Abstract
Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a global concern involves infections in multiple organs. Much of the research up to now has been descriptive on neurological manifestations followed by SARS-CoV-2 infection. Despite considerable efforts on effective SARS-CoV-2 vaccine, novel therapeutic options for COVID-19 comorbidities are warranted. One of the fast ways to introduce possible effective drugs for clinical trials is bioinformatics methods. We have conducted a comprehensive enrichment analysis of genes involved in SARS-CoV-2 and neurological disorders associated with COVID-19. For this purpose, gene sets were extracted from the GeneWeaver database. To find out some significant enriched findings for common genes between SARS-CoV-2 and its neurological disorders, several practical databases were used. Finally, to repurpose an efficient drug, DrugBank databases were used. Overall, we detected 139 common genes concerning SARS-CoV-2 and their neurological disorders. Interestingly, our study predicted around 6 existing drugs (ie, carvedilol, andrographolide, 2-methoxyestradiol, etanercept, polaprezinc, and arsenic trioxide) that can be used for repurposing. We found that polaprezinc (zinc l-carnosine) drug is not investigated in the context of COVID-19 till now and it could be used for the treatment of COVID-19 and its neurological manifestations. To summarize, enrichment and network data get us a coherent picture to predict drug repurposing to speed up clinical trials.
Keywords: Bioinformatics approach, COVID-19, neurological disorders, polaprezinc, neurotropism
Introduction
As of January 28, 2021, the largest pandemic after the Spanish influenza pandemic, the Coronavirus disease 2019 (COVID-19) pandemic caused by the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to more than 102 million confirmed cases and more than 2.19 million deaths. 1 Although patients infected with SARS-CoV-2 usually have respiratory symptoms, a wide range of neurological manifestations have been recognized and reports their severity and persistence are growing.2–8 A wide range of neurological manifestations and disorders, such as headache, dizziness, impaired consciousness, hyposmia, anosmia, hypogeusia, ageusia, ischemic stroke, cerebral vasculitis, meningitis, encephalopathy epilepsy, and other serious neurological complications have been reported in COVID-19 patients.3,9–13 Also, our previous findings suggested 4 possible routes, such as primary olfactory neurons, infected crossed monocytes, angiotensin-converting enzyme 2 (ACE2) receptors on blood-brain barrier endothelial cells, and peripheral nerves for the invasion of SARS-CoV-2 into the central nervous system. 14 This evidence will conduct clinicians, neurologists, neuroscientists, and other researchers to bring up and prove the neuroinvasion properties and neurotropism hypotheses by the SARS-CoV-2.14-17 Therefore, to minimize long-lasting neurological disorders caused by SARS-CoV-2, clinical studies are needed to improve the manifestations. To get this point, a fast way to find out a critical drug that can affect the virus and its manifestation signaling pathways is bioinformatics analyses. Here, we conducted a comprehensive enrichment analysis on biological processes, molecular functions, and cellular components of target organs of SARS-CoV-2, especially nervous tissue. Finally, to identify drug repurposing for common genes involved in SARS-CoV-2 and neurological disorders associated with COVID-19, a computational approach was performed.
Materials and Methods
Gene set selection
All genes used in this study were extracted from the GeneWeaver database (https://www.geneweaver.org/). GeneWeaver is a web-based tool for integrating and analysis of functional genomics data among cross-species. 18 To find out gene sets associated with main intended neurological disorders, the following MESH terms in GeneWeaver were selected: Stroke, Epilepsy, Central Nervous System Infections, Meningitis, Neuralgia, Encephalitis-Viral, Vasculitis-Central Nervous System, and Guillain-Barre Syndrome (Table 1). We considered these gene sets as the main neurological disorders which are related to COVID-19 according to previous studies.10,11,19,20 Afterward, we also selected and extracted gene sets that were directly associated with COVID-19 in GeneWeaver (Table 2). All genes into the COVID-19 gene sets are directly taken from current studies on patients with SARS-CoV-2 infection.21,22 Subsequently, we exported all gene sets into an excel file to identify shared genes between our separated gene sets (Figure 1). All subsequent analyses were performed on shared genes between neurological disorders and SARS-CoV-2 associated genes (Figure 2).
Table 1.
Neurological disorders–associated gene sets extracted from GeneWeaver.
| ID. | Gene count | Title | Description and reported in COVID-19 |
|---|---|---|---|
| GS242018 | 381 | Stroke | A group of pathological conditions characterized by sudden, non-convulsive loss of neurological function due to BRAIN ISCHEMIA or INTRACRANIAL HEMORRHAGES. Stroke is classified by the type of tissue NECROSIS, such as the anatomic location, vasculature involved, cause, age of the affected individual, and hemorrhagic vs. non-hemorrhagic nature. PMID: 32343504, PMID: 32354768, PMID: 32568626, PMID: 32453685, PMID: 32822622, PMID: 32362244, PMID: 33101164. |
| GS237840 | 380 | Epilepsy | A disorder characterized by recurrent episodes of paroxysmal brain dysfunction due to a sudden, disorderly, and excessive neuronal discharge. Epilepsy has been reported as a comorbidity factor in COVID-19 caused by SARS-CoV-2 infection: PMID: 32799337, PMID: 32275288, PMID: 32484990, PMID: 32563170, PMID: 32484418 . |
| GS241032 | 232 | Central Nervous System Infections | Pathogenic infections of the brain, spinal cord, and meninges. Neuroinvasion and neurotropism have been reported in COVID-19 caused by SARS-CoV-2 infection: PMID: 32753756, PMID: 32367205, PMID: 32935108. |
| GS237037 | 87 | Meningitis | Inflammation of the coverings of the brain and/or spinal cord, which consist of the PIA MATER; ARACHNOID; and DURA MATER. Infections (viral, bacterial, and fungal) are the most common causes of this condition. Meningitis has been reported as a comorbidity factor in COVID-19 caused by SARS-CoV-2 infection: PMID: 32251791, PMID: 32926584, PMID: 32926675, PMID: 33072684, PMID: 32843469. |
| GS242985 | 72 | Neuralgia | Intense or aching pain that occurs along the course or distribution of a peripheral or cranial nerve. PMID: 32275288, PMID: 32572380, PMID: 32588367, PMID: 32896463. |
| GS241015 | 50 | Encephalitis, Viral | Inflammation of brain parenchymal tissue as a result of viral infection. Encephalitis has been reported as a comorbidity factor in COVID-19 caused by SARS-CoV-2 infection: PMID: 32251791, PMID: 32283294, PMID: 32611761, PMID: 32636212, PMID: 32661082, PMID: 32387508. |
| GS245651 | 16 | Vasculitis, Central Nervous System | Inflammation of blood vessels within the central nervous system. Primary vasculitis is usually caused by autoimmune or idiopathic factors, while secondary vasculitis is caused by existing disease process. Vasculitis has been reported as a comorbidity factor in COVID-19 caused by SARS-CoV-2 infection: PMID: 32554425, PMID: 33070922, PMID: 32618041, PMID: 32609196. |
| GS235625 | 12 | Guillain-Barre Syndrome | An acute inflammatory autoimmune neuritis caused by T cell-mediated cellular immune response directed toward peripheral myelin. Demyelination occurs in peripheral nerves and nerve roots. The process is often preceded by a viral or bacterial infection, surgery, immunization, lymphoma, or exposure to toxins. Common clinical manifestations include progressive weakness, loss of sensation, and loss of deep tendon reflexes. Weakness of respiratory muscles and autonomic dysfunction may occur. PMID: 32312628, PMID: 32302082, PMID: 32350026, PMID: 32540883, PMID: 32840686, PMID: 32399950. |
Table 2.
SARS-CoV-2 associated gene sets extracted from GeneWeaver.
| ID. | Gene count | Title | Description and reported in COVID-19 |
|---|---|---|---|
| GS380581 | 35 | Upregulated genes in host transcriptional response to SARS-CoV-2 in Human adenocarcinomic alveolar basal epithelial (A549) cells | This gene set describes genes that are upregulated by the host transcriptional response to SARS-CoV-2 infection in human adenocarcinomic alveolar basal epithelial (A549) cells. COVID-19 is a disease caused by the SARS-CoV-2 virus. We define upregulated as those genes that show a (log 2 fold change) of ⩾1.5. These data are from the supplementary materials associated with a publication that, as of 5/5/2020, has not yet been peer-reviewed: https://www.biorxiv.org/content/10.1101/2020.03.24.004655v1 |
| GS380583 | 22 | Upregulated genes in host transcriptional response to SARS-CoV-2 in Normal human bronchial epithelial (NHBE) cells | This gene set describes genes that are upregulated by the host transcriptional response to SARS-CoV-2 infection in normal human bronchial epithelial (NHBE) cells. COVID-19 is a disease caused by the SARS-CoV-2 virus. We define upregulated as those genes that show a (log 2 fold change) of ⩾1.5. These data are from the supplementary materials associated with a publication that, as of 5/5/2020, has not yet been peer-reviewed: https://www.biorxiv.org/content/10.1101/2020.03.24.004655v1 |
| GS398287 | 4 | Genes that are overexpressed in severe compared to mild cases of COVID-19 | People with severe cases of COVID-19 express these proteins at significantly higher levels than people with mild cases of COVID-19. Data from Figure 2 of the paper: plasma cytokine levels in patients with COVID-19. PMID: 32217835 |
| GS398321 | 5 | Apoptosis-related pathway in peripheral blood, autophagy (- animal species) signal pathway overexpressed genes from peripheral blood of healthy volunteers and COVID-19 patients | RNA-seq data were analyzed from peripheral blood of 3 healthy volunteers and 2 COVID-19 patients. Apoptosis-related pathway genes were overexpressed in COVID-19 patients vs healthy volunteers. PMID: 32228226 |
| GS398334 | 587 | Upregulated genes in post-mortem lung samples from COVID-19-positive patients | This gene set describes genes that are upregulated in post-mortem lung samples from COVID-19-positive patients relative to biopsied healthy lung tissue from uninfected individuals. COVID-19 is a disease caused by the SARS-CoV-2 virus. We define upregulated as those genes that show a (log 2 fold change) of ⩾2. These data are from the supplementary materials associated with the publication. Note: the following HGNC id is part of this data set but was not recognized HGNC:13378. PMID: 32416070 |
| GS398533 | 14 | Upregulated genes in host transcriptional response to SARS-CoV-2 in normal human bronchial epithelium (NHBE) cells | This gene set describes genes that are upregulated by the host transcriptional response to SARS-CoV-2 infection in normal human bronchial epithelial cells (NHBE). COVID-19 is a disease caused by the SARS-CoV-2 virus. We define upregulated as those genes that show a (log 2 fold change) of ⩾2. These data are from the supplementary materials associated with the publication. PMID: 32416070 |
| GS398534 | 333 | Upregulated genes in host transcriptional response to SARS-CoV-2 in Human lung adenocarcinoma epithelial (Calu3) cells | This gene set describes genes that are upregulated by the host transcriptional response to SARS-CoV-2 infection in human lung adenocarcinoma epithelial cells derived from a pleural effusion (Calu3). COVID-19 is a disease caused by the SARS-CoV-2 virus. We define upregulated as those genes that show a (log 2 fold change) of ⩾2. These data are from the supplementary materials associated with the publication. PMID: 32416070 |
| GS398539 | 102 | Upregulated genes in host transcriptional response to SARS-CoV-2 in Human adenocarcinomic alveolar basal epithelial | This gene set describes genes that are upregulated by the host transcriptional response to SARS-CoV-2 infection in human adenocarcinomic alveolar basal epithelial (A549) cells. COVID-19 is a disease caused by the SARS-CoV-2 virus. We define upregulated as those genes that show a (log 2 fold change) of ⩾2. These data are from the supplementary materials associated with the publication. PMID: 32416070 |
| GS398329 | 119 | Upregulated angiogenesis and inflammation genes in lungs from patients who died from COVID-19 | This gene set describes genes that are upregulated in lungs from patients who died from COVID-19. COVID-19 is a disease caused by the SARS-CoV-2 virus. Note that this expression analysis includes only the angiogenesis-associated and inflammation-associated genes available on NanoString panels. The authors define upregulated as those genes that show an (FDR) of ⩽0.05. These data are from the publication (angiogenesis) and supplementary (inflammation) materials associated with the publication. PMID: 32437596 |
Figure 1.
The computational analysis flowchart for conducting over-representation analysis and repurposing potential therapeutic options for SARS-CoV-2-induced neurological disorders.
Figure 2.
Schematic overview of proposed work. All genes involved in SARS-CoV-2 and neurological disorders associated with COVID-19 were extracted from GeneWeaver. Then, common genes in both categories were isolated and proceeded for further computational analysis. Finally, cellular and molecular basis, and signaling pathways were enriched to repurpose the drug and predict possible mechanisms of neurotropism by SARS-CoV-2.
Genetic network reconstruction
To predict gene-gene interactions, we submitted our target shared genes into the STRING (https://string-db.org/). STRING generally is a free access database that provides functional interactions between proteins/genes as comprehensive networks for different species.23,24 The obtained network was then uploaded into the Cytoscape version 3.7.0 to interpret the physical and functional interactions between the desired genes. Cytoscape is a free and practical software for visualizing, integrating, and analyzing molecular connections and genetic interaction networks. 25 We finally reconstructed our networks according to main topological features, such as degree, closeness centrality, and betweenness centrality. In a network, these parameters determine which genes are more powerful than the other genes. Most connections nodes (degree), shorter path lengths to reach the other nodes (closeness), and the most central position (betweenness) are 3 advantages for desired gene/genes in a network. 26 To classify the involvement of each COVID-19-related genes in each neurological disorders, we submitted our desired shared genes in GraphPad PRISM version 8 and demonstrated them through a heat map.
Gene enrichment analysis and drug repurposing
To evaluate and visualize all related phenotypes for our intended genes, we used an integrative enrichment analysis through multiple sources. Using WebGestalt (WEB-based GEne SeT AnaLysis Toolkit), we conducted an over-representation analysis to identify biological processes, cellular components, and molecular functions (Gene Ontology) for our targeted shared genes. Finally, the significant level of false discovery rate (FDR) < 0.05 was considered. To predict cell type/cell marker-genetic interaction, we submitted our identified genes in cell types—Human Gene Atlas panel through Enrichr. 27 To reveal the phenotype and pathway category, we uploaded the intended genes into the Reactome pathway database. Our analysis was proceeded in a suggested functional database (ie, Drug Bank) through WebGestalt to predict gene-drug interactions and potential therapeutic options for candidate genes. To predict the main significant drugs for our target genes, we selected drug and DrugBank as the functional database and enrichment category, respectively. We considered our analysis as the significance level of FDR < 0.05.
Results
Genetic networks reconstruction and analysis
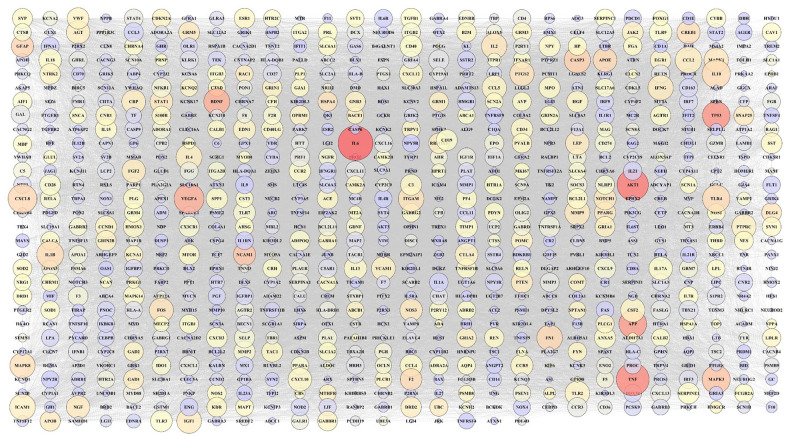
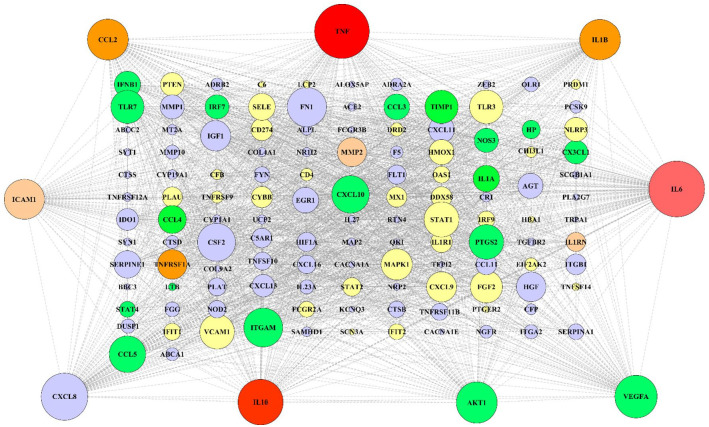
According to extracted data from GeneWeaver, 846 genes from a total of 9 gene sets associated with COVID-19-related neurological disorders were identified (Table 2). Furthermore, 1011 genes associated with SARS-CoV-2 were detected (in total 9 gene sets; Table 1). To determine the most significant genes in SARS-CoV-2, genetic network analysis showed interleukin-6 (IL-6), tumor necrosis factor (TNF), and RAC-alpha serine/threonine-protein kinase (AKT1) as the highest degree and maximum betweenness centrality (Figure 3). However, genes with the highest degree and maximum betweenness centrality, such as IL-6, AKT1, TNF, tumor protein P53 (TP53), and amyloid-beta precursor protein (APP) were detected in neurological disorders associated genes (Figure 4). After obtaining the gene networks separately, a common network containing 139 genes between COVID-19 and its neurological disorders was constructed (Figure 5). Among the shared genes, TNF and interleukin-10 (IL-10) were involved in all predetermined neurological disorders. Besides that, the IL-6 had effective nodes with 7 neurological disorders. Moreover, interleukin-1 beta (IL-1β), chemokine (C-C motif) ligand 2 (CCL2), and tumor necrosis factor receptor superfamily member 1A (TNFRSF1A) were involved in 5 neurological disorders (Figure 6). Furthermore, network analysis using Cytoscape indicated that TNF, IL-6, C-X-C motif chemokine ligand 8 (CXCL8), IL-10, vascular endothelial growth factor A (VEGFA), AKT1, CCL2, IL-1β, and intercellular adhesion molecule 1 (ICAM1) were the most significant nodes in terms of closeness centrality and degree (Figure 7; refer to supplementary Table 1 to see topological features of other genes into the network). Following this step, topological parameters, such as degree, betweenness centrality, and closeness centrality for significant genes were included. Among them, IL-6, degree (D) = 99; betweenness centrality (B) = 0.10586358, closeness centrality (C) = 0.75141243, TNF (D = 96; B = 0.14707984; C = 0.75568182), CXCL8 (D = 80, B = 0.03480363, C = 0.6751269), IL-10 (D = 77; B = 0.0311957; C = 0.665), VEGFA (D = 71; B = 0.02983814; C = 0.64563107), AKT1 (D = 68; B = 0.05121735; C = 0.63636364), CCL2 (D = 68; B = 0.01949683; C = 0.63333333), IL-1β (D = 67; B = 0.02801374; C = 0.63033175), and ICAM1 (D = 66; B = 0.02154069; C = 0.63033175) were detected as the highest topological parameters
Figure 3.
SARS-CoV-2 associated genetic network. The large nodes indicate a higher degree. The color of each node is adjusted based on its betweenness centrality. Red, orange, yellow, and purple nodes represent a spectrum from greater to lesser betweenness centrality (red nodes have greater betweenness centrality). Unconnected nodes have been excluded.
Figure 4.
Neurological disorders genetic network. The large nodes indicate a high degree. The color of each node is adjusted based on its betweenness centrality. Red, orange, yellow, and purple nodes represent a spectrum from greater to lesser betweenness centrality (red nodes have greater betweenness centrality). Unconnected nodes have been excluded.
Figure 5.
Reconstructed genetic network for shared genes between neurological disorders and SARS-CoV-2 associated genes. The current network comprised 139 nodes (genes) and 451 edges (interactions). Dark red nodes involve in all predetermined neurological disorders. The light red, dark orange, light orange, green, and yellow nodes involve 7, 5, 4, 3, and 2 neurological disorders, respectively. Purple nodes involve in one neurological disorder. The size of all nodes adjusted based on their degrees into the network (larger nodes indicate higher degree). Nine peripheral nodes have a higher degree and greater closeness and betweenness centrality in the network.
Figure 6.
Heat map of differential expression of genes. The involvement of each SARS-CoV-2-related gene in each neurological disorder is adjusted with a color map. The columns represent SARS-CoV-2 associated genes, while rows represent the most important reported neurological disorders in COVID-19 patients. The dark red color indicates positive enrichment and light red indicates negative enrichment.
Figure 7.
Highest topological features of 9 shared genes between COVID-19 and its neurological manifestations. Quantitative data represented 3 main topological features including degree, closeness centrality, and betweenness centrality.
Over-representation analysis
In this step, we analyzed biological processes, cell components, molecular functions, and signaling pathways associated with SARS-CoV-2, and its neurological disorders. Our results indicated that cytokine-mediated signaling pathway, FDR = 5.45E-45, cellular response to cytokine (FDR = 2.10E-42) and chemical stimulus (FDR = 5.79E-51), immune (FDR = 1.33E-39) and inflammatory response (FDR = 7.86E-33), and a response to another organism (FDR = 1.35E-40) are remarkable processes that are involved in SARS-CoV-2-induced neurological disorders (Figure 8A). Gene Ontology (GO) enrichment analysis also displayed the most pathological processes. Among all, cell surface (related genes to this process include CX3CL1, CD4, TNFRSF1A, NGFR, PLAT, ICAM1, VCAM1, ITGA2, NOD2, PCSK9, CXCL10, TNFRSF12A, FGG, C5AR1, TGFBR2, CXCL9, CR1, ABCC2, PLAU, ABCA1, CD274, KCNQ3, ITGB1, IL1R1, ACE2, TNF, ITGAM, VEGFA, TNFRSF9), extracellular space (predicted genes to this microenvironment include CX3CL1, TNFRSF1A, HMOX1, TIMP1, MMP2, PLAT, HGF, SERPINE1, CCL2, IL23A, CTSD, CFP, CHI3L1, IL1RN, IL1A, IL-1B, FGF2, ICAM1, PLA2G7, SCGB1A1, MMP10, FLT1, CXCL13, CXCL16, VCAM1, CSF2, TNFRSF11B, CCL11, IGF1, PCSK9, CXCL10, CXCL8, CXCL11, HBA1, SELE, FGG, CTSB, FN1, HP, IL27, CXCL9, AGT, F5, CTSS, PLAU, IFNB1, CD274, IL-6, ACE2, TNF, LTB, IL-10, SERPINA1, ITGAM, TNFSF14, CCL5, CCL3, VEGFA, TNFRSF9, CCL4), membrane raft (related genes to this structure include CD4, LCP2, TNFRSF1A, MAPK1, HMOX1, CTSD, ICAM1, NOS3, OLR1, SELE, FYN, TGFBR2, PTGS2, ABCA1, ITGB1, ACE2, TNF, ITGAM), dendritic growth cone (related genes to this structure include RTN4, MAP2), axon initial segment (related genes to this phenomenon include MAP2, KCNQ3), and glial cell projection (related genes to this process include FYN, ITGB1) were detected as the most important dysfunctional cellular components in COVID-19-induced neurological disorders (Figure 8B).
Figure 8.
Biological process, cellular component, Reactome pathway, and molecular function enrichment analysis of our target genes of SARS-CoV-2 and its neurological disorders. (A) The most significant biological processes that may involve in SARS-CoV-2-induced neurological disorders. (B) The important cellular components which can be interrupted by SARS-CoV-2. (C) The Reactome pathway enrichment analysis of shared genes between SARS-CoV-2 and its neurological manifestations. (D) The molecular function enrichment analysis of our target genes. All parameters were sorted according to the enrichment FDR from GO analysis.
Besides that, most important immunological and neural responses including neutrophil degranulation (FDR = 1.35E-05), collagen degradation (FDR = 2.34E-05), degradation of the extracellular matrix (FDR = 3.31E-05), platelet activation and aggregation (FDR = 2.81E-06), activation of matrix metalloproteinases (FDR = 0.0022), metabolism of angiotensinogen to angiotensins (FDR = 0.0048), axonal growth inhibition (FDR = 0.0198), presynaptic depolarization (FDR = 0.0251), deubiquitination (FDR = 0.0251), and axon guidance (FDR = 0.0298) were detected in COVID19-induced neurological disorders (Figure 8C).
Furthermore, some dominant signaling pathways, such as interferon-alpha/beta signaling (FDR = 9.21E-09), signaling by interleukins (FDR = 2.61E-28), toll-like receptors (FDR = 5.78E-05), tumor necrosis factor receptor 2 (TNFR2) non-canonical nuclear factor kappa B (NF-kB) pathway (FDR = 0.0002), mitogen-activated protein kinase 1 (MAPK1)/mitogen-activated protein kinase 3 (MAPK3; FDR = 0.0044), death receptor (FDR = 0.0231), and tumor necrosis factor receptor 1 (TNFR1)-induced proapoptotic signaling (FDR = 0.0275) were enriched in our target shared genes (Figure 8C). Finally, to target cellular lines, our cell type/specific marker analysis revealed CD14+ monocytes (P = .0003518), CD33+ myeloid (P = .0009167), BDCA4+ dendritic cells (P = .02248), and CD56+ NK cells (P = .01594) as the most affected cell types by SARS-CoV-2.
Drug repurposing
At the final step, gene-drug predicted analysis targeted 6 existing drugs, such as carvedilol (P = 9.6150e-7, FDR = 0.00096261), andrographolide (P = .000092022, FDR = 0.026985), 2-methoxyestradiol (P = .00018122, FDR = 0.037239), etanercept (P = .00022223, FDR = 0.037239), polaprezinc (zinc l-carnosine; P = .00022223, FDR = 0.037239), and arsenic trioxide (P = .00029155, FDR = 0.040699) for SARS-CoV-2 and neurological disorders followed by COVID-19. Among our desired shared genes, carvedilol was repurposed by 7 genes (ie, VEGFA, adrenoceptor alpha 2A [ADRA2A], vascular cell adhesion protein 1 (VCAM1), selectin E (SELE), hypoxia-inducible factor 1-alpha (HIF1A), cytochrome P450 family 1 subfamily A member 1 (CYP1A1), and beta-2 adrenergic receptor (ADRB2)). Based on our results, 3 out of 6 drugs were repurposed by 4 genes. For example, polaprezinc was predicted by TNF, IL-6, fms-related receptor tyrosine kinase 1 (FLT1), and heme oxygenase 1 (HMOX1) genes. Also, the MAPK1, ATP binding cassette subfamily C member 2 (ABCC2), AKT1, and CYP1A1 genes targeted Arsenic trioxide. Four genes including TNF, prostaglandin-endoperoxide synthase 2 (PTGS2), Fc fragment of IgG receptor IIIb (FCGR3B), and low-affinity immunoglobulin gamma Fc region receptor II-a (FCGR2A) represented Etanercept. Andrographolide (eg, TNF, IL-6, and IL-1β genes) and 2-Methoxyestradiol (eg, CYP1A1, HIF1A, and cytochrome P450 family 19 subfamilies A member 1 [CYP19A1] genes) were repurposed by 3 genes (Figure 9A). Among shared genes, TNF and IL-6 genes that are involved in COVID-19 pathogenesis and neurological manifestations pathology were targeted by polaprezinc and andrographolide in our analysis. To find out genes related to polaprezinc, further analyses were performed. Our results showed that several common genes, such as TNF, IL-6, FLT1, and HMOX1, can be targeted by polaprezinc (Figure 9B). To continue, the possible signaling pathways related to the above-mentioned genes followed by polaprezinc were predicted. As shown in Figure 9B, several signaling cascades, such as interleukin-4 (IL-4), interleukin-13 (IL-13), IL-6, VEGF, TNFR2 non-canonical NF-kB pathway, and TNFR1-induced proapoptotic signaling were targeted by polaprezinc in the context of COVID-19 and its neurological manifestations.
Figure 9.
The gene-drug network and the schematic diagram of the possible effect of Polaprezinc on signaling pathways involved in SARS-CoV-2 and its neurological manifestation. (A) The relation between genes and their target drugs has been shown as a network. The size of each drug (orange diamond nodes) was adjusted based on its FDR significance level (biggest nodes indicate smallest FDR levels). (B) Based on drug repurposing and Reactome pathway analysis, 4 target genes, such as Fms related receptor tyrosine kinase 1 (FLT1), tumor necrosis factor (TNF), interleukin-6 (IL-6), and heme oxygenase 1 (HMOX1), as well as their mechanisms, were predicted by Polaprezinc in the context of COVID-19.
Discussion
Our findings showed that 139 genes were shared between SARS-CoV-2 and neurological disorders, which appeared after the first COVID-19 symptoms. In this study, common neurological manifestations of SARS-CoV-2, such as stroke, epilepsy, meningitis, neuralgia, encephalitis, Guillain-Barre Syndrome, vasculitis, and CNS infections were included. To repurpose a drug, sequential computational steps from shared genes between SARS-CoV-2 and neurological to signaling pathways were performed. To our study, high closeness centrality and degree were observed in genes related to cytokines (eg, TNFα, IL-6, IL-10, and IL-1β), chemokines (eg, CXCL8 and CCL2), growth factor (eg, VEGFA), cell-cell interaction (eg, ICAM1), and signal transduction (eg, AKT1). Based on our enrichment analysis, different impairments, such as extracellular matrix degradation, axonal and synaptic dysfunction, and metabolism destruction were seen in SARS-CoV-2 and neurological disorders associated with COVID-19. Prominent signaling pathways behind the above-mentioned tissue impairments were inflammatory signaling pathways (ie, Interferon alpha/beta signaling, signaling by interleukins, toll-like receptor, and TNFR2 non-canonical NF-kB pathway) and intracellular signaling pathways, ie, MAPK1/MAPK3 and protein tyrosine kinase 2 (PTK2). To dig deep insight, transcription and epigenetic factors played role in shared genes were analyzed by miRNA-predicted and TRANSFAC analysis. Concerning these data, 6 miRNAs and 8 transcription factors were predicted. In the main part of our analyses, 7 potential drugs, such as carvedilol, andrographolide, 2-methoxyestradiol, etanercept, polaprezinc, arsenic trioxide, and clenbuterol were repurposed for SARS-CoV-2 and neurological disorders associated with COVID-19. We found that polaprezinc is not investigated in the context of COVID-19 and its neurological manifestations. Therefore, polaprezinc can be a potential candidate for further clinical studies.
Since SARS-CoV-2 was reported in December 2019, neurological manifestations followed by COVID-19 and the neuroinvasive potential of SARS-CoV-2 have been attracting a lot of interest.28-30 Most clinical studies have been only conducted in a cross-sectional design to describe neurological manifestations infected with COVID-19.3,7 Several attempts have been made to explain the neurotropic characteristics of SARS-CoV-2 in post-mortem samples and cerebrospinal fluid analyses.31-33 However, much of the research up to now has been descriptive in nature and SARS-CoV-2-associated neuropathogenesis to identify novel therapeutic targets very little is known. This study seeks to obtain genetic data that are common between SARS-CoV-2 and neurological disorders associated with COVID-19 which will help to address these research gaps.
As shown by previous data in the literature, infected patients with COVID-19 display high levels of pro-inflammatory cytokines (IFNα, IFNγ, IL-1β, IL-6, IL-12, IL-18, IL-33, TNFα, TGFβ), anti-inflammatory cytokines (IL-4 and IL-10), and chemokines (CXCL10, CXCL8, CXCL9, CCL2, CCL3, CCL5).34,35 Our bioinformatics analyses confirmed previous clinical results that the cytokine storm triggers and maintains the abnormal systemic inflammatory response. This phenomenon causes Acute Respiratory Distress Syndrome (ARDS) and multiple organ failure and participates in death in the most severe cases of SARS-CoV-2 infection. 36 These similarities between clinical data and our bioinformatics results encouraged us to continue further analyses on the signaling process and cellular dysfunction in COVID-19 and neurological manifestations.
As we all know, it takes more than 10 years to bring a drug from the trial stages to market availability; therefore, repurpose various approved drugs against COVID-19 to speed up clinical trial are warranted. Recently, several FDA-approved drugs were repurposed by computational studies for COVID-19.37-39 Among all, Atazanavir and Amprenavir can be repurposed for COVID-19 with high inhibitory potency against SARS-CoV-2.40,41 Our study hypothesizes that the prediction of existing drugs based on genetic and epigenetic interactions data between SARS-COV-2 and neurological disorders associated with COVID-19 may be a potential candidate to treat virus neurological comorbidities. Our computational analysis repurposed different drug categories, including the anti-inflammatory agents (eg, etanercept, andrographolide, polaprezinc, and carvedilol) and anti-tumoral effects (eg, arsenic trioxide and 2-methoxyestradiol). Among all, we found that polaprezinc is not used in COVID-19 till now and it could be used for the treatment of COVID-19 and its neurological manifestations.
Among different types of treatment strategies, there are 3 major views on the effects of existing drugs on the clinical course of severe cases of COVID-19. The first and main strategy is to inhibit the development of overwhelming inflammatory responses (ie, inhibition of cytokines storm). The second strategy is to control hypoxemia in patients hospitalized with COVID-19. The third plan is to manage coagulopathy in severe cases of COVID-19. Among predicted existing drugs, polaprezinc has anti-inflammatory, antioxidant effects, and scavenges free radicals (ROS; reactive oxygen species).42-45 Also, this drug reduces the activity of the transcription factor NF-kB and diminishes the expression of several pro-inflammatory cytokines, such as 1L-1β, IL-6, IL-8, and TNFα.45,46 Moreover, several studies have been reported that the beneficial effects of polaprezinc in the course of gastric mucosal injury may be ascribed to its anti-oxidative (ie inhibition of ROS generation) and anti-inflammatory properties (ie suppression of IL-6 and TNF).43,45,47,48
Based on our analysis, several key genes, such as FLT1, TNF, HMOX1, and IL-6 involved in SARS-COV-2 and its neurological manifestations can be targeted by polaprezinc. As stated above, SARS-CoV-2 infection can be associated with cytokine storms, especially in its severe form. The most surprising aspect of our data indicated that polaprezinc can inhibit different inflammatory signaling pathways. Besides that, we found that VEGF, IGF, and MAPK signaling pathways may play important roles in the course of SARS-COV-2 with its neurological manifestations. Furthermore, it has been reported that the HMOX1 pathway can reduce platelet aggregation and can have anti-thrombotic and anti-inflammatory properties. 49 It would be interesting to note potential molecular therapeutics that could modulate the HMOX1 pathway to enhance therapeutic intervention and control the cytokine cascade usually observed in SARS-CoV-2 patients. Data from our computational results indicated that polaprezinc can modulate the expression of HMOX1 gene; therefore, the outcome of COVID-19 patients may be improved by polaprezinc.
Interestingly, our computational results predicted the effect of polaprezinc on these growth factors and intracellular signaling pathways. Therefore, we speculate that polaprezinc may be effective in COVID-19 and its neurological manifestations through different mechanisms. However, it is unfortunate that the study did not include downregulated genes of SARS-CoV-2. Therefore, more information on downregulated genes would help us to establish a greater degree of accuracy on this matter. Furthermore, it should be noted that our results were taken from a computational approach; therefore, to prove the efficacy of polaprezinc in the course of SARS-Cov-2 and its neurological manifestations, clinical trials must be designed.
Conclusions
Most studies provide evidence for the neurotropism and neuroinvasion of SARAS-CoV-2. Furthermore, numerous reports are describing the neurological manifestations of SARS-CoV-2 in patients with COVID-19. At the current stage, due to the lack of efficient cure strategies for COVID-19, state-of-the-art methods to speed up the clinical trials need to be considered. Our findings repurposed polaprezinc drug as an effective drug for the treatment of COVID-19 and neurological manifestations followed by COVID-19 based on bioinformatics data and their mechanism of actions; however, to prove the beneficial effects on human subjects, clinical trials would be conducted.
Supplemental Material
Supplemental material, sj-pdf-1-bbi-10.1177_11779322211026728 for A Computational-Based Drug Repurposing Method Targeting SARS-CoV-2 and its Neurological Manifestations Genes and Signaling Pathways by Ali Sepehrinezhad, Fariborz Rezaeitalab, Ali Shahbazi and Sajad Sahab-Negah in Bioinformatics and Biology Insights
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: AS contributed to the conception of the analysis, designed the tables and graphs, conceived the study, and contributed to the writing of the manuscript. FR contributed to the interpretation of the neurological results. ASH contributed to the analysis. SSN conceived the study, contributed to the interpretation of the results, wrote the manuscript, and supervised the work. All authors provided critical feedback and helped shape the manuscript.
Availability of Data and Materials: All data sets generated/analyzed for this study are included in the manuscript.
ORCID iD: Sajad Sahab-Negah  https://orcid.org/0000-0002-2242-9794
https://orcid.org/0000-0002-2242-9794
Supplemental material: Supplemental material for this article is available online.
References
- 1. Guan W-J, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020;295:715-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatr. 2020;7:875-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kremer S, Lersy F, Anheim M, et al. Neurologic and neuroimaging findings in patients with COVID-19: a retrospective multicenter study. 2020;95:e1868-e1882. [DOI] [PubMed] [Google Scholar]
- 6. Xiong W, Mu J, Guo J, et al. New onset neurologic events in people with COVID-19 in 3 regions in China. Neurology. 2020;95:e1479-e1487. [DOI] [PubMed] [Google Scholar]
- 7. Nepal G, Rehrig JH, Shrestha GS, et al. Neurological manifestations of COVID-19: a systematic review. Critical Care. 2020;24:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corrêa DG, Hygino da Cruz LC, Jr, Lopes FCR, et al. Magnetic resonance imaging features of COVID-19-related cranial nerve lesions. J Neurovirol. 2021;27:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zayet S, Klopfenstein T, Kovẚcs R, Stancescu S, Hagenkötter B. Acute Cerebral Stroke with Multiple Infarctions and COVID-19, France, 2020. Emerg Infect Dis. 2020;26:2258-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fonseca E, Quintana M, Lallana S, et al. Epilepsy in time of COVID-19: a survey-based study. Acta Neurol Scand. 2020;142:545-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahalaxmi I, Kaavya J, Mohana Devi S, Balachandar V. COVID-19 and olfactory dysfunction: a possible associative approach towards neurodegenerative diseases. J Cell Physiol. 2021;236:763-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prasad K, Al Omar SY, Alqahtani SAM, Malik MZ, Kumar V. Brain disease network analysis to elucidate the neurological manifestations of COVID-19. Mol Neurobiol. 2021;58:1875-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sepehrinezhad A, Shahbazi A, Negah SS. COVID-19 virus may have neuroinvasive potential and cause neurological complications: a perspective review. J Neurovirol. 2020;26:324-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bougakov D, Podell K, Goldberg E. Multiple neuroinvasive pathways in COVID-19. Molec Neurobiol. 2020;58:564-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y-C, Bai W-Z, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumari P, Rothan HA, Natekar JP, et al. Neuroinvasion and encephalitis following intranasal inoculation of SARS-CoV-2 in K18-hACE2 Mice. Viruses. 2021;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baker EJ, Jay JJ, Bubier JA, Langston MA, Chesler EJ. GeneWeaver: a web-based system for integrative functional genomics. Nucleic Acids Res. 2011;40:D1067-D1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang BZ, Chu H, Han S, et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020;30:928-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanafi R, Roger PA, Perin B, et al. COVID-19 neurologic complication with CNS vasculitis-like pattern. AJNR. 2020;41:1384-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. SARS-CoV-2 launches a unique transcriptional signature from in vitro, ex vivo, and in vivo systems [published online ahead of print March 24, 2020]. Biorxiv. doi: 10.1101/2020.03.24.004655. [DOI] [Google Scholar]
- 22. Chua RL, Lukassen S, Trump S, et al. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nat Biotechnol. 2020;38:970-979. [DOI] [PubMed] [Google Scholar]
- 23. Szklarczyk D, Morris JH, Cook H, et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2016;45:D362-D368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hillenmeyer S, Davis LK, Gamazon ER, et al. STAMS: STRING-assisted module search for genome wide association studies and application to autism. Bioinformatics. 2016;32:3815-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Y, Goldenberg A, Wong K-C, Zhang Z. A probabilistic approach to explore human miRNA targetome by integrating miRNA-overexpression data and sequence information. Bioinformatics. 2013;30:621-628. [DOI] [PubMed] [Google Scholar]
- 26. Hanneman RA, Riddle M. Centrality and Power. Introduction to social network methods (pp. 60-76). University of California Riverside, 2005. [Google Scholar]
- 27. Hillje R, Pelicci PG, Luzi L. Cerebro: interactive visualization of scRNA-seq data. Bioinformatics. 2019;36:2311-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yashavantha Rao HC, Jayabaskaran C. The emergence of a novel coronavirus (SARS-CoV-2) disease and their neuroinvasive propensity may affect in COVID-19 patients. J Med Virol. 2020;92:786-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu J, Lazartigues E. Expression of ACE2 in human neurons supports the neuro-invasive potential of COVID-19 virus [published online ahead of print July 4, 2020]. Cell Molec Neurobiol. doi: 10.1007/s10571-020-00915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Freni F, Meduri A, Gazia F, et al. Symptomatology in head and neck district in coronavirus disease (COVID-19): a possible neuroinvasive action of SARS-CoV-2. Am J Otolaryngol. 2020;41:102612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mukerji SS, Solomon IH. What can we learn from brain autopsies in COVID-19? Neurosci Lett. 2021;742:135528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee M-H, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with Covid-19. N Engl J Med. 2020;384:481-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang C, Wu Z, Li J-W, Zhao H, Wang G-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;2020:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beck BR, Shin B, Choi Y, Park S, Kang K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput Struct Biotechnol J. 2020;18:784-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ekins S, Mottin M, Ramos PRPS, et al. Déjà vu: stimulating open drug discovery for SARS-CoV-2. Drug Discov Today. 2020;25:928-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arshad U, Pertinez H, Box H, et al. Prioritisation of potential anti-sars-cov-2 drug repurposing opportunities based on ability to achieve adequate target site concentrations derived from their established human pharmacokinetics [published online ahead of print April 22, 2020]. medRxiv. doi: 10.1101/2020.04.16.20068379. [DOI] [Google Scholar]
- 40. Fintelman-Rodrigues N, Sacromento CQ, Lima CR, et al. Atazanavir inhibits SARS-CoV-2 replication and pro-inflammatory cytokine production. Biorxiv. 2020;64:e00825-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mohapatra S, Nath P, Chatterjee M, et al. Repurposing therapeutics for COVID-19: rapid prediction of commercially available drugs through machine learning and docking. PLoS ONE. 2020;15:e0241543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Watari I, Oka S, Tanaka S, et al. Effectiveness of polaprezinc for low-dose aspirin-induced small-bowel mucosal injuries as evaluated by capsule endoscopy: a pilot randomized controlled study. BMC Gastroenterol. 2013;13:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yoshikawa T, Naito Y, Tanigawa T, Yoneta T, Kondo M. The antioxidant properties of a novel zinc-carnosine chelate compound, N-(3-aminopropionyl)-L-histidinato zinc. Biochim Biophys Acta. 1991;1115:15-22. [DOI] [PubMed] [Google Scholar]
- 44. Naito Y, Yoshikawa T. Molecular and cellular mechanisms involved in Helicobacter pylori-induced inflammation and oxidative stress. Free Radical Biol Med. 2002;33:323-336. [DOI] [PubMed] [Google Scholar]
- 45. Omatsu T, Naito Y, Handa O, et al. Reactive oxygen species-quenching and anti-apoptotic effect of polaprezinc on indomethacin-induced small intestinal epithelial cell injury. J Gastroenterol. 2010;45:692-702. [DOI] [PubMed] [Google Scholar]
- 46. Choi HS, Lim J-Y, Chun JH, et al. The effect of polaprezinc on gastric mucosal protection in rats with ethanol-induced gastric mucosal damage: comparison study with rebamipide. Life Sci. 2013;93:69-77. [DOI] [PubMed] [Google Scholar]
- 47. Ueda K, Ueyama T, Oka M, Ito T, Tsuruo Y, Ichinose M. Polaprezinc (Zinc L-Carnosine) is a potent inducer of anti-oxidative stress enzyme, Heme Oxygenase (HO)-1 — a new mechanism of gastric mucosal protection. J Pharmacol Sci. 2009;110:285-294. [DOI] [PubMed] [Google Scholar]
- 48. Naito Y, Yoshikawa T, Yagi N, et al. Effects of polaprezinc on lipid peroxidation, neutrophil accumulation, and TNF-alpha expression in rats with aspirin-induced gastric mucosal injury. Dig Dis Sci. 2001;46:845-851. [DOI] [PubMed] [Google Scholar]
- 49. Batra N, De Souza C, Batra J, Raetz AG, Yu AM. The HMOX1 pathway as a promising target for the treatment and prevention of SARS-CoV-2 of 2019 (COVID-19). Int J Molec Sci. 2020;21:6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-bbi-10.1177_11779322211026728 for A Computational-Based Drug Repurposing Method Targeting SARS-CoV-2 and its Neurological Manifestations Genes and Signaling Pathways by Ali Sepehrinezhad, Fariborz Rezaeitalab, Ali Shahbazi and Sajad Sahab-Negah in Bioinformatics and Biology Insights