Abstract
Background:
Outcomes following hepatitis C virus (HCV)-viremic heart transplantation into HCV-negative recipients with HCV treatment are good. We assessed cost-effectiveness between cohorts of transplant recipients willing and unwilling to receive HCV-viremic hearts.
Methods:
Markov model simulating long-term outcomes among HCV-negative patients on the transplant waitlist. We compared costs (2018 US$) and health outcomes (quality-adjusted life-years, QALYs) between cohorts willing to accept any heart and those only willing to accept HCV-negative hearts. We assumed 4.9% HCV-viremic donor prevalence. Patients receiving HCV-viremic hearts were treated, assuming $39,600/treatment with 95% cure. Incremental cost-effectiveness ratios (ICERs) were compared to a $100,000/QALY gained willingness-to-pay threshold. Sensitivity analyses included stratification by blood type or region, and potential negative consequences of receipt of HCV-viremic hearts.
Results:
Compared to accepting only HCV-negative hearts, accepting any heart gained 0.14 life-years and 0.11 QALYs, while increasing costs by $9,418/patient. Accepting any heart was cost-effective (ICER $85,602/QALY gained). Results were robust to all transplant regions and blood types, except type AB. Accepting any heart remained cost-effective provided post-transplant mortality and costs among those receiving HCV-viremic hearts was not >7% higher compared to HCV-negative hearts.
Conclusions:
Willingness to accept HCV-viremic hearts for transplantation into HCV-negative recipients is cost-effective and improves clinical outcomes.
INTRODUCTION
Heart failure is a major medical problem affecting nearly 5.7 million Americans, and is projected to increase over the next decade1. While advances in pharmacological and resynchronization therapy have led to symptomatic and survival benefit, many with heart failure continue to progress to end-stage disease with poor quality of life and high mortality rates2. The gold standard for treatment of end-stage heart failure remains heart transplantation for those who are eligible. Over the last decade, the number of new listings for heart transplant (HT) increased by 50%, and the number of candidates actively awaiting transplant has more than doubled3. Advances in heart failure management are allowing more patients to survive long enough to undergo transplant and are offering the possibility of transplant to a greater proportion of older patients2–5. With a shortage of viable organs, transplant rates have not paralleled this rise in candidates. Between 2005 and 2017, HT rates have fluctuated slightly but have overall stayed the same, and the median wait time in 2016–2017 was 7.9 months, varying considerably with listing status and blood type3. Due to a shortage of available donor hearts and an increasing pool of eligible recipients, ventricular assist devices (VAD) have become increasingly accepted and utilized as a life-saving bridge to transplant, but these remain costly with high rates of adverse events2,4,5.
The scarcity of hearts available for transplantation and high mortality rate while awaiting transplant have led some institutions to begin transplanting organs that previously have not been used, including those from donors with hepatitis C virus (HCV) viremia3. Traditionally, hearts from HCV-viremic donors have not been transplanted into HCV negative recipients due to concerns of viral transmission, with an accelerated risk of cirrhosis and liver failure in the setting of immunosuppression. In addition, up until recently, interferon-based HCV treatments had low sustained virologic response (SVR) rates (~50%) and poor tolerability. The development of direct-acting antivirals (DAAs) changed the HCV treatment landscape with high rates of SVR (exceeding 90%) including for patients with solid-organ transplants6–18, yet treatments are expensive ($40,000 or greater per treatment course)19. Single center data utilizing HCV-viremic donors for heart transplantation demonstrate 100% SVR rates with treatment following transplant with DAA20–24. While additional long-term data on safety and efficacy are being accumulated, the cost-effectiveness of such a strategy has not been evaluated.
With projected increasing rates of advanced heart failure, and supply and demand imbalance, it is ever more necessary to evaluate strategies to optimize the donor pool and allocation process to improve health and economic outcomes. Our aim was to compare the cost-effectiveness between cohorts of HCV-negative recipients who are willing and unwilling to receive HCV-viremic hearts for transplantation, followed by 12 weeks of DAA treatment for those receiving HCV-viremic hearts.
METHODS
Model Overview
We developed a Markov model of health and economic outcomes among cohorts of patients who are negative for HCV infection on the HT waitlist (WL). We created a virtual trial to compare the cost-effectiveness between cohorts of patients willing to accept any (HCV-viremic and HCV-negative) heart and those only willing to accept HCV-negative hearts from a health payer perspective with a lifetime horizon. Our analysis is limited to the healthcare sector only, and neglects potential broader societal benefits on economic productivity, etc. Individuals who received HCV-viremic hearts were treated with DAAs for 12 weeks, and long-term health outcomes (including liver disease progression if remaining HCV-infected) and costs were simulated.
Baseline Heart Transplantation Cohort Characteristics
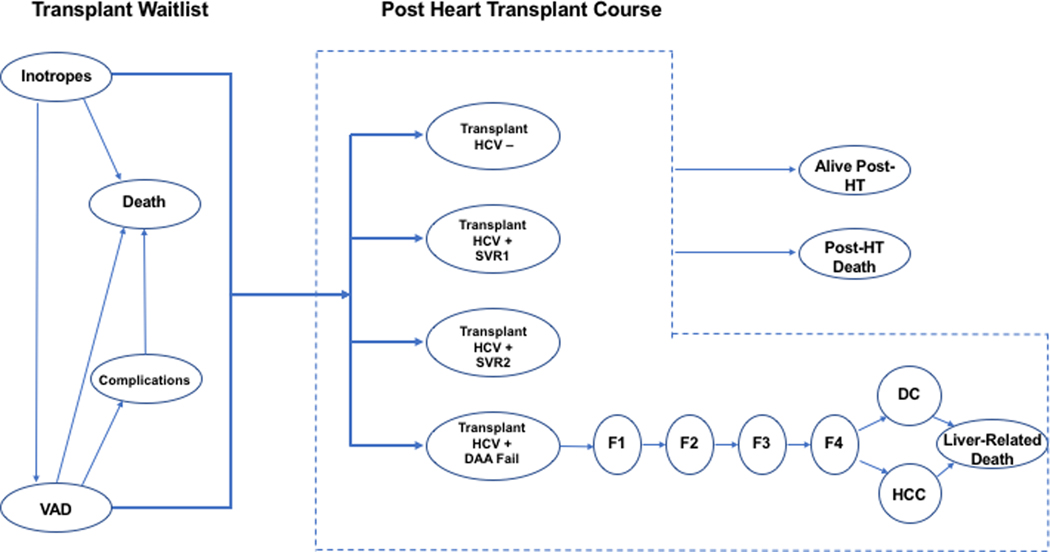
We created a hypothetical cohort of patients with end-stage heart failure who were active on the HT waitlist. We stratified our population by those managed with inotrope-dependent therapy (IDT) and with VADs, incorporating transition from IDT to VAD. Based on 2017 data from the Organ Procurement and Transplantation Network (OPTN), at baseline, we simulated a cohort aged 50 years, 65% were waitlisted with a VAD and 35% on IDT. We simulated the lifetime course of patients with advanced heart failure waiting for HT. While on the WL patients could transition from IDT to VAD, undergo HT, or die from either heart failure-related mortality from background mortality (Figure 1). We used published studies and 2017 United Network for Organ Sharing (UNOS) data to estimate transplant transition probabilities.
Figure 1.
Model schematic showing the flow of patient pre- and post-heart transplant. The model simulates cohorts on inotrope dependent therapy (IDT) and with ventricular assist devices (VAD) in two scenarios: (1) accept HIV-viremic or HCV-negative heart, and (2) accept only HCV-negative heart. Patients willing to accept any heart had a higher likelihood of receiving a heart transplant (HT). Patients receiving HCV-viremic hearts were treated with 12 weeks of DAAs. Patients could either achieve SVR after one course of DAA (SVR1), achieve SVR after a second course of DAA (SVR2) or fail both courses of therapy. If patients failed the second course of DAAs, they developed chronic HCV infection and progressed to liver fibrosis (METAVIR fibrosis stages F1-F4), decompensated cirrhosis (DC), hepatocellular carcinoma (HCC), and liver-related mortality.
Cohort comparisons
We simulated two scenarios for the HT cohort (Figure 1): (1) “accept any” heart (HCV-viremic or HCV-negative), followed by DAA treatment if an HCV-viremic heart was received, and (2) accept HCV-negative heart only. For both groups, the probability of receiving a HT was dependent on management status (VAD versus IDT), and based on national averages pooling across blood types and UNOS geographic regions for our base case analysis.
In the “negative only” scenario, patients moved to the non-viremic post-transplant health state after successful heart transplantation. In the “accept any” scenario, patients could receive HCV-negative hearts and follow traditional outcomes. However, if the heart received was HCV-viremic, the patient was subsequently treated with DAA therapy, a maximum of 2 times. If patients were successfully treated (or re-treated) they moved to the SVR state and followed a standard post-transplant course. If patients failed the second course of therapy, we tracked natural history of HCV-related liver disease as categorized by METAVIR scoring system (F0, F1, F2, F3, F4/compensated cirrhosis, decompensated cirrhosis, hepatocellular carcinoma). When patients reached decompensated cirrhosis, they began to experience mortality from liver-attributable causes. We assume patients receiving HCV-viremic and HCV-negative hearts received the same surgical procedure and the same post-transplant heart-related care, aside from additional HCV-related care.
Model parameterization
Transition probabilities, costs and health utilities were attached to each disease stage (Table 1). We assumed all patients who received HCV-viremic hearts were treated with 12 weeks of DAAs with 95% primary and retreatment cure rates.
Table 1.
Model parameterization for the base-case and probabilistic sensitivity analysis.
| Parameter | Base | Sample distribution for probabilistic sensitivity analysis | References | |
|---|---|---|---|---|
| HCV-viremic organ prevalence among heart donors (%) | 4.9% | Uniform (min=2.5, max= 7.3) | ||
| Monthly Transition Probabilities | ||||
| Inotropes | VAD | 0.0084 | Sampled annual rate uniformly +/− 50% (min=0.051, max=0.152) | 27,28 |
| Transplant (HCV Negative organ) | 0.2330 | Sampled WL time in months uniformly +/− 50% (min=1.9; max=5.7) and converted to TP | 3 | |
| Death | 0.0740 | Sampled annual rate uniformly +/− 50% (min=0.461; max=1.384) | 33,34 | |
| VAD | Complications/Delist | 0.0760 | Sampled annual rate uniformly +/− 50% (min=0.474; max=1.423) | 31,33 |
| Transplant (HCV Negative organ) | 0.1123 | Sampled WL time in months uniformly +/− 50% (min=4.2, max=12.6) and converted to TP | 3 | |
| Death | 0.009 | Sampled annual rate uniformly +/− 50% (min=0.054, max=0.163) | 31,33,86 | |
| Complications | Death | 0.64 | Sampled annual rate uniformly +/− 50% (min=6.13, max=18.390) | 33 |
| Transplant Yr1 | Death | 0.0076 | Sampled annual rate uniformly +/− 50% (min=0.077, max=0.096) | 3,87 |
| Transplant Yr2+ | Death | 0.0020 | Sampled annual rate uniformly +/− 50% (min=0.023, max=0.025) | 3,87 |
| F0 | F1 | 0.0109 | Sampled annual rate from Beta (α=446.989, β=2906.45), converted to monthly TP | 36,42 |
| F1 | F2 | 0.0074 | Sampled annual rate from Beta (α=394.323, β=3971.41), converted to monthly TP | 36,42 |
| F2 | F3 | 0.0109 | Sampled annual rate from Beta (α=2.852, β=44.384), converted to monthly TP | 36,42 |
| F3 | F4 | 0.0105 | Sampled annual rate from Beta α=666.175, β=4584.86), converted to monthly TP | 36,42 |
| CC | DC | 0.0055 | Sampled annual rate from Beta (α=33.587, β=487.785), converted to monthly TP | 36,39 |
| CC | HCC | 0.0017 | Sampled annual rate from Beta (α=12.959, β=617.161), converted to monthly TP | 36,37 |
| DC | Liver Death | 0.0151 | Sampled annual rate from Beta (α=8.5332, β=26.132), converted to monthly TP | 38,39 |
| DC | HCC | 0.0058 | Sampled annual rate from Beta (α=18.953, β=263.478), converted to monthly TP | 39–41 |
| HCC | Liver Death | 0.0742 | Sampled annual rate from Beta (α=7.597, β=6.016), converted to monthly TP | 38,39,50,52 |
| Health State Utilities | ||||
| Inotropes | 0.53 | 33,50,52 | ||
| VAD | 0.72 | 31,33,52,55 | ||
| Complications | 0.63 | 33,62 | ||
| Heart Transplant | 0.76 | 33,52 | ||
| F0-F2 | 0.53 | 64,65,88 | ||
| F3-F4 | 0.42 | 64,65,88 | ||
| DC | 0.21 | 64,65,88 | ||
| HCC | 0.21 | 64,65,88 | ||
| Costs (2018 USD$) | ||||
| Inotrope therapy (monthly) | 5668 | Uniform +/− 50% point value | 33,50,51 | |
| VAD Index Hospitalization | 307593 | Uniform +/− 50% point value | 33,44,45,49,53,55,58 | |
| Post-VAD care (monthly) | 14136 | Uniform +/− 50% point value | 33,47,48,55,56 | |
| VAD Complication (one-time cost) | 21800 | Uniform +/− 50% point value | 33,45,46,53,54 | |
| Heart Transplant Index Hospitalization | 251232 | Uniform +/− 50% point value | 33,49 | |
| Post-Transplant Care (monthly) | 13337 | Uniform +/− 50% point value | 33,48,56 | |
| DAA 12-week course | 39600 | Uniform +/− 50% point value | 19,89 | |
| Chronic HCV F0-F2 (monthly) | 252 | Uniform +/− 50% point value | 59,61 | |
| Chronic HCV F3 (monthly) | 453 | Uniform +/− 50% point value | 59,61 | |
| Compensated Cirrhosis/F4 (monthly) | 854 | Uniform +/− 50% point value | 36 | |
| Decompensated Cirrhosis (monthly) | 2906 | Uniform +/− 50% point value | 36,60 | |
| HCC (monthly) | 4637 | Uniform +/− 50% point value | 36,60 | |
VAD=ventricular assist device; METAVIR F0-F4; CC=compensated cirrhosis; DC=decompensated cirrhosis; HCC=hepatocellular carcinoma
Transplant probabilities:
UNOS data on median WL times until transplant accounting for competing risks of death from 2017 were used to estimate the monthly probability of receiving a HT (Table 1) for wait-listed patients on IDT (average 3.8 months on WL until transplant) and with VADs (average 8.4 months on WL until transplant). We assumed that willingness to accept any heart (HCV-viremic or HCV-negative) would increase the probability of transplant by increasing the number of eligible organs for transplant, by increasing the transplant probabilities for cohorts willing to receive HCV-positive organ rates by 4.9%, equating to the proportion of HCV-viremic organs among all heart donors in the U.S based on 2018 UNOS data. As such, consistent with other studies examining the impact of accepting HCV donor organs25, we calculated the overall transplant probability for those willing to accept any heart as = 1 – (1 – transplant probability) (1+HCV viremic organ prevalence)
Disease transition probabilities:
Transition probabilities for patients on the HT wait list were obtained from previously published studies and cost analyses of heart failure26–34. All individuals experienced age-specific background (non-transplant, non-HCV related) mortality based on data from the US Centers for Disease Control. Transplant-related mortality rates were estimated based on UNOS survival data by month. Because UNOS survival data reflects all-cause mortality, we calculated the transplant-specific mortality by subtracting the background mortality expected for a patient aged 50. We confirmed our model-generated survival matched reported OPTN data (Supplementary Figure S1)3. Transition probabilities for HCV disease progression were obtained from prior studies and cost analyses35–42.
Costs:
Costs of heart failure care while on the wait list (including the cost of VAD implantation), transplantation, and post-transplant care were obtained from previously published clinical trials and cost analyses33,43–58. These costs reflect a payer mix representing the mixed insurance status of the population, including Medicare and private insurance as well as costs from the National Inpatient Sample and clinical trial data, in line with previous economic evaluations of HT interventions in the U.S. We assumed a $39,600/treatment cost for HCV DAA therapy based on recent wholesale acquisition cost for a 12-week course of glecaprevir/pibrentasvir, a commonly used agent due to lowest cost, pan-genotypic activity, and minimal drug-drug interactions 19. Health state costs, including those for untreated HCV disease stages, were taken from previous economic evaluations and inflated to 2018 USD using hospital and related services component of the Consumer Price Index36,59–61.
Utilities:
For each health state, including those for heart failure31,33,50,52,55,62 and liver disease63–65, we assigned health-related quality-of-life weights which were obtained from previously published studies and cost analyses. For post-transplant health states with subsequent liver disease, the decrement of the liver disease state was subtracted from the post-transplant state (combined health utility= heart transplant utility – (1-liver disease utility).
Model Outcomes
Costs (in 2018 USD$) and U.S. based health utilities were attached to each stage and discounted at 3% per year. For each scenario, we estimated total costs and total quality-adjusted life years (QALYs). Incremental cost-effectiveness ratios (difference in costs divided by the difference in QALYs) were calculated comparing cohorts of those willing to accept any heart compared to only HCV negative hearts, and assessed for cost-effectiveness under a willingness-to-pay (WTP) threshold of $100,000 per QALY gained66.
Sensitivity analyses:
We performed several one-way and multivariate sensitivity analyses to test model assumptions on cost-effectiveness, focusing on those that were most likely to affect costs, WL time, and health outcomes associated with willingness to receive an HCV-viremic heart. Due to variability in transplant time by blood type, we performed one-way sensitivity analyses examining specific blood types (Table S1). We also examined the impact of variability in transplant WL times by geographical region, by adjusting the national transplant probabilities by a region-specific ratio (Region-specific-probability / National probability) based on 2017 UNOS data (Table S2). We examined the impact of variations in HCV prevalence among organ donors (2.5% or 10% compared to 4.9% at baseline), lower DAA SVR (90% compared to 95%), or doubled DAA cost ($80,000 compared to $39,600). We also examined the impact of variations on the baseline cohort age (40 to 65 years, compared to 50 at baseline). While early data have not demonstrated significant differences in mortality, graft function, rejection, or fibrosing hepatitis among recipients of HCV-viremic compared to HCV-negative organs20–24,67–75, we performed a sensitivity analysis to evaluate the potential for negative outcomes in patients receiving hearts from HCV-viremic donors by incrementally varying the post-transplant mortality and costs in this group by a relative 1–10% compared to those receiving HCV-negative hearts. Finally, we undertook a probabilistic sensitivity analysis where we varied all model input parameters simultaneously to determine uncertainty in the ICER (sampling distributions in Table 1), plotting the proportion of simulations which fell under various WTP thresholds.
RESULTS
Our simulations indicated that compared to those who only accepted HCV-negative donor hearts, patients who accepted any heart (HCV-viremic or HCV-negative), had an increase in survival of 0.14 life-years per patient, which translated to 0.11 QALYs gained per patient, due to reduction in WL time. This was associated with a lifetime increase in costs of $9,418/patient. Overall, compared to only accepting HCV-negative hearts accepting any heart yielded an ICER of $85,602/QALY gained and was cost-effective (Table 2).
Table 2.
Cost effectiveness analysis results for cohorts accepting any heart (HCV-viremic or negative) compared to accepting only HCV-negative hearts.
| Strategy | Cost (2018 $USD) | Incremental Cost | Quality-adjusted life-years (QALYs) | Incremental QALYs | ICER ($/QALY gained) |
|---|---|---|---|---|---|
| Accept HCV Negative Heart Only | $589,467 | 6.6 | |||
| Accept any heart (HCV RNA positive or negative) | $598,885 | $9,418 | 6.7 | 0.11 | $85,602 |
ICER: Incremental cost-effectiveness ratio
Impact of varying transplant wait time by blood type or UNOS region
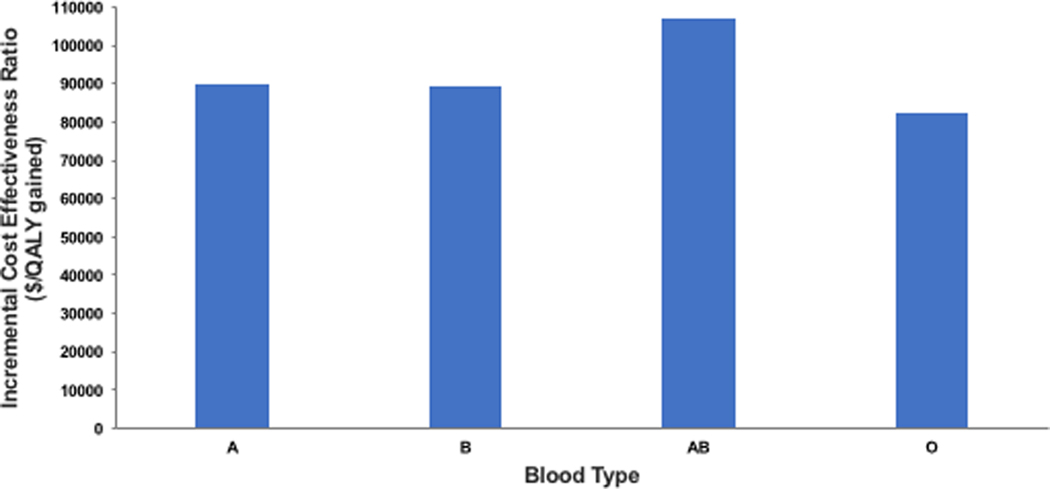
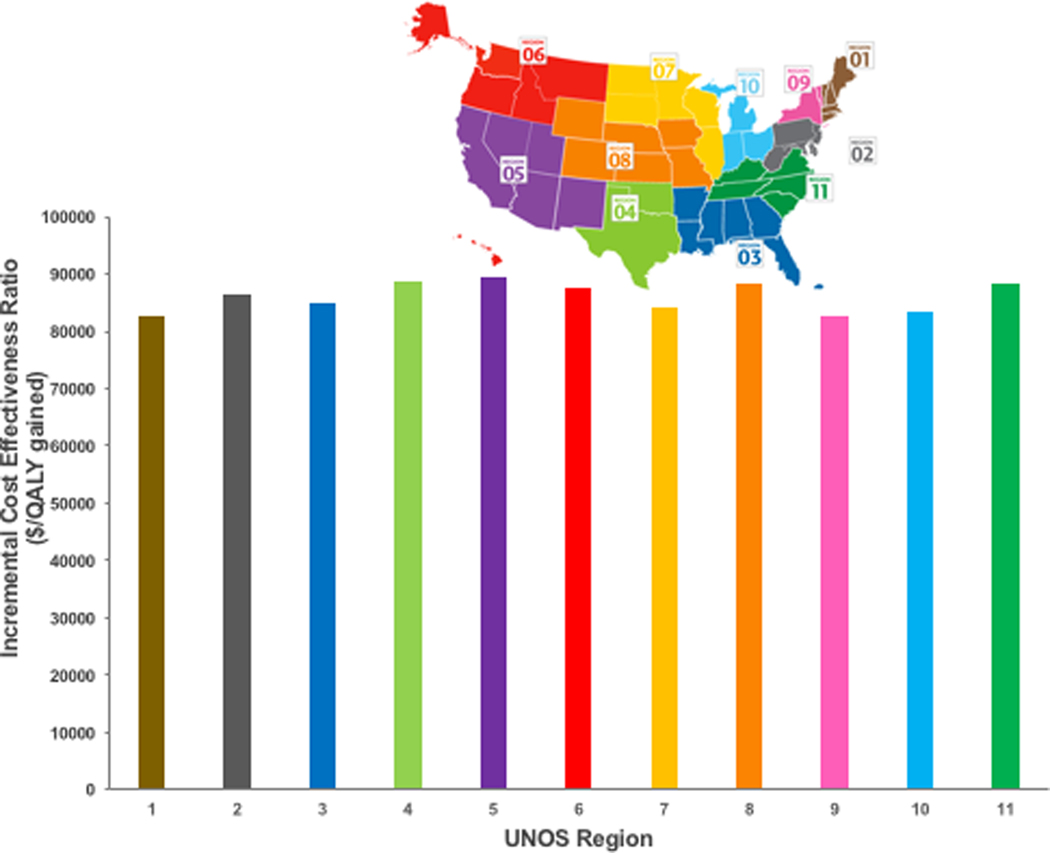
Accepting an HCV-viremic or negative heart remained cost-effective for sensitivity analyses examining impact of variations in assumptions regarding transplant wait times by blood type and UNOS region. When varying transplant time by blood type, accepting any heart (HCV-viremic or negative) remained cost-effective (ICER<$100,000/QALY gained) for all types except for the blood type AB (borderline cost-effective at $107,168/QALY gained) compared to accepting only HCV-negative hearts (Figure 2). Clinical outcomes varied, from an increase in 0.09 life-years for blood type AB to 0.15 life-years for blood type O, due to differences in WL time which were shortest for blood type AB. Similarly, when examining impact of variations in assumptions regarding transplant wait times across UNOS regions, accepting any heart remained cost-effective compared to accepting only HCV-negative hearts in all regions (Figure 3). Clinical outcomes varied, from an increase in 0.13 life-years for regions 4, 8 and 11 (region 4 with shortest WL time of 5.4 months), to 0.15 life-years for regions 1, 3, 7 and 9 (region 1 with longest WL time of 14 months).
Figure 2.
Incremental cost-effectiveness ratios for cohorts accepting any heart (HCV-viremic or negative only HCV-negative hearts, by blood type (A, B, AB, or O).
Figure 3.
Incremental cost-effectiveness ratios for cohorts accepting any heart (HCV-viremic or negative) compared to accepting only HCV-negative hearts, by transplantation wait list times across UNOS Regions Subpanel shows the corresponding region associated with UNOS Region number.
Impact of HCV prevalence among heart donors, SVR rate, and DAA costs
Accepting any heart remained cost-effective with a lower donor HCV-viremia prevalence of 2.5% ($85,623/QALY gained, Figure S2). With an HCV-viremia prevalence of 10%, the ICER was $85,561/QALY gained. When examining impact of a lower SVR rate of 90% at an HCV-viremia prevalence of 4.9%, accepting any heart remained cost-effective (ICER $86,890/QALY gained). Accepting any heart was borderline cost-effective with a higher DAA cost of $80,000 per treatment course (ICER $98,094/QALY gained).
Impact of baseline cohort age
Accepting any heart was more cost-effective with lower baseline cohort ages, and remained cost-effective with a cohort age of 65 years (ICER $94,160/QALY gained, Figure S3).
Impact of increased costs and mortality in patients receiving HCV-viremic hearts
Accepting any heart was more cost-effective (WTP <$100,000/QALY) provided that receipt of an HCV- viremic organ was associated with a less than 7% relative increase in post-transplant mortality and associated post-transplant costs among those individuals compared to those who did not receive an HCV-viremic organ (Figure S4).
Probabilistic Sensitivity Analyses
Over 10,000 iterations, accepting any heart was cost-effective (mean ICER of $90,530/QALY gained) over accepting an HCV-negative heart only, yielding an average gain of 0.1 QALYs per person (2.5–97.5% percentiles 0.1–0.1) at an average incremental cost of $9,053 (2.5–97.5% percentiles $6,279-$11,904). At a willingness-to-pay threshold of $100,000 accepting any heart was cost-effective for 75% of simulations (Figure S5).
DISCUSSION
We found important clinical and economic benefits among cohorts willing to accept HCV-viremic hearts for transplantation. Not only is SVR achievable for majority of patients infected with HCV, DAA therapy has opened the possibility of utilizing once discarded, but otherwise viable organs from HCV-infected donors for transplantation into HCV-negative recipients by allowing treatment in the early post-operative period. Early studies suggest this is clinically feasible and demonstrate excellent SVR rates even in the setting of immunosuppression21–24,70,73,74,76–80. Accepting HCV-viremic organs may allow patients to be transplanted faster, and avoid not only complications and mortality of end-stage heart failure while on the WL, but avoid additional costs of medical treatment as well81. The concerns of risk associated with HCV infection and the costs associated with DAA therapy have persisted during the early stages of this approach and may potentially limit widespread adoption. In our opinion, this is the first study to evaluate the cost-effectiveness of accepting HCV-viremic hearts for transplant into HCV-negative recipients, finding it cost-effective (ICER <$100,000/QALY) and resulting in improvement of survival and reduction in WL time.
It is unknown what proportion of individuals on the HT waitlist would be willing to receive HCV-viremic hearts. However, it has been reported that between 29% and 82% of patients awaiting kidney transplant would accept HCV-positive kidneys under certain circumstances82. In a recent study evaluating transplantation of HCV-viremic hearts, 57% of identified candidates were willing to enroll21. More data are needed to assess willingness of heart failure patients to receive HCV-viremic organs, and what information can support their decision-making of the risks and benefits.
Our analysis has several limitations, mostly due to uncertainty in the model parameterization. First, we conservatively assumed that all patients receiving an HT from a HCV-viremic donor would acquire HCV infection. It is possible, that some may avoid HCV acquisition or spontaneously clear the disease on their own, thus improving outcomes after transplantation and improving cost-effectiveness. Second, we assumed DAA therapy would result in cure in 99% of patients overall after 1 or 2 treatment courses, based on prior studies using DAA therapy to treat HCV after kidney transplant6–18. Early studies in HT, demonstrate 100% SVR rates thus far for donor-derived HCV20–24. Nevertheless, sensitivity analyses with variations in SVR rates to as low as 90% demonstrated cost-effectiveness. Third, there is now clinical trial data showing feasibility of early shorter course of DAA therapy with similar SVR rates though long term follow-up is needed and the real-world applicability outside a clinical trial is pending; this may be even more cost effective or even cost-saving; however we did not test this in our model24,83,84. Fourth, we assumed in our baseline model that patients who received an HCV-viremic heart and then successfully achieved SVR would have no negative consequences on further health outcomes. Thus far in early trials, there have been no reports of increased rates of rejection, increased infectious complications, or increased mortality among those receiving HCV-viremic organs20–24,67–75. However, these outcomes are early and so in our sensitivity analysis we incrementally increased both costs and mortality to account for this possibility, finding that willingness to accept a heart was cost-effective provided it is not associated with a relative increase of more than 7% post-transplant mortality compared to those receiving HCV-negative hearts. Fifth, as the organ distribution system has recently changed (now six medical urgency statuses rather than three, and more distinction between various types of mechanical circulatory support), we were unable to assess precise effects within specific (and now outdated) staging groups and geographical regions. Nevertheless, our numerous sensitivity analyses indicated that our results were robust to variations in assumptions regarding WL time, blood type, and HCV-prevalence among donors, indicating that our results are likely to hold regardless of these changes. Sixth, our analysis uses a health care payer perspective, and neglects the broader potential societal benefits of this approach. For example, willingness to receive an HCV-viremic organ may result in reduction in transplant WL time, and therefore potential increases in economic productivity for those who receive a transplant earlier81. Including these broader societal benefits could further improve cost-effectiveness. Finally, our analysis focused on the cost-effectiveness of these management strategies and neglects the budgetary impact. National data suggest that there are 300–500 unrealized opportunities for HCV-viremic organ donations, yet the numbers willing to accept an HCV-viremic heart are unknown17,85. Future studies exploring the potential budgetary impact of use of these organs, which could avert substantial cost in pre-transplant management, is warranted but outside the scope of our analysis.
In conclusion, willingness to accept hearts from HCV-viremic donors among HCV-negative recipients followed by DAA therapy if an HCV-viremic heart is transplanted is a clinically useful and cost-effective strategy in heart transplantation among patients with end-stage heart failure.
Supplementary Material
ACKNOWLEGMENTS:
This work was supported in part by Health Resources and Services Administration contract 234–2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. NM was supported by the National Institute for Allergy and Infectious Diseases and National Institute for Drug Abuse [grant number R01 AI147490] and the University of California San Diego Center for AIDS Research (CFAR), a National Institute of Health (NIH) funded program [grant number P30 AI036214]. JC was supported by the National Institute for Drug Abuse [grant number K01DA043421].
Disclosures
NM has received unrestricted research grants and honoraria from Gilead and Merck unrelated to this work. SA serves as a consultant to Merck for work unrelated to this project.
Abbreviations
- DAA
Direct-acting antiviral
- HT
Heart transplant
- HCV
Hepatitis C virus
- IDT
Inotrope-dependent therapy
- ICER
Incremental cost-effectiveness ratio
- OPTN
Organ Procurement and Transplantation Network
- QALY
Quality-adjusted life-year
- SVR
Sustained virologic response
- UNOS
United Network for Organ Sharing
- VAD
Ventricular assist device
- WL
Waitlist
REFERENCES
- 1.Writing Group M, Mozaffarian D, Benjamin EJ, et al. Executive Summary: Heart Disease and Stroke Statistics−−2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):447–454. [DOI] [PubMed] [Google Scholar]
- 2.Sajgalik P, Grupper A, Edwards BS, et al. Current Status of Left Ventricular Assist Device Therapy. Mayo Clin Proc. 2016;91(7):927–940. [DOI] [PubMed] [Google Scholar]
- 3.Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2017 Annual Data Report: Heart. Am J Transplant. 2019;19 Suppl 2:323–403. [DOI] [PubMed] [Google Scholar]
- 4.Silva Enciso J. Mechanical Circulatory Support: Current Status and Future Directions. Prog Cardiovasc Dis. 2016;58(4):444–454. [DOI] [PubMed] [Google Scholar]
- 5.Sunagawa G, Koprivanac M, Karimov JH, Moazami N, Fukamachi K. Current status of mechanical circulatory support for treatment of advanced end-stage heart failure: successes, shortcomings and needs. Expert Rev Cardiovasc Ther. 2017;15(5):377–387. [DOI] [PubMed] [Google Scholar]
- 6.Gupta G, Zhang Y, Carroll NV, Sterling RK. Cost-effectiveness of hepatitis C-positive donor kidney transplantation for hepatitis C-negative recipients with concomitant direct-acting antiviral therapy. Am J Transplant. 2018;18(10):2496–2505. [DOI] [PubMed] [Google Scholar]
- 7.Chen K, Lu P, Song R, et al. Direct-acting antiviral agent efficacy and safety in renal transplant recipients with chronic hepatitis C virus infection: A PRISMA-compliant study. Medicine (Baltimore). 2017;96(30):e7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chute DF, Chung RT, Sise ME. Direct-acting antiviral therapy for hepatitis C virus infection in the kidney transplant recipient. Kidney international. 2018;93(3):560–567. [DOI] [PubMed] [Google Scholar]
- 9.Colombo M, Aghemo A, Liu H, et al. Treatment With Ledipasvir-Sofosbuvir for 12 or 24 Weeks in Kidney Transplant Recipients With Chronic Hepatitis C Virus Genotype 1 or 4 Infection: A Randomized Trial. Annals of internal medicine. 2017;166(2):109–117. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez I, Munoz-Gomez R, Pascasio JM, et al. Efficacy and tolerability of interferon-free antiviral therapy in kidney transplant recipients with chronic hepatitis C. Journal of hepatology. 2017;66(4):718–723. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Ruiz M, Polanco N, Garcia-Santiago A, et al. Impact of anti-HCV direct antiviral agents on graft function and immunosuppressive drug levels in kidney transplant recipients: a call to attention in the mid-term follow-up in a single-center cohort study. Transpl Int. 2018;31(8):887–899. [DOI] [PubMed] [Google Scholar]
- 12.Lin MV, Sise ME, Pavlakis M, et al. Efficacy and Safety of Direct Acting Antivirals in Kidney Transplant Recipients with Chronic Hepatitis C Virus Infection. PloS one. 2016;11(7):e0158431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawinski D, Kaur N, Ajeti A, et al. Successful Treatment of Hepatitis C in Renal Transplant Recipients With Direct-Acting Antiviral Agents. Am J Transplant. 2016;16(5):1588–1595. [DOI] [PubMed] [Google Scholar]
- 14.Saxena V, Khungar V, Verna EC, et al. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: Results from the HCV-TARGET study. Hepatology. 2017;66(4):1090–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwo PY, Mantry PS, Coakley E, et al. An interferon-free antiviral regimen for HCV after liver transplantation. The New England journal of medicine. 2014;371(25):2375–2382. [DOI] [PubMed] [Google Scholar]
- 16.Lawitz E, Gane E, Pearlman B, et al. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385(9973):1075–1086. [DOI] [PubMed] [Google Scholar]
- 17.Levitsky J, Formica RN, Bloom RD, et al. The American Society of Transplantation Consensus Conference on the Use of Hepatitis C Viremic Donors in Solid Organ Transplantation. Am J Transplant. 2017;17(11):2790–2802. [DOI] [PubMed] [Google Scholar]
- 18.Martin MT, Koppe S. Elbasvir/Grazoprevir Use in Postliver Transplantation Patients on Hemodialysis. Transplantation. 2017;101(9):2088–2091. [DOI] [PubMed] [Google Scholar]
- 19.Horn T. FPC Welcomes FDA Approval of AbbVie’s New Pangenotypic HCV Combination Mavyret. https://fairpricingcoalition.org/2017/08/04/fair-pricing-coalition-welcomes-fda-approval-of-abbvies-new-pangenotypic-hcv-combination-mavyret/. Published 2017. Accessed April 29,2019. [Google Scholar]
- 20.Aslam S, Yumul I, Mariski M, Pretorius V, Adler E. Outcomes of heart transplantation from hepatitis C virus-positive donors. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2019;38(12):1259–1267. [DOI] [PubMed] [Google Scholar]
- 21.McLean RC, Reese PP, Acker M, et al. Transplanting hepatitis C virus-infected hearts into uninfected recipients: A single-arm trial. Am J Transplant. 2019. [DOI] [PubMed] [Google Scholar]
- 22.Moayedi Y, Gulamhusein AF, Ross HJ, Teuteberg JJ, Khush KK. Accepting hepatitis C virus-infected donor hearts for transplantation: Multistep consent, unrealized opportunity, and the Stanford experience. Clin Transplant. 2018;32(7):e13308. [DOI] [PubMed] [Google Scholar]
- 23.Schlendorf KH, Zalawadiya S, Shah AS, et al. Early outcomes using hepatitis C-positive donors for cardiac transplantation in the era of effective direct-acting anti-viral therapies. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2018;37(6):763–769. [DOI] [PubMed] [Google Scholar]
- 24.Woolley AE, Singh SK, Goldberg HJ, et al. Heart and Lung Transplants from HCV-Infected Donors to Uninfected Recipients. The New England journal of medicine. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chhatwal J, Samur S, Bethea ED, et al. Transplanting hepatitis C virus-positive livers into hepatitis C virus-negative patients with preemptive antiviral treatment: A modeling study. Hepatology. 2018;67(6):2085–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aggarwal A, Pant R, Kumar S, et al. Incidence and management of gastrointestinal bleeding with continuous flow assist devices. Ann Thorac Surg. 2012;93(5):1534–1540. [DOI] [PubMed] [Google Scholar]
- 27.Alba AC, Alba LF, Delgado DH, Rao V, Ross HJ, Goeree R. Cost-effectiveness of ventricular assist device therapy as a bridge to transplantation compared with nonbridged cardiac recipients. Circulation. 2013;127(24):2424–2435. [DOI] [PubMed] [Google Scholar]
- 28.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Lung and Heart-Lung Transplant Report−−2011. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30(10):1104–1122. [DOI] [PubMed] [Google Scholar]
- 29.Demirozu ZT, Radovancevic R, Hochman LF, et al. Arteriovenous malformation and gastrointestinal bleeding in patients with the HeartMate II left ventricular assist device. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30(8):849–853. [DOI] [PubMed] [Google Scholar]
- 30.John R, Kamdar F, Eckman P, et al. Lessons learned from experience with over 100 consecutive HeartMate II left ventricular assist devices. Ann Thorac Surg. 2011;92(5):1593–1599; discussion 1599–1600. [DOI] [PubMed] [Google Scholar]
- 31.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32(2):141–156. [DOI] [PubMed] [Google Scholar]
- 32.Lietz K, Miller LW. Improved survival of patients with end-stage heart failure listed for heart transplantation: analysis of organ procurement and transplantation network/U.S. United Network of Organ Sharing data, 1990 to 2005. J Am Coll Cardiol. 2007;50(13):1282–1290. [DOI] [PubMed] [Google Scholar]
- 33.Long EF, Swain GW, Mangi AA. Comparative survival and cost-effectiveness of advanced therapies for end-stage heart failure. Circ Heart Fail. 2014;7(3):470–478. [DOI] [PubMed] [Google Scholar]
- 34.Singh TP, Almond CS, Taylor DO, Graham DA. Decline in heart transplant wait list mortality in the United States following broader regional sharing of donor hearts. Circ Heart Fail. 2012;5(2):249–258. [DOI] [PubMed] [Google Scholar]
- 35.Degos F, Christidis C, Ganne-Carrie N, et al. Hepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and death. Gut. 2000;47(1):131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckman MH, Woodle ES, Thakar CV, Paterno F, Sherman KE. Transplanting Hepatitis C Virus-Infected Versus Uninfected Kidneys Into Hepatitis C Virus-Infected Recipients: A Cost-Effectiveness Analysis. Annals of internal medicine. 2018;169(4):214–223. [DOI] [PubMed] [Google Scholar]
- 37.Fattovich G, Pantalena M, Zagni I, et al. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. The American journal of gastroenterology. 2002;97(11):2886–2895. [DOI] [PubMed] [Google Scholar]
- 38.Hutchinson SJ, Bird SM, Goldberg DJ. Modeling the current and future disease burden of hepatitis C among injection drug users in Scotland. Hepatology. 2005;42(3):711–723. [DOI] [PubMed] [Google Scholar]
- 39.Njei B, McCarty TR, Fortune BE, Lim JK. Optimal timing for hepatitis C therapy in US patients eligible for liver transplantation: a cost-effectiveness analysis. Alimentary pharmacology & therapeutics. 2016;44(10):1090–1101. [DOI] [PubMed] [Google Scholar]
- 40.Saab S, Hunt DR, Stone MA, McClune A, Tong MJ. Timing of hepatitis C antiviral therapy in patients with advanced liver disease: a decision analysis model. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2010;16(6):748–759. [DOI] [PubMed] [Google Scholar]
- 41.Sweeting MJ, De Angelis D, Neal KR, et al. Estimated progression rates in three United Kingdom hepatitis C cohorts differed according to method of recruitment. Journal of clinical epidemiology. 2006;59(2):144–152. [DOI] [PubMed] [Google Scholar]
- 42.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48(2):418–431. [DOI] [PubMed] [Google Scholar]
- 43.Briasoulis A, Inampudi C, Akintoye E, et al. Regional Variation in Mortality, Major Complications, and Cost After Left Ventricular Assist Device Implantation in the United States (2009 to 2014). The American journal of cardiology. 2018;121(12):1575–1580. [DOI] [PubMed] [Google Scholar]
- 44.Briasoulis A, Inampudi C, Akintoye E, Adegbala O, Bhama J, Alvarez P. Effect of Hospital Ownership on Outcomes After Left Ventricular Assist Device Implantation in the United States. Ann Thorac Surg. 2019;107(2):527–532. [DOI] [PubMed] [Google Scholar]
- 45.Chung JJ, Stetson R, Gordon J, et al. Better With Time: An Economic Assessment of Long-Term Mechanical Circulatory Support in a Population Surviving at Least 1 Year with a Left Ventricular Assist Device. Semin Thorac Cardiovasc Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 46.Engel-Nitz NM, Sander SD, Harley C, Rey GG, Shah H. Costs and outcomes of noncardioembolic ischemic stroke in a managed care population. Vasc Health Risk Manag. 2010;6:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreno SG, Novielli N, Cooper NJ. Cost-effectiveness of the implantable HeartMate II left ventricular assist device for patients awaiting heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2012;31(5):450–458. [DOI] [PubMed] [Google Scholar]
- 48.Moskowitz AJ, Rose EA, Gelijns AC. The cost of long-term LVAD implantation. Ann Thorac Surg. 2001;71(3 Suppl):S195–198; discussion S203–194. [DOI] [PubMed] [Google Scholar]
- 49.Mulloy DP, Bhamidipati CM, Stone ML, Ailawadi G, Kron IL, Kern JA. Orthotopic heart transplant versus left ventricular assist device: a national comparison of cost and survival. J Thorac Cardiovasc Surg. 2013;145(2):566–573; discussion 573–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogers JG, Bostic RR, Tong KB, Adamson R, Russo M, Slaughter MS. Cost-effectiveness analysis of continuous-flow left ventricular assist devices as destination therapy. Circ Heart Fail. 2012;5(1):10–16. [DOI] [PubMed] [Google Scholar]
- 51.Russo MJ, Gelijns AC, Stevenson LW, et al. The cost of medical management in advanced heart failure during the final two years of life. J Card Fail. 2008;14(8):651–658. [DOI] [PubMed] [Google Scholar]
- 52.Sharples LD, Dyer M, Cafferty F, et al. Cost-effectiveness of ventricular assist device use in the United Kingdom: results from the evaluation of ventricular assist device programme in the UK (EVAD-UK). The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2006;25(11):1336–1343. [DOI] [PubMed] [Google Scholar]
- 53.Slaughter MS, Bostic R, Tong K, Russo M, Rogers JG. Temporal changes in hospital costs for left ventricular assist device implantation. J Card Surg. 2011;26(5):535–541. [DOI] [PubMed] [Google Scholar]
- 54.Whelan CT, Chen C, Kaboli P, Siddique J, Prochaska M, Meltzer DO. Upper versus lower gastrointestinal bleeding: a direct comparison of clinical presentation, outcomes, and resource utilization. J Hosp Med. 2010;5(3):141–147. [DOI] [PubMed] [Google Scholar]
- 55.Baras Shreibati J, Goldhaber-Fiebert JD, Banerjee D, Owens DK, Hlatky MA. Cost-Effectiveness of Left Ventricular Assist Devices in Ambulatory Patients With Advanced Heart Failure. JACC Heart Fail. 2017;5(2):110–119. [DOI] [PubMed] [Google Scholar]
- 56.Digiorgi PL, Reel MS, Thornton B, Burton E, Naka Y, Oz MC. Heart transplant and left ventricular assist device costs. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2005;24(2):200–204. [DOI] [PubMed] [Google Scholar]
- 57.Mehra MR, Salerno C, Cleveland JC, et al. Healthcare Resource Use and Cost Implications in the MOMENTUM 3 Long-Term Outcome Study. Circulation. 2018;138(18):1923–1934. [DOI] [PubMed] [Google Scholar]
- 58.Chimanji N, Kilic A, Hasan A, Higgins RS, Whitson BA, Kilic A. Institutional Cost Comparison Between Heart Transplants and Left Ventricular Assist Device Implantations. Exp Clin Transplant. 2016;14(6):656–659. [DOI] [PubMed] [Google Scholar]
- 59.Davis KL, Mitra D, Medjedovic J, Beam C, Rustgi V. Direct economic burden of chronic hepatitis C virus in a United States managed care population. Journal of clinical gastroenterology. 2011;45(2):e17–24. [DOI] [PubMed] [Google Scholar]
- 60.McAdam-Marx C, McGarry LJ, Hane CA, Biskupiak J, Deniz B, Brixner DI. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: a managed care perspective. J Manag Care Pharm. 2011;17(7):531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shelton BA, Sawinski D, Linas BP, et al. Population level outcomes and cost-effectiveness of hepatitis C treatment pre- vs postkidney transplantation. Am J Transplant. 2018;18(10):2483–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Post PN, Stiggelbout AM, Wakker PP. The utility of health states after stroke: a systematic review of the literature. Stroke; a journal of cerebral circulation. 2001;32(6):1425–1429. [DOI] [PubMed] [Google Scholar]
- 63.Hartwell D, Shepherd J. Pegylated and non-pegylated interferon-alfa and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and meta-analysis. Int J Technol Assess Health Care. 2009;25(1):56–62. [DOI] [PubMed] [Google Scholar]
- 64.Martin NK, Vickerman P, Dore GJ, et al. Prioritization of HCV treatment in the direct-acting antiviral era: An economic evaluation. Journal of hepatology. 2016;65(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wright M, Grieve R, Roberts J, Main J, Thomas HC, Investigators UKMHCT. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess. 2006;10(21):1–113, iii. [DOI] [PubMed] [Google Scholar]
- 66.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. The New England journal of medicine. 2014;371(9):796–797. [DOI] [PubMed] [Google Scholar]
- 67.Bethea E, Arvind A, Gustafson J, et al. Immediate Administration of Antiviral Therapy after Transplantation of Hepatitis C-infected Livers into Uninfected Recipients: Implications for Therapeutic Planning. Am J Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crismale JF, Khalid M, Bhansali A, et al. Liver, simultaneous liver-kidney, and kidney transplantation from hepatitis C-positive donors in hepatitis C-negative recipients: A single-center study. Clin Transplant. 2020;34(1):e13761. [DOI] [PubMed] [Google Scholar]
- 69.Cypel M, Feld JJ, Galasso M, et al. Prevention of viral transmission during lung transplantation with hepatitis C-viraemic donors: an open-label, single-centre, pilot trial. Lancet Respir Med. 2019. [DOI] [PubMed] [Google Scholar]
- 70.Durand CM, Bowring MG, Brown DM, et al. Direct-Acting Antiviral Prophylaxis in Kidney Transplantation From Hepatitis C Virus-Infected Donors to Noninfected Recipients: An Open-Label Nonrandomized Trial. Annals of internal medicine. 2018;168(8):533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kapila N, Menon KVN, Al-Khalloufi K, et al. HCV NAT positive solid organ allografts transplanted into HCV negative recipients: A real-world experience. Hepatology. 2019. [DOI] [PubMed] [Google Scholar]
- 72.Kilic A, Hickey G, Mathier M, et al. Outcomes of Adult Heart Transplantation Using Hepatitis C-Positive Donors. J Am Heart Assoc. 2020;9(2):e014495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kwong AJ, Wall A, Melcher M, et al. Liver transplantation for hepatitis C virus (HCV) non-viremic recipients with HCV viremic donors. Am J Transplant. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reese PP, Abt PL, Blumberg EA, et al. Twelve-Month Outcomes After Transplant of Hepatitis C-Infected Kidneys Into Uninfected Recipients: A Single-Group Trial. Annals of internal medicine. 2018;169(5):273–281. [DOI] [PubMed] [Google Scholar]
- 75.Wang JH, Gustafson SK, Skeans MA, et al. OPTN/SRTR 2018 Annual Data Report: Hepatitis C. Am J Transplant. 2020;20 Suppl s1:542–568. [DOI] [PubMed] [Google Scholar]
- 76.Durand CM, Garonzik-Wang J, Desai NM. Reclaiming missed opportunities: a strategy of targeted direct-acting antiviral prophylaxis for HCV-seronegative recipients of HCV-seropositive donor kidneys. Transpl Int. 2019. [DOI] [PubMed] [Google Scholar]
- 77.Wettersten N, Tran H, Mekeel K, Pretorius V, Adler E, Aslam S. Successful heart-kidney transplantation from a Hepatitis C viremic donor to negative recipient: One year of follow-up. Transpl Infect Dis. 2019;21(1):e13002. [DOI] [PubMed] [Google Scholar]
- 78.Goldberg DS, Abt PL, Blumberg EA, et al. Trial of Transplantation of HCV-Infected Kidneys into Uninfected Recipients. The New England journal of medicine. 2017;376(24):2394–2395. [DOI] [PubMed] [Google Scholar]
- 79.Khan B, Singer LG, Lilly LB, et al. Successful Lung Transplantation From Hepatitis C Positive Donor to Seronegative Recipient. Am J Transplant. 2017;17(4):1129–1131. [DOI] [PubMed] [Google Scholar]
- 80.Saberi B, Hamilton JP, Durand CM, et al. Utilization of hepatitis C virus RNA-positive donor liver for transplant to hepatitis C virus RNA-negative recipient. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2018;24(1):140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gernhofer YK, Brambatti M, Greenberg BH, Adler E, Aslam S, Pretorius V. The impact of using hepatitis c virus nucleic acid test-positive donor hearts on heart transplant waitlist time and transplant rate. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2019;38(11):1178–1188. [DOI] [PubMed] [Google Scholar]
- 82.McCauley M, Mussell A, Goldberg D, et al. Race, Risk, and Willingness of End-Stage Renal Disease Patients Without Hepatitis C Virus to Accept an HCV-Infected Kidney Transplant. Transplantation. 2018;102(4):e163–e170. [DOI] [PubMed] [Google Scholar]
- 83.Gupta G, Yakubu I, Bhati CS, et al. Ultra-short duration direct acting antiviral prophylaxis to prevent virus transmission from hepatitis C viremic donors to hepatitis C negative kidney transplant recipients. Am J Transplant. 2019. [DOI] [PubMed] [Google Scholar]
- 84.Levitsky J, Verna EC, O’Leary JG, et al. Perioperative Ledipasvir-Sofosbuvir for HCV in Liver-Transplant Recipients. The New England journal of medicine. 2016;375(21):2106–2108. [DOI] [PubMed] [Google Scholar]
- 85.Klassen DK, Edwards LB, Stewart DE, Glazier AK, Orlowski JP, Berg CL. The OPTN Deceased Donor Potential Study: Implications for Policy and Practice. Am J Transplant. 2016;16(6):1707–1714. [DOI] [PubMed] [Google Scholar]
- 86.Kirklin JK, Naftel DC, Kormos RL, et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2012;31(2):117–126. [DOI] [PubMed] [Google Scholar]
- 87.Arias E, Xu J. United States Life Tables, 2015. Natl Vital Stat Rep. 2018;67(7):1–64. [PubMed] [Google Scholar]
- 88.Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N. Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2007;11(11):1–205, iii. [DOI] [PubMed] [Google Scholar]
- 89.Bethea ED, Samur S, Kanwal F, et al. Cost Effectiveness of Transplanting HCV-Infected Livers Into Uninfected Recipients With Preemptive Antiviral Therapy. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.