Abstract
Background
During induction of general anaesthesia a ‘cannot intubate, cannot oxygenate’ (CICO) situation can arise, leading to severe hypoxaemia. Evidence is scarce to guide ventilation strategies for small-bore emergency front of neck airways that ensure effective oxygenation without risking lung damage and cardiovascular depression.
Methods
Fifty virtual subjects were configured using a high-fidelity computational model of the cardiovascular and pulmonary systems. Each subject breathed 100% oxygen for 3 min and then became apnoeic, with an obstructed upper airway. When arterial haemoglobin oxygen saturation reached 40%, front of neck airway access was simulated with various configurations. We examined the effect of several ventilation strategies on re-oxygenation, pulmonary pressures, cardiovascular function, and oxygen delivery.
Results
Re-oxygenation was achieved in all ventilation strategies. Smaller airway configurations led to dynamic hyperinflation for a wide range of ventilation strategies. This effect was absent in airways with larger internal diameter (≥3 mm). Intrapulmonary pressures increased quickly to supra-physiological values with the smallest airways, resulting in pronounced cardio-circulatory depression (cardiac output <3 L min−1 and mean arterial pressure <60 mm Hg), impeding oxygen delivery (<600 ml min−1). Limiting tidal volume (≤200 ml) and ventilatory frequency (≤8 bpm) for smaller diameter cannulas reduced dynamic hyperinflation and gas trapping, preventing cardiovascular depression.
Conclusions
Dynamic hyperinflation can be demonstrated for a wide range of front of neck airway cannulae when the upper airway is obstructed. When using small-bore cannulae in a CICO situation, ventilation strategies should be chosen that prevent gas trapping to prevent severe adverse events including cardio-circulatory depression.
Keywords: airway management, airway obstruction, apnoea, cannot intubate, cannot oxygenate, front of neck airway, oxygenation simulation
Editor's key points.
-
•
In cannot intubate, cannot oxygenate scenarios, emergency front of neck airway access can be lifesaving in the face of hypoxaemia.
-
•
Optimal ventilation strategies are not clear for small-bore emergency front of neck airways that ensure effective oxygenation without risking lung damage and cardiovascular depression.
-
•
This computational simulation study examined the effects of various ventilation strategies on re-oxygenation, pulmonary pressures, cardiovascular function, and oxygen delivery in 50 virtual subjects.
-
•
Re-oxygenation was achieved in all ventilation strategies, but smaller airway configurations led to dynamic hyperinflation.
-
•
When using small-bore cannulae for emergency front of neck access, ventilation strategies should be chosen that prevent gas trapping and cardiovascular depression.
During a ‘cannot intubate, cannot oxygenate’ (CICO) situation, the anaesthetist is unable to establish a patent airway through manipulation or conventional airway adjuncts and devices. In the apnoeic patient, failure to manage the airway effectively for an extended period can result in severe hypoxaemia, progressing potentially to organ injury and death. In clinical practice, this scenario is rare: approximately 1:12 500 cases of general anaesthesia and 3–8:1000 tracheal intubation attempts in the emergency department.1,2
Guidance on airway management generally includes emergency tracheostomy/cricothyroidotomy as a rescue intervention.2,3 Despite evidence that the insertion of larger-diameter airway devices is safer in these settings, the anatomical topography of insertion below the cricoid cartilage and the invasiveness and urgency of the procedure necessary to establish the airway often leads to use of small diameter rescue airway cannulas and tubes. Small-diameter airways produce high airflow resistances.4 Owing to incomplete exhalation, dynamic hyperinflation (commonly referred to as ‘gas trapping’ or ‘stacking’) can occur.5,6 This results in accumulation of intrathoracic gas volume, with increasing intrathoracic pressure, leading to cardiovascular depression and pulmonary injury. Because of known complications associated with over-distension and excessive ventilatory pressures,1,7 concerns have been raised regarding the safety of ventilation through small airway devices when upper airway obstruction impedes the escape of excess gas from the lungs.1,8, 9, 10, 11, 12, 13, 14 Clinically, this scenario can occur where a tumour, swelling, haematoma, or other causes of airway crowding leads to obliteration of the periglottic airspace; the need for urgent front of neck access is more likely to arise in these settings.
Because CICO scenarios are rare, it is difficult to perform structured clinical research on optimal ventilation strategies for these situations. As a result, there is scarce evidence to provide specific guidance on how to achieve rapid re-oxygenation without risking lung damage and cardiovascular depression caused by pulmonary over-distension. Animal studies,15, 16, 17 mechanical lung models,4,18, 19, 20 and clinical data13 have been used to investigate the mechanics of ventilation via small airways and identify possible pitfalls in achieving safe oxygenation in this extreme situation. However, systematic clinical investigations to assess this scenario would be difficult to recruit and ethically challenging to perform. In addition, mechanical models are limited in their capacity to reproduce human physiology. Large animal models present ethical limitations and intricate differences in their physiology, making it difficult to draw conclusions for extreme cases.
To address these challenges, high-fidelity computer simulation of pathophysiological states and specific clinical interventions can be a powerful tool to inform future investigations or practice while circumventing the need to put patients at risk or to use animal models for research.
We evaluated strategies for achieving re-oxygenation after airway rescue (i.e. re-opening the airway through a front of neck access with ongoing upper airway obstruction) using computer simulation of the integrated respiratory and cardiovascular systems. In particular, we investigated which combinations of tidal ventilation modalities assured safe intrapulmonary pressures when ventilating via narrow bore, front of neck airway devices.
Methods
Computational model
This study uses the Interdisciplinary Collaboration in Systems Medicine (ICSM) simulation suite based upon the Nottingham Physiology Simulator.21, 22, 23, 24 The ICSM computer simulation is a highly integrated high-fidelity computational model of a number of organ systems. The model has been extensively validated, including in the simulation of apnoea and hypoxaemia in adults.25, 26, 27 Recently, the ICSM simulation suite has been extended in order to include the physiological mechanisms underlying apnoeic oxygenation28; we previously used the updated model for an investigation of the influence of supraglottic oxygen supplementation on the effectiveness of airway rescue.29 A detailed description of the ICSM simulation suite is provided in the Supplementary material.
Virtual subjects and protocol
A cohort of 50 in silico subjects was created, as described,29 to represent the spectrum of physiology in an otherwise healthy population. These subjects were developed by establishing credible physiological ranges for key model parameters based on data reported in the literature. The virtual subjects were generated using randomly permutated configurations of the parameters within these ranges (Supplementary Table S1).
For all virtual subjects, we simulated rescue ventilation with five different airway sizes (varying internal diameter [ID] and length based on real-world medical devices), as follows:
-
A.
ID 1.8 mm, length 6.3 cm, representing the Ravussin 13G cannula (VBM Medical, GmbH, Sulz, Germany)
-
B.
ID 2.0 mm, length 7.5 cm, representing the Emergency Transtracheal Airway Catheter (Cook Medical Inc., Bloomington, IN, USA)
-
C.
ID 3.0 mm, length 5.8 cm, representing Patil's Airway30
-
D.
ID 4.0 mm, length 6.3 cm, representing the Quicktrach cannula 4 mm ID (VBM Medical, GmbH)
-
E.
ID 6.0 mm, length 9.0 cm, representing the Melker cannula 6 mm ID (Cook Medical Inc.)
The 50 virtual subjects underwent pulmonary denitrogenation (pre-oxygenation) during resting, tidal breathing with an inspired oxygen fraction (FiO2) of 100% for 3 min. Induction of general anaesthesia was simulated, with apnoea and upper airway obstruction commencing simultaneously. Apnoea continued until the arterial oxygen saturation (SaO2) reached 40%. At that time, the airway was opened (via front of neck access with each of the above device permutations) and various patterns of tidal ventilation with 100% oxygen were provided for 5 min.
The ventilation patterns comprised:
-
•
Tidal volumes: 20, 50, 100, 200, 300, 400, and 500 ml
-
•
Ventilatory frequencies: 4, 8, 12, 16, and 20 bpm
-
•
Inspiratory/expiratory ratio (I/E): 1:2
The above patterns of tidal ventilation, in every combination, were repeated in all small-bore configurations considered (devices A, B, and C). For the large-bore configurations (devices D and E) gas trapping was highly unlikely to occur.31 As our initial results indicated that gas trapping was indeed absent for all combinations of tidal volumes and ventilatory frequency in configurations D and E, the maximum tidal ventilation with a tidal volume of 500 ml and ventilatory frequency of 20 bpm was simulated as a control with the full bank of virtual subjects.
In a second step, we assessed safe intrapulmonary pressures for a wider range of airway size. To study the relationship between airway length and diameter, we ran a separate protocol with generic airway configurations (lengths 4–10 cm and IDs 1–4 mm). A grid of these configurations was assessed for resulting ventilator and airway pressures during ventilation with a tidal volume of 100 ml and ventilatory frequency of 8 bpm.
A total of 7100 individual simulations were conducted to examine all the above scenarios.
Data collection
The following simulation outputs were recorded every 5 ms: end-inspiratory and end-expiratory lung pressures (calculated as the volume-weighted mean of alveolar compartmental pressures), ventilator pressure, lung volume, arterial haemoglobin oxygen saturation (SaO2), arterial partial pressure of oxygen (Pao2), arterial partial pressure of carbon dioxide (Paco2), arterial oxygen delivery (DO2), cardiac output (CO), and mean arterial pressure (MAP). Data are presented as mean (standard deviation).
Model simulations were executed on a 64-bit Intel Core i7 3.7 GHz Windows 10 personal computer, running Matlab version R2018a.v9 (MathWorks Inc., Natick, MA, USA).
Results
Figure 1 shows the mean values, calculated over the cohort of subjects, of end-inspiratory and end-expiratory lung pressures, and ventilator pressures for the small-bore configurations (A, B, C), during the application of the various patterns of tidal ventilation examined. When tidal volume was ≤200 ml and ventilator frequency was ≤8 bpm, the mean end-inspiratory lung pressure remained ‘safe’ (i.e. less than 30 cm H2O) in all airway configurations. In the smallest configuration (A) when tidal volume was 200 ml and ventilatory frequency was 8 bpm, although the ventilator pressure increased to 63.2 (0.7) cm H2O, end-inspiratory lung pressure was 21.8 (0.8) cm H2O. For larger tidal volumes (i.e. 300 and 400 ml) only a ventilatory frequency of 4 bpm assured a mean end-inspiratory lung pressure <30 cm H2O in all airway configurations. The largest tidal volume studied (500 ml) with a ventilatory frequency of 4 bpm resulted in a mean end-inspiratory lung pressure <30 cm H2O in all airway configurations except in A.
Fig. 1.
Airway pressures calculated over the cohort of 50 in silico subjects. Mean values are shown for end-expiratory lung pressure, end-inspiratory lung pressure and ventilator pressure (bottom panels) for airway configurations A (inner diameter 1.8 mm, length 6.3 cm), B (inner diameter 2.0 mm, length 7.5 cm), and C (inner diameter 3.0 mm, length 5.8 cm). Standard deviations have not been reported for graphical reasons and because they were not significant. Levels of pressures were represented with different colour coding; blue represented ‘safe’ pressures (<30 cm H2O),32 purple represents pressures 30–60 cm H2O, and green represented high pressures (>60 cm H2O). These thresholds have been chosen because of clinical consideration and studies showing a proportional relationship between driving pressures and potential patient harm in other patient cohorts33 where a plateau pressure >30 cm H2O might be acceptable under certain conditions.
The oxygenation goal after airway rescue of SaO2 95% was achieved in <1 min for all patterns of ventilation studied, even when small tidal volumes and ventilatory frequencies were used (Fig. 2). In the smallest airway configurations (A and B), using larger tidal volumes ≥300 ml with ventilatory frequency 20 bpm caused re-oxygenation to be slower, whereas in airway configuration C the time to reach SaO2 95% was similar in all tidal ventilation patterns used.
Fig. 2.
Time taken to achieve arterial haemoglobin oxygen saturation (SaO2) of 95% in the small-bore airway configurations (A, B, and C) during all patterns of tidal ventilation studied. Vf, ventilatory frequency (bpm); Vt, tidal volume (ml).
In the large-bore configurations (D and E), despite a large minute ventilation (500 ml × 20 bpm), no excessive intrapulmonary pressure was observed.
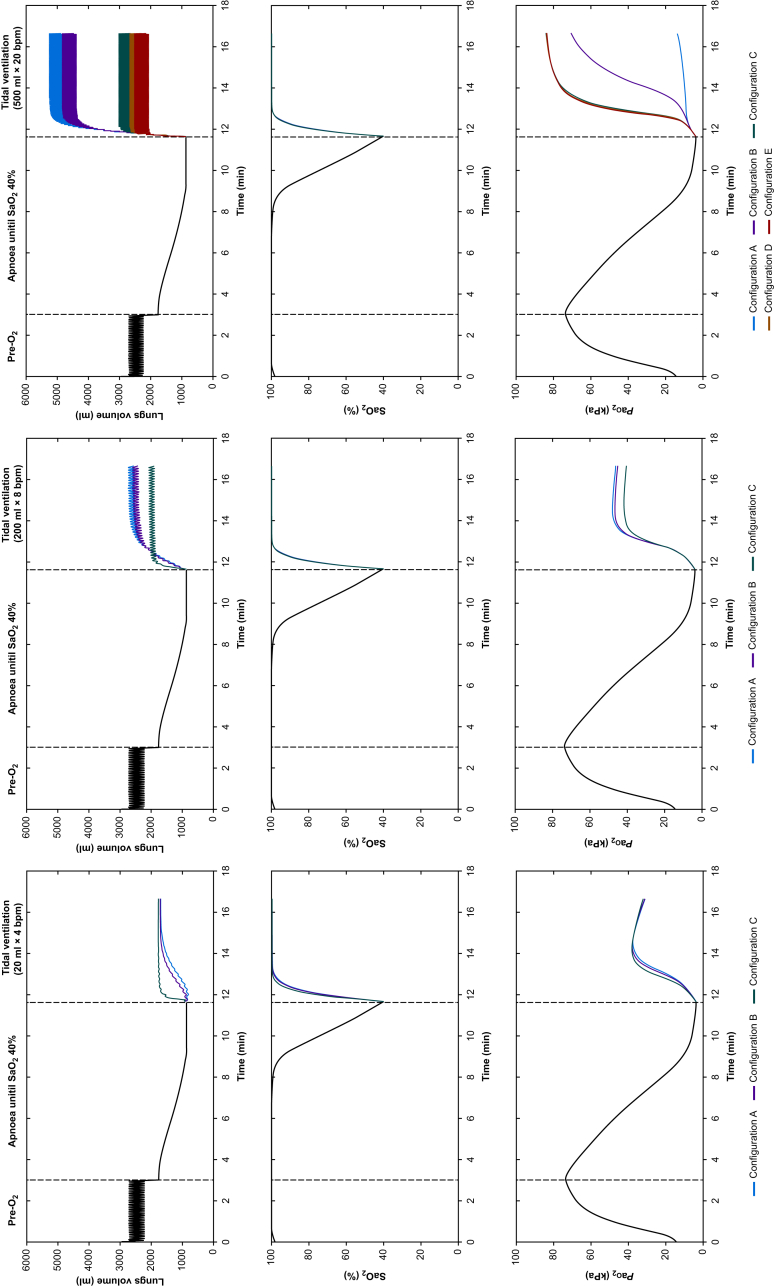
Example time courses of lung volume, Pao2 and SaO2 in one representative subject under varying ventilation strategies and airway sizes are illustrated in Figure 3. The different experimental phases of pre-oxygenation, obstructed apnoea, and ventilation via the rescue airway are demonstrated. Right and left panels show courses with the provision of the maximum (500 ml × 20 bpm) and minimum (20 ml × 4 bpm) patterns of the tidal ventilation, respectively. The middle panel shows the pattern of 200 ml × 8 bpm that still assured end-inspiratory pressure <30 cm H2O in all airway configurations. When the maximum tidal ventilation pattern (500 ml × 20 bpm) was applied, lung volumes showed a pronounced increase above functional residual capacity only in the smallest-bore airway configurations (A and B), indicating that for airway configurations with a diameter <3 mm, smaller tidal ventilation patterns should be used to avoid gas trapping and over-distension of the lung.
Fig. 3.
Time-course of lung volumes, Pao2 and SaO2, during pre-oxygenation, apnoea, and airway opening with different provisions of tidal ventilation in a representative subject; right panel: tidal volume 20 ml and ventilatory frequency 4 bpm; middle panel: tidal volume 200 ml and ventilatory frequency 8 bpm; left panel: tidal volume 500 ml and ventilatory frequency 20 bpm.
In all configurations, re-oxygenation was achieved even with the minimum pattern of tidal ventilation. Comparable time courses occurred in all modelled subjects.
Figure 4 shows the mean values, calculated over the cohort of the subjects, of end-inspiratory lung pressures (top panel), end-expiratory lung pressures (middle panel), and ventilator pressures (bottom panel) for a variation of airway lengths (4–10 cm) and diameters (1–4 mm) with a fixed minute ventilation (tidal volume, 100 ml; ventilatory frequency, 8 bpm). When airway diameter was 2 mm or more, end-inspiratory lung pressure remained <30 cm H2O for all airway lengths.
Fig. 4.
End-inspiratory lung pressure, end-expiratory lung pressure and ventilator pressure for various airway lengths and diameters, with tidal volume 100 ml and ventilatory frequency 8 bpm. Blue areas: pressure <30 cm H2O; purple areas: pressures 30–60 cm H2O and green areas: pressures >60 cm H2O.
Haemodynamic parameters (i.e. DO2, CO, and MAP), calculated during the 5 min of the tidal ventilation, for the small-bore airway configurations (A, B and C) are reported in Figure 5. DO2 was >800 ml min−1 in configuration C for all patterns of tidal ventilation, whereas it dropped to 600 ml min−1 in configurations A and B as tidal volume and ventilatory frequency increased with a correspondent decrease in CO <3 L min−1 and in MAP <60 mm Hg. In particular, in configuration A, DO2 dropped to 600 ml min−1 for tidal volume of 300 ml and ventilatory frequencies of 16 and 20 bpm, and for tidal volumes of 400 and 500 ml and ventilatory frequencies of 12, 16, and 20 bpm. In configuration B, DO2 dropped for tidal volume of 300 ml and ventilatory frequency of 20 bpm, and for tidal volumes of 400 and 500 ml and ventilatory frequencies of 16 and 20 bpm.
Fig. 5.
Arterial oxygen delivery (DO2), cardiac output (CO), and mean arterial pressure (MAP) in the small-bore configurations (A, B, and C) at the end of tidal ventilation provision. Before opening of the obstructed airway (at the end of apnoea), DO2, CO, and MAP were 227.2 (22.9) ml min−1, 2.7 (0.1) L min−1, and 57.2 (2.7) mm Hg, respectively. Vf, ventilatory frequency (bpm); Vt, tidal volume (ml).
In the large-bore configurations (D and E), DO2, CO, and MAP remained >900 ml min−1, 4.3 L min−1, and 85 mm Hg, respectively, in all configurations.
As shown in Figure 6, although there was a significant increase in CO2 when small tidal volumes were applied (i.e. ≤200 ml), oxygenation was assured in all airway configurations and for any pattern of ventilation considered at the end of the 5 min rescue ventilation period.
Fig. 6.
Arterial partial pressure of oxygen (Pao2) and carbon dioxide (Paco2) calculated in the small-bore configurations (A, B, and C) at the end of tidal ventilation provision. Before opening of the obstructed airway (at end of apnoea), Pao2 and Paco2 were 3.8 (0.1) kPa and 11.1 (1) kPa, respectively. Vf, ventilatory frequency (bpm); Vt, tidal volume (ml).
Discussion
In this computational modelling study, we simulated a CICO emergency scenario in a robust and representative cohort of 50 virtual subjects. Both choosing larger airway diameters for front of neck access, or limiting minute ventilation for smaller diameter cannulae, reduced gas trapping. In vivo, dynamic lung hyperinflation leads to alveolar over-distension and consequent barotrauma, which are common complications of this rescue approach.1,2
Our study provides new evidence that, in the case of front of neck airway rescue with an obstructed upper airway, larger airway configurations (ID ≥3 mm) do not lead to increased airway pressures, gas trapping, or excessive ventilation pressures (<30 cm H2O).34 For ID <3 mm, gas trapping becomes more pronounced as minute ventilation increases. In configurations A and B, ventilation caused excessive intrapulmonary pressures because of over-distension. This increase in lung volumes is a result of dynamic hyperinflation in the context of the use of small-bore cannulae, but not in the larger-gauge airway configurations. This behaviour reflects clinical experience, in which reports from transtracheal jet ventilation note barotrauma as a known adverse event associated with front of neck access.8, 9, 10, 11, 12 The gas trapping behaviour in the current study matches the findings of previous studies in mechanical lung models that investigated delayed expiration because of smaller diameters.4 The increase in intrapulmonary pressure resulted in a decrease in mean arterial pressure, CO, and oxygen delivery (Fig. 5). Particularly for the maximum minute ventilation scenarios in configurations A and B, severe cardiovascular depression was observed. This is in agreement with clinical experience where excessive intrapulmonary pressures can precipitate drastic cardio-circulatory compromise.
High ventilator pressures did not necessarily translate to high intrapulmonary pressures (e.g. in configuration A), using a tidal volume of 200 ml and a ventilatory frequency of 8 bpm, ventilatory pressure was 63.2 (0.7) cm H2O whereas end-inspiratory lung pressure was 21.8 (0.8) cm H2O. Other authors quote pre-systemic pressures in bar or psi. For consistency of units within the simulation, we used cm H2O. Ward and colleagues35 showed that in a canine model a pre-systemic pressure translating to 3163 cm H2O resulted in an airway pressure of only 22 cm H2O with 80% upper airway obstruction. Biro and colleagues36 made similar observations in a clinical study of 10 patients undergoing jet ventilation. In their case, driving pressures of 2099 and 3099 cm H2O resulted in a peak inspiratory pressure of 12.9 and 17.7 cm H2O in complete upper airway obstruction, respectively. The significantly greater discrepancy between the driving pressure and the pressure delivered to the airway could be explained by different airflow dynamics. In the work of Biro and colleagues,36 the jet ventilation port of a rigid bronchoscope was used, the geometry of which was not specified. However, owing to the tracheobronchial geometry of an adult patient, significantly longer tubing can be expected. In Ward and colleagues'35 work, a longer 13G cannula was used that has a higher resistance. A linear relationship between cannula length and resistance can be inferred from Figure 4. Incomplete airway obstruction in the study of Ward and colleagues would also have allowed passive gas escape. This was shown by Sasano and colleagues18 to prevent build-up of airway pressures on a mechanical lung model.
Considering that safe oxygenation was possible via passive provision of high FiO2,29 it is not surprising that even with minimal minute ventilation, safe oxygenation (SaO2 95%)37 was achieved. As CO2 removal is efficiently achieved with higher minute ventilation (Fig. 6) pursuing this goal would likely result in more pronounced gas trapping in smaller airway configurations. As re-oxygenation is the primary goal in an emergency front of neck access scenario, a volume-restricted ventilation strategy using FiO2 of 100% might be safer in small-bore cannulas, with tolerance of CO2 accumulation.
The main limitation of our modelling approach is the inability to account for variations of airflow dynamics, such as dislodgement of the cannula/device, misplacement into surrounding soft tissue or dynamic fluctuations of airway resistance, such as bronchial secretions that could fully or partially occlude the small-bore devices. Similarly, we cannot simulate geometry of the airway devices as curvature or kinks might contribute to airflow resistance.38 Also, the orientation of the airway device within the trachea cannot be simulated. It is difficult to gauge the effect these variations might have on the safety evaluation of the tested ventilation strategies. Furthermore, the simulated patients are assumed to be free of cardiovascular morbidity; the effect of the more extreme intrathoracic pressures on pre-existing cardiac disease cannot be derived from our investigation. The simulated complete upper airway obstruction is a specific scenario that might clinically not be as frequent as partial airway patency, allowing some escape of gas through a largely obstructed upper airway.
Unlike previous work,29 we used 50 virtual subjects instead of 100, because in this study the protocol considered a very large number of simulations owing to the large number of permutations of rescue ventilation strategies. However, as the virtual subjects were generated using wide-ranging, random configurations of the key model parameters, 50 virtual subjects should be considered suitable and adequate to describe a wide spectrum of pathophysiology. Similarly, concessions were made in the simulated combinations of tidal volume and ventilatory frequency to deal with limitations in computational power. The permutations presented cover a wide range of clinically feasible ventilation targets reaching moderately high minute ventilation, and volumes less than series dead-space volume. Simulations were conducted on a desktop PC; use of high-performance computing would allow for wider coverage of potential treatment and subject permutations.
Future studies should investigate more extensive variations in the I/E ratio. Additionally, adaptation of the model allowing active inhalation and exhalation15, 16, 17,39 could inform the discussion about devices that facilitate this type of ventilation, potentially reducing gas trapping. Finally, it will be useful to study partial (and absent) upper airway obstruction while simulating small-bore airway ventilation, in particular the dynamic venting of pressure from the lungs against an increased airway resistance from partial obstruction. Simulation of incomplete upper airway obstruction will most likely affect the tendency for gas trapping, the efficiency of ventilation/oxygenation and of CO2 removal, as suggested previously.40
Author's contributions
Design of study: ML, CN, AD, JGH
Simulation runs: ML
Interpretation of data: ML, CN, AD, DGB, JGH
Writing and final approval of manuscript: ML, CN, AD, DGB, JGH.
Funding
UK Engineering and Physical Sciences Research Council (grants EP/P023444/1). Fisher and Paykel Healthcare (New Zealand) sponsorship of research into apnoea and mechanisms of gas exchange.
Declarations of interest
JGH is associate editor-in-chief of the British Journal of Anaesthesia. JGH accepts fees for the provision of advice to the police, crown prosecution service, coroners and solicitors. The other authors have no conflicts to declare.
Handling editor: Hugh C Hemmings Jr
Footnotes
This article is accompanied by an editorial: New evidence to inform decisions and guidelines in difficult airway management by McNarry & Asai, Br J Anaesth 2021:126:1094–1097, doi: 10.1016/j.bja.2021.03.003
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2021.01.030.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Duggan L.V., Ballantyne Scott B., Law J.A., Morris I.R., Murphy M.F., Griesdale D.E. Transtracheal jet ventilation in the ‘can’t intubate can’t oxygenate’ emergency: a systematic review. Br J Anaesth. 2016;117:i28–38. doi: 10.1093/bja/aew192. [DOI] [PubMed] [Google Scholar]
- 2.Cook T.M., Woodall N., Frerk C. Major complications of airway management in the UK: results of the fourth national audit project of the royal college of anaesthetists and the difficult airway society. Part 1. Anaesthesia. Br J Anaesth. 2011;106:617–631. doi: 10.1093/bja/aer058. [DOI] [PubMed] [Google Scholar]
- 3.Frerk C., Mitchell V.S., McNarry A.F. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth. 2015;115:827–848. doi: 10.1093/bja/aev371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dworkin R., Benumof J.L., Benumof R., Karagianes T.G. The effective tracheal diameter that causes air trapping during jet ventilation. J Cardiothorac Anesth. 1990;4:731–736. doi: 10.1016/s0888-6296(09)90012-6. [DOI] [PubMed] [Google Scholar]
- 5.Blanch L., Bernabé F., Lucangelo U. Measurement of air trapping, intrinsic positive end-expiratory pressure, and dynamic hyperinflation in mechanically ventilated patients. Respir Care. 2005;50:110–124. [PubMed] [Google Scholar]
- 6.Gemma M., Nicelli E., Corti D. Intrinsic positive end-expiratory pressure during ventilation through small endotracheal tubes during general anesthesia: incidence, mechanism, and predictive factors. J Clin Anesth. 2016;31:124–130. doi: 10.1016/j.jclinane.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 7.Tuxen D.V., Lane S. The effects of ventilatory pattern on hyperinflation, airway pressures, and circulation in mechanical ventilation of patients with severe air-flow obstruction. Am Rev Respir Dis. 1987;136:872–879. doi: 10.1164/ajrccm/136.4.872. [DOI] [PubMed] [Google Scholar]
- 8.Benumof J.L., Scheller M.S. The importance of transtracheal jet ventilation in the management of the difficult airway. Anesthesiology. 1989;71:769–778. doi: 10.1097/00000542-198911000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Monnier P.H., Rauvissin P., Savary M., Freeman J. Percutaneous transtracheal ventilation for laser endoscopic treatment of laryngeal and subglottic lesions. Clin Otolaryngol Allied Sci. 1988;13:209–217. doi: 10.1111/j.1365-2273.1988.tb01120.x. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs H.B., Smyth N.P.D., Witorsch P. Transtracheal catheter ventilation: clinical experience in 36 patients. Chest. 1974;65:36–40. doi: 10.1378/chest.65.1.36. [DOI] [PubMed] [Google Scholar]
- 11.Chandradeva K., Palin C., Ghosh S.M., Pinches S.C. Percutaneous transtracheal jet ventilation as a guide to tracheal intubation in severe upper airway obstruction from supraglottic oedema. Br J Anaesth. 2005;94:683–686. doi: 10.1093/bja/aei088. [DOI] [PubMed] [Google Scholar]
- 12.Craft T.M., Chambers P.H., Ward M.E., Goat V.A. Two cases of barotrauma associated with transtracheal jet ventilation. Br J Anaesth. 1990;64:524–527. doi: 10.1093/bja/64.4.524. [DOI] [PubMed] [Google Scholar]
- 13.Bourgain J.L., Desruennes E., Cosset M.F., Mamelle G., Belaiche S., Truffa-Bachi J. Measurement of end-expiratory pressure during transtracheal high frequency jet ventilation for laryngoscopy. Br J Anaesth. 1990;65:737–743. doi: 10.1093/bja/65.6.737. [DOI] [PubMed] [Google Scholar]
- 14.Cook T.M., Bigwood B., Cranshaw J. A complication of transtracheal jet ventilation and use of the Aintree intubation catheter® during airway resuscitation. Anaesthesia. 2006;61:692–697. doi: 10.1111/j.1365-2044.2006.04686.x. [DOI] [PubMed] [Google Scholar]
- 15.de Wolf M.W.P., Gottschall R., Preussler N.P., Paxian M., Enk D. Emergency ventilation with the Ventrain® through an airway exchange catheter in a porcine model of complete upper airway obstruction. Can J Anaesth. 2017;64:37–44. doi: 10.1007/s12630-016-0760-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamaekers A.E.W., Götz T., Borg P.A.J., Enk D. Achieving an adequate minute volume through a 2 mm transtracheal catheter in simulated upper airway obstruction using a modified industrial ejector. Br J Anaesth. 2010;104:382–386. doi: 10.1093/bja/aep391. [DOI] [PubMed] [Google Scholar]
- 17.Hamaekers A.E., van der Beek T., Theunissen M., Enk D. Rescue ventilation through a small-bore transtracheal cannula in severe hypoxic pigs using expiratory ventilation assistance. Anesth Analg. 2015;120:890. doi: 10.1213/ANE.0000000000000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasano N., Tanaka A., Muramatsu A. Tidal volume and airway pressure under percutaneous transtracheal ventilation without a jet ventilator: comparison of high-flow oxygen ventilation and manual ventilation in complete and incomplete upper airway obstruction models. J Anesth. 2014;28:341–346. doi: 10.1007/s00540-013-1733-2. [DOI] [PubMed] [Google Scholar]
- 19.Meissner K., Iber T., Roesner J.-P. Successful transtracheal lung ventilation using a manual respiration valve: an in vitro and in vivo study. Anesthesiology. 2008;109:251–259. doi: 10.1097/ALN.0b013e31817f3de6. [DOI] [PubMed] [Google Scholar]
- 20.Flint N.J., Russell W.C., Thompson J.P. Comparison of different methods of ventilation via cannula cricothyroidotomy in a trachea–lung model. Br J Anaesth. 2009;103:891–895. doi: 10.1093/bja/aep264. [DOI] [PubMed] [Google Scholar]
- 21.Hardman J.G., Bedforth N.M., Ahmed A.B., Mahajan R.P., Aitkenhead A.R. A physiology simulator: validation of its respiratory components and its ability to predict the patient’s response to changes in mechanical ventilation. Br J Anaesth. 1998;81:327–332. doi: 10.1093/bja/81.3.327. [DOI] [PubMed] [Google Scholar]
- 22.Hardman J.G., Bedforth N.M. Estimating venous admixture using a physiological simulator. Br J Anaesth. 1999;82:346–349. doi: 10.1093/bja/82.3.346. [DOI] [PubMed] [Google Scholar]
- 23.Bedforth N.M., Hardman J.G. Predicting patients' responses to changes in mechanical ventilation: a comparison between physicians and a physiological simulator. Intensive Care Med. 1999;25:839–842. doi: 10.1007/s001340050961. [DOI] [PubMed] [Google Scholar]
- 24.Das A., Gao Z., Menon P.P., Hardman J.G., Bates D.G. A systems engineering approach to validation of a pulmonary physiology simulator for clinical applications. J R Soc Interface. 2011;8:44–55. doi: 10.1098/rsif.2010.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNamara M.J., Hardman J.G. Hypoxaemia during open-airway apnoea: a computational modelling analysis. Anaesthesia. 2005;60:741–746. doi: 10.1111/j.1365-2044.2005.04228.x. [DOI] [PubMed] [Google Scholar]
- 26.McClelland S.H., Bogod D.G., Hardman J.G. Pre-oxygenation and apnoea in pregnancy: changes during labour and with obstetric morbidity in a computational simulation. Anaesthesia. 2009;64:371–377. doi: 10.1111/j.1365-2044.2008.05785.x. [DOI] [PubMed] [Google Scholar]
- 27.Pillai A., Chikhani M., Hardman J.G. Apnoeic oxygenation in pregnancy: a modelling investigation. Anaesthesia. 2016;71:1077–1080. doi: 10.1111/anae.13563. [DOI] [PubMed] [Google Scholar]
- 28.Laviola M., Das A., Chikhani M., Bates D.G., Hardman J.G. Computer simulation clarifies mechanisms of carbon dioxide clearance during apnoea. Br J Anaesth. 2019;122:395–401. doi: 10.1016/j.bja.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Laviola M., Niklas C., Das A., Bates D.G., Hardman J.G. Effect of oxygen fraction on airway rescue: a computational modelling study. Br J Anaesth. 2020;125:69–74. doi: 10.1016/j.bja.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vadodaria B.S., Gandhi S.D., McIndoe A.K. Comparison of four different emergency airway access equipment sets on a human patient simulator. Anaesthesia. 2004;59:73–79. doi: 10.1111/j.1365-2044.2004.03456.x. [DOI] [PubMed] [Google Scholar]
- 31.Craven R.M., Vanner R.G. Ventilation of a model lung using various cricothyrotomy devices. Anaesthesia. 2004;59:595–599. doi: 10.1111/j.1365-2044.2004.03735.x. [DOI] [PubMed] [Google Scholar]
- 32.Network T.A.R.D.S. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 33.Amato M.B., Meade M.O., Slutsky A.S. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 34.Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 35.Ward K.R., Menegazzi J.J., Yealy D.M., Klain M.M., Molner R.L., Goode J.S. Translaryngeal jet ventilation and end-tidal PCO2 monitoring during varying degrees of upper airway obstruction. Ann Emerg Med. 1991;20:1193–1197. doi: 10.1016/s0196-0644(05)81469-6. [DOI] [PubMed] [Google Scholar]
- 36.Biro P., Layer M., Becker H.D. Influence of airway-occluding instruments on airway pressure during jet ventilation for rigid bronchoscopy. Br J Anaesth. 2000;85:462–465. doi: 10.1093/bja/85.3.462. [DOI] [PubMed] [Google Scholar]
- 37.Beachey W. Respiratory care anatomy and physiology foundations for clinical practise. Elsevier; St. Louis, MO: 2012. Clinical assessment of acid-base and oxygenation status. [Google Scholar]
- 38.Manczur T., Greenough A., Nicholson G.P., Rafferty G.F. Resistance of pediatric and neonatal endotracheal tubes: influence of flow rate, size, and shape. Crit Care Med. 2000;28:1595–1598. doi: 10.1097/00003246-200005000-00056. [DOI] [PubMed] [Google Scholar]
- 39.Hamaekers A., Borg P., Enk D. The importance of flow and pressure release in emergency jet ventilation devices. Paediatr Anaesth. 2009;19:452–457. doi: 10.1111/j.1460-9592.2008.02830.x. [DOI] [PubMed] [Google Scholar]
- 40.de Wolf M., van der Beek T., Hamaekers A., Theunissen M., Enk D. A prototype small-bore ventilation catheter with a cuff: cuff inflation optimizes ventilation with the Ventrain. Acta Anaesthesiol Scand. 2018;62:328–335. doi: 10.1111/aas.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.