infection.54–56 In these studies, it was found that high neutralization titers correlate with the severity of disease and older age of patients.54 They also correlate with a high increment of inflammatory factors found in severely ill patients.
There are few studies addressing the immunogenicity, durability, and efficacy of natural or vaccine-acquired natural immunity toward new emerging variants. A serological cross-reactivity study with D614G mutants57 showed that the presence of antibodies provides cross-protection with parental strains but there was marginal drop when cross-recognizing B.1.1.7 and a further drop with B.1.351.22 The best way to understand the immune response further is through virus challenge studies, which cannot be performed due to the lack of proper treatment and the possible severity of the consequences. The UK government has recently approved the first human challenge study to take place among a healthy young population. The controlled human infection trial will help to understand more about host responses to the organism and further shed light on the pathogenesis of Covid-19.
Pathophysiology of Disease and Clinical Management
Positive feedback initiated by delayed IFNγ production, cytokine secretion, IL-6 overproduction, and neutrophil and macrophage activation will cause a cytokine storm, acute respiratory distress, sepsis, and multiple organ dysfunction in critical cases.58 The disease pathophysiology can also involve the development of severe macro- and micro-vascular disorders, which result in a systemic hypercoagulable state and severe vascular injury, leading to thrombosis.27,59 This can either be due to immune dysregulation and a cytokine storm or the high expression of ACE2 in endothelial tissue (the site of entry for SARS-CoV-2).60 The combination of these disease manifestations will lead to a ventilation/perfusion mismatch and severe dyspnea.
The underlying pathophysiology of the disease described previously herein requires a combination of different drugs, including anti-inflammatory, anticoagulants, antiviral, antibacterial, and supplemental oxygen.61 Such drugs might not directly fight the virus but instead are used to prevent or reduce its complications. Drugs such as chloroquine and hydroxychloroquine, previously approved as antimalarial drugs, have shown promising results in preventing viral multiplication; however, clinical trials did not confirm their efficacy.62,63 Currently, the first FDA-approved drug, remdesivir, has not shown any decrease in the death rate, but improvements in morbidity related to kidney or liver failure have been reported.64 All other drugs under investigation for SARS-CoV-2 treatment fall into two different categories, antiviral drugs to stop viral growth and immune system boosters, such as convalescent plasma from survivors, used to modulate recipient immunity. Different clinical trials using convalescent plasma conducted in the UK and Canada (Concor-1 clinical trial https://clinicaltrials.gov/ct2/show/NCT04348656 and REMAP-CAP https://www.remapcap.org/) did not show promising results for critical inpatients; however, patient lung damage might have hindered the efficacy. Both trials stopped recruiting patients, although patients who were not intubated did show improvements in mortality rates.65 Thus, this type of treatment can be helpful during the course of disease, especially if upcoming variants continue to evolve and erode the currently available vaccine and drug therapies.
Based on the latest update on the WHO website (https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines), there are more than 80 candidate vaccines undergoing clinical trials and 180 preclinical vaccines in development. Those approved largely rely on genetic information of the S protein as the target antigen, such as mRNA (Pfizer and BioNTech and Moderna), adenovirus vector (Oxford University and AstraZeneca), peptide (EpiVacCorona), or protein subunit (Novavax) vaccines. Other vaccines are being produced using classical methods either via the inactivation or attenuation of the wild-type virus, such as BBIBP-CorV developed by the Beijing Institute of Biological Products and CoronaVac by Sinovac.66
Future Directions
There are several basic questions regarding the molecular biology of SARS-CoV-2 that need to be answered. First, the role of the insertion of a polybasic site in increasing the binding affinity of the SARS-CoV-2 S protein to the human ACE2 receptor and how this facilitates disease transmission and spread are unknown.67 Second, the functional significance of the accessory proteins and their impact on both in vivo growth and virulence of the virus are unclear.68 The roles of other non-structural and host proteins in orchestrating the sophisticated replication/transcription complex and coordinating the viral life cycle in time and space are also not fully understood. Finally, it is not known what enables SARS-CoV-2 to maintain a sufficient mutation rate during trans-species movement and host-adaptation, despite its relatively large RNA genome.
Conclusions
During the past 60 years, many zoonotic coronaviruses have emerged, crossing barriers between mammalian species and causing concern for the future. The current coronavirus pandemic is considered the most contagious of this century, infecting 150 million people worldwide and causing nearly 3 million deaths so far. To date, different SARS-CoV-2 variants have evolved, and the implications of these mutations on the detection, transmission, vaccination, and severity of disease are yet to be determined. Acquiring the highest herd immunity threshold among the population via natural or vaccinal immunity is critical to prevent and reduce the infectious disease, as well to halt viral evolution. This review covered the current state of the literature regarding the molecular mechanisms by which the virus infects, replicates, and evolves, as well as the pathogenesis and immune response of the host. It is important, more than ever before, to understand more about viral RNA synthesis, immune evasion, and pathogenesis as we encounter ever more emerging viruses in host populations.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Funding Statement
There is no funding to report.
Data Sharing Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author Contributions
Sarah F. Alsobaie is the sole author of this article. The author made substantial contribution to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The author declared that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper and reported no conflicts of interest for this work.
References
- 1.Pal M, Berhanu G, Desalegn C, Kandi V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update. Cureus. 2020. doi: 10.7759/cureus.7423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunha BA. The atypical pneumonias: clinical diagnosis and importance. Clin Microbiol Infect. 2006;12:12–24. doi: 10.1111/j.1469-0691.2006.01393.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perlman S, Mcintosh K. Coronaviruses, Including Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS). 2020. [Google Scholar]
- 5.Lam TT-Y, Jia N, Zhang Y-W, et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583(7815):282–285. doi: 10.1038/s41586-020-2169-0 [DOI] [PubMed] [Google Scholar]
- 6.Sohrabi C, Alsafi Z, O’Neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He F, Deng Y, Li W. Coronavirus disease 2019: what we know? J Med Virol. 2020;92(7):jmv.25766. doi: 10.1002/jmv.25766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20(6):669–677. doi: 10.1016/S1473-3099(20)30243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imai N, Cori A, Dorigatti I, et al. Report 3: transmissibility of 2019-nCoV. Imp Coll London. 2020. Available from: https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-transmissibility-25-01-2020.pdf. [Google Scholar]
- 11. Wu GZ, Wang JW, Xu JQ. Voice from China: nomenclature of the novel coronavirus and related diseases. Chin Med J (Engl). 2020;133(9):1012–1014. doi: 10.1097/CM9.0000000000000787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorbalenya AE, Baker SC, Baric RS, et al. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020. doi: 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu A, Peng Y, Huang B, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siu YL, Teoh KT, Lo J, et al. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J Virol. 2008;82(22):11318–11330. doi: 10.1128/jvi.01052-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D, Lee JY, Yang JS, Kim JW, Kim VN, Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020. doi: 10.1016/j.cell.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan M, Wu NC, Zhu X, et al. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368(6491):630–633. doi: 10.1126/science.abb7269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Zhang Y, Wu L, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020. doi: 10.1016/J.CELL.2020.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walls AC, Tortorici MA, Bosch BJ, et al. Cryo-electron microscopy structure of a coronavirus spike glycoprotein trimer. Nature. 2016;531(7592):114–117. doi: 10.1038/nature16988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortega JT, Serrano ML, Pujol FH, Rangel HR. Role of changes in SARS-CoV-2 spike protein in the interaction with the human ACE2 receptor: an in silico analysis. EXCLI J. 2020. doi: 10.17179/excli2020-1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rambaut A, Holmes EC, O’Toole Á, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5(11):1403–1407. doi: 10.1038/s41564-020-0770-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rambaut A, Loman N, Pybus O, Barclay W. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. Virological. 2020. Available from: https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563. [Google Scholar]
- 22.Faulkner N, Ng K, Wu M, et al. Reduced antibody cross-reactivity following infection with B.1.1.7 than with parental SARS-CoV-2 strains. bioRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naveca F, Nascimento V, Souza V, et al. Phylogenetic relationship of SARS-CoV-2 sequences from Amazonas with emerging Brazilian variants harboring mutations E484K and N501Y in the spike protein. virological.org. Available from: https://virological.org/t/phylogenetic-relationship-of-sars-cov-2-sequences-from-amazonas-with-emerging-brazilian-variants-harboring-mutations-e484k-and-n501y-in-the-spike-protein/585. Accessed May12, 2021. [Google Scholar]
- 24.Millet JK, Whittaker GR. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J. Pathogenicity and transmissibility of 2019-nCoV—a quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22(2):69–71. doi: 10.1016/j.micinf.2020.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69(4):635–664. doi: 10.1128/mmbr.69.4.635-664.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2020;19(3):155–170. doi: 10.1038/s41579-020-00468-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S, Channappanavar R, Kanneganti T-D. Coronaviruses: innate immunity, inflammasome activation, inflammatory cell death, and cytokines. Trends Immunol. 2020;41(12):1083–1099. doi: 10.1016/j.it.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stech O, Veits J, Weber S, et al. Acquisition of a polybasic hemagglutinin cleavage site by a low-pathogenic avian influenza virus is not sufficient for immediate transformation into a highly pathogenic strain. J Virol. 2009;83(11):5864–5868. doi: 10.1128/jvi.02649-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang N, Shang J, Jiang S, Du L. Subunit vaccines against emerging pathogenic human coronaviruses. Front Microbiol. 2020;11:298. doi: 10.3389/fmicb.2020.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagawa K, Lokugamage KG, Makino S. Viral and cellular mRNA translation in coronavirus-infected cells. Adv Virus Res. 2016. doi: 10.1016/bs.aivir.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sola I, Almazán F, Zúñiga S, Enjuanes L. Continuous and discontinuous RNA synthesis in coronaviruses. Annu Rev Virol. 2015;2(1):265–288. doi: 10.1146/annurev-virology-100114-055218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viehweger A, Krautwurst S, Lamkiewicz K, et al. Direct RNA nanopore sequencing of full-length coronavirus genomes provides novel insights into structural variants and enables modification analysis. Genome Res. 2019;29(9):1545–1554. doi: 10.1101/gr.247064.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wijegoonawardane PKM, Sittidilokratna N, Petchampai N, Cowley JA, Gudkovs N, Walker PJ. Homologous genetic recombination in the yellow head complex of nidoviruses infecting Penaeus monodon shrimp. Virology. 2009;390(1):79–88. doi: 10.1016/j.virol.2009.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forni D, Cagliani R, Clerici M, Sironi M. Molecular evolution of human coronavirus genomes. Trends Microbiol. 2017;25(1):35–48. doi: 10.1016/j.tim.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao Y, Yan L, Huang Y, et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368(6492):779–782. doi: 10.1126/science.abb7498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirchdoerfer RN, Ward AB. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat Commun. 2019;10(1):1–9. doi: 10.1038/s41467-019-10280-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becares M, Pascual-Iglesias A, Nogales A, Sola I, Enjuanes L, Zuñiga S. Mutagenesis of coronavirus nsp14 reveals its potential role in modulation of the innate immune response. J Virol. 2016;90(11):5399–5414. doi: 10.1128/JVI.03259-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckerle LD, Becker MM, Halpin RA, et al. Infidelity of SARS-CoV Nsp14-exonuclease mutant virus replication is revealed by complete genome sequencing. PLoS Pathog. 2010;6(5):e1000896. doi: 10.1371/journal.ppat.1000896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eckerle LD, Lu X, Sperry SM, Choi L, Denison MR. High fidelity of murine hepatitis virus replication is decreased in nsp14 exoribonuclease mutants. J Virol. 2007;81(22):12135–12144. doi: 10.1128/jvi.01296-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferron F, Subissi L, De Morais ATS, et al. Structural and molecular basis of mismatch correction and ribavirin excision from coronavirus RNA. Proc Natl Acad Sci U S A. 2017;115(2):E162–E171. doi: 10.1073/pnas.1718806115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu C, Zhou Q, Li Y, et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci. 2020;6(3):315–331. doi: 10.1021/acscentsci.0c00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Den Boon JA, Ahlquist P. Organelle-like membrane compartmentalization of positive-strand RNA virus replication factories. Annu Rev Microbiol. 2010;64(1):241–256. doi: 10.1146/annurev.micro.11240.134012 [DOI] [PubMed] [Google Scholar]
- 44.Tseng YT, Wang SM, Huang KJ, Lee AI-R, Chiang C-C, Wang C-T. Self-assembly of severe acute respiratory syndrome coronavirus membrane protein. J Biol Chem. 2010;285(17):12862–12872. doi: 10.1074/jbc.M109.030270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angelini MM, Akhlaghpour M, Neuman BW, Buchmeier MJ, Moscona A. Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. MBio. 2013;4(4). doi: 10.1128/mBio.00524-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagemeijer MC, Monastyrska I, Griffith J, et al. Membrane rearrangements mediated by coronavirus nonstructural proteins 3 and 4. Virology. 2014;458–459(1):125–135. doi: 10.1016/j.virol.2014.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: current state of the science. Immunity. 2020. doi: 10.1016/j.immuni.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type i interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon B, Ansari IH, Pattnaik AK, Osorio FA. Identification of virulence determinants of porcine reproductive and respiratory syndrome virus through construction of chimeric clones. Virology. 2008;380(2):371–378. doi: 10.1016/j.virol.2008.07.030 [DOI] [PubMed] [Google Scholar]
- 50.Yang Z, Zhang X, Wang F, Wang P, Kuang E, Li X. Suppression of MDA5-mediated antiviral immune responses by NSP8 of SARS-CoV-2. bioRxiv. 2020. [Google Scholar]
- 51.Wurm T, Chen H, Hodgson T, Britton P, Brooks G, Hiscox JA. Localization to the nucleolus is a common feature of coronavirus nucleoproteins, and the protein may disrupt host cell division. J Virol. 2001;75(19):9345–9356. doi: 10.1128/jvi.75.19.9345-9356.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu X, Pan J, Tao J, Guo D. SARS-CoV nucleocapsid protein antagonizes IFN-β response by targeting initial step of IFN-β induction pathway, and its C-terminal region is critical for the antagonism. Virus Genes. 2011;42(1):37–45. doi: 10.1007/s11262-010-0544-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gharbharan A, Jordans CCE, Geurtsvankessel C, et al. Convalescent plasma for COVID-19. A randomized clinical trial. medRxiv. 2020. doi: 10.1101/2020.07.01.20139857 [DOI] [Google Scholar]
- 54.Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020. doi: 10.1101/2020.03.30.20047365 [DOI] [Google Scholar]
- 55.Lumley SF, O’Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384(6):533–540. doi: 10.1056/nejmoa2034545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hall VJ, Foulkes S, Charlett A, et al. Do antibody positive healthcare workers have lower SARS-CoV-2 infection rates than antibody negative healthcare workers? Large multi-centre prospective cohort study (the SIREN Study), England: June to November 2020. SSRN Electron J. 2021. doi: 10.2139/ssrn.3768524 [DOI] [Google Scholar]
- 57.Garcia-Beltran WF, Lam EC, Astudillo MG, et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184(2):476–488.e11. doi: 10.1016/j.cell.2020.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Synowiec A, Szczepański A, Barreto-Duran E, Lie LK, Pyrc K. Severe acute respiratory syndrome coronavirus 2 (SAR S-CoV-2): a systemic infection. Clin Microbiol Rev. 2021;34(2):e00133–20. doi: 10.1128/CMR.00133-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larson AS, Savastano L, Kadirvel R, Kallmes DF, Hassan AE, Brinjikji W. Coronavirus disease 2019 and the cerebrovascular-cardiovascular systems: what do we know so far? J Am Heart Assoc. 2020;9(13). doi: 10.1161/JAHA.120.016793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marik PE, Kory P, Varon J, Iglesias J, Meduri GU. MATH+ protocol for the treatment of SARS-CoV-2 infection: the scientific rationale. Expert Rev Anti Infect Ther. 2021;19(2):129–135. doi: 10.1080/14787210.2020.1808462 [DOI] [PubMed] [Google Scholar]
- 62.Kashour Z, Riaz M, Garbati MA, et al. Efficacy of chloroquine or hydroxychloroquine in COVID-19 patients: a systematic review and meta-analysis. J Antimicrob Chemother. 2021;76(1):30–42. doi: 10.1093/jac/dkaa403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gasmi A, Peana M, Noor S, et al. Chloroquine and hydroxychloroquine in the treatment of COVID-19: the never-ending story. Appl Microbiol Biotechnol. 2021;105(4):1333–1343. doi: 10.1007/s00253-021-11094-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wise J. Covid-19: convalescent plasma may cut deaths in patients not on ventilation, study indicates. BMJ. 2021. doi: 10.1136/bmj.n130 [DOI] [PubMed] [Google Scholar]
- 66.Li -D-D, Li Q-H. SARS-CoV-2: vaccines in the pandemic era. Mil Med Res. 2021;8(1):1. doi: 10.1186/s40779-020-00296-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Z, Zheng H, Yuan R, et al. Identification of a common deletion in the spike protein of SARS-CoV-2. bioRxiv. 2020. doi: 10.1101/2020.03.31.015941 [DOI] [Google Scholar]
- 68.Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J Microbiol Immunol Infect. 2020;54(2):159–163. doi: 10.1016/j.jmii.2020.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li W, Kuhn JH, Moore MJ, et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24(8):1634–1643. doi: 10.1038/sj.emboj.7600640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Q, Wong G, Lu G, Yan J, Gao GF. MERS-CoV spike protein: targets for vaccines and therapeutics. Antiviral Res. 2016;133:165–177. doi: 10.1016/j.antiviral.2016.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Volz E, Mishra S, Chand M, et al. Transmission of SARS-CoV-2 Lineage B.1.1.7 in England: insights from linking epidemiological and genetic data. medRxiv. 2021. [Google Scholar]
- 72.Sabino EC, Buss LF, Carvalho MPS, et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397(10273):452–455. doi: 10.1016/S0140-6736(21)00183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.WHO. Weekly epidemiological update on COVID-19 - 20 April 2021. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---20-april-2021. Accessed 20 April 2021. [Google Scholar]