Abstract
PDAC (pancreatic ductal adenocarcinoma) is among the most deadly of human malignances. A hallmark of the disease is a pronounced collagen-rich fibrotic extracellular matrix known as the desmoplastic reaction. Intriguingly, it is precisely these areas of fibrosis in which human PDAC tumours demonstrate increased expression of a key collagenase, MT1-MMP [membrane-type 1 MMP (matrix metalloproteinase); also known as MMP-14]. Furthermore, a cytokine known to mediate fibrosis in vivo, TGF-β1 (transforming growth factor-β1), is up-regulated in human PDAC tumours and can promote MT1-MMP expression. In the present review, we examine the regulation of PDAC progression through the interplay between type I collagen (the most common extracellular matrix present in human PDAC tumours), MT1-MMP and TGF-β1. Specifically, we examine the way in which signalling events through these pathways mediates invasion, regulates microRNAs and contributes to chemoresistance.
Keywords: chemoresistance, fibrosis, microRNA, membrane–type 1 matrix metalloproteinase (MT1-MMP), pancreatic cancer, Snail, transforming growth factor-β (TGF-β)
INTRODUCTION
PDAC (pancreatic ductal adenocarcinoma) is among the most aggressive of human malignancies and a leading cause of cancer-related mortality. Worldwide, there are over 200000 deaths from PDAC each year, with 38000 annual deaths in the U.S.A. alone, as well as over 60000 deaths in Europe [1-3]. Yet what is perhaps most striking about this disease, among other cancers with a dismal prognosis, is the rapidity of its inexorable progression [4,5]. Despite the use of intense chemotherapy and targeted therapies, the median survival of patients with PDAC is less than 1 year. Overall 5-year survival is less than 5% [4,5]. Over 80% of patients with PDAC present with disease that is not amenable to surgical resection because of either advanced local tumour growth or spread to distant sites. Even for those who do undergo surgery, treatment remains arduous with a 5-year survival of only 20% [6]. Several factors are thought to contribute to the aggressive nature of PDAC. The location of the pancreas deep within the abdomen means patients are often asymptomatic until the disease is too advanced for curative options. Unlike some malignancies in which patients present on screening examinations with small tumours that can be easily excised without harming surrounding tissue, many pancreatic cancer patients come to medical attention because of jaundice from obstruction of the bile ducts or pain from invasion of the surrounding nerves [4,5]. By this stage, the tumour has typically infiltrated the pancreaticobiliary system, often encasing vital blood vessels in the vicinity, thereby excluding surgical resection. In addition, in a unique molecular hallmark, PDAC is also known to be associated with a dense collagen-rich fibrotic reaction (Figure 1) [7-9]. This so-called desmoplastic reaction is known to contribute to disease progression and chemoresistance [10,11].
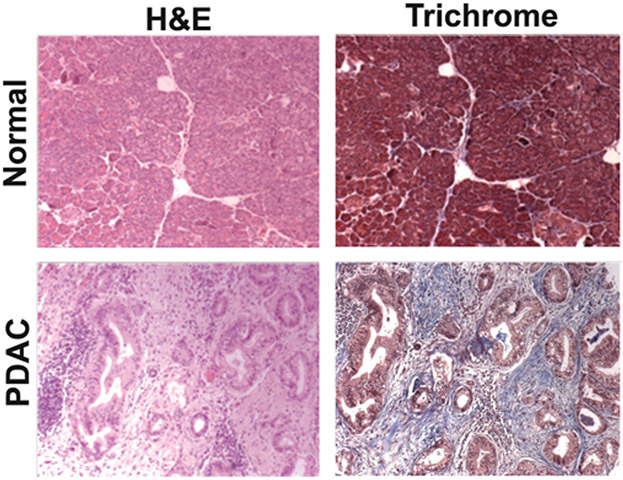
Figure 1. Desmoplastic reaction and pancreatic cancer.
H&E (haematoxylin and eosin) and trichrome stains of normal pancreas and a human PDAC tumour. Note the increased fibrosis (blue staining) in the PDAC tumour compared with the normal pancreas.
ROLE OF THE DESMOPLASTIC REACTION IN PANCREATIC CANCER
Human PDAC tumours are predominantly composed of non-neoplastic inflammatory and fibroblastic cells and contain tremendous quantities of collagen-rich ECM (extracellular matrix) [7,8]. Unlike most other tumours, this desmoplastic reaction is a characteristic feature of human PDAC and is most striking when seen side-by-side with areas of relatively normal pancreas in which only minimal fibrosis is observed [7,8]. Importantly, transgenic mouse models have been able to recapitulate the stromal changes seen in human PDAC tumours. For example, PDAC tumours developing in mice expressing activated K-RasG12D together with either inactivation of the Ink-4a/Arf tumour suppressor locus or p53 demonstrate significant desmoplasia with increased collagen expression [12-15]. In human disease, the correlate for this finding is an increased amount of interstitial fibrillar collagen (types I and III) as compared with normal pancreas [16,17]. Furthermore, during PDAC progression the normal basement membrane architecture is lost and malignant cells are directly exposed to interstitial collagen [16-18].
Myofibroblasts and the desmoplastic reaction
Beyond changes in the composition of the ECM, the desmoplastic reaction is also associated with accelerated proliferation of fibroblasts, in some cases outnumbering local tumour cells [19,20]. A more detailed molecular analysis demonstrates that these proliferating cells are mesenchymal (or stellate) cells that have differentiated into an activated myofibroblastic phenotype [19-21]. As activated myofibroblasts, they exhibit increased expression of the cytoskeletal protein α-smooth muscle actin and have been identified as the primary source of type I collagen that is present in the desmoplastic reaction. Importantly, there is significant cross-talk between fibroblasts and cancer cells, as the invasive potential of PDAC cells is greatly enhanced by coculture with stromal fibroblasts [22,23]. Co-injection of PDAC tumour cells with human stellate cells in an orthotopic mouse model results in increased primary tumour incidence, size and metastasis, notably with a direct correlation with the proportion of stellate cells present [23]. Genes encoding for proteins already known to be associated with tumour invasion, metastasis and angiogenesis are also differentially expressed in both PDAC cells and fibroblasts as a consequence of this interaction [24].
Fibrillar collagen and PDAC progression
PDAC is a malignancy with a widely recognized metastatic potential, yet one of the hallmarks of the disease is a dense fibrotic ECM that would seem to serve as a barrier to invasion. Indeed, interstitial collagens are highly resistant to proteolysis owing to their triple helical structure and fibrillar organization [25-27]. Breakdown of fibrillar collagen helices requires the enzymatic activity of specific collagenases, including interstitial collagenase [MMP (matrix metalloproteinase)-1], neutrophil collagenase (MMP-8), collagenase 3 (MMP-13) and MT1 (membrane type 1)-MMP [28,29]. Cathepsins have also been implicated in collagen cleavage, particularly under conditions of acidic pH [30]. On the basis of structural assessment alone, it would seem that the desmoplastic reaction should be a barrier and not an aid to PDAC progression. However, in a paradoxical twist, multiple studies have shown that type I collagen enhances tumour progression. Instead of blocking tumour cell motility, it is now clear that collagen fibres can act as highways for migration and facilitate metastasis by directing cancer cells to the all-important vasculature needed for local proliferation and distant spread [31,32]. In keeping with these findings, increased expression of type I collagen is detected in gene expression signatures associated with increased risk of metastasis [33-35]. Our own work has also shown that type I collagen can affect PDAC behaviour by increasing cellular motility and promoting expression of MT1-MMP [36,37].
It is also possible that the desmoplastic reaction in pancreatic tumours contributes to increased tumour migration and invasion through increased expression of an MMP-resistant isoform of type I collagen [38,39]. The normal isoform of type I collagen is an α12α2 heterotrimer consisting of two α1(I) chains and one α2(I) chain [25,40]. However, an α13 homotrimeric isoform of type I collagen composed of three α1(I) chains has been found in carcinomas in vivo and in cancer cells grown in vitro [41-46]. Although it remains unknown whether or not human PDACs express the α13 homotrimeric collagen isoform, these homotrimers are resistant to all collagenolytic MMPs and to degradation by cancer cells and fibroblasts [38,39]. Importantly, cancer cells demonstrate enhanced migration atop such atypical homotrimeric type I collagen fibres when compared with heterotrimetic type I collagen fibres found in normal tissue [38,47]. However, in the multicellular milieu of a developing carcinoma, collagen homotrimers are produced primarily by cancer cells and not by cancer-associated fibroblasts [38], which primarily contribute to the stromal reaction in pancreatic cancer [8,48]. As a result, collagen homotrimers probably comprise only a small, yet significant, pro-tumorigenic fraction of the different collagens found in the heterogenous environment of tumour tissue.
Type I collagen can also regulate cellular proliferation by modulating intracellular signals transduced by cell-surface receptors [49]. Notably, upon binding to integrins or discoidin domain receptors, type I collagen activates a series of intracellular signalling events with profound effects on cell-cycle regulatory proteins, cyclins and CDKs (cyclin-dependent kinases) [50,51]. The activation of these signalling pathways is highly dependent upon the physical state of type I collagen: fibrillar collagen may inhibit cell growth, whereas non-fibrillar collagen may have no effect. More specifically, fibrillar type I collagen inhibits vascular smooth muscle cell proliferation by increasing the levels of the CDK inhibitors p21Cip1 and p27Kip1 [52]. Moreover, type I collagen can induce opposing cellular effects based upon the tensile strength and structure of the extracellular milieu [53]. When grown in two-dimensional collagen films, hepatocytes demonstrate enhanced cyclin D1 expression and DNA synthesis, yet, when grown in three-dimensional collagen gels, cyclin D1 expression and DNA synthesis are inhibited [54]. This difference in cyclin D1 expression between the two-dimensional and three-dimensional microenvironments was shown to result from differential activation of MAPK (mitogen-activated protein kinase) signalling. Hepatocytes on two-dimensional collagen films demonstrate increased ERK (extracellular-signal-regulated kinase) 1/2 phosphorylation compared with cells in three-dimensional collagen gel; inhibiting ERK1/2 activation with PD98059 blocks both cyclin D1 expression and DNA synthesis [54]. Furthermore, as the structural rigidity of the ECM increases, integrin-mediated intracellular signalling can be induced [55-57]. As the ECM becomes less flexible, force-dependent integrin clustering in breast cancer cells increases, in turn enhancing ERK1/2 activation and cell motility [55,56]. Since the desmoplastic reaction in pancreatic tumours is associated with increased cross-linking of fibrillar collagen (and thereby increased rigidity of the ECM), the collagen-rich desmoplastic reaction may also contribute to PDAC progression by enhancing tension-dependent integrin-mediated signalling in PDAC cells.
The desmoplastic reaction and chemoresistance
The fibrotic extracellular environment that develops in the setting of PDAC does not appear to prevent tumour cell invasion out of the site of the primary tumour, but more and more data have now implicated the desmoplastic reaction as a substantial barrier to the effective targeting of chemotherapeutic agents into the site of primary disease. In a transgenic mouse model of PDAC, both delivery and efficacy of gemcitabine were improved following treatment with Hedgehog signalling pathway inhibitors which deplete the tumour-associated stromal reaction [10]. We have found that pancreatic cancer cells grown in three-dimensional collagen demonstrate decreased sensitivity to gemcitabine chemotherapy and continue to proliferate despite drug treatment [11]. In contrast with the effects of three-dimensional collagen on ERK1/2 signalling in hepatocytes, PDAC cells embedded in three-dimensional collagen show increased ERK1/2 signalling and subsequent attenuation of the effects of gemcitabine. Inhibiting ERK1/2 phosphorylation sensitizes PDAC cells in three-dimensional collagen to gemcitabine and causes growth arrest. ERK1/2 signalling is known to promote chemotherapy resistance in several different cancers, including multiple myeloma [58], HCC (hepatocellular carcinoma) [59] and PDAC [60]. Interestingly, in PDAC models, the prochemoresistance effect of collagen is only seen when the cells are grown in three-dimensional collagen and not atop three-dimensional collagen gels [11]. We have found that this is not due to differences in drug delivery to cancer cells, but rather to the increased DNA damage response induced in the three-dimensional collagen microenvironment (S. Dangi-Garimella and H. G. Munshi, unpublished work). This finding of type I collagen directly protecting cancer cells against chemotherapy has also been noted in other malignancies; previous studies in lung cancer models have shown that collagen provides survival signals to attenuate the effects of chemotherapy and to allow for continued tumour cell proliferation [61].
ROLE OF MT1-MMP IN PDAC PROGRESSION
Results of gene expression studies of stromal and neoplastic cells at the site of primary PDAC invasion have shown that MT1-MMP, a primary interstitial collagenase, is overexpressed by pancreatic cancer cells [62]. Immunohistochemistry and in situ hybridization studies have further clarified that MT1-MMP is overexpressed in pancreatic tumours relative to normal pancreas and that expression of MT1-MMP is enhanced in metastatic PDAC lesions as compared with their primary tumour of origin [36,63-65]. Genetic studies support the role of MT1-MMP as a primary regulator of interstitial collagenolysis, as mice genetically deficient in MT1-MMP have severe growth defects due to their subsequent inability to process interstitial collagens during critical stages of bone and soft tissue formation [66,67]. Moreover, in vitro studies using organotypic and three-dimensional culture systems demonstrate that MT1-MMP is the only collagenase to confer a three-dimensional growth advantage, presumably via removing the structural confines of the ECM and enabling the structural changes in the geometry of the cellular microenvironment necessary to drive proliferative responses [68,69]. Interestingly, in these studies cells failed to proliferate when embedded in collagenase-resistant three-dimensional type I collagen gels, suggesting that cleavage of type I collagen by MT1-MMP is necessary for cellular proliferation in a collagen-dominated microenvironment [68]. Indeed, overexpression of MT1-MMP alone may be sufficient to induce tumour formation. Overexpression of MT1-MMP in mouse mammary gland using a transgenic model induced adenocarcinoma of the breast [70]. Similarly, overexpression of MT1-MMP in non-tumorigenic well-differentiated MDCK (Madin–Darby canine kidney) cells was sufficient to drive formation of invasive tumours in nude mice [71].
Beyond its role in facilitating growth and invasion in the collagen microenvironment, MT1-MMP has also been implicated in the generation of the collagen-rich stromal reaction. It was previously noted that expression of MT1-MMP in the mouse mammary gland led to significant fibrosis [70], whereas expression of MT1-MMP in MDCK, squamous cell carcinoma and pancreatic cancer cells caused significant fibrosis when the cells were injected in vivo [11,70-72]. Our own work has shown that expression of MT1-MMP in the mouse pancreas causes large lesions associated with a pronounced stromal reaction [73]. These lesions are further associated with an increased number of α-smooth muscle actin(+) cells, findings consistent with the increased numbers of myofibroblasts found in human pancreatic tumours. The increased fibrosis is also associated with increased Smad2 phosphorylation [73], indicating increased TGF-β (transforming growth factor β) signalling in MT1-MMP-expressing mice. We have also shown that the positive association between MT1-MMP, fibrosis and increased Smad2 phosphorylation is present in human pancreatic cancer specimens [36], further suggesting significant cross-talk between MT1-MMP and TGF-β signalling during PDAC progression (also see below).
MT1-MMP and chemoresistance
In the complex interplay between the extracellular microenvironment and the proliferation and metastatic potential of pancreatic cancer cells, MT1-MMP can function to regulate the nuances of chemotherapy resistance generated by the collagen microenvironment. We recently showed that blocking MT1-MMP expression or function in three-dimensional collagen sensitizes PDAC cells to gemcitabine [11]. Although MT1-MMP does not affect the response to gemcitabine when cells are grown on two-dimensional surfaces, modulation of MT1-MMP does significantly affect the chemotherapy response in cells grown in three-dimensional collagen. It is now well established that intercellular focal adhesions in the three-dimensional setting have a different molecular composition when compared with intercellular two-dimensional focal adhesions, further underscoring the role of microenvironment-dependent signalling in the global context of cellular behaviour [74]. The functional implications of the extracellular milieu for cancer cell signalling and behaviour are only recently becoming more notable. In breast cancer cells, MT1-MMP can increase the functionality of the collagen-binding α2β1 integrin [75]. Additionally, the ILK (integrin-linked kinase), a kinase capable of interaction with the cytoplasmic tail of β1 integrin, has been shown to contribute to gemcitabine resistance in PDAC cells [76]. Taken together, these findings suggest that it may be possible that MT1-MMP potentiates integrin signalling only in the three-dimensional microenvironment, thus enhancing PDAC gemcitabine resistance.
Mechanistically, it has been established that MT1-MMP in the collagen microenvironment increases ERK1/2 signalling to promote gemcitabine resistance. Overexpression of MT1-MMP increases ERK1/2 phosphorylation and increases gemcitabine resistance, whereas siRNA (small interfering RNA) against MT1-MMP decreases ERK1/2 phosphorylation and sensitizes pancreatic cancer cells to gemcitabine [11]. Despite the fact that MT1-MMP has been reported to increase ERK1/2 signalling via the cytoplasmic tail [77], pancreatic cancer cells expressing MT1-MMP devoid of the cytoplasmic tail nonetheless demonstrate robust ERK1/2 phosphorylation in the collagen microenvironment [11]. Although it has also been reported that TIMP (tissue inhibitor of metalloproteinases)-2 binding to MT1-MMP induces ERK1/2 phosphorylation in the absence of MT1-MMP proteolytic activity [78], MT1-MMP-induced ERK1/2 phosphorylation in the collagen microenvironment does require the catalytic activity of this proteinase. MT1-MMP enhances ERK1/2 phosphorylation in HSC-4 oral cancer cells grown in three-dimensional collagen, with the increased ERK1/2 phosphorylation blocked by MMP inhibitors [79]. Increased ERK1/2 phosphorylation in three-dimensional collagen can also be blocked by both an αvβ3 function-blocking antibody and siRNAs directed against Src and paxillin [79], suggesting that MT1-MMP in three-dimensional collagen enhances integrin signalling. Additionally, we have found that the increased ERK1/2 phosphorylation in three-dimensional collagen can be attenuated by blocking TGF-β signalling [80], indicating that MT1-MMP may also modulate growth factor signalling in three-dimensional collagen to increase ERK1/2 activation. Consistent with this hypothesis, PDGF-β (platelet-derived growth factor-β-induced ERK1/2 phosphorylation in VSMCs (vascular smooth muscle cells) in the collagen microenvironment is blocked by TIMP-2 and is significantly attenuated in MT1-MMP-null VSMCs [81]. Interestingly, PDGF-β-induced ERK1/2 phosphorylation in MT1-MMP-null VSMCs can be restored by introducing either full-length MT1-MMP or MT1-MMP devoid of its cytoplasmic tail, but not by introducing the catalytically inactive form of MT1-MMP [81]. Taken together, these results suggest that MT1-MMP modulates growth factor and/or integrin signalling in the collagen microenvironment to enhance ERK1/2 phosphorylation, and thereby promote chemoresistance.
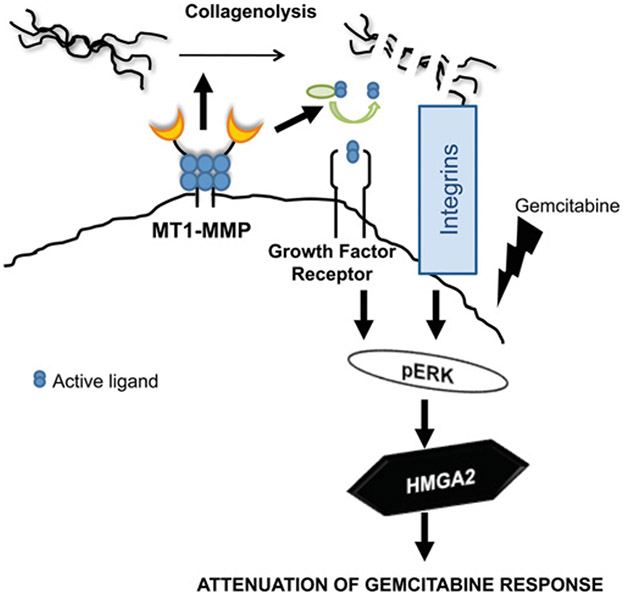
MT1-MMP also increases expression of another key protein associated with chemoresistance: the HMGA2 (high-mobility group A2) protein (Figure 2) [11]. HMGA2 is regulated by let-7 family microRNAs (see below) and is a non-histone DNA-binding protein involved in chromatin remodelling and gene transcription [82,83]. HMGA proteins induce conformational changes in bound DNA and promote recruitment of regulators of transcription [83]. In addition to their role in chromatin remodelling, HMGA proteins have also been shown to be involved in chemoresistance, specifically as a determinant of chemoresistance in PDAC [84]. HMGA2 is part of the base end-joining repair machinery that removes small damaged bases from DNA, and has an apurinic/apyrimidinic lyase activity which can protect against the effects of chemotherapy agents designed to target tumour cell DNA [85]. HMGA2 can also increase expression of ATM (ataxia telangiectasia mutated), the main cellular sensor of genotoxic stress, and thereby increasing resistance to DNA-damaging agents [86]. In addition, through a mechanism that remains as yet unknown, HMGA2 can also maintain cancer stem cells in the undifferentiated state [82]. Stem cells are resistant to most cytotoxic chemotherapy [87], thus, in an example of the complexity of molecular circuitry, the extracellular collagenase MT1-MMP may ultimately play a role in inducing chemotherapy resistance in PDAC by increasing expression of an intracellular protein HMGA2 that subsequently modulates cancer stem cells.
Figure 2. MT1-MMP in three-dimensional collagen contributes to chemotherapy resistance by increasing ERK1/2 activity and HMGA2 levels.
MT1-MMP through proteolytic processing of collagen or activation of growth factors modulates integrin and/or growth factor receptor signalling to enhance ERK1/2 phosphorylation and HMGA2 expression in pancreatic cancer cells. This signalling pathway functions to attenuate the effect of gemcitabine chemotherapy in the three-dimensional collagen microenvironment.
Regulation of MT1-MMP
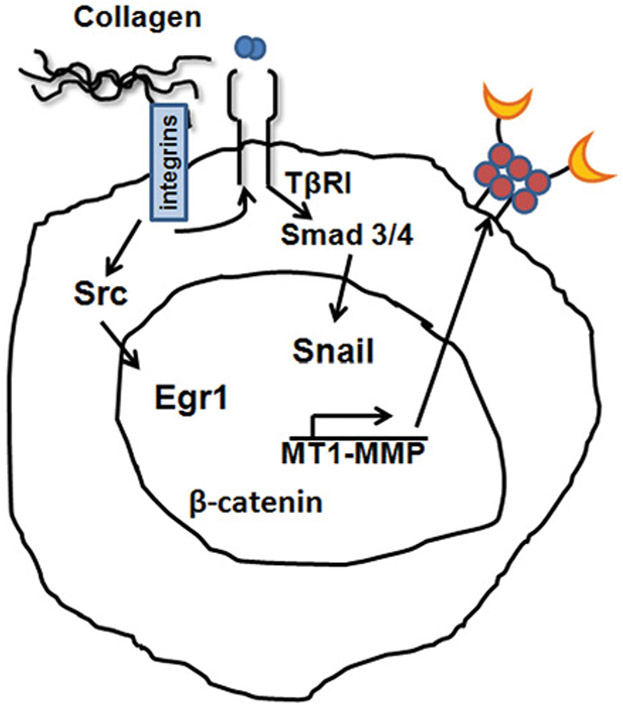
MT1-MMP plays a critical physiological role as a major interstitial collagenase. Accordingly, its expression is highly regulated by several key transcription factors (Figure 3). Earlier reports have shown that culturing endothelial cells in three-dimensional collagen gels induced expression of Egr-1 leading to transcriptional activation of the MT1-MMP promoter, an association mediated in part by displacing the pre-bound basal transcription factor Sp1 [88,89]. Previously, it was demonstrated that three-dimensional collagen also induces Egr1 expression to subsequently promote MT1-MMP expression and invasion of ovarian cancer cells [90]. The three-dimensional collagen-induced Egr1 expression in ovarian cancer cells was mediated by β1-integrin signalling through a Src kinase-dependent pathway [90]. MT1-MMP transcription can also be up-regulated by the well-known β-catenin–Tcf4 complex [91], placing MT1-MMP as a direct downstream target of the canonical Wnt signalling pathway. In addition, the transcription factor Snail, already known to be involved in the repression of E-cadherin during the pivotal EMT (epithelial–mesenchymal transition), can also up-regulate MT1-MMP expression [92,93]. Previous studies have shown that stable transfection of Snail in liver cancer cells increased MT1-MMP expression [92]. More recently, inducible expression of Snail in MCF7 breast cancer cells was also shown to increase MT1-MMP levels, thereby promoting growth and invasion in a chick chorioallantoic membrane assay [94]. Furthermore, fibroblasts in which Snail had been conditionally knocked out showed a decrease in MT1-MMP levels with attenuation of invasion in the collagen microenvironment [95]. Our own studies have shown that Snail also increases MT1-MMP in pancreatic cancer cells, and blocking MT1-MMP with siRNA attenuates Snail-induced invasion [37]. Mechanistically Snail increases ERK1/2 phosphorylation, whereas blocking ERK1/2 activation inhibits both Snail-induced MT1-MMP expression and collagen invasion. Taken together, these findings demonstrate that crucial signalling pathways known to be important in tumour progression and invasion co-ordinately regulate MT1-MMP expression.
Figure 3. Collagen regulation of MT1-MMP mRNA expression.
Pancreatic cancer cells in three-dimensional collagen up-regulate MT1-MMP through activation of the TGF-β/TβRI/Smad3/Snail pathway. MT1-MMP can also be induced in the collagen microenvironment through the β1-integrin/Src/Egr1 signalling pathway. Although β-catenin signalling can regulate MT1-MMP, it is not known whether collagen regulation of MT1-MMP also involves β-catenin signalling.
ROLE OF TGF-β IN THE DESMOPLASTIC REACTION
TGF-β is frequently overexpressed in PDAC and is associated with both advanced tumour stage and a significant decrease in survival [8,96,97]. TGF-β signals through cell-surface serine/threonine kinases to initiate cellular responses [98,99]. TGF-β binding to its type II receptor (TβRII) promotes TβRII association with the type I receptor (TβRI), followed by a phosphorylation event which then leads to subsequent phosphorylation of the R-Smads (receptor-associated Smads) Smad2 and Smad3. The phosphorylated R-Smads then bind to Smad4 and the complex translocates to the nucleus, wherein it can associate with transcription factors to regulate expression of target genes. Mutations in Smad4 are seen in an estimated 50% of PDAC tumours [100,101] and have been shown to affect tumour suppressive effects of TGF-β signalling; restoration of Smad4 suppresses in vivo tumorigenicity [102]. Interestingly, it has been shown that, in a transgenic model of PDAC, selective deletion of Smad4 in the mouse pancreas alone has no effect on PDAC development [103]. Similarly, selective loss of TβRII in the mouse pancreas also shows no discernible phenotype [104]. However, loss of Smad4 in combination with activated K-RasG12D and other PDAC-relevant mutations (loss of Ink4a/Arf or p53) does result in rapid tumour development, providing genetic confirmation that Smad4 functions to block progression of K-Ras-initiated tumours [103,105]. In a similar fashion, loss of TβRII in combination with activated K-RasG12D also rapidly results in well-differentiated PDAC with 100% penetrance [104]. Interestingly, loss of Smad4 in the K-RasG12D/Ink4a/Arf heterozygous mice not only leads to tumour development, but also results in an altered tumour phenotype, characterized by more well-differentiated tumours [103,105]. On the other hand, tumours with intact Smad4 show increased evidence of EMT, whereas a subset of advanced tumours with poor differentiation demonstrate TGF-β-dependent growth[103,105]. Taken together, these findings indicate that TGF-β and Smad4 may act further downstream as tumour promoters in the K-RasG12D/Ink4a/Arf heterozygous mouse model.
In human PDAC specimens, there is a strong and well-known correlation between mRNA expression of TGF-β1 and type I collagen, suggesting that TGF-β1 may be directly responsible for the fibrotic reaction that develops in PDAC tumours [106,107]. Consistent with this hypothesis, TGF-β1 overexpression in PDAC cells induces the desmoplastic reaction in an experimental model of human pancreatic carcinoma [108]. Since fibroblasts are responsible for the synthesis, degradation and remodelling of much of the ECM, this finding indicates that TGF-β1 synthesized by pancreatic cancer can modulate fibroblast behaviour. Moreover, these fibroblasts can, in turn, modulate the behaviour of cancer cells themselves through the secretion of additional cytokines and through further modification of the ECM microenvironment [109-115].
Cross-talk involving collagen, TGF-β1 and MT1-MMP
Deciphering the nature of the complex signalling events resulting from cross-talk between components of the extracellular microenvironment, the proteinases capable of modulating this extracellular milieu and canonical cytokine signalling families well-known to regulate tumour cell growth and development is a challenging, but important, component of ongoing efforts to better understand the initiation and development of PDAC. Our own work has shown that human tumours with increased stromal reaction demonstrate increased TGF-β signalling characterized by significant cross-talk between collagen, TGF-β1 and MT1-MMP [36]. Previously, we have published that pancreatic cancer cells induce TGF-β1 expression and signalling to promote MT1-MMP expression upon exposure to type I collagen [36]. Pancreatic cancer cells grown in three-dimensional collagen demonstrate increased TGF-β1 protein and mRNA levels, and increased Smad2 phosphorylation [36,80]. It was also previously shown that human mesangial cells cultured on type I collagen show increased TGF-β1 mRNA and protein levels [116]. Increased TGF-β1 production was diminished by a function-blocking anti-β1-integrin antibody and also by a dominant-negative ILK [116], indicating that type I collagen-induced TGF-β1 expression is regulated by β1-integrins. However, beyond regulating TGF-β expression, integrins can also promote activation of latent complexes of TGF-β [117]. The αv integrins, in particular αvβ3, αvβ5, αvβ6 and αvβ8, have been shown to liberate active TGF-β1 [118-121]. Significantly, mice carrying a single point mutation in the RGD integrin-binding motif of latent TGF-β1, thereby expressing latent TGF-β1 in a form that cannot be bound or activated by integrins, develop defects identical with those seen in mice completely lacking TGF-β1 [122]. Furthermore, generation of active TGF-β in αvβ8-expressing cells requires proteolytic cleavage of LAP (latency-associated peptide) by MT1-MMP [121].
Our own work has also identified MT1-MMP/TGF-β1 cross-talk in a MT1-MMP transgenic mouse model. Expression of MT1-MMP in the mouse pancreas results in increased pancreatic fibrosis that is also associated with increased TGF-β signalling [73]. Consistent with our observation, other groups have shown that expression of MT1-MMP in cardiac tissue causes significant fibrosis which is likewise associated with increased TGF-β signalling [123]. Previous work has demonstrated that MT1-MMP can increase levels of the active-form of TGF-β by cleaving latent TGF-β-binding protein-1 to release TGF-β bound to the ECM, an event which serves to increase the overall biological availability of TGF-β [124-126]. As indicated above, MT1-MMP can also cleave the LAP itself to release the active-form of TGF-β [121,123,126-128].
Cross-talk involving collagen, TGF-β1 and Snail
Further complexity in the role of TGF-β signalling in PDAC is introduced by considering the effects of this cytokine on the Snail family of transcription factors. TGF-β is a well-defined inducer of Snail in a variety of cell lines, including human PDAC cells [129-131]. However, more recent studies have identified the additional mechanistic link: in a PDAC model system, collagen itself can activate TGF-β signalling [36,37,80,116,132], in turn leading to increased Snail expression (Figure 3) [37]. We have demonstrated that treatment with either a highly specific inhibitor of TβRI or siRNA against TβRI blocks collagen-induced Snail expression [37]. Moreover, knocking down Smad4 using siRNA also abrogates collagen-induced Snail expression in our model system. Interestingly, TGF-β1 can signal through both Smad2 and Smad3 to regulate Snail expression [131], but, in pancreatic cancer cells, collagen-induced Snail expression involves Smad3, but not Smad2 [37]. We have found that siRNA-mediated down-regulation of Smad3, but not Smad2, abrogates collagen-induced Snail expression [37]. A closer analysis of Smad2 and Smad3 reveals a differential role for these two transcription factors during both embryonic development and in functional adult tissue. Liver-specific knock down of Smad3 or Smad2 in adult mice has shown that Smad3, but not Smad2, is required for TGF-β-stimulated EMT and growth arrest [133]. Similarly, TGF-β1-mediated repression of E-cadherin in kidney epithelial cells also requires Smad3, but not Smad2 [134,135]. On the basis of multiple studies in different tissue types, it appears that Smad3 may play a more significant role in regulating EMT than Smad2. However, other signalling events with the collagen microenvironment may also play a role in regulating this family of transcription factors, as collagen is also known to signal through ILK to regulate Snail expression [136-138]. Importantly, both integrins and integrin-associated proteins have been shown to regulate various growth factor signalling pathways including TGF-β signalling [139,140]. The collagen integrin receptor α2β1, known to associate with ILK [141], can also interact with the TβRI–TβRII heterodimeric complex to modulate Smad signalling [132,142].
Snail regulation of fibrosis
Several studies in non-PDAC systems have implicated Snail as a regulator of extracellular fibrosis. Collagen is known to promote Snail expression, but fibrogenesis itself can also be initiated by Snail. CCl4 (carbon tetrachloride)-induced fibrosis in the liver is associated with increased Snail expression, whereas liver-specific ablation of Snail attenuates this chemical-induced fibrosis [143]. Snail also promotes the activation of liver stellate cells, themselves important for activation of the fibrotic repair response during liver injury [144]. In adult renal cells, Snail activation is sufficient to drive both deposition of type I collagen and disruption of tissue architecture owing to the repression of cadherin-16 and the kidney differentiation factor HNF1 (hepatocyte nuclear factor 1) [145]. Given the clear role of Snail in regulating fibrosis in the kidney and liver, it is conceivable that Snail may also play an active role in signalling events driving the desmoplastic reaction found in PDAC tumours. In conjunction with the previously discussed cross-talk between Snail and other components of the extracellular PDAC milieu, this raises several intriguing questions for further study of the precise role played by this key transcription factor in the proliferative and metastatic events that characterize pancreatic malignancies.
MicroRNA AND PANCREATIC CANCER
MicroRNAs are small, single-stranded, non-coding RNAs that can post-transcriptionally regulate gene expression [146,147]. The identification of microRNAs and subsequent efforts to understand their role in regulating the proliferation, differentiation and invasion events that characterize tumour cell behaviour represent a major focus of molecular biology research during the last decade [148-151]. Not surprisingly, the role played by microRNAs in pancreatic cancer has been a rich area of investigation [152-155].
Collagen regulation of microRNAs
We have previously shown that collagen represses the let-7 family of microRNAs in pancreatic cancer cells. Let-7 family members, which were initially identified as key regulators of embryonic development in Caenorhabditis elegans [156], can target HMGA2, K-Ras and number of other proteins [157-159]. They have also been shown to play an important role in human cancers. Expression of let-7 increases with cell differentiation and is highest in mature tissues [160], in which microRNAs function to inhibit proliferation [161]. As with other key regulators of cell proliferation, aberrant expression of microRNA has been associated with human malignancies, including a noted decrease in the anti-proliferative let-7 family in human PDAC samples [162]. Overexpression of let-7 in mutant K-Ras-positive lung cancer cells inhibits tumour growth in vivo [163], and overexpression of let-7 in breast tumour-initiating cells inhibits tumour formation and metastasis in vivo [164]. Interestingly, collagen-mediated repression of let-7 involves MT1-MMP and TGF-β signalling [80], both clearly established mediators of the pathogenesis of pancreatic malignancies. Our own work has shown that siRNA-mediated knock down of MT1-MMP attenuates collagen repression of let-7 in PDAC cells [80]. Furthermore, blocking TGF-β signalling similarly attenuates collagen-induced repression of let-7. Collagen repression of let-7 in pancreatic cancer cells also involves ERK1/2 signalling [80], which our own work has shown is enhanced by MT1-MMP and TGF-β signalling in the collagen microenvironment (Figure 2) [80]. Consistent with the report that ERK1/2 signalling regulates the human microRNA-generating complex to reduce let-7 expression [165], collagen through increased ERK1/2 signalling also decreases let-7 levels by inhibiting let-7 processing to the mature form in pancreatic cancer cells [80]. These results further demonstrate the nuances of the significant cross-talk between collagen, MT1-MMP and TGF-β signalling in the regulation of PDAC progression.
MicroRNAs and fibrosis
MicroRNAs have also been implicated in the regulation of extracellular fibrosis, with several prominent examples standing out among previous studies (Table 1). First, let-7d expression is significantly decreased in lung samples from patients with IPF (idiopathic pulmonary fibrosis) [166]. In vitro inhibition of let-7d in lung epithelial cell lines increases the mesenchymal markers N-cadherin and vimentin, while decreasing expression of the epithelial marker cytokeratin [166]. Furthermore, inhibition of let-7d in vivo using antagomirs increases mesenchymal markers, decreases epithelial markers and increases collagen production, thus resulting in overall alveolar septal thickening. Let-7g, another member of the let-7 family, is reduced in HCC and has been shown to inhibit expression of type I collagen [167]. Low levels of let-7g expression in HCC tumours were subsequently found to predict poor patient survival, and in vitro studies have shown that let-7g inhibits HCC cell growth and migration [167]. Expression of miR-29, an additional microRNA not associated with the let-7 family, is reduced both in patients with advanced hepatic fibrosis and in a mouse model of CCl4-induced liver fibrosis [168]. This microRNA can also function to repress collagen production by stellate cells. Furthermore, overexpression of miR-29b in immortalized murine hepatic stellate cells leads to a dose-dependent decrease in the expression of the collagen Col1a1, Col4a5 and Col5a3 genes [168]. In addition, in human stellate cells miR-29b was shown to be an effective suppressor of type I collagen at the mRNA level through direct binding to the COL1A1 3′ UTR (untranslated region) [169]. Interestingly, Smad3 can also bind to the promoter region of let-7d to repress its expression [166], whereas TGF-β represses miR-29 expression [168].
Table 1.
Examples of microRNA modulating fibrosis or chemoresistance
| MicroRNA | Organ/cancer | Phenotype | Target (direct or indirect) | Reference(s) |
|---|---|---|---|---|
| let-7d | Lung | Decreases fibrosis | Inhibits COL1A1 | [166] |
| let-7g | HCC | Decreases fibrosis; reduces migration and cell growth | Inhibits COL1A2 | [167] |
| miR-29b | Liver | Reduces collagen production | Inhibits COL1A1, COL4A5 and COL5A3 | [168,169] |
| miR-200 | Kidney | Reduces fibrosis | Inhibits collagen I and III | [172] |
| miR-192 | Kidney | Promotes fibrosis and collagen I downstream of TGF-β/Smad3 | Induces collagen I | [173] |
| miR-21 | PDAC | Gemcitabine resistance | Inhibits PTEN and Bax; induces Bcl-2 | [175,176] |
| miR-21 | Bladder cancer | Doxorubicin resistance | Inhibits PTEN | [177] |
The miR-200 family of microRNAs also function to regulate fibrosis and have been identified as inhibitors of EMT in a number of different malignancies [170,171]. Recent work has shown that this family, in particular the miR-200b member, inhibits TGF-β-induced EMT of renal tubular cells [172]. Intravenous injection of a miR-200b precursor in obstructed kidneys inhibits collagen I, collagen III and fibronectin, thereby reducing fibrosis [172]. Interestingly, the miR-192 family member is a mediator of TGF-β/Smad3-driven renal fibrosis through Smad3 binding to the miR-192 promoter to increase collagen expression as a downstream effect [173]. Clearly, microRNAs share the same nuanced signalling events that characterize other families of intracellular and extracellular signalling modulators, even down to apparently paradoxical effects from very similar effectors.
MicroRNAs and chemoresistance
Our own work has shown that let-7 microRNA does not mediate gemcitabine resistance in pancreatic cancer either on two-dimensional surfaces or in the three-dimensional collagen microenvironment [80], but other microRNAs have been shown to mediate chemoresistance. For example, miR-21, overexpressed in PDAC tumours and a predictor of poor outcomes [174], contributes to gemcitabine resistance in PDAC, partially through modulation of PI3K (phosphoinositide 3-kinase)/Akt signalling [175,176]. miR-21 increases pro-survival PI3K signalling through repression of PTEN (phosphatase and tensin homologue deleted on chromosome 10). It also up-regulates the pro-survival Bcl-2 protein and inhibits the pro-apoptotic Bax protein to promote gemcitabine resistance in PDAC cells. The effects of miR-21 on PI3K/Akt and Bcl-2 signalling are not unique to pancreatic cancer cells, as miR-21 can also mediate doxorubicin resistance in bladder cancer cells [177]. Interestingly, stem cells isolated from several different tumour types demonstrate up-regulation of both miR-21 and its upstream regulator AP-1 (activator protein 1) [178]. Even more notable, a small-molecule inhibitor of AP-1 or an anti-microRNA against miR-21 were both found to sensitize cancer stem cells to topotecan and decrease colony formation [178].
CONCLUSIONS
The well-characterized desmoplastic reaction of pancreatic cancer has been described as a ‘fortress-like’ barrier that impairs drug delivery to tumour cells [179]. However, there is now an increasing body of evidence that suggests that, in addition to inanimate structural effects, the desmoplastic reaction is in fact a dynamic contributor to pancreatic cancer progression through active modulation of key signalling events regulating both tumour cell proliferation and invasion. As discussed above and summarized in Figure 4, the intricate interplay between collagen, MT1-MMP and TGF-β promotes pancreatic cancer invasion, represses tumour-suppressive microRNAs, and provides critical survival and repair signals to mediate chemoresistance in PDAC cells. Feed-forward amplification loops involving collagen, MT1-MMP and TGF-β signalling further promote the establishment and maintenance of the desmoplastic reaction. On the basis of results from several in vivo studies, targeting the key collagenase MT1-MMP may help to not only limit pancreatic cancer progression, but also paradoxically attenuate the pronounced stromal reaction that is both a characteristic descriptive hallmark of the disease and now an increasingly recognized functional component of pancreatic cancer’s aggressive molecular and clinical trajectory. A highly selective anti-MT1-MMP antibody has been shown to inhibit growth and metastasis in a mouse model of breast cancer [180]. Evaluating this antibody in similar mouse models of pancreatic cancer will provide proof-of-principle evidence that targeting MT1-MMP can prevent pancreatic cancer growth and invasion. Moving forward, a key objective in the effective treatment of pancreatic carcinoma is thus the development of strategies to breach the stromal fortress that heretofore has not only enabled metastatic forays into normal tissue, but also acted as a nearly impenetrable barrier for therapeutic drug delivery.
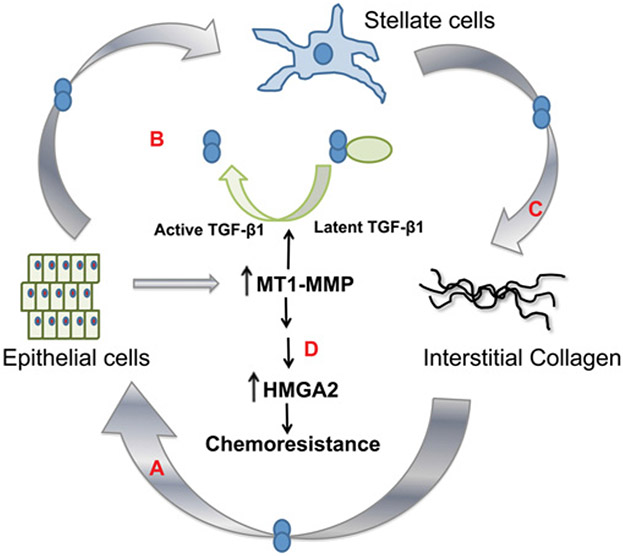
Figure 4. A feed-forward amplification loop involving collagen, MT1-MMP and TGF-β.
(A) Pancreatic cancer cells in the collagen microenvironment up-regulate TGF-β signalling to induce MT1-MMP expression. (B) Expression of MT1-MMP in cancer cells can in turn cause stellate cell activation also through increased TGF-β signalling. (C) The activated stellate cells deposit more collagen, resulting in a further increase in MT1-MMP expression in the cancer cells. As detailed in the text, this forward amplification loop contributes to pancreatic cancer invasion and generation of the desmoplastic reaction. (D) MT1-MMP expression in the collagen microenvironment also contributes to chemoresistance, in part, through up-regulation of HMGA2 (see also Figure 2). An animated version of this Figure is available at http://www.BiochemJ.org/bj/441/0541/bj4410541add.htm.
Supplementary Material
FUNDING
Our work is supported by the National Institutes of Health/National Cancer Institute [grant numbers R01CA126888, U54CA151880].
Abbreviations used:
- AP-1
activator protein 1
- CDK
cyclin-dependent kinase
- ECM
extracellular matrix
- EMT
epithelial–mesenchymal transition
- ERK
extracellular-signal-regulated kinase
- HCC
hepatocellular carcinoma
- HMGA2
high-mobility group A2
- ILK
integrin-linked kinase
- LAP
latency-associated peptide
- MDCK
Madin–Darby canine kidney
- MMP
matrix metalloproteinase
- MT1-MMP
membrane-type 1 MMP
- PDAC
pancreatic ductal adenocarcinoma
- PDGF-β
platelet-derived growth factor-β
- PI3K
phosphoinositide 3-kinase
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
- R-Smad
receptor-associated Smad
- siRNA
small interfering RNA
- TGF-β
transforming growth factor-β
- TβR
TGF-β receptor
- TIMP
tissue inhibitor of metalloproteinases
- VSMC
vascular smooth muscle cell
REFERENCES
- 1.Malvezzi M, Arfe A, Bertuccio P, Levi F, La Vecchia C and Negri E (2011) European cancer mortality predictions for the year 2011. Ann. Oncol 22, 947–956 [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E and Forman D (2011) Global cancer statistics. CA Cancer J. Clin 61, 69–90 [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ward E, Brawley O and Jemal A (2011) Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin 61, 212–236 [DOI] [PubMed] [Google Scholar]
- 4.Hidalgo M (2010) Pancreatic cancer. N. Engl. J. Med 362, 1605–1617 [DOI] [PubMed] [Google Scholar]
- 5.Vincent A, Herman J, Schulick R, Hruban RH and Goggins M (2011) Pancreatic cancer. Lancet 378, 607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS et al. (2006) 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J. Gastrointest. Surg 10, 1199–1210 [DOI] [PubMed] [Google Scholar]
- 7.Maitra A and Hruban RH (2008) Pancreatic cancer. Annu. Rev. Pathol 3, 157–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu GC, Kimmelman AC, Hezel AF and DePinho RA (2007) Stromal biology of pancreatic cancer. J. Cell. Biochem 101, 887–907 [DOI] [PubMed] [Google Scholar]
- 9.Mahadevan D and Von Hoff DD (2007) Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol. Cancer Ther 6, 1186–1197 [DOI] [PubMed] [Google Scholar]
- 10.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D et al. (2009) Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dangi-Garimella S, Krantz SB, Barron MR, Shields MA, Heiferman MJ, Grippo PJ, Bentrem DJ and Munshi HG (2011) Three-dimensional collagen I promotes gemcitabine resistance in pancreatic cancer through MT1-MMP-mediated expression of HMGA2. Cancer Res. 71, 1019–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS and DePinho RA (2003) Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 17, 3112–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA et al. (2003) Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4, 437–450 [DOI] [PubMed] [Google Scholar]
- 14.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S and Tuveson DA (2005) Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7, 469–483 [DOI] [PubMed] [Google Scholar]
- 15.Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, Feng B, Brennan C, Weissleder R, Mahmood U et al. (2006) Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc. Natl. Acad. Sci. U.S.A 103, 5947–5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imamura T, Iguchi H, Manabe T, Ohshio G, Yoshimura T, Wang ZH, Suwa H, Ishigami S and Imamura M (1995) Quantitative analysis of collagen and collagen subtypes I, III, and V in human pancreatic cancer, tumor-associated chronic pancreatitis, and alcoholic chronic pancreatitis. Pancreas 11, 357–364 [DOI] [PubMed] [Google Scholar]
- 17.Mollenhauer J, Roether I and Kern HF (1987) Distribution of extracellular matrix proteins in pancreatic ductal adenocarcinoma and its influence on tumor cell proliferation in vitro. Pancreas 2, 14–24 [DOI] [PubMed] [Google Scholar]
- 18.Linder S, Castanos-Velez E, von Rosen A and Biberfeld P (2001) Immunohistochemical expression of extracellular matrix proteins and adhesion molecules in pancreatic carcinoma. Hepatogastroenterology 48, 1321–1327 [PubMed] [Google Scholar]
- 19.Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC and Wilson JS (1998) Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 43, 128–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grunert A and Adler G (1998) Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 115, 421–432 [DOI] [PubMed] [Google Scholar]
- 21.Yen TW, Aardal NP, Bronner MP, Thorning DR, Savard CE, Lee SP and Bell RH Jr (2002) Myofibroblasts are responsible for the desmoplastic reaction surrounding human pancreatic carcinomas. Surgery 131, 129–134 [DOI] [PubMed] [Google Scholar]
- 22.Maehara N, Matsumoto K, Kuba K, Mizumoto K, Tanaka M and Nakamura T (2001) NK4, a four-kringle antagonist of HGF, inhibits spreading and invasion of human pancreatic cancer cells. Br. J. Cancer 84, 864–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB and Logsdon CD (2008) Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 68, 918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato N, Maehara N and Goggins M (2004) Gene expression profiling of tumor-stromal interactions between pancreatic cancer cells and stromal fibroblasts. Cancer Res. 64, 6950–6956 [DOI] [PubMed] [Google Scholar]
- 25.Kadler K (1995) Extracellular matrix 1: fibril-forming collagens. Protein Profile 2, 491–619 [PubMed] [Google Scholar]
- 26.Munshi HG and Stack MS (2002) Analysis of matrix degradation. Methods Cell Biol. 69, 195–205 [DOI] [PubMed] [Google Scholar]
- 27.Kadler KE, Baldock C, Bella J and Boot-Handford RP (2007) Collagens at a glance. J. Cell Sci 120, 1955–1958 [DOI] [PubMed] [Google Scholar]
- 28.Munshi HG and Stack MS (2006) Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev. 25, 45–56 [DOI] [PubMed] [Google Scholar]
- 29.Sternlicht MD and Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol 17, 463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song F, Wisithphrom K, Zhou J and Windsor LJ (2006) Matrix metalloproteinase dependent and independent collagen degradation. Front. Biosci 11, 3100–3120 [DOI] [PubMed] [Google Scholar]
- 31.Condeelis J and Segall JE (2003) Intravital imaging of cell movement in tumours. Nat. Rev. Cancer 3, 921–930 [DOI] [PubMed] [Google Scholar]
- 32.Provenzano P, Eliceiri K, Campbell J, Inman D, White J and Keely P (2006) Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL and Massague J (2008) Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451, 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramaswamy S, Ross KN, Lander ES and Golub TR (2003) A molecular signature of metastasis in primary solid tumors. Nat. Genet 33, 49–54 [DOI] [PubMed] [Google Scholar]
- 35.Erkan M, Michalski CW, Rieder S, Reiser-Erkan C, Abiatari I, Kolb A, Giese NA, Esposito I, Friess H and Kleeff J (2008) The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin. Gastroenterol. Hepatol 6, 1155–1161 [DOI] [PubMed] [Google Scholar]
- 36.Ottaviano AJ, Sun L, Ananthanarayanan V and Munshi HG (2006) Extracellular matrix-mediated membrane-type 1 matrix metalloproteinase expression in pancreatic ductal cells is regulated by transforming growth factor-β1. Cancer Res. 66, 7032–7040 [DOI] [PubMed] [Google Scholar]
- 37.Shields MA, Dangi-Garimella S, Krantz SB, Bentrem DJ and Munshi HG (2011) Pancreatic cancer cells respond to type I collagen by inducing Snail expression to promote membrane type 1 matrix metalloproteinase-dependent collagen invasion. J. Biol. Chem 286, 10495–10504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makareeva E, Han S, Vera JC, Sackett DL, Holmbeck K, Phillips CL, Visse R, Nagase H and Leikin S (2010) Carcinomas contain a matrix metalloproteinase-resistant isoform of type I collagen exerting selective support to invasion. Cancer Res. 70, 4366–4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han S, Makareeva E, Kuznetsova NV, DeRidder AM, Sutter MB, Losert W, Phillips CL, Visse R, Nagase H and Leikin S (2010) Molecular mechanism of type I collagen homotrimer resistance to mammalian collagenases. J. Biol. Chem 285, 22276–22281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadler KE, Holmes DF, Trotter JA and Chapman JA (1996) Collagen fibril formation. Biochem. J 316, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pucci-Minafra I, Andriolo M, Basirico L, Alessandro R, Luparello C, Buccellato C, Garbelli R and Minafra S (1998) Absence of regular α2(I) collagen chains in colon carcinoma biopsy fragments. Carcinogenesis 19, 575–584 [DOI] [PubMed] [Google Scholar]
- 42.Shapiro FD and Eyre DR (1982) Collagen polymorphism in extracellular matrix of human osteosarcoma. J. Natl. Cancer Inst 69, 1009–1016 [PubMed] [Google Scholar]
- 43.Minafra S, Luparello C, Rallo F and Pucci-Minafra I (1988) Collagen biosynthesis by a breast carcinoma cell strain and biopsy fragments of the primary tumour. Cell Biol. Int. Rep 12, 895–905 [DOI] [PubMed] [Google Scholar]
- 44.DeClerck YA, Bomann ET, Spengler BA and Biedler JL (1987) Differential collagen biosynthesis by human neuroblastoma cell variants. Cancer Res. 47, 6505–6510 [PubMed] [Google Scholar]
- 45.Rupard JH, Dimari SJ, Damjanov I and Haralson MA (1988) Synthesis of type I homotrimer collagen molecules by cultured human lung adenocarcinoma cells. Am. J. Pathol 133, 316–326 [PMC free article] [PubMed] [Google Scholar]
- 46.Schillaci R, Luparello C and Minafra S (1989) Type I and I-trimer collagens as substrates for breast carcinoma cells in culture. Effect on growth rate, morphological appearance and actin organization. Eur. J. Cell Biol 48, 135–141 [PubMed] [Google Scholar]
- 47.Luparello C, Sheterline P, Pucci-Minafra I and Minafra S (1991) A comparison of spreading and motility behaviour of 8701-BC breast carcinoma cells on type I, I-trimer and type V collagen substrata. Evidence for a permissive effect of type I-trimer collagen on cell locomotion. J. Cell Sci 100, 179–185 [DOI] [PubMed] [Google Scholar]
- 48.Apte MV and Wilson JS (2004) Mechanisms of pancreatic fibrosis. Dig. Dis 22, 273–279 [DOI] [PubMed] [Google Scholar]
- 49.Van Hoorde L, Van Aken E and Mareel M (2000) Collagen type I: a substrate and a signal for invasion. Prog. Mol. Subcell. Biol 25, 105–134 [DOI] [PubMed] [Google Scholar]
- 50.Henriet P, Zhong ZD, Brooks PC, Weinberg KI and DeClerck YA (2000) Contact with fibrillar collagen inhibits melanoma cell proliferation by up-regulating p27KIP1. Proc. Natl. Acad. Sci. U.S.A 97, 10026–10031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schocklmann HO, Lang S, Kralewski M, Hartner A, Ludke A and Sterzel RB (2000) Distinct structural forms of type I collagen modulate cell cycle regulatory proteins in mesangial cells. Kidney Int. 58, 1108–1120 [DOI] [PubMed] [Google Scholar]
- 52.Taki M, Verschueren K, Yokoyama K, Nagayama M and Kamata N (2006) Involvement of Ets-1 transcription factor in inducing matrix metalloproteinase-2 expression by epithelial-mesenchymal transition in human squamous carcinoma cells. Int. J. Oncol 28, 487–496 [PubMed] [Google Scholar]
- 53.Li S, Lao J, Chen BP, Li YS, Zhao Y, Chu J, Chen KD, Tsou TC, Peck K and Chien S (2003) Genomic analysis of smooth muscle cells in 3-dimensional collagen matrix. FASEB J. 17, 97–99 [DOI] [PubMed] [Google Scholar]
- 54.Fassett JT, Tobolt D, Nelsen CJ, Albrecht JH and Hansen LK (2003) The role of collagen structure in mitogen stimulation of ERK, cyclin D1 expression, and G1-S progression in rat hepatocytes. J. Biol. Chem 278, 31691–31700 [DOI] [PubMed] [Google Scholar]
- 55.Paszek MJ and Weaver VM (2004) The tension mounts: mechanics meets morphogenesis and malignancy. J. Mammary Gland Biol. Neoplasia 9, 325–342 [DOI] [PubMed] [Google Scholar]
- 56.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D et al. (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 [DOI] [PubMed] [Google Scholar]
- 57.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W et al. (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai Y, Chen S, Pei XY, Almenara JA, Kramer LB, Venditti CA, Dent P and Grant S (2008) Interruption of the Ras/MEK/ERK signaling cascade enhances Chk1 inhibitor-induced DNA damage in vitro and in vivo in human multiple myeloma cells. Blood 112, 2439–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsumoto K, Nagahara T, Okano J and Murawaki Y (2008) The growth inhibition of hepatocellular and cholangiocellular carcinoma cells by gemcitabine and the roles of extracellular signal-regulated and checkpoint kinases. Oncol. Rep 20, 863–872 [PubMed] [Google Scholar]
- 60.Zhao Y, Shen S, Guo J, Chen H, Greenblatt DY, Kleeff J, Liao Q, Chen G, Friess H and Leung PS (2006) Mitogen-activated protein kinases and chemoresistance in pancreatic cancer cells. J. Surg. Res 136, 325–335 [DOI] [PubMed] [Google Scholar]
- 61.Hodkinson PS, Mackinnon AC and Sethi T (2007) Extracellular matrix regulation of drug resistance in small-cell lung cancer. Int. J. Radiat. Biol 83, 733–741 [DOI] [PubMed] [Google Scholar]
- 62.Iacobuzio-Donahue CA, Ryu B, Hruban RH and Kern SE (2002) Exploring the host desmoplastic response to pancreatic carcinoma: gene expression of stromal and neoplastic cells at the site of primary invasion. Am. J. Pathol 160, 91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imamura T, Ohshio G, Mise M, Harada T, Suwa H, Okada N, Wang Z, Yoshitomi S, Tanaka T, Sato H et al. (1998) Expression of membrane-type matrix metalloproteinase-1 in human pancreatic adenocarcinomas. J. Cancer Res. Clin. Oncol 124, 65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bramhall SR, Neoptolemos JP, Stamp GW and Lemoine NR (1997) Imbalance of expression of matrix metalloproteinases (MMPs) and tissue inhibitors of the matrix metalloproteinases (TIMPs) in human pancreatic carcinoma. J. Pathol 182, 347–355 [DOI] [PubMed] [Google Scholar]
- 65.Ellenrieder V, Alber B, Lacher U, Hendler SF, Menke A, Boeck W, Wagner M, Wilda M, Friess H, Buchler M et al. (2000) Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int. J. Cancer 85, 14–20 [DOI] [PubMed] [Google Scholar]
- 66.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I et al. (1999) MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99, 81–92 [DOI] [PubMed] [Google Scholar]
- 67.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y and Tryggvason K (2000) Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci. U.S.A 97, 4052–4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW and Weiss SJ (2003) Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell 114, 33–45 [DOI] [PubMed] [Google Scholar]
- 69.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S et al. (2004) Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP J. Cell Biol. 167, 769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ha HY, Moon HB, Nam MS, Lee JW, Ryoo ZY, Lee TH, Lee KK, So BJ, Sato H, Seiki M et al. (2001) Overexpression of membrane-type matrix metalloproteinase-1 gene induces mammary gland abnormalities and adenocarcinoma in transgenic mice. Cancer Res. 61, 984–990 [PubMed] [Google Scholar]
- 71.Soulie P, Carrozzino F, Pepper MS, Strongin AY, Poupon MF and Montesano R (2005) Membrane-type-1 matrix metalloproteinase confers tumorigenicity on non-malignant epithelial cells. Oncogene 24, 1689–1697 [DOI] [PubMed] [Google Scholar]
- 72.Dangi-Garimella S, Redig AJ, Shields MA, Siddiqui MA and Munshi HG (2010) Rho-ROCK-myosin signaling mediates membrane type 1 matrix metalloproteinase-induced cellular aggregation of keratinocytes. J. Biol. Chem 285, 28363–28372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krantz SB, Shields MA, Dangi-Garimella S, Cheon E, Barron MR, Hwang RF, Rao MS, Grippo PJ, Bentrem DJ and Munshi HG (2011) MT1-MMP cooperates with KrasG12D to promote pancreatic fibrosis through increased TGF-β signaling. Mol. Cancer Res 9, 1294–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cukierman E, Pankov R, Stevens DR and Yamada KM (2001) Taking cell-matrix adhesions to the third dimension. Science 294, 1708–1712 [DOI] [PubMed] [Google Scholar]
- 75.Baciu PC, Suleiman EA, Deryugina EI and Strongin AY (2003) Membrane type-1 matrix metalloproteinase (MT1-MMP) processing of pro-αv integrin regulates cross-talk between αvβ3 and α2β1 integrins in breast carcinoma cells. Exp. Cell Res 291, 167–175 [DOI] [PubMed] [Google Scholar]
- 76.Duxbury MS, Ito H, Benoit E, Waseem T, Ashley SW and Whang EE (2005) RNA interference demonstrates a novel role for integrin-linked kinase as a determinant of pancreatic adenocarcinoma cell gemcitabine chemoresistance. Clin. Cancer Res 11, 3433–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gingras D, Bousquet-Gagnon N, Langlois S, Lachambre MP, Annabi B and Beliveau R (2001) Activation of the extracellular signal-regulated protein kinase (ERK) cascade by membrane-type-1 matrix metalloproteinase (MT1-MMP). FEBS Lett. 507, 231–236 [DOI] [PubMed] [Google Scholar]
- 78.D’Alessio S, Ferrari G, Cinnante K, Scheerer W, Galloway AC, Roses DF, Rozanov DV, Remacle AG, Oh ES, Shiryaev SA et al. (2008) Tissue inhibitor of metalloproteinases-2 binding to membrane-type 1 matrix metalloproteinase induces MAPK activation and cell growth by a non-proteolytic mechanism. J. Biol. Chem 283, 87–99 [DOI] [PubMed] [Google Scholar]
- 79.Takino T, Tsuge H, Ozawa T and Sato H (2010) MT1-MMP promotes cell growth and ERK activation through c-Src and paxillin in three-dimensional collagen matrix. Biochem. Biophys. Res. Commun 396, 1042–1047 [DOI] [PubMed] [Google Scholar]
- 80.Dangi-Garimella S, Strouch MJ, Grippo PJ, Bentrem DJ and Munshi HG (2011) Collagen regulation of let-7 in pancreatic cancer involves TGF-β1-mediated membrane type 1-matrix metalloproteinase expression. Oncogene 30, 1002–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lehti K, Allen E, Birkedal-Hansen H, Holmbeck K, Miyake Y, Chun TH and Weiss SJ (2005) An MT1-MMP-PDGF receptor-β axis regulates mural cell investment of the microvasculature. Genes Dev. 19, 979–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hammond SM and Sharpless NE (2008) HMGA2, microRNAs, and stem cell aging. Cell 135, 1013–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pfannkuche K, Summer H, Li O, Hescheler J and Droge P (2009) The high mobility group protein HMGA2: a co-regulator of chromatin structure and pluripotency in stem cells? Stem Cell Rev. 5, 224–230 [DOI] [PubMed] [Google Scholar]
- 84.Liau SS and Whang E (2008) HMGA1 is a molecular determinant of chemoresistance to gemcitabine in pancreatic adenocarcinoma. Clin. Cancer Res 14, 1470–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Summer H, Li O, Bao Q, Zhan L, Peter S, Sathiyanathan P, Henderson D, Klonisch T, Goodman SD and Droge P (2009) HMGA2 exhibits dRP/AP site cleavage activity and protects cancer cells from DNA-damage-induced cytotoxicity during chemotherapy. Nucleic Acids Res. 37, 4371–4384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palmieri D, Valentino T, D’Angelo D, De Martino I, Postiglione I, Pacelli R, Croce CM, Fedele M and Fusco A (2011) HMGA proteins promote ATM expression and enhance cancer cell resistance to genotoxic agents. Oncogene 30, 3024–3035 [DOI] [PubMed] [Google Scholar]
- 87.Boman BM and Wicha MS (2008) Cancer stem cells: a step toward the cure. J. Clin. Oncol 26, 2795–2799 [DOI] [PubMed] [Google Scholar]
- 88.Haas TL, Stitelman D, Davis SJ, Apte SS and Madri JA (1999) Egr-1 mediates extracellular matrix-driven transcription of membrane type 1 matrix metalloproteinase in endothelium. J. Biol. Chem 274, 22679–22685 [DOI] [PubMed] [Google Scholar]
- 89.Yun S, Dardik A, Haga M, Yamashita A, Yamaguchi S, Koh Y, Madri JA and Sumpio BE (2002) Transcription factor Sp1 phosphorylation induced by shear stress inhibits membrane type 1-matrix metalloproteinase expression in endothelium. J. Biol. Chem 277, 34808–34814 [DOI] [PubMed] [Google Scholar]
- 90.Barbolina MV, Adley BP, Ariztia EV, Liu Y and Stack MS (2007) Microenvironmental regulation of membrane type 1 matrix metalloproteinase activity in ovarian carcinoma cells via collagen-induced EGR1 expression. J. Biol. Chem 282, 4924–4931 [DOI] [PubMed] [Google Scholar]
- 91.Takahashi M, Tsunoda T, Seiki M, Nakamura Y and Furukawa Y (2002) Identification of membrane-type matrix metalloproteinase-1 as a target of the β-catenin/Tcf4 complex in human colorectal cancers. Oncogene 21, 5861–5867 [DOI] [PubMed] [Google Scholar]
- 92.Miyoshi A, Kitajima Y, Sumi K, Sato K, Hagiwara A, Koga Y and Miyazaki K (2004) Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br. J. Cancer 90, 1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miyoshi A, Kitajima Y, Kido S, Shimonishi T, Matsuyama S, Kitahara K and Miyazaki K (2005) Snail accelerates cancer invasion by upregulating MMP expression and is associated with poor prognosis of hepatocellular carcinoma. Br. J. Cancer 92, 252–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ota I, Li XY, Hu Y and Weiss SJ (2009) Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proc. Natl. Acad. Sci. U.S.A. 106, 20318–20323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rowe RG, Li XY, Hu Y, Saunders TL, Virtanen I, Garcia de Herreros A, Becker KF, Ingvarsen S, Engelholm LH, Bommer GT et al. (2009) Mesenchymal cells reactivate Snail1 expression to drive three-dimensional invasion programs. J. Cell Biol 184, 399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Friess H, Yamanaka Y, Buchler M, Ebert M, Beger HG, Gold LI and Korc M (1993) Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology 105, 1846–1856 [DOI] [PubMed] [Google Scholar]
- 97.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N and Depinho RA (2006) Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 20, 1218–1249 [DOI] [PubMed] [Google Scholar]
- 98.Shi Y and Massague J (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113, 685–700 [DOI] [PubMed] [Google Scholar]
- 99.Akhurst RJ and Derynck R (2001) TGF-β signaling in cancer: a double-edged sword. Trends Cell Biol. 11, S44–S51 [DOI] [PubMed] [Google Scholar]
- 100.Hansel DE, Kern SE and Hruban RH (2003) Molecular pathogenesis of pancreatic cancer. Annu. Rev. Genomics Hum. Genet 4, 237–256 [DOI] [PubMed] [Google Scholar]
- 101.Moore PS, Beghelli S, Zamboni G and Scarpa A (2003) Genetic abnormalities in pancreatic cancer. Mol. Cancer 2, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Freeman JW, DeArmond D, Lake M, Huang W, Venkatasubbarao K and Zhao S (2004) Alterations of cell signaling pathways in pancreatic cancer. Front. Biosci 9, 1889–1898 [DOI] [PubMed] [Google Scholar]
- 103.Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D et al. (2006) Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 20, 3130–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S, Wright CV and Moses HL (2006) Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-β signaling in cooperation with active Kras expression. Genes Dev. 20, 3147–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Izeradjene K, Combs C, Best M, Gopinathan A, Wagner A, Grady WM, Deng CX, Hruban RH, Adsay NV, Tuveson DA et al. (2007) Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell 11, 229–243 [DOI] [PubMed] [Google Scholar]
- 106.Aoyagi Y, Oda T, Kinoshita T, Nakahashi C, Hasebe T, Ohkohchi N and Ochiai A (2004) Overexpression of TGF-β by infiltrated granulocytes correlates with the expression of collagen mRNA in pancreatic cancer. Br. J. Cancer 91, 1316–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Armstrong T, Packham G, Murphy LB, Bateman AC, Conti JA, Fine DR, Johnson CD, Benyon RC and Iredale JP (2004) Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin. Cancer Res 10, 7427–7437 [DOI] [PubMed] [Google Scholar]
- 108.Lohr M, Schmidt C, Ringel J, Kluth M, Muller P, Nizze H and Jesnowski R (2001) Transforming growth factor-β1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 61, 550–555 [PubMed] [Google Scholar]
- 109.Hendrix MJ, Seftor EA, Kirschmann DA, Quaranta V and Seftor RE (2003) Remodeling of the microenvironment by aggressive melanoma tumor cells. Ann. N. Y. Acad. Sci 995, 151–161 [DOI] [PubMed] [Google Scholar]
- 110.Mueller MM and Fusenig NE (2004) Friends or foes: bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer 4, 839–849 [DOI] [PubMed] [Google Scholar]
- 111.Bhowmick NA, Zent R, Ghiassi M, McDonnell M and Moses HL (2001) Integrin β1 signaling is necessary for transforming growth factor-β activation of p38MAPK and epithelial plasticity. J. Biol. Chem 276, 46707–46713 [DOI] [PubMed] [Google Scholar]
- 112.Matrisian LM, Cunha GR and Mohla S (2001) Epithelial-stromal interactions and tumor progression: meeting summary and future directions. Cancer Res. 61, 3844–3846 [PubMed] [Google Scholar]
- 113.Lynch CC, Hikosaka A, Acuff HB, Martin MD, Kawai N, Singh RK, Vargo-Gogola TC, Begtrup JL, Peterson TE, Fingleton B et al. (2005) MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell 7, 485–496 [DOI] [PubMed] [Google Scholar]
- 114.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL and Weinberg RA (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335–348 [DOI] [PubMed] [Google Scholar]
- 115.Radisky DC, Kenny PA and Bissell MJ (2007) Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J. Cell. Biochem 101, 830–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ortega-Velazquez R, Gonzalez-Rubio M, Ruiz-Torres MP, Diez-Marques ML, Iglesias MC, Rodriguez-Puyol M and Rodriguez-Puyol D (2004) Collagen I upregulates extracellular matrix gene expression and secretion of TGF-β1 by cultured human mesangial cells. Am. J. Physiol. Cell Physiol 286, C1335–C1343 [DOI] [PubMed] [Google Scholar]
- 117.Worthington JJ, Klementowicz JE and Travis MA (2011) TGFβ: a sleeping giant awoken by integrins. Trends Biochem. Sci 36, 47–54 [DOI] [PubMed] [Google Scholar]
- 118.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y and Tamaki K (2005) Increased expression of integrin αvβ3 contributes to the establishment of autocrine TGF-β signaling in scleroderma fibroblasts. J. Immunol 175, 7708–7718 [DOI] [PubMed] [Google Scholar]
- 119.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y and Tamaki K (2005) Involvement of αvβ5 integrin-mediated activation of latent transforming growth factor β1 in autocrine transforming growth factor β signaling in systemic sclerosis fibroblasts. Arthritis Rheum. 52, 2897–2905 [DOI] [PubMed] [Google Scholar]
- 120.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA et al. (1999) The integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, 319–328 [DOI] [PubMed] [Google Scholar]
- 121.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC and Nishimura SL (2002) The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J. Cell Biol 157, 493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X and Munger JS (2007) Absence of integrin-mediated TGFβ1 activation in vivo recapitulates the phenotype of TGFβ1-null mice. J. Cell Biol 176, 787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Spinale FG, Escobar GP, Mukherjee R, Zavadzkas JA, Saunders SM, Jeffords LB, Leone AM, Beck C, Bouges S and Stroud RE (2009) Cardiac-restricted overexpression of membrane type-1 matrix metalloproteinase in mice: effects on myocardial remodeling with aging. Circ. Heart Failure 2, 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karsdal MA, Larsen L, Engsig MT, Lou H, Ferreras M, Lochter A, Delaissé J-M and Foged NT (2002) Matrix metalloproteinase-dependent activation of latent transforming growth factor-β controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J. Biol. Chem 277, 44061–44067 [DOI] [PubMed] [Google Scholar]
- 125.Freudenberg J and Chen W (2007) Induction of Smad1 by MT1-MMP contributes to tumor growth. Int. J. Cancer 121, 966–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kessenbrock K, Plaks V and Werb Z (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tatti O, Vehviläinen P, Lehti K and Keski-Oja J (2008) MT1-MMP releases latent TGF-β1 from endothelial cell extracellular matrix via proteolytic processing of LTBP-1. Exp. Cell Res 314, 2501–2514 [DOI] [PubMed] [Google Scholar]
- 128.Böttinger EP, Factor VM, Tsang ML, Weatherbee JA, Kopp JB, Qian SW, Wakefield LM, Roberts AB, Thorgeirsson SS and Sporn MB (1996) The recombinant proregion of transforming growth factor β1 (latency-associated peptide) inhibits active transforming growth factor β1 in transgenic mice. Proc. Natl. Acad. Sci. U.S.A 93, 5877–5882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peinado H, Quintanilla M and Cano A (2003) Transforming growth factor β-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J. Biol. Chem 278, 21113–21123 [DOI] [PubMed] [Google Scholar]
- 130.Sun L, Diamond ME, Ottaviano AJ, Joseph MJ, Ananthanarayan V and Munshi HG (2008) Transforming growth factor-β1 promotes matrix metalloproteinase-9-mediated oral cancer invasion through snail expression. Mol. Cancer Res 6, 10–20 [DOI] [PubMed] [Google Scholar]
- 131.Horiguchi K, Shirakihara T, Nakano A, Imamura T, Miyazono K and Saitoh M (2009) Role of Ras signaling in the induction of snail by transforming growth factor-β. J. Biol. Chem 284, 245–253 [DOI] [PubMed] [Google Scholar]
- 132.Garamszegi N, Garamszegi SP, Samavarchi-Tehrani P, Walford E, Schneiderbauer MM, Wrana JL and Scully SP (2010) Extracellular matrix-induced transforming growth factor-β receptor signaling dynamics. Oncogene 29, 2368–2380 [DOI] [PubMed] [Google Scholar]
- 133.Ju W, Ogawa A, Heyer J, Nierhof D, Yu L, Kucherlapati R, Shafritz DA and Bottinger EP (2006) Deletion of Smad2 in mouse liver reveals novel functions in hepatocyte growth and differentiation. Mol. Cell. Biol 26, 654–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Phanish MK, Wahab NA, Colville-Nash P, Hendry BM and Dockrell ME (2006) The differential role of Smad2 and Smad3 in the regulation of pro-fibrotic TGFβ1 responses in human proximal-tubule epithelial cells. Biochem. J 393, 601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S and Flanders KC (2006) Smad3 is key to TGF-β-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev. 17, 19–27 [DOI] [PubMed] [Google Scholar]
- 136.Medici D and Nawshad A (2010) Type I collagen promotes epithelial-mesenchymal transition through ILK-dependent activation of NF-κB and LEF-1. Matrix Biol. 29, 161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pogribny IP, Filkowski JN, Tryndyak VP, Golubov A, Shpyleva SI and Kovalchuk O (2010) Alterations of microRNAs and their targets are associated with acquired resistance of MCF-7 breast cancer cells to cisplatin. Int. J. Cancer 127, 1785–1794 [DOI] [PubMed] [Google Scholar]
- 138.Tan C, Costello P, Sanghera J, Dominguez D, Baulida J, de Herreros AG and Dedhar S (2001) Inhibition of integrin linked kinase (ILK) suppresses β-catenin-Lef/Tcf-dependent transcription and expression of the E-cadherin repressor, snail, in APC−/− human colon carcinoma cells. Oncogene 20, 133–140 [DOI] [PubMed] [Google Scholar]
- 139.Margadant C and Sonnenberg A (2010) Integrin-TGF-β crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 11, 97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nishimura SL (2009) Integrin-mediated transforming growth factor-β activation, a potential therapeutic target in fibrogenic disorders. Am. J. Pathol 175, 1362–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hannigan G, Troussard AA and Dedhar S (2005) Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat. Rev. Cancer 5, 51–63 [DOI] [PubMed] [Google Scholar]
- 142.Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, Frank JA, Brumwell AN, Wheeler SE, Kreidberg JA et al. (2009) Epithelial cell α3β1 integrin links β-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J. Clin. Invest 119, 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rowe RG, Lin Y, Shimizu-Hirota R, Hanada S, Neilson EG, Greenson JK and Weiss SJ (2011) Hepatocyte-derived snail1 propagates liver fibrosis progression. Mol. Cell. Biol 31, 2392–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Scarpa M, Grillo AR, Brun P, Macchi V, Stefani A, Signori S, Buda A, Fabris P, Giordani MT, De Caro R et al. (2011) Snail1 transcription factor is a critical mediator of hepatic stellate cell activation following hepatic injury. Am. J. Physiol. Gastrointest. Liver Physiol 300, G316–G326 [DOI] [PubMed] [Google Scholar]
- 145.Boutet A, De Frutos CA, Maxwell PH, Mayol MJ, Romero J and Nieto MA (2006) Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J 25, 5603–5613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lewis BP, Burge CB and Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20 [DOI] [PubMed] [Google Scholar]
- 147.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM (2005) Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433, 769–773 [DOI] [PubMed] [Google Scholar]
- 148.Olson P, Lu J, Zhang H, Shai A, Chun MG, Wang Y, Libutti SK, Nakakura EK, Golub TR and Hanahan D (2009) MicroRNA dynamics in the stages of tumorigenesis correlate with hallmark capabilities of cancer. Genes Dev. 23, 2152–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dykxhoorn DM (2010) MicroRNAs and metastasis: little RNAs go a long way. Cancer Res 70, 6401–6406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Inui M, Martello G and Piccolo S (2010) MicroRNA control of signal transduction. Nat. Rev. Mol. Cell Biol 11, 252–263 [DOI] [PubMed] [Google Scholar]
- 151.Calin GA and Croce CM (2006) MicroRNA signatures in human cancers. Nat. Rev. Cancer 6, 857–866 [DOI] [PubMed] [Google Scholar]
- 152.Szafranska AE, Davison TS, John J, Cannon T, Sipos B, Maghnouj A, Labourier E and Hahn SA (2007) MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene 26, 4442–4452 [DOI] [PubMed] [Google Scholar]
- 153.Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C and Croce CM (2007) MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA, J. Am. Med. Assoc 297, 1901–1908 [DOI] [PubMed] [Google Scholar]
- 154.Rachagani S, Kumar S and Batra SK (2010) MicroRNA in pancreatic cancer: pathological, diagnostic and therapeutic implications. Cancer Lett. 292, 8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang Z, Li Y, Ahmad A, Banerjee S, Azmi AS, Kong D and Sarkar FH (2011) Pancreatic cancer: understanding and overcoming chemoresistance. Nat. Rev. Gastroenterol. Hepatol 8, 27–33 [DOI] [PubMed] [Google Scholar]
- 156.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G (2000) The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403, 901–906 [DOI] [PubMed] [Google Scholar]
- 157.Lee YS and Dutta A (2007) The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 21, 1025–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D and Slack FJ (2005) RAS is regulated by the let-7 microRNA family. Cell 120, 635–647 [DOI] [PubMed] [Google Scholar]
- 159.Boyerinas B, Park SM, Shomron N, Hedegaard MM, Vinther J, Andersen JS, Feig C, Xu J, Burge CB and Peter ME (2008) Identification of let-7-regulated oncofetal genes. Cancer Res. 68, 2587–2591 [DOI] [PubMed] [Google Scholar]
- 160.Lee YS, Kim HK, Chung S, Kim KS and Dutta A (2005) Depletion of human micro-RNA miR-125b reveals that it is critical for the proliferation of differentiated cells but not for the down-regulation of putative targets during differentiation. J. Biol. Chem 280, 16635–16641 [DOI] [PubMed] [Google Scholar]
- 161.Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J, Shingara J et al. (2007) The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 67, 7713–7722 [DOI] [PubMed] [Google Scholar]
- 162.Torrisani J, Bournet B, du Rieu MC, Bouisson M, Souque A, Escourrou J, Buscail L and Cordelier P (2009) let-7 microRNA transfer in pancreatic cancer-derived cells inhibits in vitro cell proliferation but fails to alter tumor progression. Hum. Gene Ther 20, 831–844 [DOI] [PubMed] [Google Scholar]
- 163.Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA and Jacks T (2008) Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc. Natl. Acad. Sci. U.S.A 105, 3903–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J et al. (2007) let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 131, 1109–1123 [DOI] [PubMed] [Google Scholar]
- 165.Paroo Z, Ye X, Chen S and Liu Q (2009) Phosphorylation of the human microRNA-generating complex mediates MAPK/Erk signaling. Cell 139, 112–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pandit KV, Corcoran D, Yousef H, Yarlagadda M, Tzouvelekis A, Gibson KF, Konishi K, Yousem SA, Singh M, Handley D et al. (2010) Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med 182, 220–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ji J, Zhao L, Budhu A, Forgues M, Jia HL, Qin LX, Ye QH, Yu J, Shi X, Tang ZY et al. (2010) Let-7g targets collagen type I α2 and inhibits cell migration in hepatocellular carcinoma. J. Hepatol 52, 690–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M et al. (2011) Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology 53, 209–218 [DOI] [PubMed] [Google Scholar]
- 169.Ogawa T, Iizuka M, Sekiya Y, Yoshizato K, Ikeda K and Kawada N (2010) Suppression of type I collagen production by microRNA-29b in cultured human stellate cells. Biochem. Biophys. Res. Commun 391, 316–321 [DOI] [PubMed] [Google Scholar]
- 170.Mongroo PS and Rustgi AK (2010) The role of the miR-200 family in epithelial-mesenchymal transition. Cancer Biol. Ther 10, 219–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Peter ME (2009) Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle 8, 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Oba S, Kumano S, Suzuki E, Nishimatsu H, Takahashi M, Takamori H, Kasuya M, Ogawa Y, Sato K, Kimura K et al. (2010) miR-200b precursor can ameliorate renal tubulointerstitial fibrosis. PLoS ONE 5, e13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chung AC, Huang XR, Meng X and Lan HY (2010) miR-192 mediates TGF-β/Smad3-driven renal fibrosis. J. Am. Soc. Nephrol 21, 1317–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Giovannetti E, Funel N, Peters GJ, Del Chiaro M, Erozenci LA, Vasile E, Leon LG, Pollina LE, Groen A, Falcone A et al. (2010) MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 70, 4528–4538 [DOI] [PubMed] [Google Scholar]
- 175.Moriyama T, Ohuchida K, Mizumoto K, Yu J, Sato N, Nabae T, Takahata S, Toma H, Nagai E and Tanaka M (2009) MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresistance. Mol. Cancer Ther 8, 1067–1074 [DOI] [PubMed] [Google Scholar]
- 176.Dillhoff M, Liu J, Frankel W, Croce C and Bloomston M (2008) MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J. Gastrointest. Surg 12, 2171–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tao J, Lu Q, Wu D, Li P, Xu B, Qing W, Wang M, Zhang Z and Zhang W (2011) microRNA-21 modulates cell proliferation and sensitivity to doxorubicin in bladder cancer cells. Oncol. Rep 25, 1721–1729 [DOI] [PubMed] [Google Scholar]
- 178.Misawa A, Katayama R, Koike S, Tomida A, Watanabe T and Fujita N (2010) AP-1-dependent miR-21 expression contributes to chemoresistance in cancer stem cell-like SP cells. Oncol. Res 19, 23–33 [DOI] [PubMed] [Google Scholar]
- 179.Olson P and Hanahan D (2009) Cancer. Breaching the cancer fortress. Science 324, 1400–1401 [DOI] [PubMed] [Google Scholar]
- 180.Devy L, Huang L, Naa L, Yanamandra N, Pieters H, Frans N, Chang E, Tao Q, Vanhove M, Lejeune A et al. (2009) Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res. 69, 1517–1526 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.