ABSTRACT
The Developmental Origins of Health and Disease (DOHaD) theory predicts that prenatal and early life events shape adult health outcomes. Birth weight is a useful indicator of the foetal experience and has been associated with multiple adult health outcomes. DNA methylation (DNAm) is one plausible mechanism behind the relationship of birth weight to adult health. Through data linkage between Generation Scotland and historic Scottish birth cohorts, and birth records held through the NHS Information and Statistics Division, a sample of 1,757 individuals with available birth weight and DNAm data was derived. Epigenome-wide association studies (EWAS) were performed in two independently generated DNAm subgroups (nSet1 = 1,395, nSet2 = 362), relating adult DNAm from whole blood to birth weight. Meta-analysis yielded one genome-wide significant CpG site (p = 5.97x10−9), cg00966482. There was minimal evidence for attenuation of the effect sizes for the lead loci upon adjustment for numerous potential confounder variables (body mass index, educational attainment, and socioeconomic status). Associations between birth weight and epigenetic measures of biological age were also assessed. Associations between lower birth weight and higher Grim Age acceleration (p(FDR) = 3.6x10−3) and shorter DNAm-derived telomere length (p(FDR) = 1.7x10−3) are described, although results for three other epigenetic clocks were null. Our results provide support for an association between birth weight and DNAm both locally at one CpG site, and globally via biological ageing estimates.
KEYWORDS: DNA methylation, Generation Scotland, birth weight, depression, EWAS
Introduction
The Developmental Origins of Health and Disease theory (DOHaD) states that through developmental plasticity, the foetal experience can permanently influence adult health [1]. The theory’s main proponent, David Barker, originally relied on birth weight as an index of foetal nutrition – an assumption that has been contested by the awareness that multiple factors can influence birth weight [2]. Maternal stress, illness, and socioeconomic status [3–5] are among modifiable influences over offspring birth weight. In addition, foetal genetics and maternal genetics both influence birthweight, the latter acting through the intrauterine environment [6,7]. Thus, birth weight can be seen as an index of the general foetal experience.
There is strong, well-replicated evidence for an association between birth weight and adult health. There is particularly consistent evidence for associations between low birth weight and poor cardiovascular outcomes, such as heart disease, type II diabetes, stroke, and hypertension [8–11], as well as poorer cognitive ability and a raised risk for mood disorders [12,13]. Birth weight is clinically conceptualized as ‘low’ below 2.5 kg [14], however it can also be analysed on a continuous scale. These associations are found after accounting for adult lifestyle factors, such as smoking and body mass index (BMI), indicating a residual association between birth weight and adult health outcomes.
Prenatal factors affect the foetus in its highly plastic state, giving rise to birth weight variability, and also to developmental changes which permanently affect the function and health of organs and systems [1]. Given the genetic and environmental contributions to birth weight variability, epigenetic modifications such as DNA methylation (DNAm) might provide insights into the pathways and mechanisms through which these associations with health become manifest. DNAm is typically characterized by the addition of a methyl group to cytosine nucleotides in the context of cytosine-guanine (CpG) dinucleotides. DNAm changes are linked to the regulation of gene expression, providing a possible mechanism through which environmental influences may have lasting biological effects [15]. Therefore, DNAm is one putative mechanism through which developmental experience may relate to adult health.
Birth weight associations with DNAm have been previously described in neonatal blood [16–18] and during childhood [19], seeming to diminish into adolescence and beyond [16,18]. The established association between differential DNAm and birth weight in these highly plastic early years raises the possibility of a direct effect of transcriptional up- or down-regulation on the development of organs and tissues [20]. This is one way in which DNAm may mediate the association between birth weight and adult health. It is possible, however, that examining only differential DNAm patterns which persist from birth into the later years ignores the full adult epigenome which, despite having changed since birth, may provide information on a relationship between birth weight and downstream health outcomes nevertheless. Many of the health phenotypes associated with birthweight present in adulthood, so the question remains whether we can see biological marks of these associations laid down in the methylome. It has, for instance, been demonstrated that prenatal famine exposure is associated with differential methylation in late adulthood [21]. It is therefore plausible that other prenatal factors may continue to influence DNAm into adulthood. While none of the longitudinal DNAm analysis studies have yet identified any epigenome-wide significant evidence of persistent CpG methylation in adulthood, there are some associations which do have p < 1x10−5 [18] but do not withstand Bonferroni correction for multiple testing. This may be tentatively interpreted as evidence that the adult epigenome holds information relating to birth weight, so a full, unbiased scan of the adult methylome seems a logical step. We would hope for this agnostic approach to confirm any genuine DNAm relationships in loci identified in other samples at birth, as well as revealing any new sites which might provide information on biological pathways through which the birth weight/health relationship is operating.
In addition to the stand-alone importance of discovering DNAm associations with birth weight in adults, recent work has revealed the utility of so-called ‘epigenetic clocks’ in demonstrating elements of vulnerability to disease and mortality [22]. These clocks attempt to calculate an individual’s biological age, and the residual of biological age regressed on chronological age (i.e. age acceleration) can be used to indicate health vulnerabilities. The earliest versions of epigenetic clocks, such as the pan-tissue Horvath clock [23] and the leukocyte-based Hannum clock [24], were trained simply on chronological age. The second generation of these clocks added clinical information on top of chronological age, to improve the biological relevance of the measure. The PhenoAge clock is of this second generation, it is based on clinical biomarkers known to associate with mortality [25], as is GrimAge (a predictor combining DNAm proxies for smoking and seven plasma proteins and trained on time-to-death [26]). Finally, a DNAm-derived estimate of telomere length outperforms phenotypic measures of telomere length [27], with validated utility in predicting lifespan and other aspects of health [27,28]. DNAm associations with birthweight, together with epigenetic clock analyses, have the potential to describe the known associations between birthweight and many aspects of whole-body health in adulthood.
Here, we report an Epigenome-Wide Association Study (EWAS) of birthweight using whole blood DNA derived in an adult sample, and associations between birth weight and five epigenetic clocks. While previous work has attempted to find persistent DNAm relationships to birth weight from infancy to adulthood [16,18], none have performed an agnostic, genome-wide study of DNAm in adults. We, therefore, hypothesize that while birth weight associated DNAm patterns may change over time, differences will exist in adulthood.
Materials and methods
This study was performed on a subgroup of the Generation Scotland (GS) cohort whose birthweight data were collected at parturition in historical cohort studies. Data linkage strategies were employed to ascertain birthweight in grams from those historical cohorts. A subgroup of these individuals for whom DNA methylation data were processed formed the sample for EWAS and epigenetic clock analyses (Figure 1).
Figure 1.
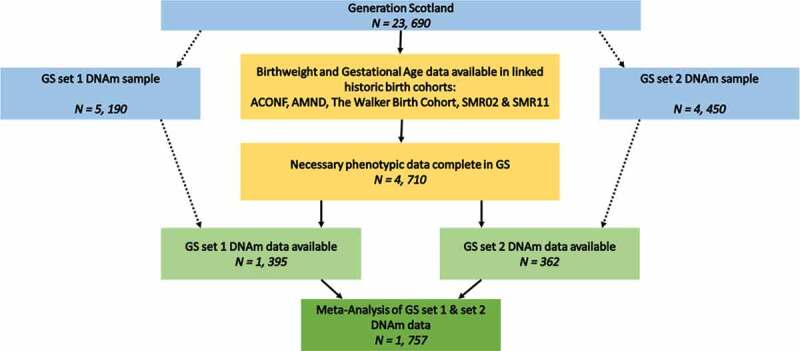
Inclusion flow diagram detailing the selection of samples for the current study
Generation Scotland and other cohorts
Generation Scotland (GS) is a Scottish family-based cohort n = 23,690 [29]. Data were collected from participants between 2006 and 2011. GS is a deeply-phenotyped cohort, allowing examination of many aspects of adult health, alongside genomic and biometric measures. In addition, 98% of GS participants gave informed consent for data linkage to routinely collected health data and to information from other Scottish population cohort studies, both current and historical. These include several with neonatal and maternity information: the Aberdeen Children of the 1950s [30]; the Aberdeen Maternity and Neonatal Databank [31]; the Walker Birth Cohort [32]; and the Scottish Morbidity Records ([33], SMR02 – the Maternity Inpatient and Day Case record, and SMR11 – the Neonatal Inpatient dataset). Birth weight in grams, alongside gestational age at birth and twin information, was collated from these sources and linked to adult GS records for 4,710 participants (Supplementary File 1, Figure 1). Birth weight data derived from health records taken at birth has been shown to improve slightly on self-reported birth weight, which is often used in population cohorts [34].
Statistical analyses
All analyses were conducted in R version 3.5.1 [35].
To control for the known effects of gestational age and sex on birthweight [36], we considered the scaled residuals (mean=0, SD=1) from a regression model in place of raw birth weight throughout:
These are referred to as ‘birth weight residuals’ hereafter.
Epigenome-wide association study
Peripheral whole blood genome-wide DNA methylation was profiled in Generation Scotland in two different sets of ~5,000 samples. First, data were generated for 5,190 individuals, using the Illumina HumanMethylationEPIC BeadChip (Illumina Inc., San Diego, CA). Quality control and normalization were carried out as described elsewhere ([37,38]; Supplementary File 2). Birth weight and gestational age information were available for n = 1,395 of this methylation set (Figure 1), which will hereafter be referred to as ‘set 1’.
The second set of DNAm data used a near identical protocol (Supplementary File 2), creating an independent set of methylation data for an additional 4,450 GS participants. In this replication sample, a further 362 participants with both birth weight and gestational age information were used, hereafter called 'set 2'.
The birth weight residuals described above were used in the EWAS model, which was run using the ‘limma’ package in R (empirical Bayes moderated t-statistics). The set 1 EWAS model used CpGs corrected for relatedness (Supplementary File 2), as the first batch of DNAm data were collected on related individuals:
CpG ~ birth weight residuals + age + sex + smoking [ever/never] + smoking pack years + 20 methylation PCs
The 20 methylation PCs were included to try and eliminate unmeasured confounders in the DNAm data. Additional covariates (estimated white blood cell proportions – CD4T, CD8T, Granulocytes, BCells, Natural Killer cells – and methylation batch), which were regressed out during the relatedness pre-correction for the set 1 dataset, were also included in the set 2 dataset of unrelated individuals.
EWAS findings are considered epigenome-wide significant if p < 3.6x10−8 [39].
Meta-analysis of set 1 and set 2 samples
An inverse variance-weighted meta-analysis of the set 1 and set 2 EWASs was performed using the METAL software package. Summary statistics from the set 1, set 2, and meta-analysis EWASs are available at https://doi.org/10.7488/ds/2876.
Subsample analysis excluding preterm births
To account for the possible differences between preterm- and term-born infants, we re-ran the analyses detailed above on a subsample excluding those with gestational age <37 weeks. This is in line with the World Health Organization’s guidelines on preterm birth which states that birth before 37 completed weeks of gestation should be defined as preterm [40]. The total subsample had nSet1 = 1,346, nSet2 = 351, resulting in a total nMeta = 1,697. By contrast, the full sample discussed in this report comprises both preterm and term born individuals.
Sensitivity analysis
As a sensitivity analysis, the EWAS meta-analysis was re-examined, fitting additional covariates individually, and together in a fully-adjusted model. Covariates were selected to account for additional lifestyle factors which may associate with DNAm or birth weight. These variables could represent confounding or mediating factors on the relationship between birthweight and adult DNAm. One of these covariates was socioeconomic status as ranked by the postcode-derived Scottish Index of Multiple Deprivation (SIMD). Socioeconomic status is positively associated with birth weight [41], and has been associated with DNAm variability [42]. BMI was included as a covariate in the sensitivity analyses, as is has been shown to associate with birth weight [43], and with DNAm [44]. The number of years of education was also included as educational attainment associates with DNAm in adulthood (most likely via its links to smoking) [45,46], and birth weight has been associated with educational attainment [47]. For detail on acquisition of covariate data see Supplementary File 1. Correction for smoking and 20PCs were already included in the EWAS model. Set 1 DNAm data were pre-corrected for batch, cell counts, and relatedness. The fully-adjusted model was:
Epigenetic clock analyses
Detail on the estimation of the five epigenetic clocks used have previously been reported elsewhere [22]. Briefly, penalized regression models were used to identify subsets of CpG sites that predict chronological age [23,24], ’phenotypic age’ [25], telomere length [27], and survival [26]. The clocks either trained predictors directly on the outcome (e.g., chronological age or telomere length), or via surrogate markers (e.g., protein levels and biomarkers that are known to associated with biological/health processes). The Horvath clock uses DNAm from 51 tissues and cell types to yield a pan-tissue predictor of chronological age [23]; the Hannum clock also predicts chronological age, but using blood-based DNAm [24]. The more recently developed PhenoAge incorporates blood-based DNAm proxies for clinical biomarkers found to associate with mortality risk, inflammatory and blood-based markers, as well as chronological age, in a ‘phenotypic age’ predictor that associates with healthspan and lifespan [25]. GrimAge takes a similar approach, using blood-based DNAm estimates of seven plasma proteins, as well as smoking pack years and chronological age to predict time to death [26]. Finally, DNAmTL is a blood-based DNAm estimator of telomere length (a measure of cellular ageing), found to outperform measured telomere length in prediction of mortality [27]. The residuals from regressions of these predictors on chronological age gives an index of biological age acceleration. Here, we used linear regression to examine the association between birth weight (outcome) and the five sets of age acceleration residuals, with covariate adjustment for age, sex, methylation ‘set’, and estimated white cell proportions. Correction for multiple testing was carried out using false discovery rate p < 0.05 for epigenetic clock analyses.
Ethics approval and consent to participate
All components of GS received ethical approval from the NHS Tayside Committee on Medical Research Ethics (REC Reference Number: 05/S1401/89). GS has also been granted Research Tissue Bank status by the Tayside Committee on Medical Research Ethics (REC Reference Number: 10/S1402/20), providing generic ethical approval for a wide range of uses within medical research.
Results
Population characteristics
There were 1,757 Generation Scotland (GS) participants with both birth weight and DNAm information available (Table 1). The set 1 population was 59.2% female, with a mean birth weight of 3,377 g (SD = 518 g), and gestational age of 40 weeks (SD = 1.8). The set 2 population was 55.8% female, with a mean birth weight of 3,421 g (SD = 535.4 g), and mean gestational age of 39.7 weeks (SD = 1.5). The minimum birth weight in the sample was 907 g, the maximum was 5,090 g. Using the clinical cut-off of 2,500 g, 4.8% (n = 84) of the sample were of clinically ‘low’ birth weight [14]. This means the data describe DNAm relationships to birth weight across the full spectrum.
Table 1.
Population characteristics of the Set 1 and Set 2 EWAS samples
| Phenotype Sample | EWAS Sample | Replication Sample | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Age (years) | 4, 710 | 29.5 | 10.9 | 1, 395 | 37.1 | 14.7 | 362 | 25.8 | 5.2 |
| Birthweight (g) | 4, 710 | 3, 399 | 516 | 1, 395 | 3, 377 | 518 | 362 | 3, 421 | 535.4 |
| Gestation (weeks) | 4, 710 | 39.8 | 1.7 | 1, 395 | 40 | 1.8 | 362 | 39.7 | 1.5 |
| BMI (kg/m2) | 4, 385 | 25.2 | 5.2 | 1, 387 | 26.1 | 5.4 | 360 | 24.9 | 4.8 |
| Education* | 4, 411 | 5 | 4–6 | 1, 317 | 5 | 4–6 | 350 | 5 | 4–6 |
| N | % | N | % | N | % | ||||
| Sex – Male | 2, 025 | 43 | 569 | 40.8 | 160 | 44.2 | |||
| Female | 2, 688 | 57 | 826 | 59.2 | 202 | 55.8 | |||
| Socieconomic Status** | (4, 389) | (1, 310) | (337) | ||||||
| Quintile 1 (most deprived) | 621 | 14.2 | 207 | 15.8 | 65 | 19.3 | |||
| Quintile 2 | 717 | 16.3 | 210 | 16 | 57 | 16.9 | |||
| Quintile 3 | 715 | 16.3 | 183 | 14 | 62 | 18.4 | |||
| Quintile 4 | 1, 064 | 24.2 | 301 | 23 | 72 | 21.4 | |||
| Quintile 5 (least deprived) | 1, 272 | 29 | 409 | 31.2 | 81 | 24 | |||
| Smoking | (4, 525) | (1, 344) | (357) | ||||||
| Current Smoker | 918 | 20.3 | 257 | 19.1 | 88 | 24.6 | |||
| Ex-Smoker (<12 months) | 237 | 5.2 | 56 | 4.2 | 26 | 7.3 | |||
| Ex-Smoker (>12 months) | 662 | 14.6 | 273 | 20.3 | 48 | 13.4 | |||
| Never Smoker | 2, 708 | 59.8 | 758 | 56.4 | 195 | 54.6 | |||
* Median and Interquartile range reported. Education was coded as an ordinal variable: 0 = 0 yrs, 1 = 1–4 yrs, 2 = 5–9 yrs, 3 = 10–11 yrs, 4 = 12–13 yrs, 5 = 14–15 yrs, 6 = 16–17 yrs, 7 = 18–19 yrs, 8 = 20–21 yrs, 9 = 22–23 yrs, 10 = ≥24 yrs.
**SIMD Quintile. SIMD is the Scottish Index of Multiple Deprivation, a postcode-derived index of socioeconomic status. The quintiles derived on the full Generation Scotland cohort ranged from 1 (most deprived) to 5 (least deprived).
Epigenome-wide association study of birthweight
The set 1 EWAS of birth weight revealed no CpGs significant at the genome-wide level (p < 3.6x10−8 [39];), although 19 CpGs had P < 1x10−5 (minimum p-value of 6.05 × 10−8 for cg00966482) (Figure 2(a); Supplementary Table 1). The 19 CpGs were largely uncorrelated, with the exception of three CpGs (located within CASZ1 – two within 200 base pairs of each other, with the third site around 11kb away – Supplementary Table 1) that had absolute r ≥ 0.6.
Figure 2.
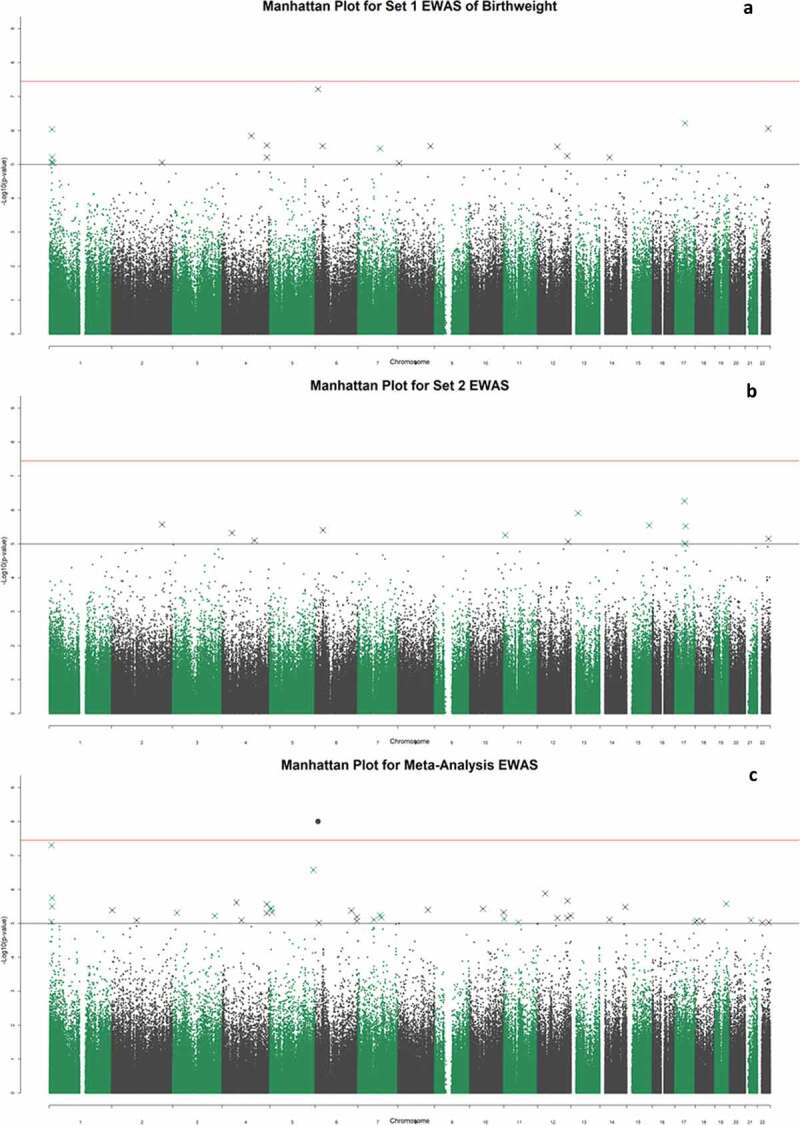
Manhattan plots for the set 1 epigenome-wide association study of birth weight (a); the set 2 sample EWAS (b); and the meta-analysis EWAS (c). The black and red lines represent the suggestive, and genome-wide significant p-value thresholds of P = 1x10−5 and 3.6x10−8, respectively
There were no genome-wide significant associations in the set 2 sample (n = 362) (Figure 2(b); Supplementary Table 2). Of the 19 CpGs that exceeded p < 1x10−5 significance threshold in the set 1 EWAS, two reached nominal significance (p < 0.05) in the set 2 analysis: cg00590817 (p = 0.0069), and cg00966482 (p = 0.031). The latter CpG was the most significantly associated site from the set 1 EWAS. There was moderate concordance between the effect sizes of the top 19 DNAm associations with birthweight in set 1, and the same CpGs in the set 2 analysis (r = 0.59) (Figure 3).
Figure 3.
Effect sizes for 18 of the top 19 CpG sites in the set 1 sample plotted against the effect sizes in the set 2 sample (cg04988918 did not pass quality control in the set 2 array). The point size is determined by the -log10 of the p-values for these hits in the set 2 analysis. The two points labelled in black are the two CpG sites which achieved nominal significance in the set 2 study, and the three highlighted in red are the three co-methylated CpG sites within the CASZ1 gene
Meta-analysis EWAS
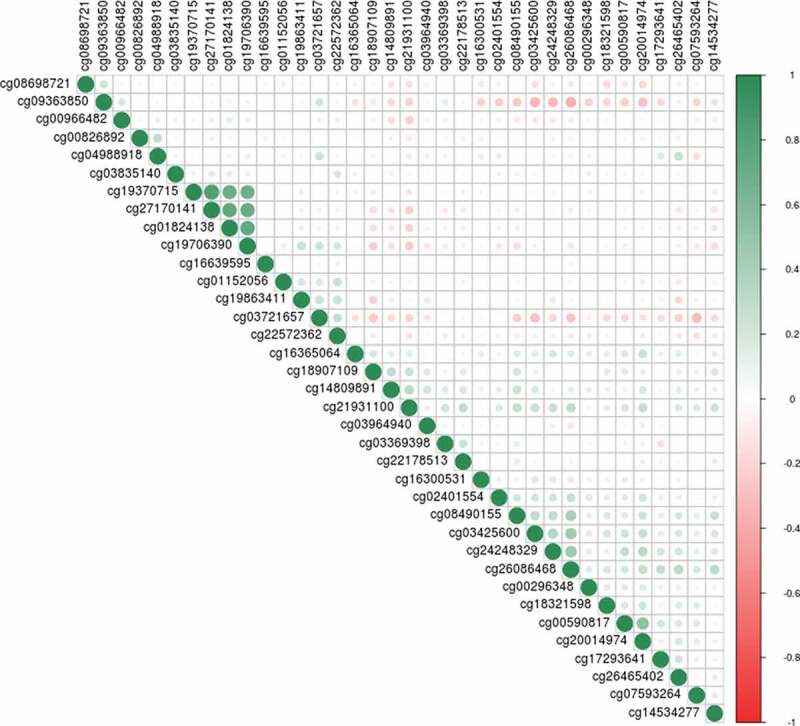
Meta-analysis of the set 1 and set 2 EWAS samples resulted in a genome-wide significant association between birthweight and DNAm at the CpG site cg00966482 mapping to the HERV-FRD and LOC221710/SMIM13 genes (β = 0.0206, SE = 0.0035, p = 5.97x10−9; Figure 2(c); Supplementary Table 3). There were 36 CpG sites with p < 1x10−5, including four CpGs located within the gene CASZ1. These four sites in CASZ1 were co-methylated, absolute r ≥ 0.92 (Figure 4)
Figure 4.

Correlation plot between methylation at the top 36 CpG sites from the meta-analysis EWAS. The shade and scale of the dots represent the magnitude and direction of the correlation between pairs of CpGs
Subsample analysis excluding preterm births
To address the view that preterm births may represent a distinct group, we ran the EWASs on a subgroup (nSet1 = 1,346, nSet2 = 351) excluding those with less than 37 complete weeks gestational age at birth (see Supplementary Table 4 for population characteristics). In the meta-analysis EWAS performed in this subgroup, cg00966482 remained significant, with a slightly greater effect (β = 0.0213; SE = 0.0037; p = 5.68x10−9). There were 36 CpG sites with p < 1x10−5, 23 of which appeared in the list of 36 lead sites in the main meta-analysis EWAS (Supplementary Table 5).
Adjusting the EWAS model for lifestyle factors
As a sensitivity analysis, we re-ran the EWAS pipeline, with corrections for BMI, education, and SIMD separately, and all three covariates together. This fully-adjusted model resulted in some changes to the effect sizes for the 36 sites with p < 1x10−5 in the basic meta-analysis output (mean attenuation of 2.2%, range 12.2% attenuation to 15.3% increase in effect size; Supplementary Table 6). There was, however, good concordance between the effect sizes for the top 36 CpG sites identified in the main model, and the same sites in this fully-adjusted sensitivity model (r = 0.998; Figure 5). Full summary statistics for the sensitivity analyses are available at https://doi.org/10.7488/ds/2876.
Figure 5.

Effect sizes for the main meta-analysis EWAS, and the fully-adjusted sensitivity analysis model. The hollow point labelled in black is cg00966482, in the HERV-FRD/SMIM13 gene which achieved epigenome-wide significance in the main model meta-analysis. The points in red are in the four CpG sites in the gene CASZ1 which had p < 1x10−5 in the meta-analysis EWAS
Relationship of birth weight to epigenetic signatures of age and telomere length
The mean values for the five epigenetic clocks were: Horvath age 44.7 yrs (SD = 10.6); Hannum age 36.1 yrs (SD = 10.2); PhenoAge 32.8 yrs (SD = 12); GrimAge 45.7 yrs (SD = 14.1); DNAm telomere length 7.6 kilobase pairs (SD = 0.29). Linear regression models of birth weight residuals (outcome) against five signatures of epigenetic age acceleration (the scaled residuals from regression of the clock predictors on chronological age) revealed significant associations between higher birth weight and lower Grim Age acceleration (β = −0.083; SE = 0.026, p(FDR) = 3.6x10−3) and longer DNAm telomere length (β = 0.098; SE = 0.027, p(FDR) = 1.7x10−3; Table 2). Associations between birth weight and the other three measures of epigenetic age acceleration were non-significant (Table 2).
Table 2.
Outputs of linear regression models between birth weight residuals and five epigenetic signatures of accelerated biological ageing – Horvath (Intrinsic Epigenetic Age Acceleration; IEAA), Hannum, PhenoAge, GrimAge, and DNAmTL
| Clock | Standardised Beta | SE | P | P.adj |
|---|---|---|---|---|
| Horvath | 3.2x10−3 | 0.025 | 0.90 | 0.90 |
| Hannum | −6.2x10−3 | 0.027 | 0.82 | 0.90 |
| GrimAge | −0.083 | 0.026 | 1.4x10−3 | 3.6x10−3 |
| PhenoAge | −0.042 | 0.027 | 0.12 | 0.20 |
| DNAmTL | 0.098 | 0.027 | 3.3x10−4 | 1.7x10−3 |
Discussion
We identified one epigenome-wide significant association between birth weight and blood-based DNA methylation in adulthood, and significant associations between birth weight and two epigenetic age measures.
In the EWAS meta-analysis we observed a genome-wide significant association between higher birthweight and higher methylation levels at cg00966482 (mapping to HERV-FRD and LOC221710/SMIM13). HERV-FRD encodes syncitin-2, a protein involved in placental embedding, whereas LOC221710/SMIM13 is a gene with unknown functions [48]. This site was not identified in a previous EWAS meta-analysis of birthweight in newborns [18]. Of the 36 CpG sites with P < 1x10−5 in the meta-analysed EWAS, 20 were located within known genes. Eighteen of the 20 sites were within regions with evidence for transcription factor binding, open chromatin, or DNase hypersensitivity. These may be attractive candidates for further investigation of the relationship between birth weight, DNA methylation, and transcriptional activity (Supplementary Table 3). Several of these genes contain SNPs that have genome-wide significant associations (GWAS P < 5x10−8) with cardiovascular, psychiatric, and developmental pathways (Supplementary Table 7). Four of the 36 CpG sites exceeding p < 1x10−5 identified in the meta-analysis EWAS were highly correlated (min r = 0.92, Figure 4). Higher birthweight was associated with higher methylation levels at these sites, which were located within CASZ1, a gene encoding the zinc finger protein castor homolog 1, a transcriptional activator involved in vascular morphogenesis [49]. A differentially methylated region in the CASZ1 gene was recently identified in placental tissue between infants born small vs. large for gestational age [17]. Additionally, a recent epigenome-wide meta-analysis of gestational age in children and infants reports several significant sites within CASZ1 [50]. In these studies CASZ1 methylation was positively associated with both size and gestational age. The findings presented here, although non-significant (p ≥ 4.37x10−7), suggest that future studies might wish to focus on studying this relationship between birth weight and CASZ1 methylation in further detail. Genetic variants in CASZ1 have previously been implicated by GWAS in various aspects of cardiovascular health (Supplementary Table 7). These have included studies in multi-ethnic populations on blood pressure [51,52], and on other cardiovascular health issues such as atrial fibrillation [53] and stroke [54].
Sensitivity analyses that excluded preterm births or adjusted individually and collectively for a series of possible confounder/mediator lifestyle variables resulted in only minor changes to the effect sizes of the primary model. However, the covariate adjustments did result in increased p-values for all CpG sites, suggesting that replication in larger samples and further work to investigate the possibility of mediation by lifestyle factors should be considered. The analysis excluding preterm births identified the same epigenome-wide significant site (cg00966482, HERV-FRD/SMIM13 locus) as the primary analysis. However, there was an increase in the effect size, resulting in a lower p-value for this CpG (p = 5.68x10−9). There were some differences between the CpGs identified at a suggestive epigenome-wide threshold of p < 1x10−5 (36 in both analyses). This may indicate differences in the biological pathways associated with birth weight in adults, according to the length of gestation, and would be an interesting topic for further study.
Significant associations are demonstrated between birth weight and epigenetic predictors of ageing, mortality, and cellular senescence. Higher birth weight is associated with lower GrimAge acceleration [26] and longer telomeres as estimated by DNAm data [27]. The effect sizes are relatively small; for instance, with astandardised Beta value of −0.083 between GrimAge acceleration and birth weight. These results, however modest, are supportive of the known associations between low birth weight and a broad range of adverse health outcomes. We did not find significant associations between birth weight and Horvath Age [23], Hannum Age [24], or PhenoAge [25] here. We have previously found that GrimAge outperforms the other clocks in the prediction of self-reported disease burden and clinical traits in GS [22], and the creators of GrimAge reported its superiority in predicting onset of a host of health conditions [26]. The DNAm estimate of telomere length performs a different function than the other epigenetic age estimators, as it specifically predicts telomere length – a measure of cellular age. The DNAmTL result suggests a relationship between lower birth weight and accelerated ageing at a cellular level, while the GrimAge result demonstrates this at a whole-body level. It is of interest that an association exists between birth weight and some, but not all, of the epigenetic age predictors tested. It is possible that some of the specific variables included in the different clocks might underlie this observation. For instance, GrimAge contains a methylation-based proxy for leptin [26], a hormone involved in regulating food intake and energy expenditure, which is likely to be linked to adult weight. In turn, adult weight associates with birth weight [43]. In contrast, PhenoAge is calculated based on methylation proxies primarily of inflammatory processes, which may not associate with birth weight as strongly; meanwhile the Hannum and Horvath clocks are more broadly trained on age, and so may not be picking up the same level of detail on susceptibility to ill-health. Maternal GrimAge acceleration during pregnancy has recently been associated with shorter gestation and, independently, lower birthweight of the offspring in a small sample of pregnant women [55]. This indicates the potential utility of GrimAge in describing both processes relevant to foetal development, and the later-life consequences of the foetal experience.
Strengths and limitations
This study exploited rarely available data linkage capacity to acquire neonatal information from birth medical records. This was then linked to DNAm in a large adult sample. There are, however, some limitations inherent to the design of this study. Longitudinal data would allow analysis of the persistence of DNAm signatures across time, although this has been investigated in previous studies [18]. A further limitation to this study is the lack of maternal characteristics available for inclusion in our models. As GS is a cross-sectional study, and birth information was derived from multiple historical sources, this information was not consistently available for our sample.
DNAm data were derived from whole blood taken during adulthood, so our findings might not generalize to other tissue types. However, there are advantages to interrogating blood-based methylation levels: first, it is the only tissue type that is readily available in large epidemiological cohorts; and second, it is systemic and tracks multiple biological processes including biomarkers of inflammation, cardiovascular disease, cardiometabolic disease, all of which are relevant processes related to birthweight.
Conclusions
This study presents the first epigenome wide association study of birth weight on DNA methylation in adulthood, alongside associations between birth weight and both epigenetic age acceleration and telomere length. The Developmental Origins of Health and Disease theory predicts that birth weight, which may be seen as a marker of the intrauterine environment, is associated with outcomes in many domains of adult health, and it has been proposed that these associations may be reflected in the epigenome. We found evidence to support this at both the level of single CpG site methylation, and at the level of broader mortality and health-related DNAm phenotypes.
Supplementary Material
Acknowledgments
The authors thank all individuals and project team members who have contributed to both GS and to the ‘STRADL: Stratifying Resilience and Depression Longitudinally’ follow-up study.
Funding Statement
GS received core support from the Chief Scientist Office of the Scottish Government Health Directorates (CZD/16/6) and the Scottish Funding Council (HR03006). Genotyping and DNA methylation profiling of the GS samples was carried out by the Genetics Core Laboratory at the Wellcome Trust Clinical Research Facility, Edinburgh, Scotland and was funded by the Medical Research Council UK and the Wellcome Trust (Wellcome Trust Strategic Award “STratifying Resilience And Depression Longitudinally” ((STRADL) Reference 104036/Z/14/Z). Data linkage was supported by MRC Mental Health Pathfinder Award (Reference MC_PC_17209). This work was conducted in the Centre for Cognitive Ageing and Cognitive Epidemiology, which is supported by the Medical Research Council and Biotechnology and Biological Sciences Research Council (MR/K026992/1). RAM and RFH are supported by funding from the Wellcome Trust 4-year PhD in Translational Neuroscience – training the next generation of basic neuroscientists to embrace clinical research [108890/Z/15/Z].
Author contributions
Conception and design: RAM, JH, AMM, and REM. Data analysis: RAM and DLM. Interpretation: RAM, DLM, JH, AMM, REM. Drafting the article: RAM. Revision of the article: all authors.
Availability of data and materials
According to the terms of consent for Generation Scotland participants, access to data must be reviewed by the Generation Scotland Access Committee. Applications should be made to access@generationscotland.org. Summary statistics for the EWAS models will be made available on the University of Edinburgh DataShare facility upon acceptance https://doi.org/10.7488/ds/2876.
Disclosure statement
AMM has received research support from Eli Lilly, Janssen and the Sackler Trust. AMM has also received speaker fees from Janssen and Illumina. The other authors declare that they have no competing interests.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(6Suppl):588s–95s. [DOI] [PubMed] [Google Scholar]
- [2].Paneth N, Susser M. Early origin of coronary heart disease (the “Barker hypothesis”). Br Med J. 1995;310(6977):411–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rondo PHC, Ferreira RF, Nogueira F, et al. Maternal psychological stress and distress as predictors of low birth weight, prematurity and intrauterine growth retardation. Eur J Clin Nutr. 2003;57(2):266–272. [DOI] [PubMed] [Google Scholar]
- [4].Grote NK, Bridge JA, Gavin AR, et al. Meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rosen D, Seng JS, Tolman RM, et al. Intimate partner violence, depression, and posttraumatic stress disorder as additional predictors of low birth weight infants among low-income mothers. J Interpers Violence. 2007;22(10):1305–1314. [DOI] [PubMed] [Google Scholar]
- [6].Horikoshi M, Beaumont RN, Day FR, et al. Genome-wide associations for birth weight and correlations with adult disease. Nature. 2016;538(7624):248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Warrington NM, Beaumont RN, Horikoshi M, et al. Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors. Nat Genet. 2019;51(5):804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Huxley R, Owen CG, Whincup PH, et al. Is birth weight a risk factor for ischemic heart disease in later life? Am J Clin Nutr. 2007;85(5):1244–1250. [DOI] [PubMed] [Google Scholar]
- [9].Zanetti D, Tikkanen E, Gustafsson S, et al. Birthweight, type 2 diabetes mellitus, and cardiovascular disease addressing the barker hypothesis with mendelian randomization. Circ Genomic Precis Med. 2018;11(6). DOI: 10.1161/CIRCGEN.117.002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mu M, Wang SF, Sheng J, et al. Birth weight and subsequent blood pressure: A meta-analysis. Arch Cardiovasc Dis. 2012;105(2):99–113. [DOI] [PubMed] [Google Scholar]
- [11].Rich-Edwards JW, Kleinman K, Michels KB, et al. Longitudinal study of birth weight and adult body mass index in predicting risk of coronary heart disease and stroke in women. Br Med J. 2005;330(7500):1115–8A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shenkin SD, Starr JM, Deary IJ. Birth weight and cognitive ability in childhood: A systematic review. Psychol Bull. 2004;130(6):989–1013. [DOI] [PubMed] [Google Scholar]
- [13].Wojcik W, Lee W, Colman I, et al. Foetal origins of depression? A systematic review and meta-analysis of low birth weight and later depression. Psychol Med. 2013;43(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kiserud T, Piaggio G, Carroli G, et al. The world health organization fetal growth charts: a multinational longitudinal study of ultrasound biometric measurements and estimated fetal weight. PLoS Med. 2017;14(1):1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33:245–254. [DOI] [PubMed] [Google Scholar]
- [16].Simpkin AJ, Suderman M, Gaunt TR, et al. Longitudinal analysis of DNA methylation associated with birth weight and gestational age. Hum Mol Genet. 2015;24(13):3752–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen P-Y, Chu A, Liao -W-W, et al. Prenatal growth patterns and birthweight are associated with differential DNA methylation and gene expression of cardiometabolic risk genes in human placentas: a discovery-based approach. Reprod Sci. 2018;25(4):523–539. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Küpers LK, Monnereau C, Sharp GC, et al. Meta-analysis of epigenome-wide association studies in neonates reveals widespread differential DNA methylation associated with birthweight. Nat Commun. 2019;10(1):1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Agha G, Hajj H, Rifas-Shiman SL, et al. Birth weight-for-gestational age is associated with DNA methylation at birth and in childhood. Clin Epigenetics. 2016;8. DOI: 10.1186/s13148-016-0285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Felix JF, Cecil CAM. Population DNA methylation studies in the developmental origins of health and disease (DOHaD) framework. J Dev Orig Health Dis. 2019;10(3):306–313. [DOI] [PubMed] [Google Scholar]
- [21].Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Nat Acad Sci. 2008;105(44):17046–17049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hillary RF, Stevenson AJ, McCartney DL, et al. Epigenetic clocks predict prevalence and incidence of leading causes of death and disease burden. bioRxiv. 2020. 2020.01.31.928648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49(2):359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10(4):573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11(2):303–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lu AT, Seeboth A, Tsai P-C, et al. DNA methylation-based estimator of telomere length. Aging (Albany NY). 2019;11(16):5895–5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang Q, Zhan Y, Pedersen NL, et al. Telomere length and all-cause mortality: a meta-analysis. Ageing Res Rev. 2018;48:11–20. [DOI] [PubMed] [Google Scholar]
- [29].Smith BH, Campbell A, Linksted P, et al. Cohort profile: generation Scotland: Scottish family health study (GS:SFHS). The study, its participants and their potential for genetic research on health and illness. Int J Epidemiol. 2013;42(3):689–700. [DOI] [PubMed] [Google Scholar]
- [30].Leon DA, Lawlor DA, Clark H, et al. Cohort profile: the Aberdeen children of the 1950s study. Int J Epidemiol. 2006;35(3):549–552. [DOI] [PubMed] [Google Scholar]
- [31].Ayorinde AA, Wilde K, Lemon J, et al. Data resource profile: the aberdeen maternity and neonatal databank (AMND). Int J Epidemiol. 2016;45(2):389–394. [DOI] [PubMed] [Google Scholar]
- [32].Libby G, Smith A, F McEwan N, ChienP, GreeneS, Forsyth S, et al. The Walker Project: A longitudinal study of 48 000 children born 1952-1966 (aged 36-50 years in 2002) and their families. Paediatric and perinatal epidemiology. 2004;18(8):302–12 [DOI] [PubMed] [Google Scholar]
- [33].Ndc.scot.nhs.uk . ISD Scotland | information services division. 2018. [cited November22]. Available from: http://www.ndc.scot.nhs.uk/Dictionary-A-Z/index.asp.
- [34].Cairns BJ, Liu B, Clennell S, et al. Lifetime body size and reproductive factors: comparisons of data recorded prospectively with self reports in middle age. BMC Med Res Methodol. 2011;11(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Team RC . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. Available from: https://www.R-project.org/ [Google Scholar]
- [36].Norris T, Seaton SE, Manktelow BN, et al. Updated birth weight centiles for England and Wales. Arch Dis Childhood-Fetal Neonatal Ed. 2018;103(6):F577–F82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McCartney DL, Stevenson AJ, Walker RM, et al. Investigating the relationship between DNA methylation age acceleration and risk factors for Alzheimer’s disease. Alzheimer’s Dementia Diagnosis Assess Dis Monit. 2018;10:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bermingham ML, Walker RM, Marioni RE, et al. Identification of novel differentially methylated sites with potential as clinical predictors of impaired respiratory function and COPD. bioRxiv. 2018;473629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Saffari A, Silver MJ, Zavattari P, et al. Estimation of a significance threshold for epigenome-wide association studies. Genet Epidemiol. 2018;42(1):20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].World Health Organization . Preterm birth [Fact Sheet]. 2018.
- [41].Martinson ML, Reichman NE. Socioeconomic inequalities in low birth weight in the United States, the United Kingdom, Canada, and Australia. Am J Public Health. 2016;106(4):748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lam LL, Emberly E, Fraser HB, et al. Factors underlying variable DNA methylation in a human community cohort. Proc Nat Acad Sci. 2012;109(Supplement 2):17253–17260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Curhan GC, Chertow GM, Willett WC, et al. Birth weight and adult hypertension and obesity in women. Circulation. 1996;94(6):1310–1315. [DOI] [PubMed] [Google Scholar]
- [44].Dick KJ, Nelson CP, Tsaprouni L, et al. DNA methylation and body-mass index: a genome-wide analysis. Lancet. 2014;383(9933):1990–1998. [DOI] [PubMed] [Google Scholar]
- [45].Karlsson Linnér R, Marioni RE, Rietveld CA, et al. An epigenome-wide association study meta-analysis of educational attainment. Mol Psychiatry. 2017;22(12):1680–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].McCartney DL, Hillary RF, Stevenson AJ, et al. Epigenetic prediction of complex traits and death. Genome Biol. 2018;19(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Richards M, Hardy R, Kuh D, et al. Birthweight, postnatal growth and cognitive function in a national UK birth cohort. Int J Epidemiol. 2002;31(2):342–348. [PubMed] [Google Scholar]
- [48].Chen C-P, Chen L-F, Yang S-R, et al. Functional characterization of the human placental fusogenic membrane protein syncytin 21. Biol Reprod. 2008;79(5):815–823. [DOI] [PubMed] [Google Scholar]
- [49].Charpentier Marta S, Christine Kathleen S, Amin Nirav M, et al. CASZ1 promotes vascular assembly and morphogenesis through the direct regulation of an EGFL7/RhoA-mediated pathway. Dev Cell. 2013;25(2):132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Merid SK, Novoloaca A, Sharp GC, et al. Epigenome-wide meta-analysis of blood DNA methylation in newborns and children identifies numerous loci related to gestational age. Genome Med. 2020;12(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kato N, Takeuchi F, Tabara Y, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet. 2011;43(6):530–U57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ehret GB, Ferreira T, Chasman DI, et al. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat Genet. 2016;48(10):1171–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Roselli C, Chaffin MD, Weng LC, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018;50(9):1225-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Malik R, Chauhan G, Traylor M, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50(4):524-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ross KM, Carroll JE, Horvath S, et al. Epigenetic age and pregnancy outcomes: grimAge acceleration is associated with shorter gestational length and lower birthweight. Clin Epigenetics. 2020;12(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
According to the terms of consent for Generation Scotland participants, access to data must be reviewed by the Generation Scotland Access Committee. Applications should be made to access@generationscotland.org. Summary statistics for the EWAS models will be made available on the University of Edinburgh DataShare facility upon acceptance https://doi.org/10.7488/ds/2876.


