Abstract
Objective:
To use cluster analysis (CA) to identify different clinical phenotypes among antiphospholipid antibodies (aPL)-positive patients.
Methods:
Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION) Registry includes persistently positive aPL of any isotype based on the Sydney APS classification criteria. We performed CA on the baseline characteristics of the first 500 patients included in the registry. Thirty clinical data points were included in the primary CA to cover the broad spectrum of aPL-positive patients.
Results:
A total of 497 patients from international centers were analyzed resulting in three main exclusive clusters: a) female patients with no other autoimmune diseases, but with venous thromboembolism (VTE) and triple-aPL positivity; b) female patients with systemic lupus erythematosus, VTE, aPL-nephropathy, thrombocytopenia, hemolytic anemia, and positive lupus anticoagulant test; and c) older men with arterial thrombosis, heart valve disease, livedo, skin ulcers, neurological manifestations, and cardiovascular disease (CVD) risk factors.
Conclusions:
Based on our hierarchical cluster analysis, we identified different clinical phenotypes of aPL-positive patients discriminated by aPL profile, lupus, or CVD risk factors. Our results, while supporting the heterogeneity of aPL-positive patients, also provide a foundation to understand disease mechanisms, create new approaches for APS classification, and ultimately to develop new management approaches.
Keywords: Antiphospholipid Syndrome, APS ACTION, Cardiovascular Risk Factors, Systemic Lupus Erythematosus, Triple Positivity
Introduction:
Persistent antiphospholipid antibodies (aPL) are recognized risk factors for thrombosis or obstetrical morbidity leading to a diagnosis of antiphospholipid syndrome (APS). Furthermore, aPL are associated with several non-thrombotic manifestations also known as “non-criteria” manifestations, e.g., thrombocytopenia, autoimmune hemolytic anemia, livedo, aPL-related nephropathy, heart valve disease, and neurological manifestations [1]. Antiphospholipid syndrome can be either associated with another autoimmune disease (mainly systemic lupus erythematosus [SLE]), or being referred to as “primary APS” when no other concomitant autoimmune disease exists. Thus, clinical presentations of aPL-positive patients represent a wide spectrum including asymptomatic carriers of aPL, arterial/venous/microvascular thrombosis, obstetrical morbidity, non-thrombotic manifestations, and the most severe form of the disease, catastrophic APS [2].
Antiphospholipid Syndrome Alliance for Clinical Trials and International Networking (APS ACTION) was created to design and conduct large-scale, multicenter studies and clinical trials in persistently aPL-positive patients [3]. The APS ACTION clinical database and repository (“registry”) was created to study the natural course of persistently aPL-positive patients with or without concomitant autoimmune disorders over at least 10 years; the registry allows us to perform large-scale cross-sectional and prospective analyses, which will eventually help us better understand the clinical characteristics of APS patients.
Cluster analysis (CA) is a data driven method that can group patients in a way that patients in the same group (cluster) are more like each other than to those in other groups. Several studies have used CA to identify phenotypes in chronic diseases such as Parkinson’s Disease, asthma, inflammatory bowel disease, or SLE [4]. This method help unravel the complex etiologies of medical conditions which may have pathogenic and therapeutic implications. For example, therapeutic response in IgG4-related disease was predicted using cluster analysis [5]. In aPL-positive patients, CA has not been used to identify different clinical phenotypes.
Therefore, to improve our understanding of APS disease characteristics and facilitate potential targeted therapies, our primary objective was to use CA to identify different clinical phenotypes among aPL-positive patients. The secondary objective was to identify homogeneous groups of aPL-related clinical manifestations and cardiovascular disease (CVD) risk factors occurring in similar patients.
Material and Methods:
The checklist of items that should be included in reports of observational studies is available in the supplemental table (STROBE Statement).
APS ACTION Registry:
An international web-based application, the REDCap (Research Electronic Data Capture) [6], captures data on patient demographics, aPL-related clinical and laboratory characteristics, and medications. The inclusion criteria are: a) age between 18 and 60 years; and b) persistent (at least 12 weeks apart) aPL-positivity within 12 months prior to screening; positivity is defined as anticardiolipin antibodies (aCL) IgG/M/A (> 40 GPL/MPL/APL, medium-to-high titer, and/or greater than the 99th percentile), anti-β2-glycoprotein-I (aβ2GPI) IgG/M/A (> 40 units, medium-to-high titer), positive lupus anticoagulant (LA) test based on International Society on Thrombosis and Hemostasis and other current guidelines [7–9]. Patients are followed every 12 ± 3 months with clinical data and blood collection.
Study Cohort and Data Points:
The primary CA was performed on the first 500 persistently aPL-positive patients with or without other systemic autoimmune diseases included in the APS ACTION registry. The goal was to identify variables that could discriminate groups of patients. We used 30 baseline (collected retrospectively at the time of the registry entry) demographic and clinical data points representative of the whole clinical spectrum of aPL-positive patients to generate clusters (Table 1). The choice of these data points was based on all available variables related to aPL in the APS ACTION registry. In a subgroup analysis, we limited our CA to female patients with a history of pregnancy; we only used 15 baseline demographic and clinical data points (Table 1).
Table 1:
Variables Used For Cluster Analysis
| Variables | |
|---|---|
| Demographics | - gender (male/female) |
| - race (white/non-white) | |
| Clinical Criteria For Definite Antiphospholipid Syndrome | - arterial thrombosis (yes/no)* |
| - venous thromboembolism (yes/no)* | |
| - biopsy-proven microvascular thrombosis (pulmonary, skin, kidney, and “other”) (yes/no)* | |
| - fetal death after 10th week of gestation (yes/no)* | |
| - premature birth due to preeclampsia, eclampsia, or placental insufficiency before 34th week of gestation (yes/no)* | |
| - three or more consecutive pre-embryonic or embryonic losses before 10th week of gestation (yes/no)* | |
| Non-Criteria Manifestations | - superficial vein thrombosis (yes/no) |
| - transient ischemic attack (yes/no) | |
| - livedo reticularis/racemosa (past or current/never) | |
| - persistent thrombocytopenia defined as platelets < 100,000x109 tested twice at least 12 weeks apart (past or current/never) | |
| - autoimmune hemolytic anemia (past or current/never) | |
| - echocardiography-proven heart valve disease (yes/no or unknown) | |
| - biopsy-proven aPL-related nephropathy (yes/no or unknown) | |
| - neuropsychiatric test-proven cognitive impairment (abnormal/normal or unknown) | |
| - chorea (yes/no) | |
| - seizure (yes/no) | |
| - skin ulcer (yes/no) | |
| - brain white matter abnormalities (yes/no or unknown) | |
| Cardiovascular Risk Factors | - body-mass index > 30 (yes/no)* |
| - hypertension requiring treatment (yes/no)* | |
| - diabetes mellitus requiring treatment (yes/no)* | |
| - hyperlipidemia requiring treatment (yes/no)* | |
| - smoking (past or current/never)* | |
| aPL Profile | - positive LA test (yes/no)* |
| - positive aCL IgG/IgM/IgA (yes/no)* | |
| - positive aβ2GPI IgG/IgM/IgA (yes/no)* | |
| Associated Auto-Immune Diseases | - SLE based on the American College of Rheumatology Classification Criteria (yes/no)* |
| - other autoimmune disease, e.g. lupus-like disease, rheumatoid arthritis, Sjogren’s syndrome, systemic sclerosis, idiopathic inflammatory myopathy, or vasculitis (yes/no) |
Variables used for the cluster analysis restricted to female aPL-positive patients with a history of pregnancy.
aβ2GPI: Anti-β2-Glycoprotein I antibodies; aCL: Anticardiolipin Antibodies; aPL: antiphospholipid antibodies; LA: Lupus Anticoagulant; SLE: Systemic Lupus Erythematosus.
For the secondary CA, clinical criteria for definite APS according to Sydney criteria (arterial thrombosis, venous thromboembolism, small vessel thrombosis, more than 3 recurrent early fetal losses, late fetal death, premature birth due to preeclampsia/eclampsia), “non-criteria” manifestations (aPL-related nephropathy, livedo, superficial vein thrombosis, heart valve disease, hemolytic anemia, thrombocytopenia, transient ischemic attack, chorea, cognitive impairment), as well as CVD risk factors (hypertension, hyperlipidemia, diabetes, smoking, obesity) were analyzed. The goal was to identify groups of clinical characteristics instead of groups of patients.
Statistical Analysis:
Characteristics of sample were described by percentage for categorical variables and mean, standard deviation, median, quartiles, and min/max values for continuous variables. Pearson’s χ2 (or Fisher’s Exact test when assumption of expected frequency is violated) and Student’s t-test were applied to compare qualitative variables and quantitative variables, respectively.
To identify clinical phenotypes, the CA method we used was the hierarchical ascending classification method based on Ward’s criterion considered as the most relevant. From a statistical point of view, the objective of Ward’s method is to find at each stage those two clusters whose fusion gives the minimum increase in the total within-groups error sum of squares. This method optimizes the variance criterion [10].
Regarding the robustness of the primary CA analysis, the Cubic Clustering Criterion and the SPRSQ (Semiparial R2) were used to identify the optimal number of patient clusters (к coefficient). The results of this analysis were validated by the bootstrap method (1,000 iterations) [11]. To identify differences between clusters, ANOVA, and χ2 test of independence were used. Tests were adjusted for all pairwise comparisons within a row using the Bonferroni correction to identify predominant and discriminant variables. The variable with the highest percentage, which is significantly more common compared to one other cluster only is defined as “Predominant Variable”, and to all other clusters as “Discriminant Variable”. Alpha risk was fixed to 5% for all analysis. These statistical analyses were done with SPPS software, Version 22.0.
Results:
After excluding three patients with missing data, 497 persistently aPL-positive patients from international centers were analyzed (female: 384 (77%), mean age: 44.5±12.9, primary aPL/APS: 324, and aPL/APS associated with other systemic autoimmune diseases: 173).
Primary Cluster Analysis - Clinical Phenotypes of Patients within the Entire Cohort:
Table 2 demonstrates the demographic, clinical, and laboratory characteristics of the patients, clustered in three main groups following a dendrogram analysis (Figure 1). The number of clusters was validated through the visual inspection of the dendrogram and confirmed by the computation of the к coefficient, which indicated a robust classification (к=0.716 [95% CI; 0.567–0.863]). Discriminant variables in the three clusters were: a) female patients with no other autoimmune diseases, but with VTE and triple-aPL positivity (Cluster 1); b) female patients with SLE, VTE, “non-criteria” manifestations (aPL-nephropathy, thrombocytopenia, and hemolytic anemia), positive LA test, and positive SLE serology (Cluster 2); and c) older men with arterial thrombosis, heart valve disease, livedo, skin ulcer, neurological manifestations, and CVD risk factors (Cluster 3). Discriminant variables were triple aPL positivity (Cluster 1), SLE (Cluster 2), and gender, older age, arterial thrombosis, heart valve disease, neurological manifestations, and CVD risk factors (except diabetes mellitus) (Cluster 3).
Table 2:
Identification of Three Distinct Clusters of Patients Among Those Included in the APS ACTION Registry
| Variables, n (%) | Cluster 1 (n=179) | Cluster 2 (n=180) | Cluster 3 (n=138) |
|---|---|---|---|
| Demographics | |||
| Mean Age, year±SD | 41.9±11.6 | 42.3±12.5 | 51.0±12.4 a,b |
| Female | 145 (81.0) c | 145 (80.6) c | 92 (66.7) |
| Male | 34 (19) | 35 (19.4) | 46 (33.3) a,b |
| Past Medical History | |||
| Clinical Criteria* | |||
| Arterial Thrombosis | 28 (15.6) | 51 (28.3)a | 95 (68.8) a,b |
| Venous Thromboembolism | 84 (46.9) c | 85 (47.2) c | 45 (32.6) |
| Small vessel thrombosis | 9 (5.0) | 11 (6.1) | 10 (7.2) |
| Pregnancy morbidity** | 73/97 (75.3) | 67/103 (65.0) | 42/66 (63.6) |
| Non-Criteria Manifestations | |||
| Heart Valve Disease | 9 (5.0) | 6 (3.3) | 23 (16.7) a,b |
| Livedo | 15 (8.4) | 26 (14.4) | 30 (21.7) a |
| Skin Ulcer | 6 (3.4) | 11 (6.1) | 14 (10.1) a |
| Neurological Manifestations | 22 (12.3) | 26 (14.4) | 58 (42.0) a,b |
| aPL Nephropathy | 2 (1.1) | 10 (5.6) c | 0 (0) |
| Thrombocytopenia | 22 (12.3) | 45 (25.0) a | 22 (15.9) |
| AutoImmune Diseases | |||
| None or unknown | 145 (81.0) b | 99 (55.0) | 102 (73.9) |
| SLE | 25 (14.0) | 74 (41.1) a,c | 26 (18.8) |
| Other | 9 (5.0) | 7 (3.9) | 10 (7.2) |
| Cardiovascular Risk Factors | |||
| Hypertension | 14 (7.8) | 33 (18.3)a | 99 (71.7) a,b |
| Diabetes | 4 (2.2) | 5 (2.8) | 12 (8.7) a |
| Hyperlipidemia | 12 (6.7) | 31 (17.2)a | 65 (47.1) a,b |
| Obesity | 31 (17.3) | 49 (27.2) | 60 (43.5) a,b |
| Smoking | 44 (24.6) | 61 (33.9) | 74 (53.6) a,b |
| Laboratory Parameters | |||
| Antiphospholipid Antibodies | |||
| Lupus Anticoagulant | 129 (72.1) | 152 (84.4) a | 105 (76.1) |
| Anticardiolipin Antibodies | 166 (92.7) b,c | 63 (35.0) | 115 (83.3)b |
| Anti-β2-GPI Antibodies | 138 (77.1) b,c | 25 (13.9) | 73 (52.9)b |
| Triple aPL-positivity | 99 (55.3) b,c | 13 (7.2) | 56 (40.6)b |
| Other Laboratory Parameters | |||
| Hemolytic Anemia | 2 (1.1) | 18 (10.0) a | 6 (4.3) |
| Antinuclear Antibodies | 104 (58.4) | 117 (65.7) c | 72 (52.2) |
| dsDNA Antibodies | 43 (24.0) | 61 (33.9) c | 23 (16.7) |
| Low C3 | 20 (29.9) | 39 (49.4) a | 18 (48.6) |
| Low C4 | 24 (35.8) | 36 (45.6) | 15 (40.5) |
Significantly (p<0.05) more prevalent than Cluster 1, 2, and 3, respectively.
Anti-β2-GPI: Anti-β2-Glycoprotein I antibodies; aPL: antiphospholipid antibodies; SD: Standard Deviation; SLE: Systemic Lupus Erythematosus.
Several clinical manifestations can occur in the same patient.
Among 266 aPL-positive female patients who have been pregnant.
The variable with the highest percentage, which is significantly more common compared to one other cluster only is defined as “Predominant Variable (bold)”, and to two other clusters as “Discriminant Variable (bold & underlined)” (NB: for each variable, when both discriminant and predominant variables are present, only the discriminant variable is shown in bold to facilitate the reading).
Figure 1: Dendrogram.
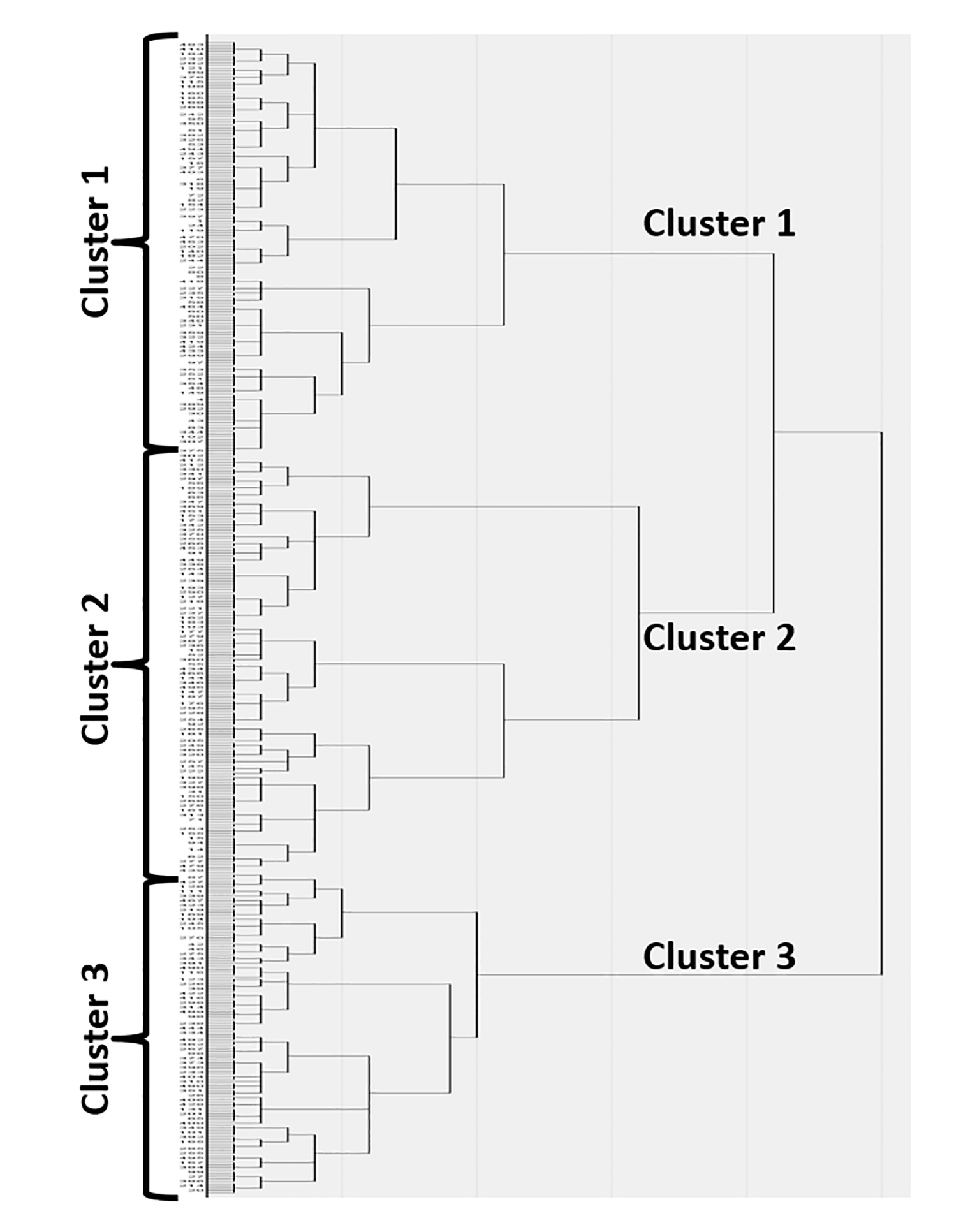
Using Ward’s minimum-variance hierarchical clustering method, 497 subjects were clustered to a single final group. At each generation of clusters, samples were merged into larger clusters to minimize the within-cluster sum of squares or maximize between-cluster sum of squares. With successive clustering, three balanced groups became obvious.
Primary Cluster Analysis Subgroup Analysis - Clinical Phenotypes of Female Patients with Pregnancy History:
Table 3 demonstrates the demographic, clinical, and laboratory characteristics of 290 female patients with pregnancy history clustered in four main groups: a) older female patients with arterial thrombosis, CVD risk factors, statin treatment (Cluster 1); b) female patients with pregnancy morbidity only (Cluster 2); c) asymptomatic aPL-positive female patients with aCL/aβ2GPI treated with aspirin (Cluster 3); and d) female patients with VTE, obesity, SLE, positive LA test, and warfarin treatment (Cluster 4). Discriminant variables were fetal death (Cluster 2), asymptomatic aPL, particularly aβ2GPI positivity (Cluster 3), and SLE, VTE, and obesity (Cluster 4).
Table 3:
Identification of Four Distinct Clusters of Patients With a History of Pregnancy Among The APS ACTION Registry
| Variables, n (%) | Cluster 1 (n=85) | Cluster 2 (n=69) | Cluster 3 (n=92) | Cluster 4 (n=44) | ||||
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Mean Age, year±SD | 47.95 | ± 9.63 b | 38.94 | ± 11.67 | 44.86 | ± 11.74 b | 42.94 | ± 11.30 |
| White | 52 | (65.8) | 35 | (54.7) | 53 | (66.3) | 22 | (50.0) |
| Asian | 3 | (3.8) | 11 | (17.2) a | 8 | (10.0) | 4 | (9.1) |
| Latin American | 22 | (27.8) | 16 | (25.0) | 12 | (15.0) | 12 | (27.3) |
| Black | 1 | (1.3) | 2 | (3.1) | 5 | (6.3) | 5 | (11.4) |
| Past Medical History | ||||||||
| Clinical Criteria* | ||||||||
| Arterial Thrombosis | 40 | (47.1) b,d | 12 | (17.4) | 31 | (33.7) | 8 | (18.2) |
| Venous Thromboembolism | 37 | (43.5)c | 27 | (39.1)c | 16 | (17.4) | 34 | (77.3) a,b,c |
| Small Vessel Thrombosis | 8 | (9.4) | 1 | (1.4) | 4 | (4.3) | 2 | (4.5) |
| ≥ 3 Fetal Losses | 7 | (8.2) | 5 | (7.2) | 8 | (8.7) | 3 | (6.8) |
| Fetal Death > 10th Week | 30 | (35.3)c | 58 | (84.1) a,c,d | 3 | (3.3) | 11 | (25.0)c |
| Premature Birth** | 12 | (14.1) | 21 | (30.4) d | 18 | (19.6) d | 1 | (2.3) |
| Classification | ||||||||
| Asymptomatic aPL-carriers | 11 | (12.9) | 3 | (4.3) | 33 | (35.9) a,b,d | 5 | (11.4) |
| Obstetrical APS | 7 | (8.2) | 29 | (42.0) a,c,d | 15 | (16.3) | 3 | (6.8) |
| Thrombotic & obstetrical APS | 30 | (35.3) c | 25 | (36.2) c | 13 | (14.1) | 12 | (27.3) |
| Thrombotic APS | 37 | (43.5) b | 12 | (17.4) | 31 | (33.7) | 24 | (54.5) b |
| Other AutoImmune Disease | ||||||||
| SLE | 21 | (24.7) | 11 | (15.9) | 20 | (21.7) | 25 | (56.8) a,b,c |
| Lupus-Like Disease | 7 | (8.2) | 4 | (5.8) | 15 | (16.3) | 0 | (0.0) |
| Cardiovascular Risk Factors | ||||||||
| Hypertension | 42 | (49.4) b,c | 12 | (17.4) | 17 | (18.5) | 13 | (29.5) |
| Diabetes | 4 | (4.7) | 3 | (4.3) | 5 | (5.4) | 2 | (4.5) |
| Hyperlipidemia | 29 | (34.1) b,c | 6 | (8.7) | 13 | (14.1) | 6 | (13.6) |
| Obesity | 21 | (24.7) | 10 | (14.5) | 21 | (22.8) | 25 | (56.8) a,b,c |
| Smoking | 14 | (16.5) b | 2 | (2.9) | 16 | (17.4) b | 5 | (11.4) |
| Treatments | ||||||||
| Aspirin | 32 | (38.1) | 32 | (46.4)d | 57 | (62.0) a,d | 9 | (20.5) |
| Warfarin | 56 | (65.9)c | 31 | (44.9) | 35 | (38.0) | 33 | (75.0) b,c |
| LMWH | 7 | (8.2) | 4 | (5.8) | 7 | (7.6) | 2 | (4.5) |
| Statins | 29 | (34.1) b,d | 5 | (7.2) | 16 | (17.4) | 5 | (11.4) |
| Hydroxychloroquine | 35 | (41.7) | 23 | (33.3) | 37 | (40.2) | 23 | (52.3) |
| Laboratory Parameters | ||||||||
| Antiphospholipid Antibodies | ||||||||
| Lupus Anticoagulant | 75 | (88.2) c | 55 | (79.7) c | 53 | (57.6) | 39 | (88.6) c |
| Anticardiolipin Antibodies | 63 | (74.1) d | 47 | (68.1) d | 78 | (84.8) d | 7 | (15.9) |
| Anti-β2-GPI Antibodies | 37 | (43.5)d | 28 | (40.6)d | 59 | (64.1) a,b,d | 1 | (2.3) |
| Other Parameters | ||||||||
| Anti-Ro | 6 | (7.1) | 6 | (8.7) | 11 | (12.0) | 10 | (22.7) |
| Anti-La | 1 | (1.2) | 2 | (2.9) | 2 | (2.2) | 4 | (9.1) |
Significantly (p<0.05) more prevalent than Cluster 1, 2, 3 and 4, respectively.
Several clinical manifestations can occur in the same patient.
Due to preeclampsia, eclampsia, or placental insufficiency.
Anti-β2-GPI: Anti-β2-Glycoprotein I; LMWH: Low Molecular Weight Heparin; SLE: Systemic Lupus Erythematosus.
The variable with the highest percentage, which is significantly more common compared to one other cluster only is defined as “Predominant Variable (bold)”, and to three other clusters as “Discriminant Variable (bold & underline (NB: for each variable, when both discriminant and predominant variables are present, only the discriminant variable is shown in bold to facilitate the reading).
Secondary Cluster Analysis - Clusters of Clinical Characteristics Occurring Together
Three main clusters with different combinations of manifestations were identified (Figure 2): a) obstetrical morbidity, “non-criteria” manifestations, and diabetes (Cluster A); b) arterial thrombosis with CVD risk factors (hypertension, hyperlipidemia, and smoking) (Cluster B); c) venous thromboembolism and obesity (Cluster C). When excluding patients with any associated autoimmune disease (mainly SLE), results from 279 patients remained unchanged (Figure 3).
Figure 2: Cluster Analysis of Antiphospholipid Antibody Related Clinical Manifestations and Cardiovascular Risk Factors.
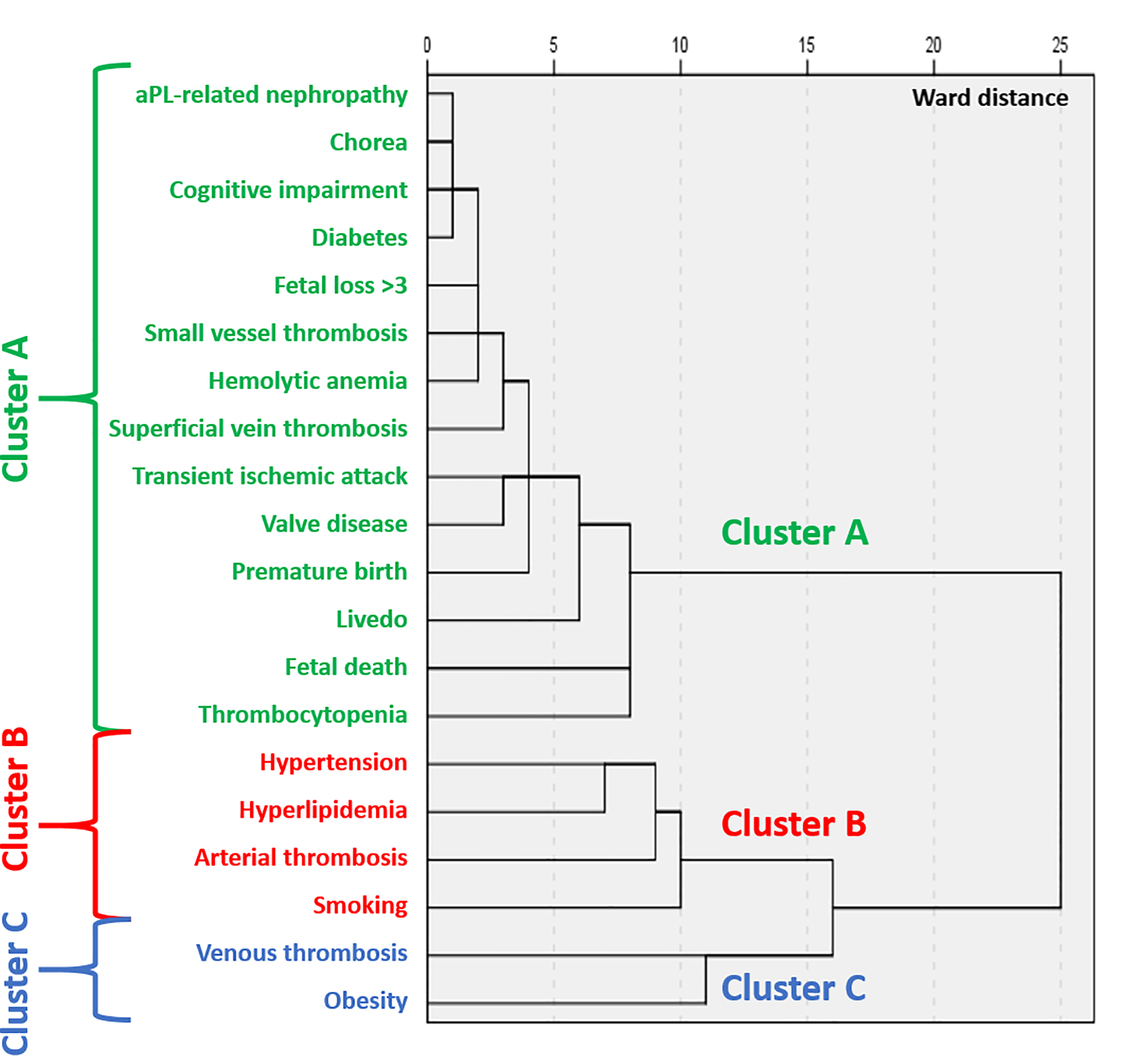
Using Ward’s minimum-variance hierarchical clustering method (n=500), three main clusters of manifestations were identified (arterial thrombosis and cardiovascular risk factors; venous thromboembolism and obesity; non-criteria manifestations, diabetes and obstetrical morbidity).
Figure 3: Cluster Analysis of Clinical Manifestations and Cardiovascular Risk Factors in aPL-positive patients with no autoimmune disease.
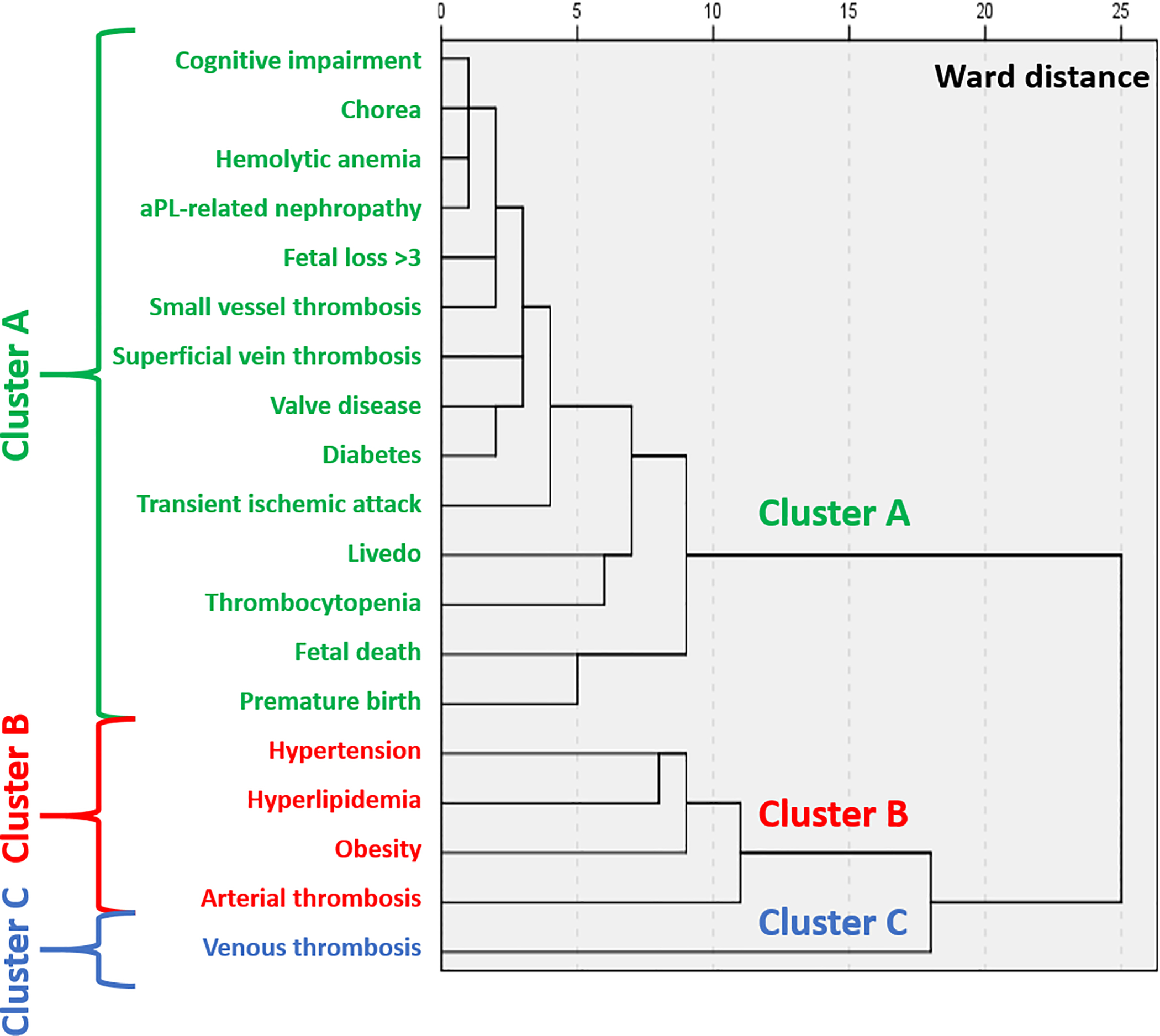
Using Ward’s minimum-variance hierarchical clustering method (n=279), three main clusters of manifestations were identified (arterial thrombosis and cardiovascular risk factors; venous thromboembolism only; non-criteria manifestations, diabetes and obstetrical morbidity).
Discussion
According to our hierarchical primary and secondary CA, we confirmed the heterogeneity of clinical phenotypes of aPL-positive patients including aPL-positive females with a history of pregnancy; factors resulting in this heterogeneity were mainly aPL profile, SLE diagnosis, and CVD risk factors. Furthermore, we identified specific clusters in asymptomatic aPL-positive patients and women with obstetrical APS only and that non-criteria manifestations do not share the same cluster of clinical APS criteria.
Antiphospholipid antibody profile, especially triple aPL-positivity, is considered as the most clinically significant laboratory profile that expose patients to a higher risk for developing aPL-related clinical events [12]. Furthermore, the additive impact of CVD risk factors on the development of thrombosis in aPL-positive patients [13] is well accepted; a similar effect of CVD risk factors (mainly smoking, hypertriglyceridemia, and obesity) on obstetrical outcomes are also identified in women with a history of pregnancy [14]. In fact, CVD risk factors are now incorporated in thrombosis prediction models [15,16]. Lastly, overlapping manifestations exist between SLE and APS; while aPL modify the clinical presentation of SLE patients [17–19], conversely, SLE could also modify the clinical presentation of aPL-positive patients [20]. Thus, as supported by our findings, the identification of triple aPL positivity, CVD risk factors, and SLE in aPL-positive patients is critical for a precise clinical phenotyping allowing a better risk stratification in aPL-positive patients [21].
Since 2010, new data confirmed the significant association between some of the non-criteria manifestations and aPL especially in SLE patients [19]. Indeed, current classification criteria are suboptimal due to several factors, the most relevant being the lack of representation of many heterogeneous manifestations of aPL. In parallel with an international collaborative effort to develop new APS classification criteria [22], our finding of the significant associations between non-criteria and classical criteria manifestations reinforce the need to take into account these manifestations in the global clinical assessment of aPL-positive patients.
From a pathogenic point of view, several non-criteria manifestations share the same underlying pathogenic process [23]: vascular wall involvement with proliferation and endothelium impairment has been demonstrated in the kidneys of APS patients with aPL-related nephropathy (thrombotic microangiopathy, intimal hyperplasia), in the brain of patients with cognitive decline, in the lungs of patients with pulmonary arterial hypertension (plexiform lesion), in placentas of women with placental-mediated complications (decidual vasculopathy), and in vessels of patients with arterial stenosis (coronary and renal artery). This “aPL-related vasculopathy” is not completely understood however there were indications of the AKT/mTORC pathway activation by aPL in cultured endothelial cells in vitro leading to aPL-related nephropathy lesions [24], although the activation of this pathway in other organs is still to be demonstrated. We found that – regardless of any underlying autoimmune diseases – all non-criteria manifestations were gathered in one cluster suggesting that patients with these manifestations could share a common phenotype supporting the hypothesis of a common underlying pathologic mechanism. Together with previous data [25], our results contributes to the understanding of the heterogeneity of clinical phenotypes of APS patients.
The limitations of this study include a potential lack of generalizability to other patient populations. However, the APS ACTION “registry” represents the largest ongoing prospective collaborative clinical database and repository gathering a large number of aPL positive patients followed regularly. In fact, confounding factors may impact the results. CA is an exploratory analysis that is used to identify subsets of cases if the grouping is not previously known. Therefore, it does not make any distinction between dependent and independent variables. The CA can identify groups of patients that present with similar symptoms/manifestations and simultaneously maximize the difference between the groups. Thus, even if potential confounding factors are not addressed in a classical fashion, e.g., multivariate analysis, the identification of a clinical heterogeneity between aPL-positive patients can be considered as the major confounding factor that could help understand different outcomes [26]. Another issue is that time is not analyzed in this CA. Indeed, we can hypothesize that disease duration could impact the results; several risk factors could have started after the aPL events took place and therefore apparent differences in attributed aPL events could be due to differences in duration of exposure and to heterogeneity of treatment.
In conclusion, our results confirm the heterogeneity of aPL-positive patients and provide a foundation to identify different disease mechanisms, create new approaches for APS classification, and ultimately develop new tailored management tactics. Furthermore, our results open new research avenues such as monitoring the long-term follow-up of patients based on their initial clusters, or conducting randomized controlled studies based on different clusters segregations.
Supplementary Material
Supplemental Table: STROBE Statement—checklist of items that should be included in reports of observational studies
Funding
This work was supported by the Clinical and Translational Science Center at Weill Cornell Medicine (Data management using REDCAP) [UL1 TR000457 to D.E.] and the National Institute of Health [AR 069572 to M.P.].
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RHWM, DE Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4: 295–306. [DOI] [PubMed] [Google Scholar]
- 2.Abreu MM, Danowski A, Wahl DG, Amigo M-C, Tektonidou M, Pacheco MS, Fleming N, Domingues V, Sciascia S, Lyra JO, Petri M, Khamashta M, Levy RA. The relevance of “non-criteria” clinical manifestations of antiphospholipid syndrome: 14th International Congress on Antiphospholipid Antibodies Technical Task Force Report on Antiphospholipid Syndrome Clinical Features. Autoimmun Rev 2015; 14: 401–14. [DOI] [PubMed] [Google Scholar]
- 3.Barbhaiya M, Andrade D, Erkan D, APS ACTION. AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION): 5-Year Update. Curr Rheumatol Rep 2016; 18: 64. [DOI] [PubMed] [Google Scholar]
- 4.Font J, Cervera R, Ramos-Casals M, García-Carrasco M, Sents J, Herrero C, del Olmo J-A, Darnell A, Ingelmo M. Clusters of clinical and immunologic features in systemic lupus erythematosus: analysis of 600 patients from a single center. Semin Arthritis Rheum 2004; 33: 217–30. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto M, Takano K-I, Kamekura R, Suzuki C, Tabeya T, Murakami R, Honda S, Mukai M, Nojima M, Ichimiya S, Himi T, Nakase H, Takahashi H. Predicting therapeutic response in IgG4-related disease based on cluster analysis. Immunol Med 2018; 41: 30–3. [DOI] [PubMed] [Google Scholar]
- 6.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL, Galli M, De Groot PG, Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009; 7: 1737–40. [DOI] [PubMed] [Google Scholar]
- 8.Keeling D, Mackie I, Moore GW, Greer IA, Greaves M, British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol 2012; 157: 47–58. [DOI] [PubMed] [Google Scholar]
- 9.Moore GW. Commonalities and contrasts in recent guidelines for lupus anticoagulant detection. Int J Lab Hematol 2014; 36: 364–73. [DOI] [PubMed] [Google Scholar]
- 10.Vogt W, Nagel D. Cluster analysis in diagnosis. Clin Chem 1992; 38: 182–98. [PubMed] [Google Scholar]
- 11.Mandara J The typological approach in child and family psychology: a review of theory, methods, and research. Clin Child Fam Psychol Rev 2003; 6: 129–46. [DOI] [PubMed] [Google Scholar]
- 12.Pengo V, Ruffatti A, Legnani C, Gresele P, Barcellona D, Erba N, Testa S, Marongiu F, Bison E, Denas G, Banzato A, Padayattil Jose S, Iliceto S. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost 2010; 8: 237–42. [DOI] [PubMed] [Google Scholar]
- 13.Urbanus RT, Siegerink B, Roest M, Rosendaal FR, de Groot PG, Algra A. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: a case-control study. Lancet Neurol 2009; 8: 998–1005. [DOI] [PubMed] [Google Scholar]
- 14.Bouvier S, Cochery-Nouvellon E, Lavigne-Lissalde G, Mercier E, Marchetti T, Balducchi J-P, Marès P, Gris J-C. Comparative incidence of pregnancy outcomes in treated obstetric antiphospholipid syndrome: the NOH-APS observational study. Blood 2014; 123: 404–13. [DOI] [PubMed] [Google Scholar]
- 15.Zuily S, de Laat B, Mohamed S, Kelchtermans H, Shums Z, Albesa R, Norman gary, Lamboux-Matthieu C, Rat A-C, Ninet J, Magy-Bertrand N, Pasquali J-L, Lambert M, Lorcerie B, Kaminsky P, Guillemin F, Regnault V, Wahl D, on Behalf of the TAC(I)T investigators. Validity Of The Global Anti-Phospholipid Syndrome Score To Predict Thrombosis. A Prospective Multicentre Cohort Study. Rheumatology (Oxford, England) 2015;. [DOI] [PubMed] [Google Scholar]
- 16.Radin M, Sciascia S, Erkan D, Pengo V, Tektonidou MG, Ugarte A, Meroni P, Ji L, Belmont HM, Cohen H, Ramires de Jesús G, Branch DW, Fortin PR, Andreoli L, Petri M, Rodriguez E, Rodriguez-Pinto I, Knight JS, Atsumi T, Willis R, et al. The adjusted global antiphospholipid syndrome score (aGAPSS) and the risk of recurrent thrombosis: Results from the APS ACTION cohort. Semin Arthritis Rheum 2019;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuily S, Regnault V, Selton-Suty C, Eschwège V, Bruntz J-F, Bode-Dotto E, De Maistre E, Dotto P, Perret-Guillaume C, Lecompte T, Wahl D. Increased risk for heart valve disease associated with antiphospholipid antibodies in patients with systemic lupus erythematosus: meta-analysis of echocardiographic studies. Circulation 2011; 124: 215–24. [DOI] [PubMed] [Google Scholar]
- 18.Zuily S, Domingues V, Suty-Selton C, Eschwège V, Bertoletti L, Chaouat A, Chabot F, Regnault V, Horn EM, Erkan D, Wahl D. Antiphospholipid antibodies can identify lupus patients at risk of pulmonary hypertension: A systematic review and meta-analysis. Autoimmun Rev 2017; 16: 576–86. [DOI] [PubMed] [Google Scholar]
- 19.Unlu O, Stéphane Z, Doruk E. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol 2016; 3: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unlu O, Erkan D, Barbhaiya M, Andrade D, Nascimento I, Rosa R, Banzato A, Pengo V, Ugarte A, Gerosa M, Ji L, Efthymiou M, Branch DW, de Jesus GR, Tincani A, Belmont HM, Fortin PR, Petri M, Rodriguez E, Pons-Estel GJ, et al. The Impact of Systemic Lupus Erythematosus on the Clinical Phenotype of Antiphospholipid Antibody-Positive Patients: Results From the AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Clinical Database and Repository. Arthritis Care Res (Hoboken) 2019; 71: 134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tektonidou MG, Andreoli L, Limper M, Amoura Z, Cervera R, Costedoat-Chalumeau N, Cuadrado MJ, Dörner T, Ferrer-Oliveras R, Hambly K, Khamashta MA, King J, Marchiori F, Meroni PL, Mosca M, Pengo V, Raio L, Ruiz-Irastorza G, Shoenfeld Y, Stojanovich L, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis 2019; 78: 1296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbhaiya M, Zuily S, Ahmadzadeh Y, Naden R, Costenbader K, Erkan D, On Behalf of the New APS Classification Criteria Collaborators. Development of New International Classification Criteria for Antiphospholipid Syndrome: Phase II Results. Arthritis Rheumatol 71. [Google Scholar]
- 23.Siddique S, Risse J, Canaud G, Zuily S. Vascular Manifestations in Antiphospholipid Syndrome (APS): Is APS a Thrombophilia or a Vasculopathy? Curr Rheumatol Rep 2017; 19: 64. [DOI] [PubMed] [Google Scholar]
- 24.Canaud G, Bienaimé F, Tabarin F, Bataillon G, Seilhean D, Noël L-H, Dragon-Durey M-A, Snanoudj R, Friedlander G, Halbwachs-Mecarelli L, Legendre C, Terzi F. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med 2014; 371: 303–12. [DOI] [PubMed] [Google Scholar]
- 25.Ripoll VM, Pregnolato F, Mazza S, Bodio C, Grossi C, McDonnell T, Pericleous C, Meroni PL, Isenberg DA, Rahman A, Giles IP. Gene expression profiling identifies distinct molecular signatures in thrombotic and obstetric antiphospholipid syndrome. J Autoimmun 2018; 93: 114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henríquez-Hernández LA, Valenciano A, Foro-Arnalot P, Alvarez-Cubero MJ, Cozar JM, Suárez-Novo JF, Castells-Esteve M, Ayala-Gil A, Fernández-Gonzalo P, Ferrer M, Guedea F, Sancho-Pardo G, Craven-Bartle J, Ortiz-Gordillo MJ, Cabrera-Roldán P, Herrera-Ramos E, Lara PC. Polymorphisms in DNA-repair genes in a cohort of prostate cancer patients from different areas in Spain: heterogeneity between populations as a confounding factor in association studies. PLoS ONE 2013; 8: e69735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table: STROBE Statement—checklist of items that should be included in reports of observational studies


