Abstract
The purpose was to compare time-based vs anti-Xa-based anticoagulation strategies in patients on ECMO. We conducted a systematic review and meta-analysis using multiple electronic databases and included studies from inception to July 19, 2019. The proportion of bleeding, thrombosis, and mortality were evaluated.
Twenty-six studies (2,086 patients) were included. Bleeding occurred in 34.2% (95%CI 25.1;43.9) of the patients with anti-Xa-based versus 41.6% (95%CI 24.9;59.4) of the patients with time-based anticoagulation strategies. Thrombosis occurred in 32.6% (95%CI 19.1;47.7) of the patients with anti-Xa-based versus 38.4% (95%CI 22.2;56.1) of the patients with time-based anticoagulation strategies. And mortality rate was 35.4% (95%CI 28.9;42.1) of the patients with anti-Xa-based versus 42.9% (95%CI 36.9;48.9) of the patients with time-based anticoagulation strategies. Among the seven studies providing results from both anticoagulation strategies, significantly fewer bleeding events occurred in the anti-Xa-based anticoagulation strategy (adjusted OR 0.49 (95%CI 0.32;0.74), p < 0.001) and a significantly lower mortality rate (adjusted OR 0.61 (95%CI 0.40;0.95), p = 0.03). There was no significant difference in thrombotic events (adjusted OR 0.91 (95%CI 0.56;1.49), p = 0.71). In these seven observational studies, only a small fraction of the patients were adults, and data were insufficient to analyze the effect of the type of ECMO.
In this meta-analysis of observational studies of patients on ECMO, an anti-Xa-based anticoagulation strategy, when compared to a time-based strategy, was associated with fewer bleeding events and mortality rate, without an increase in thrombotic events.
Keywords: Extracorporeal membrane oxygenation, anticoagulation, bleeding, thrombosis, mortality, anti-Xa, ACT, aPTT, thromboelastometry
Introduction
Extracorporeal membrane oxygenation (ECMO) is a continuously evolving and invaluable life-support technique for patients with life-threatening cardiac and/or respiratory failure.1,2 The interaction between blood and an artificial circuit and the presence of shear stress on blood components induces hemostatic disturbances during ECMO making anticoagulation mandatory to maintain circuit patency and to prevent thrombotic complications.3,4 However, the use of anticoagulation in this critically ill population significantly increases the risk of bleeding. Although greater experience, refined treatment strategies (sedation, ventilation, etc.), and technological advances have contributed to improved survival on ECMO, significant morbidity and mortality remain from hemorrhagic and thrombotic complications.5,6 Maintaining the delicate balance between hemostasis and anticoagulation is challenging but essential for favorable outcome. In most institutions, unfractionated heparin remains the anticoagulation of choice due to its fast onset, short half-life, easy reversal with protamine, and experience of use.3,7–9 Different tests are used to monitor heparin anticoagulation and the optimal approach is still debated.10,11 Global assays are based on the time required to generate a clot. Those include activated clotting time (ACT), activated partial thromboplastin time (aPTT), and clotting times generated from viscoelastic testing. Anti-factor Xa (anti-Xa) is a specific test that directly measures heparin inhibition of factor Xa.7,11 All of the assays present specific advantages and limitations. 12 Furthermore, there are disparities among the different tests in measuring the anticoagulation effect; different studies have shown poor correlations or concordance among them, as they measure different aspects of very complex hemostatic systems.13–16 Traditionally, ACT and aPTT have been the most widely utilized monitoring assays. 17 However, both may be influenced by different factors independent of heparin activity, such as hemodilution, anticoagulation factor deficiency, platelet function, medication, and many others.11,18 For this reason, there has been increasing interest in using anti-Xa to monitor and titrate heparin. Multiple studies have demonstrated its reliability as well as a clear heparin dose–response association.3,7,12 However, few studies have done a direct comparison of the two monitoring strategies and their effect on clinical outcomes.11,14,15 The goal of this systematic review was to compare the rates of bleeding, thrombosis and mortality in patients on ECMO monitored by clotting time-based anticoagulation strategies versus anti-Xa-based strategies.
Materials and methods
Design
This is a systematic review of observational studies or randomized trials to determine the prevalence of bleeding, thrombosis, and mortality, according to the anticoagulation strategy. The review followed recommendations contained within the PRISMA statement. 19
Types of studies
We included case series, retrospective or prospective observational studies, and randomized controlled trials, which enrolled critically ill patients on ECMO and reported both the anticoagulation strategy as well as at least one outcome of interest. As the field of ECMO management is rapidly evolving, we included “grey literature,” that is, abstracts of unpublished studies and studies published in non-peer reviewed journals, to insure we assess the most recent data. We excluded case reports, case series of 20 patients or less, reviews not providing original results, studies not in English, studies whose anticoagulation strategy was changed to a drug other than heparin during the course of ECMO support, studies evaluating anticoagulation on other circuits (CRRT) or during bypass, and animal studies.
Types of participants
We included studies that enrolled subjects on ECMO. Patients were further categorized as neonates (less than 28 days), children (28 days to 18 years), and/or adults.
Types of interventions
Based on the primary test used to adapt anticoagulation on ECMO, anticoagulation strategies were categorized as either time-based [ACT, aPTT, rotational thromboelastometry (ROTEM) or thromboelastography (TEG)] or anti-Xa-based, when the anticoagulation strategy was adapted according to the anti-Xa levels.
Types of outcome measures
Our primary outcome was bleeding events on ECMO, as defined by the authors of each study. Our secondary objectives were thrombotic events on ECMO, also as defined by the authors of each study, as well as mortality rate (either mortality on ECMO or mortality at hospital discharge, as defined by the authors).
Search methods for identification of studies
For this systematic review, we performed a search in Ovid MEDLINE, Ovid Embase, and Cochrane from the inception of the database to July 19, 2019. The search included keywords and controlled vocabulary for ECMO and anticoagulation (supplemental Table A). We imported the results to Covidence (version v1238, Melbourne, Australia), which automatically detected duplicates.
Selection of studies
Six reviewers (AW, PR, MN, LS, JM, and OK) independently examined all potential studies and decided on their inclusion in the review (Figure 1). We independently evaluated each study based on its methods and outcomes. We performed this process without blinding of study authors, institutions, journals of publication, or results. We resolved disagreements by additional evaluation by another author.
Figure 1.
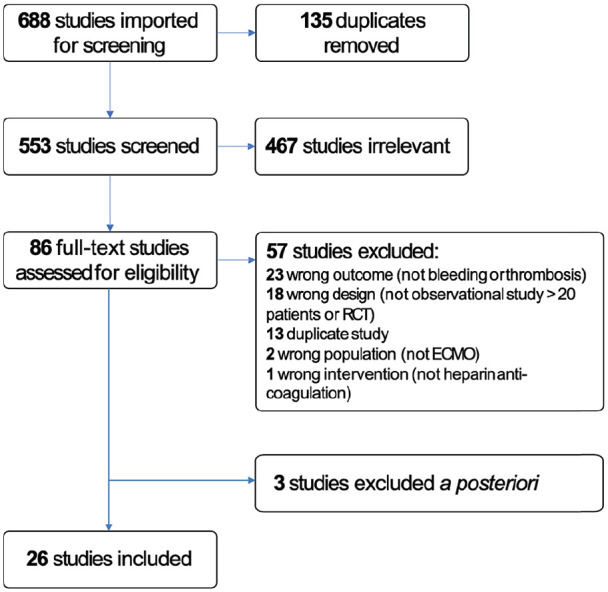
PRIMSA diagram detailing the search and selection process applied during the systematic analysis.
Data extraction and management
For each study included in the systematic review, two authors independently extracted data. We resolved disagreements by discussion. If required, we contacted study authors to ask for relevant data (e.g. specifying the primary test used to adapt anticoagulation, definition of clinical outcomes). The corresponding authors were emailed twice, within a 2-week period. If they did not respond, we then tried a final time to contact them via LinkedIn or Doximity.
Assessment of risk of bias in included studies
We evaluated the validity and design characteristics of each study looking for aspects of major potential biases (study participation, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis). 20 Two authors reviewed and ranked each study’s quality factor separately and defined studies as having low risk of bias only if they adequately fulfilled all of the criteria.
Assessment of proportions
We reported the proportions and their 95% Confidence Interval (95%CI) as the number of patients with the outcomes of interest (bleeding, thrombosis, or death) over the total number of patients enrolled in the study. To account for small proportions and interval confidences close to 0, we pooled the individual proportions using arcsin transformation. 21
Assuming each study estimated a study-specific true effect, we used random-effect models to pool odds ratios. Such models assume no a priori knowledge about the association between the real, or apparent, intervention effects; the differences between the studies are considered to be random. These models account for heterogeneity, with the center of this distribution describing the average of the effects, and its width describing the degree of heterogeneity. We used the DerSimonian-Laird random-effect method in the presence of significant heterogeneity. 22
Assessment of heterogeneity
We explored heterogeneity using the I2 statistic. An I2 statistic higher than 50% represents substantial heterogeneity. 23
Funnel plot
We planned to explore funnel plot asymmetry, as a surrogate for potential publication biases, solely in randomized controlled trial, as the performance of funnel plots is observational studies has not been thoroughly validated. 24 As our systematic review did not include randomized controlled trials, we were not able to compute funnel plots.
Subgroup analysis
To further explore heterogeneity, we planned to conduct two subgroup analyses, comparing studies according to the age category (neonates vs children vs adults) and according to the type of ECMO support (veno-venous (V-V) versus veno-arterial (V-A)). A posteriori, we also conducted a subgroup analysis of abstracts-only versus peer-reviewed full manuscripts.
Sensitivity analysis
To further explore the effect of risk of bias, we conducted a sensitivity analysis, removing studies with a high risk of bias.
Statistical analyses
Pooled proportion with 95% CI and random-effects model were undertaken using OpenMeta(Analyst) (http://www.cebm.brown.edu/openmeta/).
Results
Description of studies
We identified a total of 688 references, of which 135 were duplicates and therefore removed from review, leaving a total of 553 studies that were screened. 467 studies were irrelevant leaving 86 full text articles. We reached out to the corresponding authors of eleven studies, nine of which provided the requested data,25–32 two of which did not respond within the predefined time,33,34 and one of which responded to our first email, but not to follow-up questions. 35 The latter three were excluded. There were also five abstracts 28,36–39 that were duplicates of other abstracts 40 or full texts.41–43
Therefore, 26 studies met eligibility criteria (Figure 1). Of these, 11 were only available as abstracts.26,29–32,40,44–48
Overall, the 26 studies enrolled 2,086 patients, of which 127 (6%) were specifically in neonates, 605 (29%) in neonates and children, 1,055 (51%) in children, and 299 (14%) in adults.
All of the studies were observational studies. Seven (27%) provided outcomes for both anticoagulation strategy groups, reporting a comparison of outcome before versus after change in their anticoagulation protocol. Among these, six enrolled only neonates and/or children,26,29,43,46,47,49 whereas one enrolled both children and adults (although there were only 17/349 (5%) adults). 50 The remaining 19 (73%) studies provided outcomes for only one anticoagulation strategy. One study enrolled V-V patients only, 32 two V-A patients only,30,51 and 15 a mix of both supports,7,27,40–43,47,49,50,52–56 whereas eight did not report the type of support.26,29,31,44–46,48,57 The studies are described in supplemental Table B.
Anticoagulation strategies
Eight of the 26 studies (31%) included in our analysis did not fully describe the goals of their anticoagulation strategies.26,29,30,32,44–47 Five studies used the ELSO guidelines,7,27,51,56,57 that is, in non-bleeding patients, anticoagulation with unfractionated heparin to achieve an ACT time between 180 and 220 seconds or an anti-Xa of 0.3–0.7 IU/mL (https://www.elso.org/portals/0/files/elsoanticoagulationguideline8-2014-table-contents.pdf). Two studies used thromboelastogram, in some 31 or all patients. 48 The remaining studies reported either different ACT, aPTT and anti-Xa goals than those specified by ELSO, differentiating between bleeding status and/or type of ECMO support.25,31,40,41,43,49,50,52–55 Of these, only one study compared two different anticoagulation goals (aPTT <45 vs aPTT 50–70) which was designed to support the feasibility of a larger randomized controlled trial. And although it was not adequately powered, it reported no differences in bleeding or thrombotic events, and mortality. 41
Assessment of the risk of bias
We assessed that the risk of bias was low in 10 studies,7,25,41–43,49–51,53,56 moderate in 12 studies,27,29–32,40,46–48,52,54,57 and high in 4 studies.26,44,45,55 The full assessment is shown in Table 1.
Table 1.
Assessment of the risk of bias.
| Studies | Study participation | Prognostic factor measurement | Outcome measurement | Study confounding | Statistical analysis and presentation | Overall quality |
|---|---|---|---|---|---|---|
| Anton-Martin 53 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊖ | ⊕⊕⊕ | ⊕⊕⊕ |
| Arnouk 56 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊖ | ⊕⊕⊕ | ⊕⊕⊕ |
| Aubron 41 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ |
| Bembea 7 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊕⊕ | ⊕⊕⊕ |
| Bingham 25 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊕⊕ | ⊕⊕⊕ |
| Buttram 26 | ⊕⊖⊖ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊖⊖ | ⊕⊖⊖ | ⊕⊖⊖ |
| Byrnes 51 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊖ | ⊕⊕⊕ | ⊕⊕⊕ |
| Diaz 44 | ⊕⊕⊖ | ⊕⊕⊖ | ⊕⊖⊖ | ⊕⊖⊖ | ⊕⊖⊖ | ⊕⊖⊖ |
| Henderson 55 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊖ | ⊕⊖⊖ | ⊕⊖⊖ | ⊕⊖⊖ |
| Heyrend 45 | ⊕⊖⊖ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊖⊖ | ⊕⊖⊖ | ⊕⊖⊖ |
| Hobson 46 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊕⊖ | ⊕⊕⊖ |
| Hundalani 27 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊕⊖ | ⊕⊕⊖ |
| Irby 57 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊕⊖ | ⊕⊕⊖ |
| Kamdar 29 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊕⊖ | ⊕⊕⊖ |
| Kilgallon 47 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊕⊕ | ⊕⊕⊖ |
| McMichael 42 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊖ | ⊕⊕⊕ | ⊕⊕⊕ |
| Moynihan 54 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊖ | ⊕⊖⊖ | ⊕⊕⊕ | ⊕⊕⊖ |
| Niebler 43 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊕⊕ | ⊕⊕⊕ |
| Northrop 50 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊕⊕ | ⊕⊕⊕ |
| O’Meara 52 | ⊕⊕⊖ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊕⊕ | ⊕⊕⊖ |
| Sleeper 30 | ⊕⊕⊖ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊕⊕ | ⊕⊕⊖ |
| Vandenbriele 40 | ⊕⊕⊖ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊖⊖ | ⊕⊕⊕ | ⊕⊕⊖ |
| Venado 31 | ⊕⊕⊖ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊖⊖ | ⊕⊕⊖ | ⊕⊕⊖ |
| Wallskog 48 | ⊕⊕⊖ | ⊕⊖⊖ | ⊕⊖⊖ | ⊕⊕⊖ | ⊕⊕⊕ | ⊕⊕⊖ |
| Yu 49 | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊕⊕ | ⊕⊕⊕ |
| Zogheib 32 | ⊕⊕⊖ | ⊕⊕⊕ | ⊕⊕⊕ | ⊕⊖⊖ | ⊕⊕⊕ | ⊕⊕⊖ |
⊕⊕⊕: Low risk of bias; ⊕⊕⊖: Moderate risk of bias; ⊕⊖⊖: High risk of bias.
Proportion of bleeding
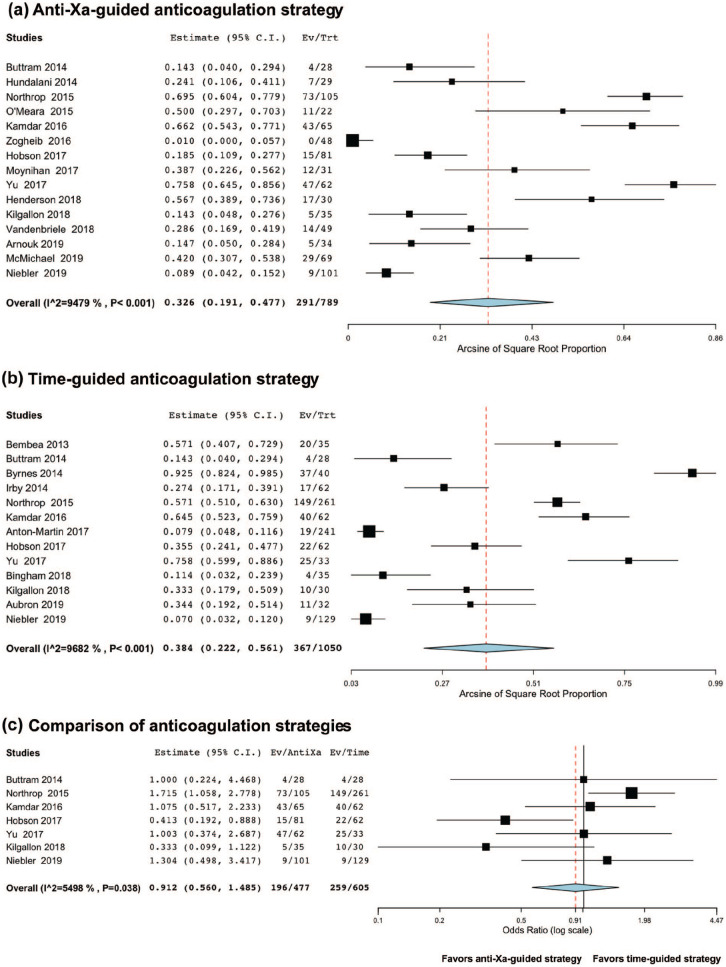
Among anti-Xa-based anticoagulation series, bleeding occurred in 34.2% of the patients (95%CI 25.1;43.9, I2 = 88.93, n = 897, Figure 2(a)). Among time-based anticoagulation series, bleeding occurred in 41.6% of the patients (95%CI 24.9;59.4, I2 = 96.86, n = 1,064, Figure 2(b)). Using a random-effect on the seven studies providing results from both strategies,26,29,43,46,47,49,50 there were significantly fewer bleeding events in the anti-Xa-based anticoagulation strategy: adjusted OR 0.49 (95%CI 0.32;0.74), p < 0.001, I2 = 47.14, n = 1,082, Figure 2(c).
Figure 2.
Meta-analysis of the proportion of bleeding according to the anticoagulation strategy (anti-Xa-guided vs time-guided). Overall, there were fewer bleeding events among anti-Xa-based anticoagulation series, bleeding occurring in 34.2% of the patients (Figure 2(a)) versus 41.6% of the patients in the time-guided studies (Figure 2(b)). Using a random-effect on the seven studies providing results from both strategies, there was significantly fewer bleeding events in the anti-Xa-based anticoagulation strategy (adjusted OR 0.49, p < 0.001), Figure 2(c)).
Proportion of thrombosis
Among anti-Xa-based anticoagulation series, thrombosis occurred in 32.6% of the patients (95%CI 19.1;47.7, I2 = 94.79, n = 789, Figure 3(a)). Among time-based anticoagulation series, thrombosis occurred in 38.4% of the patients (95%CI 22.2;56.1, I2 = 96.82, n = 1,050, Figure 3(b)). Using a random-effect on the seven studies providing results from both strategies,26,29,43,46,47,49,50 there was no significant difference in thrombotic events: adjusted OR 0.91 (95%CI 0.56;1.49), p = 0.71, I2 = 54.98, n = 1,082, Figure 3(c).
Figure 3.
Meta-analysis of the proportion of thrombosis according to the anticoagulation strategy (anti-Xa-guided vs time-guided). Overall, there were fewer thrombotic events among anti-Xa-based anticoagulation series, thrombosis occurring in 32.6% of the patients (Figure 3(a)) versus 38.4% of the patients in the time-guided studies (Figure 3(b)). Using a random-effect on the seven studies providing results from both strategies, there was no significant difference in thrombotic events (adjusted OR 0.91, p = 0.71, Figure 3(c)).
Mortality rate
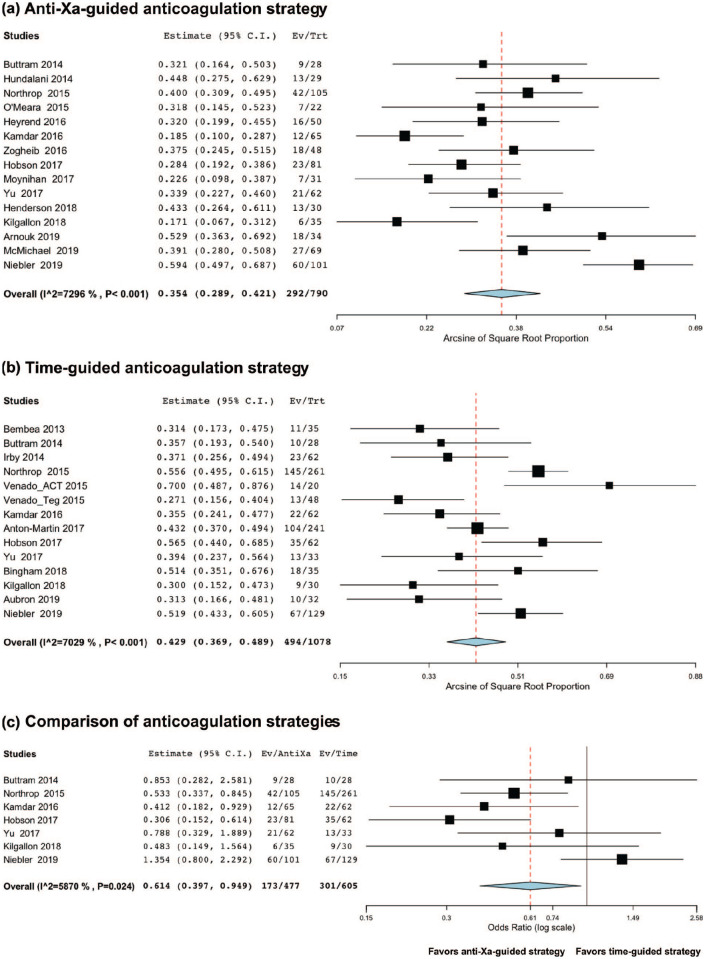
Among anti-Xa-based anticoagulation series, the mortality rate was 35.4% (95%CI 28.9;42.1, I2 = 72.96, n = 790, Figure 4(a)). Among time-based anticoagulation series, the mortality rate was 42.9% (95%CI 36.9;48.9, I2 = 70.29, n = 1,078, Figure 4(b)). Using a random-effect on the seven studies providing results from both strategies,26,29,43,46,47,49,50 there was a significantly lower mortality rate: adjusted OR 0.61 (95%CI 0.40;0.95), p = 0.03, I2 = 58.70, n = 1,082, Figure 4(c).
Figure 4.
Meta-analysis of the mortality rate according to the anticoagulation strategy (anti-Xa-guided vs time-guided). Overall, there was a lower mortality rate among anti-Xa-based anticoagulation series, bleeding occurring in 35.4% of the patients (Figure 4(a)) versus 42.9% of the patients in the time-guided studies (Figure 4(b)). Using a random-effect on the seven studies providing results from both strategies, there was significantly lower mortality rate in the anti-Xa-based anticoagulation strategy (adjusted OR 0.61, p < 0.03), Figure 4(c)).
Subgroup analysis based on age categories
As shown in supplemental Figure A, there is a wide heterogeneity among the outcomes for each age category (adults, children, neonates, and both neonates and children). Among the seven studies providing results from both strategies, there was one study enrolling only neonates (127 subjects), 29 two enrolling only children (325 subjects)43,47 and one enrolling 332 children and 17 adults (349 subjects), 50 and three enrolling both neonates and children (264 subjects).26,46,47 None were enrolling only adults.
Among those seven studies, there were significantly fewer bleeding events associated with the anti-Xa based strategy in the subgroup of studies enrolling only children (adjusted OR 0.39 (95%CI 0.20;0.76); supplemental Figure B1)). There were significantly fewer thrombotic events in the subgroup of studies enrolling both neonates and children (adjusted OR 0.45 (95%CI 0.25;0.82)) but significantly more thrombotic events in the subgroup of studies enrolling only children (adjusted OR 1.50 (95%CI 1.01;2.23); supplemental Figure B2)). There was a significantly decreased mortality rate for the subgroup of studies enrolling both neonates and children (adjusted OR 0.44 (95%CI 0.24;0.80)) and in the subgroup of neonates only (adjusted OR 0.41 (95%CI 0.18–0.93); supplemental Figure B3)).
Subgroup analysis based on type of ECMO
Among the seven studies providing results from both strategies, three did not specify the type of ECMO,26,29,46 and four enrolled both patients supported by V-V and V-A ECMO.43,47,49,50 Therefore, we were unable to analyze the potential interaction of the type of ECMO on the association between anticoagulation strategies and clinical outcomes.
Subgroup analysis of abstract-only versus peer-reviewed full manuscripts
Among the seven studies providing results from both strategies, four were abstracts-only26,29,46,47 and three were full texts.43,49,50 There were significantly fewer bleeding events associated with the anti-Xa based strategy in both subgroups (adjusted OR 0.61 (95%CI 0.37;0.95, I2 = 0, p = 0.03) for abstracts-only versus adjusted OR 0.39 (95%CI 0.20;0.76, I2 = 65.71, p = 0.006) for full manuscripts; supplemental Figure C1)). There were significantly more thrombotic events in the subgroup of full manuscript studies (adjusted OR 1.50 (95%CI 1.01;2.23, I2 = 0, p = 0.04); supplemental Figure C2)). There was a significantly lower mortality rate in the subgroup of abstract-only studies (adjusted OR 0.42 (95%CI 0.27;0.65, I2 = 0, p < 0.001); supplemental Figure C3)).
Sensitivity analysis based on quality of evidence
Among the seven studies providing results from both strategies, three had a low risk of bias,43,49,50 three had a moderate risk of bias,29,46,47 and one had a high risk of bias. 26 For this sensitivity analysis, we removed the latter. Using a random-effect on the remaining studies, there were significantly fewer bleeding events in the anti-Xa-based anticoagulation strategy: adjusted odds ratio 0.44 (95%CI 0.29;0.67), p < 0.001, I2 = 44.52, n = 1,026, supplemental Figure C1. There was no significant difference in thrombotic events: adjusted odds ratio 0.89 (95%CI 0.52;1.54), p = 0.69, I2 = 62.47, n = 1,026, supplemental Figure C2. There was a significant lower mortality rate: adjusted odds ratio 0.56 (95%CI 0.36;0.96), p = 0.03, I2 = 64.92, n = 1,026, supplemental Figure C3.
Discussion
In this systematic review and meta-analysis, we compared time-guided versus anti-Xa-guided anticoagulation strategies for heparin titration in patients on ECMO. We found that there were significantly fewer bleeding events and a lower mortality rate in the anti-Xa-based anticoagulation strategy. In these observational studies, only a small fraction of the subjects were adults, and data were insufficient to analyze separately V-V and V-A ECMOs. Furthermore, the overall quality of evidence was moderate.
To our knowledge, this is the first meta-analysis comparing time-based guided anticoagulation with anti-Xa-guided heparin anticoagulation on ECMO in neonates, children and adults for both V-A- and V-V-ECMO. A recent systematic review evaluated different laboratory measures of heparin anticoagulation in children on ECMO. 58 The authors showed that anti-Xa was the only test to strongly correlate with heparin dose. These results are not surprising if we consider the physiologic background of these different tests. The anti-Xa concentration directly measures heparin inhibition of factor Xa and indirectly “estimates” the plasmatic unfractionated heparin concentration contrary to the ACT and the aPTT. Anti-Xa is a pharmacokinetic test. ACT is a whole-blood test that measures the time for initial fibrin formation. Similar to the ACT, aPTT evaluates contact-activation in the intrinsic pathway, in plasma. These are both pharmacodynamic tests, which are prolonged in the presence of heparin, but also influenced by various other factors, such as the level of coagulation factors and platelet count in the case of the ACT. 11 In this recent study, none of the coagulation tests were significantly associated with hemorrhagic and thrombotic complications; this might be due to scarce clinical data in the studies that were included in this systematic review. 58 Two other systematic reviews evaluating anticoagulation on ECMO looked at the relationship between different tests and goals in specific ECMO populations.17,59 Unfortunately, none evaluated anti-Xa anticoagulation strategies. The first was undertaken in adults on V-A-ECMO and found fewer major bleeding events in the lower ACT goal (ACT<180 seconds) with similar thrombotic events and in-hospital mortality compared with the higher goal (ACT>180 seconds). 17 In this analysis, most included studies were assessed as being at high risk of bias and did not assess the association between anticoagulation strategies and complications. The second, undertaken in adults on V-V-ECMO for respiratory failure, compared ACT versus aPTT anticoagulation strategies and showed a significantly lower bleeding and thrombotic rate in the aPTT approach group. 59 Here too, the results must be interpreted with caution as there were only few studies, the overall quality of evidence was low, and major thrombosis usually consisted of circuit clotting, with little information on the clinical consequences of these events. In addition, mortality could not be evaluated as most of the aPTT studies did not report this outcome. However, the authors reported that within aPTT-targeted protocols, patients for which the aPTT goal was <60 seconds, experienced fewer bleeding episodes but more clotting events than in patients where the aPTT goal was >60 seconds. In our review, we did not compare different anticoagulation goals as this was beyond our scope. Although this study suggests that anti-Xa-guided heparin titration in ECMO is associated with decreased bleeding and mortality rates, the current evidence does not investigate the outcomes associated with anticoagulation protocols coupling anti-Xa with traditional coagulation tests and/or point-of-care coagulation tests (TEG or ROTEM) or even thrombin generation tests. Some experts believe the latter test evaluates other aspects of anticoagulation, that are not assessed with the anti-Xa test, which could lead to excessive anticoagulation in specific subgroups of patients. 11 Management of anticoagulation on ECMO using point-of-care coagulation tests (TEG or ROTEM) have been studied. The current evidence suggests these tests have a poor to moderate correlation with traditional coagulation tests. Furthermore, the concordance between TEG and ROTEM is poor, yielding sometimes contradictory results. 60 Studies assessing the benefits of a combined approach to manage anticoagulation on ECMO are also needed. We strongly recommend additional studies, including randomized controlled trials, to further investigate the optimal anticoagulation strategy.
Limitations
Some limitations have to be recognized. First, the main limitation of this meta-analysis is the huge heterogeneity between studies, including both patient and outcome variability. The definitions of bleeding and thrombotic events were heterogeneous. Furthermore, it was difficult to distinguish between patient and circuit complications, and it was not possible to stratify patients based on surgical versus percutaneous, or central versus peripheral cannulation. Additionally, interventions were heterogeneous between studies, as we grouped time-based anticoagulation strategies (ACT, aPTT, and R on TEG), each having different thresholds for each test. Of note, although TEG provides various measures, the only TEG studies included in the systematic review looked solely at the R time for anticoagulation management. Second, our study included only few high-quality studies (supplemental Table B). Moreover, there were no randomized controlled trials comparing a time-based to an anti-Xa based anticoagulation therapy, only observational studies, most having a retrospective design, for which selection biases could not be excluded. Third, the subgroup analyses were performed in pediatric studies only, with few studies within each age group, and a wide heterogeneity within each category, so these results should be viewed as hypothesis-generating only. Fourth, inclusion of abstracts further worsened the heterogeneity, as the definitions of outcomes and goals of anticoagulation were often not available, due to limitations in the number of words. In addition, inclusion of abstracts, that often lack of peer review and contain less information, might hinder their validity and the results of our meta-analysis. However, as suggested in the literature, we decided to include them to guard against publication bias, 61 although it is possible that only the studies with the most extreme results are published. Because of small numbers of ECMO patients in individual centers resulting in small studies, our topic is particularly vulnerable to publication bias and because of the delay between completing a study and publication, published abstracts may represent more current information certainly in the rapidly evolving field of ECMO management and research. Furthermore, meta-analyses that exclude grey literature likely overrepresent studies with statistically significant findings, inflate effect size estimates, and provide less precise effect size estimates than meta-analyses including grey literature. 62 A subgroup analysis comparing abstract-only versus peer-reviewed full manuscripts confirmed the decreased risk of bleeding in the anti-Xa-based anticoagulation strategy in the peer-reviewed studies. As this result remain consistent with the overall results, it can be considered robust. However, the decreased mortality rate was not observed in the full manuscripts, probably due to a lack of power, whereas the risk for thrombosis was increased in that subgroup. Therefore, these results should be interpreted with caution. Fifth, more than half of the studies included in this systematic review did not specifically compare different anticoagulation strategies. We attempted to address this heterogeneity by performing sub-analyses and sensitivity analyses, which led to similar results. Finally, we included all eligible studies irrespective of the date of publication, although ECMO technology and indications have considerably evolved the last decade. However, all of the included studies were published within the last 8 years.
Conclusion
In a meta-analysis of observational studies of patients on ECMO, an anti-Xa-based anticoagulation strategy, when compared to a time-based strategy, was overall associated with fewer bleeding events and a decreased mortality rate, without an increase in thrombotic events in patients on ECMO. Only large pragmatic trials will result in definitive conclusions on optimal anticoagulation monitoring during ECMO. Further research, including large pragmatic randomized controlled trials, are urgently needed to identify optimal anticoagulation strategies on ECMO. In addition, there is a need for new testing methods as the current knowledge is based tests with significant limitations. A combined approach including point-of-care tests could refine anticoagulation management but needs rigorous assessments.
Supplemental Material
Supplemental material, Supplemental_Files_-_R1_V2 for Anti-Xa versus time-guided anticoagulation strategies in extracorporeal membrane oxygenation: a systematic review and meta-analysis by Ariane Willems, Peter P Roeleveld, Sonia Labarinas, John W Cyrus, Jennifer A Muszynski, Marianne E Nellis and Oliver Karam in Perfusion
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ariane Willems  https://orcid.org/0000-0003-1303-5942
https://orcid.org/0000-0003-1303-5942
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Barbaro RP, Boonstra PS, Moler FW, et al. Hospital-level variation in inpatient cost among children receiving extracorporeal membrane oxygenation. Perfusion 2017; 32: 538–546. [DOI] [PubMed] [Google Scholar]
- 2. Kwak J, Majewski MB, Jellish WS. Extracorporeal membrane oxygenation: the new jack-of-all-trades? J Cardiothorac Vasc Anesth 2020; 34: 192–207. [DOI] [PubMed] [Google Scholar]
- 3. Oliver WC. Anticoagulation and coagulation management for ECMO. Semin Cardiothorac Vasc Anesth 2009; 13: 154–175. [DOI] [PubMed] [Google Scholar]
- 4. Doyle AJ, Hunt BJ. Current understanding of how extracorporeal membrane oxygenators activate haemostasis and other blood components. Front Med 2018; 5: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rehder KJ, Turner DA, Bonadonna D, et al. Technological advances in extracorporeal membrane oxygenation for respiratory failure. Expert Rev Respir Med 2012; 6: 377–384. [DOI] [PubMed] [Google Scholar]
- 6. Dalton HJ, Reeder R, Garcia-Filion P, et al. Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med 2017; 196: 762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bembea MM, Schwartz JM, Shah N, et al. Anticoagulation monitoring during pediatric extracorporeal membrane oxygenation. ASAIO J 2013; 59(1): 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Penk JS, Reddy S, Polito A, et al. Bleeding and thrombosis with pediatric extracorporeal life support: a roadmap for management, research, and the future from the pediatric cardiac intensive care society: part 2. Pediatr Crit Care Med 2019; 20: 1034–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Protti A, Iapichino GE, Di Nardo M, et al. Anticoagulation management and antithrombin supplementation practice during veno-venous extracorporeal membrane oxygenation: a worldwide survey. Anesthesiology 2020; 132: 562–570. [DOI] [PubMed] [Google Scholar]
- 10. Bembea MM, Annich G, Rycus P, et al. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med 2013; 14: e77–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chlebowski MM, Baltagi S, Carlson M, et al. Clinical controversies in anticoagulation monitoring and antithrombin supplementation for ECMO. Crit Care 2020; 24: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Annich GM, Zaulan O, Neufeld M, et al. Thromboprophylaxis in extracorporeal circuits: current pharmacological strategies and future directions. Am J Cardiovasc Drugs 2017; 17: 425–439. [DOI] [PubMed] [Google Scholar]
- 13. Owings JT, Pollock ME, Gosselin RC, et al. Anticoagulation of children undergoing cardiopulmonary bypass is overestimated by current monitoring techniques. Arch Surg 2000; 135: 1042–1047. [DOI] [PubMed] [Google Scholar]
- 14. Liveris A, Bello RA, Friedmann P, et al. Anti-factor Xa assay is a superior correlate of heparin dose than activated partial thromboplastin time or activated clotting time in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med 2014; 15: e72–e79. [DOI] [PubMed] [Google Scholar]
- 15. Atallah S, Liebl M, Fitousis K, et al. Evaluation of the activated clotting time and activated partial thromboplastin time for the monitoring of heparin in adult extracorporeal membrane oxygenation patients. Perfusion 2014; 29: 456–461. [DOI] [PubMed] [Google Scholar]
- 16. Ranucci M, Baryshnikova E, Cotza M, et al. Coagulation monitoring in postcardiotomy ECMO: conventional tests, point-of-care, or both? Minerva Anestesiol 2016; 82: 858–866. [PubMed] [Google Scholar]
- 17. Sy E, Sklar MC, Lequier L, et al. Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation: a systematic review and meta-analysis. J Crit Care 2017; 39: 87–96. [DOI] [PubMed] [Google Scholar]
- 18. Finley A, Greenberg C. Review article: heparin sensitivity and resistance: management during cardiopulmonary bypass. Anesth Analg 2013; 116: 1210–1222. [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: B2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013; 158: 280–286. [DOI] [PubMed] [Google Scholar]
- 21. Barendregt JJ, Doi SA, Lee YY, et al. Meta-analysis of prevalence. J Epidemiol Community Health 2013; 67: 974–978. [DOI] [PubMed] [Google Scholar]
- 22. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007; 28: 105–114. [DOI] [PubMed] [Google Scholar]
- 23. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997; 127: 820–826. [DOI] [PubMed] [Google Scholar]
- 24. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: D4002. [DOI] [PubMed] [Google Scholar]
- 25. Bingham KR, Riley JB, Schears GJ. Anticoagulation management during first five days of infant-pediatric extracorporeal life support. J Extra Corpor Technol 2018; 50: 30–37. [PMC free article] [PubMed] [Google Scholar]
- 26. Buttram SDW, Garcia-Filion P, Schmidt A, et al. Case-control study of anticoagulation regimen effects on bleeding complications and blood product administration during extracorporeal life support. Pediatr Crit Care Med 2014; 15: 116. [Google Scholar]
- 27. Hundalani SG, Nguyen KT, Soundar E, et al. Age-based difference in activation markers of coagulation and fibrinolysis in extracorporeal membrane oxygenation. Pediatr Crit Care Med 2014; 15: e198–e205. [DOI] [PubMed] [Google Scholar]
- 28. Jayakody Arachchillage D, Dhillon E, Vandenbriele C, et al. Heparin anti-Xa assay versus activated partial thromboplastin time to monitor unfractionated heparin during extracorporeal membrane oxygenation. Res Pract Thromb Haemost 2018; 2: 282.30046730 [Google Scholar]
- 29. Kamdar A, Rintoul N, Friedman DF, et al. Comparative effectiveness of different strategies for monitoring neonates on extra corporeal membrane oxygenation (ECMO). Blood 2016; 128: 1007. [Google Scholar]
- 30. Sleeper L, Mulone M, Diallo F, et al. Thermoelastography testing provides effective risk stratification for bleeding in patients on extracorporeal membrane oxygenation support. World J Ped Cong Heart Surg 2018; 9: NP27–NP28. [Google Scholar]
- 31. Venado A, Acosta-Lara P, Bellot S, et al. Comparison of anti-Xa activity and thromboelastogram analysis for guiding anticoagulation management in adult patients supported with extracorporeal membrane oxygenation. Am J Respir Crit Care Med 2015; 191: A4562. [Google Scholar]
- 32. Zogheib E, Nader J, Villeret L, et al. VV-ECMO on ARDS: toward a lower anticoagulation ratio? Ann Intens Care 2016; 6: P10. [Google Scholar]
- 33. Krusenoski S, Beckman E, Thomas C. Impact of antithrombin III supplementation in pediatric ECMO patients. Crit Care Med 2017; 46: P888. [Google Scholar]
- 34. Sacco A, Lopez N, Phillips K, et al. Monitoring heparin and associated adverse events in adults on extracorporeal membrane oxygenation. Crit Care Med 2018; 46: 435. [Google Scholar]
- 35. Cervio C, Sciuccati G, Frontroth JP, et al. Extracorporeal membrane oxigenation (ECMO) in neonates and children: prospective single center study of Argentina. Res Pract Thromb Haemost 2017; 1: 2018–2019. [Google Scholar]
- 36. McQuilten Z, Aubron C, Bailey M, et al. Low-dose heparin in critically ill patients undergoing extracorporeal membrane oxygenation - the help-ECMO pilot randomised controlled trial. Blood 2016; 128: 3822. [Google Scholar]
- 37. Gordon S, Heath T, Bourguet-Vincent A, et al. Evaluation of antithrombin supplementation on heparin anticoagulation in pediatric ECMO patients. Crit Care Med 2015; 43: 153. [Google Scholar]
- 38. Dhillon E, Laffan M, Jayakody Arachchillage D. Comparing activated partial thromboplastin time and heparin anti-Xa levels to monitoring anticoagulant intensity of unfractionated heparin in extracorporeal membrane oxygenation. Brit J Haemat 2018; 181: 138. [Google Scholar]
- 39. Parker H, Simpson P, Dasgupta M, et al. Impact of anticoagulation strategy on ECMO bleeding and thrombotic complications. Artif Organs 2015; 39: A12. [Google Scholar]
- 40. Vandenbriele C, Budhia N, Dhillon E, et al. Heparin anti-Xa assay versus activated partial thromboplastin time to monitor unfractionated heparin during extra-corporeal-membrane-oxygenation. Eur Heart J 2018; 39: 358. [Google Scholar]
- 41. Aubron C, McQuilten Z, Bailey M, et al. Low-dose versus therapeutic anticoagulation in patients on extracorporeal membrane oxygenation: a pilot randomized trial. Crit Care Med 2019; 47: e563–e571. [DOI] [PubMed] [Google Scholar]
- 42. McMichael ABV, Hornik CP, Hupp SR, et al. Correlation among antifactor Xa, activated partial thromboplastin time, and heparin dose and association with pediatric extracorporeal membrane oxygenation complications. ASAIO J 2020; 66: 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Niebler RA, Parker H, Hoffman GM. Impact of anticoagulation and circuit technology on complications during extracorporeal membrane oxygenation. ASAIO J 2019; 65: 270–276. [DOI] [PubMed] [Google Scholar]
- 44. Diaz R, Moffet BS, Mahoney D, et al. Off-label antithrombin use in pediatrics: evaluation of safety, efficacy and usage patterns. Blood 2013; 122: 1137.23843495 [Google Scholar]
- 45. Heyrend C, Olson J, Stidham C, et al. Antithrombin III supplementation for pediatric patients on ECMO and heparin anticoagulation. Crit Care Med 2016; 44: 290. [Google Scholar]
- 46. Hobson MJ. An anti-Xa driven anticoagulation algorithm combined with routine replacement of antithrombin III is associated with reduced hemorrhagic and thrombotic complications as well as improved survival in post-operative pediatric patients supported with ECMO. ASAIO J 2017; 63: 24.27660902 [Google Scholar]
- 47. Kilgallon K, Moore-Clingenpeel M, Hensley J, et al. Anticoagulation management guided by anti-Xa in neonatal and pediatric ECMO. ASAIO J 2018; 64: 73. [Google Scholar]
- 48. Wallskog K, Heih J, Rinka J, et al. Use of thromboelastography for monitoring heparin in extracorporeal membrane oxygenation therapy. Crit Care Med 2017; 46: 50. [Google Scholar]
- 49. Yu JS, Barbaro RP, Granoski DA, et al. Prospective side by side comparison of outcomes and complications with a simple versus intensive anticoagulation monitoring strategy in pediatric extracorporeal life support patients. Pediatr Crit Care Med 2017; 18: 1055–1062. [DOI] [PubMed] [Google Scholar]
- 50. Northrop MS, Sidonio RF, Phillips SE, et al. The use of an extracorporeal membrane oxygenation anticoagulation laboratory protocol is associated with decreased blood product use, decreased hemorrhagic complications, and increased circuit life. Pediatr Crit Care Med 2015; 16: 66–74. [DOI] [PubMed] [Google Scholar]
- 51. Byrnes JW, Swearingen CJ, Prodhan P, et al. Antithrombin III supplementation on extracorporeal membrane oxygenation: impact on heparin dose and circuit life. ASAIO J 2014; 60: 57–62. [DOI] [PubMed] [Google Scholar]
- 52. O’Meara LC, Alten JA, Goldberg KG, et al. Anti-Xa directed protocol for anticoagulation management in children supported with extracorporeal membrane oxygenation. ASAIO J 2015; 61: 339–344. [DOI] [PubMed] [Google Scholar]
- 53. Anton-Martin P, Journeycake J, Modem V, et al. Coagulation profile is not a predictor of acute cerebrovascular events in pediatric extracorporeal membrane oxygenation patients. ASAIO J 2017; 63: 793–801. [DOI] [PubMed] [Google Scholar]
- 54. Moynihan K, Johnson K, Straney L, et al. Coagulation monitoring correlation with heparin dose in pediatric extracorporeal life support. Perfusion 2017; 32: 675–685. [DOI] [PubMed] [Google Scholar]
- 55. Henderson N, Sullivan JE, Myers J, et al. Use of thromboelastography to predict thrombotic complications in pediatric and neonatal extracorporeal membranous oxygenation. J Extra Corpor Technol 2018; 50: 149–154. [PMC free article] [PubMed] [Google Scholar]
- 56. Arnouk S, Altshuler D, Lewis TC, et al. Evaluation of anti-Xa and activated partial thromboplastin time monitoring of heparin in adult patients receiving extracorporeal membrane oxygenation support. ASAIO J 2020; 66: 300–306. [DOI] [PubMed] [Google Scholar]
- 57. Irby K, Swearingen C, Byrnes J, et al. Unfractionated heparin activity measured by anti-factor Xa levels is associated with the need for extracorporeal membrane oxygenation circuit/membrane oxygenator change: a retrospective pediatric study. Pediatr Crit Care Med 2014; 15: e175–e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Padhya DP, Prutsky GJ, Nemergut ME, et al. Routine laboratory measures of heparin anticoagulation for children on extracorporeal membrane oxygenation: systematic review and meta-analysis. Thromb Res 2019; 179: 132–139. [DOI] [PubMed] [Google Scholar]
- 59. Sklar MC, Sy E, Lequier L, et al. Anticoagulation practices during venovenous extracorporeal membrane oxygenation for respiratory failure. a systematic review. Ann Am Thorac Soc 2016; 13: 2242–2250. [DOI] [PubMed] [Google Scholar]
- 60. Giani M, Russotto V, Pozzi M, et al. Thromboelastometry, thromboelastography, and conventional tests to assess anticoagulation during extracorporeal support: a prospective observational study. ASAIO J. Epub Ahead of Print 2020. DOI: 10.1097/MAT.0000000000001196. [DOI] [PubMed] [Google Scholar]
- 61. McAuley L, Pham B, Tugwell P, et al. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet 2000; 356: 1228–1231. [DOI] [PubMed] [Google Scholar]
- 62. Conn VS, Valentine JC, Cooper HM, et al. Grey literature in meta-analyses. Nurs Res 2003; 52(4): 256–261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Files_-_R1_V2 for Anti-Xa versus time-guided anticoagulation strategies in extracorporeal membrane oxygenation: a systematic review and meta-analysis by Ariane Willems, Peter P Roeleveld, Sonia Labarinas, John W Cyrus, Jennifer A Muszynski, Marianne E Nellis and Oliver Karam in Perfusion