Abstract
OBJECTIVES:
Methotrexate (MTX) is a broadly used anticancer. Its major side effect is hepatotoxicity. Gossypin is a flavonoid has a hepatoprotective effect as well as antitumor property. The study aimed at inspecting the protective effect of gossypin against MTX hepatotoxicity.
MATERIALS AND METHODS:
Twenty-four adult male rats arranged into four groups (six rats each): control, gossypin control, MTX, and MTX+ gossypin. Animals were orally administered gossypin at 10 mg kg-1 day-1 for 7 days. MTX was injected i.p. (20 mg/kg-1 once) on 5th day. Liver enzyme and oxidative stress markers were assessed. BAX, transforming growth factor-beta (TGF-β) gene expressions, and P-glycoprotein (P-gp) were assessed. The histopathological study as well as the immunohistochemical study for hepatic caspase 3 and nuclear factor kappa-B (NFκ-B) was done.
RESULTS:
MTX produced a significant increase of liver enzymes and distortion of hepatic architecture alongside with increased the hepatic collagen content. MTX administration significantly increased the oxidative stress markers and upregulated the pro-apoptotic BAX and the pro-fibrogenic TGF-β. MTX increased caspase 3 and NFκ-B expression, while diminished the expression of P-gp. Gossypin pretreatment improved the previous parameters, restored the normal hepatic architecture, reduced the hepatic fibrosis, and regained nearly normal expressions for BAX, TGF-β, caspase 3, and NFκ-B. Gossypin caused more reduction in P-gp hepatic expression.
CONCLUSIONS:
Gossypin may be a valuable adjuvant therapy that protects the liver against MTX toxicity through antioxidant, anti-inflammatory, antiapoptotic mechanisms, and mediated P-gp expression reduction.
Keywords: Gossypin, hepatic fibrosis, methotrexate hepatotoxicity
Introduction
Methotrexate (MTX) is considered a basis in the therapy of diverse malignant tumors and autoimmune diseases, especially in developing countries. MTX-induced liver injury is a common side effect.[1] Approximately 50% of users develop liver injury ends in fibrosis and cirrhosis.[2] How MTX induced hepatotoxicity is still unidentified, but there are different mechanisms that were postulated. The prolonged intracellular accumulation of MTX caused folic acid depletion that badly affects hepatocytes by interfering with DNA synthesis. Furthermore, MTX activates Ito cells that sited in the peri-sinusoidal spaces of the hepatic lobules. When activated, they transformed into myofibroblasts, which secrete collagen and matrix protein. This is the initiation step in liver fibrosis.[1] However, previous studies reported that MTX induced oxidative damage, an inflammatory response, and apoptosis in the hepatocyte causing liver toxicity.[3,4] MTX elimination depends mainly on the kidney and liver. MTX is excreted actively into bile by the bile canalicular membrane transporters.[5] The hepatic transporters system is implicated in the uptake of MTX in and out of the hepatocyte; thus, it modulates MTX concentration and toxicity into the hepatocyte.[6]
The natural flavonoids displayed a benefit against MTX toxicity.[7] Gossypin is a flavonoid of plant source (Hibiscus vitifolius).[8] It demonstrated antioxidant and anti-inflammatory as well as antitumor effects that broaden its experimental applications in various disease models.[8,9,10,11] Gossypin reduced the hepatic oxidative damage and the inflammatory cytokines such as tumor necrosis factor-alpha in antimicrobial-induced nephrotoxicity in rats.[12] Añón et al. have revealed a hepatoprotective effect for gossypin among other phenolic compounds on rat hepatocytes.[10] Another publication reported an antioxidant effect for gossypin against sulfur mustard-induced systemic and hepatotoxicity in mice.[13] Still, limited studies were conducted on the gossypin hepatoprotective effect. Thus, our aim was to explore the potential effect of gossypin on MTX-induced liver injury and to clarify the potential mechanisms.
Materials and Methods
Chemicals
We bought the powder of gossypin (CAS 652-78-8) from Sigma-Aldrich (St. Louis, MO, USA). MTX was got from Ebewe Co.(Unterach, Austria). Rabbit Polyclonal caspase 3 (catalog number RB-1197-B0) and nuclear factor kappa-B (NFκ-B) (catalog number RB-9034-R7) antibodies were obtained from Lab Vision Corp. USA. Quantitative real-time polymerase chain reaction (qRT-PCR) for transforming growth factor-beta (TGF-β), BAX, and NADPHD genes were conducted by primers bought from Kappa, Biosystems, USA. Enzyme-linked immunosorbent assay (ELISA) P-glycoprotein (P-gp) kit (catalog number 366408) was purchased from USBiological life Sciences (Salem, MA, USA). Other chemicals were got commercially.
Experimental design
Adult male Wistar rats were brought from the National Research Center at El-Giza, Egypt. Twenty-four animals weighed (200 ± 10 g) were arranged randomly in plastic cages and were allowed to eat a standard chow and tap water freely. After acclimatization for 1 week, four groups (six rats each) were assigned as follows: control, gossypin control, MTX, and MTX + gossypin. Gossypin 10 mg/kg was administered by oral gavage after its suspension in 1% carboxymethylcellulose daily for 7 days according to a previous study.[12] On the 5th day, 20 mg/kg MTX was injected i.p. once.[4] Control rats received the vehicle.
At the end of 7 days, animals were fasted overnight and then anesthetized by injection of urethane hydrochloride 1 g/kg i.p. Then, they sacrificed by decapitation. Three milliliters of the trunk blood was collected directly in glass tubes at the decapitation site. We left the blood to coagulate then centrifuged at 3000 g for 10 min to get clear sera. Hepatic tissue was dipped in 10% formalin for 24 h. Other liver parts were put in liquid nitrogen stored at −80 ∘ C for further manipulations. The experiment was approved by the Faculty of Medicine, Research Ethics Committee at Minia University, Egypt, that matched with EU Directive 2010/63/EU for experimental animal use and care.
Measurements
Biochemical parameters
Hepatic tissue was homogenized, centrifuged by a cold phosphate buffer (W/V 1:5) to get a supernatant. The hepatic malondialdehyde (MDA) expresses a lipid peroxidation product. MDA reacts with the thiobarbituric acid and yields compounds with wavelength detected at 535 nm.[14] The hepatic content of reduced glutathione (GSH) was measured by a method prescribed previously.[15] The method depends on the reduction of thiol groups of glutathione through Elman's reagent. The resultant colored compound measured at 412 nm. Superoxide dismutase (SOD) activity was assessed by a previously prescribed method that depends on inhibition of pyrogallol autoxidation. The produced color was estimated kinetically.[16]
Quantitative gene expression of BAX and transforming growth factor-beta
Extraction of total RNA for hepatic tissue and qRT-PCR were performed according to the manufacturer's instructions (Amresco, Solon, USA), (Kappa, Biosystems, USA). The relative gene expression was calculated proportional to controls where the latter was fixed at a value of 1. The gene primers were listed at Table 1.[17]
Table 1.
Primers of genes
| Gene | Primer sequence |
|---|---|
| BAX | Sense: 5’- GGAGACACCTGAGCTGACCT-3’ Antisense: 5’- CTCAGCCCATCTTCTTCCAG-3’ |
| TGF-β | Sense: 5’- TTGCCCTCTACAACCAACACAA -3’ Antisense: 5’- GCTTGCGACCCACGTAGTA -3’ |
| GAPDH | Sense: 5’- GTCGGTGTGAACGGATTTG-3’ Antisense: 5’- CTTGCCGTGGGTAGAGTCAT-3’ |
BAX=Bcl-2-associated X protein, TGF-β=Transforming growth factor-beta, GAPDH=Glyceraldehyde 3-phosphate dehydrogenase
Assessment of P-glycoprotein expression
Using the ELISA technique, we firmly followed the instructions of the manufacturer.
The histopathology and the immunohistochemical studies
After fixation of hepatic tissue in formalin, sections of hepatic tissue were processed routinely. H and E stain was applied. The hepatic injury was evaluated according to the necro-inflammatory score of Ishak modified HAI grading.[18] Briefly, four classes of histological findings were assessed: periportal hepatitis (0–4); confluent necrosis (0–6); apoptosis and focal inflammation (0–4); and portal inflammation (0–4). The value of each finding is summated for each field. We assessed three microscopic fields for each rat and divided by three to obtain an average score. Masson's Trichrome Stain was used to detect collagen fibers.[19] Other deparaffinized sections were processed for immunostaining of caspase 3 and NFκ-B. The histologist took into consideration the intensity and extent of positive cells during grading.[20]
Statistical analysis
Results were calculated as means ± standard error of mean. Data were analyzed by one-way ANOVA, followed by Tukey's posttest. The software used is GraphPad Prism 5.01. Significance is considered if P value was <0.05.
Results
Liver function and tissue oxidative status
Table 2 demonstrates a significant rise of serum alanine aminotransferase after MTX administration as compared to the control group. However, their levels returned to normal with cotreatment of gossypin. The hepatic content of GSH and SOD activity determine the antioxidant capacity, whereas MDA represents the oxidative damage on cells. MTX induced significant elevation of MDA alongside with depletion of hepatic GSH and reduced SOD activity as compared to the control group. Gossypin treatment increased the hepatic GSH concentration, the SOD activity, and decreased lipid peroxidation products significantly as compared to the MTX group.
Table 2.
Effect of gossypin and methotrexate on serum alanine transaminase and hepatic oxidative parameters of rats
| Groups | Serum ALT (U/L) | Hepatic malondialdehyde (nmol/g tissue) | Hepatic glutathione reduced (nmol/g tissue) | Hepatic superoxide dismutase (U/g tissue) |
|---|---|---|---|---|
| Control | 20.0±1.53 | 31.94±03.0 | 500.2±45.6 | 1213.0±062.7 |
| Gossypin | 22.0±0.60 | 28.55±00.6 | 488.5±33.1 | 1268.0±111.4 |
| MTX | 57.1±4.76* | 78.39±18.6* | 281.1±24.5* | 0521.1±065.1* |
| MTX + gossypin | 24.7±2.33# | 37.07±03.9# | 418.4±32.9# | 1131.0±234.5# |
*,# Significantly different from the control group and MTX group, respectively, at P<0.05. All parameters are expressed as mean±SEM (n=6). MTX=Methotrexate, ALT=Alanine transaminase, SEM=Standard error of mean
Expressions of BAX, transforming growth factor-beta, and P-glycoprotein
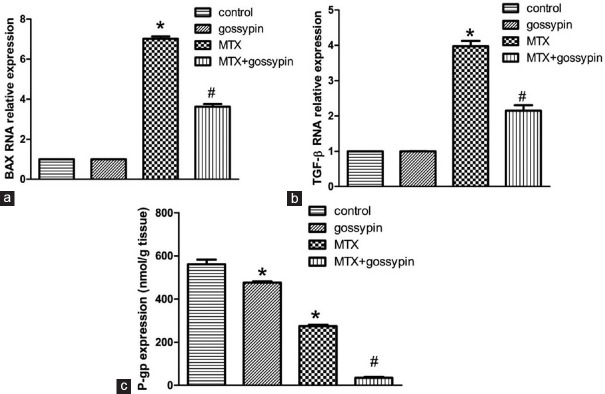
Results of qRT-PCR demonstrated significant upregulation of gene expressions of BAX and TGF-β after administration of MTX in comparison to control groups. Gossypin pretreatment significantly suppressed their expressions in the hepatic tissue [Figure 1a and b]. The protein expression of P-gp showed a significant decrease in the MTX group. However, gossypin potentiated the decrease in P-gp induced by MTX significantly when they were concurrently administered [Figure 1c].
Figure 1.
qRT-PCR for relative expression of hepatic BAX (a), TGF-β (b), and protein expression of P-glycoprotein by ELISA (c), respectively. *,#Significantly different from control, MTX, and MTX-gossypin-treated groups, respectively, at P < 0.05. TGF-β=transforming growth factor-beta, MTX = methotrexate, QRT-PCR = Quantitative real-time polymerase chain reaction
Histopathology and immunohistochemical studies
Figure 2 demonstrates disrupted hepatic lobular architecture with vacuolization and inflammatory cell infiltration after MTX injection (2C, D). Gossypin pretreatment preserved the normal liver structure with a significant decrease in cellular infiltration and degeneration.
Figure 2.
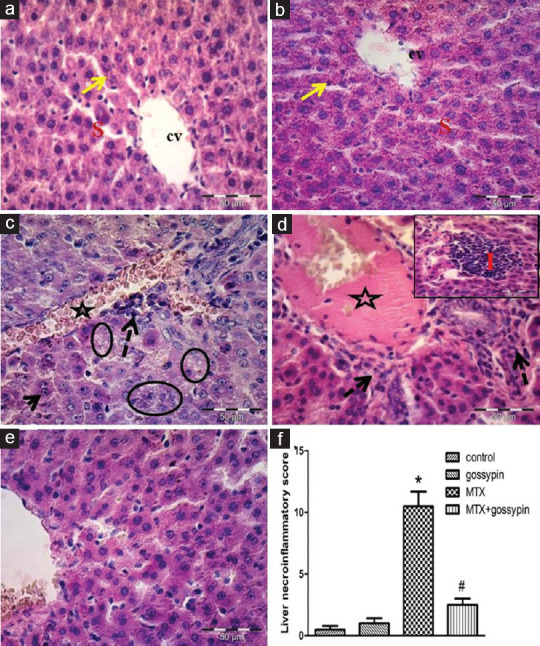
A picture of hepatic tissue at magnification × 400 for control rat shows normal cords of hepatocytes (arrow) around the central vein (cv) and blood sinusoids (s) (a and b). Liver tissue of toxic (c and d) and treated (e) groups showing dilated congested central and portal veins (star), inflammatory cells were observed (dashed arrows), vacuolization (oval), disorganization (i), and a binuclear hepatocyte could be seen (arrow). (f) Necro-inflammatory score. *,#Significantly different from control, MTX, and MTX-gossypin-treated groups, respectively, at P < 0.05. MTX = methotrexate
Masson's trichrome stain in Figure 3 demonstrates normal fine collagen fibers distribution at the liver of the control group. However, the toxic group showed a significant increase of collagen fibers in-between the hepatocytes, pericentral, and periportal [Figures 3c and d]. The treated group displayed a significant regression of the amount of collagen fibers.
Figure 3.

A Photomicrographs of Masson's trichrome stain at × 400 for liver sections. Control groups (a and b) display fine collagen fibers (arrow) around the central vein (c) and between the hepatic cords. Sections of the toxic group showing apparent rise of collagen fibers pericentrally (star) (c), periportal (d), and extending in-between the cords of hepatocytes (arrow). Treated group (e) shows apparent improvement of the amount of collagen fibers. (f) Fibrosis score. *,#Significantly different from control, MTX, and MTX-gossypin-treated groups, respectively, at P < 0.05. MTX = methotrexate
Figure 4 demonstrates very minimal positive cells for caspase 3 and also NFκ-B in the liver of control groups. MTX group showed significant caspase 3 and NFκ-B positive cells. Both nuclear and cytoplasmic expressions were seen for caspase 3. NFκ-B expression was observed as granular in the cytoplasm of hepatocyte. Gossypin significantly decreased the expression of caspase 3 and NFκ-B as compared to MTX.
Figure 4.
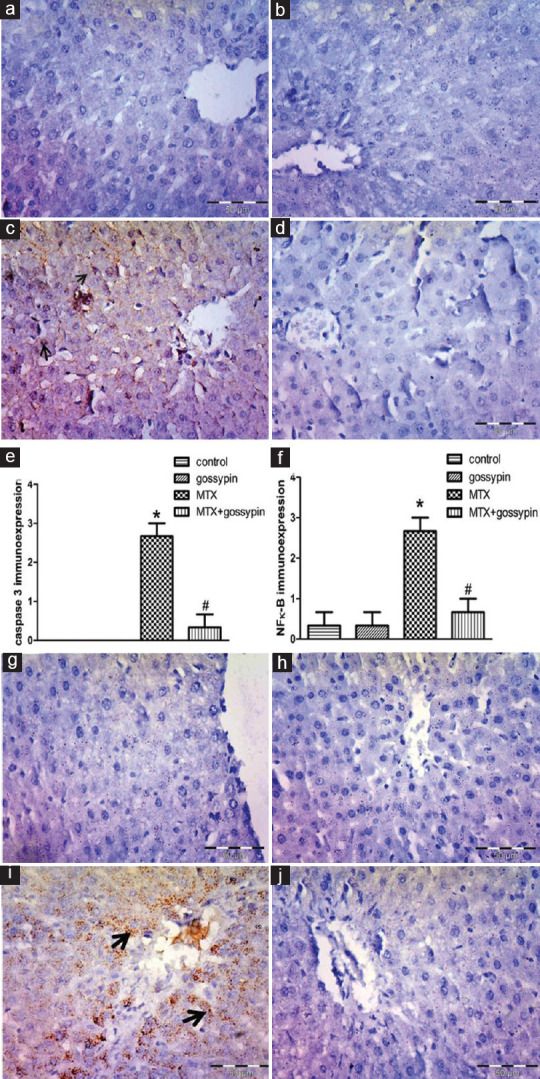
Control groups (a and b) normal control, (g and h gossypin control) show no or minimal expression of caspase 3and NFκ-B respectively. Anticaspase 3 and anti-NFκ-B immune-stained sections in the liver, ×400. Toxic group (c and i) showing intense brown nuclear immunostaining of caspase 3 and NFκ-B (arrows). Treated group (d and j) shows a marked decrease in the expression × 400. (e and f) caspase 3 and NFκ-B immunostaining scores, respectively. *,#Significantly different from control, MTX, and MTX-gossypin-treated groups, respectively, at P < 0.05. MTX = methotrexate, NFκ-B = Nuclear factor kappa-B
Discussion
MTX is a widely used anticancer and immunosuppressant. However, hepatotoxicity is the major drawback of its use. The present study offered a safe hepatoprotective modality with well-documented antitumor activity.[21] The present study proved that gossypin reduced MTX hepatotoxicity evident biochemically and by histopathology. MTX causes liver injury with the release of liver enzymes in agreement with others.[3,4] The obvious cytoplasmic vacuolization and degeneration in the hepatocyte of MTX rats are considered a footprint of typical oxidative damage.[22] Thus, our results documented the disruption of the oxidative hepatic status after MTX administration. Gossypin has antioxidant properties that could ameliorate MTX-induced oxidative injury and its sequelae in hepatocyte structures.[10,13] However, the actual morbidity of MTX use is a consequence of stellate cell activation and hepatic fibrosis.[1] The present study reported early fibrotic changes in the liver of the MTX group with concomitant upregulation of TGF-β gene expression. TGF-β is considered a pro-fibrogenic cytokine that is capable of causing hepatic stellate cells transformation into myofibroblasts and initiating of collagen deposition. Thus, interference with its signaling pathway inhibits liver disease progression.[23] Gossypin caused a decrease in TGF-β in line with the previous data[9] and prevented collagen accumulation in the liver of the treated group. We suggest an antifibrotic mechanism for gossypin against MTX-induced liver fibrosis based on its ameliorative effect on TGF-β. Cross talk was reported between TGF-β and NFκ-B. Both of these factors were shown to be involved in hepatic inflammation up to the point at which hepatocellular carcinoma develops.[24] Our study revealed hepatic infiltration by inflammatory cells that coincided with upregulation of NFκ-B in the MTX group in agreement with El-Sheikh et al.[3] Gossypin treatment nearly normalized NFκ-B expression. The ameliorative effects of gossypin on the TGF-β/NFκ-B pathway were previously described as the basis for its anti-inflammatory and antitumor effects.[9] Increase markers of apoptosis, BAX, and caspase 3 expressions proved apoptosis in the liver, in the MTX group. The levels of BAX and caspase 3 showed reduction with gossypin treatment as shown by other hepatoprotective modalities in MTX hepatotoxicity.[3,7] Despite the gossypin-induced apoptosis in most tumor cells, gossypin appears to behave in the opposite direction with respect to hepatoprotection as demonstrated by use of another flavonoid in the MTX hepatotoxicity study.[7] The current study revealed the role of P-gp in the protection against MTX hepatotoxicity. P-gp protein expression was decreased by MTX and gossypin in agreement with the previous studies, respectively.[25,26] Co-administration of gossypin and MTX resulted in enhancement of the decrease of P-gp expression with decreased toxicity as reported before with resveratrol and MTX.[27] According to literature, the disposition of MTX can be modified by the modulation of P-gp expression, as this efflux transporter is expressed along the liver canalicular membrane, where it extrudes its substrates, including MTX, from inside the hepatocytes into the bile,[28] where MTX is subjected to enterohepatic circulation and is re-absorbed to continue harming the liver. This process augments hepatotoxic effect not just of MTX, but also for other anticancer P-gp substrates as cisplatin.[29] In the present study, decreasing P-gp expression by MTX alone may be a feedback mechanism to reduce hepatotoxicity, and a further decrease of P-gp by concomitant administration of gossypin was revealed to diminish the hepatotoxicity. Furthermore, inhibiting P-gp with a subsequent decrease in MTX biliary excretion may subject the drug to the action of metabolizing enzymes in the liver for detoxification.[30] Consequently, gossypin reduced MTX uptake and toxicity into the hepatocyte. We postulated that gossypin-induced decrease in P-gp might offer a beneficial effect in deteriorating MTX hepatotoxicity and decreasing its resistance.
Conclusion
Gossypin offered a hepatoprotective effect depends on its antioxidant, anti-inflammatory, antiapoptotic, antifibrotic effect, and modulation of P-gp expression.
Financial support and sponsorship
This study was financially supported by the Science and Technology Development Fund, STDF, Egypt, Grant No. 22934.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Dr. Nagwa Zenhom (lecturer of biochemistry) for help in qRT-PCR measurements.
References
- 1.Bath RK, Brar NK, Forouhar FA, Wu GY. A review of methotrexate-associated hepatotoxicity. J Dig Dis. 2014;15:517–24. doi: 10.1111/1751-2980.12184. [DOI] [PubMed] [Google Scholar]
- 2.Conway R, Carey JJ. Risk of liver disease in methotrexate treated patients. World J Hepatol. 2017;9:1092–100. doi: 10.4254/wjh.v9.i26.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Sheikh AA, Morsy MA, Abdalla AM, Hamouda AH, Alhaider IA. Mechanisms of thymoquinone hepatorenal protection in methotrexate-induced toxicity in rats. Mediators Inflamm. 2015;2015:859383. doi: 10.1155/2015/859383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samdanci ET, Huz M, Ozhan O, Tanbek K, Pamukcu E, Akatli AN, et al. Cytoprotective effects of molsidomine against methotrexate-induced hepatotoxicity: An experimental rat study. Drug Des Devel Ther. 2019;13:13–21. doi: 10.2147/DDDT.S181550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masuda M, I’izuka Y, Yamazaki M, Nishigaki R, Kato Y, Ni’inuma K, et al. Methotrexate is excreted into the bile by canalicular multispecific organic anion transporter in rats. Cancer Res. 1997;57:3506–10. [PubMed] [Google Scholar]
- 6.Chu X, Korzekwa K, Elsby R, Fenner K, Galetin A, Lai Y, et al. Intracellular drug concentrations and transporters: Measurement, modeling, and implications for the liver. Clin Pharmacol Ther. 2013;94:126–41. doi: 10.1038/clpt.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samarghandian S, Farkhondeh T, Azimi-Nezhad M. Protective effects of chrysin against drugs and toxic agents. Dose Response. 2017;15 doi: 10.1177/1559325817711782. 1559325817711782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandrashekhar VM, Ganapaty S, Ramkishan A, Narsu ML. Neuroprotective activity of gossypin from Hibiscus vitifolius against global cerebral ischemia model in rats. Indian J Pharmacol. 2013;45:575–80. doi: 10.4103/0253-7613.121367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunnumakkara AB, Nair AS, Ahn KS, Pandey MK, Yi Z, Liu M, et al. Gossypin, a pentahydroxy glucosyl flavone, inhibits the transforming growth factor beta-activated kinase-1-mediated NF-kappaB activation pathway, leading to potentiation of apoptosis, suppression of invasion, and abrogation of osteoclastogenesis. Blood. 2007;109:5112–21. doi: 10.1182/blood-2007-01-067256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Añón MT, Ubeda A, Alcaraz MJ. Protective effects of phenolic compounds on CCl4-induced toxicity in isolated rat hepatocytes. Z Naturforsch C J Biosci. 1992;47:275–9. doi: 10.1515/znc-1992-3-417. [DOI] [PubMed] [Google Scholar]
- 11.Ganapaty S, Chandrashekhar V, Chitme H, Narsu M. Free radical scavenging activity of gossypin and nevadensin: An in-vitro evaluation. Indian J Pharmacol. 2007;39:281–3. [Google Scholar]
- 12.Katary M, Salahuddin A. Ameliorative effect of gossypin against gentamicin-induced nephrotoxicity in rats. Life Sci. 2017;176:75–81. doi: 10.1016/j.lfs.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Gautam A, Vijayaraghavan R. Prophylactic effect of gossypin against percutaneously administered sulfur mustard. Biomed Environ Sci. 2007;20:250–9. [PubMed] [Google Scholar]
- 14.Zeb A, Ullah F. A simple spectrophotometric method for the determination of thiobarbituric acid reactive substances in fried fast foods. J Anal Methods Chem. 2016;2016:9412767. doi: 10.1155/2016/9412767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giustarini D, Tsikas D, Colombo G, Milzani A, Dalle-Donne I, Fanti P, et al. Pitfalls in the analysis of the physiological antioxidant glutathione (GSH) and its disulfide (GSSG) in biological samples: An elephant in the room. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1019:21–8. doi: 10.1016/j.jchromb.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramasarma T, Rao AV, Devi MM, Omkumar RV, Bhagyashree KS, Bhat SV. New insights of superoxide dismutase inhibition of pyrogallol autoxidation. Mol Cell Biochem. 2015;400:277–85. doi: 10.1007/s11010-014-2284-z. [DOI] [PubMed] [Google Scholar]
- 17.VanGuilder HD, Vrana KE, Freeman WM. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques. 2008;44:619–26. doi: 10.2144/000112776. [DOI] [PubMed] [Google Scholar]
- 18.Brunt EM. Grading and staging the histopathological lesions of chronic hepatitis: The Knodell histology activity index and beyond. Hepatology. 2000;31:241–6. doi: 10.1002/hep.510310136. [DOI] [PubMed] [Google Scholar]
- 19.Germani G, Hytiroglou P, Fotiadu A, Burroughs AK, Dhillon AP. Assessment of fibrosis and cirrhosis in liver biopsies: An update. Semin Liver Dis. 2011;31:82–90. doi: 10.1055/s-0031-1272836. [DOI] [PubMed] [Google Scholar]
- 20.Xia L, Xue XZ. Immunohistochemical study of NF-κB p65, c-IAP2 and caspase-3 expression in cervical cancer. Oncol Lett. 2012;3:839–44. doi: 10.3892/ol.2012.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sjöstedt N, Holvikari K, Tammela P, Kidron H. Inhibition of breast cancer resistance protein and multidrug resistance associated protein 2 by natural compounds and their derivatives. Mol Pharm. 2017;14:135–46. doi: 10.1021/acs.molpharmaceut.6b00754. [DOI] [PubMed] [Google Scholar]
- 22.Del Monte U. Swelling of hepatocytes injured by oxidative stress suggests pathological changes related to macromolecular crowding. Med Hypotheses. 2005;64:818–25. doi: 10.1016/j.mehy.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Dooley S, ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res. 2012;347:245–56. doi: 10.1007/s00441-011-1246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freudlsperger C, Bian Y, Contag Wise S, Burnett J, Coupar J, Yang X, et al. TGF-β and NF-κB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene. 2013;32:1549–59. doi: 10.1038/onc.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibayama Y, Takeda Y, Yamada K. Effect of methotrexate treatment on expression levels of organic anion transporter polypeptide 2, P-glycoprotein and bile salt export pump in rats. Biol Pharm Bull. 2009;32:493–6. doi: 10.1248/bpb.32.493. [DOI] [PubMed] [Google Scholar]
- 26.Rajnarayana K, Venkatesham A, Krishna DR. Influence of some bioflavonoids on the transport of nitrendipine. Drug Metabol Drug Interact. 2008;23:299–310. doi: 10.1515/dmdi.2008.23.3-4.299. [DOI] [PubMed] [Google Scholar]
- 27.Jia Y, Liu Z, Wang C, Meng Q, Huo X, Liu Q, et al. P-gp, MRP2 and OAT1/OAT3 mediate the drug-drug interaction between resveratrol and methotrexate. Toxicol Appl Pharmacol. 2016;306:27–35. doi: 10.1016/j.taap.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 28.Hernández Lozano I, Bauer M, Wulkersdorfer B, Traxl A, Philippe C, Weber M, et al. Measurement of hepatic ABCB1 and ABCG2 transport activity with [11C] tariquidar and PET in humans and mice. Mol Pharm. 2020;17:316–26. doi: 10.1021/acs.molpharmaceut.9b01060. [DOI] [PubMed] [Google Scholar]
- 29.El-Sheikh AA. P-Glycoprotein/ABCB1 might contribute to morphine/cisplatin-induced hepatotoxicity in rats. Sci Pharm. 2020;88:1–4. [Google Scholar]
- 30.Kong LL, Shen GL, Wang ZY, Zhuang XM, Xiao WB, Yuan M, et al. Inhibition of P-glycoprotein and multidrug resistance-associated protein 2 regulates the hepatobiliary excretion and plasma exposure of thienorphine and its glucuronide conjugate. Front Pharmacol. 2016;7:242. doi: 10.3389/fphar.2016.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]