Abstract
Nanotechnology provides synthetic carriers for cancer drug delivery that protect cargos from degradation, control drug release and increase local accumulation at tumors. However, these non-natural vehicles display poor tumor targeting and potential toxicity and are eliminated by the immune system. Recently, biomimetic nanocarriers have been widely developed based on the concept of ‘mimicking nature.’ Among them, cell-derived biomimetic vehicles have become the focus of bionics research because of their multiple natural functions, such as low immunogenicity, long circulation time and targeting ability. Cell membrane-coated carriers and extracellular vesicles are two widely used cell-based biomimetic materials. Here, this review summarizes the latest progress in the application of these two biomimetic carriers in targeted cancer therapy. Their properties and performance are compared, and their future challenges and development prospects are discussed.
Keywords: Biomimetic nanocarriers, targeting, cell membranes, extracellular vesicles, exosomes, cancer therapy
1. Introduction
Cancer is one of the most invasive diseases and the leading cause of death in humans, and a large amount of effort and money has been devoted to fighting it over the past few decades (Roma-Rodrigues et al., 2019). In the struggle with cancer, early detection is important for successful treatment, and surgery, phototherapy, chemotherapy, radiotherapy (RT) and multimodal combination therapy are the principal methods of cancer treatment used currently. However, surgical resection causes relapse easily after the operation due to the limitation of the tumor distribution. Phototherapy, chemotherapy and RT may have serious side effects due to the lack of specificity (Hu et al., 2016).
In recent years, drug delivery systems (DDSs) based on nanocarriers have been widely developed, and antitumor drug carriers (such as liposomes (Wang et al., 2020), micelles (Guan et al., 2020), polymer nanogels (Maiti et al., 2018), magnetic nanoparticles (NPs) (Avval et al., 2020), and nanocapsules (de Cristo Soares Alves et al., 2020)) are usually produced on the nanoscale to increase their permeability and retention in tumor tissues. However, these carriers are identified as ‘nonself’ and are still quickly cleared by the immune system with a short half-life. They are usually unable to actively sense the disease environment and do not effectively accumulate in the tumor site; thus, their targeting rate is extremely low. In addition, NPs dispersed in vivo adsorb proteins to form a protein corona on their surfaces, which reduce the targeting rate of NPs and mediate the clearance of NPs by the reticuloendothelial system (RES). The polyethylene glycol (PEG) modification can delay the clearance time, but repeated use will induce the production of its specific antibody IgM and accelerate the clearance of NPs (Li et al., 2018). Therefore, traditional nanocarriers still have many defects and are unable to meet the current need for cancer treatment.
Based on the concept of ‘mimicking nature,’ biomimetic nanocarriers have been widely developed due to their advantages in reproducing the functions of natural materials, among which cell-derived biomimetic carriers are currently a hot spot. Due to the different proteins and carbohydrates on different cell membranes, cells perform a variety of specific functions in the body, and thus the nanocarriers formed by cell-derived membranes that encapsulate cargoes may inherit the functions of the source cells, such as immune escape, long circulation, and recognition ability. Therefore, this cell-derived biomimetic carrier is a ‘natural treasure,’ among which the cell membrane and extracellular vesicles (EVs) are widely used.
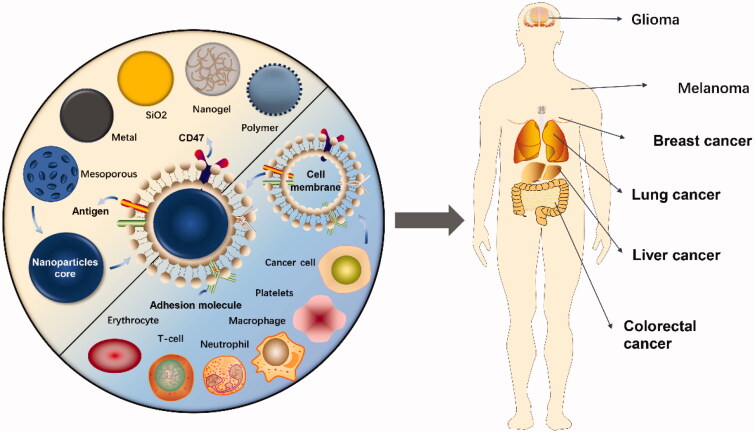
Cell membrane coating technology involves organic/inorganic synthetic NPs wrapped in a layer of natural cell membrane, and the prepared nanocarriers not only function as synthetic NPs but also have the natural complex characteristics of the source cells (Figure 1). Since researchers have successfully prepared red blood cell membrane (RBCM)-coated NPs that prolonged the circulation time in vivo in 2011, cell membrane camouflage technology has developed rapidly (Hu et al., 2011). The function of the cell membrane varies by cell type; for example, red blood cells (RBCs) evade the immune system due to the expression of CD47 on the membrane but have no targeting ability, whereas white cell membranes display the characteristic of tumor homing (Gao et al., 2020; Huang et al., 2018). Therefore, for different application purposes, researchers began to utilize a variety of cell membranes to camouflage NPs, such as the immune cell membrane, platelet membrane (PLTM), and even cancer cell membrane (CCM). The coating of the cell membrane endows NPs with good biocompatibility and adjustable surface properties, suggesting that this biomimetic carrier coated with a cell membrane is extremely promising for cancer therapy (Xu et al., 2020).
Figure 1.
Cell membrane-coated nanoparticles for cancer drug delivery. Cell membranes extracted from different types of cells are used to encapsulate different types of nanoparticles for cancer treatment.
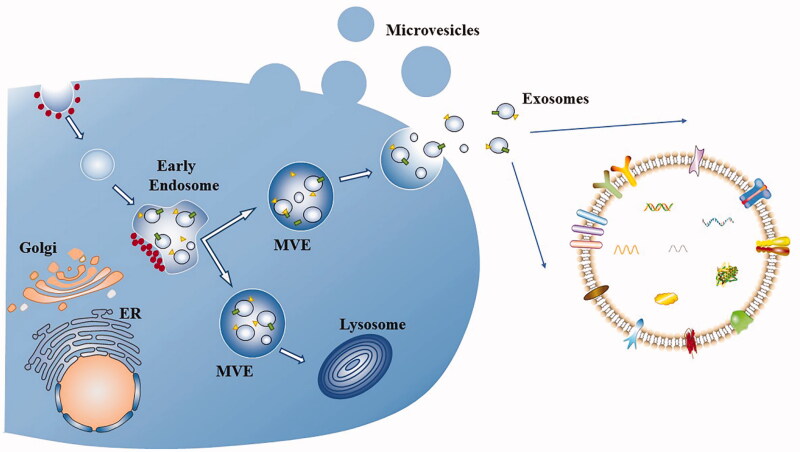
EVs are phospholipid bilayer vesicles that are secreted by almost all cells, are responsible for intercellular communication and cargo transport, and potentially alter the phenotype of recipient cells. EVs are classified according to their intracellular sources, from small to large, as exosomes (40–120 nm), microvesicles (50 nm–1 μm), and apoptotic bodies (50 nm–5 μm) (Willms et al., 2018). Exosomes and microvesicles originate from endosomes and plasma membranes, respectively, while apoptotic bodies are produced through sprouting and abscission mechanisms and autophagosome formation mechanisms, among which exosomes are the most comprehensively studied vesicles (Figures 2 and 3). However, due to their small size and overlapping dimensions, a precise method is unavailable to distinguish them, and thus these vesicles are collectively referred to as EVs (Zaborowski et al., 2015). EVs have been used as carriers of various biomolecules, such as DNA, RNA and protein. Usually, after the release of EVs wrapped in unique biomolecules, EVs are internalized by their targeted recipient cells and then deliver their contents and transmit genetic information. EVs protect the cargo from degradation during delivery and cross various barriers, such as the blood-brain barrier (BBB), spread to the interior of the body and reach distant tissues (Gargiulo et al., 2019). Importantly, autologous EVs lack immunogenicity and are extremely safe as drug delivery carriers. Building on the advantages described above, EVs have become a ‘new star’ in the field of drug delivery carriers in recent years.
Figure 2.
Biogenesis of extracellular vesicles and exosomes. Microvesicles bud from the plasma membrane. Exosomes are small vesicles that form early endosomes and multivesicular endosomes (MVEs), which are released through the fusion of MVEs with the plasma membrane. Other MVEs enter the lysosome. Dots represent clathrin-coated vesicles or clathrin coats, rectangles and triangles represent transmembrane proteins and membrane-associated proteins, respectively.
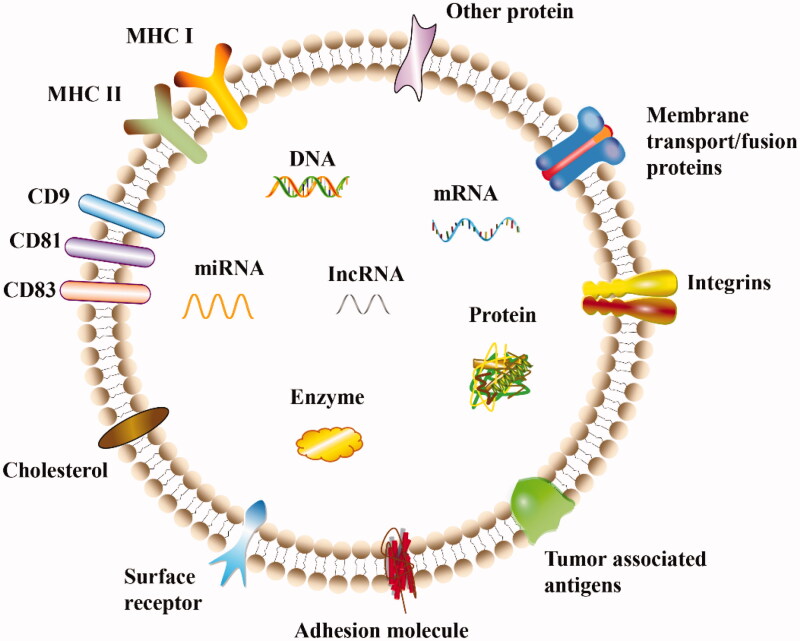
Figure 3.
Structure and composition of exosomes. Exosomes are approximately round vesicles secreted by cells that contain various cellular components, including proteins, miRNAs, mRNAs, lncRNAs, enzymes, carbohydrates, and lipids. Various proteins are present on the surface of exosomes and are responsible for different pathophysiological functions.
In this review, we summarize the latest applications of cell-derived biomimetic nanocarriers (cell membrane and EVs) as targeted cancer therapy delivery vehicles, compare the differences between these two types of carriers, and discuss their challenges and future development prospects.
2. Cell membrane-coated nanoparticles
2.1. Preparation of cell membrane-coated nanoparticles
According to the different treatments of diseases, the cell membranes of RBCs, immune cells, platelets (PLTs) and cancer cells can be used to camouflage drugs or NPs as needed. The basic production method is similar and mainly includes the extraction of the cell membrane, the preparation of the nanocore and the assembly of the ‘shell-core’.
2.1.1. Isolation of the cell membrane
Cells perform various complex functions in the body by interacting with the surrounding environment, and most of the responsibilities are performed by functional surface proteins. The properties of proteins on the membrane are easily changed, and thus the extraction and separation of the cell membrane should be carried out carefully. The separation of the cell membrane is the process of separating the membrane and intramembrane mixture, and the extraction of all cell membranes requires cell fragmentation in hypotonic solution, but the separation process for enucleated cells is slightly different from that for eukaryotic cells. The former are often mentioned as RBCs and PLTs, which are directly separated from the blood, and after the cells are lysed, the cell membranes are separated from the mixture by centrifugation. The latter, such as cancer cells and white blood cells (WBCs), are first ruptured with a hypotonic solution, and then their contents are thoroughly separated from the membrane by ultrasound and homogenization. Finally, pure cell membranes are extracted by high-speed differential centrifugation (Su et al., 2017; Fang et al., 2018). Importantly, membrane extraction should be performed under suitable conditions to ensure that the proteins on the membrane maintain their original activity, and protease inhibitors are usually used in extraction.
2.1.2. Fusion of membrane vesicles to the core nanoparticles
The membrane is wrapped around the core through various methods, all with the ultimate goals of maintaining the membrane in the right direction and exposing the proteins on the membrane for communication. Shell-core fusion was first performed using a physical extrusion method that extends from the preparation of liposomes. The extrusion force can destroy the membrane structure and cause it to reform around NPs (Hu et al., 2015). Ultrasonic treatment is also a feasible method. The breaking force generated by ultrasonic energy can cause the cell membrane to spontaneously reshape on NPs (Copp et al., 2014). Ultrasonic treatment exerts the same effect as physical extrusion, but it results in less raw material loss and is easier to expand to the production scale, suggesting that the application prospects are brighter. The semistable characteristics of the membrane and the core, as well as the asymmetry of the charge on the surface of the membrane, help the membrane wrap the nanocore. The special right-side-out membrane orientation makes the combination of the two extremely thermodynamically stable.
Usually, the nanocore is destroyed easily by ultrasonic treatment and time and labor are wasted when using physical extrusion, while microfluidic electroporation, a popular method, can remedy these deficiencies. A microfluidic chip reduces the applied voltage of electroporation. Rao et al. developed a microfluidic chip. When flowing through the electroporation area, the electric pulse promoted Fe3O4 magnetic NPs to enter the RBC vesicle, which effectively combined the two. The biomimetic carrier produced using this method shows excellent application potential in tumor diagnosis and treatment (Rao et al., 2017). In addition, an emerging cell membrane-templated polymerization technology achieves efficient membrane wrapping and controls the size of the formed biomimetic NPs, but related research is limited and requires further development (Zhang et al., 2015).
2.2. Cell membrane-coated nanoparticles for targeted cancer therapy
2.2.1. Red blood cell membrane
RBCs are abundant in the blood and are responsible for transporting oxygen. They have variable shapes and no nuclei and are easily separated from the blood, which facilitates the extraction and purification of cell membranes. RBCs also have a long life span (120 days) and can serve as carriers to improve the biosafety of DDSs and prolong their blood circulation time. However, the lack of cell adhesion molecules on RBCMs prevents them from targeting the tumor tissue, leading to hamper cellular internalization.
RBCMs are often modified with ligands (such as folic acid) to expand the applications of this natural carrier, and two main methods of active targeting modification have been developed. One is the direct modification method of binding ligands to the RBCM with active groups through covalent bonds, but the reagent used to induce binding may destroy the function of RBCM (Chai et al., 2017). Another method is the indirect modification, in which positively charged ligands (lipids or proteins) are inserted into the membrane, but the ligands are easily absorbed by negatively charged RBCMs, and thus the targeting ability is difficult to guarantee (Fang et al., 2013). A recent study cleverly avoided the charge problem by using RBCM to achieve targeted drug delivery for gliomas. In this study, a novel biomimetic carrier (T7/NGR-RBCSLNs) with double modification was constructed by modifying the RBCM with the negatively charged T7 peptide and negatively charged polypeptide NGR through lipid insertion. The former targets transferrin receptors on both the BBB and glioma surface, and the latter targets CD13, which is expressed at high levels in tumor cells. Compared with biomimetic NPs modified with only one ligand, the endocytosis of T7/NGR-RBCSLNs by glioma cells was the most significant. T7/NGR-RBCSLNs take advantage of the dual targeting effect of modified RBCMs to cross the BBB and the blood-brain tumor barrier (BBTB) and significantly enhance the anti-glioma effect in vivo (Fu et al., 2019).
In addition to ligand modification, the hybridization of RBCMs with other cell membranes also improves the targeting ability. Because the membrane proteins on PLTs bind to biomolecules expressed at high levels in some tumors, Kim et al. prepared a new biomimetic carrier (R/P-cGNS) that used gold nanostars loaded with curcumin (Cur) as the core, and the cloak was a mixture of RBCMs and PLTMs. R/P-cGNS has two membrane functions, because the carrier not only escapes phagocytosis but also effectively targets tumors (Kim et al., 2020). Natural cell membranes are affected by temperature. Combined with photothermal therapy (PTT), R/P-cGNS achieves the controlled release of Cur with increasing temperature to achieve the expected anticancer effect (Ebrahimi et al., 2018).
RBCMs were natural, abundant and safe, and can be used as a favorable antitumor tool after being endowed with target ability (Yu et al., 2019). However, besides that, the quality control of RBCs is also a challenge. It is necessary to ensure that the RBCMs will not be contaminated by pyrogens and viruses, to remove the deformed proteins, and to avoid the potential immune reaction of endogenous antigens (Li et al., 2018). For further clinical studies, the RBCMs should be matched to the patient's blood type and RH compatibility (Han et al., 2018).
2.2.2. White blood cell membrane
WBCs, also known as immune cells, are nucleated, colorless, spherical blood cells that migrate freely inside and outside blood vessels, widely exist in blood, lymph and various tissues, and affect the progression of various diseases. WBC membrane-camouflaged NPs, which endow NPSs with both an immune escape ability and active targeting ability, have been widely used as drug delivery carriers in recent years (Li et al., 2018). Macrophages and neutrophils (NEs) are the most commonly utilized WBCs.
According to the different activation states, macrophages are divided into M1 and M2 macrophages. M1 macrophages exert proinflammatory effects, induce a positive immune response and destroy tumor tissue, while M2 macrophages exert anti-inflammatory effects, downregulate the immune response and promote tumor growth (Shapouri-Moghaddam et al., 2018). The antitumor effect of M1 macrophages is mainly derived from their surface markers, such as major histocompatibility complex II (MHC-II), CD80, and CD86, and thus antitumor carriers based on macrophage membranes have been widely developed (Najafi et al., 2019). However, macrophages are affected by the complex tumor microenvironment (TME), and the antitumor effect must often be enhanced by combining macrophages with other therapies. Hu et al. prepared biomimetic nanocarriers encapsulated by the M1 macrophage membrane [(C/I)BP@B-A(D)&M1m]. Various molecules involved in costimulatory signal transduction and high expression of MHC on the cell membrane allowed (C/I)BP@B-A(D)&M1m to effectively target tumor tissues. Combined with laser irradiation, (C/I)BP@B-A(D)&M1m released drugs efficiently at the target site as needed (Hu et al., 2020). Liu et al. developed a mixed micelle with photosensitizer chlorin e6 (Ce6) and reactive oxygen species (ROX) responsive bilirubin, loaded with modified paclitaxel (PTX) dimer, and coated with macrophage membrane (I-P@NPs@M). I-P@NPs@M effectively combining chemotherapy and photodynamic therapy (PDT) by co-delivering Ce6 and PTX. Macrophage membrane can protect drugs from the capture by mononuclear macrophage system, which makes I-P@NPs@M more to be absorbed and retained by tumor cells (Liu et al., 2019; Liu et al., 2020).
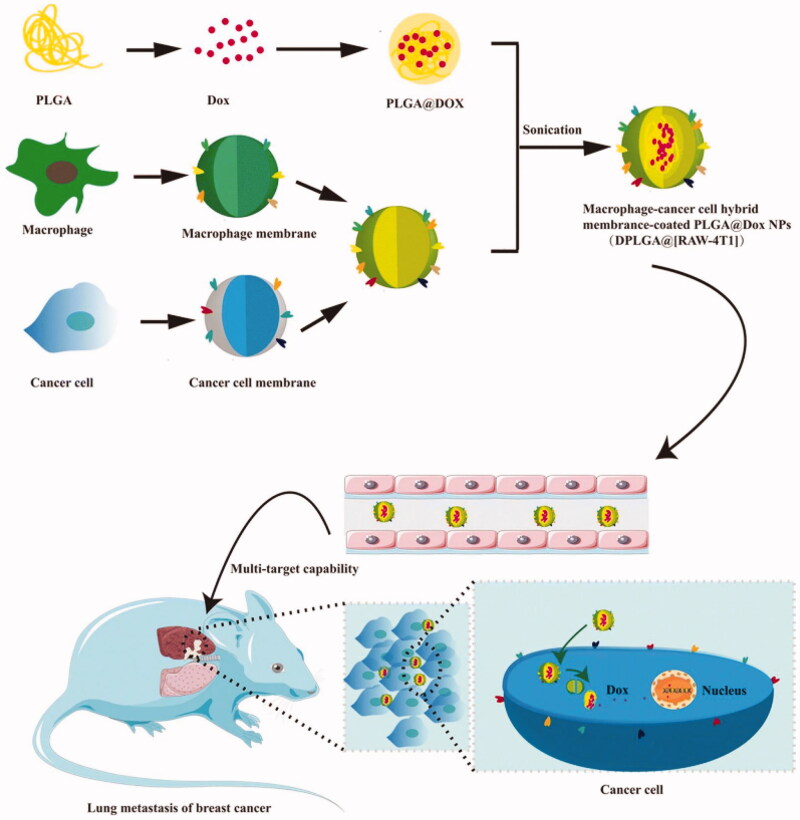
Macrophages regulate various functions in tumor immunity, not only participating in early cancer but also affecting the metastasis of terminal cancer (DeNardo and Ruffell, 2019; Jäppinen et al., 2019). Gong et al. loaded doxorubicin (Dox) into poly(lactic-co-glycolic acid) (PLGA) NPs and coated them with a hybrid coating of macrophage (RAW264.7) membranes and breast cancer cell (4T1) membranes to form new biomimetic nanocarriers (DPLGA@[RAW-4T1] NPs) (Figure 4). The α4β1 integrin on the RAW264.7 membrane is activated by vascular cell adhesion molecule-1 (VCAM-1), which is expressed at high levels on metastatic cancer cells, thereby increasing the ability of DPLGA@[RAW-4T1] NPs to specifically target metastatic cancer tissue. The 4T1 membrane enables DPLGA@[RAW-4T1] NPs to target homologous cancer cells, efficiently track the tumor and kill the tumor tissue (Gong et al., 2020). This biomimetic carrier is the first attempt to combine the macrophage cell membrane with CCM, which assists in the treatment of metastatic breast cancer and prolongs the life of patients, indicating its promising application prospects. However, whether macrophages from different individuals or races will produce individual immune rejection remains to be explored.
Figure 4.
Formation and release of RAW-4T1 hybrid membrane-coated doxorubicin (Dox)-loaded PLGA nanoparticles (DPLGA@[RAW-4T1] NPs). Reproduced with permission from Reference (Gong et al., 2020).
NEs, the most abundant immune cells, are the first to respond to infection or tumors and are closely related to tumor progression; thus, they are potentially useful as excellent carriers of antitumor drugs (Han et al., 2018; Zhang et al., 2020). Glioblastoma grows rapidly and has a high fatality rate, increasing the difficulty of complete surgical resection, and the BBB and BBTB also prevent common chemotherapy drugs from easily passing through and reaching the tumor site, resulting in a high tumor recurrence rate. Typically, postoperative inflammation occurs at the glioma site, and inflammatory cytokines [interleukin-8 (IL-8) and tumor necrosis factor α (TNF-α)] at the inflammation site activate NEs and induce their migration to the inflammatory site. Zhao et al. prepared a novel NE-based biomimetic carrier (PTX-CL/NEs) by wrapping neutrophil membranes (NEMs) around liposomes loaded with PTX. PTX-CL/NEs effectively target postoperative tumor sites where inflammatory signals are amplified, release drugs effectively, and slow tumor recurrence and growth (Xue et al., 2017). In another study, NEMs were wrapped on Celastrol-loaded PEG-PLGA nanoparticles. NEMs allow the nanoparticles to be recruited by chemokines, cross the blood-pancreas barrier and metastasize to the tumor site, thereby effectively exerting an antitumor effect (Cao et al., 2019). Although NEs are rich in content and fast in recruitment, they have a short lifespan, so they are often used in acute treatment environments (Combes et al., 2020).
In summary, immune cell membrane-based carriers with good tumor recognition ability and tumor penetration play a major role in regulating tumor occurrence and metastasis. Combined therapy with other methods is more effective. However, WBCs are highly heterogeneous. If allogeneic blood is used as a membrane source, blood type compatibility tests and screening for infectious diseases are required (Wu et al., 2019). In addition, the complexity of the TME makes it difficult to accurately grasp the mechanism of immune cell recruitment and polarization, which is still a challenge for effective drug delivery.
2.2.3. Platelet membrane
PLTs are disc-shaped and changeable, with a diameter of approximately 1-4 μm, and express membrane proteins such as p-selectin and CD47, which identify injured blood vessels and circulating tumor cells (CTCs). Because PLTs aggregate at the tumor site and their cell membranes are easy to extract and purify, the number of PLT-based drug delivery schemes has increased rapidly in recent years (Chen et al., 2018). Wang et al. encapsulated black phosphorus quantum dots with drug-carrying PLTM to form a new carrier (PLT@BPQDs-HED). The fluorescence signal of PLT@BPQDs-HED was stronger at the tumor site, and the retention rate was significantly higher after 48 hours than the control group. Because p-selectin on PLTs selectively binds to the overexpressed CD44 receptor on the tumor surface, PLT@BPQDs-HED has a higher efficiency of tumor drug delivery and enables the drug remain in the target site (Shang et al., 2019).
PTT is an invasive therapy used in combination therapy (Chen et al., 2019; Liu et al., 2019), but nanocarriers loaded with photothermal materials are unable escape the recognition of immune cells and are quickly cleared, resulting in low therapeutic efficiency. Wu et al. camouflaged NPs containing polypyrrole (PPy) and the anticancer drug Dox with the PLTM (PLT-PPy-Dox). The membrane protein enables PLT-PPy-Dox to evade the attack of immune cells and precisely target the tumor, laser irradiation causes PPy to produce hyperthermia and ablate the tumor cells, and Dox is also released from the NPs to effectively destroy the tumor. In addition, after three consecutive PTT cycles, the temperature of the tumor tissue increases, and then the tumor cells are burned; Due to PLTM recognize and accumulate in the injured site, PLT-PPy-Dox is more gathered in the tumor site, which promotes the efficiency of PTT (Wu et al., 2020). Therefore, the strategy of using biomimetic materials and combination therapy in the treatment of tumors has significant effects and promising prospects.
Cancer immunotherapy is a new method to stimulate the autoimmune response to destroy tumors, including monoclonal antibodies, cancer vaccines, and immune checkpoint inhibitors, among which the application of immune checkpoint blockade therapy in cancer treatment has been gradually developed in recent years (Friedman et al., 2020; Veldman et al., 2020). However, the TME is extremely complex, and tumor cells protect themselves from attack in various ways, limiting the effectiveness of immunotherapy (Phuengkham et al., 2019). Jiang et al. combined mild immunogenic ferroptosis with programmed cell death 1 (PD-1) immune checkpoint blockade therapy to treat cancer, and his team prepared Fe3O4 magnetic NPs loaded with sulfasalazine (SAS) and coated with PLTM (Fe3O4-SAS@PLT). The expression of p-selectin on PLTM enables Fe3O4-SAS@PLT to target tumors in mice and accumulate in the tumor site, thereby inducing ferroptosis and triggering the immune response. Twenty-four hours after injection, a high level of signal was still detected in the tumor site, indicating that the protection of PLTM enabled Fe3O4-SAS@PLT to escape the ‘pursuit’ of immune cells and facilitated a long circulation time. In addition, the immune response induced by Fe3O4-SAS@PLT enhanced the efficacy of PD-1 inhibitors, and almost no tumor metastasis occurred in metastatic mice (Jiang et al., 2020).
PLTs are closely related to tumor cells, and carriers that wrap the PLTM are not only treated as ‘self’ by the immune system to avoid clearance but also target tumors through their membrane surface proteins. PLTM-based DDSs may be combined with phototherapy, immunotherapy and other methods to effectively target CTCs to control the development of tumors and have broad application prospects in the treatment of tumors. However, the relationship between PLTs and tumor cells has not been fully elucidated, especially the role of PLTs on cancer cells beyond the function of blood metastasis; thus, sophisticated models that accurately mimic human disease are needed to unravel the variable interactions of PLTs in different cancers (Hyslop and Josefsson, 2017).
2.2.4. Cancer cell membrane
Cancer cells can replicate indefinitely, are easy to culture in vitro, and a large amount of membranes can be isolated from these cells. The CCM is rich in functional proteins, and its molecular repertoire is divided into three categories according to its use: (1) membrane proteins that mediate homotypic binding, such as selectin, tissue factor-antigen, and integrins (2) markers that promote immune escape, such as CD47; and (3) unique tumor antigens that stimulate the immune response of the body (Fang et al., 2018; He et al., 2020; Janiszewska et al., 2020). Given these advantages, the CCM has attracted the attention of researchers, and the use of CCM-modified NPs for drug delivery in tumor therapy is promising.
Although many treatments have been developed for cancer, chemotherapy is still the most common treatment. However, the efficiency of chemotherapy is often reduced by multidrug resistance (MDR), which is a difficulty that researchers have been attempting to overcome (Dei et al., 2019; Negi et al., 2019). CCM encapsulates calcium channel antagonists to overcome MDR by regulating intracellular channels in tumor cells. A recent study developed multidrug-resistant cervical cancer cell (HeLa/Dox) membrane-decorated silica NPs for the codelivery of siRNA and Dox (CCM/CS/R-D). The siRNA replaces the commonly used Cav antagonist, reduces the Ca2+ level, and increases the number of cells in the DNA synthesis stage, thus increasing drug retention. Ability of multidrug resistant CCM to bind to homotypic cell membranes and the integrin-associated protein CD47 on CCM endows CCM/CS/R-D with an excellent targeting ability and in vivo escape ability, which are conducive to the efficient arrival and function of DOX and siRNA in tumors (Zhao et al., 2020).
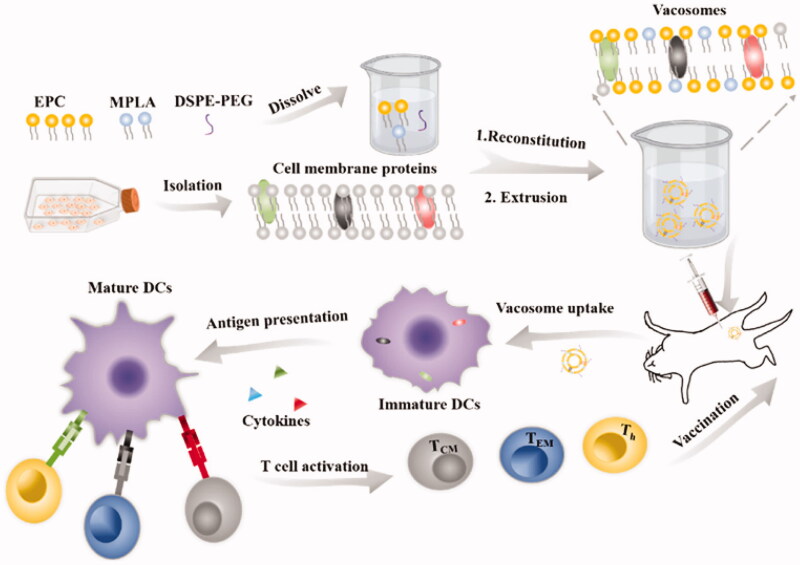
Cancer vaccines usually resist cancer cells by stimulating the human immune system, which is an effective method of cancer immunotherapy (Hu et al., 2018; Fusciello et al., 2019). CCM-coated nanoscale drugs have significant potential as cancer vaccines due to the unique tumor antigens of source cells (Zhu et al., 2017). A cancer vaccine (Vacosome) was prepared by mixing the 4T1 cell membrane, Toll-like receptor agonist monophosphoryl lipid A (MPLA) and common lipids in a certain proportion (Figure 5). The presence of tumor-specific antigens on the CCM enables MPLA to activate the corresponding receptors and increase the activity of antigen-presenting cells (APCs), thereby activating CD8+ T lymphocytes (TLs) to destroy tumors. The CCM in Vacosome is obtained from the tumor cells of patients after surgery, and thus the development of Vacosome is more personalized and more widely used in cancer immunotherapy (Cheng et al., 2020). At present, nanovaccines are still in the early stage, some problems remain to be solved (for example, the antigens on the membrane may be degraded in the complex physiological environment), and the effectiveness of their use must be optimized.
Figure 5.
Process used to fabricate the vacosome and the immune response induced by the vacosome in vivo. Reproduced with permission from reference (Cheng et al., 2020).
This unique yolk-shell structure based on the CCM has promoted progress in various cancer treatment methods with high application flexibility, and its unique membrane components endow the carrier with various capabilities, improve the transport path of the carrier in the body, and improve the efficiency of tumor treatment. In addition, the use of primary tumor cell membranes to develop more personalized and novel treatments is one of the important directions for the future development of CCM systems. The challenge is the reproducible synthesis of CCMs under good medical practice conditions and a deep understanding of their underlying homologous targeting mechanisms. Other important characteristics, such as purity, safety, and integrity, need further clarification (Jin and Bhujwalla, 2019).
2.2.5. Other cell membranes
The most commonly used types of cell membranes have been discussed above. With the development of biomimetic science, an increasing number of cell membranes have been employed for drug delivery. Researchers have also used activated fibroblast (AF) membranes to camouflage NPs in cancer treatment. Because tumor-associated AFs secrete growth factors and cytokines, interact with tumor cells and promote the progression of cancer, the modification of the AF cell membrane on the surface of NPs enables NPs to target tumor-associated AFs and improve the efficiency of various cancer treatment methods (Li et al., 2018). In addition, mesenchymal stem cells (MSCs) with strong self-renewal capacity and myeloid-derived suppressor cells (MDSCs) have also been used to carry nanodrugs to target the TME (Liu et al., 2020; Tesi, 2019).In addition, scientists are increasingly focusing on bacterial cell membranes. Ye et al. prepared a new nanomedicine using PC7A and CPG oligodeoxynucleotides as the core and a maleimide-modified Mycobacterium smegmatis (MS) membrane as the shell. MS are nonpathogenic bacteria with strong immunogenicity, and after RT treatment, the MS membrane captures neoantigens produced by tumor cells, promotes the uptake of these antigens by dendritic cells (DCs), and stimulates the antitumor response of TLs (Patel et al., 2019).
Research on membrane-based DDSs is developing rapidly, and researchers are aggressively exploiting the function of cell membranes (Table 1). In recent years, hybrid membrane-camouflaged nanocarriers have also attracted much attention, and the fusion of different membranes enables carriers to inherit the specific functions and key proteins of the source cells, thus having greater advantages in terms of effectiveness and safety (Jiang et al., 2019). As mentioned above, RBCM can prolong the circulation time of the carrier in vivo but lacks targeting ability. Sun et al. have constructed a RBCs-cancer cells hybrid membrane coated Dox gold nanocage for the combined PTT/RT/chemotherapy of breast cancer (Sun et al., 2020). The carrier encapsulated with hybrid membrane inherits the excellent homologous tumor-homing and the immune escape ability of RBCs, and can gather efficiently in the target sites. Bu et al. coated the hybrid membrane of PLTs and tumor stem cells on magnetic iron oxide NPs for the treatment of head and neck squamous cell carcinoma (Bu et al., 2019). PLTM evades the recognition of the immune system through the signal expression of surface markers, while the surface adhesion molecules of tumor stem cells enable the carrier to target tumor cells. In TME, this carrier enhances the tumor magnetic resonance imaging characteristics of NPs and shows a good photothermal effect, which could be an excellent delivery tool for tumor therapy. Therefore, hybrid membrane-wrapped NPs can make up the defects of single-cell membrane and give full play to its advantages in a ‘natural modification’ way, so that the NPs can be endowed with at least two biological functions by the cell membrane, of which targeting is crucial.
Table 1.
Cell membrane coated nanocarriers as carriers for targeted cancer therapy.
| Source cell | Nanoparticles | Cargo | Cancer type | Outcome | Ref |
|---|---|---|---|---|---|
| RBCM | Prussian blue nanoparticles |
Folic acid and compound(J5) | Cervical cancer |
Significantly enhancing the synergistic antitumor effect of phototherapy/chemotherapy |
(Daniyal et al., 2020) |
| RBCM | Upconversion nanoparticles |
DSPE-PEG-FA | Breast cancer |
Successfully realizing the tumor PET imaging | (Li et al., 2020) |
| RBCM | Bovine serum albumin nanoparticles |
Indocyanine green and gambogic acid |
Breast cancer |
Significantly improving the antitumor effect of synergistic chemotherapy-photothermal therapy | (Wang et al., 2020) |
| RBCM | Prussian blue nanoparticles |
Hyaluronic acid and gamabufotalin |
Breast cancer |
Accurately, efficiently and safely treat breast cancer | (Liu et al., 2019) |
| WBC membrane |
Gallium nano-swimmer |
Dox | Cervical cancer |
Enhanced photothermal and chemical cancer therapy | (Wang et al., 2020) |
| WBC membrane |
Lipid nanovector | Dox and siRNA | Esophageal cancer |
Realized targeted therapy of esophageal cancer | (Jun et al., 2020) |
| WBC membrane |
Fe3O4 magnetic nanoclusters |
DSPE-modified SYL3C aptamer | Cancer | Realized the rapid and specific detection of CTCs | (Zhang et al., 2019) |
| WBC membrane |
Bimetallic nanoparticles |
Epithelial cell adhesion molecule antibody | Epithelial cancer |
Realized to capture and analyze CTCs | (Chang et al., 2020) |
| Macrophage plasma membrane |
Albumin nanoparticles |
PTX | Melanoma | Accumulating more at the tumor and exerting stronger antitumor effect | (Cao et al., 2020) |
| Macrophage membrane |
Biomimetic superparticle |
Dox hydrochloride and quaternary quantum dots | Lung cancer | Specifically targeting metastatic nodules in the lung | (Liang et al., 2020) |
| PLTM | Porous nanoparticles |
Bufalin | Liver cancer | Inhibiting tumor growth | (Wang et al., 2019) |
| PLTM | Liposome | Dox | Cancer | Improving antitumor effect | (Liu et al., 2019) |
| PLTM | Nanostructured lipid nanoparticles |
PTX | Ovarian cancer |
Targeting and treating tumors effectively | (Bang et al., 2019) |
| Tumor cell membrane |
Aluminum phosphate nanoparticles |
CpG | Melanoma | Suppressing tumor progression | (Gan et al., 2020) |
| Tumor cell membrane |
Mesoporous silica nanoparticle | Dox and mefuparib hydrochloride | Breast cancer | Enhanced antitumor activity | (Nie et al., 2020) |
| Tumor cell membrane |
Zeolitic-imidazolate framework hybrid nanoparticle | Cisplatin and oleanolic acid |
Bladder cancer |
Promoting cell apoptosis and reversing MDR in tumor cells | (Chen et al., 2020) |
| Tumor cell membrane |
PLGA nanoparticles | PTX and siRNA | Cervical cancer |
Precisely treating of cervical cancer through chemo-gene combined therapy | (Xu et al., 2020) |
Currently, PLTs-RBCs hybrid membranes (Liu et al., 2018), WBCs-cancer cells hybrid membranes (He et al., 2018), macrophage-cancer cells hybrid membranes (Gong et al., 2020), and bacterial outer membrane vesicles (OMVs)-cancer cells hybrid membranes (Chen et al., 2020) have been successfully developed for cancer therapy. However, current studies have focused on the coating of single-cell membranes, and there are still some challenges before functionalizing using multiple cell membranes. This novel hybrid membrane coating strategy opens up a new way to overcome the limitations of the current tumor treatment.
3. Extracellular vesicles
3.1. Extracellular vesicle isolation and drug loading
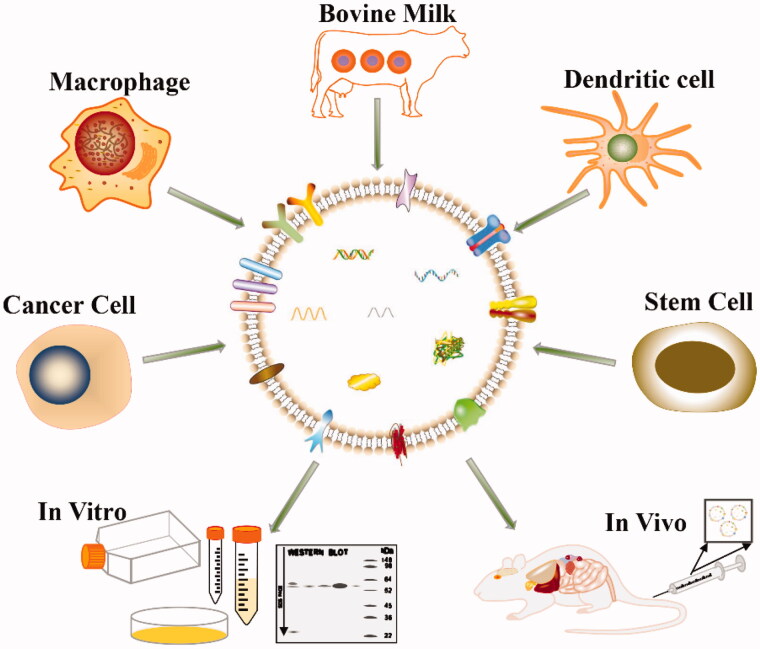
When EVs are employed for research, appropriate separation methods are often designed according to the research purpose. When EVs are used for diagnosis, a sufficient quantity is more important than purity, and thus a separation method that produces a high yield must be chosen. However, when EVs are used for drug delivery, their structural integrity is extremely important because substances such as proteins on EVs may have roles in targeting. In addition, many types of cells are commonly used to extract EVs (Figure 6), and therefore the separation method should also consider the characteristics of the sample, such as the viscosity of the sample and the concentration of EVs (Abramowicz et al., 2016). Overall, the separation method expected by researchers is simple and less expensive, and EVs are quickly extracted from larger samples. Most traditional separation methods are based on the size and buoyant density of EVs, among which the most commonly used method is ultracentrifugation. Ultracentrifugation has been used to separate EVs from a large number of samples with the consumption of very few reagents and good reproducibility, but the purity of EVs is not high. Density gradient ultracentrifugation improves the efficiency of particle separation and the purity of EVs, but it has the disadvantages of requiring expensive equipment, a long time and a large amount of labor; thus, its clinical application is limited (Tauro et al., 2012). Ultrafiltration is also a commonly used method that saves time and money, but the number of EVs isolated is limited, and the purity is low (Alvarez et al., 2012). However, the combination of ultrafiltration and ultracentrifugation effectively divides different subgroups of EVs, which has promising application prospects (Xu et al., 2017).
Figure 6.
EVs secreted by different cells for targeted cancer therapy.
The isolated EVs are composed of lipid bilayers that can be used as drug carriers to wrap the cargo and protect it from degradation in vivo. However, the introduction of cargo may destroy the contents and membrane structure of EVs, and thus effective strategies for loading therapeutic cargo into EVs are a challenge. Currently, two main ways for EVs to carry cargo have been developed. One is to combine the source cells of EVs with therapeutic drugs using an endogenous loading mechanism ensuring that the vesicles secreted by cells contain target drugs, of which the most common is the direct transfection of a therapeutic small RNA into cells, and then cells secrete the desired type of EVs (Kosaka et al., 2010). A recent study modified α-fetoprotein (AFP) on DCs, which secrete exosomes carrying AFP, for use in the immunotherapy of hepatocellular carcinoma (HCC) (Lu et al., 2017). The other is the direct combination of curative drugs and EVs, also known as exogenous loading. Hydrophobic drugs, such as Cur and PTX, directly combine with EVs through a coincubation, but hydrophilic compounds, such as RNA, are affected by lipid bilayers, preventing them from being encapsulated in EVs. At present, hydrophilic compounds are loaded into EVs by electroporation and coupled with membrane stabilizers to reduce the effect of the loading process on the structural integrity of EVs (Hood et al., 2014). In summary, the loading method should be selected according to the cargo and specific application purpose, and the cargo loading capacity of different EVs requires further study.
3.2. EV-based drug delivery systems
3.2.1. Dendritic cells
DCs are a ‘scout’ among immune cells that accumulate around cancer cells based on the attraction provided by immune signals (such as proinflammatory cytokines and pathogen-associated molecular patterns). These immune signals trigger MCH-1 and MCH-2 on DCs to interact with costimulatory molecules and transport tumor-associated antigens (TAAs) to lymph nodes, where they transmit information to naive TLs, which differentiate into mature TLs and attack cancer cells (Chen and Mellman, 2013). Briefly, the ultimate goal of DCs is to activate TLs to fight tumors, and therefore a promising approach is to design tumor immunotherapy based on the characteristics of DCs. However, the problems that limit the application of DCs, such as high price, short efficacy period and complicated preparation method, still remain to be solved (Palmer et al., 2009). DC-derived exosomes (DEXs) contain the membrane components (for example, DC-originating molecules) of DCs and possess the targeting and immune stimulation capabilities of their source cells. The composition of their membrane is easy to control and stable, and these exosomes can be frozen for half a year (Zhang et al., 2014).
The feasibility of dendritic cell-derived EVs (DC-EVs) for cancer treatment has been proven, but antitumor experiments showed that DC-EVs do not eradicate tumors, and the insufficient induction of the immune response leads to the ability of tumors to overcome immune attack, which is the main reason for the limited therapeutic effect (Viaud et al., 2010). Therefore, DC-EVs with higher immune activity must be developed to combat tumors. In a recent study, ovalbumin (OVA) was added to DC cultures as an antigen, followed by interferon-γ (IFN-γ) and lipopolysaccharide stimulation to activate DCs, and then activated exosomes (DCOVA-sEVs) containing ovalbumin were collected. DCOVA-sEVs activated by IFN-γ and lipopolysaccharide polarize M2 macrophages to M1 macrophages that are involved in a positive immune response. Because DCOVA-sEVs contain antigens, MHC I, MHC II and other molecules, activated DCOVA-sEVs interact with TLs, macrophages and DCs to improve antitumor immunity in vivo. DCOVA-sEVs increase antigen levels through APC-dependent mechanisms and APC-independent mechanisms, but the former is the major pathway, indicating that the delivery of APCs is the key to obtaining immunity. In addition, DCOVA-sEVs do not induce negative phenomena, such as promoting angiogenesis, and the dose used does not cause systemic toxicity, suggesting that they have bright development prospects as a type of DC-EV with high immune activity (Matsumoto et al., 2020).
In addition to delivering proteins as tumor vaccines, DC-EVs can also be directly loaded with drugs for antitumor therapy. In a recent study, DEXs with specific membrane proteins led their loaded fluorouracil (Fu) to directly target cancer cells, fuze with the cell membrane and increase drug internalization, which is expected to replace long-term intravenous administration of Fu and reduce side effects (Xu et al., 2020). However, few studies have examined the direct loading of therapeutic drugs in DC-EVs, and the therapeutic effect of the loaded drug still must be judged based on the results of practical experiments.
3.2.2. Stem cells
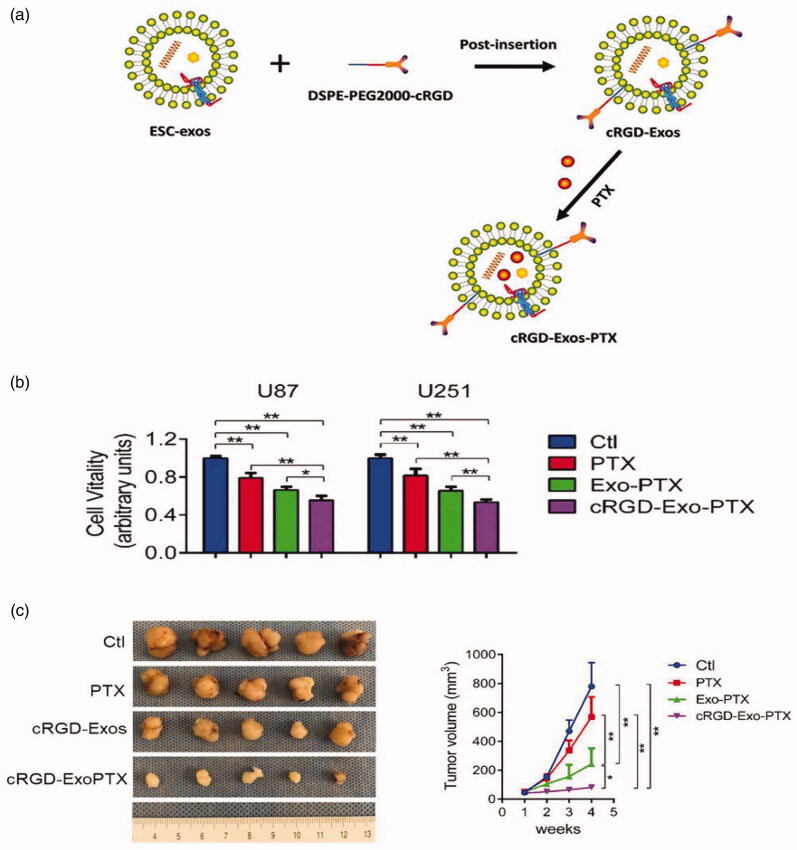
One of the important reasons why the problem of GBM is difficult to overcome is that the BBB prevents drugs from reaching the tumor, and the efficiency of various drug carriers at penetrating the BBB is very low. Naturally, exosomes, natural endogenous carriers, are widely used as ideal carriers for treatment due to their nontoxicity, protective drug-carrying capacity and strong penetration of biological barriers. A study took advantage of the unlimited proliferation of embryonic stem cells (ESCs) to mass produce ESC-derived exosomes (ESC-exos) for the treatment of GBM. ESC-exos contain ESC-specific reprogramming factors with antitumor function that reprogram malignant tumors to a benign phenotype (Costa et al., 2009; Díez-Torre et al., 2009; Zhou et al., 2017). A peptide (C (RGKyK)) was modified on the surface of ESC-exos to target the αvβ3 integrin receptor that is overexpressed on tumor cells to improve the targeting of these carriers, and then PTX was loaded (Figure 7(a)). The new carrier (cRGD-Exo-PTX) transports drugs to target tumors through the BBB and then releases drugs. Compared with the control group, the cRGD-Exo-PTX group decreased the vitality of GBM more effectively and inhibited the growth of GBM (Figure 7(b,c)). The use of ESC-exos as a drug delivery carrier is a promising treatment for GBM that may play an unexpected role in the treatment of other cancers (Zhu et al., 2019).
Figure 7.
Targeting capability of cRGD-Exo-PTX in vitro and in vivo. (a) Schematic diagram of the synthesis of cRGD-Exo-PTX. (b) Cell viability of PBS (CTL group), PTX, Exo-PTX, and cRGD-Exo-PTX groups during targeted therapy was determined using the CCK-8 assay. U87 and U251 are two human GBM cell lines. (c) Schematic diagram of subcutaneous U87 GBM in different groups and tumor volumes measured at specified time points during in vitro targeted therapy. Reproduced with permission from reference (Zhu et al., 2019).
MSCs are present in numerous tissues, such as fat and bone marrow, and MSCs from different sites also have distinct gene expression patterns and differentiation potential. Many studies have shown that MSCs are involved in tumor growth (Xie et al., 2019), angiogenesis (Zhu et al., 2012), metastasis (de Araujo Farias et al., 2018), drug resistance (Kabashima-Niibe et al., 2013) and other processes; therefore, MSCs have become a hot topic in cancer treatment. Bone marrow mesenchymal stem cells (BM-MSCs) have unique properties, such as immune regulation and strong self-renewal ability, and BM-MSC-derived exosomes (BM-MSCs-EXOs) have also been proved to have good therapeutic effects. As a messenger, BM-MSCs-EXOs transport various cargoes to receptor cells, including therapeutic drugs and a variety of biological macromolecules, such as RNA (Kabashima-Niibe et al., 2013; Błogowski et al., 2016; Zhou et al., 2020). Recently, antimiR-142-3P was modified with locked nucleic acid (LNA-antimiR-142-3p) and then loaded into BM-MSC-EXOs in an attempt to inhibit the growth of breast cancer stem cells. Due to the homologous targeting of BM-MSCs, the modified BM-MSC-EXOs delivered LNA-antimiR-142-3p to tumor stem cells and suppressed miR-142-3p expression, subsequently reducing the expression of miR-150 and ultimately significantly reducing the proliferation and tumor-initiating capability of tumor stem cells (Naseri et al., 2020). In addition, BM-MSCs-EXOs have low immunogenicity and can modulate the TME that affects tumor growth and development. Ying et al. transfected miR-193a into BM-MSCs-EXOs to target focal adhesion kinase in colon cancer, which downregulated the expression of focal adhesion kinase and inhibited the invasion, migration and proliferation of colon cancer cells (François et al., 2019; Ying et al., 2020). In another study, BM-MSCs-EXOs carrying miR-193A promoted apoptosis in drug-resistant lung cancer cells by downregulating LRRC1 (Wu et al., 2020). However, few studies have examined the transport RNA of BM-MSCs-EXOs to tumor stem cells, which creates new and feasible carriers for tumor drug delivery and has the value of further exploration.
In MSCs, the clinical application of adipose-derived mesenchymal stem cells (ADSCs) is widely used in the clinic, and it is one of the hotspots of tumor treatment (Miana and González, 2018). ADSCs are involved in regulating the TEM, interact with tumors, and have achieved satisfactory results in the treatment of bladder cancer and breast cancer (Rigotti et al., 2009; Wang et al., 2019). ADSC-derived EVs (ADSC-EVs) have no immunogenicity and have heterogeneous subsets of derived cells, thus obtaining good antitumor properties and representing a good tool for the treatment of tumors. Studies have proven that CD90lowADSC-EVs have anticancer activity (Zeng et al., 2019), Li and coauthors loaded miR-16-5p with an anticancer effect into CD90lowADSC-EVs and observed that it significantly promoted cancer cell apoptosis and slowed tumor growth (Li et al., 2020). Notably, miR-199a-3p is also an important miRNA involved in tumor regulation that is expressed at high levels in normal liver cells but downregulated in HCC (Callegari et al., 2018). After the inclusion of miR-199a-3p, exosomes secreted by ADSCs (ADSC-exo-199a) effectively targeted mammalian target of rapamycin (mTOR), which increased the sensitivity of tumors to chemotherapeutic drugs and significantly reduced the tumor volume (Lou et al., 2020). ADSC-derived exosome administration provides a new strategy for overcoming chemotherapeutic resistance.
3.2.3. Macrophages
According to many studies, macrophage-derived exosomes (M-EXOs) are indispensable in the communication between cancer cells and macrophages (Liu et al., 2020). As mentioned in the section on macrophage membranes above, macrophages differentiate into M1 and M2 phenotypes with different functions in response to different stimuli (Binenbaum et al., 2018).
Recently, Wang and colleagues compared the differences between M1 exosomes (M1-Exos) and M2 exosomes (M2-Exos). When macrophages were coincubated with these two exosomes, IL-12 and TNF-α levels were increased in the M1-Exos group, while the opposite results were obtained from the M2-Exos group, indicating the proinflammatory effect of M1-Exos. When PTX was loaded into M1-Exos, M1-Exos were used as carriers to deliver chemotherapeutic drugs, and the stimulation of M1-Exos with IFN-γ activated the NF-κB pathway, which created a proinflammatory environment, enhanced the antitumor effect of the drug, and fully exploited the advantages of M1-Exos. The drug-loaded exosomes (PTX-M1-Exos) were more likely to accumulate in the tumor than PTX because M1-Exos replicated the function of the source cells, effectively targeting tumors and activating macrophages to destroy tumors. In addition, the safety of PTX-M1-Exos was so high that it had no discernible organic toxicity, even if double the therapeutic dose was administered (Wang et al., 2019). In another study, an anti-CD47 antibody and anti-SIRP α antibody were modified on M1-Exos. When the drug was administered to the tumor site, it not only blocked CD47 on tumor cells to eliminate the ‘do not eat me’ signal but also targeted SIRP α on macrophages and enhanced the phagocytic activity of macrophages. In addition, M1-Exos also transform M2 macrophages into M1 macrophages, suggesting that M1-Exos have a bright future as an antitumor treatment (Nie et al., 2020).
In addition to the direct drug delivery method described above, M-Exos can also load NPs, improving the properties of NPs and enhancing the delivery of the drug to the desired site. Due to the lack of targets, many treatments for triple-negative breast cancer (TNBC) are ineffective, and the commonly used drug-loaded NPs are also ineffective because of their high toxicity and ability to activate systemic immunity (Yadav et al., 2014; Xu et al., 2018). A new study modified peptides targeting mesenchymal–epithelial transition factor on TNBC on M-Exos and then loaded PLGA NPs (MEP-D) containing the anticancer drug Dox, taking full advantage of the nontoxicity and low immunogenicity of these endogenous vesicles. The exosomes produced by immune cells inherit the tumor targeting ability of their parent cells and efficiently deliver large quantities of drugs to the target tissue. After administration, no tissue damage was observed in sections of the principal organs, and no significant changes were observed in the serum levels of liver-related enzymes, indicating low hepatotoxicity after MEP-D administration (Li et al., 2020).
In recent years, in-depth studies of macrophage-derived EVs have been conducted, which also provides more recent ideas for cancer treatment. M2-Exos were modified to inhibit tumors, and downregulating lncRNA SBF2-AS1 in M2-Exos promoted the expression of miR-122-5p and then inhibited the X-linked inhibitor of apoptosis protein, which ultimately prevented the development of pancreatic cancer (Yin et al., 2020). Therefore, M-Exos have a complex mechanism, and their more effective utilization still requires further research.
3.2.4. Cancer cells
Compared to normal cells, tumor cells contain more lipids, proteins, and nucleic acids, and exosomes secreted by tumor cells also contain these substances and play an important role in the communication between cancer tissues and distant organs. Tumor cell-derived exosomes (TEXs) are similar to their parent cells and interact with their parent cells. Lipids on the tumor cell membrane surface determine the fusion of TEXs with their parent cells (Parolini et al., 2009), and unique proteins on TEXs facilitate their fusion with parent cells (Smyth et al., 2014). When the drug Doxil (a Dox liposome) was loaded into HT1080 exosomes and used to treat HT1080 tumor-bearing mice, the drug-loaded HT1080 exosomes (D-HT1080 exos) displayed strong tumor targeting in vivo. Compared with the group that was directly administered Doxil, the concentration of Dox in the tumor site of the group treated with D-HT1080 exos increased by 2.3 times, and the retention time was also significantly increased (Qiao et al., 2020).
The metastasis of cancer cells not only depends on the characteristics of cells but is also closely related to the microenvironment of the premetastatic niche (PMN), which is conducive to the metastasis of primary tumors. S100A4 advances the formation of PMNs and builds a microenvironment suitable for the survival of malignant tumors, while an S100A4 siRNA (siS100A4) blocks the expression of S100A4 and inhibits tumor growth (Liu et al., 2018; van den Brand et al., 2018). Using the advantages of bionics, Zhao et al. conjugated cationic bovine serum albumin and siS100A4 (CBSA/siS100A4) and then encapsulated them within autologous breast cancer-derived exosomes (OCC@EXOs) to form a novel biomimetic carrier (CBSA/siS100A4@Exosomes). Due to the lung targeting properties of integrins on the OCC@EXO membrane, CBSA/siS100A4@Exosomes had a stronger ability to target lung tissue than liposome-coated CBSA/siS100A4, increasing drug release and adhesion to lung PMNs (Wortzel et al., 2019). In addition, OCC@EXOs reduced the cytotoxicity and immunogenicity of DDSs to a very low level and exhibited strong affinity for the parent cancer cells, which increased the release of drugs at the target and enhanced the anticancer effect (Zhao et al., 2020).
Cancer immunotherapy is an emerging treatment method. In contrast to chemotherapy and RT, cancer immunotherapy works by enhancing the ability of the immune system to fight against tumors, with minimal side effects. Proteins on TEXs, such as CD63, CD81, liposome-associated membrane protein 1 (LAMP1), MHC-I, MHC-II, and Annexin II, interact with ligands on DCs to promote the binding of DCs to TEXs (Hong et al., 2014; Gu et al., 2015). Under ideal conditions, the combination of an immune adjuvant with TEXs improves the immunogenicity of DCs and the efficacy of immunotherapy. High mobility group nucleosome-binding protein 1 (HMGN1) is a potent immune adjuvant that activates DCs and induces a sustained immune response (Yang et al., 2012). In a recent study, TEXs (TEX-N1ND) were modified with the functional N-terminus of HMGN1 (N1ND) to deliver TAAs and N1ND to DCs together, thereby activating DCs, promoting DC migration to lymph nodes, and increasing the generation of memory TLs. This treatment substantially increased the strength of antitumor immunity, remodeled the TME of orthotopic HCC in mice with a deficiency in original immunogenicity, and delayed tumor growth (Zuo et al., 2020). In addition, some miRNAs, such as miR-142 and let-7i, also activate DCs and TLs, and tumor cell-derived EVs loaded with multiple miRNAs have been used to deliver tumor-specific antigens to DCs, significantly affecting the maturation of DCs and the activity of CTLs, reducing tumor volume and prolonging the survival of mice (Khani et al., 2021). This attempt to activate DCs using TEXs transporting antigens and exogenous substances has promoted the development of DC immunotherapy.
3.2.5. Other sources
In addition to EVs produced by these cells (Table 2), several other sources of EVs have been attempted to be used for targeted cancer therapy in recent years, such as HEK293 cell-derived exosomes (HEK293-Exos), milk-derived exosomes secreted by mammary gland epithelial cells, and OMVs. HEK293 cells were transfected with miR-204-5p, which inhibits tumor growth and metastasis, and secreted exosomes stably expressing miR-204-5p. Epidermal growth factor and GE11 peptides on HEK293-Exos recognize epidermal growth factor ligand on tumor cells, enabling the carrier to target tumors, and then miR-204-5p effectively inhibited tumor growth in mice, increased apoptosis induced by 5-fluorouracil, and reversed drug resistance to chemotherapy (Yao et al., 2020).
Table 2.
Extracellular vesicles as delivery carriers for targeted cancer therapy.
| Donor | Cargo | Cancer type | Target | Outcome | Ref |
|---|---|---|---|---|---|
| DCs | Ovalbumin, LPS and IFN-γ |
T cell carcinoma |
Macrophages, DCs and T cells | Boosted both innate and adaptive immunity | (Matsumoto et al., 2020) |
| Breast cancer cells |
miR-126 | Lung cancer | A549 cells | Inhibiting lung metastasis | (Nie et al., 2020) |
| BM-MSCs | miR-375 | Cervical cancer |
Cervical cancer cells | Discover new biomarkers for cervical cancer treatment | (Ding et al., 2020) |
| Human liver stem cells |
miR-145 and miR-200 |
Renal cell carcinoma |
Renal cancer stem cells | Inhibiting tumor growth | (Brossa et al., 2020) |
| HEK-293 cells | HN3 protein | Liver cancer | GPC3 + HuH-7 cancer cells | Effectively targeting liver cancer cells and inhibiting tumor growth | (He et al., 2020) |
| DCs | CD9 and CD63 | Lung cancer | T cells and T cell subset populations | Induced immune responses | (Than et al., 2020) |
| DCs | E749-57 peptide |
Cervical cancer |
CD8+ T cells | Induced protective immunity responses to cervical cancer | (Chen et al., 2018) |
| Human breast cancer cells |
PTX-linoleic acid prodrug and CuB |
Breast cancer | CTCs | Inhibiting tumor regression and metastasis | (Wang et al., 2020) |
| BM-MSCs | Let-7 | Lung cancer | KDM3A/DCLK1/FXYD3 axis | Significantly suppressing cancer proliferation, migration and invasion | (Liu et al., 2021) |
| Breast cancer cells |
miRNAs (Let-7i, miR-142 and, miR-155) | Breast cancer | DCs and T cells | Inhibiting of solid tumors | (Khani et al., 2021) |
| Hepatocellula carcinoma cells |
miR30a-3p | Hepatocellular carcinoma |
SNAP23 gene | Effectively attenuating HCC migration, invasion, and metastasis | (Liu et al., 2021) |
Exosomes derived from bovine milk have the advantages of a low cost, high yield, easy extraction, and good biological and physical stability and are good tools for oncology drug delivery (Admyre et al., 2007; Chen et al., 2020). However, the lack of targeting is also an issue that must be addressed (Melnik et al., 2014). Bovine milk exosomes were modified with hyaluronic acid (HA), which targets CD44 overexpressed on tumors, and then loaded with Dox to form a new carrier (HA-mExo-Dox) that specifically targets CD44-positive tumor cells and allow tumor cells to efficiently take up Dox (Li et al., 2020).
OMVs facilitate communication between bacteria and the environment and are biodegradable and targeted. OMVs, as attractive carriers, carry immunostimulatory factors and induce appropriate immune responses, showing great potential in tumor immunotherapy. Dox-carrying OMVs (Dox-OMVs) from attenuated Klebsiella pneumoniae not only interact with epithelial cells to initiate immune signals and recruit immune cells but also directly interact with macrophages to activate the immune system, induce antitumor immunity and increase the toxicity toward non-small cell lung cancer (Kuerban et al., 2020).
4. Future challenges
Many advantages of cell membrane-coated nanoparticles and EVs have been reported, especially in terms of targeting and biocompatibility. Current synthetic DDSs are essentially foreign materials with potential toxicity and immunogenicity, while cell membranes and EVs are endogenous and are deemed to be biocompatible and have multiple biological functions that are similar to the source cell. However, some issues remain to be addressed for these carriers to continue to evolve and make the transition from the laboratory to the clinic.
First, the question of yield must be addressed. Existing separation technologies not only produce a small amount of EVS but are also expensive for large-scale production. Therefore, more advanced large-scale production methods are needed to continue to expand the application of EVs. Initially, scientists increased the release of vesicles by adding exogenous compounds to the cells from which EVs are derived (Allan et al., 1980). In recent years, an increasing number of studies on EV mimetics, which are artificial vesicles obtained from the membrane broken by extrusion, have been conducted to solve the yield problem (Sil et al., 2020). The properties of EV mimetics are similar to those of natural EVs, with better scalability and higher bioavailability. The same number of THP-1 cells produces 2.5 times more simulated exosomes than natural exosomes, and the former has higher encapsulation and drug release rates (Pisano et al., 2020). The process used to prepare cell membranes is mature and results in a much higher yield than the preparation of exosomes, but the separation and purification schemes also must be adjusted and optimized because a large number of cells still need to be cultured to obtain a sufficient number of membranes, and the preparation process still needs to be simplified (Li et al., 2018).
The processes of modification and loading may alter the original properties of the cell membrane. For RBCMs that lack a targeting capability, the membranes must be endowed with the ability to reach the target site for the release of therapeutic cargoes, but the modification of the membrane is likely to change its original structure and reduce the biocompatibility of the carrier. PLTM is highly sensitive, and a suitable loading scheme must be identified to ensure sufficient drug loading and safe delivery of the drug to the target site (Wang et al., 2020). The stability and toxicity of modified or drug-loaded EVs also require further exploration, especially as carriers for cancer nanomedicines. The appropriate drug loading method should be selected to effectively load the drug into the EVs with the minimum ratio of carrier to drug to achieve the desired dose and release profile (Susa et al., 2019).
In addition, the complex mechanism of the transport of cell membranes from different sources as carriers in vivo is not completely understood and requires further study (Li et al., 2020). For example, the delivery of therapeutic molecules by carriers based on white cell membranes may activate components of the immune system and trigger inflammation (Jin et al., 2018). When CCM is used, it may induce cancer development in the body if the genetic material from the parent cancer cells is not completely eliminated. Methods for the purification and characterization of EVs vary from laboratory to laboratory, and different methods may result in confusion regarding the subgroups and physicochemical properties of EVs. Therefore, researchers can share data and reasonably develop a unified standardized procedure with excellent repeatability for the quality control of EVs.
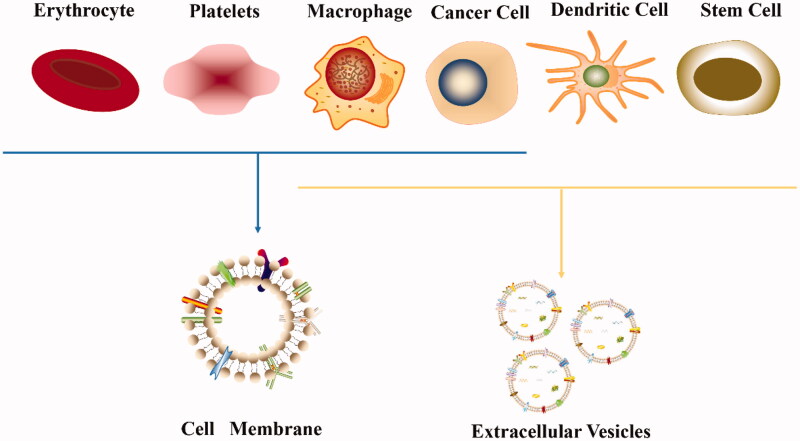
Both the cell membrane and EVs enable carriers to effectively cross biological barriers and target cancer tissues. Some cells can not only be used to extract membranes to prepare carriers but also for the isolation of their EVs to transport drugs (Figure 8). The extraction and preparation of the cell membrane is relatively easy, but the targeting ability may be impaired due to the loss of proteins during membrane extraction. Although the preparation of EVs is challenging, they generally retain the complete membrane components, and thus they have excellent targeting ability (Xia et al., 2020). Therefore, the appropriate carrier must be selected according to the purpose of the experiment to improve the therapeutic effect as much as possible. Differences between cell membrane vesicles and EVs are summarized in Table 3.
Figure 8.
Common cell types of carriers for tumor targeted therapy.
Table 3.
Comparison of cell membrane vesicles and extracellular vesicles.
| Cell membrane vesicles | Extracellular vesicles | |
|---|---|---|
| Source | Extrusion of cell membrane | Secretion by cells |
| Size (diameter) | 100–400 nm | 20 nm–2 μm |
| Separation | Separation process of membrane and intramembrane mixture | Separation of extracellular vesicles and source cells |
| Cargo loading | Nanoparticles or direct loading of drugs | Drugs are usually loaded directly. |
| Source of cells | Red blood cell, white blood cell, platelet, cancer cell, fibroblast, bacterial, etc. | Dendritic cell, stem cell, macrophage, cancer cell, HEK293 cell, bacterial outer membrane vesicles, etc. |
| Advantages | Long-term circulation; Good biological barrier permeability; Efficient cell fusion; Large production. | Low immunogenicity, non-cytotoxicity, high biocompatibility; Intrinsic tumor targeting; Efficient cellular uptake. |
5. Conclusions
Although cell-based DDSs face many challenges, their powerful advantage of ‘mimicking nature’ still overcomes many of the disadvantages of traditional DDSs and provides a more effective strategy for cancer treatment. The substance on the surface of the new DDS takes advantage of the natural characteristics of cells, such as the enrichment of targeted proteins, long-term circulation in the body, ability to pass through biological barriers, interactions with other cells, and reduced tissue and cell toxicity, which effectively protect the cargo carried and substantially improve the therapeutic effect. With the rapid development of pharmacology, material science, bioinformatics, proteomics and nanotechnology, the combination of DDSs and cells is expected to overcome many obstacles, change the current medical technology, and provide new horizons for targeted cancer therapy.
Funding Statement
The work was supported by the projects of the National Natural Science Foundation of China [81873011, 82074272], the Science and Technology Commission of Shanghai Municipality [20S21900300], the Outstanding Talents Program of Shanghai Health and Family Planning Commission [2018BR27]
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abramowicz A, Widlak P, Pietrowska M. (2016). Proteomic analysis of exosomal cargo: the challenge of high purity vesicle isolation. Mol Biosyst 12:1407–19. [DOI] [PubMed] [Google Scholar]
- Admyre C, Johansson SM, Qazi KR, et al. (2007). Exosomes with immune modulatory features are present in human breast milk. J Immunol 179:1969–78. [DOI] [PubMed] [Google Scholar]
- Allan D, Thomas P, Limbrick AR. (1980). The isolation and characterization of 60 nm vesicles ('nanovesicles') produced during ionophore A23187-induced budding of human erythrocytes. Biochem J 188:881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez ML, Khosroheidari M, Kanchi Ravi R, et al. (2012). Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int 82:1024–32. [DOI] [PubMed] [Google Scholar]
- Avval ZM, Malekpour L, Raeisi F, et al. (2020). Introduction of magnetic and supermagnetic nanoparticles in new approach of targeting drug delivery and cancer therapy application. Drug Metab Rev 52:157–84. [DOI] [PubMed] [Google Scholar]
- Bang KH, Na YG, Huh HW, et al. (2019). The delivery strategy of paclitaxel nanostructured lipid carrier coated with platelet membrane. Cancers 11:807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binenbaum Y, Fridman E, Yaari Z, et al. (2018). Transfer of miRNA in macrophage-derived exosomes induces drug resistance in pancreatic adenocarcinoma. Cancer Res 78:5287–99. [DOI] [PubMed] [Google Scholar]
- Błogowski W, Bodnarczuk T, Starzyńska T. (2016). Concise review: pancreatic cancer and bone marrow-derived stem cells. Stem Cells Transl Med 5:938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossa A, Fonsato V, Grange C, et al. (2020). Extracellular vesicles from human liver stem cells inhibit renal cancer stem cell-derived tumor growth in vitro and in vivo. Int J Cancer 147:1694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu LL, Rao L, Yu GT, et al. (2019). Cancer stem cell-platelet hybrid membrane-coated magnetic nanoparticles for enhanced photothermal therapy of head and neck squamous cell carcinoma. Adv Funct Mater 29:1807733. [Google Scholar]
- Callegari E, D’Abundo L, Guerriero P, et al. (2018). miR-199a-3p modulates MTOR and PAK4 pathways and inhibits tumor growth in a hepatocellular carcinoma transgenic mouse model. Mol Ther Nucleic Acids 11:485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Hu Y, Luo S, et al. (2019). Neutrophil-mimicking therapeutic nanoparticles for targeted chemotherapy of pancreatic carcinoma. Acta Pharm Sin B 9:575–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Tan T, Zhu D, et al. (2020). Paclitaxel-loaded macrophage membrane camouflaged albumin nanoparticles for targeted cancer therapy. Int J Nanomedicine 15:1915–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Z, Hu X, Wei X, et al. (2017). A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery. J Control Release 264:102–11. [DOI] [PubMed] [Google Scholar]
- Chang ZM, Zhou H, Yang C, et al. (2020). Biomimetic immunomagnetic gold hybrid nanoparticles coupled with inductively coupled plasma mass spectrometry for the detection of circulating tumor cells. J Mater Chem B 8:5019–25. [DOI] [PubMed] [Google Scholar]
- Chen D, Cai L, Guo Y, et al. (2020). Cancer cell membrane-decorated zeolitic-imidazolate frameworks codelivering cisplatin and oleanolic acid induce apoptosis and reversed multidrug resistance on bladder carcinoma cells. ACS Omega 5:995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DS, Mellman I. (2013). Oncology meets immunology: the cancer-immunity cycle. Immunity 39:1–10. [DOI] [PubMed] [Google Scholar]
- Chen Q, Hu Q, Dukhovlinova E, et al. (2019). Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells. Adv Mater 31:e1900192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Huang G, Wu W, et al. (2020). A hybrid eukaryotic-prokaryotic nanoplatform with photothermal modality for enhanced antitumor vaccination. Adv Mater 32:e1908185. [DOI] [PubMed] [Google Scholar]
- Chen S, Lv M, Fang S, et al. (2018). Poly(I:C) enhanced anti-cervical cancer immunities induced by dendritic cells-derived exosomes. Int J Biol Macromol 113:1182–7. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang Q, Liu L, et al. (2018). Double-sided effect of tumor microenvironment on platelets targeting nanoparticles. Biomaterials 183:258–67. [DOI] [PubMed] [Google Scholar]
- Chen Z, Xie Y, Luo J, et al. (2020). Milk exosome-derived miRNAs from water buffalo are implicated in immune response and metabolism process. BMC Vet Res 16:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R, Fontana F, Xiao J, et al. (2020). Recombination monophosphoryl lipid A-derived vacosome for the development of preventive cancer vaccines. ACS Appl Mater Interfaces 12:44554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes F, Meyer E, Sanders NN. (2020). Immune cells as tumor drug delivery vehicles. J Control Release 327:70–87. [DOI] [PubMed] [Google Scholar]
- Copp JA, Fang RH, Luk BT, et al. (2014). Clearance of pathological antibodies using biomimetic nanoparticles. Proc Natl Acad Sci USA 111:13481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa FF, Seftor EA, Bischof JM, et al. (2009). Epigenetically reprogramming metastatic tumor cells with an embryonic microenvironment. Epigenomics 1:387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniyal M, Jian Y, Xiao F, et al. (2020). Development of a nanodrug-delivery system camouflaged by erythrocyte membranes for the chemo/phototherapy of cancer. Nanomedicine 15:691–709. [DOI] [PubMed] [Google Scholar]
- de Araujo Farias V, O'Valle F, Serrano-Saenz S, et al. (2018). Exosomes derived from mesenchymal stem cells enhance radiotherapy-induced cell death in tumor and metastatic tumor foci. Mol Cancer 17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cristo Soares Alves A, Lavayen V, Figueiró F, et al. (2020). Chitosan-coated lipid-core nanocapsules functionalized with Gold-III and bevacizumab induced in vitro cytotoxicity against C6 cell line and in vivo potent antiangiogenic activity. Pharm Res 37:91. [DOI] [PubMed] [Google Scholar]
- Dei S, Braconi L, Trezza A, et al. (2019). Modulation of the spacer in N,N-bis(alkanol)amine aryl ester heterodimers led to the discovery of a series of highly potent P-glycoprotein-based multidrug resistance (MDR) modulators. Eur J Med Chem 172:71–94. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Ruffell B. (2019). Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol 19:369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez-Torre A, Andrade R, Eguizábal C, et al. (2009). Reprogramming of melanoma cells by embryonic microenvironments. Int J Dev Biol 53:1563–8. [DOI] [PubMed] [Google Scholar]
- Ding F, Liu J, Zhang X. (2020). microRNA-375 released from extracellular vesicles of bone marrow mesenchymal stem cells exerts anti-oncogenic effects against cervical cancer. Stem Cell Res Ther 11:455. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ebrahimi A, Csonka LN, Alam MA. (2018). Analyzing thermal stability of cell membrane of Salmonella using time-multiplexed impedance sensing. Biophys J 114:609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang RH, Hu CM, Chen KN, et al. (2013). Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles. Nanoscale 5:8884–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang RH, Kroll AV, Gao W, et al. (2018). Cell membrane coating nanotechnology. Adv Mater 30:e1706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François S, Usunier B, Forgue-Lafitte ME, et al. (2019). Mesenchymal stem cell administration attenuates colon cancer progression by modulating the immune component within the colorectal tumor microenvironment. Stem Cells Transl Med 8:285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Moore EC, Zolkind P, et al. (2020). Neoadjuvant PD-1 immune checkpoint blockade reverses functional immunodominance among tumor antigen-specific T cells. Clin Cancer Res 26:679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Liang M, Wang Y, et al. (2019). Dual-modified novel biomimetic nanocarriers improve targeting and therapeutic efficacy in glioma. ACS Appl Mater Interfaces 11:1841–54. [DOI] [PubMed] [Google Scholar]
- Fusciello M, Fontana F, Tähtinen S, et al. (2019). Artificially cloaked viral nanovaccine for cancer immunotherapy. Nat Commun 10:5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan J, Du G, He C, et al. (2020). Tumor cell membrane enveloped aluminum phosphate nanoparticles for enhanced cancer vaccination. J Control Release 326:297–309. [DOI] [PubMed] [Google Scholar]
- Gao C, Chu X, Gong W, et al. (2020). Neuron tau-targeting biomimetic nanoparticles for curcumin delivery to delay progression of Alzheimer's disease. J Nanobiotechnol 18:71. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gargiulo E, Paggetti J, Moussay E. (2019). Hematological malignancy-derived small extracellular vesicles and tumor microenvironment: the art of turning foes into friends. Cells 8:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C, Yu X, You B, et al. (2020). Macrophage-cancer hybrid membrane-coated nanoparticles for targeting lung metastasis in breast cancer therapy. J Nanobiotechnol 18:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Erb U, Büchler MW, et al. (2015). Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int J Cancer 136:E74–84. [DOI] [PubMed] [Google Scholar]
- Guan S, Zhang Q, Bao J, et al. (2020). Phosphatidylserine targeting peptide-functionalized pH sensitive mixed micelles for enhanced anti-tumor drug delivery. Eur J Pharm Biopharm 147:87–101. [DOI] [PubMed] [Google Scholar]
- Han X, Wang C, Liu Z. (2018). Red blood cells as smart delivery systems. Bioconjug Chem 29:852–60. [DOI] [PubMed] [Google Scholar]
- Han Y, Zhao R, Xu F. (2018). Neutrophil-based delivery systems for nanotherapeutics. Small 14:e1801674. [DOI] [PubMed] [Google Scholar]
- He C, Jaffar Ali D, Li Y, et al. (2020). Engineering of HN3 increases the tumor targeting specificity of exosomes and upgrade the anti-tumor effect of sorafenib on HuH-7 cells. PeerJ 8:e9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Guo C, Wang J, et al. (2018). Leutusome: a biomimetic nanoplatform integrating plasma membrane components of leukocytes and tumor cells for remarkably enhanced solid tumor homing. Nano Lett 18:6164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Zhang Y, Feng N. (2020). Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: a review. Mater Sci Eng C Mater Biol Appl 106:110298. [DOI] [PubMed] [Google Scholar]
- Hong CS, Muller L, Boyiadzis M, et al. (2014). Isolation and characterization of CD34+ blast-derived exosomes in acute myeloid leukemia. PLOS One 9:e103310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JL, Scott MJ, Wickline SA. (2014). Maximizing exosome colloidal stability following electroporation. Anal Biochem 448:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Lei T, Wang Y, et al. (2020). Phagocyte-membrane-coated and laser-responsive nanoparticles control primary and metastatic cancer by inducing anti-tumor immunity. Biomaterials 255:120159. [DOI] [PubMed] [Google Scholar]
- Hu CM, Fang RH, Wang KC, et al. (2015). Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526:118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CM, Zhang L, Aryal S, et al. (2011). Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci USA 108:10980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Sheng Z, Gao G, et al. (2016). Activatable albumin-photosensitizer nanoassemblies for triple-modal imaging and thermal-modulated photodynamic therapy of cancer. Biomaterials 93:10–9. [DOI] [PubMed] [Google Scholar]
- Hu Z, Ott PA, Wu CJ. (2018). Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol 18:168–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Mei CM, Tian YQ, et al. (2018). Bioinspired tumor-homing nanosystem for precise cancer therapy via reprogramming of tumor-associated macrophages. NPG Asia Mater 10:1002–15. [Google Scholar]
- Hyslop SR, Josefsson EC. (2017). Undercover agents: targeting tumours with modified platelets. Trends Cancer 3:235–46. [DOI] [PubMed] [Google Scholar]
- Janiszewska M, Primi MC, Izard T. (2020). Cell adhesion in cancer: beyond the migration of single cells. J Biol Chem 295:2495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäppinen N, Félix I, Lokka E, et al. (2019). Fetal-derived macrophages dominate in adult mammary glands. Nat Commun 10:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Liu Y, Guo R, et al. (2019). Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials 192:292–308. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Wang K, Zhang X, et al. (2020). Platelet membrane-camouflaged magnetic nanoparticles for ferroptosis-enhanced cancer immunotherapy. Small 16:e2001704. [DOI] [PubMed] [Google Scholar]
- Jin J, Bhujwalla ZM. (2019). Biomimetic nanoparticles camouflaged in cancer cell membranes and their applications in cancer theranostics. Front Oncol 9:1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Luo Z, Zhang B, et al. (2018). Biomimetic nanoparticles for inflammation targeting. Acta Pharm Sin B 8:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun Y, Tang Z, Luo C, et al. (2020). Leukocyte-mediated combined targeted chemo and gene therapy for esophageal cancer. ACS Appl Mater Interfaces 12:47330–41. [DOI] [PubMed] [Google Scholar]
- Kabashima-Niibe A, Higuchi H, Takaishi H, et al. (2013). Mesenchymal stem cells regulate epithelial-mesenchymal transition and tumor progression of pancreatic cancer cells. Cancer Sci 104:157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khani AT, Sharifzad F, Mardpour S, et al. (2021). Tumor extracellular vesicles loaded with exogenous Let-7i and miR-142 can modulate both immune response and tumor microenvironment to initiate a powerful anti-tumor response. Cancer Lett 501:200–9. [DOI] [PubMed] [Google Scholar]
- Kim MW, Lee G, Niidome T, et al. (2020). Platelet-like gold nanostars for cancer therapy: the ability to treat cancer and evade immune reactions. Front Bioeng Biotechnol 8:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Yoshioka Y, et al. (2010). Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285:17442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerban K, Gao X, Zhang H, et al. (2020). Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm Sin B 10:1534–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Wang F, Gui L, et al. (2018). The potential of biomimetic nanoparticles for tumor-targeted drug delivery. Nanomedicine 13:2099–118. [DOI] [PubMed] [Google Scholar]
- Li B, Yuan Z, Zhang P, et al. (2018). Zwitterionic nanocages overcome the efficacy loss of biologic drugs. Adv Mater 30:e1705728. [DOI] [PubMed] [Google Scholar]
- Li D, Yao S, Zhou Z, et al. (2020). Hyaluronan decoration of milk exosomes directs tumor-specific delivery of doxorubicin. Carbohydr Res 493:108032. [DOI] [PubMed] [Google Scholar]
- Li J, Zhen X, Lyu Y, et al. (2018). Cell membrane coated semiconducting polymer nanoparticles for enhanced multimodal cancer phototheranostics. ACS Nano 12:8520–30. [DOI] [PubMed] [Google Scholar]
- Li M, Fang H, Liu Q, et al. (2020). Red blood cell membrane-coated upconversion nanoparticles for pretargeted multimodality imaging of triple-negative breast cancer. Biomater Sci 8:1802–14. [DOI] [PubMed] [Google Scholar]
- Li R, He Y, Zhang S, et al. (2018). Cell membrane-based nanoparticles: a new biomimetic platform for tumor diagnosis and treatment. Acta Pharm Sin B 8:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Wu Y, Ding F, et al. (2020). Engineering macrophage-derived exosomes for targeted chemotherapy of triple-negative breast cancer. Nanoscale 12:10854–62. [DOI] [PubMed] [Google Scholar]
- Li T, Qin X, Li Y, et al. (2020). Cell membrane coated-biomimetic nanoplatforms toward cancer theranostics. Front Bioeng Biotechnol 8:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Zhou X, Wang J, et al. (2020). Adipose-derived mesenchymal stem cells and extracellular vesicles confer antitumor activity in preclinical treatment of breast cancer. Pharmacol Res 157:104843. [DOI] [PubMed] [Google Scholar]
- Liang B, Deng T, Li J, et al. (2020). Biomimetic theranostic strategy for anti-metastasis therapy of breast cancer via the macrophage membrane camouflaged superparticles. Mater Sci Eng C Mater Biol Appl 115:111097. [DOI] [PubMed] [Google Scholar]
- Liu B, Wang W, Fan J, et al. (2019). RBC membrane camouflaged Prussian blue nanoparticles for gamabutolin loading and combined chemo/photothermal therapy of breast cancer. Biomaterials 217:119301. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhou X, Long Q, et al. (2021). Small extracellular vesicles containing miR-30a-3p attenuate the migration and invasion of hepatocellular carcinoma by targeting SNAP23 gene. Oncogene 40:233–45. [DOI] [PubMed] [Google Scholar]
- Liu G, Zhao X, Zhang Y, et al. (2019). Engineering biomimetic platesomes for pH-responsive drug delivery and enhanced antitumor activity. Adv Mater 31:e1900795. [DOI] [PubMed] [Google Scholar]
- Liu J, Feng Y, Zeng X, et al. (2021). Extracellular vesicles-encapsulated let-7i shed from bone mesenchymal stem cells suppress lung cancer via KDM3A/DCLK1/FXYD3 axis. J Cell Mol Med 25:1911–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wu F, Zhou H. (2020). Macrophage-derived exosomes in cancers: biogenesis, functions and therapeutic applications. Immunol Lett 227:102–8. [DOI] [PubMed] [Google Scholar]
- Liu R, An Y, Jia W, et al. (2020). Macrophage-mimic shape changeable nanomedicine retained in tumor for multimodal therapy of breast cancer. J Control Release 321:589–601. [DOI] [PubMed] [Google Scholar]
- Liu R, Yu MN, Yang XT, et al. (2019). Linear chimeric triblock molecules self-assembled micelles with controllably transformable property to enhance tumor retention for chemo-photodynamic therapy of breast cancer. Adv. Funct. Mater 29:16. [Google Scholar]
- Liu Y, Bhattarai P, Dai Z, et al. (2019). Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem Soc Rev 48:2053–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Geng YH, Yang H, et al. (2018). Extracellular ATP drives breast cancer cell migration and metastasis via S100A4 production by cancer cells and fibroblasts. Cancer Lett 430:1–10. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang X, Ouyang B, et al. (2018). Erythrocyte-platelet hybrid membranes coating polypyrrol nanoparticles for enhanced delivery and photothermal therapy. J Mater Chem B 6:7033–41. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhao J, Jiang J, et al. (2020). Doxorubicin delivered using nanoparticles camouflaged with mesenchymal stem cell membranes to treat colon cancer. Int J Nanomedicine 15:2873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou G, Chen L, Xia C, et al. (2020). MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res 39:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Zuo B, Jing R, et al. (2017). Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol 67:739–48. [DOI] [PubMed] [Google Scholar]
- Maiti D, Chao Y, Dong Z, et al. (2018). Development of a thermosensitive protein conjugated nanogel for enhanced radio-chemotherapy of cancer. Nanoscale 10:13976–85. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Asuka M, Takahashi Y, et al. (2020). Antitumor immunity by small extracellular vesicles collected from activated dendritic cells through effective induction of cellular and humoral immune responses. Biomaterials 252:120112. [DOI] [PubMed] [Google Scholar]
- Melnik BC, John SM, Schmitz G. (2014). Milk: an exosomal microRNA transmitter promoting thymic regulatory T cell maturation preventing the development of atopy? J Transl Med 12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miana VV, González EAP. (2018). Adipose tissue stem cells in regenerative medicine. ecancer 12:822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi M, Hashemi Goradel N, Farhood B, et al. (2019). Macrophage polarity in cancer: a review. J Cell Biochem 120:2756–65. [DOI] [PubMed] [Google Scholar]
- Naseri Z, Oskuee RK, Forouzandeh-Moghadam M, et al. (2020). Delivery of LNA-antimiR-142-3p by mesenchymal stem cells-derived exosomes to breast cancer stem cells reduces tumorigenicity. Stem Cell Rev Rep 16:541–56. [DOI] [PubMed] [Google Scholar]
- Negi LM, Talegaonkar S, Jaggi M, et al. (2019). Hyaluronated imatinib liposomes with hybrid approach to target CD44 and P-gp overexpressing MDR cancer: an in-vitro, in-vivo and mechanistic investigation. J Drug Target 27:183–92. [DOI] [PubMed] [Google Scholar]
- Nie D, Dai Z, Li J, et al. (2020). Cancer-cell-membrane-coated nanoparticles with a yolk-shell structure augment cancer chemotherapy. Nano Lett 20:936–46. [DOI] [PubMed] [Google Scholar]
- Nie H, Xie X, Zhang D, et al. (2020). Use of lung-specific exosomes for miRNA-126 delivery in non-small cell lung cancer. Nanoscale 12:877–87. [DOI] [PubMed] [Google Scholar]
- Nie W, Wu G, Zhang J, et al. (2020). Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angew Chem Int Ed Engl 59:2018–22. [DOI] [PubMed] [Google Scholar]
- Palmer DH, Midgley RS, Mirza N, et al. (2009). A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology 49:124–32. [DOI] [PubMed] [Google Scholar]
- Parolini I, Federici C, Raggi C, et al. (2009). Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem 284:34211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RB, Ye M, Carlson PM, et al. (2019). Development of an in situ cancer vaccine via combinational radiation and bacterial-membrane-coated nanoparticles. Adv Mater 31:e1902626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuengkham H, Ren L, Shin IW, et al. (2019). Nanoengineered immune niches for reprogramming the immunosuppressive tumor microenvironment and enhancing cancer immunotherapy. Adv Mater 31:e1803322. [DOI] [PubMed] [Google Scholar]
- Pisano S, Pierini I, Gu J, et al. (2020). Immune (cell) derived exosome mimetics (IDEM) as a treatment for ovarian cancer. Front Cell Dev Biol 8:553576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Hu S, Huang K, et al. (2020). Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics 10:3474–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao L, Cai B, Bu LL, et al. (2017). Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano 11:3496–505. [DOI] [PubMed] [Google Scholar]
- Rigotti G, Marchi A, Sbarbati A. (2009). Adipose-derived mesenchymal stem cells: past, present, and future. Aesthetic Plast Surg 33:271–3. [DOI] [PubMed] [Google Scholar]
- Roma-Rodrigues C, Pombo I, Raposo L, et al. (2019). Nanotheranostics targeting the tumor microenvironment. Front Bioeng Biotechnol 7:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Wang Q, Wu B, et al. (2019). Platelet-membrane-camouflaged black phosphorus quantum dots enhance anticancer effect mediated by apoptosis and autophagy. ACS Appl Mater Interfaces 11:28254–66. [DOI] [PubMed] [Google Scholar]
- Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. (2018). Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 233:6425–40. [DOI] [PubMed] [Google Scholar]
- Sil S, Dagur RS, Liao K, et al. (2020). Strategies for the use of extracellular vesicles for the delivery of therapeutics. J Neuroimmune Pharmacol 15:422–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth TJ, Redzic JS, Graner MW, et al. (2014). Examination of the specificity of tumor cell derived exosomes with tumor cells in vitro. Biochim Biophys Acta 1838:2954–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Sun H, Meng Q, et al. (2017). Enhanced blood suspensibility and laser-activated tumor-specific drug release of theranostic mesoporous silica nanoparticles by functionalizing with erythrocyte membranes. Theranostics 7:523–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Duan Y, Ma Y, et al. (2020). Cancer cell-erythrocyte hybrid membrane coated gold nanocages for near infrared light-activated photothermal/radio/chemotherapy of breast cancer. Int J Nanomedicine 15:6749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susa F, Limongi T, Dumontel B, et al. (2019). Engineered extracellular vesicles as a reliable tool in cancer nanomedicine. Cancers 11:1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauro BJ, Greening DW, Mathias RA, et al. (2012). Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 56:293–304. [DOI] [PubMed] [Google Scholar]
- Tesi RJ. (2019). MDSC; the most important cell you have never heard of. Trends Pharmacol Sci 40:4–7. [DOI] [PubMed] [Google Scholar]
- Than UTT, Le HT, Hoang DH, et al. (2020). Induction of antitumor immunity by exosomes isolated from cryopreserved cord blood monocyte-derived dendritic cells. Int J Mol Sci 21:1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand D, Mertens V, Massuger L, et al. (2018). siRNA in ovarian cancer – delivery strategies and targets for therapy. J Control Release 283:45–58. [DOI] [PubMed] [Google Scholar]
- Veldman J, Visser L, Berg AVD, et al. (2020). Primary and acquired resistance mechanisms to immune checkpoint inhibition in Hodgkin lymphoma. Cancer Treat Rev 82:101931. [DOI] [PubMed] [Google Scholar]
- Viaud S, Théry C, Ploix S, et al. (2010). Dendritic cell-derived exosomes for cancer immunotherapy: what's next? Cancer Res 70:1281–5. [DOI] [PubMed] [Google Scholar]
- Wang D, Gao C, Zhou C, et al. (2020). Leukocyte membrane-coated liquid metal nanoswimmers for actively targeted delivery and synergistic chemophotothermal therapy. Research 2020:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Wu B, Li Q, et al. (2020). Active transportation of liposome enhances tumor accumulation, penetration, and therapeutic efficacy. Small 16:e2004172. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu Y, He R, et al. (2020). Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater Sci 8:552–68. [DOI] [PubMed] [Google Scholar]
- Wang H, Wu J, Williams GR, et al. (2019). Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. J Nanobiotechnology 17:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Ye H, Zhang X, et al. (2020). An exosome-like programmable-bioactivating paclitaxel prodrug nanoplatform for enhanced breast cancer metastasis inhibition. Biomaterials 257:120224. [DOI] [PubMed] [Google Scholar]
- Wang P, Jiang F, Chen B, et al. (2020). Bioinspired red blood cell membrane-encapsulated biomimetic nanoconstructs for synergistic and efficacious chemo-photothermal therapy. Colloids Surf B Biointerfaces 189:110842. [DOI] [PubMed] [Google Scholar]
- Wang P, Wang H, Huang Q, et al. (2019). Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics 9:1714–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Yu X, Lin J, et al. (2019). Adipose-derived stem cells inhibited the proliferation of bladder tumor cells by S phase arrest and Wnt/β-catenin pathway. Cell Reprogram 21:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willms E, Cabañas C, Mäger I, et al. (2018). Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol 9:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortzel I, Dror S, Kenific CM, et al. (2019). Exosome-mediated metastasis: communication from a distance. Dev Cell 49:347–60. [DOI] [PubMed] [Google Scholar]
- Wu H, Mu X, Liu L, et al. (2020). Bone marrow mesenchymal stem cells-derived exosomal microRNA-193a reduces cisplatin resistance of non-small cell lung cancer cells via targeting LRRC1. Cell Death Dis 11:801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Xie W, Zan HM, et al. (2020). Platelet membrane-coated nanoparticles for targeted drug delivery and local chemo-photothermal therapy of orthotopic hepatocellular carcinoma. J Mater Chem B 8:4648–59. [DOI] [PubMed] [Google Scholar]
- Wu M, Le W, Mei T, et al. (2019). Cell membrane camouflaged nanoparticles: a new biomimetic platform for cancer photothermal therapy. Int J Nanomedicine 14:4431–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Rao L, Yao H, et al. (2020). Engineering macrophages for cancer immunotherapy and drug delivery. Adv Mater 32:e2002054. [DOI] [PubMed] [Google Scholar]
- Xie C, Du LY, Guo F, et al. (2019). Exosomes derived from microRNA-101-3p-overexpressing human bone marrow mesenchymal stem cells suppress oral cancer cell proliferation, invasion, and migration. Mol Cell Biochem 458:11–26. [DOI] [PubMed] [Google Scholar]
- Xu C, Feng Q, Yang H, et al. (2018). A light-triggered mesenchymal stem cell delivery system for photoacoustic imaging and chemo-photothermal therapy of triple negative breast cancer. Adv Sci 5:1800382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Liu W, Hu Y, et al. (2020). Bioinspired tumor-homing nanoplatform for co-delivery of paclitaxel and siRNA-E7 to HPV-related cervical malignancies for synergistic therapy. Theranostics 10:3325–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CH, Ye PJ, Zhou YC, et al. (2020). Cell membrane-camouflaged nanoparticles as drug carriers for cancer therapy. Acta Biomater 105:1–14. [DOI] [PubMed] [Google Scholar]
- Xu M, Chen Q, Li J, et al. (2020). Dendritic cell-derived exosome-entrapped fluorouracil can enhance its anti-colon cancer effect. J BUON 25:1413–22. [PubMed] [Google Scholar]
- Xu R, Simpson RJ, Greening DW. (2017). A protocol for isolation and proteomic characterization of distinct extracellular vesicle subtypes by sequential centrifugal ultrafiltration. Methods Mol Biol 1545:91–116. [DOI] [PubMed] [Google Scholar]
- Xue J, Zhao Z, Zhang L, et al. (2017). Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol 12:692–700. [DOI] [PubMed] [Google Scholar]
- Yadav BS, Sharma SC, Chanana P, et al. (2014). Systemic treatment strategies for triple-negative breast cancer. World J Clin Oncol 5:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Postnikov YV, Li Y, et al. (2012). High-mobility group nucleosome-binding protein 1 acts as an alarmin and is critical for lipopolysaccharide-induced immune responses. J Exp Med 209:157–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Yin Y, Jin G, et al. (2020). Exosome-mediated delivery of miR-204-5p inhibits tumor growth and chemoresistance. Cancer Med 9:5989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Zhou Y, Ma T, et al. (2020). Down-regulated lncRNA SBF2-AS1 in M2 macrophage-derived exosomes elevates miR-122-5p to restrict XIAP, thereby limiting pancreatic cancer development. J Cell Mol Med 24:5028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H, Lin F, Ding R, et al. (2020). Extracellular vesicles carrying miR-193a derived from mesenchymal stem cells impede cell proliferation, migration and invasion of colon cancer by downregulating FAK. Exp Cell Res 394:112144. [DOI] [PubMed] [Google Scholar]
- Yu W, He X, Yang Z, et al. (2019). Sequentially responsive biomimetic nanoparticles with optimal size in combination with checkpoint blockade for cascade synergetic treatment of breast cancer and lung metastasis. Biomaterials 217:119309. [DOI] [PubMed] [Google Scholar]
- Zaborowski MP, Balaj L, Breakefield XO, et al. (2015). Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience 65:783–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Li B, Li T, et al. (2019). CD90(low) MSCs modulate intratumoral immunity to confer antitumor activity in a mouse model of ovarian cancer. Oncotarget 10:4479–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Yin Y, Lai RC, et al. (2014). Immunotherapeutic potential of extracellular vesicles. Front Immunol 5:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wu L, Nie W, et al. (2019). Biomimetic microfluidic system for fast and specific detection of circulating tumor cells. Anal Chem 91:15726–31. [DOI] [PubMed] [Google Scholar]
- Zhang J, Gao W, Fang RH, et al. (2015). Synthesis of nanogels via cell membrane-templated polymerization. Small 11:4309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guoqiang L, Sun M, et al. (2020). Targeting and exploitation of tumor-associated neutrophils to enhance immunotherapy and drug delivery for cancer treatment. Cancer Biol Med 17:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Gu C, Gan Y, et al. (2020). Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J Control Release 318:1–15. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Ji M, Wang Q, et al. (2020). Ca(2+) signaling modulation using cancer cell membrane coated chitosan nanoparticles to combat multidrug resistance of cancer. Carbohydr Polym 238:116073. [DOI] [PubMed] [Google Scholar]
- Zhou S, Abdouh M, Arena V, et al. (2017). Reprogramming malignant cancer cells toward a benign phenotype following exposure to human embryonic stem cell microenvironment. PLOS One 12:e0169899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhou W, Chen X, et al. (2020). Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm Sin B 10:1563–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Zhang F, Ni Q, et al. (2017). Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano 11:2387–92. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Ling X, Yang Y, et al. (2019). embryonic stem cells-derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy. Adv Sci 6:1801899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Huang L, Li Y, et al. (2012). Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett 315:28–37. [DOI] [PubMed] [Google Scholar]
- Zuo B, Qi H, Lu Z, et al. (2020). Alarmin-painted exosomes elicit persistent antitumor immunity in large established tumors in mice. Nat Commun 11:1790. [DOI] [PMC free article] [PubMed] [Google Scholar]