Abstract
Chimeric antigen receptor T cells (CAR-Ts) constitute a novel therapeutic strategy for relapsed/refractory B-cell malignancies. With the extensive application of CAR-T therapy in clinical settings, CAR-T-associated toxicities have become increasingly apparent. However, information regarding the associated infections is limited. We aimed to evaluate the incidence of infection during CAR-T therapy and identify the potential risk factors. Especially, we evaluated infections and the associated risk factors in 92 patients. The cohort included patients with acute lymphoblastic leukemia (n = 58) and non-Hodgkin lymphoma (n = 34). Fifteen cases of infection (predominantly bacterial) were observed within 28 days of CAR-T therapy, with an infection density of 0.5 infections for every 100 days-at-risk. Neutropenia before CAR-T therapy (P = .005) and prior infection (P = .046) were independent risk factors associated with infection within 28 days after CAR-T therapy; corticosteroid treatment during cytokine release syndrome (P = .013) was an independent risk factor during days 29–180 after CAR-T infusions. Moreover, the 2-year survival duration was significantly shorter in patients with infections than in those without (126 vs 409 days; P = .006). Our results suggested that effective anti-infection therapies may improve prognosis of patients who have a high infection risk. The risk of bacterial infections during the early stages of CAR-T therapy and the subsequent risk of viral infections thereafter should be considered to provide the appropriate treatment and improve patient prognosis.
Keywords: bacterial infection; chimeric antigen receptor T cell therapy, B-cell malignancies, prognosis, risk
Introduction
Although novel drugs have been developed recently, patients with relapsed/refractory (R/R) B-cell malignancies have a very poor prognosis. Thus, therapeutic strategies are urgently required to treat these patients. Recently, chimeric antigen receptor T cells (CAR-Ts) have successfully improved the treatment outcomes of individuals with B-cell malignancies. High complete remission (CR) rates of 70–95% and 50–70% for CAR-T treatment of patients with R/R acute lymphoblastic leukemia (ALL) 1 –6 and B-cell lymphoma, respectively, have been reported in independent clinical trials 7 –9 . Therefore, the National Comprehensive Cancer Network guidelines recommend the performance of CAR-T cell therapy for B-cell malignancies.
With the widespread application of CAR-T therapy and increasing data obtained from long-term follow-up studies, CAR-T-associated unique toxicities, including cytokine release syndrome (CRS), B-cell aplasia, and immune effector cell-associated neurotoxicity syndrome 10 –15 , have become increasingly apparent. Infections occur frequently in individuals receiving chemotherapy or allogeneic hematopoietic stem cell transplantation (HSCT) 16 –19 ; however, such cases rarely been reported after CAR-T therapy 20 –25 .
Before receiving CAR-T treatment, patients with R/R B-cell malignancies usually undergo chemotherapy courses, such as high-dose salvage chemotherapy. Some patients relapse after autologous or allogeneic HSCT. During these treatments, patients usually experienced pancytopenia leading to severe infection, which may have increased the bacterial, viral, or fungal infection risk. Moreover, patients with R/R B-cell malignancies have poor immune function because of prior cytotoxic treatments for their primary malignancies, which also increase the infection risk. Generally, patients receive conditioning chemotherapy, such as fludarabine and cyclophosphamide administration, before their CAR-T infusions (CTIs), which may have exacerbated their immunosuppression. After CTI, some patients experience complications, including severe CRS, corticosteroid and/or tocilizumab administration, or admission to the intensive care unit (ICU) as part of severe CRS management that may lead to increased infection risk.
To date, limited systematic studies focused on the complications after CAR-T therapy, including infections, have been conducted 20 –25 . Additionally, data from systemic studies on Asian natives and children remain limited 22 . In this study, we investigated the incidence of infections during the first 180 days after CAR-T cell infusions in 92 Chinese patients with R/R B-cell malignancies to identify the risk factors for infection.
Materials and Methods
The study design, timeline, and outcome measures were explained to all eligible patients, and they were informed that they were free to discontinue participation at any time without consequences. All patients signed the written informed consent form prior to examination. The study was approved by the Institutional Review Boards of the First Affiliated Hospital, School of Medicine, Zhejiang University (No. 2018-747-2).
Patients and Clinical Protocols
We reviewed the data of 92 Chinese patients (10 children, 82 adults) with R/R B-cell malignancies who were treated with lymphodepletion chemotherapy and CTI from July 2015 to May 2019 in a phase 1/2 open-label clinical trial (ChiCTR-ORN-16008948, ChiCTR-OIC-17011310, ChiCTR1800015575) 3 . We reviewed their medical records to examine the presence of infections after chemotherapy for B-cell malignancies. All patients received a single lymphodepletion chemotherapy cycle, followed by CTI at doses of 1.0×106–10.0×106 cells/kg. All patients were admitted to a laminar flow ward after CTI. When their absolute neutrophil counts (ANCs) were < 500 cells/mm3, the granulocyte colony-stimulating factor was administered daily. The serum immunoglobulin G (IgG) levels were evaluated prior to and approximately weekly after CTI. Immunoglobulin (400 mg/kg, IV) was recommended when the serum IgG levels were < 600 mg/dL. Patients with a body temperature ≥ 38°C and neutropenia were treated immediately with 1 g of meropenem/imipenem every 8 h. Repeated blood cultures were performed where necessary. In cases where a patient had symptoms of urinary tract irritation or diarrhea, urine or stool cultures were performed. Cytomegalovirus, Epstein-Barr virus, adenovirus, and influenza virus infections were monitored each week using polymerase chain reaction, while the fungal infections were monitored using the Galactomannan tests, with an increase in the frequency of testing according to the patients’ condition. Patients with respiratory symptoms were tested using lung computed tomography and sputum cultures that were repeated when necessary. CRS was graded according to a revised grading system 15 . Moreover, treatment for CRS, including the use of tocilizumab and/or steroids, was recorded. Especially, they received methylprednisolone 80 mg every 12 h or tocilizumab 8 mg once. When the symptoms were not improved after 2 days, increasing the dose, repeating tocilizumab administration, or administrating these two drugs combined was considered.
In general, patients in the ALL group remained under our center’s care for a minimum of 28 days after therapy, as this is the time-frame during which most complications are expected. After discharge, they visited our center for follow-up examinations every 15 days until a new treatment, such as HSCT, was prescribed. Patients’ medical information was recorded in our center. Patients with lymphoma were discharged from the hospital after their symptoms had subsided after 1 week. They visited our center for follow-up examinations every month for 1–2 years.
Definition of infection
An episode of infection was defined as the presence of clinical symptoms along with corroborating radiographical, microbiological, or histopathological results. The infection-onset day was defined as the day on which the diagnostic test was performed. Bacterial infections were categorized as bacteremia or site-specific infections. Multiple positive blood cultures for different microorganisms on the same day were considered as separate events. Patients with typical clinical symptoms (temperature > 38°C and purulent cough) and radiographical evidence without CAR-T expansion were considered to have an infection even if their microbiological findings were negative. These infections were classified as unknown, and the infection-onset day was defined as the day on which lung computed tomography was performed.
Data collection and statistical analysis
Patients’ data, including demographic data, previous treatment history, morphological disease status, duration of neutropenia and fever, and CRS grade, were obtained from their medical records. Infections were retrospectively identified from the day of the first CTI (day 0) up to day 180 after CTI. Patients were censored on the date of de novo antitumor therapy, last clinical contact at our hospital, or death.
Summary statistics were calculated for categorical variables, along with medians and ranges for continuous variables. Infection densities were determined and compared between the first 28 days and days 28–180, using the mid-P method (an efficient method for computing the exact confidence limits for the common rate ratio in a series of two χ2 tables with person-time denominators). To evaluate the risk factors for infection post-CTI, we performed univariate and stepwise multivariate analyses using a competing risk model. Neutrophil recovery was defined from the 1st of 3 consecutive days with an ANC ≥ 500 cells/mm3. Patients with a body temperature ≥ 38°C within 72 h before CTI were considered to have fever before undergoing CTI. The body temperature of every patient was recorded from CTI day 1 to death or discharge. The duration of fever was defined from the 1st of 3 consecutive days with a body temperature > 38°C to the 1st of 3 consecutive days when the body temperature returned to normal. Analyses were performed for infections occurring between CTI days 0 and 180 upon standard follow-up evaluations at our center.
Results
Clinical and Treatment Characteristics
Ninety-two patients, of which 10 were children, had R/R ALL or non-Hodgkin lymphoma (NHL) and received CTI during the study period. Eighteen patients (one child) received CTI twice or thrice (15 and 3 patients because of relapse and consolidation therapy, respectively). When the interval period between each treatment was > 3 months, these treatments were considered independent; hence, 113 CTIs were administered in total. The patient and treatment characteristics are presented in Table 1. Infections, which occurred in each time period, are presented in Table 2. Twenty-five patients developed infections during the previous treatment; among them, there were 5, 14, and 4 cases of sepsis, respiratory tract infections (five, seven, and two cases of bacterial, viral, and fungal infection, respectively), and gastrointestinal tract infections (two cases of bacterial and fungal infection each), respectively. Of these, 10 cases were clinically diagnosed. In the ALL and NHL cohorts, 62 and 25 patients developed neutropenia, respectively. The median and range values of recovery time are listed in Table 1.
Table 1.
Clinical and Treatment Characteristics Before and after Chimeric Antigen Receptor T Cell Therapy.
| ALL (n = 75)a | NHL (n = 38)a | Total (n = 113)a | |
|---|---|---|---|
| Baseline characteristics | |||
| Age, median (range), years | 29 (7–76) | 45.5 (23–71) | 35 (7–71) |
| Female | 38 (50.7%) | 14 (36.8%) | 52 (46%) |
| Prior antitumor treatment regimens | 5 (1–24) | 8 (5–24) | 6 (1–24) |
| IgG <600 mg/dL | 3 (4%) | 4 (10.5%) | 36 (31.9%) |
| ANC <500 cells/mm3 before CTI | 26 (34.7%) | 2 (5.3%) | 28 (24.8%) |
| Fever before CTI | 34 (45.3%) | 8 (21.1%) | 42 (37.2%) |
| Prior autologous and/or allogeneic HSCT | 24 (32.0%) | 4 (10.5%) | 28 (24.8%) |
| Infection in prior treatment | 20 (26.7%) | 5 (13.2%) | 25 (22.1%) |
| Disease status | |||
| ≤20% blasts b/I-IIc | 27 (36%) | 11 (28.9%) | 38 (33.6%) |
| >20% blasts b/III-IVc | 48 (64%) | 27 (71.1%) | 75 (66.4%) |
| Post CAR-T cell characteristics | |||
| Time to neutrophil recovery ≥500 cells per mm3 median (range)d | 11 (1–78) | 4 (1–36) | 6 (1–78) |
| Duration of fevere | 8 (2–45) | 7 (2–63) | 7 (2–63) |
| CRS grade | |||
| 0 | 14 (18.7%) | 5 (13.2%) | 19 (16.8%) |
| 1–2 | 35 (46.7%) | 25 (65.8%) | 60 (53.1%) |
| 3–5 | 26 (34.7%) | 8 (21.1%) | 34 (30.1%) |
| Corticosteroid | 17 (22.7%) | 7 (18.4%) | 24 (21.2%)f |
| Tocilizumab | 24 (32%) | 5 (13.2%) | 29 (25.7%)f |
| ICU admission by day 28 | 3 (4.0%) | 0 (0%) | 3 (2.7%) |
a In our study, 92 patients received CTI; 19 patients received CTI more than once (ALL, n = 15; NHL, n = 4). The clinical characteristics of the same patients on different courses were similar; hence, we considered each CTI as an independent clinical and treatment characteristic.
b Blasts referred to the blast cells in the bone marrow.
c Patients with NHL were examined via positron emission tomographic/computed tomographic scanning before CTI and staged in accordance with the results.
d Patients who did not display an ANC of ≥500 cells/mm3 before CTI (ALL, n=1) or did not have an ANC of <500 cells/mm3 were excluded from the analysis.
e Patients who did not have a fever (ALL, n = 8; NHL, n = 4) were excluded from the analysis.
f16, 9, and 15 patients received only tocilizumab, corticosteroids, or both.
ALL, acute lymphoblastic leukemia; ANC, absolute neutrophil count; CAR-T, chimeric antigen receptor T cells; CTI, CAR-T infusion; CRS, cytokine release syndrome; HSCT, hematopoietic stem cell transplantation; IgG, immunoglobulin G; NHL, non-Hodgkin lymphoma.
Table 2.
Clinical Characteristics of Infection and the Occurrence of Time.
| No. of patients | Age | Disease type | Infections | Occurrence of infection (days after CTI) |
|---|---|---|---|---|
| 1 | 53 | ALL | Clinical diagnosis | 7 |
| 2 | 39 | ALL | Pseudomonas aeruginosa | 17 |
| 3 | 7 | ALL | Kreber Pneumonia | 16 |
| 4 | 45 | ALL | Staphylococcus | 4 |
| 5 | 17 | ALL | Candida sp. | 1 |
| Salmonella | 1 | |||
| 6 | 20 | ALL | Candida sp. | 89 |
| 7 | 66 | ALL | Syncytial | 12 |
| 8 | 57 | ALL | Clinical diagnosis | 1 |
| 9 | 20 | ALL | Clinical diagnosis | 1 |
| 10 | 32 | ALL | Pseudomonas aeruginosa | 24 |
| 11 | 47 | ALL | Influenza viremia | 94 |
| 12 | 67 | ALL | Clinical diagnosis | 1 |
| 13 | 42 | ALL | Staphylococcus | 34 |
| Enterococcus faecium | 39 | |||
| 14 | 66 | ALL | Clinical diagnosis | 1 |
| 15 | 49 | NHL | Influenza viremia | 137 |
| 16 | 53 | NHL | Corynebacterium | 19 |
| 17 | 36 | NHL | Salmonella | 30 |
| 18 | 47 | NHL | Staphylococcus sp. | 17 |
| Aspergillus sp. | 37 | |||
| Fusarium sp. | 52 | |||
| 19 | 41 | NHL | Enterococcus faecium | 9 |
| 20 | 59 | NHL | Herpes zoster | 39 |
ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin lymphoma.
Incidence of infection after CTI
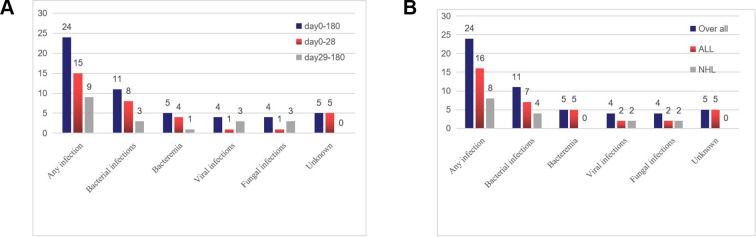
Within the first 28 days after CTI, we analyzed the data on days at risk of infection in 2,943 patients. Initial infection was detected within a median duration of 7 days (range, 1–24 days) after CTI; 60% of the initial infections occurred within the first 10 days. Infection density during the first 28 days after CTI was 0.476 infections per 100 days at risk. Fifteen infections were recorded in 14 patients among the 113 CTI treatments (13.2%) during this period (Fig. 1A). Bacterial infections (eight infection events) were predominant (four systemic infections and four localized infections). The incidence of viral and fungal infections was very low (one viral and one fungal). Five patients presented with typical clinical symptoms, including fever and cough, but without positive microbiological results. These patients could not tolerate bronchoscopies or biopsies. Moreover, CAR-T expansion was not detected via flow cytometry; these patients were classified as having unknown infections. Moreover, infections occurred in eight out of 28 patients who had neutropenia before CTI.
Figure 1.
Incidence of infections after CAR-T infusion within 180 days. (A) Number of events in different periods. (B) Number of events in different disease groups. Bacterial infections (n = 4) observed on days 0–29 included the following types: (Staphylococcus sp., n = 1; Pseudomonas aeruginosa, n=2; Klebsiella pneumoniae, n = 1); site of bacterial infection (n = 4) (respiratory tract: Staphylococcus sp., n = 1; Corynebacterium, n = 1; digestive tract: Enterococcus, n = 1, salmonella, n = 1); viral infections (respiratory: respiratory syncytial virus, n = 1); and fungal infections (respiratory: Candida sp., n = 1). Bacterial infections (n = 1) observed on days 29–180 included the following: Staphylococcus sp. (n = 1); site of bacterial infection (n = 2) (digestive tract Enterococcus faecium, n = 1; salmonella, n = 1); viral infections (n = 3) (influenza viremia, n = 2; herpes zoster virus, n = 1 [one patient presented with herpes with pain, and the symptoms improved after antiviral treatment]; fungal infections (n = 3) (Candida sp., n = 1; Aspergillus sp., n = 1; Fusarium sp., n = 1). CAR-T, chimeric antigen receptor T cell.
Nine infection events (three bacterial, three fungal, and three viral events) occurred in seven patients between days 29 and 180 (Fig. 1). The infection density between days 29 and 180 after CTI was 0.111 infections per 100 days at risk. Statistical analysis revealed that the incidence of infection events within 28 days was greater than that between days 29 and 180 (relative risk [RR], 4.30; 95% confidence interval [CI], 1.99–9.25; P < .001), and the late pathogens (days 29–180) were primarily viruses and fungi, while bacteria were dominant within 28 days.
The two cohorts (ALL and NHL) showed that the infection incidence was similar (17.3% vs 15.8%, P = .531), but bacteremia occurred only in the ALL cohort, and the infection incidence during days 0–28 in the ALL group seemed to be higher than that in the NHL group (14.7% vs 7.9%, respectively, P = .237) (Fig. 1B).
Three infection events (i.e., two bacterial and one fungal) occurred in two out of 11 children within 28 days after receiving CTI. In contrast, there were only 12 infection events in 102 adults following CTI treatment (27.3% [children] vs 11.8% [adults], P = .162). No late infection events occurred in the children’s cohort.
Risk Factors Associated with Infection After CTI
We analyzed various pre- and post-treatment clinical factors to identify risk factors associated with infections. Risk factors associated with infections were identified using univariate and multivariate analyses and are presented in Table 3. As there were different distributions for early and late pathogens, we analyzed the risk factors separately. Patients’ sex and age, prior chemotherapy, prior autologous and/or allogeneic HSCT, disease type, CRS grade, and serum hypogammaglobulinemia were not correlated with the infection risk (Table 3). An ANC < 500 cells/mm3 before CTI (hazard ratio [HR], 5.914; 95% CI, 1.720–20.331; P = .005) and infection during prior treatment (HR, 3.732; 95% CI, 1.025–13.597; P = .046) were independent risk factors associated with a significantly increased infection density within 28 days after CTI. Similarly, corticosteroid treatment during CRS (HR, 7.711, 95% CI, 1.534–38.759; P = .013) was an independent risk factor during days 29–180 after CTI.
Table 3.
Risk Factors for the Initial Infection Determined using a Competing Risks Model.
| Variables | Day 0-28 | Day 29-180 | ||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | P | Adjusted 95% CI | P c | 95% CI | P | Adjusted 95% CI | P c | |
| Age | 1.037 (0.994–1.081) | .09 | 1.019 (0.991–1.049) | .186 | ||||
| Sex | 1.306 (0.414–4.116) | .649 | 1.906 (0.357-10.177) | .450 | ||||
| Disease type | 0.331 (0.086–1.274) | .108 | 3.518 (0.716-17.296) | .122 | ||||
| Prior treatments | 1.001 (0.878–1.141) | .986 | 1.027 (0.905–1.165) | .680 | ||||
| Prior autologous and/or allogenic HSCT | 0.887 (0.243–3.242) | .856 | 0.147 (0.018–1.190) | .072 | 0.078 (0.006 -1.012) | .051 | ||
| Hypogammaglobulinemia (IgG <600 mg/dL) | 1.607 (0.522–4.942) | .408 | 2.342 (0.496–11.053) | .282 | ||||
| ANC <500 cells/mm3 before CTI | 4.930 (1.607–15.123) | .005 | 5.914 (1.720–20.331) | .005 | 0.232 (0.029–1.865) | .170 | ||
| Fever before CTI | 1.929 (0.632–5.888) | .249 | 2.090 (0.444–9.852) | .351 | ||||
| High disease burden | 2.583 (0.753-8.865) | .132 | 4.936 (0.849-28.685) | .075 | ||||
| Infection in prior treatment | 3.239 (1.058–9.918) | .040 | 3.732 (1.025–13.597) | .046 | 3.165 (0.652-15.361) | .153 | ||
| CRS ≥3 | 2.157 (0.706–6.594) | .178 | 1.538 (0.287–8.249) | .616 | ||||
| Corticosteroida | 1.363 (0.394–4.717) | .625 | 8.810 (1.869–41.541) | .006 | 7.711 (1.534-38.759) | .013 | ||
| Tocilizumaba | 0.710 (0.190–2.648) | .610 | 1.873 (0.349–10.053) | .464 | ||||
| ICU admission by day 28b | 3.980 (0.521–30.427) | .183 | ||||||
a The use of corticosteroid or tocilizumab after infection, the analysis considered this as not to be used.
b As there were no patients admitted to the ICU during day 29-180, we did not perform the analysis.
ALL, acute lymphoblastic leukemia; ANC, absolute neutrophil count; NHL, non-Hodgkin lymphoma; CI, confidence interval; CRS, cytokine release syndrome; CTI, CAR-T infusion; HSCT, hematopoietic stem cell transplantation; IgG, immunoglobulin G.
Treatment and Clinical Outcomes of Patients with Infection
Empirical anti-infective treatment was administered when clinically suspected infections were considered. The anti-infective drugs were adjusted according to the drug sensitivity test via bacterial culturing. Post-treatment, 15 out of 20 patients with infections after CTI recovered from infections, while five patients including three patients with bacteremia died because of the infection. Two patients were readmitted to the hospital because of infection after discharge. After CTI, 12 patients died in the first 28 days. Of the 12 patients with early mortality, eight patients died from infection. The 28-day cumulative incidence of infection and death from infection was 12.4% and 7.1%, respectively (Fig. 2A). At the end of the follow-up period, 13 and 40 patients with and without infection, respectively, died, and the 2-year overall survival rate was lower in the infection than in the non-infection group (P = .006; median survival duration, 106 vs 409 days; Fig. 2B).
Figure 2.

The 28-day cumulative incidence of infection, death from infection, and the 2-year survival curve of patients receiving CTI is presented. (A) The 28-day cumulative incidence of infection and death from infection is presented. (B) The 2-year survival curve of patients receiving CTI (P = .006) is presented. CTI, chimeric antigen receptor T cell infusion.
Discussion
We described cases of infectious complications, including the incidence and distribution of infections, in patients with R/R ALL and NHL during the first 6 months after CTI. We identified that neutropenia before CTI and treatment with corticosteroids during CRS were the two independent risk factors associated with an increased incidence of infection after CTI. Moreover, patients with infection had a shorter survival duration than those without infection.
A high infection rate related to chemotherapy or allogeneic HSCT has been reported 16 –19,26 . Slade et al. 26 reported that approximately 89% of the patients experienced at least one infection and that bacterial infections were predominant during the pre-engraftment phase, whereas viral infections were predominant in the early post-engraftment period. In general, the infection rate following chemotherapy for patients with R/R ALL was found to be very high 18,26 . Kantarjian et al. 18 reported that infection was an adverse event and found that approximately 34.1% and 57% of patients were treated with blinatumomab and chemotherapy, respectively. Performing CD19 CAR-T, as a novel therapy for R/R ALL, led to a lower incidence of infection than salvage chemotherapy or allogeneic HSCT 23–24 . Hill et al. 23 reported 43 instances of infections in 30 out of 133 patients (23%) within 28 days after CTI with an infection density of 1.19 infections for every 100 days at risk, while Park et al. 24 reported that 26 infection instances developed in 22 patients (42%). Vora et al. 22 reported that infections occurred in 40% of children in the first 28 days following CTI (infection density, 2.89). The infection rate in our study was lower than those observed in these clinical trials, despite the difference observed between adults and children (27.3% vs 11.8%). The following factors may have been related: (1) High-efficiency particulate air-filtered rooms were recommended by the Centers for Disease Control for patients at high risk 27,28 . These have been shown to greatly decrease the incidence of aspergillosis 28 . Hahn et al. 29 demonstrated that high-efficiency particulate air filters were protective for highly immunocompromised patients with hematologic malignancies and were effective because of air contamination with Aspergillus conidia. Compared with the results obtained by Hill et al. and Park et al. 23–24 , the incidence of Aspergillus infections in our study was lower during the early period (0% vs 1.5% and 6%, respectively).
(2) Only three patients were admitted to the ICU, which were markedly lower than those admitted in Hill et al.’s study (20 of 133 patients), wherein ICU admission was reported as a significant risk of infection (P = .001).
(3) The incidence of bacterial infections in the aforementioned study may have been overestimated, as it was not possible to distinguish the cause of fever between infections and CRS 23 .
Furthermore, the distribution of infections is clinically important and can guide anti-infection treatment. Our results revealed that in early (days 0–28) infectious complications, bacterial infections were predominant. However, in late infectious complications (days 29–180), bacterial infections decreased and viral infections increased. The distribution of specific infection categories in the early and late periods in our study aligned with those observed after allogeneic HSCT 17,26 . The following factors may have been related: (1) Fludarabine-cyclophosphamide chemotherapy may have negatively influenced immune cell function in early periods, leading to increased bacterial infections; (2) Pancytopenia caused by chemotherapy, primary disease, or CRS in the early period may have increased the bacterial infection rate; (3) As the primary disease was well controlled, immune cells were reconstituted, especially for neutrophil recovery, thus, playing important roles in anti-bacterial infections. Patients usually have B-cell aplasia with a deficiency in humoral immunity, leading to a high viral infection risk because of CAR-T persistence. Our results provided a robust basis to improve anti-infection treatment.
Invasive mold infections (IMIs) are common in patients who receive chemotherapy or HSCT. However, reports of IMI after CAR-T-cell therapy are limited 20 . At our center, the IMI rate was 3% (4/131; one occurred early and three occurred late), which was similar to that observed in Haidar’s study 20 . Two out of four patients died of infection, which demonstrated that such infections had high morbidity and mortality rates. The patients who were enrolled in the CAR-T treatment were neutropenic and deeply immunosuppressed. As the studies performed on CAR-T-cell recipients were limited and the optimal duration of prophylaxis remains unclear, there is still a debate on whether all CAR-T-cell therapy recipients should receive anti-mold prophylaxis. Based on the presence of neutropenia or steroid use, Haidar et al. 20 proposed strategies to prevent IMIs in these patients, while Lewis et al. 30 suggested the provision of active mold prophylaxis to all patients who were going to receive CAR-T.
Similar to fungal infections, viral infections mostly occur in the late stage. In this study, there were four patients with viral infections, of which three cases occurred in the late stage. The time distribution was similar to other studies 23–24 . In our study, three patients showed respiratory symptoms. Thus, in the future, patients in the late stage with advanced respiratory symptoms should consider the possibility of viral infection.
Previous studies have reported that neutropenia was the primary risk factor associated with infections in patients with acute leukemia after chemotherapy, especially in those with chronic conditions 31 –34 . Similar results have been observed among patients after CTI. In most cases, after CTI, patients experienced cytopenia because of receiving chemotherapy and because of CRS. Park et al. reported that all, apart from four patients, had an infection while they were neutropenic after CTI 24 . Our study showed that an increased infection risk was associated with the neutropenic state before CTI. Patients who had neutropenia before CTI usually were neutropenic for a longer period. Our results revealed that prior infection was another risk factor associated with infection in the early period. In our study, 33 pathogens were identified from the previous infection records. Only one patient had the same pathogen infection prior to and after CTI, while in the other patients, this was not consistent after CTI, indicating that the infection recurrence was not a problem.
Our results revealed that corticosteroid treatment during CRS was another risk factor associated with infection in the late period. Corticosteroid exposure has been reported previously, as a risk factor for infection, especially IMIs 35,36 . Dix et al. 37 reported that corticosteroid exposure was associated with higher rates of infection, sepsis, and infection-related mortality, as opposed to our results. The adverse effects of hormones are often not manifested within 28 days, and after 28 days, most patients would have recovered from neutropenia, which explained the inconsistency of risk factors during the different periods. Hence, for patients at a high infection risk, infection prophylaxis or intensive anti-infection treatment may be necessary.
Our study also showed that the survival duration was shorter in patients with infections than in those without. The early mortality rate was high among patients with infections. To date, no study has performed a survival analysis based on the infection state after CAR-T treatment. To the best of our knowledge, our study was the first to report that infectious complications after CTI were associated with a poor prognosis. Therefore, strategies to reduce the infection rate are important to improve the overall survival after CTI.
This study had some limitations, including its retrospective nature and the limited sample size, which could have influenced the statistical analysis reliability. The choice of covariates for multivariable analysis may have been constrained by the small number of observed events.
Conclusion
We provided a broad and comprehensive description of the infectious complications that can occur in this novel cohort. The incidence of infections was relatively lower in patients who underwent CTI than in those who underwent chemotherapy and allogeneic HSCT (13.2% vs 34.1–89%, respectively). However, patients with infections after CTI had a poor prognosis. Our results suggested that the initial bacterial (days 0–28) and subsequent viral (days 29–180) infections should be considered. For patients at a high risk of infection, effective prophylaxis therapies may improve their prognosis. Further studies with larger cohorts and longer follow-up periods are recommended to obtain more extensive data on infections.
Footnotes
Authors Contribution: Feng Zhu, and Guoqing Wei, These authors contributed equally to this work.
Ethical approval: This study was conducted according to the ethical principles for medical research involving human participants as stated in the Declaration of Helsinki. The study protocol was reviewed and approved by the Institutional Review Boards of the First Affiliated Hospital, School of Medicine, Zhejiang University (No. 2018-747-2).
Statement of human and animal rights: The article contains human studies that were approved by the Institutional Review Boards of the First Affiliated Hospital, School of Medicine, Zhejiang University (No. 2018-747-2).
Statement of informed consent: The study design, timeline, and outcome measures were explained to all eligible patients, and they were informed that they were free to discontinue participation at any time without consequence. All patients signed the written informed consent form prior to examination.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the grants from the 973 Program (2015CB964900), the Natural Science Foundation of China (81470341, 81770201, 81730008), Key Project of Science and Technology Department of Zhejiang Province (2018C03016-2), and Zhejiang public welfare foundation (GF18H180002).
ORCID iDs: Feng Zhu  https://orcid.org/0000-0003-3140-4289
https://orcid.org/0000-0003-3140-4289
Yongxian Hu  https://orcid.org/0000-0001-9564-1852
https://orcid.org/0000-0001-9564-1852
References
- 1. Orlowski RJ, Porter DL, Frey NV. The promise of chimeric antigen receptor T cells (CARTs) in leukaemia. Br J Haematol. 2017;177(1):13–26. [DOI] [PubMed] [Google Scholar]
- 2. Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, Sauter C, Wang YM, Santomasso B, Mead E, Roshal M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu Y, Wu Z, Luo Y, Shi J, Yu J, Pu C, Liang Z, Wei G, Cui Q, Sun J, Jiang J, et al. Potent anti-leukemia activities of chimeric antigen receptor-modified T cells against CD19 in Chinese patients with relapsed/refractory acute lymphocytic leukemia. Clin Cancer Res. 2017;23(11):3297–3306. [DOI] [PubMed] [Google Scholar]
- 4. Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, Gea-Banacloche C, Pavletic SZ, Hickstein DD, Lu TL, Feldman SA, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34(10):1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, et al. Tisagenlecleucel in children and young adults with B-Cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, Bleakley M, Brown C, Mgebroff S, Kelly-Sprat KS, Hoglund V, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kochenderfer JN, Somerville RPT, Lu T, Shi V, Bot A, Rossi J, Xue A, Goff SL, Yang JC, Sherry RM, Klebanoff CA, et al. Lymphoma remissions caused by Anti-CD19 chimeric antigen receptor T Cells are associated with high serum interleukin-15 levels. J Clin Oncol. 2017;35(16):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak O, Brogdon JL, Pruteanu-Malicini I, Bhoj V, Landsburg D, Wasik M, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hay KA. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br J Haematol. 2018;183(3):364–374. [DOI] [PubMed] [Google Scholar]
- 11. Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, Yeung C, Liles WC, Wurfel M, Lopez JA, Chen J, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santomasso BD, Park JH, Salloum D, Riviere I, Flynn J, Mead E, Halton E, Wang X, Senechal B, Purdon T, Cross JR, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8(8):958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, Lopez JA, Chen J, Chung D, Harju-Baker S, Cherian S, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor modified T-cell therapy. Blood. 2017;130(21):2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marín M, Gudiol C, Ardanuy C, Garcia-Vidal C, Jimenez L, Domingo-Domenech E, Perez FJ, Carratala J. Factors influencing mortality in neutropenic patients with haematologic malignancies or solid tumours with bloodstream infection. Clin Microbiol Infect. 2015;21(6):583–590. [DOI] [PubMed] [Google Scholar]
- 17. Young JH, Logan BR, Wu J, Wingard JR, Weisdorf DJ, Mudrick C, Knust K, Horowitz MM, Confer DL, Dubberke ER, Pergam SA, et al. Infections after transplantation of bone marrow or peripheral blood stem cells from unrelated donors. Biol Blood Marrow Transplant. 2016;22(2):359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera JM, Wei A, Dombret H, Foa R, Bassan R, Arslan O, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia, N Engl J Med. 2017;376(9):836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ustun C, Young JH, Papanicolaou GA, Kim S, Ahn KW, Chen M, Abdel-Azim H, Aljurf M, Beitinjaneh A, Brown V, Cerny J, et al. Bacterial blood stream infections (BSIs), particularly post-engraftment BSIs, are associated with increased mortality after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2019;54(8):1254–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haidar G, Dorritie K, Farah R, Bogdanovich T, Nguyen MH, Samanta P. Invasive mold infections after chimeric antigen receptor-modified T-cell therapy: a case series, review of the literature, and implications for prophylaxis. Clin Infect Dis. 2020;71(3):672–676. [DOI] [PubMed] [Google Scholar]
- 21. Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood. 2020;136(8):925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vora SB, Waghmare A, Englund JA, Qu P, Gardner RA, Hill JA. Infectious complications following CD19 chimeric antigen receptor t-cell therapy for children, adolescents, and young adults. Open Forum Infect Dis. 2020;7(5):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hill JA, Li D, Hay KA, Green ML, Cherian S, Chen X, Riddel SR, Maloney DG, Boeckh M, Turtle CJ. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131(1):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park JH, Romero FA, Taur Y, Sadelain M, Brentjens RJ, Hohl TM, Seo SK. Cytokine release syndrome grade as a predictive marker for infections in patients with relapsed or refractory B-Cell acute lymphoblastic leukemia treated with chimeric antigen receptor T cells. Clin Infect Dis. 2018;67(4):533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cordeiro A, Bezerra ED, Hirayama AV, Hill JA, Wu QV, Voutsinas J, Sorror ML, Turtle CJ, Maloney DG, Bar M. Late events after treatment with CD19-targeted chimeric antigen receptor modified T Cells. Biol Blood Marrow Transplant. 2020;26(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Slade M, Goldsmith S, Romee R, DiPersio JF, Dubberke ER, Westervelt P, Uy GL, Lawrence SJ. Epidemiology of infections following haploidentical peripheral blood hematopoietic cell transplantation. Transplant Infect Dis. 2017:19(1):e12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Centers for Disease Control and Prevention; Infectious Disease Society of America; American Society of Blood and Marrow Transplantation. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Recomm Rep. 2000;49(RR-10):1–125. [PubMed] [Google Scholar]
- 28. Sehulster L, Chinn RY. Guidelines for environmental infection control in health-care facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR-10):1–42. [PubMed] [Google Scholar]
- 29. Hahn T, Cummings KM, Michalek AM, Lipman BJ, Segal BH, McCarthy PL, Jr. Efficacy of high-efficiency particulate air filtration in preventing aspergillosis in immunocompromised patients with hematologic malignancies. Infect Control Hosp Epidemiol. 2002;23(9):525–531. [DOI] [PubMed] [Google Scholar]
- 30. Lewis RE, Kontoyiannis DP. Chimeric antigen receptor t-cell immunotherapy and need for prophylaxis for invasive mold infections. Clin Infect Dis. 2020;71(7):1802–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Afzal S, Ethier MC, Dupuis LL, Tang L, Punnett AS, Richardson SE, Allen U, Abla O, Lillian S. Risk factors for infection-related outcomes during induction therapy for childhood acute lymphoblastic leukemia. Pediatr Infect Dis J. 2009;28(12):1064–1068. [DOI] [PubMed] [Google Scholar]
- 32. O’Connor D, Bate J, Wade R, Clack R, Dhir S, Hough R, Vora A, Goulden N, Samarasinghe S. Infection-related mortality in children with acute lymphoblastic leukemia: an analysis of infectious deaths on UKALL2003. Blood. 2014;124(7):1056–1061. [DOI] [PubMed] [Google Scholar]
- 33. Graubner UB, Porzig S, Jorch N, Kolb R, Wessalowski R, Escherich G, Janka GE. Impact of reduction of therapy on infectious complications in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;50(2):259–263. [DOI] [PubMed] [Google Scholar]
- 34. Dvorak CC, Steinbach WJ, Brown JM, Agarwal R. Risks and outcomes of invasive fungal infections in pediatric patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2005;36(7):621–629. [DOI] [PubMed] [Google Scholar]
- 35. Miceli MH, Churay T, Braun T, Kauffman CA, Couriel DR. Risk factors and outcomes of invasive fungal infections in allogeneic hematopoietic cell transplant recipients. Mycopathologia. 2017;182(5-6):495–504. [DOI] [PubMed] [Google Scholar]
- 36. Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: Changes in epidemiology and risk factors. Blood. 2002;100(13):4358–4366. [DOI] [PubMed] [Google Scholar]
- 37. Dix D, Cellot S, Price V, Gillmeister B, Ethier MC, Johnston DL, Lewis V, Michon B, Mitchell D, Stobart K, Yanofsky R, et al. Association between corticosteroids and infection, sepsis, and infectious death in pediatric acute myeloid leukemia (AML): Results from the Canadian infections in AML research group. Clin Infect Dis. 2012;55(12):1608–1614. [DOI] [PubMed] [Google Scholar]