Abstract
Energy-dense rechargeable batteries have enabled a multitude of applications in recent years. Moving forward, they are expected to see increasing deployment in performance-critical areas such as electric vehicles, grid storage, space, defense, and subsea operations. While this at first glance spells great promise for conventional lithium-ion batteries, all of these use-cases, unfortunately, share periodic and recurring exposures to extremely low-temperature conditions, a performance constraint where the lithium-ion chemistry can fail to perform optimally. Next-generation chemistries employing alternative anodes with increased solvent compatibility or altogether different operating mechanisms could present an avenue for overcoming many of the low-temperature hurdles intrinsic to the lithium-ion battery. In this article, we provide a brief overview of the challenges in developing lithium-ion batteries for low-temperature use, and then introduce an array of nascent battery chemistries that may be intrinsically better suited for low-temperature conditions moving forward. Specifically, we evaluate the prospects of using lithium-metal, lithium-sulfur, and dual-ion batteries for performance-critical low-temperature applications. These three chemistries are presented as prototypical examples of how the conventional low-temperature charge-transfer resistances can be overcome. However, these three chemistries also present their own unique challenges at low temperatures, highlighting the balance between traditional low-temperature electrolyte design and next-generation approaches.
Keywords: lithium batteries, low-temperature operation, lithium-metal anode, lithium-sulfur batteries, desolvation
Graphical Abstract

Low-temperature conditions present severe hurdles towards operation in lithium-ion batteries. Next-generation batteries can present opportunities for heightened low-temperature performance through increased solvent compatibility or unique charge-transfer mechanisms. This presents an avenue for overcoming the conventionally envisioned rate-limiting hurdles at low-temperatures, including lithium-ion desolvation. An overview and outlook are provided on the potential for advanced lithium-based batteries at low-temperature conditions.
1. Introduction
The dawn of the 21st century has brought with it the ubiquity of battery-enabled electronic devices, enabling tremendous societal advances over the course of the past 3 decades.[1] While lithium-ion batteries have been universally adopted for portable electronics applications, there is increasing need and desire for reliable storage of electrical energy in applications such as electric vehicles, grid storage, space exploration, defense applications, and subsea operations. However, each of these applications have highly diverse and varying performance requirements over the time of use, including variable rates, and pressures, and often most critical, temperatures.[2–4] The required low-temperature capabilities, shown in Figure 1, can present a critical roadblock towards the use of lithium-ion batteries. Commercial state-of-the-art batteries see noticeable drops in capacity retention and rate capability below 0 °C, and will rarely be recommended for use below −20 °C.[5] This limitation is defined by the kinetics of ion-transfer in solution; at low-temperatures, every stage of charge-transfer, from ion diffusion within the electrolyte and electrode materials to charge-transfer across the electrode-electrolyte interfaces, is significantly impeded. Low-temperature conditions, in which the environment itself has relatively limited thermal kinetic energy available, can present significant energetic hurdles within the chemical reaction pathway normally followed during charge and discharge at room temperature.
Figure 1.
Application-specific low-temperature conditions that battery systems must be capable of operating within.
In applications with recurring exposure to low-temperature conditions, particularly electric-vehicles and space applications, there is currently heavy reliance on secondary heating solutions coupled with thermal management systems in order to ensure consistent power delivery during operation.[6] Thermal management solutions can generally be classified as either internal, with thermal management elements integrated directly within the cell itself, or external, where thermal management solutions are applied to an entire pack or module. Internal solutions, including cells with integrated AC self-heating[7] or other internal self-heating structures[8], are generally not feasible or practical to integrate within every cell used in an application given the added weight and complexity of such systems.[9] More commonly, external heating solutions are applied including thermal heating jackets or heating elements, warm-air heating, or liquid heat-transfer approaches. While these strategies may be more feasible to implement in practice than internal solutions, they also are accompanied by added weight, greater complexity, and reduced overall energy efficiency.[7,9]
In space applications, there is often a reliance on radioisotope thermal generators (RTGs), which pair a thermoelectric generator with a decaying radioactive material to ensure consistent and reliable power generation energy. The waste heat from such systems is utilized to heat secondary systems, such as on-board electronics and the lithium-ion battery pack, as was in the case of the 2012 Mars Curiosity rover.[10] In this instance as well as older missions like the 2004 Mars Spirit and Opportunity rovers, the battery cells were designed to ensure stable operation from −20 to 30 °C, even though the Martian surface itself can dip below −100 °C.[10] The onboard radioisotope heating systems were thus vital to maintaining a favorable temperature environment within the battery chamber and ensuring reliable power delivery. However, future missions with substantially decreased cost or onboard functionality may lack radioisotope heating systems. Given the added complexity, cost, regulations, and weight of such systems, directly innovating and advancing the battery chemistry itself to be able to withstand lower-temperature conditions has been and will continue to be vital in enabling EVs, space missions, and other complex low-temperature applications.
In this article, we provide an overview of the low-temperature limiting mechanisms intrinsic to the lithium-ion battery chemistry, and then survey the field of next-generation battery chemistries that may potentially be better suited for performance-critical, low-temperature applications. In particular, battery chemistries with high gravimetric and volumetric energy density will be critical towards enabling low-temperature aerospace applications that place an additional priority on minimizing payload mass. Employing next-generation chemistries that deliver requisite power capabilities at a reduced mass and volume will be a large enabler in accelerating growth in this sector. Additionally, reliable low-temperature performance is paramount in such niche and performance-critical applications, and thus the development of next-generation battery chemistries should focus on and address the most significant low-temperature hurdles. We provide our perspective on the low-temperature potential of various advanced chemistries, including lithium-metal, lithium-sulfur, and dual-ion batteries, with the hopes of identifying the potential key towards enabling reliable energy storage in challenging, low-temperature conditions.
2. Low-temperature Behavior of Lithium-ion Batteries
The lithium-ion battery has intrinsic kinetic limitations to performance at low temperatures within the interface and bulk of the anode, cathode, and electrolyte. Traditionally, lithium-ion cells tend to exhibit massive overpotential at low-temperatures during charge and discharge, stunting capacity attainment and rate capability, as shown in Figure 2a. Furthermore, increased overpotential during charging can lead to the occurrence of lithium-plating on the graphite electrode, leading to accelerated instability and the likelihood of cell failure and safety hazards.[11,12] While it is commonly accepted that the poor low-temperature performance is due to impeded kinetics, it was not actually clear for some time what the exact rate-limiting step during discharge was. Initial efforts focused on targeted use of favorable co-solvents in the electrolyte to (i) lower the overall freezing point of the electrolyte and displace the use of high-freezing point ethylene carbonate (EC) and (ii) form a more favorable solid-electrolyte interphase (SEI) on the graphite electrode that could boost charge-transfer kinetics at the electrode-electrolyte interface.[13–18] On the other hand, some early work has shown that in fact the electrolyte and the SEI were not the key limiting sub-units, and rather diffusion limitations within graphite were primarily impeding the performance.[19,20] As a whole, there was a clear lack of consensus on what the exact limiting mechanisms were, and this led to an inability to implement precise mechanism-based improvements to the chemistry.
Figure 2.
(a) Low-temperature variation in the capacity of a graphite || NCA cell with an electrolyte consisting of 1.0 M LiPF6 in EC:PC:EMC (5:2:3 by weight) with 0.05 M CsPF6. (b) Low-temperature variation in the capacity with an optimized electrolyte consisting of 1.0 M LiPF6 in EC:PC:EMC (1:1:8 by weight) with 0.05 M CsPF6. Reproduced with permission.[18] Copyright 2017, American Chemical Society. (c) A listing of every step in the Li+ charge-transfer process during discharge of a lithium-ion cell. Reproduced with permission.[31] Copyright 2017, American Chemical Society.
Nonetheless, iterative engineering efforts both on the electrolyte and electrodes have remained quite an effective force in optimizing the chemistry for low-temperature performance. Electrolyte engineering, an example of which can be seen in Figure 2b, focuses on iteratively varying the ratios of solvents, salts, and additives within a liquid formulation to promote better charge-transfer kinetics. Meanwhile, electrode engineering involves deliberately modifying the cathode and anode active materials in an effort to make their lithiation and delithiation behaviors at low temperatures more kinetically amenable. Within the layered-oxide cathode material, it has been shown that the cathode-electrolyte interphase (CEI) thickness and stability is a critical determining factor for low-temperature charge transfer, with increasing Ni content lending to a heightened resistance at this interface.[21] To this end, artificially grown and modified cathode coatings and interfaces have been shown to improve lithium and delithiation kinetics by allowing for a more stable CEI and better insertion kinetics.[22,23] On the other end, graphite has also been shown to exhibit critical performance variation with SEI stability. Past work has shown how graphite can be modified for improved low-temperature performance through artificially implemented interphases and passivation coatings.[24–26] While briefly touched upon here, a more detailed chronology of specific low-temperature engineering developments for the graphite anode and layered-oxide cathode can be found in past reviews focused on traditional lithium-ion battery materials at low temperatures.[5,6,12]
As demonstrated through these engineering efforts, the key performance-inhibiting behavior of lithium-ion batteries at low-temperature conditions is fundamentally tied to the electrolyte-electrode interface, rather than the bulk electrolyte or electrodes themselves. This was further diagnosed in 2003, when Zhang and coworkers showed that charge-transfer at the graphite-electrolyte interface sharply increased as a function of decreasing temperature.[27] The authors demonstrated that Rct was substantially higher when the graphite electrode was fully delithiated, presenting an overwhelming kinetic barrier during the charging process when lithium-ions are inserted into the anode. This charge-transfer resistance can be deconvoluted into two separate contributions: (i) desolvation of individual lithium-ions, where the positive ion sheds its solvation sheath prior to (ii) subsequent migration of the bare ion through the resistive SEI. However, low temperatures impede performance not just during the charging process, but also during the discharging process. Through detailed studies employing electrochemical impedance spectroscopy, Xu and coworkers followed up this work by showing that ion-desolvation makes up the strongest contributor to charge-transfer resistance prior to insertion into the anode or cathode.[28] The solvation sheath of Li+ ions in traditional carbonate electrolytes is primarily composed of EC molecules, and the stripping of these surrounding species is found to constitute an energetic barrier on the order of 50 kJ mol-1.[29,30] This work was followed up by a 2017 study that confirmed that ion-desolvation was specifically the rate-limiting step at low-temperature conditions.[31] A listing of every charge-transfer step during discharge evaluated in this study is shown in Figure 2c.
The lithium-ion battery’s potential as a low-temperature energy storage solution is thus predicated on the ability of the electrolyte to enable a facile desolvation of Li+ ions at the electrode-electrolyte interface, on both charge and discharge. This is an important note, as it suggests that low-temperature design of battery systems is far more complex than just ensuring a low-freezing point electrolyte with adequate ionic conductivity or optimizing electrode-level kinetics.[21,24–26,32–34] While all of these factors are still bare minimum considerations, the primary lever during low-temperature charge-transfer is the coordination environment surrounding the Li+ ion in solution. This dictates the rate-limiting step during operation, and it is this mechanistic framing that will be essential for enabling superior low-temperature performance.[35] However, there are clear limits to the performance that can be achieved in lithium-ion batteries, primarily due to the compatibility of solvents with the graphite electrode. The use of graphite is accompanied by the strict requirement of maintaining a certain percentage of EC in the electrolyte; the use of EC to some degree is generally necessary with graphite anodes in order to form a favorable SEI that subsequently ensures sustained stability and compatibility with other solvents.[36] However, EC, beyond just having a high-freezing point, preferentially coordinates and makes up the tight solvation shell surrounding Li+ in solution, presenting an intrinsically high desolvation barrier.
Nonetheless, there have been many recent advancements in developing electrolyte formulations with little to no EC content, otherwise known as ‘EC-free’ electrolytes.[37–39] Such formulations can be especially beneficial for stabilizing high-voltage cathodes operating up to 4.4 V.[40] While this approach may certainly be promising for developing low-temperature capable lithium-ion batteries, other alternative carbonate formulations can still exhibit strong desolvation barriers. In fact, Dahn and coworkers showed that a favorable EC-free formulation (1 M LiPF6 in 98:2 ethyl methyl carbonate:vinylene carbonate), while sufficiently passivating graphite, still exhibited lithium-plating at low-temperatures.[39] Moreover, the absence of EC exacerbated reductive decomposition of electrolyte in contact with the plated lithium-metal, triggering significantly more gas evolution and ultimately worse performance at 10 °C. The current EC-based lithium-ion electrolyte is highly optimized for the majority of relevant conditions lithium-ion batteries face, and although there could certainly be a path forward with EC-free formulations, other factors beyond low-temperature performance (such as gas-evolution) must not be sacrificed. It may be both preferable and synergistically beneficial to approach the problem from another angle. Specifically, consideration of altogether new battery chemistries may unveil previously unseen paths for tackling the traditional rate-limiting desolvation barriers present at low temperatures.
Lithium-ion batteries have enabled tremendous progress during their incumbency as the battery of choice for the last few decades. As we exponentially advance the use-cases where low-temperature-resilient energy storage solutions are needed, however, it will be valuable to consider alternative battery chemistries with heightened solvent compatibility, alternative ion-transport strategies, and altogether new low-temperature mechanisms. Nevertheless, these next-generation chemistries can present additional challenges on par with the desolvation limitations seen in lithium-ion batteries, though this additional complexity may be surmountable with dedicated study and optimization. As we embark on the next phase of battery research and development for performance-critical applications, it will be vital to reimagine and build new low-temperature capable chemistries.
3. Low-temperature Lithium-metal Batteries
A significant majority of the recent advances in electrolyte chemistry have been designed not for the graphite electrode, but for the lithium-metal anode. Lithium-metal, possessing one of the highest theoretical capacities (3,860 mA h g−1, 2,061 mA h cm−3) and the lowest reduction potential (−3.04 V vs SHE) of all battery anode materials, has received considerable attention in recent years. Lithium-metal, unlike graphite, does not have the strict requirement of maintaining a certain percentage of EC in the electrolyte, opening the possibility of employing solvents with better desolvation kinetics. While this stringent electrolyte requirement is circumvented, lithium-metal generally has far greater problems in terms of solvent stability, including the continuous growth of high surface area dendritic deposits and unceasing consumption and decomposition of liquid electrolyte.[41–43] These drawbacks ultimately lead to poor stability and cycle life in full-cells employing lithium-metal anodes. However, the last decade has consisted of a plethora of advances towards overcoming the intrinsic drawbacks of lithium-metal anodes. Given the critical need to redesign and build from the ground up new solvents with greater low-temperature capability and desolvation kinetics, pairing with alternative anodes like lithium-metal may be an excellent strategy for enabling electrochemical energy storage fine-tuned for low-temperature, high performance, and energy-dense applications.
The electrochemical performance and chemical dynamics of the lithium-metal anode vary considerably as a function of temperature. A critical aspect of lithium-metal use is the growth and subsequent stability of the SEI formed in contact with the liquid electrolyte. This evolution is the end-result of a multi-step reduction process, the kinetics of which can be substantially altered at low-temperature conditions. As outlined in a recent study performed by McDowell and coworkers, one primary difference at low temperatures is that lithium-metal SEIs contain a much higher proportion of inorganic LiF components, with significantly diminished presence of organic polymeric decomposition products.[44] Furthermore, the formed layer is found to be much thinner and less resistive. Finally, the morphology of lithium-metal deposits is found to be much more nanoscale with substantially increased surface area, as shown Figure 3. The low particle size deposits are eluded to cause a heightened propensity of “dead lithium” formation that is electronically disconnected from the anode-bulk by resistive SEI. This leads to poor, unstable coulombic efficiencies as low as 25% when stripping and plating lithium-metal at low temperatures.
Figure 3.
Morphology of lithium-metal deposited onto a Cu substrate in DOL:DME electrolyte at 0.2 mA cm−2, at temperatures of (a) +20 °C, (b) −20 °C, (c) −40 °C, and (d) −60 °C. (e) Variations in the deposited lithium-metal particle size with temperature. (f) Galvanostatic electrodeposition profile at 0.2 mA cm−2 varying with temperature, with the inset showing a closer look at the nucleation onset. Reproduced with permission.[44] Copyright 2019, American Chemical Society.
A separate study by Cui and coworkers evaluated lithium-metal cyclability at both high and low temperatures and demonstrated similar conclusions. It was shown that the coulombic efficiency of lithium stripping and plating on a copper current collector fell to ~ 30% at −20 °C, and the morphology of deposited lithium became highly nanosized with decreasing temperature.[45] It is important to note that the electrolytes employed in both of the previously mentioned investigations consisted of 1 M lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and small percentage of LiNO3 in dioxolane (DOL) and dimethoxyethane (DME) (1:1, v/v), a formulation that has been widely shown to exhibit relatively high stability in contact with lithium-metal. The instabilities shown here would likely be exacerbated with less favorable electrolytes.
Thus, the implementation of lithium-metal anodes in low-temperature systems is an attractive prospect due to the possibility of overcoming desolvation kinetics, but there will be a variety of additional factors to consider as well. Just as in lithium-ion batteries, ensuring an ionically conductive liquid phase at low-temperatures is required. Additionally, any implementation of a lithium-metal containing battery will also need to ensure favorable growth, stabilization, and morphology of the lithium-metal anode over the course of cycling. With that framing in mind, many of the recent improvements in this area have targeted at least one of these critical areas, either improving the anode surface stability over time, optimizing the kinetics of desolvation, ensuring low-temperature phase stability of the electrolyte, or some combination of the three.
The morphology and growth of the lithium-metal surface is highly dependent on both the chemical compatibility and dielectric properties of the solvent within which it is electrochemically cycled. In general, carbonate-based electrolytes have shown to perform less than ideal at stabilizing lithium-metal, particularly compared to other functional classes of organic solvents, such as ethers like DOL and DME.[43] However, Shangguan et al. demonstrated a Li || LiNi0.5Mn0.3Co0.2O2 (NMC) cell with a low-temperature capable electrolyte framework built upon the commonly employed carbonate solvents.[46] Their solvent formulation consisted of 20% EC along with lower melting point carbonates, including 20% of propylene carbonate (PC, Tm = −48.8 °C) and 60% ethyl methyl carbonate (EMC, Tm = −53 °C). The key distinguisher of their formulation, however, was the implementation of a novel lithium trifluoro(perfluoro-tert-butyloxyl)borate (LiTFPFB) salt used in conjunction with LiTFSI and a 0.05 M LiPO2F2 additive. These additions allow for stable cycling at −20 °C, where 93.7% of the initial capacity, or about 101 mA h g−1, is retained after 50 cycles. Cycling is also demonstrated at −40 °C with a capacity attainment of ~ 65 mA h g-1. The key to this impressive behavior is attributed to LiPO2F2 forming a dense, low impedance, protective SEI that prevents high surface area dendritic growth.[46] Thus, engineering a more favorable SEI could potentially suppress the high surface area growth and dead-lithium formation characteristic to lithium-metal at sub-zero temperatures.[44]
McDowell and coworkers recently demonstrated the degree to which cycling in ether versus cyclic carbonate electrolytes affects the subsequent morphology of lithium-metal deposition at low temperatures.[47] Interestingly, they demonstrated that lithium metal deposits cycled in a mixed ether-carbonate formulation tend to be larger on average than those cycled in a pure ether solvent, and subsequently demonstrated better cycling performance at low-temperature conditions. The addition of cyclic carbonates is shown to promote the growth of LiF and Li2CO3 crystallites in the SEI, whereas on LiF is present in the corresponding SEI cycled in pure ether containing electrolyte; this distinction in SEI character is attributed as the cause of better performance in cells cycled with the mix electrolyte. Similar conclusions were also recently reported in an investigation by Wang’s group, where the SEI on lithium-metal was artificially engineered to enable cycling at low-temperatures of −15 °C.[48] They specifically employ an electrochemically active monolayer (EAM) composed of 1,3-benzenedisulfonyl fluoride on the copper current collector, which allows for the formation of a distinct SEI composed of a LiF-rich inner phase and an amorphous outer phase. This SEI varies from the highly crystalline SEI normally formed on lithium-metal at low temperatures (as also shown in the work by McDowell and coworkers),[47] allowing for much larger deposits of lithium-metal and greater low-temperature cycling stability.
In recent years, there has also been push towards developing high-concentration electrolytes, where super-concentrated amounts of supporting salt are dissolved in the electrolyte to boost electrochemical stability at both electrodes and ensure stable and constant flux of lithium-ions during deposition.[49–51] This is turn has been shown to boost coulombic efficiency and drastically improve lithium-metal morphology and life over the course of cycling. The vast presence of supporting salt at such high concentrations can also lead to a drastic freezing-point reduction in aqueous-based solvents, enabling low-temperature performance well below the standard freezing point of 0 °C.[52] However, the large salt usage in super concentrated electrolytes can deprecate the overall energy density and heighten the cost of the battery system. Nonetheless, recent works have shown how these electrolytes can be diluted with a non-coordinating fluorinated solvent to maintain the favorable aggregated ionic coordination state, all while improving viscosity and transport properties of the electrolyte and surface stability of the anode.[51,53–55] Dilution also serves to reduce the bulk salt concentration down to an acceptably low value, minimizing mass and reducing overall cost while maintaining a highly aggregated coordination state. In fact, the appeal of fluorinated electrolytes has driven several investigations into their utility for low-temperature performance. For instance, Holoubek et al. employed a 1 M LiPF6 in a methyl 3,3,3-trifluoropropionate (MTFP) : fluoroethylene carbonate (FEC) (9 : 1) electrolyte.[56] The MTFP acts as a fluorinated analogue of methyl propionate, which along with other esters has been utilized as a low-temperature co-solvent in much of the lithium-ion low-temperature literature.[57,58] Impressively, the fluorinated ester formulation maintains a high ionic conductivity of 0.75 mS cm−1 at −60 °C compared to 0.005 mS cm−1 for 1 M LiPF6 in EC/DEC, meeting the requirement for low-temperature phase stability. Furthermore, Li || NMC 811 cells with this formulation are able to stably discharge at −40, −50, and −60 °C, attaining, respectively, 80%, 75%, and 70% of the room-temperature capacity. Moreover, the Li+ and PF6− coordination state in the fluorinated electrolyte is found to exhibit rather high coordination, existing in contact-ion pair states approaching that of the aggregated state generally found in concentrated electrolytes.
Additionally, Ren et al. demonstrated a locally concentrated tetramethylene sulfone (TMS) electrolyte consisting of a 1:3:3 molar ratio of LiTFSI, TMS, and 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether (TTE) as a diluent.[59] TMS is actually not an ideal solvent to employ in dedicated low-temperature resilient batteries given its high freezing point (Tm = 27 °C), but nonetheless, the addition of TTE diluent allows a Li || NMC cell to cycle at −10 °C. This demonstrates the highly useful effects fluorinated diluents can have simultaneously on lithium-metal stability and melting point reduction.
Further insight into fluorinated co-solvents for low-temperature lithium-metal batteries was elucidated by Fan et al. in their 2019 study.[60] By using a mix of all-fluorinated electrolytes and non-polar fluorinated diluents, they precisely controlled the affinity between the solvated lithium-ion and its explicit solvation shell, demonstrating that desolvation is still among the most critical factors for low-temperature lithium-metal batteries. Through an array of experimental and computational studies, the authors quantitatively deduced that adding fluorinated non-polar solvents can actively minimize the desolvation energy of lithium-ions prior to plating on the lithium-metal surface. As shown in Figure 4a, they find that desolvation energy can be minimized to a remarkable extent using optimized blends of fluorinated solvents and diluents. This is in addition to the multitude of other benefits that fluorinated diluents can bring to lithium-metal deposition, electrochemical stability, melting point, non-flammability, and viscosity of the overall electrolyte. Through the use of a formulation consisting of 1.28 M lithium bis(fluorosulfonyl)imide (LiFSI) in FEC/ methyl (2,2,2-tri-fluoroethyl) carbonate (FEMC) diluted with nonpolar (tetrafluoro- 1-(2,2,2-trifluoroethoxy)ethane (D2), they show high ionic conductivities down to −125 °C. Additionally, they present ultra-stable cycling of a Li || LiNi0.8Co0.15Al0.05O2 (NCA) cell with 99.9% coulombic efficiency at incredibly low temperatures of −85 °C. The significantly optimized performance of this electrolyte in comparison to unoptimized EC and dimethyl carbonate (DMC) electrolyte is shown in Figure 4b-d. The low-temperature performance shown in this work is among the most impressive displayed in the lithium-metal battery field thus far and demonstrates the great potential of alternative chemistries in circumventing traditional desolvation barriers. Similar approaches utilizing nonpolar alkane solvents have also been investigated, demonstrating analogous improvements to the desolvation energy of Li+ cations.[61] Importantly, the use of meticulously designed solvents in conjunction with rational design of the ionic coordination state of lithium-ions in solution seems to be a highly promising and necessary approach moving forward.
Figure 4.
(a) Calculated solvation/desolvation energies of Li+ in various formulations of EC/DMC, FEC/FEMC, and FEC/DEC electrolytes, where DMCcc and DMCccb refer to two different cis–cis conformers of DMC. (b) The discharge at various temperatures of Li || NCA cells with 1 M LiPF6 in EC:DMC electrolyte. (c) The discharge profile at various temperatures of Li || NCA cells with optimized 1.28 M LiFSI in FEC:FEMC:D2 electrolyte. (d) Variable-temperature discharge of Li || NCA cells with each electrolyte. Reproduced with permission.[60] Copyright 2019, Nature Publishing Group.
The strategy of varying the effects of polarity and ion-coordination within electrolyte solvents has in recent work been taken to new heights by employing gaseous electrolytes.[62,63] Electrolytes are predominantly thought of as liquid or solid, allowing ionic-diffusion to occur through coordinating interaction with the surrounding dielectric medium. Gases at room temperature are generally considered to have poor intermolecular interactions between neighboring molecules, leading to their lack of a consolidated phase. However, by utilizing pressurized hermetically sealed cell-setups, Meng and co-workers demonstrated the use of liquified hydrofluorocarbons as ultra-low viscosity and ultra-low temperature-capable electrolyte solvents.[62] They found that monofluorinated solvents like fluoromethane (FM) show the ability to solvate lithium salts, and demonstrate evidence for the formation of a highly fluorinated SEI that stabilizes lithium-metal deposition. In Li || LiCoO2 cells, they show 43.5% capacity retention at −60 °C at a rate of C/10. Interestingly, even though the FM solvent shows an incredibly low Tm = −142 °C and a high ionic conductivity down to −60 °C, it is clear that the charge-transfer resistance still leads the lithium-plating process to incur a large overpotential at −60 °C, preventing realizable capacity attainments at rates of C/5 or higher. This reinforces that the considerations for low-temperature battery design extend far beyond the ionic conductivity of the electrolyte at low-temperatures, and the exact ionic coordination environment of the solvated lithium-ion often plays the most critical determining role. This study established the field of gaseous electrolytes for rechargeable batteries, presenting a framework upon which further improvements could certainly be made.
The same group followed up this work in 2019 with an enhanced FM liquified gas electrolyte tailored with additive amounts of tetrahydrofuran.[63] This additive selectively coordinates with lithium cations to boost solvation and transport, markedly boosting coulombic efficiencies of lithium-metal deposition up to 99.6% from the values of ~ 97% seen in their earlier work. In the initial work, FM could at a maximum dissolve 0.1 M LiTFSI, but with the addition of THF in a 1:1 salt to additive ratio, the LiTFSI fully coordinates with THF. More so, a highly coordinated and aggregated state of lithium-salt coordination results given the small additive amount of THF, similar to the coordination-state resulting from highly concentrated electrolytes. Lithium-metal deposition at −60 °C is shown with a high coulombic efficiency of 98.4% and dendrite-free morphology. This work was further followed up by a 2020 investigation utilizing an analogous approach with FM liquefied gas electrolyte and acetonitrile cosolvent.[64] As in the prior work, the cosolvent selectively coordinates with the lithium cation to boost ion transport and desolvation behavior. This enables lithium-metal deposition with coulombic efficiency of 99.4%, and more so, this is shown at high rates of 3 mA cm-2. The novelty of this approach coupled with the large degree of freedom for further optimization presents substantial promise for future work.
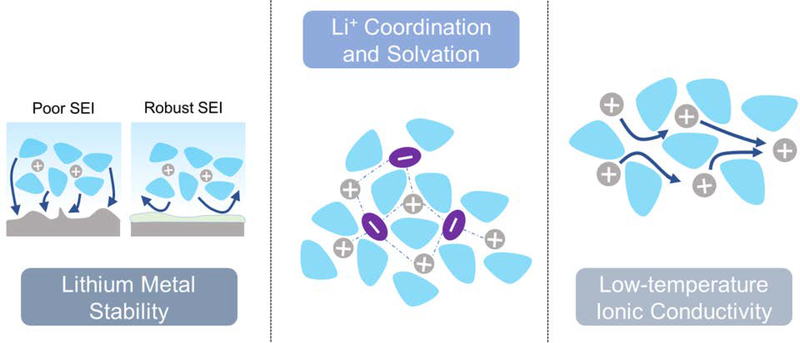
Lithium-metal represents a highly promising platform upon which to further develop low-temperature batteries with novel favorable electrolytes and rationally designed lithium protection interfaces. As seen in the work reviewed here, the performance of lithium-metal anodes at low temperature is highly associated with having a robust and stabilized SEI, adequate low-temperature conductivity, and intriguingly, sufficient coordination behavior of Li+ ions in solution. This emphasis on ion-coordination behavior in almost every lithium-metal investigation reviewed here points to what may be a universal design parameter necessary for enabling low-temperature performance. The strong dependence of charge-transfer kinetics on desolvation behavior is highly dependent on the degree of coordination and strength of interaction between the lithium cation and its solvation shell. Therefore, whether the chosen strategy introduces competing electrostatic interactions from a higher-degree of coordination with contiguous salt ions (as in the case of super-concentrated electrolytes) or just achieving weaker solvation behavior (from nonpolar fluorinated solvents), understanding and rationally engineering the solvation shell is key to unlocking the desirable kinetics necessitated at low temperatures. These essential electrolyte design aspects for lithium-metal are summarized in Figure 5.
Figure 5.
A visual outline of the three broad areas of focus with which electrolytes must be optimized and developed in order to enable lithium-metal anodes under rigorous low-temperature conditions.
4. Low-temperature Lithium-sulfur Batteries
As discussed, the low-temperature constraints within space and aerospace applications can be quite demanding, and lithium-sulfur (Li-S) batteries may in fact be quite suited for such use-cases. Alternative high-capacity cathode systems such as sulfur (1,672 mA h g−1) will be a necessity for applications with ever-increasing specific energy requirements. Additionally, the predominantly employed electrolyte solvents in Li-S batteries, DOL and DME, possess very low freezing points of −95 and −58 °C, respectively.[36] Indeed, such low freezing-point solvents retain very high ionic conductivities of > 4 mS cm−1 even at −40 °C, meeting the minimum requirements needed for low-temperature optimization.[65] However, as outlined and discussed previously, the primary considerations for low-temperature battery design can often extend far beyond just the ionic conductivity of the electrolyte at low-temperatures, and indeed, the Li-S battery chemistry is no exception. As shown in Figure 6a, Li-S batteries exhibit significant losses in discharge capacity with decreasing temperature, just as in other commonly used battery chemistries. Here, we will briefly outline the unique problems encountered by the Li-S chemistry at low-temperature conditions and present a chronology of the literature and required development path needed towards overcoming these hurdles.
Figure 6.
(a) Variations in the discharge behavior at low temperatures in Li-S cells at 25, 0, and −20 °C, in a cell with a large E/S ratio of 25 μL mg-1. (b) Variations in the discharge behavior for a Li-S cell with a lean E/S ratio of 7.5 μL mg-1. Reproduced with permission.[65] Copyright 2020, American Chemical Society.
While the Li-S battery retains many of the problems encountered by other lithium-metal anode containing systems at low temperatures, including ion-desolvation and SEI growth, it also has an additional degree of complexity stemming from the sulfur cathode. During discharge, elemental S8 is ultimately reduced to Li2S, and during this process, soluble lithium polysulfide intermediates (Li2Sx, 2 < x ≤ 8) facilitate discharge. In fact, these soluble intermediates can allow for facile charge-transfer in solution, enabling solution-mediated pathways upon which the Li-S system relies.[66–69] This is best illustrated by a commonly employed metric within the Li-S research community, the electrolyte-to-sulfur (E/S) ratio (in μLelectrolyte mg−1sulfur). The performance of Li-S cells generally exhibits strong dependencies on the total amount of electrolyte used in the cell; cells with practically-relevant, lean-electrolyte volumes tend to underperform compared to cells with abundant electrolyte amounts in terms of electrochemical utilization and cycle life. This is attributed to the solution-mediated mechanisms in Li-S discharge, displaying a dependence on the degree to which active material can be dissolved and incorporated into the solution-based reaction process.[67,70] This is clearly shown to extend to low-temperature conditions in Figure 6b, where cells with a low E/S of 7 μL mg−1 exhibit significantly deprecated capacity attainment compared to cells with an E/S of 25 μL mg−1 at both 0 °C and −20 °C.
The physical and chemical properties of the electrolyte and cathode active material are thus coupled and interdependent, and any investigation into diagnosing or optimizing the low-temperature performance requires mechanistic insights that may be fundamentally different from those for more traditional battery chemistries. However, mirroring the pathway of development in low-temperature lithium-ion systems, early investigations into Li-S low-temperature behavior were primarily concerned with iterative electrolyte engineering efforts, rather than mechanistic and kinetic studies on the critical effects of low-temperature conditions. Mikhaylik and Akridge showed in their 2003 work that low-temperature performance down to −40 °C could be boosted by employing a DOL:DME ratio of 86:14 in the electrolyte, rather than the more conventionally used 1:1 volume ratio.[71] Ryu et al. investigated the effects of adding methyl acetate and DOL to a predominantly tetraglyme (TEGDME) containing electrolyte formulation and showed beneficial improvements to low-temperature performance compared to a pure TEGDME electrolyte.[72] While both of these works approached electrolyte engineering from the lens of similar early approaches for other battery chemistries, further consideration is needed to understand the exact role the soluble active material plays in mechanistically impeding low-temperature performance. Recently, our group decided to take the mantle in investigating low-temperature Li-S battery behavior by considering the unique solution-phase mechanisms intrinsic to the system.
In our past work, we unveiled that intermediate polysulfides species like Li2S4 tend to cluster and aggregate with other polysulfide units in solution at low-temperatures, leading to an array of negative effects on attainable capacity and electrochemical kinetics.[65] By employing hybrid density functional theory calculations coupled with experimental evidence from 7Li-Nuclear Magnetic Resonance (NMR) Spectroscopy, we observed clear shifts to clustered aggregates at low temperatures, which from a chemical, conformational, and transport-based perspective impede kinetics and attainable capacity. Furthermore, we see that this kinetic hurdle is particularly detrimental during discharge within the second lower voltage plateau (as seen in Figure 6), given the tendency of Li2S4 specifically to aggregate (inhibiting conversion to Li2S). The parallels between this phenomenon and what was previously discussed regarding lithium-metal low-temperature behavior are striking; ion-coordination behavior seems to be a primary lever for modifying behavior in both cases, though aggregation of salt seems quite beneficial while aggregation of active material is critically detrimental. In this work, beyond elucidating the mechanism of polysulfide clustering, we introduced a strongly binding lithium trifluoroacetate (LiTFA) salt into the liquid electrolyte in order to improve low-temperature performance. This was implemented with the aim of introducing highly binding competing electrostatic interactions in the electrolyte solution to preferentially coordinate with Li+ cations, disrupting clustered polysulfide networks. This optimized electrolyte showed 167% improvement in capacity over analogous electrolyte with LiTFSI salt at −20 °C with a clear improvement to second plateau kinetics. This strategy of influencing and taking advantage of countercyclical ion-coordination between the lithium salt and lithium polysulfide is illustrated in Figure 7.
Figure 7.
(a) Strong Li+−Sx2– interactions can be electrostatically disrupted from competing interactions between Li+ and lithium salt anions. (b) Lithium polysulfides clusters tend to naturally form at low temperatures; this behavior can be disrupted from the influence of competing lithium salt. Reproduced with permission.[65] Copyright 2020, American Chemical Society.
Further mechanistic insights into Li-S low-temperature behavior were unveiled by Abruña and co-workers in their 2018 study.[73] This investigation employed operando x-ray-based methods to directly visualize the sulfur cathode evolution with high detail during charge, discharge, at different rates, and at different temperatures. Through the use of operando x-ray diffraction techniques at varying discharge temperatures as low as 5 °C, they showed that Li2S particle size tended to be much more nanoscale across the cathode as temperature is decreased or rate is increased. As shown in other reports, greater propensity of distributed nanoscale nuclei is more reminiscent of 2D “film-like” growth, while larger clustered nuclei (what is present at higher temperatures) is indicative of more desirable 3D growth.[69,74] These growth mechanisms dictate whether the cathode is prematurely passivated (as is often the case in 2D growth), curtailing subsequent electron transfer and adequate utilization.[75] Thus, the insight that Li2S nucleation and growth mode becomes highly nanoscale and two-dimensional at low temperatures is a highly interesting consideration, presenting another mechanism unique to the conversion-based sulfur reduction chemistry.
An avenue commonly explored in the Li-S literature, even at low-temperatures, is incorporating small quantities of electrocatalytically active materials or doped sites in the sulfur cathode-host.[76] These modifications can provide an array of benefits for discharge behavior and longevity of the sulfur cathode, including improved electronic and ionic conductivity, heightened kinetics, and the ability to adsorb lithium polysulfides to prevent detrimental polysulfide shuttling.[76–78] Furthermore, the presence of electrocatalysts can dynamically influence the nucleation and growth of Li2S, feasibly presenting a route towards addressing the detrimental growth mechanisms shown by Abruña and co-workers.[73] In 2016, Zhu et al. demonstrated a nitrogen-enriched carbon host, which allowed the sulfur active material to outperform an unmodified carbon host at low temperatures.[79] Indeed, after 100 cycles at −20 °C, the nitrogen-enriched cathode demonstrated a discharge capacity of 368 mA h g−1, compared to just 115 mA h g−1 for the unmodified cathode. In 2018, two studies were published that evaluated the low-temperature impacts of electrocatalytic host materials at low-temperatures, rather than modifications to the carbon framework itself. Deng et al. reported a graphene-supported boron nitride (BN) nanosheet, which enhanced polysulfide adsorption, leading to high electrochemical utilizations in a wide temperature range.[80] This led to an impressive capacity of 800 mA h g−1 at −20 °C, and the cell maintained a capacity of > 700 mA h g−1 for 100 cycles. Fan et al. investigated the effects of MoSe2 nanosheets on the discharge behavior of the sulfur cathode, particularly the interaction between the metal selenide edge sites and lithium polysulfides.[81] They demonstrate cycling at both 0 °C and −25 °C with high capacities of 1100 mA h g−1 and 870 mA h g−1, respectively, after 100 cycles. Recently, Wang et al. reported a MnS-carbon nanofiber composite interlayer to inhibit polysulfide shuttling and boost reaction kinetics at both high temperatures of 55 °C and low temperatures of 0 °C.[82] Their modification enables a high capacity of 850 mA h g−1 after 100 cycles at 0 °C, compared to only 500 mA h g−1 for an unoptimized cathode. In every case, the specific solid host or additive positively contributes to performance, though often, it is not clear whether the improvement is due to the chemisorption ability of the additive or the electrocatalytic strength of the additive. While these two phenomena are certainly associated, the former explicitly reduces the overall concentration of polysulfides in solution while the latter directly reduces the energetic barrier to conversion.[83] Future work in this area is needed to deconvolute which property is more critical for low-temperature performance, particularly considering the clustering and 2D growth mechanisms discussed here. One important aspect to note is that the studies summarized here did not universally operate cells under rigorous lean-electrolyte conditions. Thus, it is still to be determined how many of these electrocatalytic improvements would translate to low-temperature conditions under practically-relevant cycling parameters.[78]
In summary, there is great promise and potential in optimizing and enabling the Li-S battery for performance-critical low-temperature conditions. The polysulfide-rich chemistry, and particularly the unique mechanisms of polysulfide clustering and nanoscale growth of Li2S at low temperatures, present a key body of knowledge to further investigate and improve upon as we continue to develop this system. Indeed, it will be interesting to investigate if these two phenomena are actively related through diffusion-controlled models for nucleation and growth behavior.[84,85] Individually, both polysulfide clustering and favorable growth of Li2S have been shown to be influenced through the use of strongly-coordinating lithium salts, and thus there is great potential in further developing the theory intrinsic to these two phenomena.[65,74,86] It is intriguing that deliberately engineered ion-coordination behavior in-solution seems to be a major driver of beneficial low-temperature behavior, not just in the Li-S system, but also in lithium-metal batteries. Thus, there is great potential in exploring future rationally designed electrolytes, including those with highly fluorinated solvents that were shown to be beneficial in reducing desolvation barriers.[87–89] Coupling a highly engineered and rationally designed electrolyte (under low E/S ratios) with novel electrocatalytic materials will likely be a necessary key towards unlocking the promise of Li-S battery performance at low-temperatures.
5. Overcoming Desolvation through Anion-insertion
As enumerated throughout this article, often the most critical barrier for low-temperature performance, regardless of the battery chemistry of interest, is the large charge-transfer barrier stemming from lithium-ion desolvation. Lithium-ion and lithium-metal batteries generally rely on a “rocking-chair” mechanism, where the Li+ ions commute from the anode to the cathode upon discharge, and vice-versa upon charge. This back and forth transference requires desolvation and solvation to occur simultaneously at opposite electrodes during both charge and discharge. However, one clever approach to circumvent this kinetic barrier on discharge is by using dual-ion batteries. During charge, dual-ion systems rely upon simultaneous cation and anion desolvation and insertion into, respectively, the anode and cathode. During discharge, however, the anion and cation are de-inserted and solvated by the electrolyte.[90] This operation directs desolvation to only occur on the charge step and solvation to only occur during the discharge step at both electrodes, as shown in Figure 8a and b.[91] By eliminating the desolvation step from the discharge ion-transfer process, this strategy reimagines the conventionally envisioned hurdles experienced during Li+ migration, mechanistically avoiding the most critical pitfall to low-temperature performance. The ability of the cathode to reversibly insert and de-insert salt anions is this strategy’s key advantage, and indeed, has garnered significant attention for low-temperature applications in recent work.
Figure 8.
Diagrams illustrating the operating mechanism in (a) graphite || LiCoO2 (LCO) and (b) graphite || graphite cells. Variations in the discharge behavior as a function of temperature in (c) graphite || LCO and (d) graphite || graphite cells with 2 m LiPF6 in methyl propionate and 10 % FEC electrolyte. Reproduced with permission.[91] Copyright 2019, Wiley-VCH.
One class of systems capable of exhibiting dual-ion insertion mechanisms are those with dual-graphite electrodes. The graphite cathode is among the most commonly employed cathodes in dual-ion systems due to its ability to reversibly accommodate large anions within its interstitial sites.[90] Such a system would be highly attractive for overcoming traditional desolvation barriers, and indeed, Holoubek et al. demonstrated a dual-graphite system paired with a novel low-temperature-resilient electrolyte in late 2019.[91] Their electrolyte, consisting of 2 M LiPF6 in methyl propionate with 10% FEC, retains high concentration of their supporting salt, maintains suitable reductive stability from the FEC, and possesses a high ionic conductivity of 1.50 mS cm−1 at −60 °C. At a 1C discharge rate, the battery retains 93% and 84% of its room temperature capacity, respectively, at −40 °C and −60 °C. This is in stark contrast to a traditional graphite || LCO cell; a comparison between these two systems are shown in Figure 8c and d. This low-temperature performance is highly impressive, especially considering the dual-graphite system retained 62% of its room-temperature capacity at −60 °C even at a rate of 10C.
Another system capable of employing dual-ion insertion mechanisms are those with organic electrodes, which can undergo reversible adsorption of anions and enolization of cations at opposite electrodes upon charge. Xia and coworkers applied this concept to low-temperature conditions in their 2018 study, demonstrating a dual-ion organic battery in a concentrated ethyl acetate (EA) based electrolyte with the ability to operate at −70 °C.[92] In this respect, their strategy relies on two key advances. Firstly, the use of a high concentration 2 m (mol kg−1) LiTFSI in EA electrolyte ensures highly-coordinated ion states in a solvent with a high ionic conductivity at low temperatures and a low freezing point of −91 °C. Secondly, by employing dual-ion insertion mechanisms, they overcome the classical desolvation kinetic barrier, achieving ultra-high discharge rates at room temperature (as high as 100 C, corresponding to 10 A g−1cathode). Furthermore, they achieve 100% of the room temperature capacity (99 mA h g−1) at low temperatures of −40 °C and 70% of this capacity at −70 °C. This study demonstrates a highly promising initial approach, with the primary drawbacks and areas for improvement being the low volumetric energy density and specific energy of the organic electrodes. Moving forward, it will be interesting to see the integration of higher capacity electrode materials such as lithium-metal with higher capacity anion-inserting cathodes.[93] Such an approach could capitalize on the mechanistic advantages of dual-ion batteries, while bringing forth the high energy densities desired for next-generation batteries.
The mechanistic advantages to circumventing desolvation kinetic barriers are truly remarkable in the dual-ion systems described here, allowing for simultaneous high-rate and low-temperature performance. As this approach continues to develop, it will be necessary to demonstrate higher voltage and higher capacity cathodes capable of functioning under the dual-ion anion-insertion mechanism. This approach re-envisions the traditionally described charge-transfer mechanisms in lithium-based battery systems, and thus the novelty of the strategy merits further targeted research and exploration.
6. Conclusions and Outlook
As society progress and technology develops, battery scientists and engineers must continue to invent and develop new battery technologies capable of enabling the next generation of powered devices and applications. As we do this, it is important to keep in mind that many performance-critical applications, such as electric vehicles, space, defense, and subsea applications will require battery chemistries capable of not just tolerating, but thriving, at recurring low-temperature conditions. While the lithium-ion battery has sustained development in this area for the past few decades, this is a critical time in which to emphasize this aspect as we transition to developing the next-generation of higher energy density batteries. Moreover, beyond just retaining that focus with already promising chemistries, it will also be important to develop technologies built from the ground up to be mechanistically advantageous at low temperatures. The lithium-based chemistries covered here, including lithium-metal batteries, lithium-sulfur batteries, and dual-ion batteries all illustrate broad frameworks for thinking about the additional complexity that can be introduced in low-temperature battery design, beyond simply ionic conductivity and freezing point of the electrolyte. Lithium-metal is one of the most promising anode technologies moving forward, but its low-temperature performance must be investigated and optimized in a manner which also addresses its critical issues in stability and safety. Lithium-sulfur batteries are a natural extension of this, pairing with a high capacity cathode to have a theoretical step-change increase to specific energy. However, the intrinsic solution-based reaction paradigm introduces tremendous complexity to understanding and optimizing the performance at low temperatures, particularly the interplay between solution-coordination behavior and solid-phase growth. Dual-ion batteries, meanwhile, introduce a new framework for addressing ion-desolvation conventionally encountered during discharge. The strategy is emblematic of the sorts of approaches that will be necessary when envisioning and building a low-temperature capable battery from the ground up. However, dual-ion systems have primarily been shown using electrodes with low specific capacities, and thus their pathway to outperforming lithium-ion batteries in terms of energy density is not straightforward. Each of these battery chemistries present new paradigms and considerations for low-temperature battery design, but each also present unique hurdles towards widescale adoption. Thus, it is not currently clear which, if any, of these systems will ultimately be the most appealing for future implementation of low-temperature batteries. Each system tackles the problem from a different angle, and thus it will be interesting to see how their various intricacies will be exploited and coupled in future approaches.
Broadly speaking, addressing low-temperature performance in all systems, beyond just those listed here, will require a robust knowledge of the target chemistry and precisely designed electrolyte systems or electrode coatings that stabilize solvation behavior and charge transfer across the electrode-electrolyte interface. As has been brought up throughout the literature discussed here, lithium-ion coordination and aggregation in solution often is the most important consideration in this endeavor, since it plays a paramount role in determining the solvation energy of lithium-ions, the growth and stability of lithium-metal, and the conformational behavior of associated species in solution such as lithium polysulfides. The complexity driven from the interplay of solvated charge carriers with an array of coordinating solvents, each with their own affinities and tendencies for interaction, presents an expansive toolbox for design and optimization of low-temperature battery performance. Considering the ever-increasing need for advanced energy storage systems and the strategies outlined here, the future is ripe with countless possibilities for developing novel, low-temperature capable batteries.
Acknowledgements
This work was supported by a NASA Space Technology Research Fellowship (NSTRF) under award number 80NSSC17K0089.
Biographies

Abhay Gupta is a PhD candidate and a NASA Space Technology Research Fellow (NSTRF) studying Materials Science and Engineering at the University of Texas at Austin. He received his B.S. from the Hildebrand Department of Petroleum and Geosystems Engineering at the University of Texas at Austin in 2016. His research focuses on solution-coordination behavior in lithium-sulfur batteries, with an emphasis on low-temperature performance.

Arumugam Manthiram is the Cockrell Family Regents Chair in Engineering and the Director of Texas Materials Institute and Materials Science and Engineering Program at the University of Texas at Austin. His research interests are in the area of materials for rechargeable batteries and fuel cells, including novel synthesis approaches for nanomaterials.
Contributor Information
Abhay Gupta, Materials Science and Engineering Program & Texas Materials Institute, The University of Texas at Austin, Austin, TX 78712, USA.
Arumugam Manthiram, Materials Science and Engineering Program & Texas Materials Institute, The University of Texas at Austin, Austin, TX 78712, USA.
References
- [1].Manthiram A, Nat. Commun. 2020, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lin X, Salari M, Arava LMR, Ajayan PM, Grinstaff MW, Chem. Soc. Rev. 2016, 45, 5848. [DOI] [PubMed] [Google Scholar]
- [3].FY2015 Energy Storage R&D Annual Progress Report, 2016.
- [4].“Into the deep: electrifying subsea oil and gas operations | Saft Batteries,” can be found under https://www.saftbatteries.com/media-resources/our-stories/deep-electrifying-subsea-oil-and-gas-operations, n.d.
- [5].Zhu G, Wen K, Lv W, Zhou X, Liang Y, Yang F, Chen Z, Zou M, Li J, Zhang Y, He W, J. Power Sources 2015, 300, 29. [Google Scholar]
- [6].Hou J, Yang M, Wang D, Zhang J, Adv. Energy Mater. 2020, 10, 1904152. [Google Scholar]
- [7].Stuart TA, Hande A, J. Power Sources 2004, 129, 368. [Google Scholar]
- [8].Wang CY, Zhang G, Ge S, Xu T, Ji Y, Yang XG, Leng Y, Nature 2016, 529, 515. [DOI] [PubMed] [Google Scholar]
- [9].Wu W, Wang S, Wu W, Chen K, Hong S, Lai Y, Energy Convers. Manag. 2019, 182, 262. [Google Scholar]
- [10].Smart MC, Ratnakumar BV, Ewell RC, Surampudi S, Puglia FJ, Gitzendanner R, Electrochim. Acta 2018, 268, 27. [Google Scholar]
- [11].Zhu G, Wen K, Lv W, Zhou X, Liang Y, Yang F, Chen Z, Zou M, Li J, Zhang Y, He W, J. Power Sources 2015, 300, 29. [Google Scholar]
- [12].Rodrigues M-TF, Babu G, Gullapalli H, Kalaga K, Sayed FN, Kato K, Joyner J, Ajayan PM, Nat. Energy 2017, 2, 1. [Google Scholar]
- [13].Smart MC, Ratnakumar BV, Surampudi S, J. Electrochem. Soc. 1999, 146, 486. [Google Scholar]
- [14].Zhang SS, Xu K, Allen JL, Jow TR, J. Power Sources 2002, 110, 216. [Google Scholar]
- [15].Plichta EJ, Hendrickson M, Thompson R, Au G, Behl WK, Smart MC, Ratnakumar BV, Surampudi S, J. Power Sources 2001, 94, 160. [Google Scholar]
- [16].Smart MC, Whitacre JF, Ratnakumar BV, Amine K, J. Power Sources 2007, 168, 501. [Google Scholar]
- [17].Zhang SS, Xu K, Jow TR, Electrochem. commun. 2002, 4, 928. [Google Scholar]
- [18].Li Q, Jiao S, Luo L, Ding MS, Zheng J, Cartmell SS, Wang CM, Xu K, Zhang JG, Xu W, ACS Appl. Mater. Interfaces 2017, 9, 18826. [DOI] [PubMed] [Google Scholar]
- [19].Huang C-K, Sakamoto JS, Wolfenstine J, Surampudi S, J. Electrochem. Soc. 2000, 147, 2893. [Google Scholar]
- [20].Zhang SS, Xu K, Jow TR, Electrochim. Acta 2002, 48, 241. [Google Scholar]
- [21].Yoon S-J, Myung S-T, Sun Y-K, J. Electrochem. Soc. 2014, 161, A1514. [Google Scholar]
- [22].Park BC, Kim HB, Bang HJ, Prakash J, Sun YK, Ind. Eng. Chem. Res. 2008, 47, 3876. [Google Scholar]
- [23].Wu X-L, Guo Y-G, Su J, Xiong J-W, Zhang Y-L, Wan L-J, Adv. Energy Mater. 2013, 3, 1155. [Google Scholar]
- [24].Nobili F, Dsoke S, Mecozzi T, Marassi R, Electrochim. Acta 2005, 51, 536. [Google Scholar]
- [25].Nobili F, Mancini M, Dsoke S, Tossici R, Marassi R, J. Power Sources 2010, 195, 7090. [Google Scholar]
- [26].Gao J, Fu LJ, Zhang HP, Zhang T, Wu YP, Wu HQ, Electrochem. commun. 2006, 8, 1726. [Google Scholar]
- [27].Zhang SS, Xu K, Jow TR, Electrochim. Acta 2004, 49, 1057. [Google Scholar]
- [28].Xu K, Von Cresce A, Lee U, Langmuir 2010, 26, 11538. [DOI] [PubMed] [Google Scholar]
- [29].Xu K, Von Wald Cresce A, J. Mater. Res. 2012, 27, 2327. [Google Scholar]
- [30].Xu K, Lam Y, Zhang SS, Jow TR, Curtis TB, J. Phys. Chem. C 2007, 111, 7411. [Google Scholar]
- [31].Li Q, Lu D, Zheng J, Jiao S, Luo L, Wang CM, Xu K, Zhang JG, Xu W, ACS Appl. Mater. Interfaces 2017, 9, 42761. [DOI] [PubMed] [Google Scholar]
- [32].Smart MC, Hwang C, Krause FC, Soler J, West WC, Ratnakumar BV, Amine K, ECS Trans. 2013, 50, 355. [Google Scholar]
- [33].Li Y, Qian K, He YB, Kaneti YV, Liu D, Luo D, Li H, Li B, Kang F, J. Power Sources 2017, 342, 24. [Google Scholar]
- [34].Qin R, Wei Y, Zhai T, Li H, J. Mater. Chem. A 2018, 6, 9737. [Google Scholar]
- [35].V Amanchukwu C, Joule 2020, 4, 281. [Google Scholar]
- [36].Xu K, Chem. Rev. 2004, 104, 4303. [DOI] [PubMed] [Google Scholar]
- [37].Petibon R, Xia J, Ma L, Bauer MKG, Nelson KJ, Dahn JR, J. Electrochem. Soc. 2016, 163, A2571. [Google Scholar]
- [38].Li W, Dolocan A, Li J, Xie Q, Manthiram A, Adv. Energy Mater. 2019, 9, 1901152. [Google Scholar]
- [39].Liu QQ, Xiong DJ, Petibon R, Du CY, Dahn JR, J. Electrochem. Soc. 2016, 163, A3010. [Google Scholar]
- [40].Ma L, Glazier SL, Petibon R, Xia J, Peters JM, Liu Q, Allen J, Doig RNC, Dahn JR, J. Electrochem. Soc. 2017, 164, A5008. [Google Scholar]
- [41].Fang C, Wang X, Meng YS, Trends Chem. 2019, 1, 152. [Google Scholar]
- [42].Niu C, Lee H, Chen S, Li Q, Du J, Xu W, Zhang JG, Whittingham MS, Xiao J, Liu J, Nat. Energy 2019, 4, 551. [Google Scholar]
- [43].Liu J, Bao Z, Cui Y, Dufek EJ, Goodenough JB, Khalifah P, Li Q, Liaw BY, Liu P, Manthiram A, Meng YS, Subramanian VR, Toney MF, Viswanathan VV, Whittingham MS, Xiao J, Xu W, Yang J, Yang XQ, Zhang JG, Nat. Energy 2019, 4, 180. [Google Scholar]
- [44].Thenuwara AC, Shetty PP, McDowell MT, Nano Lett. 2019, 19, 8664. [DOI] [PubMed] [Google Scholar]
- [45].Wang J, Huang W, Pei A, Li Y, Shi F, Yu X, Cui Y, Nat. Energy 2019, 4, 664. [Google Scholar]
- [46].Shangguan X, Xu G, Cui Z, Wang Q, Du X, Chen K, Huang S, Jia G, Li F, Wang X, Lu D, Dong S, Cui G, Small 2019, 15, 1900269. [DOI] [PubMed] [Google Scholar]
- [47].Thenuwara AC, Shetty PP, Kondekar N, Sandoval SE, Cavallaro K, May R, Yang C-T, Marbella LE, Qi Y, McDowell MT, ACS Energy Lett. 2020, 17, 41. [Google Scholar]
- [48].Gao Y, Rojas T, Wang K, Liu S, Wang D, Chen T, Wang H, Ngo AT, Wang D, Nat. Energy 2020, 5, 534. [Google Scholar]
- [49].Qian J, Adams BD, Zheng J, Xu W, Henderson WA, Wang J, Bowden ME, Xu S, Hu J, Zhang J-G, Adv. Funct. Mater. 2016, 26, 7094. [Google Scholar]
- [50].Nanda S, Gupta A, Manthiram A, Adv. Energy Mater. 2020, 2000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Borodin O, Self J, Persson KA, Wang C, Xu K, Joule 2020, 4, 69. [Google Scholar]
- [52].Chen L, Zhang J, Li Q, Vatamanu J, Ji X, Pollard TP, Cui C, Hou S, Chen J, Yang C, Ma L, Ding MS, Garaga M, Greenbaum S, Lee HS, Borodin O, Xu K, Wang C, ACS Energy Lett. 2020, 5, 968. [Google Scholar]
- [53].Yu L, Chen S, Lee H, Zhang L, Engelhard MH, Li Q, Jiao S, Liu J, Xu W, Zhang J-G, ACS Energy Lett. 2018, 3, 2059. [Google Scholar]
- [54].Cao X, Ren X, Zou L, Engelhard MH, Huang W, Wang H, Matthews BE, Lee H, Niu C, Arey BW, Cui Y, Wang C, Xiao J, Liu J, Xu W, Zhang JG, Nat. Energy 2019, 4, 796. [Google Scholar]
- [55].Zheng Y, Soto FA, Ponce V, Seminario JM, Cao X, Zhang JG, Balbuena PB, J. Mater. Chem. A 2019, 7, 25047. [Google Scholar]
- [56].Holoubek J, Yu M, Yu S, Li M, Wu Z, Xia D, Bhaladhare P, Gonzalez MS, Pascal TA, Liu P, Chen Z, ACS Energy Lett. 2020, 1438. [Google Scholar]
- [57].Smart MC, Ratnakumar BV, Behar A, Whitcanack LD, Yu JS, Alamgir M, J. Power Sources 2007, 165, 535. [Google Scholar]
- [58].Smart MC, Lucht BL, Dalavi S, Krause FC, V Ratnakumar B, J. Electrochem. Soc. 2012, 159, 739. [Google Scholar]
- [59].Ren X, Chen S, Lee H, Mei D, Engelhard MH, Burton SD, Zhao W, Zheng J, Li Q, Ding MS, Schroeder M, Alvarado J, Xu K, Meng YS, Liu J, Zhang JG, Xu W, Chem 2018, 4, 1877. [Google Scholar]
- [60].Fan X, Ji X, Chen L, Chen J, Deng T, Han F, Yue J, Piao N, Wang R, Zhou X, Xiao X, Chen L, Wang C, Nat. Energy 2019, 4, 882. [Google Scholar]
- [61].Amanchukwu CV, Kong X, Qin J, Cui Y, Bao Z, Adv. Energy Mater. 2019, 9, 1902116. [Google Scholar]
- [62].Rustomji CS, Yang Y, Kim TK, Mac J, Kim YJ, Caldwell E, Chung H, Meng YS, Science (80-. ). 2017, 356. [DOI] [PubMed] [Google Scholar]
- [63].Yang Y, Davies DM, Yin Y, Borodin O, Lee JZ, Fang C, Olguin M, Zhang Y, Sablina ES, Wang X, Rustomji CS, Meng YS, Joule 2019, 3, 1986. [Google Scholar]
- [64].Yang Y, Yin Y, Davies DM, Zhang M, Mayer M, Zhang Y, Sablina ES, Wang S, Lee JZ, Borodin O, Rustomji CS, Meng YS, Energy Environ. Sci. 2020, 13, 2209. [Google Scholar]
- [65].Gupta A, Bhargav A, Jones JP, Bugga RV, Manthiram A, Chem. Mater. 2020, 32, 2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cuisinier M, Hart C, Balasubramanian M, Garsuch A, Nazar LF, Adv. Energy Mater. 2015, 5, 1401801. [Google Scholar]
- [67].Gupta A, Bhargav A, Manthiram A, Adv. Energy Mater. 2018, 1803096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zou Q, Lu Y-C, J. Phys. Chem. Lett. 2016, 7, 1518. [DOI] [PubMed] [Google Scholar]
- [69].Li Z, Zhou Y, Wang Y, Lu YC, Adv. Energy Mater. 2019, 9, 1802207. [Google Scholar]
- [70].Shin H, Baek M, Gupta A, Char K, Manthiram A, Choi JW, Adv. Energy Mater. 2020, 10, 2001456. [Google Scholar]
- [71].Mikhaylik YV, Akridge JR, J. Electrochem. Soc. 2003, 150, A306. [Google Scholar]
- [72].Ryu H-S, Ahn H-J, Kim K-W, Ahn J-H, Cho K-K, Nam T-H, Kim J-U, Cho G-B, J. Power Sources 2006, 163, 201. [Google Scholar]
- [73].Yu SH, Huang X, Schwarz K, Huang R, Arias TA, Brock JD, Abruña HD, Energy Environ. Sci. 2018, 11, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chu H, Noh H, Kim Y-J, Yuk S, Lee J-H, Lee J, Kwack H, Kim Y, Yang D-K, Kim H-T, Nat. Commun. 2019, 10, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Gerber LCH, Frischmann PD, Fan FY, Doris SE, Qu X, Scheuermann AM, Persson K, Chiang YM, Helms BA, Nano Lett. 2016, 16, 549. [DOI] [PubMed] [Google Scholar]
- [76].He J, Manthiram A, Energy Storage Mater. 2019, 20, 55. [Google Scholar]
- [77].Liu XC, Yang Y, Wu J, Liu M, Zhou S, Levin BDA, Zhou XD, Cong H, Muller DA, Ajayan PM, Abruña HD, Ke FS, ACS Energy Lett. 2018, 2. [Google Scholar]
- [78].Bhargav A, He J, Gupta A, Manthiram A, Joule 2020, 4, 285. [Google Scholar]
- [79].Zhu S, Wang Y, Jiang J, Yan X, Sun D, Jin Y, Nan C, Munakata H, Kanamura K, ACS Appl. Mater. Interfaces 2016, 8, 17253. [DOI] [PubMed] [Google Scholar]
- [80].Deng DR, Xue F, Bai CD, Lei J, Yuan R, Sen Zheng M, Dong QF, ACS Nano 2018, 12, 11120. [DOI] [PubMed] [Google Scholar]
- [81].Fan C-Y, Zheng Y-P, Zhang X-H, Shi Y-H, Liu S-Y, Wang H-C, Wu X-L, Sun H-Z, Zhang J-P, Adv. Energy Mater. 2018, 8, 1703638. [Google Scholar]
- [82].Wang X, Zhao X, Ma C, Yang Z, Chen G, Wang L, Yue H, Zhang D, Sun Z, J. Mater. Chem. A 2020, 8, 1212. [Google Scholar]
- [83].Ye H, Lee JY, Small Methods 2020, 4, 1900864. [Google Scholar]
- [84].Danner T, Latz A, Electrochim. Acta 2019, 322, 134719. [Google Scholar]
- [85].Ren YX, Zhao TS, Liu M, Tan P, Zeng YK, J. Power Sources 2016, 336, 115. [Google Scholar]
- [86].Chu H, Jung J, Noh H, Yuk S, Lee J, Lee J, Baek J, Roh Y, Kwon H, Choi D, Sohn K, Kim Y, Kim H, Adv. Energy Mater. 2020, 2000493. [Google Scholar]
- [87].Cheng L, Curtiss LA, Zavadil KR, Gewirth AA, Shao Y, Gallagher KG, ACS Energy Lett. 2016, 1, 503. [Google Scholar]
- [88].Lee C-W, Pang Q, Ha S, Cheng L, Han S-D, Zavadil KR, Gallagher KG, Nazar LF, Balasubramanian M, ACS Cent. Sci. 2017, 3, 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zheng J, Ji G, Fan X, Chen J, Li Q, Wang H, Yang Y, DeMella KC, Raghavan SR, Wang C, Adv. Energy Mater. 2019, 9, 1803774. [Google Scholar]
- [90].Rodríguez-Pérez IA, Ji X, ACS Energy Lett. 2017, 2, 1762. [Google Scholar]
- [91].Holoubek J, Yin Y, Li M, Yu M, Meng YS, Liu P, Chen Z, Angew. Chemie Int. Ed. 2019, 58, 18892. [DOI] [PubMed] [Google Scholar]
- [92].Dong X, Guo Z, Guo Z, Wang Y, Xia Y, Joule 2018, 2, 902. [Google Scholar]
- [93].Dong X, Lin Y, Li P, Ma Y, Huang J, Bin D, Wang Y, Qi Y, Xia Y, Angew. Chemie Int. Ed. 2019, 58, 5623. [DOI] [PubMed] [Google Scholar]