Abstract
Background and purpose:
Neuropathic pain is one of the most common types of chronic pain that is very difficult to treat. Numerous studies have shown the potential role of vitamins in relieving both hyperalgesia and allodynia. Based on the convincing evidence, this study was designed to evaluate the possible antinociceptive effect of biotin on neuropathic pain in rats.
Experimental approach:
This study was performed on male Sprague Dawley rats weighing 200-300 g. Neuropathic pain was induced by tying the sciatic nerve. Chronic constriction injury (CCI) of the sciatic nerve resulted in hyperalgesia and allodynia. To measure the thermal hyperalgesia, the plantar test was used. Also to evaluate the cold and mechanical allodynia, acetone test and von Frey test were applied. Biotin (4, 8, and 16 mg/kg) was administered orally as two different treatment regimens, acute and chronic.
Findings/Results:
Acute oral administration of biotin (4, 8, and 16 mg/kg p.o.) on the 7th, 14th, and 21st postoperative days couldn’t reduce pain sensitivity compared to the CCI group. However, following the oral administration of biotin (8 and 16 mg/kg p.o.) from the first day after the surgery until day 21, mechanical allodynia (P < 0.001) and heat hyperalgesia (P < 0.05) significantly relieved.
Conclusion and implications:
Our results suggest that biotin can be considered as a potential therapeutic for the treatment of neuropathic pain, and supplementation with this vitamin could reduce the required doses of analgesic drugs. However, further studies are needed to confirm this hypothesis.
Keywords: Allodynia, Biotin, Hyperalgesia, Neuropathic pain, Rat.
INTRODUCTION
There are different types of chronic pain. One of the most common types of chronic pain is neuropathic pain (1). Symptoms of this type of pain include unpleasant sensation, dysaesthesia, increased sensitivity to painful stimuli (hyperalgesia), and pain when exposed to stimuli (allodynia) (2). The most common causes of neuropathic pain are damage to the central or peripheral nervous system, infection, inflammation, and metabolic disorders (3). Various mechanisms are involved in the development of neuropathic pain, including increased NMDA receptor activity, overexpression of excitatory neurotransmitters such as glutamate, and decreased activity of inhibitory pathways (4). Despite intensive research on the neurological mechanisms involved in this type of pain, effective treatment and underlying mechanisms are poorly understood (5,6). Existing treatments, including anticonvulsants, topical anesthetics, and opiates, are often ineffective (7). The role of vitamins in control neurological disorder has been proven (8).
Vitamin H or biotin, a water-soluble vitamin, (vitamin B7) is part of the B family vitamins (9). Biotin is a cofactor for carboxylase enzymes, such as pyruvate carboxylase, acetyl-carboxylase, propionyl carboxylase, and methyl crotonyl-carboxylase, which play an important role in gluconeogenesis, fatty acid synthesis, and catabolism (10). There are various forms of biotin, but only D-biotin has vitamin activity and is naturally occurring (11). Biotin deficiency induces mild depression, fatigue, drowsiness, mood changes, myalgias, hyperesthesias, paresthesias, progressive spastic paraparesis, hypertonia, seizures, and encephalopathy (12-14). The other biotin-dependent neurological disorders include limb weakness, biotin-responsive encephalopathy, biotin-responsive Leigh syndrome, and biotin-responsive myelopathy have been identified (15-18). Biotin also plays an important role in cell signaling, gene expression, and chromatin structure as well as regulates immune and inflammatory function (19,20). Therefore, biotin can activate many anti-inflammatory and repair pathways (21). Experimental studies have shown that inflammation leads to hyperalgesia and biotin deficiency can enhance the proinflammatory cytokine responses of human dendritic cells (22,23). Biotin can affect nuclear factor-κB activity which is one of the most important transcription factors involved in immune and inflammatory functions (24). Biotin deficiency increases tumor necrosis factor (TNF)-a production and biotin supplementation reduce this overproduction (25). Biotin at high doses is able to increase cGMP production and produce neuroprotective effects (26).
Recently, the relationship between pain and vitamin B complex (B1, B6, and B12) has been investigated (27). It has been found that high doses of biotin could improve the clinical symptoms of diabetic neuropathic pain (27). Research on the relationship between peripheral neuropathic pain and biotin supplementation is still limited. Therefore, this study was designed to determine whether biotin supplementation is effective in reducing pain sensitivity in a rat model of neuropathic pain. Previous studies have shown that treatments with vitamin D which started immediately after nerve injury are more effective (28). So, in this study, we have designed two types of treatment regimens.
MATERIAL AND METHODS
Animal grouping and treatment
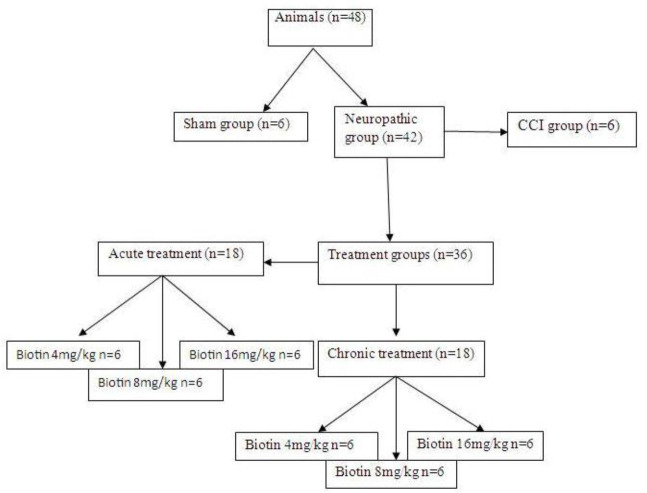
These experiments were performed on male Sprague-Dawley rats weighing 200-300 g. In each cage four rats were placed, in a room under controlled temperature (23 °C), 55% humidity, and 12/12-h light/dark cycle, with free access to water and food. In this study for acute treatment regimen, biotin was administered in different doses (4, 8, and 16 mg/kg; p.o.) only on the test days, in three groups of rats (n = 6). The effects of acute treatment on behavioral tests were assessed 30 min after drug administration, on the 7th, 14th, and 21st days after surgery. Also, for chronic treatment, biotin (4, 8, and 16 mg/kg; p.o.) was administered daily on the 1st day after nerve injury and for 21 consecutive days. Behavioral tests were done on the 7th, 14th, and 21st days after surgery. At the end of the experiment, based on the laboratory animal protocols rats were sacrificed (Fig. 1).
Fig. 1.
Schematic presentation of treatments, animal grouping, and behavioral tests of the chronic constriction injury (CCI) model.
This study ethically was approved by the Army University of Medical Sciences (Ethic No. IR.AJAUMS.REC.1398.145).
Neuropathic pain model
To induce neuropathic pain, the chronic constriction injury (CCI) model was used. Rats were anesthetized with i.p. administration of ketamine/xylazine (100 mg/kg and 15 mg/kg, respectively) injections. After complete anesthesia (no response to painful reflexes) rats were placed in the right lateral position and the eyes of the rat were covered with simple eye ointment and the left buttock was shaved and after that, a 1cm incision was made in the posterior inferior region of the leg. The skin was separated from the underlying fascia tissue and the muscle was cut into two layers and the sciatic nerve was exposed. Then nerve adhesion was separated and the nerve was completely exposed (without metal tools contacting the nerve), then in an area, about 5 mm above the nerve triceps, four ligatures (4.0 chromic gut) were tied loosely around the common sciatic nerve with a 1-1.5 mm interval between ligatures, so that the circulation through the superficial epineuria vasculature was not interrupted (29).
Behavioral tests
Cold allodynia
This test was used to determine the susceptibility of the animal to cold allodynia. With an insulin syringe inserted into a thin polypropylene tube, a drop of acetone was sprayed to the plantar surface of the hind paw. This test was performed five times. In the case of acetone spray, the animal which raised its paw was considered as a positive response, otherwise, it was considered as a negative response. The percentage of response then calculated by the number of positive animal responses to the total number of stimuli. The frequency of paw withdrawal was expressed as a percentage (the number of paw withdrawals/number of trials × 100) (30,31)
Mechanical allodynia
Animals were placed on a wired mesh in a 20 × 20 cm, 30 cm high Plexiglas housing, and after acclimatization to the new environment, various von Frey filaments (2-60 g) were used to measure mechanical allodynia. These filaments are in the range of 2-60 g. A series of von Frey filament stimuli were delivered in ascending order of forces to the central region of the plantar surface of the hind paw. The stimulation was applied three times consecutively, pushing down on the hind paw until the rat withdrew its paw or the fiber bowed. Lifting of the paw due to normal locomotor behavior was ignored. The smallest filament size which evoked at least 3 withdrawal responses during 5 consecutive applications was considered as withdrawal threshold. Each filament was applied for approximately 1 s and the interstimulus intervals were about 5 s (32,33).
Thermal hyperalgesia
Using a Plantar test apparatus (UgoBasile, Varese, Italy) and irradiating infrared beam from the surface of the Plexiglas enclosure to the mid-plantar surface of the hind paw, the animal’s tolerance to the heat stimulus was measured. With the movement of the hind paw or reflex, the delay time of the paw was recorded as paw withdrawal latency. Thermal stimuli are repeated three times at intervals of 5 min, and the test cut-off was 22 s (34,35).
Statistical analysis
Parametric data were analyzed by two-way repeated-measures ANOVA followed by Bonferroni post hoc test for multiple comparisons. Moreover, the nonparametric data were converted to parametric data by a rank transformation method, and new data were analyzed by ANOVA.
RESULTS
Behavioral tests of neuropathic pain
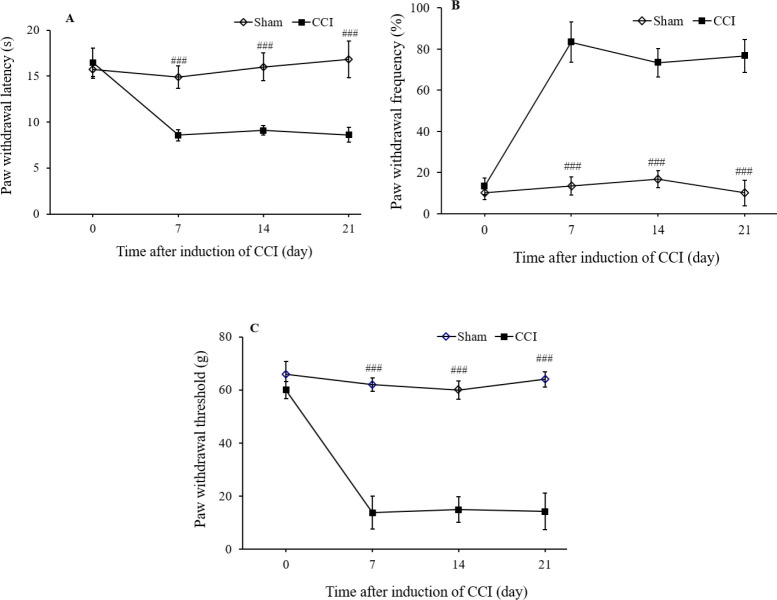
There was no sign of contralateral hyperalgesia and allodynia in CCI and treatment groups. The sciatic nerve injury reduced paw withdrawal latency (P < 0.001), but the sham group did not show any change in the heat hyperalgesia test (Fig. 2A). As shown in Fig. 2B acetone application led to a significant increase in the paw withdrawal frequency of the neuropathic group compared to the sham group (P < 0.001). Also, Fig. 2C shows the results of mechanical allodynia. Sciatic nerve injury extensively increased the sensitivity of the ipsilateral hind paw to the von Frey monofilament test in the neuropathic group compared to the sham group (P < 0.001).
Fig. 2.
The effects of chronic constriction injury (CCI) of the sciatic nerve on (A) the heat hyperalgesia; (B) the cold allodynia; and (C) mechanical allodynia. The CCI groups received saline solution according to the treatment schedule. Sham group had the same surgery, the left common sciatic nerve was exposed but no ligation was made. The results are expressed as mean ± SEM, n = 8. ###P < 0.001indicate significant differences compared with the CCI group. CCI, chronic constriction injury.
The effects of biotin administration on the heat hyperalgesia
To study the mechanisms of persistent pain, animal models of hyperalgesia that mimic human clinical pain conditions have been developed. The paw withdrawal to a thermal stimulus after nerve injury defines as thermal hyperalgesia because withdrawal occurs at a normally innocuous temperature (29). Acute administration of biotin (4, 8, and 16 mg/kg; p.o.) on the 7th, 14th, and 21st days after surgery, 30 min before the behavioral tests, didn’t show any significant attenuation in the paw withdrawal latency (Fig. 3A). As shown in Fig. 4A, chronic supplementation with biotin 8 mg/kg (F(2,10)= 11.229, mean ± SEM = 13.483 ± 0.696, P < 0.05) and 16 mg/kg (F(2,10)= 10.617, mean ± SEM = 13.34 ± 0.485, P < 0.05) from the first day after surgery until the 21st day inhibited heat hyperalgesia.
Fig. 3.
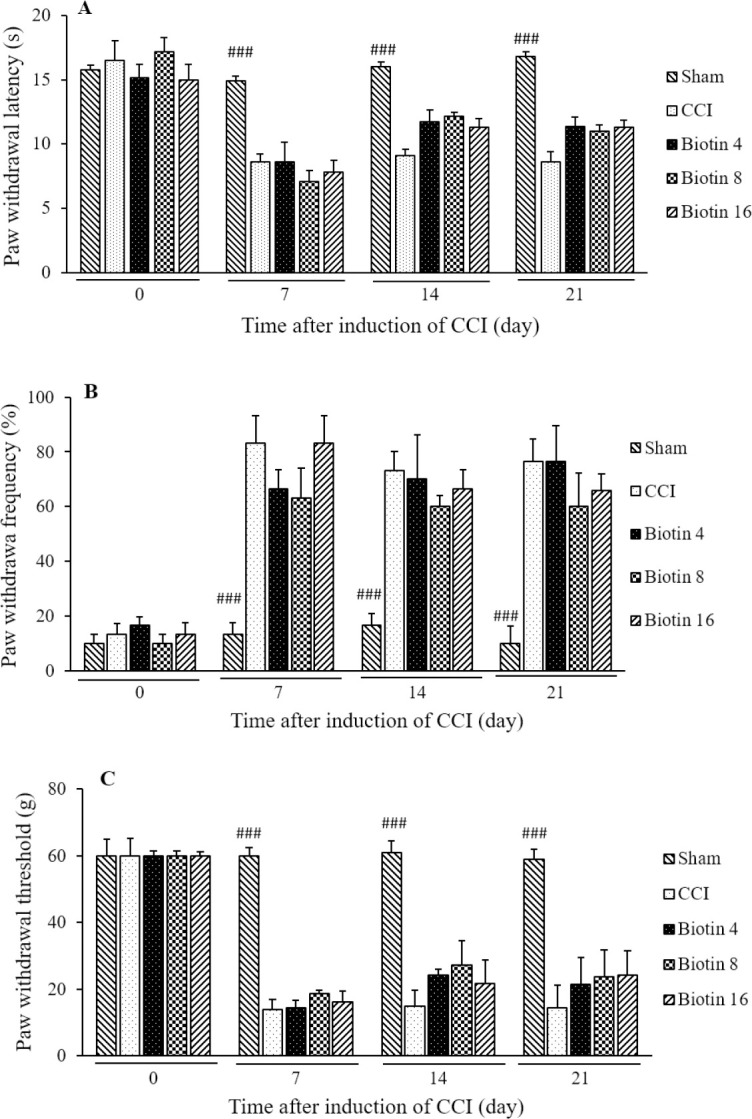
The effect of acute treatment with biotin (4, 8, and 16 mg/kg; p.o.) on the expression of (A) heat hyperalgesia, (B) cold allodynia, and (C) mechanical allodynia. The vehicle groups (CCI) received saline solution according to the treatment schedule. The results are expressed as mean ± SEM, n = 8 in all groups. ###P < 0.001 versus CCI group. CCI, chronic constriction injury.
Fig. 4.
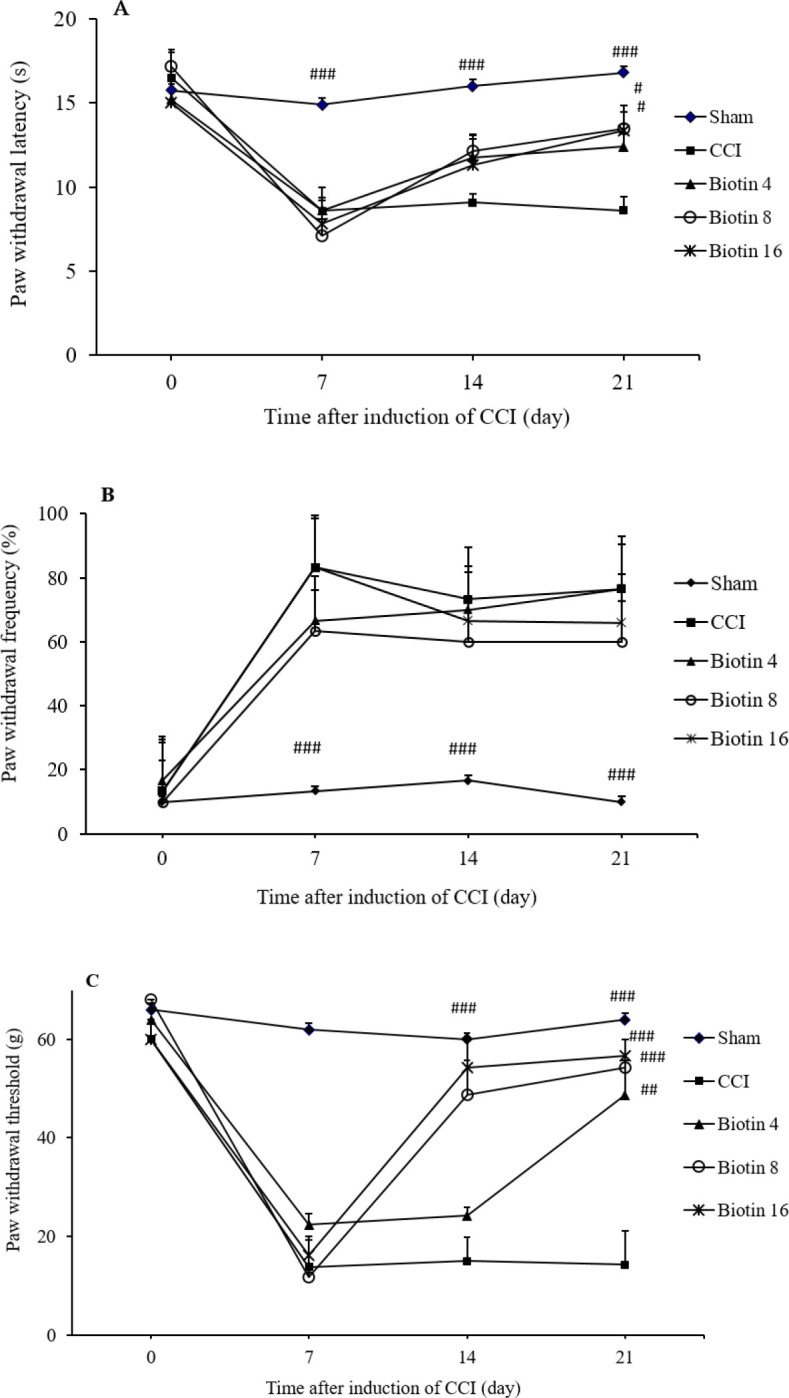
The effects of biotin on the development of neuropathic pain. The effect of chronic treatment with biotin (4, 8, and 16 mg/kg; p.o.) from 1st day to the 21st day after surgery on the development of (A) heat hyperalgesia, (B) cold allodynia, and (C) mechanical allodynia. The CCI groups received saline solution according to the treatment schedule. The results are expressed as mean ± SEM, n = 8. #P < 0.05, ##P < 0.01, and ###P < 0.001 indicate significant differences versus the CCI group. CCI, chronic constriction injury.
The effects of biotin administration on the cold allodynia
Allodynia is defined as pain due to a stimulus that does not normally provoke pain. Detection of cold allodynia is a very important aspect in the study of pain behavior. Cold allodynia and mechanical allodynia are common signs in various neuropathic conditions (36). Acute biotin (4, 8, and 16 mg/kg; p.o.) administration on the 7th, 14th, and 21st days after surgery, 30 min before the behavioral tests, didn’t show any significant improvement in the paw withdrawal frequency (Fig. 3B). Also, chronic administration of biotin (4, 8, and 16 mg/kg; p.o.) didn’t show any significant attenuation in the cold allodynia compared with the CCI group (Fig. 4B).
The effects of biotin administration on the mechanical allodynia
Mechanical allodynia is a painful sensation caused by innocuous stimuli like light touch. Specific treatments that reverse mechanical allodynia could be very useful (37). Biotin (4, 8, and 16 mg/kg; p.o.) administration on the 7th, 14th, and 21st days after surgery didn’t show any significant inhibition in the paw withdrawal threshold (Fig. 3C). As shown in Fig. 4C, the significant improvement in mechanical allodynia was observed in the animals were treated with chronic biotin administration 8 mg/kg (F(2,10)= 24.969, mean ± SEM = 48.66 ± 7.16, P < 0.001) and 16 mg/kg (F(2,10)= 19.906, mean ± SEM = 54.33 ± 5.66, P < 0.001) on the 14th day. Also chronic administration of biotin 4 mg/kg (F(2,10)= 9.637, mean ± SEM = 48.66 ± 7.167, P < 0.01), 8 mg/kg (F(2,10)= 26.916, mean ± SEM = 54.33 ± 5.66, P < 0.001) and 16 mg/kg (F(2,10)= 28.907, mean ± SEM = 56.66 ± 3.33, P < 0.001) significantly suppressed mechanical allodynia on the 21st day after surgery (Fig. 4C).
Discussion
Based on the results of this study, it was found that acute administration of biotin was not able to alter the pain threshold in neuropathic pain. But chronic administration of this vitamin significantly inhibited thermal hyperalgesia and mechanical allodynia but had no significant effect on the cold allodynia. This difference in efficacy is likely due to the different pathways involved in the transmission of pain sensation in neuropathic pain. Mechanical allodynia-induced stimulation is transmitted through Aβ myelinated fibers, but cold-induced allodynia is transmitted through non-myelinated C fibers. Thermal hyperalgesia is mainly transmitted through vanilloid receptors (38).
Considering these results, it can be deduced that biotin administration if started immediately after nerve injury and for a sufficient period of time inhibits the development of neuropathic pain. This finding is in accordance with the results obtained by Koutsikos et al. which showed that administration of biotin could reduce diabetes-induced neuropathic pain (39). It should be noted that different animal models have been developed to study neuropathic pain. In this study, CCI of the sciatic nerve model was used to induce neuropathic pain, which is one of the most common models of neuropathic pain. Sciatic nerve ligation caused allodynia and hyperalgesia (40). Evaluation of the animal models of neuropathic pain have shown that various mechanisms, such as spontaneous neuronal excitability, alterations in gene expression in neurons, and increased sensitivity of neurons in the dorsal horn of the spinal cord, are involved in producing this pain (41). Activation of inflammatory pathways and pathological changes also occur in the nerve roots and dorsal root ganglia (42). Nitric oxide production plays an important role in the development of neuropathic pain. Oxygen-free radicals such as nitric oxide and superoxide have important roles in inflammation and immune responses. These compounds are released by cells such as neutrophils, macrophages, astrocytes, and microglia (43,44).
Previous studies have shown that biotin deficiency affects the function of the immune system, especially natural killer and T cells (20). Recent research has also shown that biotin deficiency stimulates the secretion of inflammatory cytokines from dendritic cells. Biotin deficiency also promotes enhanced secretion of TNF-α in mouse macrophages (23). It has been suggested that biotin deficiency may increase TNF-α production and biotin supplementation may inhibit TNF-α releases (25). Therefore, biotin status may affect inflammatory diseases. As mentioned earlier, inflammatory mediators play an important role in neuropathic pain development. It has been shown that biotin is capable of increasing cGMP by stimulation the soluble guanylate cyclase. Several studies have reported the anti-inflammatory effects of cGMP. cGMP stimulates Schwann cells to produce neurotrophic factors. Activation of the PI3K-Akt and Ras-ERK pathways by cGMP promotes neuron survival and plasticity (45). The role of biotin in maintaining cellular energy homeostasis has been demonstrated. Previous studies provide convincing evidence that biotin plays an important role in cellular energy homeostasis as biotin and its metabolite biotinyl-AMP increase in the synthesis of cGMP (26). Subsequently, cGMP activates protein kinase G, thereby increasing the transcription of genes coding for biotin-dependent carboxylases and holocarboxylase synthetase that are essential for the metabolism of glucose, fatty acids, and amino acids (45). Biotin at high doses is able to increase cGMP production and produce neuroprotective effects.
Based on the present study it can be concluded that biotin supplementation inhibits the development of neuropathic pain. These findings indicated the potential role of this vitamin in the management of neuropathic pain.
CONCLUSION
Considering the results of the current study, biotin administration immediately after nerve injury, and for a sufficient period of time, can improve the pain-related behavior of the neuropathic pain. This may indicate the therapeutic potential for biotin administration in reducing the symptoms of neuropathic pain. Due to the ineffectiveness of usual treatments and associated adverse effects, biotin could be considered as a supplement in the treatment of neuropathic pain.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Authors’ contribution
A. Abed and A.R. Abed contributed to the conception, design, statistical analysis, and drafting of the manuscript. H.R. Banafshe, E. Shiri, S. Gorgani-firuzjaee, and A. Dadashi contributed to conception, data collection, and manuscript drafting. The final version was confirmed by all authors for submission.
Acknowledgments
This joint research project was financially supported by both the Research Council of Army University of Medical Sciences (Gran No. 998984) and Kashan University of Medical Sciences under Grant No. No.98209.
REFERENCES
- 1.Tembhurne S, Sakarkar D. Influence of Murraya koenigii on experimental model of diabetes and progression of neuropathic pain. Res Pharm Sci. 2010;5(1):41–47. [PMC free article] [PubMed] [Google Scholar]
- 2.Amin B, Hajhashemi V, Hosseinzadeh H. Minocycline potentiates the anti-hyperalgesic effect of ceftriaxone in CCI-induced neuropathic pain in rats. Res Pharm Sci. 2015;10(1):34–42. [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes JP, Chessell I, Malamut R, Perkins M, Backonja M, Baron R, et al. Understanding chronic inflammatory and neuropathic pain. Ann N Y Acad Sci. 2012;1255:30–44. doi: 10.1111/j.1749-6632.2012.06561.x. DOI: 10.1111/j.1749-6632.2012.06561.x. [DOI] [PubMed] [Google Scholar]
- 4.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51(2):240–264. doi: 10.1016/j.brainresrev.2005.11.004. DOI: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Pfau DB, Krumova EK, Treede RD, Baron R, Toelle T, Birklein F, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): reference data for the trunk and application in patients with chronic postherpetic neuralgia. Pain. 2014;155(5):1002–1015. doi: 10.1016/j.pain.2014.02.004. DOI: 10.1016/j.pain.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Dworkin RH, O’Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3–S14. doi: 10.4065/mcp.2009.0649. DOI: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schug SA, Goddard C. Recent advances in the pharmacological management of acute and chronic pain. Ann Palliat Med. 2014;3(4):263–275. doi: 10.3978/j.issn.2224-5820.2014.10.02. DOI: 10.3978/j.issn.2224-5820.2014.10.02. [DOI] [PubMed] [Google Scholar]
- 8.Mesdaghinia A, Alinejad M, Abed A, Heydari A, Banafshe HR. Anticonvulsant effects of thiamine on pentylenetetrazole-induced seizure in mice. Nutr Neurosci. 2019;22(3):165–173. doi: 10.1080/1028415X.2017.1357919. DOI: 10.1080/1028415X.2017.1357919. [DOI] [PubMed] [Google Scholar]
- 9.Said HM. Biotin: biochemical, physiological and clinical aspects. Subcell Biochem. 2012;56:1–19. doi: 10.1007/978-94-007-2199-9_1. DOI: 10.1007/978-94-007-2199-9_1. [DOI] [PubMed] [Google Scholar]
- 10.Pacheco-Alvarez D, Solórzano-Vargas RS, Del Río AL. Biotin in metabolism and its relationship to human disease. Arch Med Res. 2002;33(5):439–447. doi: 10.1016/s0188-4409(02)00399-5. DOI: 10.1016/s0188-4409(02)00399-5. [DOI] [PubMed] [Google Scholar]
- 11.McMahon RJ. Biotin in metabolism and molecular biology. Annu Rev Nutr. 2002;22:221–239. doi: 10.1146/annurev.nutr.22.121101.112819. DOI: 10.1146/annurev.nutr.22.121101.112819. [DOI] [PubMed] [Google Scholar]
- 12.Chedrawi AK, Ali A, Al Hassnan ZN, Faiyaz-Ul-Haque M, Wolf B. Profound biotinidase deficiency in a child with predominantly spinal cord disease. J Child Neurol. 2008;23(9):1043–1048. doi: 10.1177/0883073808318062. DOI: 10.1177/0883073808318062. [DOI] [PubMed] [Google Scholar]
- 13.Rathi N, Rathi M. Biotinidase deficiency with hypertonia as unusual feature. Indian Pediatr. 2009;46(1):65–67. [PubMed] [Google Scholar]
- 14.Joshi SN, Fathalla M, Koul R, Maney MA, Bayoumi R. Biotin responsive seizures and encephalopathy due to biotinidase deficiency. Neurol India. 2010;58(2):323–324. doi: 10.4103/0028-3886.63783. DOI: 10.4103/0028-3886.63783. [DOI] [PubMed] [Google Scholar]
- 15.Adhisivam B, Mahto D, Mahadevan S. Biotin responsive limb weakness. Indian Pediatr. 2007;44(3):228–230. [PubMed] [Google Scholar]
- 16.Bressman S, Fahn S, Eisenberg M, Brin M, Maltese W. Biotin-responsive encephalopathy with myoclonus, ataxia, and seizures. Adv Neurol. 1986;43:119–125. [PubMed] [Google Scholar]
- 17.Fassone E, Wedatilake Y, DeVile CJ, Chong WK, Carr LJ, Rahman S. Treatable Leigh-like encephalopathy presenting in adolescence. BMJ Case Rep. 2013;2013:200838,1–5. doi: 10.1136/bcr-2013-200838. DOI: 10.1136/bcr-2013-200838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yilmaz S, Serin M, Canda E, Eraslan C, Tekin H, Ucar SK, et al. A treatable cause of myelopathy and vision loss mimicking neuromyelitis optica spectrum disorder: late-onset biotinidase deficiency. Metab Brain Dis. 2017;32(3):675–678. doi: 10.1007/s11011-017-9984-5. DOI: 10.1007/s11011-017-9984-5. [DOI] [PubMed] [Google Scholar]
- 19.Brigolin C, McKenty N, Pindolia K, Wolf B. Differential gene expression during early development in brains of wildtype and biotinidase-deficient mice. Mol Gene Metab Rep. 2016;9:35–41. doi: 10.1016/j.ymgmr.2016.09.007. DOI: 10.1016/j.ymgmr.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuroishi T. Regulation of immunological and inflammatory functions by biotin. Can J Physiol Pharmacol. 2015;93(12):1091–1096. doi: 10.1139/cjpp-2014-0460. DOI: 10.1139/cjpp-2014-0460. [DOI] [PubMed] [Google Scholar]
- 21.Dakshinamurti K, Bagchi RA, Abrenica B, Czubryt MP. Microarray analysis of pancreatic gene expression during biotin repletion in biotin-deficient rats. Can J Physiol Pharmacol. 2015;93(12):1103–1110. doi: 10.1139/cjpp-2014-0517. DOI: 10.1139/cjpp-2014-0517. [DOI] [PubMed] [Google Scholar]
- 22.Schäfers M, Sorkin L. Effect of cytokines on neuronal excitability. Neurosci Lett. 2008;437(3):188–193. doi: 10.1016/j.neulet.2008.03.052. DOI: 10.1016/j.neulet.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal S, Agrawal A, Said HM. Biotin deficiency enhances the inflammatory response of human dendritic cells. Am J Physiol Cell Physiol. 2016;311(3):C386–C391. doi: 10.1152/ajpcell.00141.2016. DOI: 10.1152/ajpcell.00141.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Melendez R, Schwab LD, Zempleni J. Jurkat cells respond to biotin deficiency with increased nuclear translocation of NF-kB, mediating cell survival. Int J Vitam Nutr Res. 2004;74(3):209–216. doi: 10.1024/0300-9831.74.3.209. DOI: 10.1024/0300-9831.74.3.209. [DOI] [PubMed] [Google Scholar]
- 25.Kuroishi T, Endo Y, Muramoto K, Sugawara S. Biotin deficiency up-regulates TNF-α production in murine macrophages. J Leukoc Biol. 2008;83(4):912–920. doi: 10.1189/jlb.0607428. DOI: 10.1189/jlb.0607428. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Melendez R, Zempleni J. Nitric oxide signaling depends on biotin in Jurkat human lymphoma cells. J Nutr. 2009;139(3):429–433. doi: 10.3945/jn.108.101840. DOI: 10.3945/jn.108.101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mimenza Alvarado A, Aguilar Navarro S. Comparative clinical trial of safety and tolerability of gabapentin plus vitamin B1/B12 versus pregabalin in the treatment of painful peripheral diabetic neuropathy. J Pain Relief. 2014;S3:006,1–7. DOI: 10.4172/2167-0846.S3-006. [Google Scholar]
- 28.Banafshe HR, Khoshnoud MJ, Abed A, Saghazadeh M, Mesdaghinia A. Vitamin D supplementation attenuates the behavioral scores of neuropathic pain in rats. Nutr Neurosci. 2019;22(10):700–705. doi: 10.1080/1028415X.2018.1435485. DOI: 10.1080/1028415X.2018.1435485. [DOI] [PubMed] [Google Scholar]
- 29.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6. DOI: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 30.Abed A, Hajhashemi V, Banafshe HR, Minaiyan M, Mesdaghinia A. Venlafaxine attenuates heat hyperalgesia independent of adenosine or opioid system in a rat model of peripheral neuropathy. Iran J Pharm Res. 2015;14(3):843–850. [PMC free article] [PubMed] [Google Scholar]
- 31.Abed A, Khoshnoud MJ, Taghian M, Aliasgharzadeh M, Mesdaghinia A. Quetiapine reverses paclitaxel-induced neuropathic pain in mice: role of alpha2-adrenergic receptors. Iran J Basic Med Sci. 2017;20(11):1182–1188. doi: 10.22038/IJBMS.2017.9500. DOI: 10.22038/IJBMS.2017.9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamidi GA, Jafari-Sabet M, Abed A, Mesdaghinia A, Mahlooji M, Banafshe HR. Gabapentin enhances anti-nociceptive effects of morphine on heat, cold, and mechanical hyperalgesia in a rat model of neuropathic pain. Iran J Basic Med Sci. 2014;17(10):753–759. [PMC free article] [PubMed] [Google Scholar]
- 33.Naji-Esfahani H, Vaseghi G, Safaeian L, Pilehvarian AA, Abed A, Rafieian-Kopaei M. Gender differences in a mouse model of chemotherapy-induced neuropathic pain. Lab Anim. 2016;50(1):15–20. doi: 10.1177/0023677215575863. DOI: 10.1177/0023677215575863. [DOI] [PubMed] [Google Scholar]
- 34.Banafshe HR, Hajhashemi V, Minaiyan M, Mesdaghinia A, Abed A. Antinociceptive effects of maprotiline in a rat model of peripheral neuropathic pain: possible involvement of opioid system. Iran J Basic Med Sci. 2015;18(8):752–757. [PMC free article] [PubMed] [Google Scholar]
- 35.Hajhashemi V, Banafshe HR, Minaiyan M, Mesdaghinia A, Abed A. Antinociceptive effects of venlafaxine in a rat model of peripheral neuropathy: role of alpha2-adrenergic receptors. Eur J Pharmacol. 2014;738:230–236. doi: 10.1016/j.ejphar.2014.04.046. DOI: 10.1016/j.ejphar.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 36.Lindblom U, Merskey H, Mumford JM, Nathan PW, Noordenbos W, Sunderland S. Pain terms, a current list with definitions and notes on usage. Pain. 1986;3:215–221. DOI: 10.1016/0304-3959(86)90113-2. [Google Scholar]
- 37.Lolignier S, Eijkelkamp N, Wood JN. Mechanical allodynia. Pflugers Arch. 2015;467:133–139. doi: 10.1007/s00424-014-1532-0. DOI: 10.1007/s00424-014-1532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meacham K, Shepherd A, Mohapatra DP, Haroutounian S. Neuropathic pain: central vs. peripheral mechanisms. Curr Pain Headache Rep. 2017;21(6):28–38. doi: 10.1007/s11916-017-0629-5. DOI: 10.1007/s11916-017-0629-5. [DOI] [PubMed] [Google Scholar]
- 39.Koutsikos D, Agroyannis B, Tzanatos-Exarchou H. Biotin for diabetic peripheral neuropathy. Biomed Pharmacother. 1990;44(10):511–514. doi: 10.1016/0753-3322(90)90171-5. DOI: 10.1016/0753-3322(90)90171-5. [DOI] [PubMed] [Google Scholar]
- 40.Jaggi AS, Jain V, Singh N. Animal models of neuropathic pain. Fundam Clin Pharmacol. 2011;25(1):1–28. doi: 10.1111/j.1472-8206.2009.00801.x. DOI: 10.1111/j.1472-8206.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 41.Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An overview of animal models of pain: disease models and outcome measures. J Pain. 2013;14(11):1255–1269. doi: 10.1016/j.jpain.2013.06.008. DOI: 10.1016/j.jpain.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sommer C, Leinders M, Üçeyler N. Inflammation in the pathophysiology of neuropathic pain. Pain. 2018;159(3):595–602. doi: 10.1097/j.pain.0000000000001122. DOI: 10.1097/j.pain.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Z, Liang Y, Deng F, Cheng Y, Sun J, Guo L, et al. Phosphorylated neuronal nitric oxide synthase in neuropathic pain in rats. Int J Clin Exp Pathol. 2015;8(10):12748–12756. [PMC free article] [PubMed] [Google Scholar]
- 44.Mukherjee P, Cinelli MA, Kang S, Silverman RB. Development of nitric oxide synthase inhibitors for neurodegeneration and neuropathic pain. Chem Soc Rev. 2014;43(19):6814–6838. doi: 10.1039/c3cs60467e. DOI: 10.1039/c3cs60467e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tong L. Structure and function of biotin-dependent carboxylases. Cell Mol Life Sci. 2013;70(5):863–891. doi: 10.1007/s00018-012-1096-0. DOI: 10.1007/s00018-012-1096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]