Abstract
Background:
Intraplaque neovascularisation (IPN) increases the vulnerability of plaques, which makes them more likely to rupture and increases the risk of vascular events. However, it is unclear whether IPN can predict future vascular events (stroke recurrence and cardiovascular events). Previous studies on IPN have focused on patients with severe stenosis but overlooked patients with mild and moderate stenosis. This study aimed to investigate whether IPN assessed by contrast-enhanced ultrasonography (CEUS) in patients with mild and moderate degrees of stenosis is associated with future vascular events.
Methods:
One hundred and twenty-one patients participated in this study. 76 patients who met the inclusion and exclusion criteria were included in the final dataset of the study. IPN was graded from 0 to 2 according to the extent of the microbubbles assessed using CEUS. The degree of carotid stenosis was graded as mild, moderate, or severe. We recorded future vascular events during the follow-up. Univariate and multivariate logistic regression analyses were used to evaluate risk factors for future vascular events.
Results:
After a follow-up period of 30 ± 6 months, 30 patients (39.5%) experienced subsequent vascular events. Compared with the ‘non-recurrent’ group, the ‘recurrent’ group showed a higher proportion of grade 2 neovascularisation (p < 0.05), and it was an independent predictor of subsequent vascular events (odds ratio 6.066, 95% confidence interval 1.565–23.512, p < 0.05). Furthermore, in patients with mild and moderate stenosis, future vascular events occurred in an unexpectedly high proportion (up to 42.9%). In the ‘recurrent’ group, 55% of patients with mild and moderate stenosis had grade 2 neovascularisation.
Conclusion:
IPN by CEUS was an independent predictor of future vascular events in patients with recent ischemic stroke, and the high proportion of neovascularisation in patients with mild and moderate stenosis requires more attention.
Keywords: carotid intraplaque neovascularisation, carotid stenosis, contrast-enhanced ultrasonography, ischaemic stroke, vascular event recurrence
Introduction
Stroke is a leading cause of disability and mortality among adults worldwide. 1 Approximately 80% of strokes are ischaemic, and 10–25% are caused by arterial atherosclerosis, with vulnerable plaques in the carotid being the frequently involved ones.2,3 Intraplaque neovascularisation is a hallmark of plaque vulnerability, and vulnerable plaque inclines towards haemorrhage and rupture. This often leads to a thrombus and subsequent distal embolism, thus causing ischaemic stroke.2,4,5 Such lesions are diffused and systemic, have a common pathogenesis, and simultaneously affect multiple circulatory regions. Patients with recent ischaemic events have an increased risk of stroke recurrence or a high proportion of cardiac events.6,7
Contrast-enhanced ultrasonography (CEUS) is a non-invasive technique for visualising intraplaque neovascularisation and identifying plaque vulnerability in symptomatic and asymptomatic patients.8,9 The degree of enhancement of intraplaque neovascularisation is related to cardiovascular and cerebrovascular events.10–12 Some studies reported that intraplaque neovascularisation assessed by CEUS correlates significantly with histopathological findings of the same lesions. Moreover, the enhancement of intraplaque neovascularisation, which was assessed using CEUS, was consistent with the density of plaque neovascularisation, which was detected by immunohistochemistry, in patients with severe carotid stenosis undergoing endarterectomy.13–15 However, only few studies have focused on intraplaque neovascularisation in patients with mild and moderate stenosis and future vascular events.
Therefore, in this prospective study, we aimed to investigate the relationship between carotid intraplaque neovascularisation assessed by CEUS and future vascular events (stroke recurrence and cardiovascular events) in patients with mild and moderate stenosis.
Patients and methods
Study population
In total, 121 patients were prospectively recruited at the First Hospital of Jilin University between December 2015 and March 2018. Patients older than 50 years of age with no prior history of coronary disease and a recent ischaemic stroke within the last month with at least one atherosclerotic carotid plaque in the ipsilateral region were included in the study. The exclusion criteria were as follows: (1) <50 years of age; (2) prior cardioembolic or haemodynamic stroke/transient ischaemic attack (TIA) or stroke mimics; (3) the presence of calcified plaques; (4) carotid revascularisation before the CEUS examination; (5) patients with isolated posterior circulation infarcts; (6) severe infections, tumours, hepatorenal failure, or respiratory failure; and (7) patients lost to follow-up.
Imaging protocol
Standard carotid ultrasound study
All standard ultrasonography (US) examinations of both carotid systems were performed using an Aplio500 Ultrasound Machine (Toshiba, Tokyo, Japan) and a linear probe (4–11 MHz) by an experienced examiner (10 years of experience; CY). The common carotid artery, carotid bifurcation and internal carotid arteries were examined in the longitudinal and transverse planes. Subjects were asked to lie supine, with their head rotated approximately 30–40° away from the side being examined. According to the Gray–Weale classification system, plaque echogenicity was classified as follows: type I: uniformly hypoechoic; type II: predominantly hypoechoic; type III: predominantly hyperechoic; type IV: uniformly hyperechoic; and type V: calcified. 16 According to the degree of stenosis as defined by NASCET, carotid stenosis was divided into mild (<50%), moderate (50–69%) or severe (⩾70%). 17
When observing the plaques, the size, location, intraplaque echogenicity and degree of carotid stenosis were recorded. If the patient had more than one plaque, then the thickest plaque was selected as the target plaque.
CEUS of the carotid artery
We generally performed CEUS examination within 1 week after the patient was admitted to our hospital. Carotid intraplaque neovascularisation was assessed by CEUS, with the same machine as that used for the standard carotid US examination. An experienced examiner (10 years of experience; CY) performed the examinations with a linear probe (5–8 MHz), and the gain was suitably adjusted to achieve optimal microbubble visualisation. The mechanical index was maintained at 0.16, preventing destruction of the contrast microbubbles. The contrast agent SonoVue (Bracco, Milan, Italy) and saline (5 mL) were used to prepare the suspension. A 2-ml bolus of contrast agent was injected into the peripheral vein, immediately followed by 5 ml of saline for CEUS analysis. Neovascularisation was identified according to the extent of the microbubbles within the plaque. Digital recordings were made in the longitudinal plane for 10–20 s at baseline and after contrast microbubbles arrived at the carotid arteries and continued for up to 120 s. Native raw data were stored in the scanner’s hard drive, and the cine clips were later assessed offline.
Analysis of carotid intraplaque neovascularisation
The intraplaque contrast enhancement was classified as follows: grade 0: no visible microbubbles within the plaque; grade 1: moderate microbubbles confined to the shoulder and/or adventitial side of the plaque; or grade 2: extensive microbubbles throughout the plaque (Figure 1). 18 Scans were analysed by two experienced examiners in CEUS (XYQ and ZYY: each with 10 years of experience), who were blinded to the clinical information and each other’s results. Inconsistent gradings were discussed, and the final result was determined by both examiners.
Figure 1.
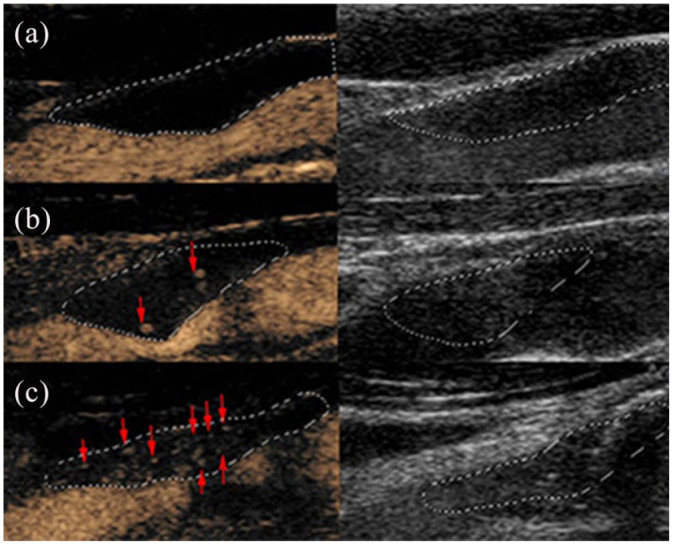
Typical examples of grades of carotid intraplaque neovascularisation based on the contrast-enhanced ultrasonography examination (left: plaque after contrast enhancement; right: plaque before contrast enhancement). (a) Grade 0: no visible microbubbles within the plaque. (b) Grade 1: moderate microbubbles on the shoulder and/or adventitial side of the plaque. (c) Grade 2: extensive microbubbles throughout the plaque.
Clinical data and follow-up
Data of the following clinical parameters were recorded: (1) age and sex; (2) risk factors: family history, diabetes mellitus, hypertension, previous ischaemic stroke, alcohol abuse (⩾120–150 g/day), and smoking; (3) serological indicators: triglyceride, cholesterol, low-density lipoprotein, high-density lipoprotein, homocysteine, high-sensitivity C-reactive protein, vitamin B12 and folic acid levels; (4) whether the patients underwent carotid revascularisation during the follow-up; and (5) medication adherence.
We followed up the clinical course of patients every 6 months. During the follow-up period of 30 ± 6 months, the occurrence of primary outcomes was recorded. The endpoint was recurrent stroke and cardiovascular events that occurred during follow-up. Recurrent ischaemic stroke was defined as a sudden focal neurological deficit lasting more than 24 h, which indicates a new ischaemic event and is confirmed by cranial computed tomography or magnetic resonance imaging. 19 Cardiovascular events included non-fatal myocardial infarction and refractory unstable angina pectoris requiring unplanned coronary revascularisation, heart failure or cardiac death. 20 Patients who took medications ⩾80% of days in the study period showed good medication adherence. 21
Statistical analysis
Statistical analysis was performed using SPSS Statistics (version 26.0; IBM Corp., Armonk, NY, USA). We used the Kolmogorov–Smirnov test (n > 50) or Shapiro–Wilk test (n ⩽ 50) in SPSS Statistics to test for the normality of the metrology data, and data with a p-value of >0.05 were considered normally distributed. For continuous variables, data of normally distributed variables were presented as mean ± standard deviation, while the data of non-normally distributed variables were presented as median and interquartile range. Frequencies and percentages (%) were used to express categorical variables. Student t-test was used to analyse normally distributed variables, and the non-parametric rank sum test was used to analyse non-normally distributed variables. The chi-square test was used to analyse categorical variables. Univariate and multivariate logistic regression analyses were used to analyse the risk factors for future vascular events. We performed univariate analysis of each variable first, and the variables that were significant in univariate analysis (p < 0.05) were then included in multivariate analysis. Inter-observer agreement and intra-observer agreement were assessed using the Cohen kappa coefficient. The level of significance was set at p < 0.05.
Results
Patient outcomes
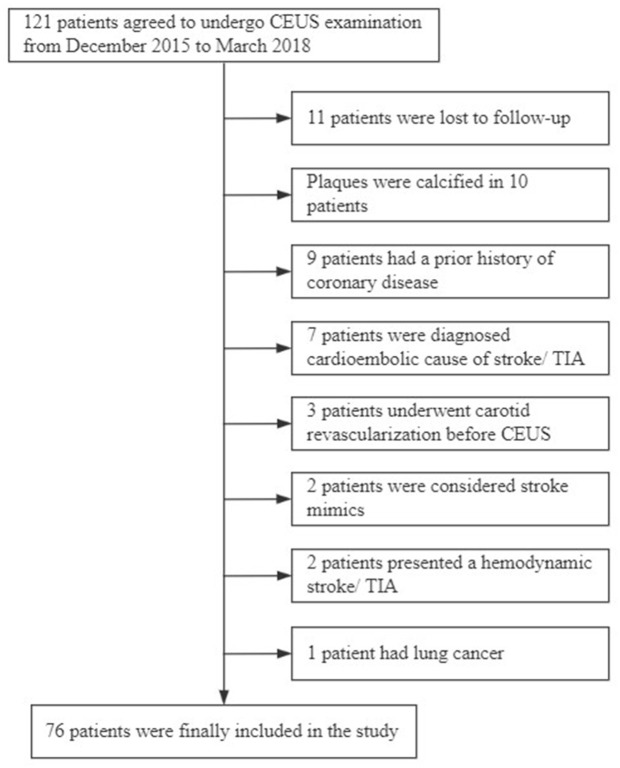
Initially, 121 patients were recruited into the study. However, 45 patients were excluded for various reasons (Figure 2), and 76 patients who met the inclusion and exclusion criteria were eventually included in the study. Their average age was 65 ± 7.9 years, and 67 patients were male (88.2%). After the follow-up period, 30 patients were in the ‘recurrent’ group and 46 were in the ‘non-recurrent’ group. In the ‘recurrent’ group, we observed isolated recurrent stroke in 23 patients (76.7%), isolated cardiovascular events in three patients (10%) and both diseases in four patients (13.3%). In particular, of the 23 patients experiencing a recurrent stroke, 19 had lesions ipsilateral to the carotid plaque with neovascularisation, three had lesions contralateral to the carotid plaque with neovascularisation, and one had both lesions bilaterally. Twenty-eight patients underwent carotid revascularisation, including 21 patients with severe stenosis and seven patients with moderate stenosis.
Figure 2.
Enrolment and analysis of the study participants.
Baseline characteristics
The baseline characteristics of the patients are shown in Table 1. There were no significant clinical differences in terms of age or sex between the groups (p > 0.05). The number of patients with diabetes mellitus in the ‘recurrent’ group was higher than that in the ‘non-recurrent’ group (p = 0.007), but other risk factors were similar between the two groups. Compared with the ‘non-recurrent’ group, the ‘recurrent’ group had higher levels of cholesterol (p = 0.030), low-density lipoprotein (LDL) (p = 0.042) and homocysteine (p = 0.006). In addition, the percentage of carotid revascularisation (p = 0.003) was significantly higher in the ‘non-recurrent’ group than in the ‘recurrent’ group.
Table 1.
Baseline characteristics of the patients (N = 76).
| Total N = 76 |
Non-recurrent n = 46 |
Recurrent n = 30 |
p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 65 (7.9) | 64 (7.2) | 67 (8.7) | 0.157 |
| Sex, men | 67 (88.2) | 42 (91.3) | 25 (83.3) | 0.293 |
| Risk factors | ||||
| Family history | 37 (48.7) | 23 (50.0) | 14 (46.7) | 0.776 |
| Diabetes mellitus | 20 (26.3) | 7 (15.2) | 13 (43.3) | 0.007 |
| Hypertension | 55 (72.4) | 33 (71.7) | 22 (73.3) | 0.879 |
| Previous ischaemicstroke | 41 (53.9) | 24 (52.2) | 17 (56.7) | 0.701 |
| Alcohol abuse | 39 (51.3) | 26 (56.5) | 13 (43.3) | 0.261 |
| Smoking | 49 (64.5) | 28 (60.9) | 21 (70.0) | 0.416 |
| Serological indicators | ||||
| Triglyceride level, mmol/l | 1.42 (1.01–1.79) | 1.28 (0.93–2.06) | 1.61 (1.25–1.75) | 0.079 |
| Cholesterol level, mmol/l | 3.96 (1.03) | 3.76 (1.09) | 4.28 (0.86) | 0.030 |
| LDL level, mmol/l | 2.43 (0.75) | 2.29 (0.79) | 2.65 (0.64) | 0.042 |
| HDL level, mmol/l | 1.06 (0.85–1.20) | 1.08 (0.88–1.21) | 0.98 (0.82–1.18) | 0.392 |
| Homocysteine level, mmol/l | 14.0 (10.90–14.90) | 12.81 (10.33–14.11) | 14.11 (12.78–18.83) | 0.006 |
| hs-CRP level, mg/l | 3.23 (3.14–3.74) | 3.23 (3.14–3.74) | 3.23 (3.14–3.79) | 0.847 |
| Vitamin B12 level, pmol/l | 237.87 (184.25–254.75) | 237.87 (224.0–278.25) | 224.0 (152.50–240.50) | 0.086 |
| Folic acid level, ng/ml | 6.50 (5.35–7.35) | 6.50 (5.34–7.44) | 6.34 (5.25–7.47) | 0.781 |
| Carotid revascularisation | 28 (36.8) | 23 (50.0) | 5 (16.7) | 0.003 |
| Medication adherence | 57 (75.0) | 38 (82.6) | 19 (63.3) | 0.058 |
Numbers are presented as n (%), mean (standard deviation) or median (interquartile range).
HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein.
Bold represents p < 0.05.
Plaque characteristics
Hypoechoic plaques (types I and II) (p = 0.031) and the proportion of diffuse neovascularisation (grade 2) (p = 0.007) were significantly higher in the ‘recurrent’ group than in the ‘non-recurrent’ group. There was no difference in the degree of carotid stenosis between the two groups (p > 0.05) (Table 2).
Table 2.
Plaque characteristics of the patients (n = 76).
| Total N = 76 |
Non-recurrent n = 46 |
Recurrent n = 30 |
p-value | |
|---|---|---|---|---|
| Plaque echogenicity | ||||
| Types I and II | 34 (44.7) | 16 (34.8) | 18 (60.0) | 0.031 |
| Types III and IV | 42 (55.3) | 30 (65.2) | 12 (40.0) | |
| CEUS | ||||
| Grades 0 and 1 | 40 (52.6) | 30 (65.2) | 10 (38.3) | 0.007 |
| Grade 2 | 36 (47.4) | 16 (34.8) | 20 (66.7) | |
| Degree of carotid stenosis | ||||
| Mild and moderate stenosis | 42 (55.3) | 24 (52.2) | 18 (60.0) | 0.502 |
| Severe stenosis | 34 (44.7) | 22 (47.8) | 12 (40.0) | |
Numbers are presented as n (%).
CEUS, contrast-enhanced ultrasonography.
Bold represents p < 0.05.
Table 3 shows the occurrence of future vascular events in patients with different degrees of carotid stenosis. In the ‘severe stenosis’ group, 12 patients (35.3%) had subsequent vascular events, whereas in the ‘mild and moderate stenosis’ groups, 18 patients (42.9%) had primary outcomes, which accounted for an unexpectedly high proportion of patients (p = 0.019). Table 4 shows the relationship between the degree of carotid stenosis and intraplaque neovascularisation (IPN) assessed by CEUS in the ‘recurrent’ group. We found that IPN (grade 2) in patients with severe stenosis accounted for 75%, and it accounted for a considerable percentage (55%) in patients with mild and moderate stenosis.
Table 3.
Future vascular event occurrence in patients with degree of carotid stenosis.
| Degree of carotid stenosis | |||
|---|---|---|---|
| Mild stenosis n = 24 |
Moderate stenosis n = 18 |
Severe stenosis n = 34 |
|
| Non-recurrent | 18 (75.0) | 6 (33.3) | 22 (64.7) |
| Recurrent | 6 (25.0) | 12 (66.7) | 12 (35.3) |
Numbers are presented as n (%).
Table 4.
Relationship between the degree of stenosis and CEUS in the recurrent group.
| CEUS | Degree of carotid stenosis | ||
|---|---|---|---|
| Mild stenosis n = 6 |
Moderate stenosis n = 12 |
Severe stenosis n = 12 |
|
| Grade 0 | 1 (16.7) | 3 (25.0) | 1 (8.3) |
| Grade 1 | 1 (16.7) | 3 (25.0) | 2 (16.7) |
| Grade 2 | 4 (66.6) | 6 (50.0) | 9 (75.0) |
Numbers are presented as n (%).
CEUS, contrast-enhanced ultrasonography.
Risk factors for carotid intraplaque neovascularisation
In terms of the CEUS examinations of 76 patients, 13 patients had no neovascularisation (grade 0), while 63 patients presented with neovascularisation (grades 1 and 2; Table 5). A significantly greater number of men showed the presence of neovascularisation, but age was similar in patients with and without neovascularisation (p > 0.05). There were no statistically significant differences in serological indicators and risk factors for future vascular events between the two groups (p > 0.05). Severe stenosis (p = 0.019) and hypoechoic plaques (types I and II) (p = 0.003) were more frequently detected in the ‘neovascularisation’ group than in the ‘non-neovascularisation’ group. The agreement for intra-observer reliability was 0.791, which corresponds to good reliability. Agreement for two-reader reliability also showed good reliability (0.726).
Table 5.
Relationship between the risk factors and carotid plaque neovascularisation.
| Absence of neovascularisation – grade 0 n = 13 |
Presence of neovascularisation – grades 1 and 2 n = 63 |
p-value | |
|---|---|---|---|
| Demographics | |||
| Age | 65 (8.66) | 65 (7.75) | 0.756 |
| Sex | 8 (61.5) | 59 (93.7) | 0.005 |
| Risk factors | |||
| Family history | 7 (53.8) | 30 (47.6) | 0.683 |
| Diabetes mellitus | 2 (15.4) | 18 (28.6) | 0.524 |
| Hypertension | 10 (76.9) | 45 (71.4) | 0.950 |
| Previous ischaemic stroke | 10 (76.9) | 31 (49.2) | 0.068 |
| Alcohol abuse | 4 (30.8) | 35 (55.6) | 0.104 |
| Smoking | 5 (38.5) | 44 (69.8) | 0.067 |
| Serological indicators | |||
| Triglyceride level, mmol/l | 1.48 (0.89–2.24) | 1.41 (1.04–1.73) | 0.079 |
| Cholesterol level, mmol/l | 4.02 (0.91) | 3.95 (1.06) | 0.839 |
| LDL level, mmol/l | 2.47 (0.79) | 2.43 (0.75) | 0.854 |
| HDL level, mmol/l | 1.17 (0.27) | 1.04 (0.29) | 0.190 |
| Homocysteine level, mmol/l | 14.11 (13.0–18.90) | 13.50 (10.70–14.11) | 0.609 |
| hs-CRP level, mg/l | 3.23 (3.09–4.82) | 3.23 (3.14–3.74) | 0.847 |
| Vitamin B12 level, pmol/l | 237.0 (165.0–239.0) | 237.87 (196.0–230.5) | 0.972 |
| Folic acid level, ng/ml | 6.55 (5.60–9.03) | 6.50 (4.98–7.09) | 0.781 |
| Carotid revascularisation | 3 (23.1) | 28 (44.4) | 0.153 |
| Medication adherence | 4 (30.8) | 53 (84.1) | <0.001 |
| Plaque echogenicity | |||
| Types I and II | 1 (7.7) | 33 (52.4) | 0.003 |
| Types III and IV | 12 (92.3) | 30 (47.6) | |
| Degree of carotid stenosis | |||
| Mild and moderate stenosis | 11 (84.6) | 31 (49.2) | 0.019 |
| Severe stenosis | 2 (15.4) | 32 (50.8) |
Numbers are presented as n (%), mean (standard deviation) or median (interquartile range).
HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein.
Bold represents p < 0.05.
Univariate and multivariate logistic regression analyses
Table 6 shows the results of univariate and multivariate logistic regression analyses of future vascular events. Univariate logistic regression analysis showed that diabetes mellitus, cholesterol, LDL, homocysteine, carotid revascularisation, CEUS (grade 2) and hypoechoic plaque (types I and II) were significant predictors of primary outcomes (p < 0.05). Multivariate logistic regression analysis revealed that CEUS (grade 2) [odds ratio (OR) 6.066, 95% confidence interval (CI) 1.565–23.512], homocysteine (OR 1.203, 95% CI 1.001–1.446) and diabetes mellitus (OR 6.686, 95% CI 1.530–29.221) remained significant independent predictors of future vascular events, and carotid revascularisation (OR 0.218, 95% CI 0.055–0.869) was a protective factor.
Table 6.
Univariate and multivariate logistic regression analyses of future vascular events.
| Univariate analysis | OR | 95% CI | p-value |
|---|---|---|---|
| Diabetes mellitus | 4.261 | 1.445–12.559 | 0.009 |
| Homocysteine | 1.197 | 1.044–1.371 | 0.010 |
| Vitamin B12 | 0.993 | 0.986–1.0 | 0.043 |
| Carotid revascularisation | 0.364 | 0.135–0.983 | 0.046 |
| Medication adherence | 0.269 | 0.091–0.798 | 0.018 |
| CEUS (grade 2) | 3.750 | 1.419–9.910 | 0.008 |
| Plaque echogenicity (types I and II) | 2.812 | 1.088–7.269 | 0.033 |
| Multivariable analysis | |||
| Diabetes mellitus | 6.686 | 1.530–29.221 | 0.012 |
| Homocysteine | 1.203 | 1.001–1.446 | 0.049 |
| CEUS (grade 2) | 6.066 | 1.565–23.512 | 0.009 |
| Carotid revascularisation | 0.218 | 0.055–0.869 | 0.031 |
CEUS, contrast-enhanced ultrasonography; CI, confidence interval; LDL, low-density lipoprotein; OR, odds ratio.
Discussion
In our study, we focused on atherosclerosis as a systemic disease and demonstrated that carotid intraplaque neovascularisation assessed by CEUS in patients with recent ischaemic events was an independent predictor of future vascular events. We found that the proportion of patients with future vascular events was high in the mild and moderate stenosis group (up to 42.9%); of those, IPN assessed by CEUS accounted for a considerable percentage.
Given that the degree of carotid stenosis is the main factor for determining whether carotid revascularisation is necessary, in the past few decades, attention has been paid to patients with severe symptomatic stenosis, while patients with mild to moderate stenosis have been ignored. 22 In the study, we observed that the proportion of patients with future vascular events was high in the mild and moderate stenosis group. This may be because in clinical practice, revascularisation, as a therapeutic procedure, affects the occurrence of future vascular events. Patients with severe carotid artery stenosis underwent more carotid revascularisation procedures, whereas patients with mild to moderate stenosis mainly received medical therapy. Furthermore, previous research indicates that not only the degree of stenosis but also the structure and composition of the carotid plaques are associated with vascular events.23,24 The present study demonstrated that plaque vulnerability is predictive of future vascular events and is a critical factor among patients with mild and moderate stenosis. A recent meta-analysis reported that patients with mild stenosis but vulnerable plaques are also at a higher risk of vascular events. 25 Our findings lead to a similar conclusion: patients with mild and moderate stenosis who have future vascular events have a higher grade of IPN (grade 2), which implies that the plaque is more vulnerable.
Vulnerable plaques often show features such as a thin fibrous cap, lipid-rich necrotic core, intraplaque neovascularity, intraplaque haemorrhage and plaque ulceration. B-mode US imaging is a useful tool for assessing the vulnerability of carotid plaques by evaluating plaque echogenicity. A meta-analysis by Gupta et al. 26 showed that patients with predominantly hypoechoic plaques had a higher future ipsilateral ischaemic stroke risk. Using integrated backscatter (IBS) analysis to characterise plaque echogenicity, investigators have shown that patients with acute coronary syndrome have lower IBS values for carotid plaque than those with stable coronary artery disease (CAD). In addition, carotid plaque hypoechogenicity was independently associated with future coronary heart disease (CHD) events in stable CHD patients, 27 which is in line with our own findings, confirmed through univariate analysis. We demonstrated that both hypoechoic plaques (types I and II) and intraplaque neovascularisation (grade 2) were significantly higher in the ‘recurrent’ group than in the ‘non-recurrent’ group. After adjusting for confounding factors, intraplaque neovascularisation (grade 2) was an independent risk factor for future vascular events, while hypoechoic plaques (types I and II) were not. Therefore, it is necessary to use CEUS detected IPN in routine carotid US to prevent future vascular events.
In the present study, we showed the importance of neovascularisation detection for predicting atherosclerosis-related events recurrence, consistent with recent research. Li et al. 11 showed that high-grade contrast enhancement assessed using CEUS is an independent risk factor for ischaemic stroke or recurrent TIA in TIA patients. However, the occurrence of TIA is transient and imaging evidence is not enough to support it to make a definite diagnosis. Furthermore, Camps-Renom et al. 12 demonstrated that when adjusting for the degree of stenosis, neovascularisation measured by CEUS was still statistically significant in predicting stroke recurrence. However, participants with complete carotid occlusion and calcified plaque were included in the study, and intraplaque neovascularisation was not clear. In our study, only patients with recent ischaemic stroke were included, and a relatively larger number of patients were included in our study to explore IPN assessed by CEUS than in previous studies. In addition, we found that IPN (grade 2) was frequently observed in patients with mild and moderate stenosis with subsequent vascular events. Moreover, we found an interesting result: the group with the presence of neovascularisation had better medication adherence than the group with the absence of neovascularisation. This is partly because when patients are at greater risk of future vascular events, they tend to cooperate with treatment. Nevertheless, we only classified the three grades detected by CEUS to show their effect on the primary outcomes. Further investigation may obtain the grades of each plaque by averaging the overall score per patient and obtaining the score as a threshold for atherosclerosis-related event risk stratification. 28
Our study demonstrated the systemic nature of atherosclerosis, which was predicted by detecting carotid intraplaque neovascularisation using CEUS. Ischaemic stroke and CAD share common risk factors and pathological mechanisms. CAD may coexist with atherosclerotic ischaemic stroke in patients. Furthermore, population data have found that CAD is the major cause of death in ischaemic stroke patients in both the short and the long term. 29 A recent meta-analysis 30 showed that in eight studies, the total risk of myocardial infarction was 3% in 47,229 ischaemic stroke patients without any history during follow-up. Previous studies reported that patients with an episode of ischaemic stroke or TIA had a higher risk of CAD, and the prevalence of CAD was particularly associated with both carotid stenosis and carotid plaque. Magnetic resonance angiography or cardiac magnetic resonance vessel wall imaging was performed to assess plaque features.31,32 However, similar studies are rare in the field of US. Published studies have focused on isolated cerebrovascular or cardiovascular diseases. Some studies have suggested that intraplaque neovascularisation is significantly related to ischaemic stroke, that carotid plaque can independently predict significant and complex CAD, and that CEUS-assessed carotid IPN is a clinically useful tool in high-risk cardiac patients.28,33 In the present study, we demonstrated that carotid intraplaque neovascularisation using CEUS can predict not only stroke recurrence but also cardiovascular events, which may represent a systematic burden of atherosclerotic disease.
We found that diabetes mellitus and homocysteine were independent risk factors for future vascular events, and hypertension was not related to future vascular events. Studies have shown that diabetes is an independent risk factor for ischaemic stroke and cardiovascular events and that controlling glycaemia significantly reduces the risk of vascular events. 34 Additionally, a high homocysteine level is a risk factor for ischaemic stroke and recurrent stroke. 22 We found similar findings, which were explained by the following potential mechanisms. Hyperglycaemia and increased plasma homocysteine levels are related to oxidative stress, dysfunction of the nitric oxide synthase system and inflammation. The potentially overall mechanisms may not only accelerate vascular injury but also introduce carotid atherosclerotic plaque formation. Plaque formation causes oxygen deprivation and stimulates neovascularisation. In addition, the presence of diabetes can break the balance between procoagulants and anticoagulants and make the body prone to a procoagulant state, promoting thrombosis. 35 Previous studies have suggested that hypertension is associated with recurrent stroke. Additionally, studies have shown that controlling blood pressure reduces the incidence of cardiovascular events. 36 However, we did not obtain similar results. The main reason for this is that hypertension is specifically related to recurrent strokes caused by small artery diseases. 37 Another possible reason is the small sample size of this study, as only four cardiovascular events occurred in patients with recurrent vascular events.
The current study has some limitations. Only the thickest plaques in the ipsilateral lesion were assessed, which may have introduced potential spectrum bias. In addition, CEUS is a two-dimensional imaging technique that may have overlooked neovascularisation in certain plaques. A semi-quantitative grading system was used to detect neovascularisation using CEUS, which has high subjectivity. However, we performed interobserver consistency analysis to obtain good reliability. The follow-up duration was short and the sample size was small. Therefore, large multicentric studies with longer follow-up times are required to validate the present findings.
Conclusions
In conclusion, our study provides evidence that carotid intraplaque neovascularisation assessed by CEUS is an independent predictor of future vascular events in patients with recent ischaemic events. Moreover, the high proportion of neovascularisation in patients with mild and moderate stenosis should receive more attention.
Acknowledgments
We thank all the staff involved in this study, as well as the patients and their families for their participation and cooperation.
Footnotes
Author contributions: Liuping Cui and Yingqi Xing contributed equally to this work. Conceptualisation: Liuping Cui, Yingqi Xing, and Ying Chen; methodology: Liuping Cui, Yingqi Xing, Yangyang Zhou, Lijuan Wang, Kangding Liu, and Daofu Zhang; formal analysis and investigation: Liuping Cui, Yingqi Xing, Yangyang Zhou, Lijuan Wang, Kangding Liu, and Daofu Zhang; writing – original draft preparation: Liuping Cui and Ying Chen; writing – review and editing: Ying Chen and Yingqi Xing; funding acquisition: Ying Chen and Yingqi Xing; resources: Ying Chen and Yingqi Xing; supervision: Ying Chen.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Grant No. 81971620), the Natural Science Foundation of Jilin Science and Technology Department (Grant No. 20200201522JC), and the Youth Development Fund of The First Hospital of Jilin University (Grant No. JDYY92018011).
Ethics statement: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the First Hospital of Jilin University (No. 2015-285).
Informed consent: Informed consent was obtained from all individual participants included in the study. Patients signed informed consent regarding publication of their data.
ORCID iDs: Kangding Liu  https://orcid.org/0000-0002-7304-347X
https://orcid.org/0000-0002-7304-347X
Ying Chen  https://orcid.org/0000-0001-6396-7815
https://orcid.org/0000-0001-6396-7815
Contributor Information
Liuping Cui, Department of Neurology, The First Hospital of Jilin University, Changchun, China.
Yingqi Xing, Department of Neurology, The First Hospital of Jilin University, Changchun, China; Department of Vascular Ultrasonography, Xuanwu Hospital, Capital Medical University, Centre of Vascular Ultrasonography, Beijing Institute of Brain Disorders, Beijing, China.
Yangyang Zhou, Department of Neurology, The First Hospital of Jilin University, Changchun, China.
Lijuan Wang, Department of Neurology, The First Hospital of Jilin University, Changchun, China.
Kangding Liu, Department of Neurology, The First Hospital of Jilin University, Changchun, China.
Daofu Zhang, Department of Neurology, The First Hospital of Jilin University, Changchun, China.
Ying Chen, Department of Neurology, The First Hospital of Jilin University, Xinmin Street 71, Changchun, 130021, China.
References
- 1. GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 2019; 18: 459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saba L, Saam T, Jäger HR, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol 2019; 18: 559–572. [DOI] [PubMed] [Google Scholar]
- 3. Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part II. Circulation 2003; 108: 1772–1778. [DOI] [PubMed] [Google Scholar]
- 4. Fleiner M, Kummer M, Mirlacher M, et al. Arterial neovascularisation and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation 2004; 110: 2843–2850. [DOI] [PubMed] [Google Scholar]
- 5. Kramer CM, Treiman GS. Vulnerable plaque in carotid arteries without “significant” stenosis: unmasking the hidden links to stroke. J Am Coll Cardiol 2020; 76: 2223–2225. [DOI] [PubMed] [Google Scholar]
- 6. Adams RJ, Chimowitz MI, Alpert JS, et al. Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association. Circulation 2003; 108: 1278–1290. [DOI] [PubMed] [Google Scholar]
- 7. Zhang Q, Wang A, Zhang S, et al. Asymptomatic polyvascular disease and the risks of cardiovascular events and all-cause death. Atherosclerosis 2017; 262: 1–7. [DOI] [PubMed] [Google Scholar]
- 8. Deyama J, Nakamura T, Takishima I, et al. Contrast-enhanced ultrasound imaging of carotid plaque neovascularisation is useful for identifying high-risk patients with coronary artery disease. Circ J 2013; 77: 1499–1507. [DOI] [PubMed] [Google Scholar]
- 9. Giannoni MF, Vicenzini E, Citone M, et al. Contrast carotid ultrasound for the detection of unstable plaques with neoangiogenesis: a pilot study. Eur J Vasc Endovasc Surg 2009; 37: 722–727. [DOI] [PubMed] [Google Scholar]
- 10. Huang R, Abdelmoneim SS, Ball CA, et al. Detection of carotid atherosclerotic plaque neovascularisation using contrast enhanced ultrasound: a systematic review and meta-analysis of diagnostic accuracy studies. J Am Soc Echocardiogr 2016; 29: 491–502. [DOI] [PubMed] [Google Scholar]
- 11. Li Z, Xu X, Ren L, et al. Prospective study about the relationship between CEUS of carotid intraplaque neovascularisation and ischemic stroke in TIA patients. Front Pharmacol 2019; 10: 672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Camps-Renom P, Prats-Sánchez L. Plaque neovascularisation detected with contrast-enhanced ultrasound predicts ischaemic stroke recurrence in patients with carotid atherosclerosis. Eur J Neuro 2020; 27: 809–816. [DOI] [PubMed] [Google Scholar]
- 13. Spanos K, Tzorbatzoglou I, Lazari P, et al. Carotid artery plaque echomorphology and its association with histopathologic characteristics. J Vasc Surg 2018; 68: 1772–1780. [DOI] [PubMed] [Google Scholar]
- 14. Müller HF, Viaccoz A, Kuzmanovic I, et al. Contrast-enhanced ultrasound imaging of carotid plaque neo-vascularization: accuracy of visual analysis. Ultrasound Med Biol 2014; 40: 18–24. [DOI] [PubMed] [Google Scholar]
- 15. Lyu Q, Tian X, Ding Y, et al. Evaluation of carotid plaque rupture and neovascularisation by contrast-enhanced ultrasound imaging: an exploratory study based on histopathology. Transl Stroke Res 2020; 12: 49–56. [DOI] [PubMed] [Google Scholar]
- 16. Gray-Weale AC, Graham JC, Burnett JR, et al. Carotid artery atheroma: comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J Cardiovasc Surg (Torino) 1988; 29: 676–681. [PubMed] [Google Scholar]
- 17. von Reutern GM, Goertler MW, Bornstein NM, et al. Grading carotid stenosis using ultrasonic methods. Stroke 2012; 43: 916–921. [DOI] [PubMed] [Google Scholar]
- 18. Staub D, Partovi S, Schinkel AF, et al. Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard US with intraplaque neovascularisation detected at contrast-enhanced US. Radiology 2011; 258: 618–626. [DOI] [PubMed] [Google Scholar]
- 19. Naylor AR, Ricco JB, de Borst GJ, et al. Editor’s choice – management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018; 55: 3–81. [DOI] [PubMed] [Google Scholar]
- 20. Nakamura J, Nakamura T, Deyama J, et al. Assessment of carotid plaque neovascularisation using quantitative analysis of contrast-enhanced ultrasound imaging is useful for risk stratification in patients with coronary artery disease. Int J Cardiol 2015; 195: 113–119. [DOI] [PubMed] [Google Scholar]
- 21. Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health 2008; 11: 44–47. [DOI] [PubMed] [Google Scholar]
- 22. Liapis CD, Kakisis JD, Kostakis AG. Carotid stenosis: factors affecting symptomatology. Stroke 2001; 32: 2782–2786. [DOI] [PubMed] [Google Scholar]
- 23. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 2160–2236. [DOI] [PubMed] [Google Scholar]
- 24. Brinjikji W, Huston J, III, Rabinstein AA, et al. Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. J Neurosurg 2016; 124: 27–42. [DOI] [PubMed] [Google Scholar]
- 25. Singh N, Marko M. The risk of stroke and TIA in nonstenotic carotid plaques: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2020; 41: 1453–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gupta A, Kesavabhotla K, Baradaran H, et al. Plaque echolucency and stroke risk in asymptomatic carotid stenosis: a systematic review and meta-analysis. Stroke 2015; 46: 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Honda O, Sugiyama S, Kugiyama K, et al. Echolucent carotid plaques predict future coronary events in patients with coronary artery disease. J Am Coll Cardiol 2004; 43: 1177–1184. [DOI] [PubMed] [Google Scholar]
- 28. Mantella LE, Colledanchise KN, Hétu MF, et al. Carotid intraplaque neovascularisation predicts coronary artery disease and cardiovascular events. Eur Heart J Cardiovasc Imaging 2019; 20: 1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dhamoon MS, Sciacca RR, Rundek T, et al. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan study. Neurology 2006; 66: 641–646. [DOI] [PubMed] [Google Scholar]
- 30. Gunnoo T, Hasan N, Khan MS, et al. Quantifying the risk of heart disease following acute ischaemic stroke: a meta-analysis of over 50,000 participants. BMJ Open 2016; 6: e009535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tagawa M, Takeuchi S, Nakamura Y, et al. Asymptomatic coronary artery disease in Japanese patients with the acute ischemic stroke. J Stroke Cerebrovasc Dis 2019; 28: 612–618. [DOI] [PubMed] [Google Scholar]
- 32. Li J, Li D, Yang D, et al. Co-existing cerebrovascular atherosclerosis predicts subsequent vascular event: a multi-contrast cardiovascular magnetic resonance imaging study. J Cardiovasc Magn Reson 2020; 22: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi SW, Kim H. Implication of ultrasound contrast-enhancement of carotid plaques in prevalence of acute coronary syndrome and occurrence of cardiovascular outcomes. J Clin Ultrasound 2018; 46: 461–466. [DOI] [PubMed] [Google Scholar]
- 34. Leong D P, Joseph P G, McKee M, et al. Reducing the global burden of cardiovascular disease, part 2: prevention and treatment of cardiovascular disease. Circ Res 2017; 121: 695–710. [DOI] [PubMed] [Google Scholar]
- 35. Rodriguez-Araujo G, Nakagami H. Pathophysiology of cardiovascular disease in diabetes mellitus. Cardiovasc Endocrinol Metab 2018; 7: 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yusuf S, Lonn E, Pais P, et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016; 374: 2032–2043. [DOI] [PubMed] [Google Scholar]
- 37. Wang Y, Xu J, Zhao X, et al. Association of hypertension with stroke recurrence depends on ischemic stroke subtype. Stroke 2013; 44: 1232–1237. [DOI] [PubMed] [Google Scholar]