Abstract
The essential goal of any adhesive restoration is to achieve a tight and long-lasting adaptation of the restorative material to enamel and dentin. The key challenge for new dental adhesives is to be simultaneously effective on two dental substrates of conflicting nature. Some barriers must be overcome to accomplish this objective. While bonding to enamel by micromechanical interlocking of resin tags within the array of microporosities in acid-etched enamel can be reliably achieved and can effectively seal the restoration margins against leakage, bonding effectively and durably to organic and humid dentin is the most puzzling task in adhesive dentistry.
Much of the research and development of dental adhesives has focused on making the clinical procedure more user-friendly by reducing the number of bottles and/or steps. Although clinicians certainly prefer less complicated and more versatile adhesive materials, there is a trade-off between simplification of dental adhesives and clinical outcomes. Likewise, new materials are launched with claims of being novel and having special properties without much supporting evidence.
This review article discusses dental adhesion acknowledging pioneer work in the field, highlights the substrate as a major challenge to obtain durable adhesive restorations, as well as analyzes the three adhesion strategies and their shortcomings. It also reviews the potential of chemical/ionic dental adhesion, discusses the issue of extensively published laboratory research that does not translate to clinical relevance, and leaves a few thoughts in regard to recent research that may have implications for future adhesive materials.
1. Introduction
Dental adhesion was responsible for a paradigm shift in dentistry (Table 1). Dental adhesives have become one of the most intriguing biomaterials in Health Sciences. Research efforts in the last 20 years have shifted from clinically-proven multi-step dental adhesives to simplified versions that do not perform adequately in laboratory and clinical studies [1,2]. The ideal goals for clinical effectiveness and durability of the restorations have been frequently neglected in favor of fewer number of bottles and quicker application of newer dental adhesives.
Table 1.
Changes that resulted from the introduction of adhesives in Dentistry.
| Positive | Negative |
|---|---|
| The use of dental adhesives has expanded across different dental disciplines (Operative Dentistry, Orthodontics, Pediatric Dentistry, Periodontology, Prosthodontics, Endodontics) | Clinicians tend to rely solely on adhesion as the source of primary retention in clinical situations without enamel margins or without enough residual tooth structure, as in core build-up composite resin restorations |
| Dental adhesives are used to retain a wide range of restorative materials – glass-matrix ceramics, oxide ceramics, pre-polymerized composite resins, direct composite resins, metal-based restorations, fiber posts, fiber splinting materials | Potential for marginal bacterial leakage when the cavo-surface margin is in dentin/cementum |
| More conservative tooth preparations (lesion-specific preparations) | Post-operative sensitivity in posterior adhesive restorations related to polymerization shrinkage stress |
| Reliable micromechanical retention to etched enamel without macro-retention features | Enamel cracks in posterior adhesive restorations related to polymerization shrinkage stress |
| Reinforcement of residual tooth structure; undermined enamel does not need to be always removed | Moisture contamination of the operatory field may be more detrimental for adhesive than for non-adhesive restorations |
| Increased resistance to recurrent caries lesions when dentin is impregnated with dental adhesive | Small monomers, such as HEMA, may easily seep into the pulp space and cause pulp inflammation |
| Increased resistance to caries lesions in sealed fissure systems of posterior teeth | Frequent open contacts in posterior composite resin restorations (compared to amalgam restorations) |
| High efficacy to treat root sensitivity | Adhesives may cause pulp necrosis if applied in preparations close to the pulp |
| Some adhesives have antibacterial properties, which may prevent recurrent caries lesions | Some of the monomers in dental adhesives may cause contact dermatitis |
| Stronger retention of glass-matrix ceramics restorations; adhesives increase the resistance to fracture of glass-matrix ceramics | |
| Stable chemical adhesion to hydroxyapatite for some adhesive materials when dentin is not etched with phosphoric acid | |
Several obstacles must be overcome to accomplish the objective of developing a dental adhesive that bonds effectively to enamel and dentin, and achieves durable restorations that seal the margins and provide some form of resistance to recurrent caries lesions.
The continuous development and frequent introduction of dental adhesives render existing materials outdated within a few years. When clinical studies are completed, often a new version of the same material has already been made available on the market. In fact, dental adhesives can be launched without proof of clinical efficacy, as the FDA usually reviews “the Section 510(k) premarket notification of intent to market the device and determines the device to be substantially equivalent to legally marketed predicate devices” used for the same indications [[3], [4], [5]].
It is extremely difficult for practicing dentists to keep updated as so many dental adhesives are constantly launched on the market and updated or relaunched within short periods of time. In addition, dentists do not have access to the latest evidence-based information. As a result, dentists rely on the information provided by the industry representatives or information disseminated in continuing education courses and dental meetings, often without solid evidence to support the claims [6].
The objective of this review article is to summarize the current evidence on dental adhesion, from the challenging substrate to the latest trends, many of which do not extrapolate to sound evidence pertinent to clinical practice.
2. Milestones in dental adhesion
In 1952, a manuscript published in the British Dental Journal by Kramer and McLean described an in-situ study that was carried out in 124 preparations of 118 teeth scheduled to be extracted for orthodontic reasons [7]. The authors used 15 combinations of restorative materials. Tooth sections were stained with hematoxylin/eosin upon extraction, and observed blindly under the optical microscope. The authors then matched the data with the experimental groups. Some of the sections displayed “an altered reaction observed as a narrow zone of material staining deeply with hæmatoxylin immediately bordering the cavity. This zone averaged about 3 μm in thickness and was seen to be composed of dentine having an intense affinity for hæmatoxylin. This change was present in all of the 28 teeth filled with Sevriton-adhesive. Similar changes were absent from all of the 96 teeth filled with other materials.” The specific chemically-cured adhesive used in this study had been developed in 1949 by Oskar Hagger, a chemist who worked for DeTrey/Amalgamated Dental Company [8]. The adhesive contained a phosphate monomer, later identified by Dr. Buonocore’s research group as glycerol phosphoric acid dimethacrylate [9], which is still used in a few dental adhesives as GPDM [10]. Remarkably, the findings of the 1952 manuscript [7] were the first reference to the concept currently known as the hybrid layer, elegantly illustrated with an image of dentin altered by the adhesive [7]. In addition, the use of the phosphate monomer GPDM as a dentin adhesive may now be part of history as the first research report of a self-etch adhesive in the literature.
In 1955, a major advance for the history of dental adhesion was published in the Journal of Dental Research [11]. Michael Buonocore used 85% phosphoric acid to change enamel surfaces and make them more suitable for mechanical adhesion, using an industrial technique that improved the adhesion of paints to metal surfaces. Buonocore later expanded his acid-etch technique to clinical dentistry to seal pits and fissures, as reported in 1967 [12]. The authors used 50% phosphoric to etch pits and fissures, followed by the application of a silica-filled methacrylate adhesive. This novel technique, which was not standard of care in 1967, resulted in a reduction of caries incidence in pits and fissures by as much as 86.3% at 1 year [12].
Etching enamel with phosphoric acid (Fig. 1) is still considered, sixty-five years later, as the gold standard for bonding resin-based materials to tooth structure. The interlocking of resin tags (Fig. 1) within the microsized porosities left by enamel chemical etching can effectively seal the restoration margins in the long-term [13]. There is clinical evidence that dental adhesives result in more reliable clinical behavior when enamel is etched prior to the application of the adhesive [14]. Nonetheless, new adhesives are still being launched without recommendations for etching enamel with phosphoric acid.
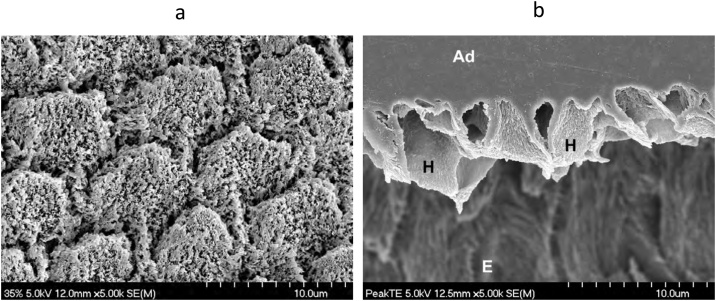
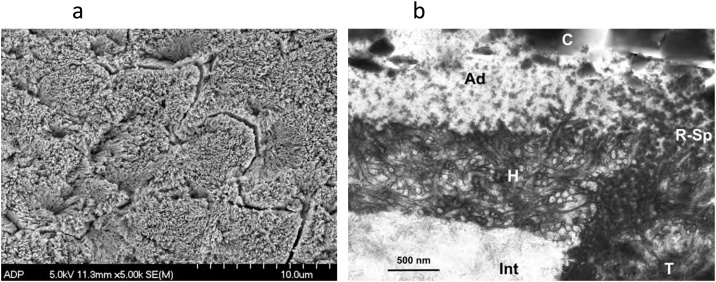
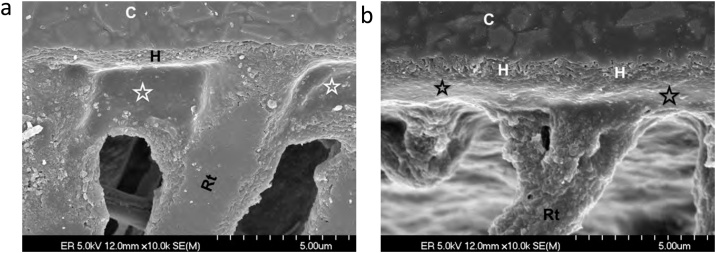
Fig. 1.
(a) SEM micrograph of human enamel etched with 35% phosphoric acid for 15 s. Original magnification = 5,000X. (b) SEM micrograph of a replica of an interface of etched enamel with a dental adhesive. After curing the adhesive, the specimen was left in 6N HCl for 12 h to dissolve the enamel. The enamel prisms at the interface were not dissolved because the etched enamel was impregnated with polymerized adhesive, creating a hybrid layer. H – hybrid layer; Ad – adhesive; E – residual enamel. Original magnification = 5,000X.
Another milestone in adhesive dentistry occurred in 1960. Rafael Bowen (who retired from the ADA Foundation Research Center in 2018 after 62 years of continuous service) and Mario Rodriguez presented a paper [15] at the IADR meeting in Chicago that reported the tensile strength of several materials, including a new silica-resin material that contained “about 70 per cent vinyl silane-treated clear fused silica combined with about 30 per cent of an adduct of glycidyl methacrylate and bisphenol A”. It is worth underlining here the inclusion of a silane to bond the inorganic filler to the new Bis-GMA resin. By 1963, the full composition of the new material had been finalized [16]. Based on Bowen’s research with the Bis-GMA molecule, the first commercial composite resin (Addent, 3 M) was introduced in 1964 as a chemically-cured paste-paste material. Interestingly, since that time most changes in composite resin technology have been in the filler particle size and distribution rather than in the resin matrix, which is still based on Bis-GMA, also known as Bowen’s resin.
Alan Wilson and Brian Kent, working at the Laboratory of the Government Chemist in the UK, invented in 1968 one the most groundbreaking materials in dentistry, for which the patent was applied for in 1969 [17]. This self-adhesive material was widely known as glass-ionomer cement (GIC), although the correct terminology is glass polyalkenoate cement [18]. The first report of their findings in the literature appeared in 1971 [19]. The commercial version was subsequently launched in Europe in 1975 under the commercial name ASPA by Amalgamated Dental International, DeTrey Division.
Takao Fusayama, defying the general belief that etching dentin caused irreversible pulp damage, in 1979 reported that etching dentin and enamel with 40% phosphoric acid for 60 sec substantially improved the adhesion of Clearfil Bond System-F (Kuraray) [20]. For the first time, the concept of “total-etch” was associated with improved dentin adhesion.
Nobuo Nakabayashi’s research team was responsible for another breakthrough in 1982, when they reported for the first time a “demineralization-resistant zone” [21] in dentin after etching dentin with 10% citric acid-3% ferric chloride (10:3 solution) for 30 sec and applying 4-META (methacryloxyethyltrimellitate anhydride) cured with tri-n-butyl borane. This concept of hybrid layer in etched dentin was identified using the scanning electron microscope (SEM). The same authors also highlighted the importance of monomers with both hydrophobic and hydrophilic groups to promote adhesion with tooth substrates by penetration and infiltration dentin as a new concept in biocompatible materials for dental use [21].
The most recent milestone was associated with the adhesion-decalcification concept (AD-concept) for adhesion to dentin [22,23]. This concept was originally described for carboxylic acids. When these acids are applied on hydroxyapatite they first form ionic bonds to calcium, which may be dependent on the pKa of each acid. While some of the carboxylic acids, such as oxalic acid, stay attached to calcium on the hydroxyapatite surface resulting in insignificant decalcification, other carboxylic acids result in a significant decalcification of hydroxyapatite with minimal or no chemical attachment. This adhesion-decalcification (AD) concept is still relevant. Etch-and-rinse (ER) adhesives follow the decalcification pathway derived from phosphoric acid etching, whereas mild self-etch (SE) adhesives (pH ≈ 2), such as those that contain 10-methacryloyloxydecyl dihydrogen phosphate or 10-MDP (MDP), tend to follow the adhesion pathway. Nevertheless, mild SE adhesives still cause minimal decalcification, which is still required for calcium release and subsequent formation of stable MDP-Ca salts and respective nanolayering, as discussed later in this article.
3. The substrate
Enamel and dentin are the dental substrates to which we bond our restorative materials. Cementum may also be involved when the cavo-surface margin is located apically to the cementum-enamel junction.
Enamel is a dry substrate without vital structures containing 92 vol% of mineral phase (hydroxyapatite), which makes enamel almost the ideal substrate to form a tight adhesive joint. The acid-etch technique [11] is still the gold standard for bonding resin-based materials to tooth structure. The micromechanical interaction of adhesives with enamel is a result of the diffusion and interlocking of resin monomers into the array of microporosities left by the acid chemical dissolution of enamel (Fig. 1). Bonding to enamel after etching with phosphoric acid is certainly the foundation for the durability of adhesive restorative procedures.
Dentin is a complex biocomposite structure, defined by some authors as a puzzle of different types of dentin and by other authors as a bone-like nanocomposite built of carbonated hydroxyapatite mineral particles, protein, and water [24,25]. As opposed to enamel, dentin is a humid and more organic substrate. Dentin adhesion has been one of the most challenging and less predictable tasks in adhesive dentistry due to the dynamic compositional differences and complex histology of dentin. The ability of adhering restorative materials intimately to dentin is affected by many factors, including biological and clinical factors. These factors include the patient’s age, location of the tooth in the mouth, dentin depth and permeability, pulpal fluid flow, presence of sclerotic and/or carious dentin, radicular versus coronal dentin, type of restorative material and procedure, isolation, parafunctional habits, dentist’s experience, among others [[26], [27], [28], [29], [30], [31]].
The mineral phase (hydroxyapatite) of dentin is on average 45 vol%, while the organic matrix is 33 vol%, the remainder being water [32]. Type I collagen is the most abundant protein in the organic phase. Dentin encloses a maze of inverted-cone shaped tubules that traverse dentin, radially oriented with the larger diameter facing the pulp [26]. Garberoglio and Brännström in 1976 [33] measured the area occupied by the tubules and the tubular diameter in 30 extracted teeth. The number of tubules near the pulp was 45,000 per square millimeter and their diameter 2.5 μm. In middle dentin, the number of tubules was 29,500/mm2 and the average diameter was 1.2 μm. In superficial dentin, the area occupied by tubules was 20,000/mm2 and the average tubule diameter was 0.9 μm [33]. The contents of water increase 20-fold from superficial to deep dentin. The mean tubule volume in coronal dentin is 10% of the entire dentin volume, while near the DEJ it is 4% and increases to 28% near the pulp [33] (Fig. 2).
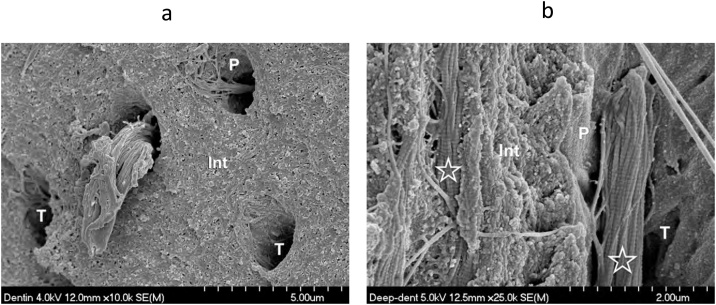
Fig. 2.
(a) SEM micrograph of fractured superficial dentin. Int – intertubular dentin; P – peritubular dentin; T – dentin tubule; Arrows – bacteria in the tubule lumen. Original magnification = 10,000X. (b) SEM micrograph of fractured deep dentin 75 μm from the pulp of the same tooth in Fig. 2a. Original magnification = 10,000X.
Dentin tubules are permeated with fluid under constant outward pulpal pressure estimated to be 25 to 30 mm Hg [34]. In addition, there is fluid present within the intertubular dentin area, making dentin an intrinsically moist hard tissue throughout its internal structure. Dentin contains extensions of the odontoblast (odontoblastic processes) and intra-tubular collagen fibers in deeper areas (Fig. 3), less frequently in middle and superficial dentin. These characteristics, which we sometimes overlook as clinicians, attest the greater challenge when an adhesive restoration is inserted in deep dentin compared to restorations placed in more superficial dentin.
Fig. 3.
(a) SEM micrograph of fractured middle dentin showing an odontoblastic process extending from the tubule Int – intertubular dentin; P – peritubular dentin; T – dentine tubule. Original magnification = 10,000X. (b) SEM micrograph of fractured deep dentin showing intratubular collagen (asterisk) with the characteristic 64 nm collagen banding pattern. Int – intertubular dentin; P – peritubular dentin; T – dentin tubule. Original magnification = 25,000X.
In addition to the compositional differences, there is a subtle difference between the enamel and dentin hydroxyapatite crystallites. Enamel crystallites are larger and have a more regular and parallel oriented arrangement, whereas dentin hydroxyapatite crystals are smaller and arranged in a crisscross pattern within the organic matrix (Fig. 4), rendering it more difficult to establish a micro-mechanical interlocking with dentin. Conversely, chemical bonding to dentin is facilitated by the smaller size and the crisscross orientation of the hydroxyapatite crystallites [35]. The different crystallite size facilitates chemical bonding to dentin to the detriment of chemical bonding to enamel [35].
Fig. 4.
SEM micrograph of fractured dentin-enamel junction (DEJ) area. Note the morphological differences between enamel and dentin hydroxyapatite. Original magnification = 5,000X. Arrows – DEJ; E – enamel; Int – intertubular dentin; T – dentin tubule.
Physiological changes resulting from dentin aging or in response to carious lesions and other aggressive stimuli increase the degree of mineralization of dentin, resulting in an increase in dentin thickness and a reduction of dentin permeability [28,29]. Reduction of permeability with age may have a direct effect on dentin bond strengths [36] as dentin permeability affects the adhesion process.
4. Relevance of in vitro testing for dental adhesion
Several peer-reviewed publications have covered this topic very well [[37], [38], [39]]. It is still important to emphasize that laboratory tests of dental adhesives usually employ extracted impacted third molars and premolars extracted for orthodontic reasons. This ‘laboratory-type’ (or unaffected dentin substrate) lacks clinical relevance, as clinicians place adhesive restorations in teeth that have had carious lesions, failed restorations, or non-carious cervical lesions with sclerotic dentin continuously exposed to the oral environment.
The clinically relevant substrates for dentin adhesion include affected dentin, which is located immediately underneath the carious dentin area. Affected dentin is slightly decalcified but sill amenable to recalcification, with odontoblastic processes, sound collagen fibers, and apatite crystals bound to the fibers [40,41]. Continuous deposition of mineral within the tubules underneath a carious lesion process results in tubular obliteration and the formation of sclerosis, and potentially reducing bond strengths [28].
Another type of clinically relevant dentin that may be found in deep caries lesions is reactionary tertiary dentin, which is formed by odontoblasts in the pulp chamber wall near the area corresponding to the carious lesion or to the occlusal trauma. In case of a pulp exposure, newly differentiated odontoblast-like or odontoblastoid cells replace the irreversibly injured odontoblasts at the exposure site and form a bridge of atubular dentin known as reparative tertiary dentin [42].
Sclerotic dentin is common in areas where dentin has been exposed to the oral environment, such as non-carious cervical lesions (NCCLs) (Fig. 5). These lesions contain a complex dentin substrate with different ultrastructural layers [43], including a hypermineralized layer on the surface with denatured collagen and bacteria (Fig. 6). The tubules appear obliterated by crystalline deposits. Etching sclerotic dentin in NCCLs is difficult to achieve [43,44], as phosphoric acid does not etch underneath the hypermineralized surface layer and is unable to dissolve the sclerotic casts in the tubules. Due to the intricacies of the substrate, restorations of NCCLs have a higher tendency to fail than restorations in other areas of the mouth [43,45]. Roughening the superficial area of hypermineralized dentin (and enamel) in NCCLs improves the survival rate of restorations [46].
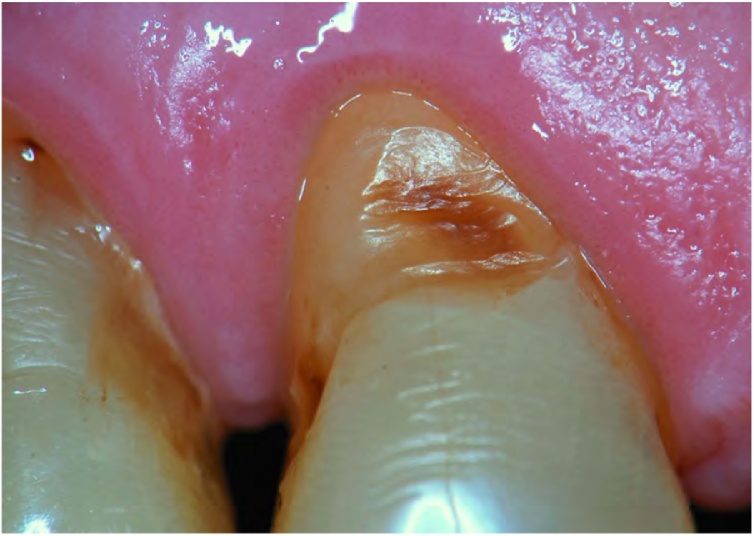
Fig. 5.
Non-carious cervical lesion (NCCL) with sclerotic dentin.
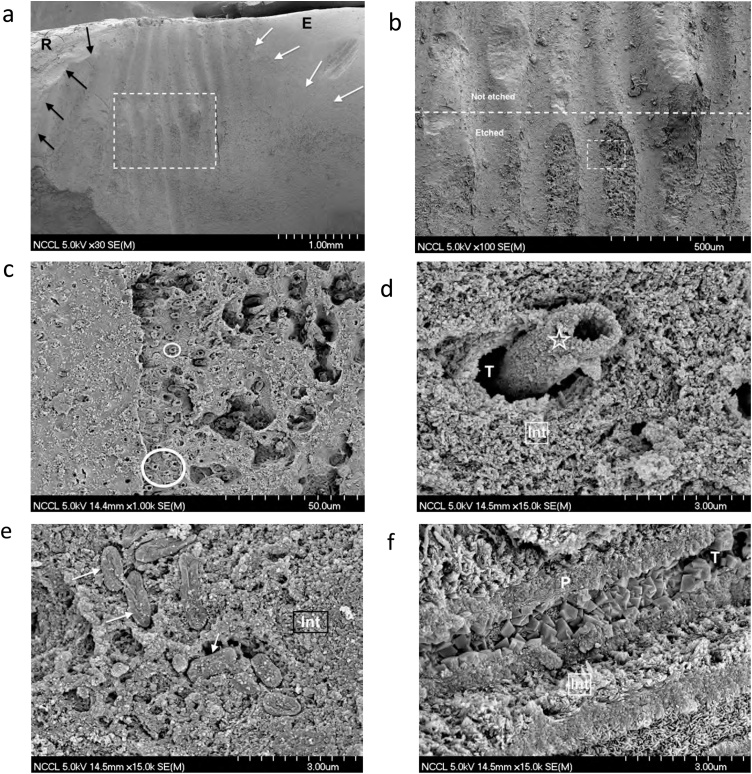
Fig. 6.
Sequence of SEM micrographs to illustrate the morphological characteristics of the bonding substrate in a NCCL of a recently extracted mandibular canine. (a) SEM micrograph depicting a general view of the NCCL. The incisal aspect is on the right side (E-enamel), with the cervical aspect on the left side (R-root). The white arrows point to the natural incisal cavo-surface margin. The dark arrows point to the natural cervical cavo-surface margin. Original magnification = 30X. (b) SEM micrograph of the area included in the rectangle in Fig. 6a. The horizontal dotted line separates the unetched area (upper half) from the area that was etched with 35% phosphoric acid for 15 s (lower half). Original magnification = 100X. (c) SEM micrograph of the area included in the rectangle of Fig. 6b (etched area). Note the sclerotic casts in the tubules (circles) and, overall, hypermineralized dentin. Original magnification = 1,000X. (d) SEM micrograph of a sclerotic cast (asterisk) obliterating the tubule (T). Note how intertubular dentin (Int) is densely mineralized. Original magnification = 15,000X. (e) SEM micrograph of bacteria (arrows) ‘fossilized’ into the mineralized area of intertubular dentin (Int). Original magnification = 15,000X. (f) SEM micrograph of a longitudinal fracture of dentin in a NCCL. Note how the tubule is obliterated with rhombohedral mineral crystals, which were elegantly described in 1989 [173]. Int – intertubular dentin; P – peritubular dentin; T – dentin tubule. Original magnification = 15,000X.
5. Current dental adhesives
Dental adhesives are currently categorized using two different classifications.
-
I
By generation – from first to eighth generations.
It is used mostly by the dental industry to highlight the latest trend. It is a confusing nomenclature, as the first dentin adhesives that used a phosphoric acid etchant on enamel and dentin are known as the fourth generation. This classification is not very informative either, especially when considering the missing components of the dental adhesive, i.e., etchant, primer and bonding resin.
-
II
By adhesion strategy – with or without etching enamel and dentin simultaneously with phosphoric acid (Table 2).
Table 2.
Classification of dental adhesives by adhesion strategy.
 |
Ac – Phosphoric acid; Pr – Hydrophilic primer; BR – Non-solvated bonding resin; GIC– glass ionomer cement; PAA -polyacrylic acid.
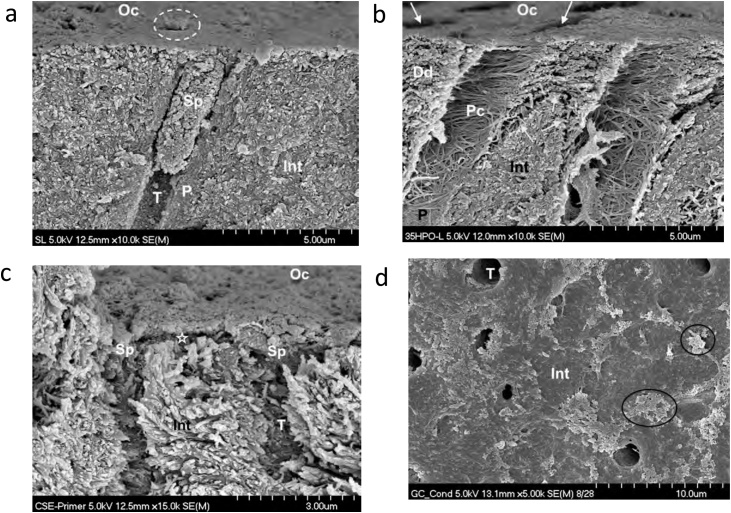
This classification is easier to understand for practicing dentists than the classification by generation, as adhesives are grouped according to their interaction with the tooth structure, more precisely according to the way they interact with the smear layer (Fig. 7a). In addition, it is informative in regards to the different steps used in the adhesive procedure. This nomenclature will be used in this manuscript (Table 2). Adhesives that include a phosphoric acid-etching step are known as etch-and-rinse (ER) adhesives. They dissolve and remove the smear layer and smear plugs (Fig. 7b). Adhesives that do not use a separate etching step are known as self-etch (SE) adhesives, as they do not remove the smear layer, but incorporate into the adhesive interface (Fig. 7c). Self-adhesive (SA) materials (dental adhesive and restorative component all-in-one material), belong to another strategy for adhesion to tooth structure, as either composite resins or GIC-based materials. The latter use a separate polyalkenoic acid (often polyacrylic) dentin conditioner that is rinsed off (Fig. 7d).
Fig. 7.
(a) SEM micrograph of a fractured human dentin specimen with smear layer and a smear plug created with diamond bur. Int – intertubular dentin; P – peritubular dentin; T – dentin tubule; Sp – smear plug; Oc –occlusal surface; Dotted circle – another tubule plugged with smear layer. Original magnification = 10,000X. (b) SEM micrograph of human dentin etched with liquid phosphoric acid for 15 sec. Int – intertubular dentin; P – peritubular dentin; T – dentin tubule; Oc – occlusal surface; Pc – exposed peritubular collagen from dissolution of the peritubular dentin; Dd – dentin demineralized by the etching agent; arrows – other tubules. Original magnification = 10,000X. (c) SEM micrograph of human dentin treated with the Clearfil SE primer (Kuraray) from the 2-step SE adhesive Clearfil SE Bond. The asterisk marks the area of dentin partially decalcified by the primer (pH = 1.8–2.0). Upon application of the respective hydrophobic bonding resin, this 0.5 μm deep area will become the hybrid layer. Int – intertubular dentin; T – dentin tubule; Oc – occlusal surface; Sp – primer-infiltrated smear plug. Original magnification = 15,000X. (d) SEM micrograph of occlusal view of human dentin treated with GC Cavity Conditioner (20% polyacrylic acid with 3% aluminum chloride hexahydrate) for 10 sec, and rinsed with water for 15 sec. Note residual smear layer (ovals) and some patent tubules (T). The intertubular dentin does not have morphological characteristics of demineralization (no visible collagen fibers). Original magnification = 5,000X.
The basic components of a dental adhesive system are
-
A)
Etchant, currently phosphoric acid in a concentration between 30% and 40%. Most phosphoric acid gels are thickened with silica microparticles, although there are a few that contain other thickeners such as xanthan gum. A color dye is always included to improve the application accuracy and ensure that all gel is washed off. Glycol is often added to improve wettability and decrease viscosity. The etchant is always rinsed off from the tooth surface.
-
B)
Primer which is a hydrophilic solution of resin monomers, organic solvent (alcohol or acetone), water, and stabilizers. The hydrophilic groups boost the wettability to the dentin surface, which is a humid environment. The role of primers in dentin adhesives is comparable to that of primers in paints. The primer adheres to surfaces and forms a binding layer that is better prepared to receive the paint, in this case, the bonding resin. Primers are not usually rinsed off nor cured once placed on the tooth surface; they are only air-dried.
-
C)
The bonding resin is a solvent-free (hydrophobic) low-viscosity resin that is applied over the primer and then light-cured. The hydrophobic groups interact and copolymerize with the restorative material and make dentin bonding more stable and more durable by sealing the bonded interface against nanoleakage [47,48]. The hydrophobic resin improves both the polymerization rate of the primer and the mechanical properties of the adhesive and hybrid layer [48]. Adhesive systems that have this separate bonding step result in better in vitro and clinical outcomes [49].
Dental adhesives may involve three separate procedures or steps, two of them merged in one, or all of them merged in one (Table 2).
5.1. Etch-and-rinse (ER) adhesives
The advantages and disadvantages of ER adhesives ae summarized in Table 3. The first ER adhesives were 3-step adhesives. They include an acid etchant applied to enamel and dentin simultaneously to remove the smear layer and demineralize the substrate. Phosphoric acid has been the standard etchant but other acids have been unsuccessfully used, namely maleic, nitric and oxalic acids [[57], [58], [59]]. The 3-step ER adhesives also include a primer and a hydrophobic bonding resin that infiltrates the demineralized dentin (Dd in Fig. 7b) to form a hybrid layer of collagen and resin (Fig. 8). Regarding the phosphoric acid etching agent, the current market tendency is for newer gels (Fig. 9a) being less viscous (runnier) than older phosphoric acid gels (Fig. 9b), which is likely to be as a result of extra glycol added to the gel. Although this detail is often overlooked, newer gels are much less aggressive on enamel and dentin than their predecessors [56] (Table 4) without reducing dentin bond strengths. However, their effect on enamel bonding durability needs to be further investigated.
Table 3.
Etch-and-rinse adhesives.
| Advantages | Disadvantages |
|---|---|
| Three-step ER adhesives have been available since the 1990s, therefore they have a long-track record. | Acetone-based adhesives need more applications than those recommended by the respective manufacturers [51]. |
| High immediate dentin and enamel bond strengths in laboratory studies | Over-etching decreases bond strengths [52]. |
| Excellent bonding to enamel in vitro and durable restorations in clinical studies. However, retention rates for 2-step ER adhesives are lower than for 3-step ER adhesives [49]. | More technique sensitive than SE adhesives, as the potential for incomplete infiltration of the adhesive into the etched dentin depends on several contributing factors occurring simultaneously in a very short time. |
| Clinical studies over 5 years with excellent results for specific 3-step ER adhesives. Optibond FL (Kerr) resulted in excellent retention at 13 years in NCCLs [50]. Optibond FL is still the reference against which all ER adhesives are compared. | Hydrolytic degradation of the bonds occurs when margins are located in dentin. |
| As these adhesives contain organic solvents such as ethanol or acetone, minor dentin contamination with saliva does not always decrease bond strengths in vitro. | The clinical and in vitro performance of 2-step ER adhesives undergo degradation faster than that of 3-step ER adhesives. |
| As opposed to 2-step ER adhesives, 3-step ER adhesives contain a hydrophobic bonding resin that prevents or delays the degradation of the resin-dentin interface by making the interface impermeable and increasing the film thickness. The lack of solvent increases the degree of conversion. | Bond strengths may vary with the degree of moisture, depending on the specific adhesive. |
| ER adhesives may result in mechanical interlocking with etched dentin provided that the dentin in not overetched, the primer/adhesive for 2-step ER adhesives is applied in an active scrubbing mode, and that there is not excessive water within the interfibrillar spaces. | Although clinical evidence demonstrates that ER adhesives do not cause more post-operative sensitivity than SE adhesives [53,54], some clinicians claim that ER adhesives cause higher incidence of post-operative sensitivity with posterior composite restorations. |
| Ability to bond composite, porcelain, fiber posts, etched or sandblasted metals, or amalgam. | Solvent air-drying time recommended by the manufacturers is insufficient [55] and must be extended. |
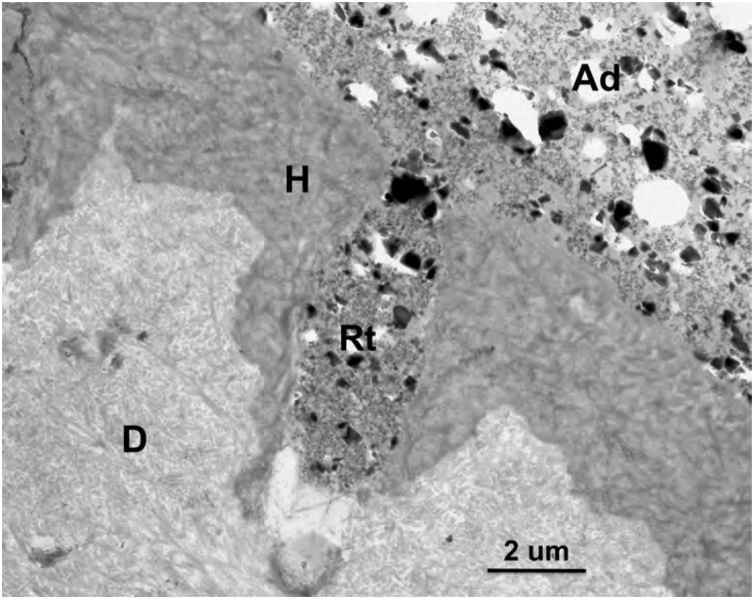
Fig. 8.
TEM micrograph of the adhesive-dentin interface formed by the 3-step ER adhesive OptiBond FL (Kerr). The particle-filled hydrophobic bonding adhesive resulted in filled resin tags (Rt). Ad – adhesive; H – hybrid layer; D – dentin. Original magnification = 6,000X.
Fig. 9.
(a) SEM micrograph of human dentin etched with 32% phosphoric acid (Scotchbond Universal Etchant, 3M). Original magnification = 7,000X. (b) SEM micrograph of human dentin etched with 35% phosphoric acid (Scotchbond Etchant, 3M). Original magnification = 7,000X. Int – intertubular dentin; P –peritubular dentin; T – dentin tubule; Oc – Occlusal surface; Pc – exposed peritubular collagen from dissolution of the peritubular dentin; Dd – dentin demineralized by the etching agent; Circles – silica thickening agent; Arrows – intertubular anastomoses.
Table 4.
Median intertubular dentin demineralization of current phosphoric acid gels [56].
| Etching gel | Intertubular dentin demineralization (μm) Etching time =15 sec |
|---|---|
| Ultra-Etch 35% (Ultradent) |
1.155A |
| Scotchbond Universal Etchant 32% (3 M Oral Care) |
1.675AB |
| Tooth Conditioning Gel 34% (Dentsply Sirona) |
2.185B |
| Total Etch 37% (IvoclarVivadent) |
2.820C |
| Select HV Etch 35% (Bisco) |
3.090C |
| Scotchbond Etchant 35% 3 M Oral Care) |
3.205C |
| Gel Etchant 37.5% (Kerr) |
4.033D |
| Liquid etchant 35% (prepared in laboratory) |
4.636D |
Three dentin disks per etching gel; 4 measurements in each half disk in identical areas, 24 measurements per etching gel; measurements taken with image analysis software (ImageJ, NIH). Statistical analysis included Kruskal-Wallis and Median tests at p < 0.05
Since the early 1990s, the concept of wet or moist dentin for ER adhesives [60,61] has been widely advocated and taught in dental schools. The collagen left in the area of demineralized dentin collapses when dentin is air-dried after rinsing off the etchant. This collapse results in incomplete infiltration of the adhesive into demineralized intertubular dentin [62] and lower bond strengths [63]. For this reason, keeping the dentin substrate moist (glistening) after rinsing off the etching gel has been recommended based on in vitro testing. However, in vitro tests are carried out in “laboratory-type” or unaffected dentin, as discussed above. When the degree of dentin moisture was tested in clinical trials with the same or similar ER adhesives, including a recent study with a universal adhesive, moist dentin was not associated with better retention rate for ER adhesives when compared to dry dentin [64,65].
Leaving the dentin moist after etching and rinsing may not be so crucial with current simplified adhesives, as agitation of the adhesive during application improves infiltration of the monomers into etched dentin. In fact, a clinical study in non-carious cervical lesions (NCCLs) found that passive application of the adhesive resulted in 82.5% retention rate after 2 years compared to 92.5% retention rate of the restorations in which the adhesive was scrubbed vigorously [66]. Furthermore, leaving dentin moist has been shown to cause degradation of the resin dentin interfaces at 6 months [67]. Dentists are advised to gently dry dentin after rinsing off the gel without inducing desiccation.
In addition, the residual water inside the resin-adhesive interface reduces the degree of conversion of the monomers and may induce hydrolytic degradation of adhesive polymers, compromising their physical properties [[68], [69], [70]]. Once the resins that envelope the collagen fibers are dissolved, the fibers become susceptible to further degradation [48]. A simple method to remove the extra water from the adhesive interface is to extend the solvent drying time. The 5 to 10 sec duration usually recommended by manufacturers may be inadequate to obtain durable bonding to dentin. as discussed under universal adhesives [55,71]. Gently air drying for at least 15 seconds is recommended for simplified adhesives with water in their composition.
5.2. Self-etch (SE) adhesives
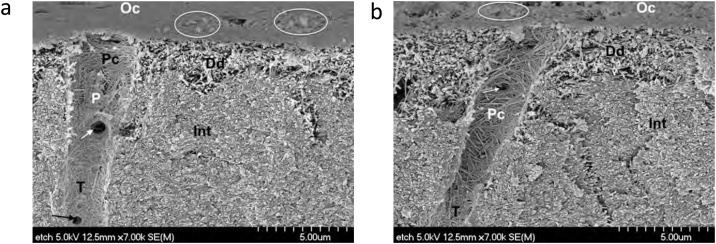
The advantages and disadvantages of SE adhesives are summarized in Table 5. The first SE adhesives were 2-step adhesives. They included an acidic primer and a hydrophobic bonding resin (Table 2). As SE adhesives do not require separate acid-etching and rinsing steps, they condition and prime enamel and dentin simultaneously, relying on their ability to infiltrate through smear layers (Fig. 7c) and partially dissolve hydroxyapatite to generate a hybrid layer with residual hydroxyapatite crystals [1] (Fig. 10). One of the positive features of SE adhesives is that they are considered more user-friendly than ER adhesives because their application time is somewhat reduced. A crucial shortcoming is their incapacity to etch enamel adequately, as they result in a poorly-defined shallow enamel etching pattern. However, the enamel etching pattern produced by the SE adhesives depends on their acidity, application time, and application mode [72,73]. The poor etching ability of self-etch adhesives may result in debonding at the margins, leading to marginal discoloration. A simple way to improve the bonding of SE adhesives is to etch enamel without etching dentin, which is known as the “selective enamel etching” technique (Fig. 11). A systematic review of clinical trials of up to 3 years showed that selective enamel etching resulted in less marginal discoloration, better marginal integrity, and increased retention rate of composite restorations bonded with SE adhesives [14].
Table 5.
Self-etch adhesives.
| Advantages | Disadvantages |
|---|---|
| Extremely easy to apply, no etching, no rinsing. | The acidic primer is not as acidic as phosphoric acid; therefore, SE adhesives do not etch enamel to the same depth as phosphoric acid. |
| SE adhesives can be used with selective enamel etching to improve clinical performance. | Some 1-step SE adhesives need more applications than those recommended by the respective manufacturers [76,77] |
| At least one 2-step SE adhesive has been available since the late 1990s, therefore they have a long-track record. | Some 1-step SE adhesives result in clinical signs of enamel leakage at 1 year, and unacceptable marginal discoloration at 2 years [78] |
| Mild SE adhesives result in a sub-micrometric resin-infiltrated calcium-rich hybrid layer allowing for simultaneous micromechanical and chemical bonding. | The acidity of 1-step SE adhesives inhibits the polymerization of chemically-cured composites. Special attention may be required to the utilization of self- or dual-cure cured composite buildup materials and self- or dual-cured luting cements with 1-step SE adhesives. |
| Two-step SE adhesives (for example, Clearfil SE Bond, Kuraray Noritake) contain a hydrophobic bonding resin that prevents or delays the degradation of the resin-dentin interface. | On dentin 1-step SE adhesives behave as permeable membranes after polymerization, allowing the permeation of fluids through the adhesive layer to the surface and subsequent degradation of the resin-dentin interface by hydrolysis. |
| The inclusion in their composition of monomers capable of bonding chemically to calcium, such as MDP, has been shown to be a major breakthrough for the durability of adhesive restorations. | On enamel, HEMA-free 1-step SE adhesives result in the formation of water blisters or droplets on the surface of the adhesive, which may compromise durability of enamel bonding. |
| Clearfil SE Bond (Kuraray Noritake) results in high dentin bond strengths in vitro [74] and excellent clinical results at 13 years [75]. Clearfil SE Bond is still the reference against which all other SE adhesives are compared. | One-step HEMA-free self-etch adhesives undergo phase separation in the form of droplets, while HEMA-rich 1-step adhesives may result in droplets in the adhesive layer induced by osmosis. |
| One-step SE adhesives are available in unidose to help complying with stricter infection control guidelines in some countries. | Residual water (from their composition) may become entrapped if not properly evaporated, which results in nanoleakage. |
| Strong 1-step SE adhesives result in enamel bond strengths comparable to ER adhesives, but cause a deep dentin decalcification similar to that of ER adhesives, which results in poor bonding to dentin [77,79]. Nevertheless, a few clinical studies up to 3 years have reported excellent retention in spite of the marginal degradation [80,81]. | |
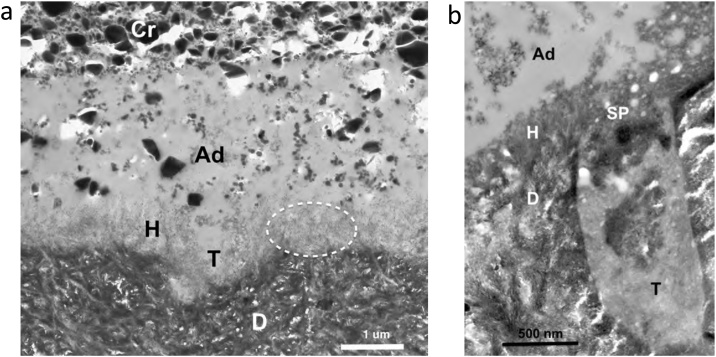
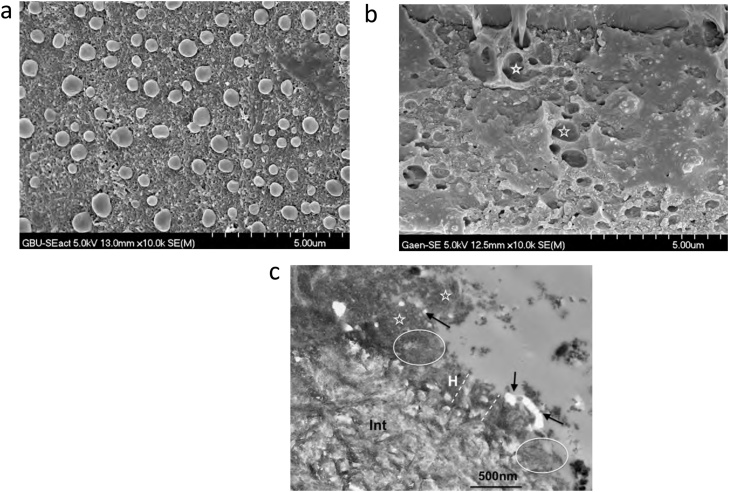
Fig. 10.
(a) TEM micrograph of the adhesive-dentin interface formed by the 2-step SE adhesive OptiBond SE (Kerr). Hydroxyapatite crystals are visible inside the entire length of the 1 μm-thick hybrid layer (dotted circle). Int – intertubular dentin; T – tubule; Ad – adhesive; Cr – composite resin; H – hybrid layer. Original magnification = 12,000X. (b) TEM micrograph of the adhesive-dentin interface formed by the 1-step SE adhesive G-ænial Bond (GC), a HEMA-free adhesive. Hydroxyapatite crystals are mixed with smear layer remnants in the 0.4 μm-thick hybrid layer. Int – intertubular dentin; T – tubule; Ad – adhesive; H – hybrid layer; SP – smear plug. Arrows – empty areas corresponding to droplets in the transition between the adhesive layer and the hybrid layer. Original magnification = 40,000X.
Fig. 11.
Selective enamel etching with 35% phosphoric acid in an occlusal preparation of a mandibular molar.
The pH of the SE adhesive priming solution is the basis for categorizing them as ultra-mild (pH ≥ 2.5), mild (pH ≈ 2), intermediately strong (pH between 1 and 2) and strong (pH < 1) [2]. Mild self-etch adhesives partially dissolve dentin to a depth under 1 μm, leaving behind a significant amount of hydroxyapatite crystallites to interact with functional monomers in the adhesive (Fig. 10). The adhesion mechanism in this case is two-fold: (A) Micromechanical entanglement of the adhesive with the partially decalcified dentin to form a sub-micrometric hybrid layer; (B) Chemical/ionic interaction of functional monomers, such as phosphate or carboxylate, with calcium in hydroxyapatite.
The dentin and enamel etching pattern of strong SE adhesives resembles that of ER adhesives (Fig. 12). While this is a positive feature for enamel bonding, it has the opposite effect on dentin adhesion. Strong SE adhesives remove hydroxyapatite that is potentially needed for chemical bonding of functional monomers to dentin. In addition, 1-step SE adhesives contain at least 20% water [82], which is required to ionize the respective monomers and enable the monomers to interact with enamel and dentin. As strong 1-step SE adhesives demineralize dentin as deep as some phosphoric acid etchants (Fig. 12b), the complete evaporation of the water from the adhesive inside the demineralized dentin is unlikely to occur in a clinical setting. In addition, being SE adhesives, they do not include a rinsing step, which may lead to accumulation of precipitates from the dissolution of hydroxyapatite within or above the hybrid layer. This was the case of Prompt L-Pop (pH ≈ 1), originally marketed by ESPE and later introduced in different versions by 3 M ESPE. The shortcomings described above may have resulted in clinical failure rates of this specific adhesive of 35% at 1 year [83].
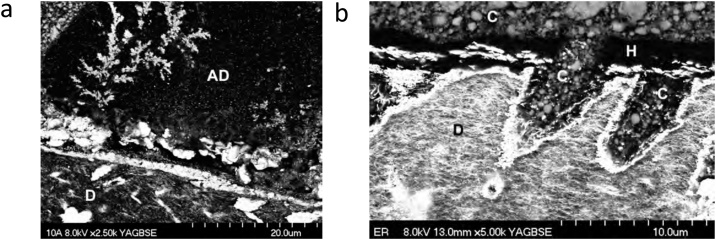
Fig. 12.
(a) SEM micrograph of human enamel conditioned with the 1-step SE adhesive Prompt L-Pop (3M ESPE). The adhesive was not light cured. It was dissolved in acetone for 12 h in a rotator. Note that the etching pattern resembles that of phosphoric acid as a result of the low pH of this adhesive (pH ≈ 1). Original magnification = 5,000X. (b) TEM micrograph the adhesive-dentin interface formed by the ‘strong’ 1-step SE adhesive Prompt L-Pop (3M). Int – intertubular dentin; T – tubule; Ad – adhesive; H – hybrid layer; R – Sp-adhesive mixed with residual smear plug; C – composite resin. Original magnification = 20,000X.
The difference between 1-step SE and 2-step SE adhesives is the extra hydrophobic bonding resin applied over the acidic primer for 2-step SE adhesives (Table 2). A 2-step SE adhesive is currently the gold standard for the SE strategy. Clearfil SE Bond (Kuraray Noritake) has resulted in excellent 13-year clinical retention rates in NCCLs [75], more specifically, 86% for SE and 93% for selective enamel etch. This adhesive was first launched in Japan in the late 1990s as Mega Bond (Kuraray) therefore it has a long track record of over 20 years.
Three factors may be responsible for the excellent clinical behavior of Clearfil SE Bond: (A) The presence of the molecule MDP to trigger chemical bonding to calcium in enamel and dentin [26,27,84]. In fact, the same 13-year clinical study [75] found that the enamel margins of restorations without previous enamel etching had some minor issues with marginal integrity, but they were still retained, most likely as a result of the chemical bonding from MDP; (B) The slight etching potential of MDP to provide micromechanical retention [84]; (C) The presence of the hydrophobic bonding resin, which prevents the degradation of the adhesive interface, as discussed above.
5.3. Simplification versus effectiveness
The 3-step ER adhesives and 2-step self-etch adhesives (Table 2) have been shown to be clinically effective over time [49,75,85]. To make the bonding procedure easier and faster, manufacturers have merged some of the three steps, i.e., etchant, hydrophilic primer and hydrophobic bonding resin. There is strong clinical evidence that the trend for simplification of the dentin bonding procedure results in lower effectiveness and compromised durability. For example, 2-step ER adhesives do not have a separate primer and bonding resin. They have a solution that is a blend of both, therefore combining hydrophilic and hydrophobic monomers in the same bottle. Unfortunately, the lack of a separate hydrophobic bonding layer in 2-step ER adhesives makes the resin-dentin interfaces more susceptible to in vitro and clinical degradation. As a result, the clinical outcomes of 2-step ER adhesives are worse than those of their predecessors, the 3-step-ER adhesives [49]. Another example is the simplification from 2-step SE to 1-step SE adhesives. The omission of the hydrophobic bonding resin in 1-step SE adhesives makes them more vulnerable to in vitro degradation and poor clinical performance [47,49,78,[86], [87], [88], [89]].
Some simplified adhesives, including 2-step ER and 1-step SE, are advertised as being easy and quick to use. Yet, several of them need more coats than those recommended by the respective manufacturer [51,76,77], making the respective clinical application more time-consuming than their less simplified counterparts. Clinicians need to use extra caution with the solvent air-drying step of simplified adhesives, especially HEMA-free adhesives or adhesives with very low HEMA content. These adhesives undergo phase-separation [90,91] forming droplets in the adhesive layer and the transition between the adhesive layer and the hybrid layer (Fig. 13), which require a strong air-drying procedure to remove water and facilitate the polymerization of the adhesive monomers. On the other hand, HEMA-rich adhesives are prone to water sorption from the substrate via osmosis, forming droplets at the adhesive-composite interface, especially when the light polymerization of the composite resin is delayed or when a chemically-cured composite resin is used over the adhesive [92,93].
Fig. 13.
(a) SEM micrograph of a replica of human enamel conditioned with G-Bond Plus (GC). The adhesive was dissolved in acetone for 12 h in a rotator. The replica of droplets is depicted throughout the surface. Original magnification = 10,000X. (b) SEM micrograph of the adhesive-dentin interface formed with G-Bond Plus (GC) in self-etch mode. This particular area corresponds to the top of the hybrid layer below the adhesive layer. The asterisks depict residual droplets. Original magnification = 10,000X. (c) TEM micrograph of the adhesive-dentin interface formed with the G-Bond Plus (GC) in self-etch mode. H – hybrid layer; Int – intertubular dentin; Arrows – residual droplets The dotted lines mark the hybrid layer thickness; Ovals – hydroxyapatite crystallites visible inside the hybrid layer denoting mild decalcification; Asterisks – residual smear layer.
5.4. Universal adhesives
A new family of dental adhesives was launched in 2011. This new generation of 1-bottle dental adhesives is known as universal adhesives (Table 2). They have become extremely popular in Restorative Dentistry [94]. Universal adhesives are indicated for a variety of clinical procedures, including direct composite restorations, indirect restorations, zirconia primers and silane coupling agent for glass-matrix ceramics, as some universal adhesives also contain a silane in their composition. They may be used with the ER or SE strategy as relevant for the individual clinical situation. The advantages and disadvantages of universal adhesives ae summarized in Table 6.
Table 6.
Advantages and disadvantages of universal adhesives.
| Advantages | Disadvantages |
|---|---|
| Extremely versatile, as they are recommended as ER and SE adhesives in addition to selective enamel etching. | Since etching dentin is not recommended with universal adhesives, a separate enamel acid-etching step is necessary, which increases the clinical application time. |
| Potential for chemical bonding to hydroxyapatite when used in SE mode. | Clinical studies have reported that the SE strategy results in poor retention rate compared to ER and selective enamel etching. |
| Seal dentin margins against nanoleakage when used in SE mode. | Most universal adhesives require mixing with the respective dual-cure activator when used with self- or dual-cure composite materials, such as build-up materials and resin cements with aromatic tertiary amines in the initiator system. |
| Application of the adhesive in SE mode with a scrubbing movement increases enamel bond strengths. | They do not seal dentin margins well when dentin is etched with phosphoric acid. |
| No need to leave dentin moist when used in ER mode. | Similar to classical 1-step SE adhesives, universal adhesives may behave as permeable membranes after polymerization, allowing the permeation of fluids through the adhesive layer to the surface and subsequent degradation of the resin-dentin interface by hydrolysis. |
| Indicated for a wider variety of restorative procedures. | Solvent evaporation time is a critical issue and must be extended, as water in their composition may become entrapped if not properly evaporated, which results in nanoleakage. |
| Indicated as zirconia primers. | The incorporation of a silane in the adhesive solution does not improve bond strengths to glass-matrix ceramics. A separate silane solution must be used. |
| Solvent evaporation time must be extended to remove the residual water that is in the composition of the adhesive [71]. | |
In the past, dentists have used dentin adhesives following one specific adhesion strategy, ER or SE. However, clinicians now demand more versatile materials. As a result, manufacturers have developed dental adhesives that are more user-friendly while providing dentists with the possibility of selecting the adhesion strategy. With the advent of these universal adhesives, dentists can use the same adhesive tailored to a specific clinical situation by selecting one of the adhesion strategies recommended by the respective manufacturers. Besides the ER and SE strategies, universal adhesives may be applied as SE adhesives on dentin and ER adhesives on enamel, i.e. the selective enamel etching technique previously mentioned.
A few years after Fusayama’s total-etch technique in 1979 (simultaneous etching of enamel and dentin with phosphoric acid), Kuraray introduced a new self-cured dental adhesive in 1984 specifically indicated for the total-etch technique. This adhesive, Clearfil New Bond, included HEMA and, for the first time, included the molecule MDP [34,95,96]. The MDP monomer has been used in the composition of the 2-step SE adhesive Clearfil SE Bond (Kuraray Noritake) since the late 1990s, when it was launched in Japan as Mega Bond (Kuraray). MDP includes a methacrylate polymerizable end, a long hydrophobic 10-carbon chain, and a short hydrophilic phosphate component. It is currently widely used in dental adhesives. In 2006, it was reported that calcium ions were leached from hydroxyapatite to form an MDP-calcium salt layered structure on the hydroxyapatite surface [97].
The addition of acidic functional monomers to universal adhesives, such as MDP, distinguishes them from the classical 1-step SE adhesives. The molecule MDP has been shown to interact with hydroxyapatite through a dual adhesion mechanism: (1) stable ionic bonding to calcium is formed through a nanolayered structure of MDP-Ca salts at the interface with hydroxyapatite [[98], [99], [100]]. Such hydrophobic nanolayering is deemed to improve the long-term durability of dentin and enamel bonding [93]; and (2) greater enamel etching potential for MDP compared to other acidic functional monomers [84].
In spite of the excellent adhesion capability of MDP as a functional monomer, it has been known for some time that adhesive monomers with pH between 1.5 and 2.5, such as HEMA and MDP, are susceptible to hydrolytic degradation [82]. This may be the reason why the presence of HEMA and MDP in the same solution inhibits nanolayering at the adhesive interface of a commercial universal adhesive [101]. In addition, many universal adhesives that contain MDP do not seem to result in significant nanolayering [102]. It is the author’s view that, while the potential for MDP to bond chemically to dentin via nanolayering exists in universal adhesives, there must be an ideal combination of adhesive components, pH, and concentration of MDP, to make this adhesion mechanism effective and durable. It is still unclear whether the presence of MDP in current universal adhesives improves their long-term clinical behavior.
Calcium is depleted from the dentin surface when dentin is etched with phosphoric acid. Without calcium it is unclear how MDP-containing adhesives are able to bond ionically to etched dentin through nanolayering. The lack of calcium may be responsible for the lower bond strengths and increase in nanoleakage observed when Scotchbond Universal Adhesive (3 M) was applied as an ER adhesive in comparison with the SE strategy up to 1-year water storage [[103], [104], [105]]. Therefore, MDP-containing SE adhesives may need to be applied only on unetched dentin, while ER adhesives are more effective on enamel. The only option is to use the selective enamel etching technique, in which only enamel is etched with phosphoric acid [106]. In fact, etching enamel may not only enable the characteristic micromechanical interlocking into the enamel microporosities, but also potentiates the chemical bonding of MDP around the enamel crystallites in etched enamel resulting in higher bond strengths than other adhesives without MDP [107].
Universal adhesives do not include the hydrophobic bonding resin as the last step of the adhesive procedure, which may render these adhesives more prone to interfacial degradation than 2-step SE adhesives such as Clearfil SE Bond. With this in mind, the addition of a non-solvated hydrophobic resin to universal adhesives has been tested in vitro with favorable results up to 6 months of aging in water [47,108,109]. However, this improvement does not seem to extrapolate to clinical behavior. A recent randomized clinical trial [110] resulted in lower retention rates in NCCLs when the hydrophobic bond resin was used with Scotchbond Universal Adhesive (3 M). This clinical outcome was unexpected, as the addition of a hydrophobic bonding resin to classical 1-step SE adhesives improved their clinical behavior in a clinical study at two years [88].
When universal adhesives are applied as 1-step SE adhesives they result in significantly lower enamel bond strengths than when used as 2-step ER adhesives [111,112]. In case dentists prefer to use these adhesives in the SE mode on enamel, it has been shown that scrubbing the adhesive solution improves enamel bond strengths for specific universal adhesives [113]. This may be particularly relevant for pediatric dentists to shorten the clinical procedure in children. However, etching enamel with phosphoric acid results in significantly improved outcomes of universal adhesives even when the bonds are challenged with artificial aging [111,114]. Clinically, the use of phosphoric acid etching on enamel has also improved the performance of universal adhesives [65].
The 5-year data of the first clinical trial of a universal adhesive in 200 restorations in NCCLs has recently been released. This specific adhesive, Scotchbond Universal Adhesive, resulted in excellent retention rate when the adhesive was used in ER mode, regardless of the degree of dentin moisture (moist or dry). However, when used in the SE mode, it resulted in statistically lower retention rate and worse marginal staining [115].
Dentists must keep in mind that universal adhesives are originally 1-step SE adhesives. Therefore, they contain up to 20% water to enable the respective monomers to ionize as described above for classical SE adhesives. Hence, the issue of leaving residual water inside the adhesive-dentin interface also applies to universal adhesives, as observed with nanoleakage studies (Fig. 14). The solvent evaporation time is therefore a crucial issue for universal adhesives [55,71] as most manufacturers only recommend 5 sec. For this reason, we currently teach extending the evaporation time to 15 sec using gentle air drying from a dental air syringe. Our analyses with FESEM show that several universal adhesives do not infiltrate etched dentin completely when used as per manufacturers’ directions. They leave behind collagen fibers not enveloped by the polymerized adhesive, which are easily dissolved in in vitro experiments using sodium hypochlorite, a deproteinizing agent, to challenge the dentin-adhesive interfaces (Fig. 15).
Fig. 14.
(a) SEM micrograph (backscattered mode) of the adhesive-dentin interface formed between the universal adhesive Single Bond Universal (3M) and dentin. This specimen was part of a nanoleakage study using ammoniacal silver nitrate as the tracer. Note the deposition of silver ions as water-trees in the adhesive layer denoting residual water. AD – adhesive; D – Dentin. Original magnification = 2,500X. (b) SEM micrograph in backscattered mode of the adhesive-dentin interface formed between the universal adhesive Prime & Bond Elect (Dentsply) and dentin. This specimen was part of a nanoleakage study using ammoniacal silver nitrate as the tracer. Note the intense deposition of silver ions in the hybrid layer and into the wall of the dentin tubules. This universal adhesive has acetone as the organic solvent. Acetone-based adhesives usually need more applications than the number recommended by the respective manufacturer. This may explain the absence of an adhesive layer at the entrance of the tubules, allowing the penetration of the composite resin (C) into the tubules. C – composite resin; H – hybrid layer; D – dentin. Original magnification = 5,000X.
Fig. 15.
(a) SEM micrograph of the adhesive-dentin interface formed between a universal adhesive applied in etch-and-rinse mode and dentin. Dentin was partially dissolved with 6N HCl for 30 sec and deproteinized with 5%NaOCl for 5 min. Note that the typical reticulation from the presence of collagen in the hybrid layer is only observed in a <1 μm deep area (H). The characteristic reticular pattern on the hybrid layer is absent (asterisks) below this area. The collagen is dissolved by the deproteinizing agent NaOCl when the collagen fibers are not fully enveloped by the resin from the adhesive. This phenomenon is known as hybridoid layer and ghost hybrid layer [62,104,174]. C – composite resin; Rt – resin tag. Original magnification = 10,000X. (b) SEM micrograph in backscattered mode of the adhesive-dentin interface formed between a universal adhesive applied in etch-and-rinse mode and dentin. Dentin was partially dissolved with 6N HCL for 30 sec and deproteinized with 5%NaOCl for 5 min. Note that reticulation from the presence of collagen in the hybrid layer is only observed in a 1 μm deep area (H). Below this area, the characteristic reticular pattern on the hybrid layer is absent (asterisks), for the same reason explained in Fig. 15a. C – composite resin; Rt – resin tag. Original magnification = 10,000X.
Regarding the use of universal adhesives as part of the luting procedure for glass-matrix restorations, four dental manufacturers have incorporated silane into their universal adhesive [94,106] and do not recommend the application of a separate silane solution. However, the efficacy of the combined adhesive/silane solution for luting glass-matrix ceramic restorations is questionable [116], as the silane may not be stable in the adhesive solution [117,118]. The low pH of universal adhesives decreases the effectiveness of the incorporated silane [118]. For this reason, the application of a separate silane solution is still recommended.
5.5. Self-adhesive (SA) materials
-
A.
Glass-ionomer cements (GICs):
A summary of the advantages and disadvantages is laid out in Table 7. GICs have been used as SA materials for over 50 years. They result from an acid-base reaction between aluminosilicate glass and a polyalkenoic acid, commonly polyacrylic acid. Although GICs have been described in the literature as the only restorative material able to truly self-adhere to tooth structure, they ‘borrowed’ the self-adhesive property from zinc polycarboxylate cement, which also has self-adhesive properties. These two materials share the same liquid, polyacrylic acid, one of several polyalkenoic acids used in current GICs. In fact, the first commercial GIC had the term ‘polyacrylate’ in its name ASPA or alumino-silicate polyacrylate (Amalgamated Dental Company DeTrey Division).
Research on GICs has confirmed that the bonding mechanism involves carboxylic groups of the GIC that replace phosphate ions of the substrate and establish ionic bonds with calcium ions of hydroxyapatite [120], slowly forming an ion-exchange layer between the tooth and the cement [121,122]. It is simultaneously an ionic bonding mechanism and a self-etching interdiffusion nanomechanical interaction, also known as surface interaction zone or surface intermediate layer [123] (Fig. 16).
The reason the present author decided to include GICs in this review is that they are often overlooked as adhesive materials. Yet, systematic reviews have produced enough evidence for type II GICs to be considered as the best dentin adhesive/restorative material in non-carious cervical lesions [49,85,124]. Resin-modified GICs have also been used as a separate adhesive layer underneath composite resin restorations. Fuji Bond LC is a liner version of the restorative resin-modified GIC (RMGIC), Fuji II LC (GC) [89]. The retention rate of Fuji Bond LC at 3 years was found to be 94% when used in conjunction with Silux Plus (3 M), and 100% with Estio LC (GC) [125] in NCCLs, which is certainly a better outcome than with many resin-based dental adhesives.
-
B.
Resin-based self-adhesive (SA) materials:
-
1)
Flowable composite resins with (claimed) self-adhesion to tooth structure, including Constic (DMG), Fusio Liquid Dentin (Pentron/Kerr), Vertise Flow (Kerr), and Surefil One (Dentsply). The respective compositions are available online in the respective manufacturer’s Safety Sata Sheets (SDS) documents.:
The in vitro and clinical outcomes of self-adhesive composite resins as direct restoratives and as pit-and-fissure-sealants are not very favorable [[126], [127], [128], [129]] and therefore their use is not encouraged. A pit-and-fissure sealant, Vertise Flow, resulted in 62.9% retention rate at 2 years versus 95.7% for a traditional flowable composite applied with a separate dental adhesive [128]. As a restorative material in NCCLs, Fusio Liquid Dentin resulted in a retention rate of only 33% at 6 months with 27 out of 40 restorations lost [129].
-
2)
“Bioactive” restorative materials:
These materials have become very popular in recent years. The commercial name that stands out is ACTIVA BioACTIVE-RESTORATIVE (Pulpdent) [130]. According to the manufacturer in 2013 [131], this material has properties such as “moisture friendly, triple cure, fluoride releasing and radiopaque, no Bisphenol A, no Bis-GMA, no BPA derivatives”. In addition, “strong, durable, ionic restorative resins that release and recharge more calcium, phosphate and fluoride than glass ionomers and traditional RMGIs, and have the physical properties and esthetics of composites. They stimulate mineral apatite formation that seals the material/tooth interface against microleakage, and they are the first dental restoratives with a bioactive resin matrix, shock-absorbing resin component, and reactive ionomer glass fillers designed to mimic the physical and chemical properties of natural teeth”. In 2015 [132], the properties claimed by the manufacturer were almost identical except that ‘triple-cure’ changed to ‘dual-cure’ and the materials ‘have a greater potential to recharge these minerals than glass ionomers and traditional RMGls’.
Table 7.
Self-adhesive materials (Type II GIC-based materials).
| Advantages | Disadvantages |
|---|---|
| Stable chemical bonding to calcium in the tooth structure without any intermediary adhesive. | Clinical studies have shown that the surface becomes rough rapidly. |
| Excellent sealing of dentinal margins, ideal restorative materials for root caries lesions. | RMGICs contain small monomers, such as HEMA, therefore there is potential for release of uncured monomers. |
| Fluoride release, potential for recharging. | Surface degradation with moisture prior to full setting especially for classical GICs. |
| No monomer release, no shrinkage (for classical GICs). | The relevant initial fluoride release only lasts a few weeks. |
| Coefficient of thermal expansion identical to that of dentin; can be used as dentin replacement in deep and wide preparations. | Water expansion of RMGICs may be an issue, especially in encased environments such as the root canal. |
| Increase resistance to fatigue and reduce the deleterious effects of composite polymerization stress (cusp flexural and resulting enamel cracks) when used underneath deep and wide posterior composite restorations [119]. | Not as esthetic nor mechanically strong as composite resins. |
| Restorative GICs are able to increase the F release in acidic, cariogenic pH, when F is most needed to inhibit caries lesions. | More porous than composite resins, susceptible to surface staining. |
| Restorative GICs are a great resource for Pediatric Dentists, especially the reinforced type. | More difficult to use clinically than composite resins. |
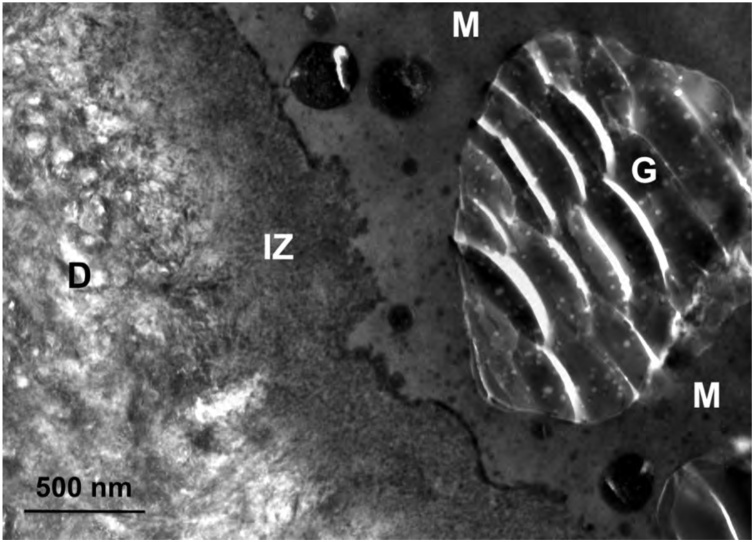
Fig. 16.
TEM micrograph of the adhesive-dentin interface formed by Fuji II LC (GC) with dentin. G – glass particle; M – matrix; IZ – interaction zone; D – dentin. Original magnification = 40,000X.
One important change from 2013 to 2015 was in the instructions for use. Whereas in the 2013 instructions [131], a bonding agent was only recommended in non-retentive restorations such as class V lesions, the 2015 instructions [132] specifically stated ‘apply a bonding agent of your choice’. This change may actually be a very relevant change and may lead to improved clinical outcomes. When this restorative material was inserted in class I and class II preparations without a separate adhesive, the failure rate at 1 year was 24.1% [133]. The authors stated that the study had to be discontinued due to the high failure rate.
Regarding the composition [134], this restorative material contains a blend of diurethane and other methacrylates with modified polyacrylic acid (44.6%), amorphous silica (6.7%), and sodium fluoride (0.75%) according to the relevant SDS information. However, the SDS information only includes components that are hazards. The terminology used to characterize these materials, i.e., ‘bioactive’ and ‘biomineralization’, has been questioned recently in a paper in which the authors suggested to limit these terms only to scientifically proven dental materials that release substantial quantities of ions for specific biomineralization in the clinical environment [135]. In a review article published in 2015 in Pediatric Dentistry [136], the authors questioned if these materials are resin-based composites with RMGI properties or a RMGI that has resin-based composite physical strengths. In fact, a similar material has been approved by the FDA as a RMGIC [137].
The same issue, i.e., whether a material is a true GIC or a composite resin, is not new. The comparative analysis of the relevant physical properties, the absence of an acid-base reaction and silica gel layer around the glass particles characteristic of true GIC materials (Fig. 17), as well as the fact that some of these supposedly GIC-based materials do not set in the dark, have been reported by several authors. It is still unclear if the nomenclature used for some materials marketed as GICs and RMGICs is accurate [18,[138], [139], [140], [141], [142]].
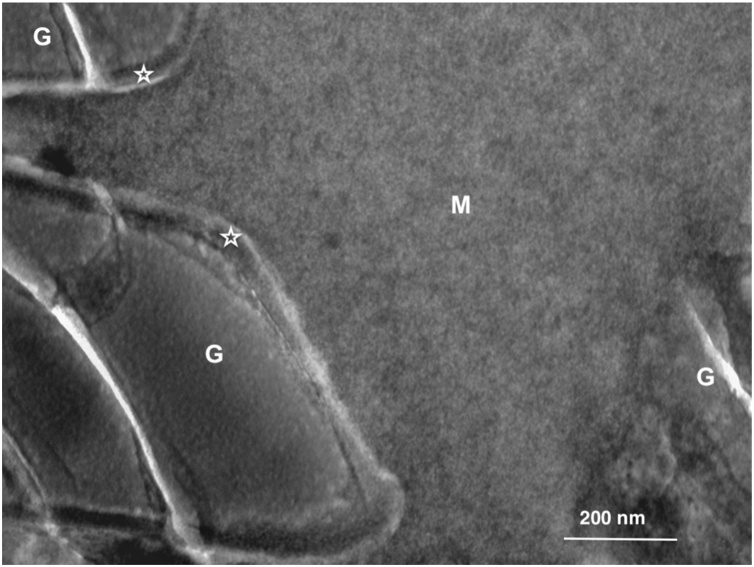
Fig. 17.
TEM micrograph of Fuji II LC (GC) after setting. G – glass particle; M – matrix; Asterisk – silica gel around the glass particles. Original magnification = 100,000X.
5.6. Dentin matrix metalloproteinase-inhibitors
Dentin collagen fibrils contain inactive forms of proteolytic enzymes called matrix metalloproteinases (MMPs) [48]. These enzymes, which are found in odontoblasts and mineralized or demineralized human dentin, may play a role on the degrADAtion of resin-dentin bonds in vitro [143,144] and in situ [145,146].
Dentin MMPs are activated by SE or by ER adhesives during adhesive procedures in vitro [48,145]. MMPs may degrade the collagen within deficiently resin-infiltrated hybrid layers, reducing the longevity of adhesive restorations in vitro [147].
The use of exogenous MMP inhibitors, such as chlorhexidine, has been advocated to help improve the longevity of adhesive restorations [48]. Despite the publication of over 200 in vitro and in situ studies within the last 15 years demonstrating the benefits of applying potential MMP inhibitors as an adjunct to dentin bonding, only a few clinical studies with follow-up over 2 years have been published [148,149]. These clinical studies have not reported any benefit from using chlorhexidine as an MMP inhibitor, which contradicts the findings of most in vitro and in situ studies. In fact, a meta-analysis concluded that there is insufficient evidence to recommend or refute the degrADAtion inhibitory effect of MMP inhibitors used for cavity pretreatment prior to inserting adhesive restorations [150]. The conclusion of this review’s author is that the relevance of the use of MMP inhibitors with dentin adhesives is merely academic, as no clinical validation has been found after all these years. In fact, it is basically an extra step added to the clinical procedure without clear clinical benefit.
5.7. What does the future hold for dental adhesives?
There have been several different new molecules and techniques within the last 10-15 years intended to improve dentin adhesion, but most were solely doable in the laboratory and not applicable to clinical dentistry. Others, such as new analogues of MDP (different carbon chain size, maintaining the methacrylate and the phosphate groups), were tested 6-7 years ago without further known developments.
Adhesives with antibacterial properties by incorporation of an antibacterial agent were first described over 25 years ago, but problems may result from the leaching of the inhibitory agent to the oral environment and to the pulp, in addition to degradation of the physical properties of the composite resin or the adhesive. The synthesis of the antibacterial quaternary ammonium methacrylate MDPB (12 methacryloyloxydodecylpyridinium bromide) resulted in the first successful antibacterial composite resin [151] and dentin primer [152]. This antibacterial monomer combines a quaternary ammonium compound, dodecylpyridinium bromide, with a methacryloyl group. MDPB covalently bonds to the resin matrix becoming immobilized to provide long-term contact-inhibition against oral bacteria. It possesses potent antibacterial activity against several bacteria, including S. mutans, and is able to eliminate bacterial inside dentinal tubules of tooth preparations. Furthermore, the cured resin does not undergo decline in mechanical properties, retaining its inhibitory effects against bacterial growth. MDPB is incorporated in a commercial dental adhesive, Clearfil SE Protect (Kuraray Noritake), formerly Clearfil Protect Bond. In fact, this 2-step SE adhesive is basically Clearfil SE Bond with MDPB and fluoride added to its composition.
Other recent experimental adhesives with antibacterial properties have been tested, including adhesives with another quaternary ammonium monomer, dimethylaminododecyl methacrylate (DMADDM), which has been recently used in several research projects with encouraging results [153,154].
Chlorhexidine has been used as an antibacterial agent in Periodontology for over 30 years [48]. More recently it has been added to phosphoric acid etchants, experimental primers and, at least, one commercial dental adhesive [48]. There are a few chlorhexidine-containing scrubbing solutions to be applied prior to the dentin adhesive. However, it is not clear if there is a clinical advantage by using chlorhexidine during the adhesive procedure.
Silver nanoparticles have also been used in several studies with promising results regarding toxicity and preservation of the bonds [155,156]. Dentin adhesives and resin cements containing nanotubes loaded with different active substances have also been used in vitro within the last 10 years with very encouraging outcomes [[157], [158], [159], [160]].
Another avenue of research includes antimicrobial peptides incorporated in dental adhesives [[161], [162], [163]]. A peptide derived from a salivary protein [164] with amphipathic and antimicrobial properties has the potential to achieve hydrophobicity and antibiofilm properties underneath adhesive restorations [161,165]. The ability to form hydrophobic coatings on dentin with intrinsic antibacterial properties would forever change the way we currently struggle with the clinical challenges related to dentin adhesion.
6. Conclusions
-
1)
Dentin in NCCLs is still the most challenging and unpredictable coronal substrate for adhesion.
-
2)
In vitro results cannot be directly extrapolated to clinical practice.
-
3)
Leaving dentin moist after rinsing the etchant may leave too much water inside the dentin surface. Scrubbing the primer or the primer/adhesive is more relevant than leaving dentin moist.
-
4)
The user-friendliness of simplified adhesives, including ease of use and quick application, is often highlighted in marketing efforts targeted at clinicians. Despite this aggressive marketing, ultra-simplified 1-step self-etch adhesives, which combine dentin-enamel conditioner, primer and bonding adhesive resin in one bottle without requiring mixing, have resulted in low dentin bond strengths [166,167], deficient bonding to intact enamel (including enamel leakage) [168,169], incompatibility with self-curing resins [170], increased nanoleakage [86], behavior as semi-permeable membranes on enamel and dentin [[44], [45], [46],87,92,171], monomer-solvent phase separation [90,91], decreased shelf-life [172], poor clinical outcomes in non-carious cervical lesions and posterior composite restorations [49,78], among other shortcomings.
-
5)
Ideal adhesion and sealing of enamel are only feasible with adhesives that rely on enamel phosphoric acid etching [14,169].
-
6)
Simplified adhesives, which do not have a hydrophobic bonding resin step, leave residual free water in the interface. It is likely that the durability of restorations is compromised. An extra solvent drying time with a gentle air stream is recommended [55].
-
7)
The clinical behavior of universal adhesives also depends on enamel etching with phosphoric acid. Therefore, selective enamel etching is recommended without etching dentin. This would allow the potential chemical bonding between the functional monomer and dentin hydroxyapatite.
-
8)
Universal adhesives may also need extra solvent drying time to ensure removal of the residual water in the interface [66].
-
9)
For adhesion to glass-matrix ceramics, the silane incorporated into the composition of universal adhesives is ineffective [116,117].
-
10)
There is no clinical evidence to recommend the use of resin-based self-adhesive restorative materials. On the other hand, polyalkenoate-based restorative materials are strongly backed by clinical evidence [49,85,124].
-
11)
It is unclear regarding what bioactive materials and materials that promote biomineralization are [135]. Most evidence available to clinicians is anecdotal without strong support by peer-reviewed clinical research.
-
12)
There is no clinical evidence to support the use of MMP inhibitors during the adhesive procedure [[148], [149], [150]].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Perdigão J. New developments in dental adhesion. Dent Clin North Am. 2007;51(2):333–357. doi: 10.1016/j.cden.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Van Meerbeek B., yhara K., Yoshida Y., Mine A., De Munck J., Van Landuyt K. State of the art of self-etch adhesives. Dent Mater. 2011;27(1):17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 3.https://www.accessdata.fda.gov/cdrh_docs/pdf10/K100347.pdf, accessed April 2, 2020.
- 4.https://www.accessdata.fda.gov/cdrh_docs/pdf15/K151619.pdf, accessed April 2, 2020.
- 5.https://www.accessdata.fda.gov/cdrh_docs/pdf19/K192961.pdf, accessed April 2, 2020.
- 6.Perdigão J. Fourteen years later! J Adhes Dent. 2016;18(4):279–280. doi: 10.3290/j.jad.a36719. [DOI] [PubMed] [Google Scholar]
- 7.Kramer I.R.H., McLean J.W. Alterations in the staining reactions of dentine resulting from a constituent of a new self-polymerising resin. Br Dent J. 1952;93:150–153. [Google Scholar]
- 8.Söderholm K.J. Dental adhesives …. how it all started and later evolved. J Adhes Dent. 2007;9(Suppl 2):227–230. [PubMed] [Google Scholar]
- 9.Brudevold F., Buonocore M., Wileman W. A report on a resin composition capable of bonding to human dentin surfaces. J Dent Res. 1956;35(6):846–851. doi: 10.1177/00220345560350060401. [DOI] [PubMed] [Google Scholar]
- 10.Sezinando A., Perdigão J., Regalheiro R. Dentin bond strengths of four adhesion strategies after thermal fatigue and 6-month water storage. J Esthet Restor Dent. 2012;24(5):345–355. doi: 10.1111/j.1708-8240.2012.00531.x. [DOI] [PubMed] [Google Scholar]
- 11.Buonocore M.G. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res. 1955;34(6):849–853. doi: 10.1177/00220345550340060801. [DOI] [PubMed] [Google Scholar]
- 12.Cueto E.I., Buonocore M.G. Sealing of pits and fissures with an adhesive resin: its use in caries prevention. J Am Dent Assoc. 1967;75(1):121–128. doi: 10.14219/jada.archive.1967.0205. [DOI] [PubMed] [Google Scholar]
- 13.Van Meerbeek B., De Munck J., Yoshida Y., Inoue S., Vargas M., Vijay P. Buonocore memorial lecture. Adhesion to enamel and dentin: Current status and future challenges. Oper Dent. 2003;28(3):215–235. [PubMed] [Google Scholar]
- 14.Szesz A., Parreiras S., Reis A., Loguercio A. Selective enamel etching in cervical lesions for self-etch adhesives: A systematic review and meta-analysis. J Dent. 2016;53:1–11. doi: 10.1016/j.jdent.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Bowen R.L., Rodriguez M.S. Tensile strength, testing method, and values for some dental materials. J Dent Res. 1960;39(4):768–769. Abstract M52, 38th General Meeting of the IADR, Chicago, IL, USA. [Google Scholar]
- 16.Bowen R.L. Properties of a silica-reinforced polymer for dental restorations. J Am Dent Assoc. 1963;66:57–64. doi: 10.14219/jada.archive.1963.0010. [DOI] [PubMed] [Google Scholar]
- 17.Wilson A.D. Glass-ionomer cement--origins, development and future. Clin Mater. 1991;7(4):275–282. doi: 10.1016/0267-6605(91)90070-v. [DOI] [PubMed] [Google Scholar]
- 18.McLean J.W., Nicholson J.W., Wilson A.D. Proposed nomenclature for glass-ionomer dental cements and related materials. Quintessence Int. 1994;25(9):587–589. [PubMed] [Google Scholar]
- 19.Wilson A.D., Kent B.E. The glass-ionomer cement, a new translucent dental filling material. J Appl Chem Biotechnol. 1971;21:313. [Google Scholar]
- 20.Fusayama T., Nakamura M., Kurosaki N., Iwaku M. Non-pressure adhesion of a new adhesive restorative resin. J Dent Res. 1979;58(4):1364–1370. doi: 10.1177/00220345790580041101. [DOI] [PubMed] [Google Scholar]
- 21.Nakabayashi N., Kojima K., Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16(3):265–273. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida Y., Van Meerbeek B., Nakayama Y., Yoshioka M., Snauwaert J., Abe Y. Adhesion to and decalcification of hydroxyapatite by carboxylic acids. J Dent Res. 2001;80(6):1565–1569. doi: 10.1177/00220345010800061701. [DOI] [PubMed] [Google Scholar]
- 23.Yoshioka M., Yoshida Y., Inoue S., Lambrechts P., Vanherle G., Nomura Y. Adhesion/decalcification mechanisms of acid interactions with human hard tissues. J Biomed Mater Res. 2002;59(1):56–62. doi: 10.1002/jbm.1216. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg M., Kulkarni A.B., Young M., Boskey A. Dentin: structure, composition and mineralization. Front Biosci. 2011;3:711–735. doi: 10.2741/e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chau M., De France K.J., Kopera B., Machado V.R., Rosenfeldt S., Reyes L. Composite hydrogels with tunable anisotropic morphologies and mechanical properties. Chem Mater. 2016;28(10):3406–3415. [Google Scholar]
- 26.Carvalho R.M., Tjäderhane L., Manso A.P., Carrilho M.R., Carvalho C.A.R. Dentin as a bonding substrate. Endod Topics. 2012;21:62–88. [Google Scholar]
- 27.Pashley D.H., Horner J.A., Brewer P.D. Interactions of conditioners on the dentin surface. Oper Dent. 1992;(Suppl 5):137–150. [PubMed] [Google Scholar]
- 28.Perdigão J. Dentin bonding-variables related to the clinical situation and the substrate treatment. Dent Mater. 2010;26(2):e24–37. doi: 10.1016/j.dental.2009.11.149. [DOI] [PubMed] [Google Scholar]
- 29.Marshall G.W., Jr, Marshall S.J., Kinney J.H., Balooch M. The dentin substrate: structure and properties related to bonding. J Dent. 1997;25(6):441–458. doi: 10.1016/s0300-5712(96)00065-6. [DOI] [PubMed] [Google Scholar]
- 30.Perdigão J., Sezinando A., Monteiro P.C. Effect of substrate age and adhesive composition on dentin bonding. Oper Dent. 2013;38(3):267–274. doi: 10.2341/12-307-L. [DOI] [PubMed] [Google Scholar]
- 31.Laske M., Opdam N.J., Bronkhorst E.M., Braspenning J.C., Huysmans M.C. Longevity of direct restorations in Dutch dental practices. Descriptive study out of a practice based research network. J Dent. 2016;46:12–17. doi: 10.1016/j.jdent.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Tjäderhane L., Carrilho M.R., Breschi L., Tay F.R., Pashley D.H. Dentin basic structure and composition—an overview. Endod Topics. 2012;20:3–29. [Google Scholar]
- 33.Garberoglio R., Brännström M. Scanning electron microscopic investigation of human dentinal tubules. Arch Oral Biol. 1976;21(6):355–362. doi: 10.1016/s0003-9969(76)80003-9. [DOI] [PubMed] [Google Scholar]
- 34.Swift E.J., Jr, Perdigão J., Heymann H.O. Bonding to enamel and dentin: a brief history and state of the art, 1995. Quintessence Int. 1995;26(2):95–110. [PubMed] [Google Scholar]
- 35.Yoshihara K., Yoshida Y., Hayakawa S., Nagaoka N., Irie M., Ogawa T. Nanolayering of phosphoric acid ester monomer on enamel and dentin. Acta Biomater. 2011;7(8):3187–3195. doi: 10.1016/j.actbio.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 36.Burrow M.F., Takakura H., Nakajima M., Inai N., Tagami J., Takatsu T. The influence of age and depth of dentin on bonding. Dent Mater. 1994;10(4):241–246. doi: 10.1016/0109-5641(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 37.De Munck J., Van Landuyt K., Peumans M., Poitevin A., Lambrechts P., Braem M. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005;84(2):118–132. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 38.Heintze S.D., Thunpithayakul C., Armstrong S.R., Rousson V. Correlation between microtensile bond strength data and clinical outcome of Class V restorations. Dent Mater. 2011;27(2):114–125. doi: 10.1016/j.dental.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Heintze S.D. Clinical relevance of tests on bond strength, microleakage and marginal adaptation. Dent Mater. 2013;29(1):59–84. doi: 10.1016/j.dental.2012.07.158. [DOI] [PubMed] [Google Scholar]
- 40.Fusayama T., Terachima S. Differentiation of two layers of carious dentin by staining. J Dent Res. 1972;51(3):866. doi: 10.1177/00220345720510032601. [DOI] [PubMed] [Google Scholar]
- 41.Kuboki Y., Ohgushi K., Fusayama T. Collagen biochemistry of the two layers of carious dentin. J Dent Res. 1977;56(10):1233–1237. doi: 10.1177/00220345770560102301. [DOI] [PubMed] [Google Scholar]
- 42.Murray P.E., Hafez A.A., Windsor L.J., Smith A.J., Cox C.F. Comparison of pulp responses following restoration of exposed and non-exposed cavities. J Dent. 2002;30(5-6):213–222. doi: 10.1016/s0300-5712(02)00021-0. [DOI] [PubMed] [Google Scholar]
- 43.Tay F.R., Pashley D.H. Resin bonding to cervical sclerotic dentin: a review. J Dent. 2004;32(3):173–196. doi: 10.1016/j.jdent.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Marshall G.W., Jr, Chang Y.J., Saeki K., Gansky S.A., Marshall S.J. Citric acid etching of cervical sclerotic dentin lesions: an AFM study. J Biomed Mater Res. 2000;49(3):338–344. doi: 10.1002/(sici)1097-4636(20000305)49:3<338::aid-jbm6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 45.van Dijken J.W., Sunnegårdh-Gronberg K., Lindberg A. Clinical long-term retention of etch-and-rinse and self-etch adhesive systems in non-carious cervical lesions. A 13 years evaluation. Dent Mater. 2007;23(9):1101–1107. doi: 10.1016/j.dental.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Heintze S.D., Ruffieux C., Rousson V. Clinical performance of cervical restorations--a meta-analysis. Dent Mater. 2010;26(10):993–1000. doi: 10.1016/j.dental.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Sezinando A., Luque-Martinez I., Muñoz M.A., Reis A., Loguercio A.D., Perdigão J. Influence of a hydrophobic resin coating on the immediate and 6-month dentin bonding of three universal adhesives. Dent Mater. 2015;31(10):e236–46. doi: 10.1016/j.dental.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Perdigão J., Reis A., Loguercio A.D. Dentin adhesion and MMPs: A comprehensive review. J Esthet Restor Dent. 2013;25(4):219–241. doi: 10.1111/jerd.12016. [DOI] [PubMed] [Google Scholar]
- 49.Peumans M., De Munck J., Mine A., Van Meerbeek B. Clinical effectiveness of contemporary adhesives for the restoration of non-carious cervical lesions. A systematic review. Dent Mater. 2014;30(10):1089–1103. doi: 10.1016/j.dental.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Peumans M., De Munck J., Van Landuyt K.L., Poitevin A., Lambrechts P., Van Meerbeek B. A 13-year clinical evaluation of two three-step etch-and-rinse adhesives in non-carious class-V lesions. Clin Oral Investig. 2012;16(1):129–137. doi: 10.1007/s00784-010-0481-z. [DOI] [PubMed] [Google Scholar]
- 51.Platt J.A., Almeida J., Gonzalez-Cabezas C., Rhodes B., Moore B.K. The effect of double adhesive application on the shear bond strength to dentin of compomers using three one-bottle adhesive systems. Oper Dent. 2001;26(3):313–317. [PubMed] [Google Scholar]
- 52.Hashimoto M., Ohno H., Kaga M., Sano H., Tay F.R., Oguchi H. Over-etching effects on micro-tensile bond strength and failure patterns for two dentin bonding systems. J Dent. 2002;30(2-3):99–105. doi: 10.1016/s0300-5712(02)00004-0. [DOI] [PubMed] [Google Scholar]
- 53.Perdigão J., Swift E.J., Jr Critical appraisal: post-op sensitivity with direct composite restorations. J EsthetRestor Dent. 2013;25(4):284–288. doi: 10.1111/jerd.12045. [DOI] [PubMed] [Google Scholar]
- 54.Reis A., DouradoLoguercio A., Schroeder M., Luque-Martinez I., Masterson D., Cople Maia L. Does the adhesive strategy influence the post-operative sensitivity in adult patients with posterior resin composite restorations? A systematic review and meta-analysis. Dent Mater. 2015;31(9):1052–1067. doi: 10.1016/j.dental.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Awad M.M., Alrahlah A., Matinlinna J.P., Hamama H.H. Effect of adhesive air-drying time on bond strength to dentin: A systematic review and meta-analysis. Int J Adhes Adhes. 2019;90:154–162. [Google Scholar]
- 56.Perdigão J. Dentin etching depth of current phosphoric acid gels. J Dent Res. 2016;95(Spec Iss A) Abstract 1338. [Google Scholar]
- 57.Berry T.G., Barghi N., Knight G.T., Conn L.J., Jr Effectiveness of nitric-NPG as a conditioning agent for enamel. Am J Dent. 1990;3(2):59–62. [PubMed] [Google Scholar]
- 58.Saunders W.P., Strang R., Ahmad I. Shear bond strength of Mirage Bond to enamel and dentin. Am J Dent. 1991;4(6):265–267. [PubMed] [Google Scholar]
- 59.Swift E.J., Jr, Cloe B.C. Shear bond strengths of new enamel etchants. Am J Dent. 1993;6(3):162–164. [PubMed] [Google Scholar]
- 60.Kanca J., 3rd Resin bonding to wet substrate. I. Bonding to dentin. Quintessence Int. 1992;23(1):39–41. [PubMed] [Google Scholar]
- 61.Gwinnett A.J. Moist versus dry dentin: its effect on shear bond strength. Am J Dent. 1992;5(3):127–129. [PubMed] [Google Scholar]
- 62.Tay F.R., Gwinnett A.J., Wei S.H.Y. Micromorphological spectrum from overdrying to overwetting acid-conditioned dentin in water-free, acetone-based, single-bottle primer/adhesives. Dent Mater. 1996;12(4):236–244. doi: 10.1016/s0109-5641(96)80029-7. [DOI] [PubMed] [Google Scholar]
- 63.Perdigão J., Frankenberger R. Effect of solvent and re-wetting time on dentin adhesion. Quintessence Int. 2001;32(5):385–390. [PubMed] [Google Scholar]
- 64.Perdigāo J., Carmo A.R., Geraldeli S. Eighteen-month clinical evaluation of two dentin adhesives applied on dry vs moist dentin. J Adhes Dent. 2005;7(3):253–258. [PubMed] [Google Scholar]
- 65.Loguercio A.D., de Paula E.A., Hass V., Luque-Martinez I., Reis A., Perdigāo J. A new universal simplified adhesive: 36-Month randomized double-blind clinical trial. J Dent. 2015;43(9):1083–1092. doi: 10.1016/j.jdent.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 66.Loguercio A.D., Raffo J., Bassani F., Balestrini H., Santo D., do Amaral R.C. 24-month clinical evaluation in non-carious cervical lesions of a two-step etch-and-rinse adhesive applied using a rubbing motion. Clin Oral Investig. 2011;15(4):589–596. doi: 10.1007/s00784-010-0408-8. [DOI] [PubMed] [Google Scholar]
- 67.Reis A., Chibinski A.C., Stanislawczuk R., Wambier D.S., Grande R.H., Loguercio A.D. The role of dentin moisture in the degradation of resin-dentin interfaces under clinical and laboratory conditions. J Am Dent Assoc. 2012;143(7):e29–36. doi: 10.14219/jada.archive.2012.0274. [DOI] [PubMed] [Google Scholar]
- 68.Ito S., Hashimoto M., Wadgaonkar B., Svizero N., Carvalho R.M., Yiu C. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005;26(33):6449–6459. doi: 10.1016/j.biomaterials.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 69.Chiaraputt S., Mai S., Huffman B.P., Kapur R., Agee K.A., Yiu C.K. Changes in resin-infiltrated dentin stiffness after water storage. J Dent Res. 2008;87(7):655–660. doi: 10.1177/154405910808700704. [DOI] [PubMed] [Google Scholar]
- 70.Spencer P., Wang Y. Adhesive phase separation at dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62(3):447–456. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 71.Luque-Martinez I.V., Perdigão J., Muñoz M.A., Sezinando A., Reis A., Loguercio A.D. Effects of solvent evaporation time on immediate adhesive properties of universal adhesives to dentin. Dent Mater. 2014;30(10):1126–1135. doi: 10.1016/j.dental.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 72.Perdigão J., Lopes M.M., Gomes G. In vitro bonding performance of self-etch adhesives: II--ultramorphological evaluation. Oper Dent. 2008;33(5):534–549. doi: 10.2341/07-133. [DOI] [PubMed] [Google Scholar]
- 73.Moura S.K., Pelizzaro A., Dal Bianco K., de Goes M.F., Loguercio A.D., Reis A. Does the acidity of self-etching primers affect bond strength and surface morphology of enamel? J Adhes Dent. 2006;8(2):75–83. [PubMed] [Google Scholar]
- 74.Burrow M.F., Kitasako Y., Thomas C.D., Tagami J. Comparison of enamel and dentin microshear bond strengths of a two-step self-etching priming system with five all-in-one systems. Oper Dent. 2008;33(4):456–460. doi: 10.2341/07-125. [DOI] [PubMed] [Google Scholar]
- 75.Peumans M., De Munck J., Van Landuyt K., Van Meerbeek B. Thirteen-year randomized controlled clinical trial of a two-step self-etch adhesive in non-carious cervical lesions. Dent Mater. 2015;31(3):308–314. doi: 10.1016/j.dental.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 76.Ito S., Tay F.R., Hashimoto M., Yoshiyama M., Saito T., Brackett W.W. Effects of multiple coatings of two all-in-one adhesives on dentin bonding. J Adhes Dent. 2005;7(2):133–141. [PubMed] [Google Scholar]
- 77.Frankenberger R., Perdigão J., Rosa B.T., Lopes M. “No-bottle” vs “multi-bottle” dentin adhesives—a microtensile bond strength and morphological study. Dent Mater. 2001;17(5):373–380. doi: 10.1016/s0109-5641(00)00084-1. [DOI] [PubMed] [Google Scholar]
- 78.Perdigão J., Dutra-Corrêa M., Anauate-Netto C., Castilhos N., Carmo A.R., Lewgoy H.R. Two-year clinical evaluation of self-etching adhesives in posterior restorations. J Adhes Dent. 2009;11(2):149–159. [PubMed] [Google Scholar]
- 79.Tay F.R., Pashley D.H., Yoshiyama M. Two modes of nanoleakage expression in single-step adhesives. J Dent Res. 2002;81(7):472–476. doi: 10.1177/154405910208100708. [DOI] [PubMed] [Google Scholar]
- 80.Loguercio A.D., Bittencourt D.D., Baratieri L.N., Reis A. A 36-month evaluation of self-etch and etch-and-rinse adhesives in noncarious cervical lesions. J Am Dent Assoc. 2007;138(4):507–514. doi: 10.14219/jada.archive.2007.0204. [DOI] [PubMed] [Google Scholar]
- 81.Kim S.Y., Lee K.W., Seong S.R., Lee M.A., Lee I.B., Son H.H. Two-year clinical effectiveness of adhesives and retention form on resin composite restorations of non-carious cervical lesions. Oper Dent. 2009;34(5):507–515. doi: 10.2341/08-006C. [DOI] [PubMed] [Google Scholar]
- 82.Salz U., Zimmermann J., Zeuner F., Moszner N. Hydrolytic stability of self-etching adhesive systems. J Adhes Dent. 2005;7(2):107–116. [PubMed] [Google Scholar]
- 83.Brackett W.W., Covey D.A., St Germain H.A., Jr One-year clinical performance of a self-etching adhesive in class V resin composites cured by two methods. Oper Dent. 2002;27(3):218–222. [PubMed] [Google Scholar]
- 84.Yoshihara K., Hayakawa S., Nagaoka N., Okihara T., Yoshida Y., Van Meerbeek B. Etching efficacy of self-etching functional monomers. J Dent Res. 2018;97(9):1010–1016. doi: 10.1177/0022034518763606. [DOI] [PubMed] [Google Scholar]
- 85.Peumans M., Kanumilli P., De Munck J., Van Landuyt K., Lambrechts P., Van Meerbeek B. Clinical effectiveness of contemporary adhesives: A systematic review of current clinical trials. Dent Mater. 2005;21(9):864–881. doi: 10.1016/j.dental.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 86.Tay F.R., King N.M., Chan K.M., Pashley D.H. How can nanoleakage occur in self-etching adhesive systems that demineralize and infiltrate simultaneously? J Adhes Dent. 2002;4(4):255–269. [PubMed] [Google Scholar]
- 87.Tay F.R., Pashley D.H., Suh B.I., Carvalho R.M., Itthagarun A. Single-step adhesives are permeable membranes. J Dent. 2002;30(7-8):371–382. doi: 10.1016/s0300-5712(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 88.Reis A., Leite T.M., Matte K., Michels R., Amaral R.C., Geraldeli S. Improving clinical retention of one-step self-etching adhesive systems with an additional hydrophobic adhesive layer. J Am Dent Assoc. 2009;140(7):877–885. doi: 10.14219/jada.archive.2009.0281. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi A., Inoue S., Kawamoto C., Ominato R., Tanaka T., Sato Y. In vivo long-term durability of the bond to dentin using two adhesive systems. J Adhes Dent. 2002;4(2):151–159. [PubMed] [Google Scholar]
- 90.Van Landuyt K.L., De Munck J., Snauwaert J., Coutinho E., Poitevin A., Yoshida Y. Monomer-solvent phase separation in one-step self-etch adhesives. J Dent Res. 2005;84(2):183–188. doi: 10.1177/154405910508400214. [DOI] [PubMed] [Google Scholar]
- 91.Van Landuyt K.L., Mine A., De Munck J., Jaecques S., Peumans M., Lambrechts P. Are one-step adhesives easier to use and better performing? Multifactorial assessment of contemporary one-step self-etching adhesives. J Adhes Dent. 2009;11(3):175–190. [PubMed] [Google Scholar]
- 92.Tay F.R., Pashley D.H., Suh B., Carvalho R., Miller M. Single-step, self-etch adhesives behave as permeable membranes after polymerization. Part I. Bond strength and morphologic evidence. Am J Dent. 2004;17(4):271–278. [PubMed] [Google Scholar]
- 93.Van Landuyt K.L., Snauwaert J., De Munck J., Coutinho E., Poitevin A., Yoshida Y. Origin of interfacial droplets with one-step adhesives. J Dent Res. 2007;86(8):739–744. doi: 10.1177/154405910708600810. [DOI] [PubMed] [Google Scholar]
- 94.Christensen G.J. Adhesives 2018: Are they getting better? Clinicians Report. 2018;11(5):1–4. [Google Scholar]
- 95.Perdigão J., Swift E.J. Fundamental Concepts of Enamel and Dentin Adhesion. In: Roberson T., Heymann H.O., Swift E.J., editors. Sturdevant’s Art and Science of Operative Dentistry. 4th edition. Mosby; St. Louis: 2002. ISBN:0-323010873. [Google Scholar]
- 96.Manabe A., Katsuno K., Itoh K., Wakumoto S., Miyasaka T. Bonding efficacy of erythritol methacrylate solutions as dentin primers. J Dent Res. 1991;70(9):1294–1298. doi: 10.1177/00220345910700091201. [DOI] [PubMed] [Google Scholar]
- 97.Fukegawa D., Hayakawa S., Yoshida Y., Suzuki K., Osaka A., Van Meerbeek B. Chemical interaction of phosphoric acid ester with hydroxyapatite. J Dent Res. 2006;85(10):941–944. doi: 10.1177/154405910608501014. [DOI] [PubMed] [Google Scholar]
- 98.Yoshihara K., Yoshida Y., Nagaoka N., Fukegawa D., Hayakawa S., Mine A. Nano-controlled molecular interaction at adhesive interfaces for hard tissue reconstruction. Acta Biomater. 2010;6(9):3573–3582. doi: 10.1016/j.actbio.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 99.Yoshihara K., Yoshida Y., Nagaoka N., Hayakawa S., Okihara T., De Munck J. Adhesive interfacial interaction affected by different carbon-chain monomers. Dent Mater. 2013;29(8):888–897. doi: 10.1016/j.dental.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 100.Yoshida Y., Nagakane K., Fukuda R., Nakayama Y., Okazaki M., Shintani H. Comparative study on adhesive performance of functional monomers. J Dent Res. 2004;83(6):454–458. doi: 10.1177/154405910408300604. [DOI] [PubMed] [Google Scholar]
- 101.Yoshida Y., Yoshihara K., Hayakawa S., Nagaoka N., Okihara T., Matsumoto T. HEMA inhibits interfacial nano-layering of the functional monomer MDP. J Dent Res. 2012;91(11):1060–1065. doi: 10.1177/0022034512460396. [DOI] [PubMed] [Google Scholar]
- 102.Tian F., Zhou L., Zhang Z., Niu L., Zhang L., Chen C. Paucity of nanolayering in resin-dentin interfaces of MDP-based adhesives. J Dent Res. 2016;95(4):380–387. doi: 10.1177/0022034515623741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marchesi G., Frassetto A., Mazzoni A., Apolonio F., Diolosa M., Cadenaro M. Adhesive performance of a multi-mode adhesive system: 1-year in vitro study. J Dent. 2014;42(5):603–612. doi: 10.1016/j.jdent.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 104.Sezinando A., Perdigão J., Ceballos L. Long-term in vitro adhesion of polyalkenoate-based adhesives to dentin. J Adhes Dent. 2017;19(4):305–316. doi: 10.3290/j.jad.a38895. [DOI] [PubMed] [Google Scholar]
- 105.Sezinando A., Perdigão J. Interfacial characterization of a new universal dentin adhesive. J Dent Res. 2012;91(Special Issue A) Abstract 469. [Google Scholar]
- 106.Perdigão J., Swift E.J., Jr Universal adhesives. J Esthet Restor Dent. 2015;27(6):331–334. doi: 10.1111/jerd.12185. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Z., Wang X., Zhang L., Liang B., Tang T., Fu B. The contribution of chemical bonding to the short- and long-term enamel bond strengths. Dent Mater. 2013;29(7):e103–12. doi: 10.1016/j.dental.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 108.Muñoz M.A., Sezinando A., Luque-Martinez I., Szesz A.L., Reis A., Loguercio A.D. Influence of a hydrophobic resin coating on the bonding efficacy of three universal adhesives. J Dent. 2014;42(5):595–602. doi: 10.1016/j.jdent.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 109.Perdigão J., Muñoz M.A., Sezinando A., Luque-Martinez I.V., Staichak R., Reis A. Immediate adhesive properties to dentin and enamel of a universal adhesive associated with a hydrophobic resin coat. Oper Dent. 2014;39(5):489–499. doi: 10.2341/13-203-LR. [DOI] [PubMed] [Google Scholar]
- 110.Perdigão J., Ceballos L., Giráldez I., Baracco B., Fuentes M.V. Effect of a hydrophobic bonding resin on the 36-month performance of a universal adhesive-a randomized clinical trial. Clin Oral Investig. 2020;24(2):765–776. doi: 10.1007/s00784-019-02940-x. [DOI] [PubMed] [Google Scholar]
- 111.Tsujimoto A., Barkmeier W.W., Takamizawa T., Watanabe H., Johnson W.W., Latta M.A. Influence of duration of phosphoric acid pre-etching on bond durability of universal adhesives and surface free-energy characteristics of enamel. Eur J Oral Sci. 2016;24(4):377–386. doi: 10.1111/eos.12284. [DOI] [PubMed] [Google Scholar]
- 112.McLean D.E., Meyers E.J., Guillory V.L., Vandewalle K.S. Enamel bond strength of new universal adhesive bonding agents. Oper Dent. 2015;40(4):410–417. doi: 10.2341/13-287-L. [DOI] [PubMed] [Google Scholar]
- 113.Loguercio A.D., Muñoz M.A., Luque-Martinez I., Hass V., Reis A., Perdigão J. Does active application of universal adhesives to enamel in self-etch mode improve their performance? J Dent. 2015;43(9):1060–1070. doi: 10.1016/j.jdent.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 114.Suzuki T., Takamizawa T., Barkmeier W.W., Tsujimoto A., Endo H., Erickson R.L. Influence of etching mode on enamel bond durability of universal adhesive systems. Oper Dent. 2016;41(5):520–530. doi: 10.2341/15-347-L. [DOI] [PubMed] [Google Scholar]
- 115.Paris Matos T., Perdigão J., de Paula E., Coppla F., Hass V., Scheffer R.F. Five-year clinical evaluation of a universal adhesive: a randomized double-blind trial. Dent Mater. 2020 doi: 10.1016/j.dental.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 116.Kalavacharla V., Lawson N., Ramp L., Burgess J. Influence of etching protocol and silane treatment with a universal adhesive on lithium disilicate bond strength. Oper Dent. 2015;40(4):372–378. doi: 10.2341/14-116-L. [DOI] [PubMed] [Google Scholar]
- 117.Dimitriadi M., Panagiotopoulou A., Pelecanou M., Yannakopoulou K., Eliades G. Stability and reactivity of γ-MPTMS silane in some commercial primer and adhesive formulations. Dent Mater. 2018;34(8):1089–1101. doi: 10.1016/j.dental.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 118.Yao C., Yu J., Wang Y., Tang C., Huang C. Acidic pH weakens the bonding effectiveness of silane contained in universal adhesives. Dent Mater. 2018;34(5):809–818. doi: 10.1016/j.dental.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 119.Magne P., Silva S., Andrada Md, Maia H. Fatigue resistance and crack propensity of novel “super-closed” sandwich composite resin restorations in large MOD defects. Int J Esthet Dent. 2016;11(1):82–97. [PubMed] [Google Scholar]
- 120.Yoshida Y., Van Meerbeek B., Nakayama Y., Snauwaert J., Hellemans L., Lambrechts P. Evidence of chemical bonding at biomaterial-hard tissue interfaces. J Dent Res. 2000;79(2):709–714. doi: 10.1177/00220345000790020301. [DOI] [PubMed] [Google Scholar]
- 121.Ngo H., Mount G.J., Peters M.C. A study of glass-ionomer cement and its interface with enamel and dentin using a low-temperature, high-resolution scanning electron microscopic technique. Quintessence Int. 1997;28(1):63–69. [PubMed] [Google Scholar]
- 122.Ngo H.C., Mount G., Mc Intyre J., Tuisuva J., Von Doussa R.J. Chemical exchange between glass-ionomer restorations and residual carious dentine in permanent molars: an in vivo study. J Dent. 2006;34(8):608–613. doi: 10.1016/j.jdent.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 123.Yip H.K., Tay F.R., Ngo H.C., Smales R.J., Pashley D.H. Bonding of contemporary glass ionomer cements to dentin. Dent Mater. 2001;17(5):456–470. doi: 10.1016/s0109-5641(01)00007-0. [DOI] [PubMed] [Google Scholar]
- 124.Santos M.J., Ari N., Steele S., Costella J., Banting D. Retention of tooth-colored restorations in non-carious cervical lesions-a systematic review. Clin Oral Investig. 2014;18(5):1369–1381. doi: 10.1007/s00784-014-1220-7. [DOI] [PubMed] [Google Scholar]
- 125.Tyas M.J., Burrow M.F. Clinical evaluation of a resin-modified glass ionomer adhesive system--results at three years. Oper Dent. 2001;26(1):17–20. [PubMed] [Google Scholar]
- 126.Perdigão J., Sezinando A., Gomes G. In vitro sealing potential of a self-adhesive pit and fissure sealant. Quintessence Int. 2011;42(5):e65–73. [PubMed] [Google Scholar]
- 127.Poitevin A., De Munck J., Van Ende A., Suyama Y., Mine A., Peumans M. Bonding effectiveness of self-adhesive composites to dentin and enamel. Dent Mater. 2013;29(2):221–230. doi: 10.1016/j.dental.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 128.Kucukyilmaz E., Savas S. Evaluation of different fissure sealant materials and flowable composites used as pit-and-fissure sealants: A 24-month clinical trial. Pediatr Dent. 2015;37(5):468–473. [PubMed] [Google Scholar]
- 129.Çelik E.U., Aka B., Yilmaz F. Six-month clinical evaluation of a self-adhesive flowable composite in noncariouscervical lesions. J Adhes Dent. 2015;17(4):361–368. doi: 10.3290/j.jad.a34556. [DOI] [PubMed] [Google Scholar]
- 130.McCollum N. The benefits of bioactive restorative materials-How ACTIVA BioACTIVE-RESTORATIVE leads to longer-lasting restorations. Dental Products Report. 2019 https://www.dentalproductsreport.com/dental/article/benefits-bioactive-restorative-materials, last accessed April 17, 2010. [Google Scholar]
- 131.ACTIVA™ BioACTIVE Products, https://www.pulpdent.com/wp-content/uploads/2013/12/IFU-English-5.pdf, accessed April 15 2020.
- 132.ACTIVATMBioACTIVE Dual Cure Products. BioACTIVE-RESTORATIVE™ BioACTIVE-BASE/LINER, https://www.pulpdent.com/wp-content/uploads/2015/03/XP-V-IN-10w.pdf, accessed April 16, 2020.
- 133.van Dijken J.W.V., Pallesen U., Benetti A. A randomized controlled evaluation of posterior resin restorations of an altered resin modified glass-ionomer cement with claimed bioactivity. Dent Mater. 2019;35(2):335–343. doi: 10.1016/j.dental.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 134.https://www.pulpdent.com/msds/activa-bioactive-restorative/?msds=activa-bioactive-restorative&sidebar=ISO, accessed April 2, 2020.
- 135.Vallittu P.K., Boccaccini A.R., Hupa L., Watts D.C. Bioactive dental materials-Do they exist and what does bioactivity mean? Dent Mater. 2018;34(5):693–694. doi: 10.1016/j.dental.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 136.Berg J.H., Croll T.P. Glass ionomer restorative cement systems: an update. Pediatr Dent. 2015;37(2):116–124. [PubMed] [Google Scholar]
- 137.https://www.accessdata.fda.gov/cdrh_docs/pdf15/K153249.pdf, accessed April 2, 2020.
- 138.Sidhu S.K. Glass-ionomer cement restorative materials: a sticky subject? Aust Dent J. 2011;56(Suppl 1):23–30. doi: 10.1111/j.1834-7819.2010.01293.x. [DOI] [PubMed] [Google Scholar]
- 139.Gladys S., Van Meerbeek B., Braem M., Lambrechts P., Vanherle G. Comparative physico-mechanical characterization of new hybrid restorative materials with conventional glass-ionomer and resin composite restorative materials. J Dent Res. 1997;76(4):883–894. doi: 10.1177/00220345970760041001. [DOI] [PubMed] [Google Scholar]
- 140.Kakaboura A.I., Eliades G.C., Palaghias G. Laboratory evaluation of three visible light-cured resinous liners. J Dent. 1996;24(3):223–231. doi: 10.1016/0300-5712(95)00058-5. [DOI] [PubMed] [Google Scholar]
- 141.Ruse N.D. What is a "compomer"? J Can Dent Assoc. 1999;65(9):500–504. [PubMed] [Google Scholar]
- 142.USAF Dental Evaluation & Consultation Service. Ketac Nano Light-Curing Glass Ionomer Restorative (3M ESPE), https://media.dentalcompare.com/m/25/Downloads/KetacNanoLightCuringGlassIonomerRestorativeProjectReports.pdf, last accessed April 14, 2020.
- 143.Mazzoni A., Mannello F., Tay F.R., Tonti G.A., Papa S., Mazzotti G. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. J Dent Res. 2007;86(5):436–440. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 144.Nishitani Y., Yoshiyama M., Wadgaonkar B., Breschi L., Mannello F., Mazzoni A. Activation of gelatinolytic/ collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci. 2006;114(2):160–161. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 145.Hebling J., Pashley D.H., Tjaderhane L., Tay F.R. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84(8):741–746. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 146.Ricci H.A., Sanabe M.E., de Souza Costa C.A., Pashley D.H., Hebling J. Chlorhexidine increases the longevity of in vivo resin–dentin bonds. Eur J Oral Sci. 2010;118(4):411–416. doi: 10.1111/j.1600-0722.2010.00754.x. [DOI] [PubMed] [Google Scholar]
- 147.De Munck J., Van den Steen P.E., Mine A., Van Landuyt K.L., Poitevin A., Opdenakker G. Inhibition of enzymatic degradation of adhesive- dentin interfaces. J Dent Res. 2009;88(12):1101–1106. doi: 10.1177/0022034509346952. [DOI] [PubMed] [Google Scholar]
- 148.Sartori N., Stolf S.C., Silva S.B., Lopes G.C., Carrilho M. Influence of chlorhexidine digluconate on the clinical performance of adhesive restorations: A 3-year follow-up. J Dent. 2013;41(12):1188–1195. doi: 10.1016/j.jdent.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 149.Favetti M., Schroeder T., Montagner A.F., Correa M.B., Pereira-Cenci T., Cenci M.S. Effectiveness of pre-treatment with chlorhexidine in restoration retention: A 36-month follow-up randomized clinical trial. J Dent. 2017;60:44–49. doi: 10.1016/j.jdent.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 150.Göstemeyer G., Schwendicke F. Inhibition of hybrid layer degradation by cavity pretreatment: Meta- and trial sequential analysis. J Dent. 2016;49:14–21. doi: 10.1016/j.jdent.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 151.Imazato S., Torii M., Tsuchitani Y., McCabe J.F., Russell R.R. Incorporation of bacterial inhibitor into resin composite. J Dent Res. 1994;73(8):1437–1443. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 152.Imazato S., Kinomoto Y., Tarumi H., Torii M., Russell R.R., McCabe J.F. Incorporation of antibacterial monomer MDPB into dentin primer. J Dent Res. 1997;76(3):768–772. doi: 10.1177/00220345970760030901. [DOI] [PubMed] [Google Scholar]
- 153.Cheng L., Weir M.D., Zhang K., Arola D.D., Zhou X., Xu H.H. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J Dent. 2013;41(4):345–355. doi: 10.1016/j.jdent.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang N., Melo M.A., Chen C., Liu J., Weir M.D., Bai Y. Development of a multifunctional adhesive system for prevention of root caries and secondary caries. Dent Mater. 2015;31(9):1119–1131. doi: 10.1016/j.dental.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dutra-Correa M., Leite A.A.B.V., de Cara S.P.H.M., Diniz I.M.A., Marques M.M., Suffredini I.B. Antibacterial effects and cytotoxicity of an adhesive containing low concentration of silver nanoparticles. J Dent. 2018;77:66–71. doi: 10.1016/j.jdent.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 156.Suzuki T.Y.U., Gallego J., Assunção W.G., Briso A.L.F., Dos Santos P.H. Influence of silver nanoparticle solution on the mechanical properties of resin cements and intrarradicular dentin. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0217750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen Q., Zhao Y., Wu W., Xu T., Fong H. Fabrication and evaluation of Bis-GMA/TEGDMA dental resins/composites containing halloysite nanotubes. Dent Mater. 2012;28(10):1071–1079. doi: 10.1016/j.dental.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ramos-Tonello C.M., Lisboa-Filho P.N., Arruda L.B., Tokuhara C.K., Oliveira R.C., Furuse A.Y. Titanium dioxide nanotubes addition to self-adhesive resin cement: Effect on physical and biological properties. Dent Mater. 2017;33(7):866–875. doi: 10.1016/j.dental.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 159.Degrazia F.W., Genari B., Leitune V.C.B., Arthur R.A., Luxan S.A., Samuel S.M.W. Polymerisation, antibacterial and bioactivity properties of experimental orthodontic adhesives containing triclosan-loaded halloysite nanotubes. J Dent. 2018;69:77–82. doi: 10.1016/j.jdent.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 160.Suo L., Li Z., Luo F., Chen J., Jia L., Wang T. Effect of dentin surface modification using carbon nanotubes on dental bonding and antibacterial ability. Dent Mater J. 2018;37(2):229–236. doi: 10.4012/dmj.2017-023. [DOI] [PubMed] [Google Scholar]
- 161.Moussa D.G., Fok A., Aparicio C. Hydrophobic and antimicrobial dentin: A peptide-based 2-tier protective system for dental resin composite restorations. Acta Biomater. 2019;88:251–265. doi: 10.1016/j.actbio.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aida K.L., Kreling P.F., Caiaffa K.S., Calixto G.M.F., Chorilli M., Spolidorio D.M. Antimicrobial peptide-loaded liquid crystalline precursor bioadhesive system for the prevention of dental caries. Int J Nanomedicine. 2018;13:3081–3091. doi: 10.2147/IJN.S155245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xie S., Boone K., VanOosten S., Yuca E., Song L., Ge X. Peptide mediated antimicrobial dental adhesive system. Appl Sci. 2019;9(3):557. doi: 10.3390/app9030557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gorr S.U., Sotsky J.B., Shelar A.P., Demuth D.R. Design of bacteria-agglutinating peptides derived from parotid secretory protein, a member of the bactericidal/permeability increasing-like protein family. Peptides. 2008;29(12):2118–2127. doi: 10.1016/j.peptides.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 165.Moussa D.G., Kirihara J.A., Ye Z., Fischer N.G., Khot J., Witthuhn B.A. Dentin priming with amphipathic antimicrobial peptides. J Dent Res. 2019;98(10):1112–1121. doi: 10.1177/0022034519863772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Goracci C., Sadek F.T., Monticelli F., Cardoso P.E., Ferrari M. Microtensile bond strength of self-etching adhesives to enamel and dentin. J Adhes Dent. 2004;6(4):313–318. [PubMed] [Google Scholar]
- 167.Perdigão J., Gomes G., Gondo R., Fundingsland J.W. In vitro bonding performance of all-in-one adhesives. Part I--microtensile bond strengths. J Adhes Dent. 2006;8(6):367–373. [PubMed] [Google Scholar]
- 168.Brackett M.G., Brackett W.W., Haisch L.D. Microleakage of Class V resin composites placed using self-etching resins: Effect of prior enamel etching. Quintessence Int. 2006;37(2):109–113. [PubMed] [Google Scholar]
- 169.Miguez P.A., Castro P.S., Nunes M.F., Walter R., Pereira P.N. Effect of acid-etching on the enamel bond of two self-etching systems. J Adhes Dent. 2003;5(2):107–112. [PubMed] [Google Scholar]
- 170.Sanares A.M., Itthagarun A., King N.M., Tay F.R., Pashley D.H. Adverse surface interactions between one-bottle light-cured adhesives and chemical-cured composites. Dent Mater. 2001;17(6):542–556. doi: 10.1016/s0109-5641(01)00016-1. [DOI] [PubMed] [Google Scholar]
- 171.Tay F.R., Lai C.N., Chersoni S., Pashley D.H., Mak Y.F., Suppa P. Osmotic blistering in enamel bonded with one-step self-etch adhesives. J Dent Res. 2004;83(4):290–295. doi: 10.1177/154405910408300404. [DOI] [PubMed] [Google Scholar]
- 172.Moszner N., Salz U., Zimmermann J. Chemical aspects of self-etching enamel–dentin adhesives: a systematic review. Dent Mater. 2005;21(10):895–910. doi: 10.1016/j.dental.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 173.Yoshiyama M., Masada J., Uchida A., Ishida H. Scanning electron microscopic characterization of sensitive vs. insensitive human radicular dentin. J Dent Res. 1989;68(11):1498–1502. doi: 10.1177/00220345890680110601. [DOI] [PubMed] [Google Scholar]
- 174.Perdigão J. An ultra-morphological study of the interaction of adhesive systems with human dentin. Van der Poorten, Leuven, Belgium. 1995 ISBN 90-801303-4-6. [Google Scholar]