Abstract
A 61-year-old man underwent total gastrectomy with esophago-jejunostomy for Borrmann type I gastric cancer. Postoperative intra-abdominal abscess made the patient unable to receive adjuvant chemotherapy. Only 23 weeks after operation, the patient developed melena and anemia, leading to the diagnosis of recurrence in the jejunum close to the anastomotic site. The patient received salvage resection of the recurrence. Pathological study showed that the tumor was composed of atypical cells similar to those of the primary gastric cancer. Normal jejunal mucosa was observed between the esophagus and the recurrent tumor. We judged that exfoliation of the gastric cancer cells caused the recurrence due to both the very short disease-free interval and pathological findings. Surgeons should pay attention to this type of recurrence especially for Borrmann type I gastric cancer. In addition to the adjuvant chemotherapy, gastric irrigation using distilled water during the operation seems to be a feasible measure to prevent this type of recurrence.
Keywords: Borrmann type I gastric cancer, Cancer cell exfoliation, Gastric irrigation with distilled water, Recurrence in the anastomotic site
Introduction
Due to both the development of novel imaging technologies and specialized endoscopic resection techniques, many patients with mucosal and submucosal gastric cancers have been treated not with conventional surgeries, but with endoscopic mucosal resection or endoscopic submucosal dissection. On the other hand, many patients with invasive gastric cancer still need laparoscopic or open surgery, and some patients unfortunately develop locoregional and/or distant recurrence.
Cancer metastases and/or dissemination is often found in the lymph node, liver, lung, and peritoneum. Although its mechanism has not yet been clarified, suture-line recurrence, especially after esophago-jejunostomy, has been observed in 3–10% of patients [1].
We herein report an extremely rare case of gastric cancer recurrence in the jejunum very close to the anastomotic site after total gastrectomy [2, 3, 4].
Case Report
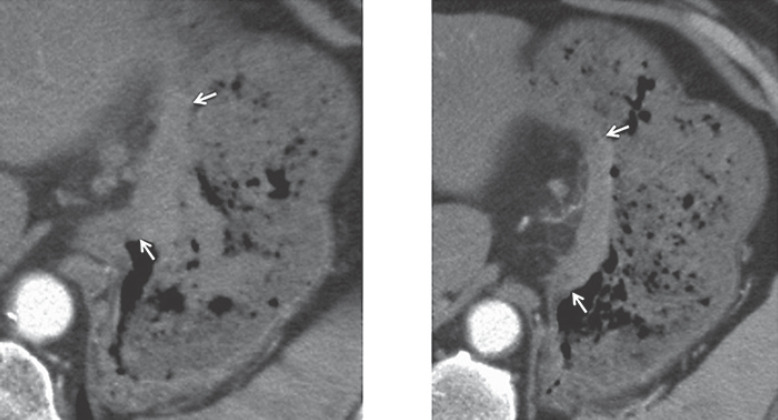
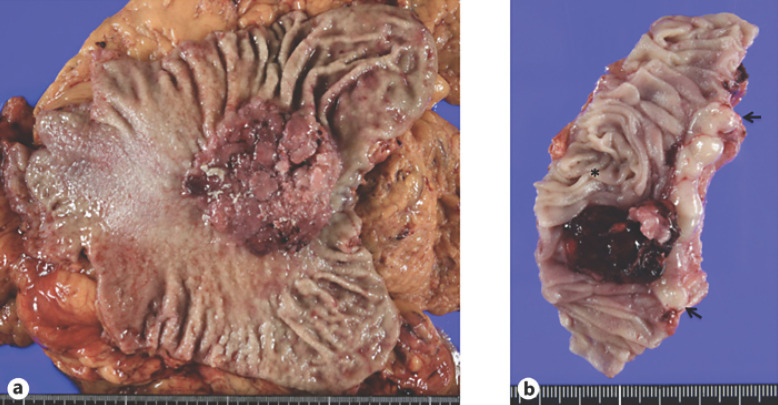
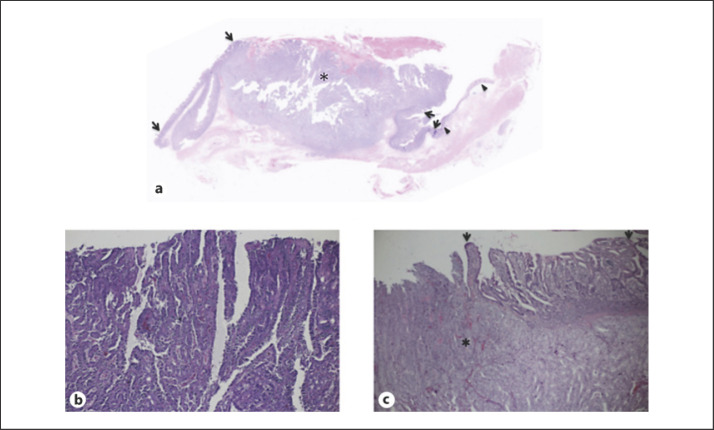
A 61-year-old man with anemia and melena was referred to our hospital. Endoscopy showed a polypoid tumor (Fig. 1) partially covered with coagula in the upper body of the stomach, leading to the diagnosis of gastric adenocarcinoma, tubular well-differentiated type, with forceps biopsies. After imaging studies for staging, the patient underwent laparoscopic total gastrectomy with esophago-jejunostomy. Pathological studies of the resected stomach showed a node-positive (n:6/57) Borrmann type I gastric cancer in the lesser curvature, 68 × 68 mm in size (Fig. 2a), with tubular and papillary growth (Fig. 3b) extending from the luminal surface to the serosa of the stomach. Both surgical margins were pathologically negative. The patient recovered uneventfully and was discharged on the 9th postoperative day. Due to the massive lymph node involvement, the patient was scheduled to receive adjuvant chemotherapy using capecitabine and oxaliplatin. The patient, however, developed intra-abdominal abscess around the spleen, leading to the readmission with antibiotics therapy and the abandonment of adjuvant chemotherapy. Only 23 weeks after the operation, the patient again developed melena and anemia (Hb 7.4 g/dL). Gastrointestinal endoscopy revealed an oozing polypoid mass around the anastomotic site. Pathological examination revealed papillary adenocarcinoma similar to that of the primary gastric carcinoma. After confirming no distant and regional metastases and normal tumor marker levels, the patient underwent resection of the presumed recurrence in the jejunum (Fig. 2b) with Roux-en-Y reconstruction. Neither peritoneal dissemination nor lymph node metastases were observed. Macroscopically, the tumor was located in the jejunum 1 cm apart from the anastomotic site (Fig. 3a). Pathological study showed that the tumor was composed of atypical cells extending from the luminal surface with papillary growth to the muscle layer with tubular growth. Normal jejunal mucosa was observed between the esophagus and the tumor (Fig. 3c). The patient recovered uneventfully and was discharged in 2 weeks after the operation.
Fig. 1.
Computed tomography of the stomach. Protruding-type gastric cancer (arrows), i.e. Borrmann type I, located in the upper part of the stomach body.
Fig. 2.
Macroscopic appearance of the resected specimen. a Primary gastric cancer; Borrmann type I. A large polypoid tumor with a broad base. b Recurrent gastric cancer; a polypoid mass covered with coagula located mainly in the jejunum (asterisk) close to the esophagus (arrows).
Fig. 3.
Pathological findings. a Recurrent tumor; low magnification view showed normal esophageal epithelium (arrowheads), intact jejunal epithelium (arrows), and recurrent tumor (asterisk). b Primary gastric cancer; magnified view showed atypical cells with tubule formation. c Recurrent tumor; recurrent gastric cancer (asterisk) similar to that of primary gastric cancer located adjacent to normal jejunal epithelium (arrows) near the anastomotic site.
Discussion
It is well-recognized that gastric cancer spreads to the liver through portal system and to the regional nodes via lymphatics. Cancer cell exfoliation from the bulging gastric cancer beyond serosa leads to peritoneal dissemination, often causing unfavorable ascites [5]. In this case, neither lymphatic nor hematogenous spread could cause the recurrence around the anastomotic site due to the following reasons. Firstly, despite the massive lymph node involvement, margin negativity of the resected stomach negates the submucosal lymphatic permeation to the jejunum. Secondly, recurrence was observed not in the esophagus but in the jejunum, far distant, i.e. 35 cm, from the primary lesion. Thirdly, hematogenous cancer cell spread specific to the jejunum is unlikely. Lastly, disease-free interval (DFI) of 23 weeks in this case is extremely short for gastric cancer recurrence. Miyoshi et al. [3] also reported a similar case of recurrent gastric cancer in the anastomotic site with very short DFI of 4 months. Short DFI may be characteristic of this type of recurrence.
Free cancer cells can be observed in more than half of the patients with gastric washing using lactated Ringer's solution [6], resulting in high cancer cell exposure to the operative field including anastomotic site(s). All the reported cases [2, 3, 4], including our present case, of gastric cancer recurrence in the anastomotic site had primary gastric cancer with protruding growth, i.e. Borrmann type I. Polypoid gastric cancer may be more exfoliative than other type gastric cancers, leading to its easy dissemination via the digestive tract.
Inevitable abandonment of adjuvant chemotherapy due to postoperative complication might facilitate the anastomotic site recurrence in this case. Meta-analyses have proven the benefit of adjuvant chemotherapy, i.e. 15% reduction of mortality, for gastric cancer [7]. Adjuvant chemotherapy, however, is often not applicable for some patients with various reasons such as comorbidity, old age, and postoperative complication like in this case.
In clinical practice, even though many exfoliated viable cancer cells could reach to the small intestine, patients with Borrmann type I gastric cancer scarcely have or develop metastasis to the small intestine. There are two possible reasons for this. Firstly, intact mucosa of the small intestine might reject the cancer cell implantation. Secondly, Ohki et al. [6] confirmed, by gastric washing, a lower detection rate of cancer cells with swollen and ruptured shape in the distilled water than that in the lactate Ringer's solution. It is well known that hypotonic solution can easily cause cell damage, leading to the prevention of cancer cell dissemination. This fact well explains why patients with gastric cancer scarcely have or develop transluminal metastasis due to the water, i.e. hypotonic solution, intake in daily life. Therefore, gastric irrigation with distilled water during the operation should be a feasible measure to prevent transluminal metastasis.
In conclusion, surgeons should pay attention to the transluminal metastasis in the gastric, especially Borrmann type I, cancer. Recurrent site in this case is not at the suture line but jejunum very close to the suture line, suggesting mucosal damage due to presumed rough operative procedures. Surgeons, therefore, should operate in a more gentle and sophisticated manner to prevent this type of recurrence. In addition, the efficacy of gastric irrigation using distilled water during the operation should be evaluated for gastric cancer operation.
Statement of Ethics
We have reported this case in compliance with the Declaration of Helsinki. Written informed consent was obtained from the family of the deceased patient for the publication of the clinical data.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
Not applicable.
Author Contributions
H.S. contributed to the design of the report and collected the data. S.O. drafted the manuscript, S.M. revised the manuscript. All authors have read and approved the final version of the manuscript.
References
- 1.Papachristou DN, Karas M, Fortner JG. Anastomotic recurrence in the oesophagus complicating gastrectomy for adenocarcinoma of the stomach. Br J Surg. 1979;66((9)):609–12. doi: 10.1002/bjs.1800660904. [DOI] [PubMed] [Google Scholar]
- 2.Namikawa T, Kobayashi M, Okamoto K, Okabayashi T, Akimori T, Sugimoto T, et al. Recurrence of gastric cancer in the jejunal pouch after completion gastrectomy. Gastric Cancer. 2007;10((4)):256–9. doi: 10.1007/s10120-007-0441-8. [DOI] [PubMed] [Google Scholar]
- 3.Miyoshi K, Fuchimoto S, Ohsaki T, Sakata T, Takeda I, Takahashi K, et al. Suture line recurrence in jejunal pouch replaced after total gastrectomy for gastric cancer. Gastric Cancer. 1999;2((3)):194–7. doi: 10.1007/s101200050046. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura M, Honda I, Watanabe S, Nagata M, Souda H, Miyazaki M. Recurrence in jejunal pouch after proximal gastrectomy for early upper gastric cancer. Gastric Cancer. 2003;6((3)):197–201. doi: 10.1007/s10120-003-0242-7. [DOI] [PubMed] [Google Scholar]
- 5.Virgilioa E, Balduccib G, Mercantinib P, Giarnieric E, Giovagnolic MR, Montagninic M, et al. Preoperative gastric lavage in gastric cancer patients undergoing surgical, endoscopic or minimally invasive treatment: An oncological measure preventing peritoneal spillage of intragastric cancer cells and development of related metastases. Med Hypotheses. 2018;114:30–4. doi: 10.1016/j.mehy.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Ohki A, Abe N, Yoshimoto E, Hashimoto Y, Takeuchi H, Nagao G, et al. Gastric washing by distilled water can reduce free gastric cancer cells exfoliated into the stomach lumen. Gastric Cancer. 2018;21((6)):998–1003. doi: 10.1007/s10120-018-0824-z. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Nieto R, Orti-Rodríguez R, Winslet M. Post-surgical chemotherapy versus surgery alone for resectable gastric cancer. Cochrane Database Syst Rev. 2013;((9)):CD008415. doi: 10.1002/14651858.CD008415.pub2. [DOI] [PubMed] [Google Scholar]