Abstract
Introduction
In the light of the ongoing SARS-CoV-2 pandemic, convalescent plasma is a treatment option for COVID-19. In contrast to usual therapeutic plasma, the therapeutic agents of convalescent plasma do not represent clotting factor activities, but immunoglobulins. Quarantine storage of convalescent plasma as a measure to reduce the risk of pathogen transmission is not feasible. Therefore, pathogen inactivation (e.g., Theraflex®-MB, Macopharma, Mouvaux, France) is an attractive option. Data on the impact of pathogen inactivation by methylene blue (MB) treatment on antibody integrity are sparse.
Methods
Antigen-specific binding capacity was tested before and after MB treatment of plasma (n = 10). IgG and IgM isoagglutinin titers were tested by agglutination in increasing dilutions. Furthermore, the binding of anti-EBV and anti-tetanus toxin IgG to their specific antigens was assessed by ELISA, and IgG binding to Fc receptors was assessed by flow cytometry using THP-1 cells expressing FcRI and FcRII.
Results
There was no significant difference in the isoagglutinin titers, the antigen binding capacity of anti-EBV and anti-tetanus toxin IgG, as well as the Fc receptor binding capacity before and after MB treatment of plasma.
Conclusion
MB treatment of plasma does not inhibit the binding capacity of IgM and IgG to their epitopes, or the Fc receptor interaction of IgG. Based on these results, MB treatment of convalescent plasma is appropriate to reduce the risk of pathogen transmission if quarantine storage is omitted.
Keywords: Pathogen inactivation, Methylene blue, Immunoglobulin G, Immunoglobulin M
Introduction
Convalescent plasma is discussed as an option to treat patients with SARS, MERS, Ebola, and COVID-19 diseases [1, 2, 3]. Plasma of donors who have recovered from a viral infection contains pathogen-specific antibodies, which are used for passive immunization of acutely ill patients.
According to the national German guidelines, plasma donations have to be stored under quarantine for at least 4 months and can only be issued after consecutive negative testing for transfusion-transmitted pathogens [4]. Alternatively, plasma has to be pathogen inactivated. In a pandemic situation like the SARS-CoV-2 outbreak, convalescent plasma is used as supportive treatment [5, 6, 7]. Especially at the beginning of a pandemic, quarantine storage is not feasible as the plasma is required before the end of the quarantine period. Therefore, exceptions have been granted in regard to mandatory quarantine storage of non-pathogen-inactivated convalescent plasma.
Possible means of pathogen inactivation for plasma are a combination of amotosalen/UVA (e.g., Intercept® Blood System, Cerus, Concord, CA USA), riboflavin/UV (e.g., Mirasol® PRT, Terumo BCT Europe N.V., Leuven, Belgium), and methylene blue (MB)/visible light (e.g., Theraflex®-MB; Macopharma, Mouvaux, France) [8, 9, 10]. Phenothiazine-like dyes such as MB can induce the formation of singlet oxygen [11], which can alter protein structures. This is most likely the reason for the reduction in activity of some clotting factors (especially factor V and factor VIII) and fibrinogen caused by MB treatment [8, 12].
Materials and Methods
Study Design and Sample Preparation
Plasmapheresis was performed in healthy donors of different blood types. All plasma units were frozen within 6 h after collection and stored at −30°C. After storage, plasma was thawed at 37°C for 1 h and split into 3 bioequivalent subunits of approximately 220 mL each. One was treated with MB/light according to the manufacturer's instructions (Macotronic B2, Macopharma; peak wavelength 627 ± 10 nm, 120 J/cm2). Plasma samples were drawn from MB-treated and untreated plasma and stored at −80°C until analysis.
THP-1 Cells
The human monocytic cell line THP-1, expressing FcγRI (CD64) and FcγRII (CD32) but not FcγRIII (CD16) [13], was obtained from the DSMZ (Braunschweig, Germany) and cultured in RPMI-1640 (PAN Biotech, Aidenbach, Germany) supplemented with 10% FBS Gold plus (Bio&Sell, Feucht/Nürnberg, Germany). Cells were washed once (140 g, 5 min), and resuspended in PBS/BSA (PBS buffer w/o Ca, Mg; pH 7.5; 0.22% BSA; Ortho Clinical), at 10 × 106 cells/mL. Cell viability was monitored by 7AAD (flow cytometry, Beckmann Coulter Inc., Miami Lakes, FL, USA).
Antigen-Specific Binding of IgM
IgM binding was assessed by isoagglutinin testing with red blood cells (RBCs). MB-treated and non-MB-treated plasma in 1:2 dilution series were incubated (RT, 15 min) with RBCs of blood type A and B using a microcolumn gel card system (ID-Card for NaCl, enzyme test and cold agglutinins, Bio-Rad Laboratories Inc., Hercules, CA, USA) according to the manufacturer's instructions. RBCs of blood type O were used as a negative control. Agglutination strengths were evaluated by 2 independent researchers. Antibody titer was defined as the last plasma dilution inducing agglutination. Relevant differences between MB treated and non-treated plasma were defined as antibody titers differing by 2 or more dilution steps.
Antigen-Specific Binding of IgG
IgG binding was assessed by incubating MB-treated and non-MB-treated plasma, as follows. (1) In a 1:2 dilution series (37°C, 15 min) with RBCs of blood type A and B using a microcolumn gel card system with human antiglobulin phase (ID-Card LISS/Coombs, Bio-Rad Laboratories Inc.) according to the manufacturer's instructions. RBCs of blood type O were used as negative controls. Agglutination strengths were evaluated by 2 independent researchers. Antibody titer was defined as the last plasma dilution inducing agglutination. Relevant differences between MB-treated and non-treated plasma were defined as antibody titers differing by 2 or more dilution steps. (2) By reactivity of the plasma units (OD measurements) in an anti-EBV (Epstein-Barr virus)-IgG ELISA and an anti-tetanus toxin IgG ELISA (EBV VCA IgG/Tetanus IgG, Serion ELISA Classic, Virion, Würzburg, Germany) according to the manufacturer's instructions.
Fc Part of IgG Binding to Fc Receptors
The influence of MB treatment on IgG-Fc binding to Fc receptors on THP-1 cells was assessed by flow cytometry (Cytomics FC 500, Beckmann Coulter Inc., Miami Lakes, USA). IVIG (Privigen®, CSL Behring GmbH, Hattersheim am Main, Germany) was used as positive control and buffer as negative control, as previously described [14]. In brief, 100 µL (1 × 105) of THP-1 cells were incubated (30 min; 4°C) with diluted (1:1,000 to 1:64,000) plasma samples or controls, washed and stained with 100 µL of FITC-rabbit anti-Human IgG-F(ab')2 fragment (Agilent, Santa Clara, USA, 1:75 in PBS/BSA; 30 min), washed twice and resuspended in 500 µL of PBS/BSA and assessed by forward-sideward scatter gating.
Statistics
The Mann-Whitney test (GraphPad Prism 8.0.1) was used for statistical analysis of the isoagglutinin titer test results. The Wilcoxon test (GraphPad Prism 8.0.1) was used for statistical analysis of the EBV and tetanus toxin-specific IgG binding test results. The Friedman test (GraphPad Prism 8.0.1) was used for statistical analysis of the Fc part of IgG binding test results. p < 0.05 was defined as statistically significant.
Results
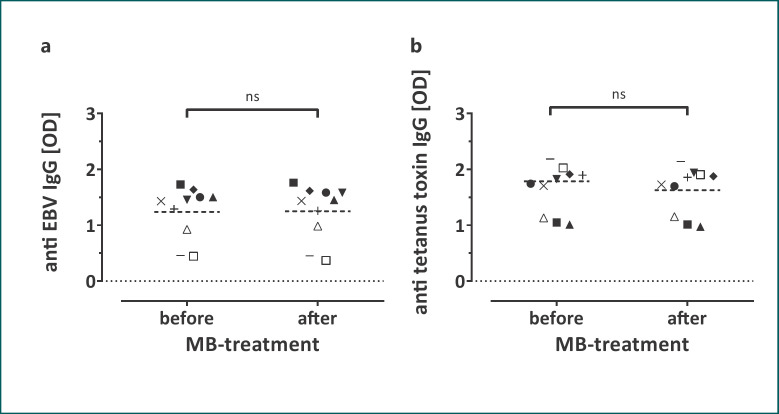
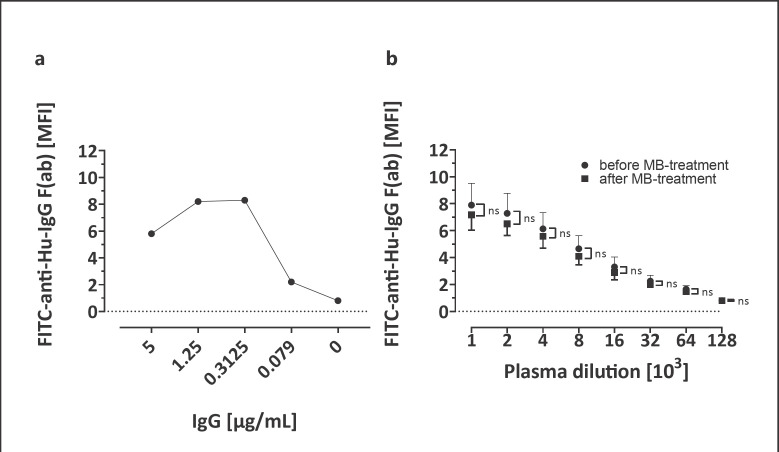
Isoagglutinin titers of plasma (blood type A, n = 4; B, n = 2; O, n = 4) before and after MB treatment did not differ (Fig. 1a, b for IgM, and c, d for IgG). Reactivity of the plasma units for anti-EBV and anti-tetanus toxin IgG before and after MB treatment did not differ (Fig. 2). IgG binding to Fc receptors did not differ between MB-treated and untreated plasma (Fig. 3).
Fig. 1.
Anti-A and anti-B isoagglutinin titers before and after treatment with MB pathogen inactivation using microcolumn gel cards with (c, d) and without (a, b) human antiglobulin serum in 10 individual donor plasma units each (blood type A, n = 4; B, n = 2; O, n = 4, medians are given). a, c Anti-A isoagglutinin titers of samples with blood type B and O. b, d Anti-B isoagglutinin titers of samples with blood type A and O. ns, not significant.
Fig. 2.
ODs measured for EBV-specific (a) and tetanus toxin-specific (b) IgG before and after MB treatment (n = 10; medians are given). ns, not significant.
Fig. 3.
IgG Fc binding to THP-1 cell Fc receptors. a IVIG was used as positive control, binding analysis was feasible with concentrations above 0.1 µg/mL (reference for plasma concentrations 7–16 × 103 µg/mL; n = 1). b IgG binding to Fc receptors on THP-1 cells did not differ before and after MB pathogen inactivation (n = 10 individual donor plasma units, means ± SD are given). ns, not significant.
Discussion
In this study we have shown that pathogen inactivation of plasma by MB treatment does not affect IgM and IgG binding to their cognitive epitopes, or IgG binding to Fc receptors. This was expected as MB shows high affinities to negatively charged macromolecules like DNA and only low affinities to neutral macromolecules like immunoglobulins [15]. Preservation of immunoglobulin function is important for the use of MB-treated convalescent plasma for the treatment of infections, such as COVID-19. To allow generalizable assessment of the integrity of IgM and IgG antibodies after MB treatment, we assessed antibodies with specificities widely present in the normal population. We used the anti-A and anti-B isoagglutinins and additionally IgG specific for EBV and tetanus toxin. Both antibody specificities are ubiquitous in the blood donor population of Western Pomerania (data not shown). These tests allow assessment of the integrity of the F(ab) part of the antibodies. Beside antigen binding, the integrity of the Fc part of IgG is important for its biological efficacy, especially when antibodies are needed for defense against pathogens. To test the integrity of the Fc part of IgG we used binding of IgG to THP-1 cells expressing Fc receptors [14]. This method has been shown to be equivalent to the European Pharmacopoeia method 2.7.9 (test for Fc binding of immunoglobulin) [16], and has been used for quality control of IVIG preparations.
Our study has some limitations. We neither tested preservation of the binding and neutralizing capacity of anti-SARS-CoV-2 IgG, nor the integrity of the Fc part of anti-SARS-CoV-2 IgG. Taking this into account, we cannot make definite assumptions on an eventual change in effectiveness of pathogen-inactivated convalescent plasma in the way shown by Tonn et al. [17] using the Intercept® system. However, there is no reason to assume that MB treatment would affect anti-SARS-CoV-2 IgG in a different way than EBV or tetanus toxin antibodies as they share the basic structure which presumably leads to the same low affinity for the intercalation of MB. In conclusion, our results indicate that MB treatment of plasma does not change the binding capacity of IgM and IgG to their cognitive antigens, or of IgG Fc receptor binding.
Statement of Ethics
Our research complies with the guidelines for human studies and the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The institutional ethics review board of Universitätsmedizin Greifswald approved the study (BB 014/14). As we used residuals of samples routinely collected during plasmapheresis, a separate written consent was not collected.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was funded by the research budget of Universitätsmedizin Greifswald.
Author Contributions
J.R. contributed to the conception of the work and the acquisition, analysis, and interpretation of the data for the work, and drafted the manuscript. K.Z. contributed to the conception of the work and the acquisition and analysis of the data for the work. Specifically, she contributed the ELISA test results showing the binding affinity of IgG to their specific antigens. J.W. contributed to the conception of the work and the acquisition, analysis and interpretation of the data for the work. Specifically, he contributed the flow cytometry results showing the binding affinity of IgG to their receptors on THP-1 cells. K.A. and A.G. contributed to the conception of the work and the interpretation of the data for the work. K.S. contributed to the conception of the work and the acquisition, analysis, and interpretation of the data for the work. Specifically, she contributed the agglutination results showing the isoagglutinin titers. All authors approved the final version of the manuscript to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
We acknowledge support for the Article Processing Charge from the DFG (German Research Foundation, 393148499) and the Open Access Publication Fund of the University of Greifswald.
References
- 1.Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020 Apr;20((4)):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020 Jun;130((6)):2757–65. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020 Apr;117((17)):9490–6. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bundesärztekammer . Richtlinie zur Gewinnung von Blut und Blutbestandteilen und zur Anwendung von Blutprodukten. Richtlinie Hämotherapie; 2019. [Google Scholar]
- 5.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, et al. Convalescent Plasma Study Group The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015 Jan;211((1)):80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020 Apr;323((16)):1582–9. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung IF, To KK, Lee CK, Lee KL, Chan K, Yan WW, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011 Feb;52((4)):447–56. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rock G. A comparison of methods of pathogen inactivation of FFP. VOX. 2011;100:169–178. doi: 10.1111/j.1423-0410.2010.01374.x. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1423-0410.2010.01374.x. [DOI] [PubMed] [Google Scholar]
- 9.Smith J, Rock G. Protein quality in Mirasol pathogen reduction technology-treated, apheresis-derived fresh-frozen plasma. Transfusion. 2010;50:926–931. doi: 10.1111/j.1537-2995.2009.02517.x. [DOI] [PubMed] [Google Scholar]
- 10.Irsch J, Pinkoski L, Corash L, Lin L. INTERCEPT plasma comparability with conventional fresh-frozen plasma based on coagulation function − an in vitro analysis. Vox Sang. 2010;98:47–55. doi: 10.1111/j.1423-0410.2009.01224.x. [DOI] [PubMed] [Google Scholar]
- 11.Seghatchian J, Walker WH, Reichenberg S. Updates on pathogen inactivation of plasma using Theraflex methylene blue system. Transfus Apheresis Sci. 2008 Jun;38((3)):271–80. doi: 10.1016/j.transci.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Thiele T, Kellner S, Hron G, Wasner C, Nauck M, Zimmermann K, et al. Storage of thawed plasma for a liquid plasma bank: impact of temperature and methylene blue pathogen inactivation. Transfusion. 2012 Mar;52((3)):529–36. doi: 10.1111/j.1537-2995.2011.03317.x. [DOI] [PubMed] [Google Scholar]
- 13.Handke W, Gravemann U, Sumian C, Reichenberg S, Seltsam A. Theraflex MB-Plasma treatment does not interfere with the antibody integrity in human plasma. 25th Regional Congress of the ISBT, London. 2015
- 14.Ramasamy I, Tran E, Charnock A, Farrugia A. Flow-cytometric method for the quantitation of the Fc function of intravenous immunoglobulin preparations. Vox Sang. 2000;78((3)):185–193. doi: 10.1159/000031178. [DOI] [PubMed] [Google Scholar]
- 15.Dardare N, Platz MS. Binding affinities of commonly employed sensitizers of viral inactivation. Photochem Photobiol. 2002 Jun;75((6)):561–4. doi: 10.1562/0031-8655(2002)075<0561:baoces>2.0.co;2. Available from: https://onlinelibrary.wiley.com/doi/full/10.1562/0031-8655%282002%290750561BAOCES2.0.CO2. [DOI] [PubMed] [Google Scholar]
- 16.Reipert BM, Ilas J, Carnewal C, Füreder SF, Bölzlbauer U, Teschner W, et al. Fc function of a new intravenous immunoglobulin product: IGIV 10% triple virally inactivated solution. Vox Sang. 2006 Oct;91((3)):256–63. doi: 10.1111/j.1423-0410.2006.00828.x. [DOI] [PubMed] [Google Scholar]
- 17.Tonn T, Corman VM, Johnsen M, Richter A, Rodionov RN, Drosten C, Bornstein SR. Stability and neutralising capacity of SARS-CoV-2-specific antibodies in convalescent plasma. The Lancet Microbe. 2020;1:e63. doi: 10.1016/S2666-5247(20)30037-9. Dev Mittar, Rosanto Paramban, Catherine McIntyre: Flow Cytometry and High-Content Imaging to Identify Markers of Monocyte-Macrophage Differentiation. BD Biosciences 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]