Abstract
INSULIN-DEPENDENT diabetes mellitus (IDDM) in non-obese diabetic (NOD) mice results from the T-lymphocyte-mediated destruction of the insulin-producing pancreatic β-cells and serves as a model for human IDDM1. Whereas a number of autoantibodies are associated with IDDM2, it is unclear when and to what β-cell antigens pathogenic T cells become activated during the disease process. We report here that a T-helper-1 (Thl) response to glutamate decarboxylase develops in NOD mice at the same time as the onset of insulitis. This response is initially limited to a confined region of glutamate decarboxylase, but later spreads intramolecularly to additional determinants. Subsequently, T-cell reactivity arises to other β-cell antigens, consistent with intermolecular diversification of the response. Prevention of the spontaneous anti-glutamate decarboxylase response, by tolerization of glutamate decarboxylase-reactive T cells, blocks the development of T-cell autoimmunity to other β-cell antigens, as well as insulitis and diabetes. Our data suggest that (1) glutamate decarboxylase is a key target antigen in the induction of murine IDDM; (2) autoimmunity to glutamate decarboxylase triggers T-cell responses to other β-cell antigens, and (3) spontaneous autoimmune disease can be prevented by tolerization to the initiating target antigen.
We tested NOD mice from birth to 28 weeks of age for T-cell reactivity to β-cell antigens that are targets of IDDM-associated autoantibodies. These included one of the two forms of glutamate decarboxylase3–5 (GAD65, an early target of autoantibodies6,9), carboxypeptidase H10, insulin2 and the immunodominant determinant of heat-shock protein (Hsp65) (refs 11, 12).
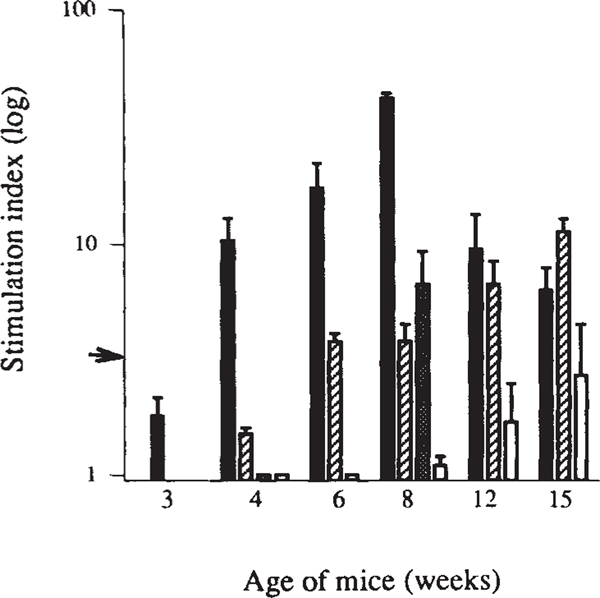
Proliferative T-cell responses to these antigens developed spontaneously in a sequential order. First, a response to GAD arose at 4 weeks of age (Fig. 1), concurrent with the onset of insulitis. This reactivity increased during the next four weeks and then declined to background levels by week 16. At 6 weeks of age, responses to heat-shock protein appeared, increased until week 15 and then diminished (Fig. 1; also ref. 12). Similarly, whereas no response was detected to carboxypeptidase H at 4 weeks old, there was a strong anti-carboxypeptidase H response by week 8. In some mice, a weak response to insulin became detectable at 12–15 weeks old.
FIG. 1.
Proliferative T-cell responses to β-cell antigens develop spontaneously in NOD mice in a defined chronological order. Antigen-induced blastogenesis was measured in spleen cells from newborn to 28-week-old female NOD mice (data from 3–15 weeks are shown). β-Cell antigens include GAD65 (black bars), Hsp65 peptide PALDSLTPANED12 (striped bars), carboxypeptidase H (grey bar) and insulin (white bars). Data are expressed as stimulation indices (SI) ± standard error of the mean (s.e.m.), calculated from 3–5 mice tested individually in 2 separate experiments for each time point. Arrow indicates SI = 3, the level of significance. Carboxypeptidase H responses were only tested at 4 and 8 weeks. None of the control antigens (hen egg-white lysozyme, human serum albumin, E. coli β-galactosidase or murine myelin basic protein) induced T-cell proliferation at any age. Also, none of the β-cell antigens or control antigens induced proliferation of T cells from age-matched control BALB/c or (NOD × BALB/c) F1 mice (data not shown).
METHODS. NOD (Taconic farms) and BALB/c mice (Jackson Laboratories) were housed under specific pathogen-free conditions. Spleen cells were tested directly ex vivo for proliferative recall responses. Cells were plated at 1 × 106 cells per well in 96-well microtitre plates in 200 μl HL-1 medium (Ventrex) containing 2 mM glutamine and 10 μg ml−1 antigen, or 7 μM peptide (the optimal concentrations for all time points tested) in triplicate. During the last 16 h of the 72 h culture period, 1 μCi [3H]thymidine was added per well. Incorporation of label was measured by liquid scintillation counting. Human GAD65 (ref. 25) and E. coli β-galactosidase were both purified from recombinant bacteria using a hexahistidine tag and metal-affinity chromatography26. Bovine carboxypeptidase H was a generous gift from L. Fricker; human insulin was purchased from Eli Lilly.
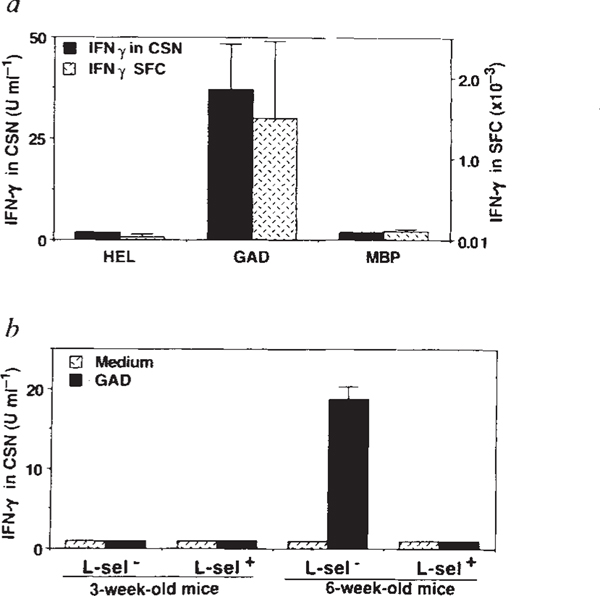
The spontaneous development of a proliferative T-cell response to GAD is consistent with, but does not prove, endogenous priming. We therefore tested GAD-reactive T cells for additional properties that distinguish activated/memory lymphocytes from resting/naive lymphocytes. First, we found that GAD-challenged freshly isolated T cells from 6–9-week-old NOD mice produce interferon-y (IFN-γ), which is secreted only by preactivated Thl lymphocytes13 (Fig. 2a). Second, whereas the frequency of T cells reactive to control antigens constituted ~1 in 105 cells in the spleen of 6–9-week-old NOD mice, the frequency of GAD-reactive T cells was almost two orders of magnitude higher, which is consistent with clonal expansion (Fig. 2a). Third, GAD reactivity resided within the L-selectin− fraction of CD4+ cells, a phenotype characteristic of activated lymphocytes14 (Fig. 2b). These findings provide independent evidence that a potentially pathogenic15 Thl-type T-cell population is spontaneously primed to GAD65 early in the development of NOD diabetes.
FIG. 2.
GAD-specific T cells in 6–9-week-old female NOD mice are primed Thl type CD4+ lymphocytes based on their production of IFN-γ (a and b), enhanced clonal size (a) and L-selectin− phenotype (b). a, Detection of IFN-γ by ELISA in culture supernatants (CSN) of spleen cells from 6–9-week-old mice 48 h after challenge with GAD or control antigens hen egg-white lysozyme (HEL) and murine myelin basic protein (MBP). High concentrations of IFN-γ were detected only in cultures containing GAD. IFN-γ production was measured by ELISA27 using IFN-γ-specific monoclonal antibodies R4–6A2 and XMG 1.2 (Pharmingen). T cells from age-matched BALB/c mice did not respond to GAD or to control antigens (data not shown). The frequency of antigen-specific IFN-γ-producing cells was determined by an ELISA spot technique28 using the complementary IFN-γ mAbs. Frequency of antigen-induced spot-forming cells (SFC) is shown. Values are the mean ±s.e.m. from 5 female NOD mice, tested in triplicate cultures, with and without antigen. Results are from a single experiment and are representative of 3 experiments. b, Detection of GAD-specific, IFN-γ-producing cells in the L-selectin (L-sel−) subpopulation of CD4+ cells. CD4+ cells were isolated from the spleens by panning on goat-anti-mouse immunoglobulin (Zymed) and on anti-CD8 (mAb 58.6–72)-coated plates. CD4+ cells were subfractionated using L-selectin-specific mAb MEL-14. CD4+ L-selectin+ and CD4+ L-selectin− fractions were seeded in triplicate at 2 × 105 cells per well with irradiated (3,000 rad) spleen cells of 3-week-old NOD mice (5 × 105 cells per well) as a source of antigen-presenting cells. GAD-induced IFN-γ production was measured after 48 h by ELISA.
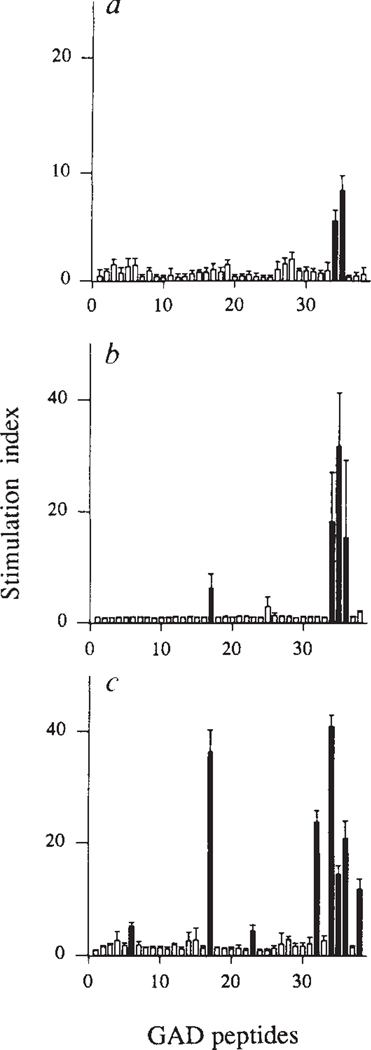
We mapped the fine specificity of the anti-GAD T-cell response using an overlapping set of peptides that span GAD65. The initial response, at 4 weeks, involved two adjacent peptides (amino acids 509–528 and 524–543, peptides 34 and 35, respectively; Fig. 3a). During the next 3 weeks, T-cell autoimmunity spread to several additional GAD determinants (including amino acids 246–266, peptide 17, which contains a region of sequence similarity with Coxsackie virus9; Fig. 3b, c). Subsequently, reactivity to GAD peptides declined (data not shown), paralleling the loss of response to the whole protein (Fig. 1).
FIG. 3.
Intramolecular spreading of autoimmunity within the GAD molecule. Spleen cells were tested from 4-week-old (a), 5-week-old (b) and 7-week-old (c) NOD mice for proliferative responses to GAD65 peptides. A set of 38 peptides, each 20–23 amino acids long, span the entire human GAD65 (ref. 25) molecule with overlaps of 5 amino acids. These peptides are numbered successively from the N terminus. Peptides that triggered stimulation indices >3 are indicated as black bars. These peptides did not induce proliferation in T cells from NOD mice <3 or >16 weeks in age (paralleling reactivity to whole GAD), or from control (BALB/c × NOD)F1 mice (data not shown). Data are represented as the mean SI ±s.e.m. calculated from 3–6 mice tested individually in two separate experiments for each age group.
METHODS. Proliferation was assayed as described for Fig. 1. Peptides were present in cultures at 7 μM and label was added during the last 16 h of a 5-day culture. Peptides were synthesized using standard F-moc chemistry and purified by reverse-phase HPLC (Advanced Chem-tech). The sequences of stimulatory peptides are: peptide 509–528 (no. 34), (IPPSLRYLED–NEERMSRLSK); peptide 524–543 (no. 35), (SRLS-KVAPVIKARMMEYGTT); and peptide 247–266 (no. 17), (NMYAMMIARFKMFPEVKEKG). Murine and human GAD65 share 95% amino-acid identity and are 98% conserved29. Underlined amino acids are conservatively substituted in murine GAD65. In separate experiments, the murine forms of these peptides were tested and produced similar results.
The gradual diversification of the primed autoreactive T-cell repertoire in this naturally occurring autoimmune disease parallels similar findings in experimentally induced autoimmune disease16,17. Apparently lymphokine secretion by the first wave of autoreactive T cells in the target organ induces loss of T-cell tolerance to additional antigens, resulting in a cascade of autoimmune responses18,20. The activation of T cells reactive to additional β-cell antigens is likely to promote β-cell destruction (for example, Hsp-reactive CD4+ T-cell clones can induce IDDM11,12).
GAD-reactive T cells were the first to arise among the autoantigens tested (Fig. 1). But did the anti-GAD response itself develop through intermolecular spreading, after a T-cell response to an unidentified β-cell antigen? If the development of T-cell reactivity to GAD is a primary event in the pathogenesis of IDDM, the inactivation of GAD-reactive T-cells before their spontaneous priming should prevent the cascade of T-cell responses to other β-cell antigens, insulitis and diabetes.
NOD mice were injected intravenously with GAD at 3 weeks of age, a treatment that causes unresponsiveness in antigen-reactive T cells21. Control groups received a similarly purified antigen (β-galactosidase), the immunodominant Hsp65 peptide, or mycobacterial heat-shock protein (which has been shown to vaccinate against murine IDDM11). The mice were examined for autoantigen-reactive T cells and insulitis at 12 weeks of age (Table 1), an age at which both reactive T cells and insulitis are clearly established in untreated NOD mice. Seventy five per cent of the GAD-treated mice (but none of the controls), displayed no T-cell reactivity to GAD, indicating complete tolerization. The GAD-tolerized mice showed no reactivity to other β-cell antigens and were completely free of insulitis (score zero). If there were another effector T-cell population in the islets, specific for an unknown β-cell antigen, that preceded the anti-GAD response, the release of cytokines by this population should have promoted T-cell responses to β-cell antigens and insulitis19,20. Twenty five per cent of the GAD-treated mice were not completely tolerized to GAD, as evidenced by weak residual GAD reactivity (stimulation index ~3) and displayed very limited peri-insulitis. In contrast, although tolerization to both of the heat-shock protein antigens was complete, these treatments reduced, but did not prevent, the development of T-cell responses to other β-cell antigens or insulitis—as would be expected if a secondary element were removed from the amplificatory cascade.
TABLE 1.
Induced tolerance to GAD prevents the development of insulitis and the spread of T-cell autoimmunity
| Treatment | Insulitis score | Spleen cell proliferation (Sl±s.e.m.) | ||||||
|---|---|---|---|---|---|---|---|---|
| β-galactosidase | GAD | GAD peptides | Hsp peptide | CPH | ||||
| No. 17 | No. 34 | No.35 | ||||||
| Uninjected | 2.4 ± 0.2 | 1.0 ± 0.2 | 9.5 ±2.1 | 4.8 ±0.4 | 6.0 ±0.1 | 2.9 ±0.2 | 6.7 ±1.0 | ND |
| β-Galactosidase | 2.6 ± 0.6 | 1.1 ± 0.1 | 15.4 ± 1.8 | 5.1 ± 0.6 | 5.1 ± 0.6 | 4.0 ± 0.2 | 6.6 ± 0.5 | 11.5 ± 0.9 |
| GAD | 0.1 ± 0.1 | 1.1 ± 0.03 | 1.6 ± 0.3 | 1.0 ± 0.05 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.02 |
| Hsp-p | 1.7 ± 0.4 | 1.1 ± 0.05 | 5.8 ± 0.2 | 4.5 ± 0.1 | 4.1 ± 0.3 | 4.2 ± 0.1 | 1.1 ± 0.04 | 4.4 ± 0.2 |
| m-Hsp | 1.8 ± 0.5 | 1.0 ± 0.1 | 4.2 ± 0.1 | 3.9 ± 0.1 | 3.9 ± 0.2 | 3.4 ± 0.2 | 1.0 ± 0.03 | 4.3 ± 0.2 |
Female NOD mice were injected intravenously at 3 weeks of age with 50 μg GAD65, β-galactosidase, mycobacterial Hsp65 (m-Hsp) or 0.1 μg immunodominant heat-shock peptide (Hsp-p) in PBS. At 12 weeks, mice were examined for insulitis and splenic T-cell responses to IDDM-associated autoantigens. Pancreatic tissue sections were stained using immunoperoxidase for insulin and counterstained with haematoxylin. Insulitis was scored blinded by examining 54 to 87 islets on 5 interrupted tissue sections from each pancreas. Severity of mononuclear cell infiltration was defined histologically: zero indicates lymphocytic infiltration; 1 indicates <25%; 2, 25–50%; 3, 50–75%; 4, >75%23. Proliferative splenic T-cell responses were measured as described for Fig. 1. Values are stimulation index (SI) ±s.e.m. Significant T-cell responses are underlined; a dashed underline is used for a borderline response. See Fig. 3 legend for GAD peptide numbering system. CPH, carboxypeptidase H. ND, not determined at this time point. Background counts in all groups was typically around 2,000 c.p.m. N = 8 for the GAD-treated group and N = 5 for all other groups.
In ongoing experiments examining the effects of GAD tolerization on diabetes incidence, all of the GAD-treated mice (n = 17; now 37 weeks old) have normal glucose levels, whereas 70% of the mice receiving control antigens developed hyperglycaemia by 19 weeks of age (n = 20). Five GAD-treated mice were examined at 30 weeks of age for insulitis. Four pancreata were completely free of insulitis (score 0.0) and one showed very limited peri-insulitis (J.T. and D.L.K., unpublished results). Thus, GAD tolerization can prevent the development of clinical diabetes in NOD mice.
Prevention of diabetes in NOD mice has also been reported following oral feeding of insulin22, immunization with complete Freund’s adjuvant23, heat-shock protein11 and heat-shock protein-specific T cells12. These treatments are thought to work by induction of active protective immune responses11,12,22,23 and have not been shown to prevent insulitis. In contrast, we have shown that in the absence of T-cell reactivity to GAD, autoimmunity to β-cells does not develop, demonstrating the pathogenic significance of this response.
Our data qualify GAD as a key antigen in the induction of murine IDDM. The initial spontaneous anti-GAD response leads to the recruitment of additional β-cell antigen-reactive T cells. This cascade of autoimmune responses can be circumvented by inactivating GAD-reactive T cells. As a similar autoimmune progression may also occur in human IDDM24 and in other T-cell-mediated autoimmune diseases (involving different autoantigens), these findings should be useful in the design of immunotherapies. □
ACKNOWLEDGEMENTS.
We thank C. Evans and D. Keith for help and advice; C. Anderson for tissue preparation; M. Kronenberg, Z. Nagy, M. Cohn, N. Maclaren, M. S. Sy and D. Kaplan for comments on the manuscript; and D. Newman, T. Phan, A. Peck, N. Kim, W. Chen, K. Jensen, N. Khodadadi and V. Arroyoso for their assistance. This work was supported by grants from the American Diabetes Association (D.L.K.), the NIH (M.C.-S., A.J.T., E.E.S.), the Juvenile Diabetes Foundation (M.C.-S., A.J.T., E.E.S.) and the Diabetes Research and Education Foundation (M.A.A.). P.V.L. was a Feftow of the Jaye Haddad/Concern Foundation and T.F. was an NIH Postdoctoral Trainee.
References
- 1.Castaño L. & Eisenbarth GS A. Rev. Immun 8, 647–679 (1990). [DOI] [PubMed] [Google Scholar]
- 2.Palmer JP Diabetes, Rev. 1,104–115 (1993). [Google Scholar]
- 3.Kaufman DL, McGinnis JF, Krieger NR & Tobin AJ Science 232, 1138–1140 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erlander MG, Tillakaratne NJK, Feldblum S, Patel N. & Tobin AJ Neuron 7, 91–100 (1991). [DOI] [PubMed] [Google Scholar]
- 5.Kaufman DL & Tobin AJ Trends pharmac. Sei 14, 107–109 (1993). [DOI] [PubMed] [Google Scholar]
- 6.Baekkeskov S. et al. Nature 298, 167–169 (1982). [DOI] [PubMed] [Google Scholar]
- 7.Baekkeskov S. et al. Nature 347, 151–156 (1990). [DOI] [PubMed] [Google Scholar]
- 8.Atkinson MA, Maclaren NK, Scharp DW, Lacy PE & Riley WJ Lancet 335,1357–1360 (1990). [DOI] [PubMed] [Google Scholar]
- 9.Kaufman DL et al. J. din. Invest 89, 283–292 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castaño L, Russo E, Zhou L, Lipes MA & Eisenbarth GS J. clin. endocrin. Metab 73, 1197–1201 (1991). [DOI] [PubMed] [Google Scholar]
- 11.Elias D, Markovits D, Reshef T, van der Zee R. & Cohen IR Proc. natn. Acad. Sci. U.S.A 87, 1576–1580 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elias D. et al. Proc. natn. Acad. Sci. U.S.A 88, 3088–3091 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croft M, Duncan D. & Swain SL J. exp. Med 176, 1431–1437 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradley LM, Atkins GG & Swain SL J. Immun 148, 324–331 (1992). [PubMed] [Google Scholar]
- 15.Ando DG, Clayton J, Kono D, Urban JL & Sercarz EE Cell. Immun 124, 132–143 (1989). [DOI] [PubMed] [Google Scholar]
- 16.Lehmann PV, Forsthuber T, Miller A. & Sercarz EE Nature 358, 155–157 (1992). [DOI] [PubMed] [Google Scholar]
- 17.Watanabe R, Wege H. & ter Meulen V. Nature 305,150–153 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehmann PV, Sercarz EE, Forsthuber T, Dayan CM & Gammon G. Immun. Today 14, 203–208 (1993). [DOI] [PubMed] [Google Scholar]
- 19.Sarvetnick N. et al. Nature 346, 844–847 (1990). [DOI] [PubMed] [Google Scholar]
- 20.Heath WR et al. Nature 359, 547–549 (1992). [DOI] [PubMed] [Google Scholar]
- 21.Gammon G. & Sercarz EE Nature 342, 183–185 (1989). [DOI] [PubMed] [Google Scholar]
- 22.Zhang ZJ, Davidson L, Eisenbarth G. & Weiner HL Proc. natn. Acad. Sci. U.S.A 88, 10252–10256 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin H-V, Sadelain MWJ, Hitchon C, Lauzon J. & Singh BJ Immun. 150, 2072–2080 (1993). [PubMed] [Google Scholar]
- 24.Atkinson MA et al. Lancet 339, 458–459 (1992). [DOI] [PubMed] [Google Scholar]
- 25.Bu DF et al. Proc. natn. Acad. Sei. U.S.A 89, 2115–2119 (1992). [Google Scholar]
- 26.Hochuli E, Bannwarth W, Döbeli H, Gentz R. & Stüber D. Bio/Technology 6, 1321–1325 (1988). [Google Scholar]
- 27.Macy E, Kemeny M. & Saxon A. FASEB J. 3003–3009 (1988). [DOI] [PubMed] [Google Scholar]
- 28.Taguchi T. et al. J. Immun 145, 68–77 (1990). [PubMed] [Google Scholar]
- 29.Lee DS, Tian J, Phan T. & Kaufman DL Biochim. biophys. Acta 1216, 157–160 (1993). [DOI] [PubMed] [Google Scholar]