Abstract
Currently, conventional treatments for metastatic RCC (mRCC) include immune-based combination regimens and/or targeted therapies, the latter mainly acting on angiogenesis, a key element of the process of tumor growth and spread. Although these agents proved able to improve patients’ outcomes, drug resistance and disease progression are still experienced by a substantial number of VEGFR-TKIs-treated mRCC patients. Following the inhibition of the VEGF/VEGFRs axis, two strategies have emerged: either specifically targeting resistance pathways, at the same time continuing to inhibit angiogenesis, or using a completely different approach aimed at re-activating the immune system through the use of inhibitors of specific negative immune checkpoints. These two approaches, practically represented by the use of either cabozantinib or nivolumab, seem to remain a rational therapeutic approach also when first-line immune-based combinations are used. The objective of this study is to design a preferential therapeutic pathway for the second-line treatment of mRCC. The procedure applied in this project was a group discussion, based on the Nominal Group Technique (NGT) method in a meeting session, aimed at defining the therapeutic choice for the second-line treatment of mRCC. The NGT process defined the most relevant parameters that, according to the interviewed panelists, clinicians should consider for the selection of the second-line therapy in the context of advanced renal cell carcinoma of mRCC. The algorithm developed for the treatment selection as a result of this process should thus be considered by clinicians as reference for therapy selection.
Plain language summary
The result of this paper was the definition of an algorithm intended to suggest a preferential therapeutic pathway considering both the outputs of the Nominal Group Technique (NGT) process and the actual clinical practice and the experience of selected panelists. During the NGT process and the discussion phase, panelists defined the most important parameters to be included in the algorithm that are important for the treatment definition. Cabozantinib and nivolumab are identified as the most reasonable therapeutic options for patients progressing after first-line treatment and are the medication options included in the algorithm for therapy selection.
Keywords: immune checkpoint inhibitors, nominal group technique, second-line treatment, target metastatic renal cell carcinoma, therapy, tyrosine kinase inhibitors
Introduction
Currently, conventional treatments for metastatic RCC (mRCC) include targeted therapies, mainly acting on vascular endothelial growth factor (VEGF)/VEGR receptors (VEGFR)-driven angiogenesis, 1 with or without immune checkpoint inhibitors (IC). Tyrosine kinase inhibitors (TKIs) were the first agents to be approved with the aim of targeting angiogenesis either through inhibition of the VEGF/VEGFR pathway (i.e. bevacizumab, sunitinib, sorafenib, pazopanib, axitinib and tivozanib), or by acting on the mammalian target of rapamycin (mTOR) anti-apoptotic pathway (i.e. everolimus and temsirolimus).2,3 Although these agents, as a whole, proved able to improve patients’ outcomes [more often progression-free survival (PFS)], drug resistance and disease progression are still experienced by a substantial number of mRCC patients treated with first-line VEGFR TKI.2,4–6
VEGFR TKIs showed significantly longer time to treatment discontinuation (i.e. time from target treatment initiation to discontinuation for any reason) compared with mTOR as second-line therapy, post a first VEGFR TKI. 7
Preclinical studies indicated that the activation of the MET and AXL receptor tyrosine kinases is often implicated in the development of resistance to VEGF/VEGFR pathway inhibition; more importantly, the capability of cabozantinib to overcome this therapeutic resistance8,9 through the combined inhibition of the VEGF/VEGFR pathway, together with MET and AXL,8,10 has been hypothesized and then confirmed within several clinical trials. 11
In particular, in the phase III METEOR trial, it has been demonstrated that cabozantinib improves PFS, objective response rate (ORR), and overall survival (OS) as compared with everolimus, in mRCC patients who had experienced disease progression following treatment with a VEGFR TKI.12,13 A PFS advantage was also observed, within a network meta-analysis, when cabozantinib was compared with other conventional treatments. 14
The immune checkpoint inhibitor nivolumab was also shown to be effective as a second-line approach (post VEGFR TKI) for mRCC. 2 Indeed, after progression on a first-line VEGFR TKI, nivolumab showed improved OS and ORR compared with everolimus in the CheckMate 025 trial, and currently competes with cabozantinib as a standard second-line treatment in the post-VEGFR TKIs setting. 15
Especially in the second-line setting, the safety profile of either cabozantinib or nivolumab is a key driver for treatment choice; as a whole, available data from METEOR and CheckMate 025 demonstrated that nivolumab has a more tolerable safety profile, as compared with everolimus, while cabozantinib adverse events can be managed with dose modifications, treatment stops, and supportive care, without losing efficacy. Finally, it should be mentioned that in second-line treatment, the CheckMate 025 shows that nivolumab also gives an advantage in quality of life. In the METEOR study, however, there are no data showing a quality of life advantage with cabozantinib.11,12,15
Considering the huge issue represented by the development of resistance to first-line treatments, patient selection is key to find the ideal, if any, second-line therapy for mRCC. It is thus fundamental to critically analyze all available data in order to identify, and possibly solve, challenges for regimen selection.
The objective of this study is to design a preferential therapeutic pathway for the second-line treatment of mRCC based on three key elements: the patient, the disease, and the treatment. The development of this therapeutic pathway for mRCC was defined by using the Nominal Group Technique (NGT) method that allowed clinical experts in the kidney cancer field (through a four-step process) to prioritize solutions or recommendations on second-line mRCC treatment through clinicians’ consensus.16,17 The final step to reach the objective will be the definition of an algorithm intended to suggest a preferential therapeutic pathway considering both the outputs of the NGT process, as well as the actual clinical practice, and the experience of selected panelists.
Results
As specified in the method section, the first part of the meeting was intended to generate a panel of parameters that are the main keys to determining the therapeutic choice for mRCC in the second-line setting. The generated variables were the results of the process in which the most important aspects for the therapy selection were identified from the meeting panelists and recorded in a flip chart by the moderator. A total of 29 parameters were selected by the participants during the NGT process and grouped in specific categories (Table 1). The most relevant categories identified, based on the clinical experience of the participants, are the features of the patients, of the disease and the characteristics of the anticancer agent itself.
Table 1.
Selection and classification (patient, disease and treatment parameters) of selected variables relevant for the second-line therapeutic choice and the relative ranking score and importance.
| Patient-dependent parameters | Ranking score | Parameter importance |
|---|---|---|
| Symptomatology of the disease in the second line | 12 | 1 |
| Comorbidities (especially cardiovascular) and immuno-oncology (I-O) characteristic of the patient | 13 | 2 |
| Prognostic class: IMDC (International Metastatic RCC Database Consortium) risk score | 23 | 3 |
| Compliance and independent management of therapy and patient perception | 24 | 4 |
| Predictable need to use steroids in the short term | 25 | 5 |
| Patient motivation (respect the survival curve) and patient awareness of the therapy | 29 | 6 |
| Disease-dependent parameters | Ranking score | Parameter importance |
| Biological aggressiveness of the tumor e type of progression | 14 | 1 |
| Presence of the sarcomatoid component | 20 | 2 |
| Disease volume | 23 | 3 |
| Symptomatology of the disease in the second line | 23 | 3 |
| Prognostic class: IMDC (International Metastatic RCC Database Consortium) risk score | 29 | 5 |
| Predictable need to use steroids in the short term | 34 | 6 |
| Sites of metastases (in particular, presence of brain and liver metastases) | 35 | 7 |
| Histology | 38 | 8 |
| Treatment-dependent parameters | Ranking score | Parameter importance |
| Selection of the first line between TKI and immunotherapy | 12 | 1 |
| Objective of the second-line treatment | 13 | 2 |
| Onset of drug action | 18 | 3 |
| Duration of response to first-line therapy (TKI) | 19 | 4 |
| Toxicity of first-line treatment | 22 | 5 |
According to the methodology, the final objective is to choose the most important parameters that represent the group’s preferences and recommendation, by eliminating the features that the board does not approve unanimously. Importantly, the parameters’ selection was the result of the discussion step in which meeting panelists commented, if required, the meaning and the relative importance of the identified items. Indeed, during this phase the variables included in Table 1 were individually discussed and several items were unified as one variable.
In particular, the clinical meaning of some parameters had been clarified to better define its importance in therapeutic selection and to guarantee that the items significance was unanimously shared among panelists.
Table 2 shows all the parameters that were selected as relevant for the second-line therapeutic choice and the category to which they belong. This selection was the result of the panelist discussion and the related comments on the consolidation or removal of items are reported below.
Table 2.
The parameters considered relevant for the choice of second-line therapy and the category to which they belong.
| Parameter | ||
|---|---|---|
| Number (#) | Description | Category (Therapy=T – Disease=D – Patient=P) |
| 1 | Duration of response to first-line therapy (TKI) | T |
| 2 | Selection of the first line between TKI and immunotherapy | T |
| 3 | Presence of nephrectomy in the patient | T |
| 4 | Objective of the second-line treatment | T |
| 5 | Onset of drug action | T |
| 6 | Toxicity of first-line treatment | T |
| 7 | Presence of a sarcomatoid component | D |
| 8 | Biological aggressiveness of the tumor | D |
| 9 | Prognostic class: IMDC (International Metastatic RCC Database Consortium) risk score | D, P |
| 10 | Performance status of the patient | P |
| 11 | Comorbidities (especially cardiovascular) | P |
| 12 | Immuno-oncology (I-O) characteristic of the patient | P |
| 13 | Compliance and autonomous management of therapy | P |
| 14 | Patient preference | P |
| 15 | Method of therapy administration (oral versus intravenous) | T |
| 16 | Patient motivation | P |
| 17 | Patient awareness of the therapy | P |
| 18 | Disease volume | D |
| 19 | Mechanism of action of the anticancer agent | T |
| 20 | Site of metastases | D |
| 21 | Presence of brain and liver metastases (bone excluded) | D |
| 22 | Type of progression | D |
| 23 | Symptomatology of the disease in the second line | D, P |
| 24 | Predictable need to use steroids in the short term | D, P |
| 25 | Histology | D |
| 26 | Vascular architecture in the absence of necrosis | D |
| 27 | Ratio of N/L (neutrophils/lymphocyte) | D |
| 28 | Imaging necrosis (CT) | D |
| 29 | MSI (state of instability of the microsatellites) | D |
Parameters consolidation
The parameters that expressed the same clinical concept have been unified following participants’ consensus (Table 2). The awareness and the patient’s emotional motivation to start the therapy are integrated in a single variable that reflects the willingness of the patient to achieve a possible therapeutic benefit and long-term survival. Additionally, patient compliance and autonomous management of treatment are combined with the parameter referring to the method of therapy administration (oral versus intravenous), integrating this concept under the patient’s perception of the therapy. The combination of these variables, shared by all clinicians, is included in the patient-dependent category. Clinicians also confirmed the consolidation of parameters concerning the site of metastases and the presence of brain and liver metastases, as these parameters are important for the treatment selection.
Panelists agreed on the unification of the parameters corresponding to the objective and the onset of drug action of the second-line treatment, and they also confirmed the combination of the comorbidity and the immuno-oncological characteristic (I-O) of the patient.
The type of tumor progression and the biological aggressiveness are also proposed to be consolidated in a unified parameter, keeping both the variable as important for the treatment selection.
In some cases, the consolidation of items did not reach consensus among clinicians during the discussion phase, as in the case of the duration of the response to first-line therapy (TKI) and the type of progression. Clinicians also disagreed to combine the variables related to the presence of a sarcomatoid component with the biological aggressiveness. Among these negative prognostic factors, the biological aggressiveness is specified as worse with respect to the presence of a sarcomatoid component. This evidence makes the therapy selection different for these two variables and for this reason cannot be unified under a single parameter.
Parameter elimination
Notably, the architecture of the vasculature in the absence of necrosis, the ratio between lymphocytes and neutrophils, the presence of necrosis evaluated with computed tomography (CT), and microsatellite instability (MSI) are the parameters that have been eliminated, as these variables do not reach consensus among all participants. Moreover, nephrectomy is not considered a criterion for the selection of the second-line therapy given that this parameter does not allow, for example, the selection of treatment between cabozantinib and immunotherapy.
Another parameter that has been eliminated is the mechanism of action of the drug that results decisive for the choice of the second-line treatment but is already expressed under the selection of first-line therapy variables.
Patient performance is also proposed as a variable to be eliminated since this item can fall within some characteristics that have already been mentioned in the previous parameters.
Finally, after a preliminary discussion regarding the meaning of the disease volume, the meeting panelists unanimously defined this parameter as the need to slow the development and extension of tumor expansion, which results as fundamental for the treatment selection.
Ranking of parameters
After the discussion phase, the individuals voted privately to prioritize the selected parameter and the moderator reported on the flip chart the obtained ranking for each variable. The assignment of relevant ranking allowed the panelists to vote individually for the fundamental parameters that, according from their clinical experience, have the greatest influence on the selection of the second-line treatment for renal cell carcinoma.
The variables that were the most highly rated by the group have been defined within three different categories:
Patient-related parameters: symptoms are the main characteristic referred to the patient for the treatment selection. Additionally, the comorbidities and the risk class (IMDC Risk score) are, respectively, the second and third patient-related parameters considered important for discriminating the best therapies for renal cell carcinoma.
Disease-related parameters: biological aggressiveness of the tumor has been defined as the main feature related to the disease and considered relevant by clinicians for the therapeutic selection. The presence of the sarcomatoid component and the volume of the disease also influence the therapeutic proposal.
Therapy-related parameters: the treatment selected in the first line, the onset of drug action and the main objective of the second-line therapy are the most important parameters to be considered for the disease progression after first-line treatment.
Algorithm definition
The algorithm definition has the objective to define a possible therapeutic pathway that should be considered by clinicians as a reference for the therapy selection in the context of mRCC.
The results obtained during the NGT process allowed us to define the variables that should mainly influence the selection of second-line treatment for renal cell carcinoma. The panelist debate was very useful to argue this selection with clinical and scientific data, also considering the latest treatment updates and the international therapeutic scenario for mRCC. However, the complexity of the selection of second-line treatment, the promptness needed for the treatment of mRCC, and the real scenario of clinical practice in which clinicians actually operate revealed deficiencyin the algorithm definition. In this context, the clinicians revised the variables defined and, taking into account the actual national clinical practice, they finally included some previously selected NGT parameters within the algorithm that, in their experience, were actually considered for the best therapy selection. Additionally, they included new variables identified that were deeply discussed and finally integrated in the algorithm reported below (Figure 1).
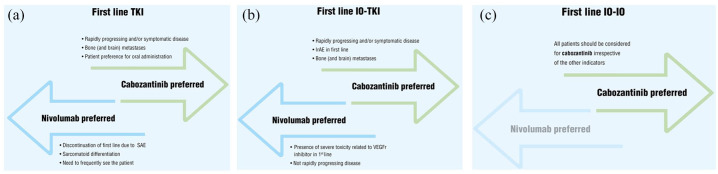
Figure 1.
(a) Therapeutic pathway algorithm which addresses a possible therapeutic pathway for the selection of second-line treatment for renal cell carcinoma. Therapy selection after tyrosine kinase inhibitor (TKI) first-line treatment. (b) Therapeutic pathway algorithm which addresses a possible therapeutic pathway for the selection of second-line treatment for renal cell carcinoma. Therapy selection after combination of TKI and immune checkpoint inhibitor (IO-TKI) in first-line treatment. (c) Therapeutic pathway algorithm which addresses a possible therapeutic pathway for the selection of second-line treatment for renal cell carcinoma. Therapy selection after combination of immune checkpoint inhibitor (IO-IO) in first-line treatment.
The defined algorithm for the second-line treatment selection for mRCC includes the identification of the three case scenarios that correspond to the possible types of first-line treatment that can be experience by mRCC patients and are reported below:
tyrosine kinase inhibitors alone (TKIs)
combination of tyrosine kinase inhibitors and immune checkpoint inhibitor (IO-TKI)
combination of immune checkpoint inhibitor (IO-IO)
Cabozantinib and nivolumab were identified as the most reasonable therapeutic options for patients progressing after first-line treatment and for this reason were included in the algorithm.
Considering the criticism previously discussed, the clinicians emphasized that the algorithm constitutes one possible therapeutic pathway for the second-line treatment, and in particular the pharmacological options proposed are not exclusive, but constitute the preferred choices identified by the experts.
After VEGFR TKIs, cabozantinib is the preferred therapeutic option for situations in which the development of a quick antitumor effect is required, as in the case of subjects characterized by bone (and brain) metastases, or in the context of rapidly progressing and/or symptomatic disease.
On the contrary, nivolumab was the preferred option in case of discontinuation of first-line TKI treatment due to a serious adverse event (SAE), in order to reduce the probability of falling into the same safety complications as per the use of cabozantinib. Furthermore, for the panelists, sarcomatoid differentiation is a feature that needs to be preferentially treated with nivolumab. During the variable revisions, the clinicians also included in the algorithm parameters that are specifically related to the therapy compliance and patient preference. In particular, cabozantinib is indicated for patients with a preference for a drug that can be orally administered, whilst nivolumab is preferred when the disease progression required frequent observation of the patient.
For subjects characterized by bone (and brain) metastases or in the situation of rapidly progressing and/or symptomatic disease, the preferred treatment is cabozantinib also when the first-line therapy is characterized by a combination of an immune checkpoint inhibitor together with a TKI. In this context, this latter treatment is also suggested in case of immune-related adverse event (irAE), whilst in the presence of severe toxicity related to VEGFr inhibitor in first-line therapy the preferred option should be nivolumab. The anticancer agent nivolumab is also the preferred selected therapy in case of not rapidly progressing disease.
In case of a subject treated in first line with a combination of immune checkpoint inhibitor (IO-IO), the only preferred therapeutic solution for all patients is TKI irrespective of the other indicators.
Discussion
The continuous implementation of several first-line therapies, initially with VEGFR-TKIs and then immune-based combinations, has contributed to significantly improve the prognosis of mRCC patients. A large amount of evidence indicates improved outcomes with novel immune-based combinations in the first-line setting of mRCC patients compared with standard-of-care, single-agent therapies, and this evidence has been recently collected in a dedicated retrospective study. Interestingly, during the European Society of Medical Oncology (ESMO 2020), Dr. Choueiri presented the results of the phase III CheckMate-9ER trial that demonstrated the superiority of nivolumab plus cabozantinib with respect to sunitinib in the first-line treatment of mRCC patients and supported this approach as a new therapeutic option for these patients. 18 Although no direct comparison is available between the two types of combinations explored to date (either the combination of two immune checkpoint inhibitors, or of an immune checkpoint inhibitor plus a VEGFR-TKI), it is clear that these novel treatments have changed, for the better, the treatment landscape of mRCC patients, yielding impressive OS results. Defining the best treatment post each of these options remains debatable, given the relative inadequate amount of available prospective data (Table 3).
Table 3.
Trials in second-line therapy.
| POST TKI | |||||
|---|---|---|---|---|---|
| Trial | Comparators | Primary endpoint | Secondary endpoints | Median OS | HR |
| Checkmate 025 (phase III) | Nivolumab (N = = 410) versus Everolimus (N = = 411) | OS | ORR, PFS, OS by PD-L1 expression, incidence of AEs | 25.8 versus 19.7 | 0.73 (95% CI 0.62–0.85) |
| METEOR (phase III) | Cabozantinib (N = = 330) versus Everolimus (N = = 328) | PFS | OS, ORR | 21.4 versus 17.1 | 0.70 (95% CI 0.58–0.85) |
| Keynote 581/CLEAR (study 307) (phase III) | Lenvatinib + Everolimus (N = = 357) or Lenvatinib + Pembrolizumab (N = = 355) versus Sunitinib (N = = 357) | PFS | OS, ORR, safety | NA | Lenvatinib + Everolimus versus Sunitinib 1.15 (95% CI 0.88–1.5); Lenvatinib + Pembrolizumab versus Sunitinib 0.66 (95% CI 0.49–0.88) |
| POST IO combinations | |||||
| McGregor et al. 19 | Retrospective study. | ||||
| Cabozantinib in patients with metastatic clear-cell renal cell carcinoma after immune checkpoint blockade 86 patients. median OS 13 months (OS rate at 12 months) 19 | |||||
| Breakpoint | Open-label phase II - prospective study | ||||
| Breakpoint Meet-uro 03 (phase II) |
Cabozantinib in patients with advanced or metastatic renal cell carcinoma pretreated with a line of treatment with immune-checkpoints (anti-PD1/PDL1) inhibitor 20 | ||||
| There are no phase III prospective trials or recommendations, only retrospective evidence and cohort studies21,22 | |||||
CR, complete response rate; HR, hazard ratio; ORR, overall response rate; OS, overall survival; PFS, progression-free survival.
The objective of this study is to design a preferential therapeutic pathway for the second-line treatment of mRCC, based on the analysis of actual clinical practice and the recommendations through the NGT method. The development of a therapeutic algorithm was the final output of this process and the drafted model should thus be considered by clinicians as reference for the therapy selection.
During the NGT process, expert panelists unanimously defined the main aspects to be considered for the treatment selection that are categorized under the clinical characteristics of the patient, the disease and the treatment.
The NGT process results indicated that the presence of symptoms is the main characteristic referred to the patient for the treatment selection. Additionally, the comorbidities and the risk class (IMDC risk score) are, respectively, the second and third patient-related parameters considered important to discriminate the best therapies for RCC. The parameters of adherence and the autonomous management of therapy showed a higher-ranking value, meaning that the variable is a priority only for some clinicians, therefore having less influence on the choice of the treatment.
Moreover, biological aggressiveness constitutes the main characteristic related to disease features considered by clinicians for the therapeutic proposal, while the ranking values associated with the presence of the sarcomatoid component and the volume of the disease show greater variability during the NGT process, suggesting a lower importance of these features for the panelists in defining treatment selection.
Notably, the treatment selected in the first line, the onset of drug action and the objective of the second-line therapy are the most important parameters to be considered for the disease progression after first-line treatment.
Even though the NGT process allowed the discussion and the selection of the most important variables to be considered for identification of the best second-line therapy, the complexity of the selection of second-line treatment, the promptness needed for the treatment of fast-progressing mRCC and the real scenario of clinical practice in which clinicians actually operate revealed deficiency in the algorithm definition. Indeed, single patient cases often show a specific clinical feature and disease characteristics, resulting in a non-standard picture for which the best therapy selection remains a difficult step even if the physician is supported by formal guidelines. 23 Moreover, the market access of oncological drugs is a dynamic field and new pharmacological options are differentially available across different countries. Due to these limitations, in some cases guidelines can only support clinical decisions, but clinical practical experience is fundamental, and physicians need to perform deep pathology evaluations of the patient in order to select the best therapeutic approach.
By considering these concepts, the algorithm was then drafted as a result of a discussion process in which panelists deeply revised and defined a possible therapeutic pathway that reflects the actual clinical practice applied for the mRCC treatment selection. The objective of this treatment flowchart design is to provide supportive material to kidney cancer physicians for therapy identification, considering that guidelines are the main instruction manual to assist practitioners’ and patients’ decisions. According to the panelists, the type of first-line agent(s) administered, either single-agent VEGFR TKI or an immune-based combo (TKI, IO-IO, IO-TKI), represents one of the most important features that should be considered in the choice of the second-line treatment.
The activity of axitinib was evaluated in a randomized phase III trial called AXIS (NCT00678392). The trial, which compared the efficacy and safety of axitinib and sorafenib as second-line treatment for mRCC, showed a significant advantage of axitinib in terms of PFS (primary endpoint), but no data highlighted improvement in OS.
Therefore, axitinib could be considered as an option for selected patients ineligible to receive immunotherapy with nivolumab or cabozantinib according to the international guidelines and recommendations.
Indeed, the defined algorithm included the identification of the three case scenarios of first-line therapies in which cabozantinib and nivolumab are identified as most reasonable therapeutic options preferred by the panelists.
In particular, cabozantinib is the preferred therapeutic option for situations in which the clinical intervention needs to be quick, that is, in the context of rapidly progressing and/or symptomatic disease. Indeed, the capability of VEGFR TKIs to show early disease control makes this class of agents the preferred second-line option after both VEGFR TKI given either alone or in combination with immune checkpoint inhibitors. Nivolumab is instead the suggested therapy when the pathology progression is slow and controlled and can wait for the long-term benefits of immunotherapy. Of note, the symptomatology of the disease in the second line was one of the most important patient-dependent parameters identified during the NGT process.
Moreover, nivolumab is considered the preferred option in the case of discontinuation of first-line TKI treatment due to SAE, in order to reduce the probability of falling into the same safety complication administering cabozantinib. In the presence of severe toxicity related to VEGFr inhibitor, the administration of nivolumab is the suggested option shared by panelists after first-line treatment characterized by combination of a VEGFR TKI and an immune checkpoint inhibitor. As a whole, adverse events associated with the administration of multitarget TKIs, as in the case of cabozantinib, need to be carefully addressed to avoid excessive and unnecessary treatment interruptions, as well as a negative impact on patient quality of life. Monitoring and proactive supportive measures should help in the management of drug tolerability, and in the context of cabozantinib, dose reductions may be required to reduce drug toxicity. Interestingly, these dose reductions do not appear to impact on the overall positive outcome of this therapy in RCC. 24
Moreover, patients treated in the first line with VEGFR TKIs and immune check point inhibitors and who experienced irAEs should be preferentially treated with cabozantinib, as per algorithm definition, to avoid the development of this severe safety complication in the second line.
For the panelists, sarcomatoid differentiation is an oncological clinical feature that needs to be considered in the therapy selection after the TKI first-line therapy. This parameter, previously identified during the NGT process, has been finally included in the algorithm, directing the preferential therapy selection toward nivolumab. Indeed, it has been demonstrated that RCCs with sarcomatoid differentiation express programmed death 1 (PD-1) and its ligand (PD-L1) at a much higher level than RCCs without sarcomatoid elements. 25 This evidence suggested that acting on the PD-1/PD-L1 axis could be the most indicated therapeutic target and, in this context, nivolumab should be the preferred treatment approach for sarcomatoid RCC.
During the revision of the chosen variables, the clinicians also included in the algorithm parameters that are specifically related to therapy compliance. Indeed, the effect of therapy nonadherence has been demonstrated to be a limitation for patient treatment outcomes, and physicians should consider the patient’s prior treatment adherence behavior. 26 As previously discussed, optimization of doses and possible control of drug-related adverse events (AEs) should be a strategy to ameliorate the compliance to treatment. 24 In this context, patient medication preference can also play a fundamental role connected with therapy compliance. To this purpose, cabozantinib is indicated for patients with a preference for a drug that can be orally administrated, whilst nivolumab is preferred when the disease progression requires frequent observation of the patient. Interestingly, adherence and the autonomous management of therapy is one of the most important parameters defined by the NGT process.
When VEGFR TKIs were used in the first line alone or in combination with an immune checkpoint inhibitor, cabozantinib is the preferred therapeutic option for subjects characterized by bone and brain metastases. The presence of brain metastases constitutes an important clinical feature to be considered, since kidney cancer tends to metastasize to the brain in about 4–11% of cases.27,28 In this context, the panelists showed preference for cabozantinib over nivolumab, in accord with the data from the Nivoren trial that demonstrated the poor activity of nivolumab against untreated brain metastases in patients with cell clear RCC. 29 Moreover, real-world data showed that cabozantinib is safe and shows preliminary antitumor activity on brain metastasis of mRCC patients. 30 Considering this evidence, the panelists expressed their preference in favor of cabozantinib in the case of aggressive and fast-progressing tumors characterized by the development of brain metastasis. Finally, as a whole, in the context of a patient treated first line with an immune-based combination, the use of cabozantinib is considered by the panelists as the most appropriate second-line therapy. In conclusion, the results obtained using the NGT process defined the most relevant parameters that, according to the interviewed panelists, clinicians should consider for the selection of the second-line therapy of mRCC. The algorithm, developed as a result of this process and optimized after a discussion–revision phase, showed the variables that the panelists use in actual clinical practice that lead to a preferred therapy selection between cabozantinib and nivolumab.
Materials and methods
Meeting process to reach consensus
The procedure applied in this project was a group discussion, based on the NGT process in a meeting session, aimed at defining the therapeutic choice for the second-line treatment of mRCC. The processes of the project were structured and handled by a moderator, who first explained the NGT methodology to the participants of the meeting. The members invited to constitute the expert panel were key opinion leaders with extensive experience in the clinical management of the mRCC patients. A medical writer was present during the meeting in order to record the ideas coming from the panelists.
During the meetings, Giovanni Pappagallo was in the moderator’s role while Valentina Guadalupi, Giacomo Cartenì, Roberto Iacovelli, Camillo Porta, Riccardo Ricotta and Giuseppe Procopio, who were invited to contribute to the meeting session, participated as clinical experts, to develop a consensus on the above topic.
Verbal evidence was the instrument utilized as systematic method to reach the objective of the meeting and to define the individual expert opinion, as specified by the NGT method. All participants were involved in the discussions and equally contributed to the scientific and clinical debate to reach the treatment consensus. The ideas coming from the experts were recorded in a flip chart and then structured in a report intended to document the evidence of the meeting. Thanks to this systematic approach, and to reach the aim and objective of the project, the individual clinical experience and the individual perspectives recorded were clear and exhaustive, and this manuscript was agreed as the final output of the whole discussion.
Methodology: nominal group technique (NGT)
NGT is a structured variation of a small-group discussion to reach consensus.16,17 NGT gathers information by asking individuals to respond to questions posed by a moderator, and then asking participants to prioritize the ideas or suggestions of all group members. The process prevents the domination of the discussion by a single person, encourages all group members to participate, and results in a set of prioritized solutions or recommendations that represent the group’s preferences.
First-level experts in the renal cancer field were involved in a NGT-panel through a four-step process. 31
Generating Ideas: The moderator presented the problem to the expert panel in written form and directed everyone to write ideas 32 in brief phrases or statements and to work silently and independently. Each person silently generated ideas and wrote them down.
Recording Ideas: Expert panelists engaged in a round-robin feedback session to concisely record each idea (without debate at this point). The moderator wrote an idea from a group member on a flip chart that is visible to the entire group and proceeded to ask for another idea from the next group member, and so on. There was no need to repeat ideas; however, if group members believed that an idea provided a different emphasis or variation, they were feeling free to include it. The session proceeded until all members’ ideas have been documented.
Discussing Ideas: Each recorded idea was then discussed to determine clarity and importance. For each idea, the moderator asked, “Are there any questions or comments group members would like to make about the item?” This step provided an opportunity for members to express their understanding of the logic and the relative importance of the item. The creator of the idea needed not feel obliged to clarify or explain the item; any member of the group could play that role.
Voting on Ideas: Individuals voted privately to prioritize the ideas. The votes were tallied to identify the ideas that are rated highest by the group as a whole. After members ranked their responses in order of priority, the moderator created a tally sheet on the flip chart with numbers down the left-hand side of the chart, which corresponded to the ideas from the round-robin. The ideas that were the most highly rated by the group were the most favored group actions or ideas in response to the question posed by the moderator.
Conclusions
The results obtained using the NGT process defined the most relevant parameters that, according to the interviewed panelists, clinicians should consider for the selection of the second-line therapy of mRCC. The algorithm, developed as a result of this process and optimized after a discussion–revision phase, showed the variables that panelists use in actual clinical practice that lead to a preferred therapy selection between cabozantinib and nivolumab.
The scenario will be completed by the first-line immunotherapy combinations that are showing interesting results for the increase of OS at least in three combinations (pembrolizumab/axitinib, nivolumab/cabozantinib, pembrolizumab/lenvatinib) and consequently different conditions also for the optimal therapeutic choice in the second-line treatment will be configured.33–35
Finally, therefore, the increasing importance of identifying a predictive biomarker to lead the therapeutic choice in clinical practice should also be noted.
Footnotes
Author contributions: Valentina Guadalupi prepared the manuscript collaboratively with input of Giacomo Cartenì, Roberto Iacovelli, Camillo Porta, Giovanni Pappagallo, Riccardo Ricotta and Giuseppe Procopio. All authors have read and agreed to the published version of the manuscript.
Conflict of interest statement: - Valentina Guadalupi in the last two years has had financial or working relationships with Janssen
- Giacomo Cartenì works as responsible for research and development at Kerubin Digital Therapeutic
- Roberto Iacovelli acted as a Consultant and/or a Speaker for Pfizer, Ipsen, MSD, Sanofi, Janssen
- Camillo Porta acted as a Consultant and/or a Speaker for Ipsen, BMS, MSD, Astra Zeneca, Pfizer, Eisai, EUSA and General Electrics; he acted also as an Expert Testimony for both Pfizer and EUSA and is a protocol steering committee member for BMS, EUSA and Eisai
- Giovanni Pappagallo acted as a Consultant and/or Speaker (training or advisory board or clinical epidemiological evaluations) for Astellas, AstraZeneca, Clovis, Ipsen, Janssen, MSD. Pierre Fabre, Pfizer, Roche, Sanofi, Servier, Teva
- Riccardo Ricotta acted as a Consultant and/or a Speaker for BMS, Ipsen, Janssen, MSD, Pfizer, Sanofi-Genzyme
- Giuseppe Procopio acted as a Consultant and/or a Speaker for Bayer, BMS, Novartis, Amgen, Pfizer, Janssen, Ipsen, Boehringer
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Giuseppe Procopio  https://orcid.org/0000-0002-2498-402X
https://orcid.org/0000-0002-2498-402X
Contributor Information
Valentina Guadalupi, Istituto Nazionale dei Tumori IRCCS Milano, Milano, Lombardia 20133, Italy.
Giacomo Cartenì, Responsible for Research and Development Kerubin Digital Therapeutic, Italy.
Roberto Iacovelli, Fondazione Policlinico Universitario A. Gemelli IRCCS Roma, Lazio, Italy.
Camillo Porta, Chair of Oncology Department of Biomedical Sciences and Human Oncology University of Bari ‘A. Moro’ and Division of Oncology AOU Consorziale Policlinico di Bari Bari, Italy.
Giovanni Pappagallo, Clinical Epidemiologist, Silea (TV), Italy.
Riccardo Ricotta, RCCS MultiMedica Sesto San Giovanni (MI), Sesto San Giovanni, Lombardia, Italy.
Giuseppe Procopio, Istituto Nazionale dei Tumori IRCCS Milano, Milano, Lombardia, Italy.
References
- 1. Ljungberg B, Albiges L, Bensalah K. EAU guidelines on renal cell carcinoma, https://uroweb.org/wp-content/uploads/EAU-RCC-Guidelines-2018-large-text.pdf (2018, accessed 10 June 2021).
- 2. Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019; 30: 706–720. [DOI] [PubMed] [Google Scholar]
- 3. Domblides C, Gross-Goupil M, Quivy A, et al. Emerging antiangiogenics for renal cancer. Expert Opin Emerg Drugs 2013; 18: 495–511. [DOI] [PubMed] [Google Scholar]
- 4. Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 2017; 376: 354–366. [DOI] [PubMed] [Google Scholar]
- 5. Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 2008; 8: 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Griffioen AW, Mans LA, de Graaf AMA, et al. Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients. Clin Cancer Res 2012; 18: 3961–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graham J, Wells JC, McKay R, et al. Clinical outcomes of patients with metastatic renal cell carcinoma (mRCC) treated with vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKI) and mammalian target of rapamycin inhibitors (mTORI) after immuno-oncology (IO) checkpoint inhibitors. Ann Oncol 2018; 29(Suppl. 8): viii303–viii331. [Google Scholar]
- 8. Zhou L, Liu X-D, Sun M, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene 2016; 35: 2687–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tannir NM, Schwab G, Grunwald V. Cabozantinib: an active novel multikinase inhibitor in renal cell carcinoma. Curr Oncol Rep 2017; 19: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011; 10: 2298–2308. [DOI] [PubMed] [Google Scholar]
- 11. Yu SS, Quinn DI, Dorff TB. Clinical use of cabozantinib in the treatment of advanced kidney cancer: efficacy, safety, and patient selection. Onco Targets Ther 2016; 9: 5825–5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016; 17: 917–927. [DOI] [PubMed] [Google Scholar]
- 13. Powles T, Motzer RJ, Escudier B, et al. Outcomes based on prior therapy in the phase 3 METEOR trial of cabozantinib versus everolimus in advanced renal cell carcinoma. Br J Cancer 2018; 119: 663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amzal B, Fu S, Meng J, et al. Cabozantinib versus everolimus, nivolumab, axitinib, sorafenib and best supportive care: a network meta-analysis of progression-free survival and overall survival in second line treatment of advanced renal cell carcinoma. PLoS One 2017; 12: e0184423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Escudier B, Sharma P, McDermott DF, et al. CheckMate 025 randomized phase 3 study: outcomes by key baseline factors and prior therapy for nivolumab versus everolimus in advanced renal cell carcinoma. Eur Urol 2017; 72: 962–971. [DOI] [PubMed] [Google Scholar]
- 16. Delbecq AL, Van de Ven AH, Gustafson DH. Group techniques for program planning: a guide to nominal group and Delphi processes. Glenview, IL: Scott Foresman and Company, 1975. [Google Scholar]
- 17. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995; 311: 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choueiri TK, Powles T, Burotto M, et al. 696O_PR Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: first results from the randomized phase III CheckMate 9ER trial. Ann Oncol 2020; 31(Suppl. 4): S1142–S1215. [Google Scholar]
- 19. McGregor BA, Lalani AA, Xie W, et al. Activity of cabozantinib after immune checkpoint blockade in metastatic clear-cell renal cell carcinoma. Eur J Cancer 2020; 135: 203–210. [DOI] [PubMed] [Google Scholar]
- 20. Procopio G. BREAKPOINT: cabozantinib after first-line ICI in RCC. ASCO GU (American Society of Clinical Oncology Genitourinary) 2021. [Google Scholar]
- 21. Shah AY, Kotecha RR, Lemke EA, et al. Outcomes of patients with metastatic clear-cell renal cell carcinoma treated with second-line VEGFR-TKI after first-line immune checkpoint inhibitors. Eur J Cancer 2019; 114: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Auvray M, Auclin E, Barthelemy P, et al. Second-line targeted therapies after nivolumab-ipilimumab failure in metastatic renal cell carcinoma. Eur J Cancer 2019; 108: 33–40. [DOI] [PubMed] [Google Scholar]
- 23. Bamias A, Escudier B, Sternberg CN, et al. Current clinical practice guidelines for the treatment of renal cell carcinoma: a systematic review and critical evaluation. Oncologist 2017; 22: 667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmidinger M, Danesi R. Management of adverse events associated with cabozantinib therapy in renal cell carcinoma. Oncologist 2018; 23: 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kawakami F, Sircar K, Rodriguez-Canales J, et al. Programmed cell death ligand 1 and tumor-infiltrating lymphocyte status in patients with renal cell carcinoma and sarcomatoid dedifferentiation. Cancer 2017; 123: 4823–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shafrin J, Sullivan J, Chou JW, et al. The effect of medication nonadherence on progression-free survival among patients with renal cell carcinoma. Cancer Manag Res 2017; 9: 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nieder C, Spanne O, Nordøy T, et al. Treatment of brain metastases from renal cell cancer. Urol Oncol 2011; 29: 405–410. [DOI] [PubMed] [Google Scholar]
- 28. Choi WH, Koh YC, Song SW, et al. Extremely delayed brain metastasis from renal cell carcinoma. Brain Tumor Res Treat 2013; 1: 99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lombardi G, Maruzzo M, Minniti G, et al. Immune–checkpoint inhibitors in brain metastases from renal cell carcinoma: a battle was lost but not the war. Ann Transl Med 2019; 7(Suppl. 6): S222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peverelli G, Raimondi A, Ratta R, et al. Cabozantinib in renal cell carcinoma with brain metastases: safety and efficacy in a real-world population. Clin Genitourin Cancer 2019; 17: 291–298. [DOI] [PubMed] [Google Scholar]
- 31. Allen J. Building consensus in health care: a guide to using the nominal group technique. Br J Community Nurs 2004; 9: 110–114. [DOI] [PubMed] [Google Scholar]
- 32. Ideas = patient/disease/treatment characteristics worthy of far-reaching discussion. [Google Scholar]
- 33. ESMO 2020. New first-line treatment option for metastatic kidney cancer, according to results of phase 3 study, https://www.esmo.org/newsroom/press-office/esmo2020-metastatic-kidney-cancer-nivolumab-cabozantinib-checkmate9er (2020, accessed 10 June 2021).
- 34. Exelixis. Study of cabozantinib in combination with nivolumab and ipilimumab in patients with previously untreated advanced or metastatic renal cell carcinoma (COSMIC-313), https://clinicaltrials.gov/ct2/show/NCT03937219 (2021, accessed 10 June 2021).
- 35. National Cancer Institute. Immunotherapy with nivolumab and ipilimumab followed by nivolumab or nivolumab with cabozantinib for patients with advanced kidney cancer, the PDIGREE study, https://clinicaltrials.gov/ct2/show/NCT03793166 (2021, accessed 10 June 2021).