Abstract
Abnormalities that characterize pulmonary arterial hypertension include impairment in the structure and function of pulmonary vascular endothelial and smooth muscle cells. Aldosterone levels are elevated in human pulmonary arterial hypertension and in experimental pulmonary hypertension, while inhibition of the aldosterone-binding mineralocorticoid receptor attenuates pulmonary hypertension in multiple animal models. We explored the role of mineralocorticoid receptor in endothelial and smooth muscle cells in using cell-specific mineralocorticoid receptor knockout mice exposed to sugen/hypoxia-induced pulmonary hypertension. Treatment with the mineralocorticoid receptor inhibitor spironolactone significantly reduced right ventricular systolic pressure. However, this is not reproduced by selective mineralocorticoid receptor deletion in smooth muscle cells or endothelial cells. Similarly, spironolactone attenuated the increase in right ventricular cardiomyocyte area independent of vascular mineralocorticoid receptor with no effect on right ventricular weight or interstitial fibrosis. Right ventricular perivascular fibrosis was significantly decreased by spironolactone and this was reproduced by specific deletion of mineralocorticoid receptor from endothelial cells. Endothelial cell-mineralocorticoid receptor deletion attenuated the sugen/hypoxia-induced increase in the leukocyte-adhesion molecule, E-selectin, and collagen IIIA1 in the right ventricle. Spironolactone also significantly reduced pulmonary arteriolar muscularization, independent of endothelial cell-mineralocorticoid receptor or smooth muscle cell-mineralocorticoid receptor. Finally, the degree of pulmonary perivascular inflammation was attenuated by mineralocorticoid receptor antagonism and was fully reproduced by smooth muscle cell-specific mineralocorticoid receptor deletion. These studies demonstrate that in the sugen/hypoxia pulmonary hypertension model, systemic-mineralocorticoid receptor blockade significantly attenuates the disease and that mineralocorticoid receptor has cell-specific effects, with endothelial cell-mineralocorticoid receptor contributing to right ventricular perivascular fibrosis and smooth muscle cell-mineralocorticoid receptor participating in pulmonary vascular inflammation. As mineralocorticoid receptor antagonists are being investigated to treat pulmonary arterial hypertension, these findings support novel mechanisms and potential mineralocorticoid receptor targets that mediate therapeutic benefits in patients.
Keywords: pulmonary hypertension, experimental, right ventricle, spironolactone, mineralocorticoid receptor, endothelial cell, vascular smooth muscle cell
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by pulmonary arterial remodeling and elevated right ventricular (RV) systolic pressure that culminates in death from RV failure. The pulmonary vasculature undergoes alterations in the structure and function of endothelial cells (ECs),1 smooth muscle cells (SMCs),2 and adventitial fibroblasts.3 In addition, perivascular inflammation has been implicated in the pathogenesis of the disease.4 In parallel with pulmonary vascular abnormalities, the RV undergoes significant changes, characterized by hypertrophy and fibrosis. Clinical outcome ultimately depends on the function of the RV, as most fatalities occur from RV failure.5 Several new therapies have recently been approved, yet mortality is still high and current treatments do not effectively restore the normal pulmonary vascular architecture or normalize RV function. Therefore, elucidating the mechanisms of both pulmonary vascular remodeling as well as RV dysfunction is of crucial importance for finding novel therapies.
The mineralocorticoid receptor (MR) is an intracellular steroid hormone receptor that was initially identified as a critical regulator of systemic blood pressure by mediating the effects of aldosterone on sodium handling in the kidney.6,7 It is now well accepted that MR is also expressed in the vasculature, including in vascular SMCs and ECs where it contributes to cardiovascular disease.8–10 Specifically MR in SMCs promotes cell proliferation and fibrosis, contributing to adverse vascular remodeling in response to hypertension or vascular injury,11–15 while EC-MR contributes to vasoconstriction and vascular inflammation in response to obesity and hyperlipidemia.9,16–18
MR has also been recently implicated in the pathogenesis of PAH.19 Elevated levels of aldosterone have been detected in PAH patients and in experimental animal models of pulmonary hypertension (PH).20,21 This may be due in part to extra-adrenal synthesis of the hormone in the pulmonary vasculature and is thought to participate in pulmonary vascular remodeling via an MR-dependent mechanism.21 Several in vivo preclinical studies have demonstrated that MR inhibition attenuates experimental PH. In three distinct PH animal models, the monocrotaline and sugen/hypoxia models in rats and the hypoxia model in mice, pharmacological MR blockade with spironolactone or eplerenone attenuates PH in both prevention and treatment protocols.21,22 In these studies, MR inhibition decreased pulmonary vessel thickening and RV fibrosis, suggesting that MR contributes to both adverse pulmonary vascular and RV remodeling in experimental PH.21,22 In vitro studies implicate SMC-MR as well as EC-MR as potential regulators of pulmonary vascular remodeling in PAH. In cultured human pulmonary artery SMCs, MR is expressed and translocates to the nucleus when stimulated by aldosterone, hypoxia, or platelet-derived growth factor and induces SMC proliferation. All of these processes are blocked by the MR antagonist, spironolactone.22 In human cultured pulmonary artery ECs, aldosterone enhances oxidant stress and induces post-translational modification of the endothelin-B receptor, resulting in diminished nitric oxide production, processes also ameliorated by treatment with the MR inhibitor spironolactone.21
However, whether the benefits of MR inhibition are mediated by blockade of MR in SMC- or EC-MR to ameliorate vascular or RV remodeling has never been tested in vivo. Therefore, in this study, we first demonstrated that spironolactone treatment is also beneficial in another experimental model of PH induced by the vascular endothelial growth factor receptor 2 blocker Sugen 5416 combined with hypoxia (“sugen/hypoxia” model) and then tested whether this protection may be mediated by MR in SMCs and/or ECs. To test this hypothesis, mice with selective MR deletion in SMCs or with selective MR deletion in ECs and their respective littermate controls were exposed to sugen/hypoxia and the impact of the lack of SMC- or EC-MR on RV pressure, hypertrophy, and fibrosis and on pulmonary vascular remodeling and inflammation was interrogated and compared to mice treated with the systemic MR antagonist spironolactone compared to placebo. For accuracy, we will refer to PAH for human disease and PH for experimental models of the disease, acknowledging the limitations of our experimental animal model in recapitulating human PAH.
Materials and methods
Animals
All mice were handled in accordance with US National Institutes of Health standards and all procedures were approved by our Medical Center Institutional Animal Care and Use Committee. Creation of tamoxifen-inducible SMC-MR knockout (KO) and EC-MR-KO mice and confirmation of inducibility and cell-type specificity of SMC-MR and EC-MR recombination and deletion have been described previously in detail.14,23,24 Briefly, the SMC-MR–/– animals were generated using the smooth-muscle-actin promoter driving Cre recombinase fused to the tamoxifen-sensitive estrogen receptor ligand-binding domain crossed to the floxed MR mouse and smooth muscle-specific deletion of the MR was confirmed in vessels and other smooth muscle containing tissues including bladder and uterus.14 Tamoxifen was given daily for five days at the age of six weeks and studies were initiated at least four weeks after completing tamoxifen. Prior published studies demonstrated recombination of MR in SMC with this regimen.14,24,25 The EC-MR–/– animals were generated using the VE-Cadherin promoter driving Cre recombinase crossed to the floxed MR mouse showing specific recombination of the MR gene in EC-containing tissues including heart, lung, and vessels, and in isolated heart ECs, with no recombination in immune cells.23,26 Ten- to 12-week-old male and female mice were used for all experiments. One group of mice had MR inducibly deleted specifically in SMCs (SMC-MR–/–), the other had MR deleted specifically in ECs (EC-MR–/–), and a third group was treated with spironolactone. Pellets containing sustained-release spironolactone (15 mg·kg−1·day−1) or identical placebo pellets (Innovative Research of America) were implanted a day before initiating exposure to experimental PH in wild-type (WT) mice, as previously described.22 This dose of spironolactone has been previously shown by our group and others to have no effect on systemic blood pressure while modulating systemic vascular function in rodent models of systemic vascular disorders,24,27,28 as well as in experimental PH.22 All mice are on a pure C57Bl6 background and each group had appropriate littermate controls: Specifically, MR floxed, Cre-negative littermates of the SMC-MR+/+ mice treated were similarly treated with tamoxifen and used as controls for tamoxifen-induced SMC-MR–/– mice; MR floxed, Cre-negative littermates (EC-MR+/+) were used for comparisons with EC-MR–/– mice; and WT mice treated with placebo pellets were included as controls for the spironolactone-treated mice (Supplemental Table S1). All control groups (normoxic and sugen/hypoxic, respectively) had similar hemodynamic measurements, proportion of muscularized vessels and inflammation in the lungs, as well as the degree of collagen deposition in the RVs. Therefore, the data from these control groups have been combined into a single normoxic/control group and in a sugen/hypoxic control group, respectively for the figures and statistical analyses. Male and female mice were used and balanced in all groups.
Experimental model of PH: sugen/hypoxia model and hemodynamic measurements
Sex-balanced mice were subjected to sugen/hypoxia PH for four weeks and hemodynamics were measured at the end of the exposure (see Supplement).29,30 All data were collected and analyzed by genotype- and treatment-blinded investigators.
Assessment of RV cardiomyocyte cross-sectional area and collagen deposition
Sections of the free wall of the RV were fixed in 10% neutral buffered formalin, imbedded in paraffin and stained with hematoxylin and eosin for quantification of cardiomyocytes size31 and with picrosirius red for quantification of collagen deposition (Supplemental data); 12–15 regions of photomicrographs covering the whole section were obtained and interrogated for myocytes cut in cross section and exposing the nucleus centrally. Cross-sectional area was measured using an Olympus CH2 microscope with a DP25 camera and DP2-BSW software (Tokyo, Japan). Perivascular and interstitial fibrosis were evaluated separately. For collagen fraction quantification, stained areas and myocyte areas from each section were determined using color-based thresholding.32 The total fibrosis area was calculated as a percentage of total surface area, using image analysis software (Image-Pro Plus 7.0), as the summed stained areas divided by total ventricular area.
Lung histology and pulmonary vascular morphometry
Paraffin-embedded lung sections were stained with Verhoeff-VanGieson (VVG) for elastin followed by morphometric analysis of the vessels by light microscopy (Zeiss, Thornwood, NY). In each animal, 80–100 intra-acinar arteries (20–100 µm diameter) were evaluated at high-power magnification (40×) and categorized as muscular (>75% of the circumference of the vessel), partially muscular (25–75%), or non-muscular (<25%).
Analysis of lung inflammation
VVG-stained lung sections were assessed for the degree of perivascular inflammation. In each animal, 40–60 intra-acinar arteries (20–100 µm diameter) were evaluated for the presence of perivascular accumulation of inflammatory cells at high-power magnification (40×). The percent of vessels with perivascular inflammation that surrounded at least one-third of the vessel was reported for each animal.
Polymerase chain reaction analysis of Nr3c2 genomic DNA
DNA was extracted from hypoxic lung and RV tissues with the DNeasy kit (Qiagen) and polymerase chain reaction (PCR) for recombined MR (454 base pair band) and intact floxed MR (364 base pair band) was performed as previously described,33 using a combination of three primers listed in Supplemental Table S2.
RNA extraction and real-time PCR analysis
Total RNA of RV tissue was isolated by TRI reagent, and real-time PCR was performed as previously described,34 using primers listed in Supplemental Table S2. Real-time PCR was performed with use of Applied Biosystems QuantStudio 3.
Statistical analysis
Data are expressed as mean ± SEM (for baseline characteristics and hemodynamics), percent of total vessels (for lung morphometry and inflammation), or percent of total area analyzed (for RV collagen deposition and RV inflammation). The six normoxic control groups (Table 1) had similar characteristics and were combined in the analysis. Similarly, pulmonary hypertensive control groups (Control/Placebo, SMC-MR+/+/tamoxifen and EC-MR+/+) responded similarly to sugen/hypoxia and thus they were combined for statistical comparison. To confirm the pooled analysis of RV fibrosis, a more detailed comparison was performed, with each experimental group being compared with their respective controls (Supplemental Table S3). Experimental groups were analyzed by one-way analysis of variance and Shapiro–Wilk post hoc test for multiple comparisons, or Kruskel–Wallis one-way analysis of variance on ranks with Dunn’s method for comparisons, if normality test failed. The data in Fig. 4 were analyzed by two-way ANOVA with Tukey HSD post hoc test. Calculations were performed using SigmaStat 3.1 software (Systat Software, CA). A p value of <0.05 was considered statistically significant.
Table 1.
Baseline right ventricular hemodynamic studies under basal normoxic conditions.
| Normoxia | Wild type | SMC-MR–/– | SMC-MR+/+ | EC-MR–/– | EC-MR+/+ |
|---|---|---|---|---|---|
| Body weight (g) | 26.4 ± 0.6 | 25.4 ± 0.8 | 26.3 ± 2.2 | 23.4 ± 1.3 | 27.2 ± 1.8 |
| RVSP (mm Hg) | 26.7 ± 1.2 | 25 ± 1.1 | 24.4 ± 0.8 | 21 ± 1.7 | 23 ± 1.1 |
| RV/weight | 0.77 ± 0.06 | 0.72 ± 0.005 | 0.74 ± 0.07 | 0.66 ± 0.01 | 0.67 ± 0.03 |
| (LV + septum)/body weight | 3.57 ± 0.2 | 3.16 ± 0.09 | 3.32 ± 0.08 | 2.99 ± 0.17 | 3.92 ± 0.1 |
| RV/LV + septum | 0.21 ± 0.005 | 0.22 ± 0.003 | 0.22 ± 0.02 | 0.22 ± 0.01 | 0.22 ± 0.009 |
| CM area (μm2) | 203 ± 8.8 | 233 ± 13 | 254 ± 65 | 202 ± 2.5 | 222 ± 66.5 |
Note: Data are presented as mean ± SEM. There are no significant differences by genotype in any of the parameters reported. N = 5–8/group.
CM: cardiomyocyte; EC-MR–/–: selective MR blockade in endothelial cells (ECs); LV: left ventricle; RV: right ventricle; RVSP: RV systolic pressure; SMC-MR–/–: selective mineralcorticoid receptor (MR) blockade in smooth muscle cells (SMCs).
Fig. 4.
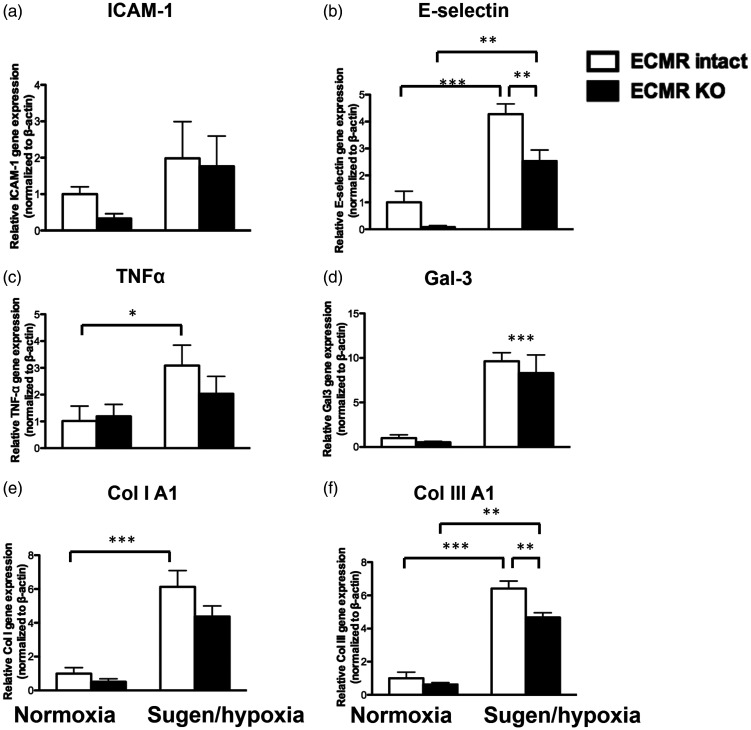
Endothelial cell MR regulates genes involved in fibrosis and inflammation in the RV. mRNA expression was quantified in EC-MR–/– mice (normoxic and sugen/hypoxic—black bars) and their respective controls with intact EC-MR (white bars). Expression of cell adhesion molecules: (a) E-selectin; (b) intercellular adhesion molecule 1 (ICAM1); inflammatory mediators (c) TNFα; and (d) Galectin-3 (Gal-3) and fibrosis genes: (e) collagen IA1; and (f) collagen IIIA1. *p < 0.05; **p < 0.01; ***p < 0.001, N = 4 animals/group.
Results
MR deletion from SMCs or ECs does not alter baseline RV size or RV hemodynamics in young adult mice
Previous reports have demonstrated that both SMC-MR–/– and EC-MR–/– mice display normal development and growth and in young adult life they exhibit essentially normal systemic vascular function and systemic blood pressure.23,35 Systemic pressures, as well as LV characteristics, have been previously published but this has not been assessed in the RV.24,36 In normoxic conditions, we found that selective deletion of MR in SMCs or ECs does not significantly alter the body weight, RV pressure, RV and LV weights, or RV cardiomyocyte size (Table 1). In addition, the degree of muscularized vessels in the lungs as well as collagen deposition in the RVs was very low and similar in all normoxic controls (not shown). Therefore, normoxic control animals’ data have been combined for the respective figures.
Spironolactone treatment attenuates RV pressure elevation independent of SMC- or EC-MR
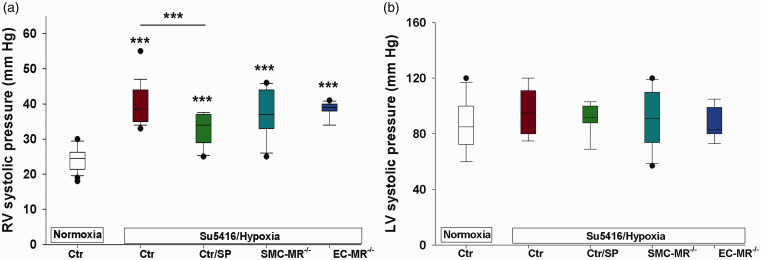
WT mice treated with placebo or spironolactone, SMC-MR–/– or EC-MR–/– mice, and respective littermate MR-intact mice were all exposed to normoxia (combined normoxia Ctr) or to sugen/hypoxia (combined Su5416/hypoxia Ctr) to induce experimental PH. Spironolactone significantly attenuated the sugen/hypoxia-induced rise in RV systolic pressures compared with the combined control group (Fig. 1(a)). This finding is in accord with prior studies using the hypoxia mouse model, the monocrotaline rat model,22 and using eplerenone in the sugen/hypoxia rat model.21 Selective deletion of MR in either SMCs or ECs had no effect on the experimental increase in RV pressure. We detected no changes in systemic pressures in any of the treatment groups as measured by the LV systolic pressure (Fig. 1(b)). The lack of effect on systemic pressures by spironolactone suggests that the dose was low enough to avoid significant systemic antihypertensive effects while still impacting RV pressure. In addition, SMC-MR and EC-MR-KO mice had similar systemic pressures compared with controls, even after exposure to sugen/hypoxia.
Fig. 1.
Spironolactone but not selective vascular MR deletion inhibits right ventricular pressure elevation in experimental PH. Mice with selective mineralocorticoid receptor (MR) deletion in smooth muscle cells (SMCs) or in endothelial cells (ECs) and their respective littermate controls (Ctr) were exposed to sugen (Su5416)/hypoxia for four weeks, or normoxia. A separate group of control mice was treated with spironolactone (SP, 15 mg/kg/day) or placebo for the four-week period. Spironolactone treatment or selective MR deletion did not affect baseline hemodynamics, therefore all normoxic controls are combined. Similarly, controls for the three groups had similar sugen/hypoxia-induced hemodynamics and are combined. (a) Spironolactone but not cell-selective MR deletion significantly attenuated right ventricular (RV) pressure elevation. (b) There were no differences in left ventricular (LV) pressure between groups, suggesting the SP dose used did not have antihypertensive effects. ***p < 0.001 control normoxia vs. hypoxia and control/hypoxia vs. SP/hypoxia. N = 20 (controls); N = 11–12/hypoxic per treatment group.
Treatment with the MR inhibitor spironolactone does not eliminate RV hypertrophy in experimental PH
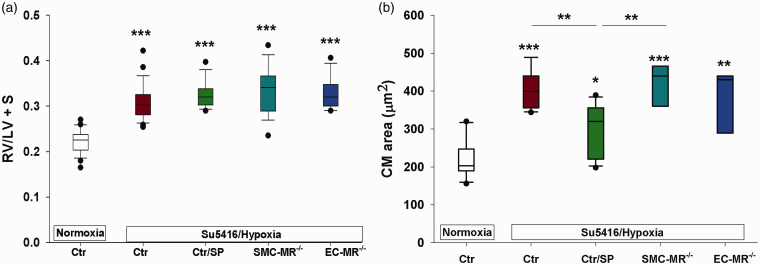
As expected, sugen/hypoxia treatment resulted in significant RV hypertrophy in control animals, as demonstrated by an increase in Fulton index and cardiomyocyte area (Fig. 2). None of the experimental treatments: spironolactone, SMC-MR deletion, or EC-MR deletion completely prevented RV hypertrophy (Fig. 2(a)). These findings are consistent with prior reports using the more selective MR antagonist eplerenone.22 Only the increase in cardiomyocyte area in PH animals was attenuated by spironolactone treatment (Fig. 2(b)), suggesting that spironolactone had a modest blunting effect on the degree of cardiomyocyte hypertrophy. Selective MR deletion from SMCs or ECs did not affect cardiomyocyte or RV size.
Fig. 2.
Spironolactone but not selective MR blockade decreases cardiomyocyte size in experimental PH. Right ventricular hypotrophy as measured by the RV/LV + septum ratio (a) and cardiomyocyte (CM) size (b) were measured in mice described in Fig. 1. RV hypertrophy was consistently elevated in all hypoxic groups. Spironolactone produced a modest, but significant decrease in CM size. *p < 0.5; **p < 0.01; ***p < 0.001. N = 12–24/group for RV hypertrophy and N = 3–4 animals/group for CM size (30–40 cardiomyocytes per animal).
RV: right ventricular; LV: left ventricular; S: septum; SP: spironolactone; SMC: smooth muscle cell; MR: mineralocorticoid receptor; EC: endothelial cell.
EC-MR contributes to RV perivascular fibrosis
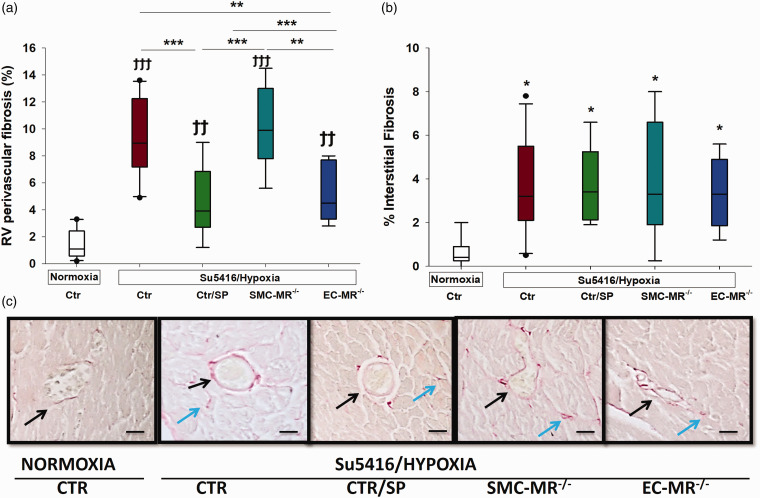
We next assessed RV fibrosis by measuring the amount of collagen deposition in the perivascular area, as well as the interstitium. Perivascular fibrosis was significantly increased in the RV of pulmonary hypertensive mice and this was attenuated by treatment with spironolactone and this was reproduced by selective MR deletion in ECs (Fig. 3(a) and (c)). SMC-MR–/– pulmonary hypertensive animals had a similar degree of perivascular RV fibrosis as control PH animals indicating no role for SMC-MR in the process. RV interstitial fibrosis was also increased in sugen/hypoxia mice and was not affected in any of the treatment groups (Fig. 3(b) and (c)). These data support the concept that RV perivascular fibrosis is mediated by MR in ECs and can be blocked by spironolactone, while RV interstitial fibrosis is independent of the MR in this model. A more detailed analysis of perivascular fibrosis was conducted without combining the control groups. These data confirm that SP treatment significantly decreased perivascular fibrosis compared to placebo-treated mice and that this finding is reproduced in EC-MR–/– mice compared to EC-MR+/+ littermate controls (Supplemental Table 3).
Fig. 3.
Effects of MR on right ventricular fibrosis. RV fibrosis was evaluated separately in the (a) perivascular and (b) interstitial areas. Spironolactone and EC-MR blockade were associated with significantly less perivascular fibrosis, as measured by collagen staining. (c) RV sections stained with mason trichrome for collagen assessment; visualization by light microscopy (40×); dark arrows point toward perivascular collagen staining, while green arrows indicate interstitial collagen staining. Ϯϯp < 0.01; Ϯϯϯp < 0.001, normoxia vs. sugen/hypoxia; *p < 0.05; **p < 0.01; ***p < 0.001. N = 6–8 animals/group.
RV: right ventricular; SP: spironolactone; SMC: smooth muscle cell; MR: mineralocorticoid receptor; EC: endothelial cell.
EC-MR regulates expression of E-selectin and fibrosis genes in the RV
We confirmed Cre-specific recombination of MR in RV tissue from sugen/hypoxic RVs and lungs (Supplemental Figure S1). To investigate the mechanism through which EC-MR may contribute to RV perivascular fibrosis, we measured mRNA expression in RV tissue of EC-MR target genes previously implicated in inflammation and fibrosis markers. Specifically, intercellular adhesion molecule 1 (ICAM1) and E-selectin are EC-MR target genes that contribute to leukocyte recruitment to the vasculature in response to injury or infection.37 Tumor necrosis factor (TNF)α and galectin-3 are inflammatory mediators known to be affected by MR,38 and MR has previously been shown to regulate the expression of inflammatory mediators as an initial step toward fibrosis.39 Sugen/hypoxia induced a significant increase in RV E-selectin expression with no significant change in ICAM-1 expression (Fig. 4(a) and (b)). This was associated with an increase in the inflammatory markers galectin 3 and TNFα (Fig. 4(c) and (d)). Selective deletion of MR in ECs specifically and significantly attenuated the increase in E-selectin and prevented the significant rise in TNFα. We next measured expression of collagen genes implicated in cardiac fibrosis. PH induced an increase in mRNA expression for collagen IA1 and collagen IIIA1 (Fig. 4(e) and (f)). Selective deletion of MR in ECs prevented the significant rise in collagen 1A1 and significantly attenuated the increase of collagen IIIA1. At this four-week termination, sugen/hypoxia was not associated with infiltration with inflammatory cells in the RV interstitium or perivascular areas (results not shown). These findings suggest that EC-MR upregulates the leukocyte adhesion molecule E-selectin in response to sugen/hypoxia and that this may contribute to induction of collagen synthesis and the observed increase in RV perivascular fibrosis in this PH model.
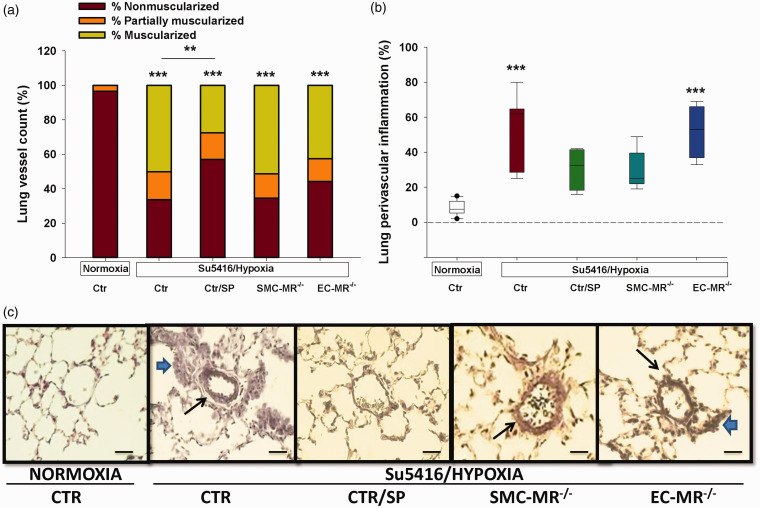
Treatment with the MR inhibitor spironolactone attenuates pulmonary vascular remodeling independent of SMC- or EC-MR
Next, sections of lung tissue were evaluated for the degree of muscularization of the pulmonary arterioles, a hallmark of PAH. In the mouse sugen/hypoxia PH model, spironolactone reduced pulmonary vascular remodeling, as demonstrated by a significant reduction in the number of muscularized small- and medium-sized pulmonary arteries compared with sugen/hypoxia controls (Fig. 5(a)) and consistent with our prior report in other PH models.22 Selective MR deletion from SMCs or ECs did not affect the degree of muscularization, supporting the concept that spironolactone attenuates pulmonary vascular remodeling independent of its role in these vascular cells.
Fig. 5.
Effects of MR on lung vasculature. (a) The degree of muscularized small- and medium-sized arterioles was evaluated. Spironolactone was the only group that showed significantly lower degree of mice/group. (b) Lung perivascular infiltration with inflammatory cells was quantified by determining the percentage of vessels with perivascular inflammation that surrounded at least one-third of the vessel. For each animal, 80–100 small- and medium-sized arterioles were assessed for the degree of muscularization and the presence of perivascular inflammatory cells. (c) Representative sections of lungs from the five groups; visualization by light microscopy (40×). The long arrows point toward muscularized arterioles, while the short arrows point toward perivascular accumulation of inflammatory cells. N = 12–22 mice/group. ***p < 0.001 sugen/hypoxia vs. normoxia.
SP: spironolactone; SMC: smooth muscle cell; MR: mineralocorticoid receptor; EC: endothelial cell.
SMC-MR contributes to lung perivascular inflammation
We next assessed the degree of perivascular lung inflammation as this has been described as an important aspect of both experimental and human PAH and since MR antagonism attenuates lung inflammation in a model of pulmonary fibrosis.39–43 Analysis of lung sections showed that sugen/hypoxia produced significant accumulation of inflammatory cells in the perivascular area of small- and medium-sized arteries. MR blockade with spironolactone prevented this increase in perivascular inflammation and this was reproduced by selective MR deletion in SMCs, while MR deletion in ECs had no effect (Fig. 5(b) and (c)). These data suggest a role for SMC-MR in mediating perivascular inflammation in the lung in PH.
Discussion
In this study, we investigated the role of vascular MR in a well-established mouse model of PH induced by the combination of sugen and hypoxia. To elucidate vascular MR cell-specific actions, we compared systemic-MR blockade (spironolactone) with a SMC-specific MR-KO mouse model and an EC-specific MR-KO model, with intact MR in all other cell lines. Specifically, we report several novel findings: (1) The systemic MR inhibitor spironolactone attenuates sugen/hypoxia-induced PH in mice (an experimental model in which it has not been previously tested); (2) EC-MR regulates RV E-selectin and collagen III expression and mediates RV perivascular fibrosis without any effects on the pulmonary vasculature in this model; and (3) SMC-MR mediates perivascular lung inflammation, without any effects on the RV.
The renin–angiotensin–aldosterone system is activated in PAH and contributes to disease pathology.19 Nevertheless, small clinical trials with the angiotensin converting enzyme inhibitor captopril provided mixed results.44,45 These findings suggest an independent contribution of the MR to PAH pathophysiology. In fact, MR’s role in the pathogenesis of PAH is being increasingly recognized. In patients with PAH, plasma levels of MR’s natural ligand, aldosterone, are increased when compared to controls.20,45 Moreover, in patients with severe PAH, aldosterone levels correlated with hemodynamic markers of PAH severity including pulmonary vascular resistance.20,46 These data suggest that PAH is associated with an active MR state.
Our results strengthen the data supporting an important contribution of MR to the development of experimental PH and expand our understanding of the role of MR in specific vascular cell types in the process. While no preclinical PH model completely reproduces the human PAH phenotype, systemic-MR blockade attenuates PH in multiple well-established animal PH models. MR inhibition is effective in preventing the development of hypoxia-induced PH in mice and in both prevention and treatment models using monocrotaline-induced PH in rats.22 In hypoxic PH mice, spironolactone attenuated the increase in RV systolic pressure, pulmonary arterial muscularization, cardiomyocyte size, and RV fibrosis. In rat monocrotaline-induced PH (prevention arm), spironolactone attenuated pulmonary vascular resistance and pulmonary vascular remodeling. In the established disease (treatment arm), spironolactone decreased RV systolic pressure and pulmonary vascular resistance with no significant effect on histological measures of pulmonary vascular remodeling, or RV fibrosis. Maron et al. showed similar findings in the sugen/hypoxia rat model.21 Our current study demonstrates the benefit of MR blockade with spironolactone in the sugen/hypoxia mouse model, a fourth distinct model of PH. Similar to our previous report, systemic-MR blockade reduced pulmonary vascular remodeling and RV pressures. In addition, spironolactone attenuated the increase in cardiomyocyte area and RV perivascular fibrosis as well as decreased perivascular inflammation in the lung, although not in the total RV mass. The effects of MR blockade are consistent with our prior report and suggest a modest blunting effect on the degree of cardiomyocyte hypertrophy, without affecting the total RV mass, suggesting that a component of RV hypertrophy in response to increased pulmonary pressure is MR-independent. Interestingly, similar effects were reported in the impact on LV size in obesity-associated LV diastolic dysfunction in the Zucker obese rat model, where spironolactone reduced LV fibrosis, but not hypertrophy, via a reduction in cardiac oxidative stress and improvement in endothelial insulin signaling, with no change in arteriolar stiffness.41 As increased pulmonary artery stiffness is also a feature of PAH, it is possible that similar mechanisms may play a role in RV hypertrophy. Also, a significant portion of the hypertrophic RV is composed of extracellular matrix.47 While treatment with spironolactone appears to significantly improve perivascular but not interstitial collagen deposition, other extracellular matrix components that are not quantified in this study may also contribute to RV mass.
EC-MR activation has been demonstrated as pivotal in causing endothelial dysfunction, vascular inflammation, and consequent vascular and cardiac fibrosis in animal models of hypertension. Interestingly, we found that EC-MR mediates RV perivascular fibrosis, without an effect on RV pressures, or pulmonary vascular remodeling. Taken together, these findings suggest that EC-MR has direct effects on the RV in response to increased afterload induced in this PH model. Once again, these results parallel findings in models of experimental LV dysfunction in which EC-MR plays a direct role. For example, in the model of deoxycorticosterone/salt-induced LV dysfunction, EC-MR knock-out mice had attenuation of macrophage recruitment, collagen deposition, and pro-inflammatory gene expression in the LV.48 In terms of the molecular mechanism for the role of EC-MR in RV fibrosis, ICAM1 was previously demonstrated to be regulated by EC-MR in primary human coronary ECs and we recently showed that EC-MR regulates ICAM1 and E-selectin to contribute to leukocyte trafficking and contributes to vascular inflammation and atherosclerosis.9,37 ICAM1 and E-selectin have been studied in various animal models including acute coronary syndromes as molecules expressed in response to an inflammatory milieu which facilitates EC adhesion of neutrophils and monocytes.49 This adhesion then stimulates profibrotic cascades.48 It has also been previously shown that ICAM1-mediated recruitment of leukocytes to the heart contributes to cardiac fibrosis in other models of heart failure.50 Here we show that E-selectin, but not ICAM1, is induced in the RV by sugen/hypoxia and this is significantly attenuated by EC-MR deficiency. The lack of effect on ICAM1 is consistent with prior studies in the left ventricle showing that the increase in ICAM1 was not mediated by EC-MR.36 The rise in RV E-selectin expression correlated with a significant increased expression of collagen IIIA1 which was also attenuated in EC-MR-KO mice. MR-mediated collagen gene regulation and fibrosis has previously been described in various cell lines including rat mesangial cells51 and fibroblasts.52 Focusing on ECs, in a deoxycorticosterone salt rodent model, EC-MR-KO was shown to attenuate cardiac collagen deposition and profibrotic gene expression including collagen III, as well as proinflammatory gene expression.53 Our results are in concordance with prior studies demonstrating that aldosterone increases oxidative stress in pulmonary artery ECs via an MR-dependent mechanism21 and promotes perivascular collagen production.54 Unlike published LV dysfunction studies, we found a lack of inflammatory cell accumulation in the RV after four weeks of exposure to our PH model. These data are consistent with a model in which early temporary recruitment of inflammatory cells with cytokine release may have contributed to late perivascular fibrosis in the RV and this recruitment may be inhibited in EC-MR-KO mice perhaps by preventing E-selectin upregulation. However, future time-course studies will be needed to explore this hypothesis. Our results are also in concordance with differential effects of EC-MR inhibition resulting in vascular-bed-specific actions. For instance, EC-MR deletion improved mesenteric but not coronary endothelial function in angiotensin II-mediated hypertension.23 The improved RV structure without any effects on the pulmonary vasculature in our model solidify a more direct role for EC-MR in the impact of PH in the heart.
Overall, the role of MR in RV hypertrophy across all three PH models appears modest despite the beneficial effects of spironolactone on fibrosis and hemodynamics. This may in part be explained by the lack of complete normalization of RV pressures which in turn prevents restoration of RV size and morphology. In addition, multiple MR-independent mechanistic pathways have been implicated in cardiomyocyte hypertrophy in PAH including, but not limited to, endothelin 1, prostaglandin, platelet-derived growth factor, and adrenomedullin-mediated pathways.47 These studies indicate that multiple cellular pathways likely act in concert at both the cardiomyocyte and in the vasculature in the RV, explaining the limitations of EC-MR deletion in restoring the RV morphology in this model. Finally, the EC-MR-KO model has a unique feature: the attenuation of perivascular RV fibrosis occurred in spite of the lack of hemodynamic or lung histologic improvement, suggesting that RV perivascular fibrosis is, at least in part, independent of the lung vascular remodeling and the elevated RV pressure. These results challenge the notion that RV changes in PH are exclusively due to pulmonary vascular changes.
In addition to the effects of EC-MR, we showed that both spironolactone and SMC-MR deletion attenuates the degree of lung perivascular inflammation, as evidenced by a decrease in the number of small- and medium-sized vessels surrounded by inflammatory cells. We have previously established MR function as a transcription factor in cultured human distal pulmonary artery smooth muscle cells (PASMCs). In quiescent PASMCs, the MR localizes in a non-specific perinuclear cytoplasmic pattern. Stimulation with aldosterone, hypoxia, or platelet-derived growth factor stimulates translocation of the receptor into the nucleus and activates its transcriptional activity, effects inhibited by spironolactone.22 Our current results expand on this knowledge and suggest that pulmonary artery SMC-MR, activated in vivo in PH, contributes to the recruitment of inflammatory cells in the lung perivascular area. These findings are consistent with a novel contribution of SMC-MR to lung inflammation. The effects of SMC-MR on the systemic vasculature are consistent with increased expression of profibrotic genes and extracellular matrix deposition, resulting in increased vascular tone, calcification, stiffening, and aging, but its direct role in inflammation has not been clearly established.55 Previous in vitro studies have shown that activation of MR in human coronary artery SMC results in production of a paracrine factor that enhances monocyte chemotaxis.56 However, in the ApoE–/– in vivo mouse model of atherosclerosis, SMC-MR deletion does not significantly alter the formation, progression, or inflammation of atherosclerotic plaques.57
In this PH model, cell-specific MR deletion was unable to recapitulate the effects of systemic-MR blockade. Given the presence of MR in multiple other cell types, this is not all together unexpected and raises the question of roles of MR on other cells involved in pathogenesis of PAH. MR is expressed in the cardiomyocyte and the role of cardiomyocyte MR in RV dysfunction in PH has not been explored. In addition, recent data demonstrate an important role for myeloid cell MR in vascular inflammation via a classic-macrophage activation pathway58 and deletion of myeloid cell MR limits macrophage accumulation and vascular inflammation in a femoral artery injury model.59 Myeloid-MR-KO mice have reduced cardiac macrophage recruitment and perivascular fibrosis when challenged with angiotensin II60 and decreased plaque size and inflammation in atherosclerosis models.61 Therefore, similar mechanisms may be at play in PH pathogenesis and exploration of the mechanism for the non-vascular benefits of MR inhibition in PH are a future area of interest and exploration.
Our study has several limitations that must be acknowledged. First, it is a general limitation in the field that there is no antibody to specifically recognize MR in the mouse. Thus, although EC-MR and SMC-MR-KO mice have been extensively evaluated to show cell-type-, tissue-, and Cre recombinase-specificity using gene recombination and mRNA quantification of the MR gene,14,23,36 this cannot currently been demonstrated at the level of protein. We show that EC-MR gene recombination persists after exposure to PH and we know that SMC-MR recombination persists long after tamoxifen induction in aging mice.24,25 Second, while we identified novel cell-specific roles for vascular MR in PH in vivo, we did not fully dissect the molecular mechanisms by which SMC-MR contributes to pulmonary inflammation. In addition, we used both male and female animals for our studies, but did not evaluate sex differences separately. Recent reports suggested that there may be sex differences in responses to both MR blockade,58,62 the role of vascular MR,17,63 and in experimental models of PH.64 This is relevant to human PAH which exerts female predominance and sexual dimorphism may also influence response to treatment.65 Finally, spironolactone was chosen because it is the most potent available MR antagonist which is currently in trials for PAH in humans (ClinicalTrials.gov Identifier: NCT01712620).66 However, spironolactone can also affect other steroid receptors including the progesterone and androgen receptors and was recently found to inhibit panexin channels67 and to suppress NF-κB and AP-1 reporter activity independent of MR.68 Another study showed that the highly MR-specific antagonist, eplerenone, has similar beneficial effects in two PH models supporting that much of the impact of spironolactone may be mediated by MR in vivo.21 However, although vascular MR clearly contributes to some benefits of MR antagonism, we cannot rule out the possibility that some of the effects of spironolactone are mediated by non-MR effects. Finally, spironolactone globally antagonizes MR, therefore we did not evaluate the contribution of MR in other cells. As detailed above, the MR in cardiomyocytes or inflammatory cells may also play a role in the disease process. Accordingly, future studies are needed to address these unanswered questions.
Despite these limitations, our study has several novel findings: we showed that the role of vascular MR in experimental PH is cell- and tissue-specific and we discovered the effect of SMC-MR on inflammatory cell recruitment in the lungs and a role for EC-MR in RV perivascular fibrosis. These novel contributions of cell-specific vascular MR to the pathogenesis of pulmonary vascular remodeling and RV adaptation in experimental PH enhance our understanding of the mechanism of disease. Given the availability of MR antagonists in clinical practice, treatment with MR inhibitors is under consideration for PAH. The ongoing clinical trial with spironolactone in PAH focuses on the effects of MR inhibition on endothelial dysfunction. In addition, the new class of non-steroidal MR antagonists currently in clinical trials may be more vascular specific with less renal effects69 and could provide novel avenues for therapy, even if spironolactone fails or is not well tolerated.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_20458940211025240 for Vascular cell-specific roles of mineralocorticoid receptors in pulmonary hypertension by Divya P. Menon, Guanming Qi, Seung K. Kim, M. Elizabeth Moss, Krishna C. Penumatsa, Rod R. Warburton, Deniz Toksoz, Jamie Wilson, Nicholas S. Hill, Iris Z. Jaffe and Ioana R. Preston: On behalf of THALES Registry Investigators in Pulmonary Circulation
Footnotes
Authors’ contributions: D.P.M. and G.Q.: study conception and design, acquisition of data, analysis and interpretation of data, and drafting of the article; R.R.W. and M.E.M.: acquisition of data and analysis and interpretation of data; S.K.K., K.C.P., D.T., J.W., and N.S.H.: analysis and interpretation of data and drafting of the article; I.R.P. and I.Z.J.: study conception and design, analysis and interpretation of data, drafting of the article, and critical revision of the article. All authors read and approved the final article.
Conflict of interest: The author(s) declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by research funding from an American Heart Association Award 16GRNT31330020 (to I.R.P.) and from the National Institutes of Health (HL119290 (to I.Z.J.), HL095590 (to I.Z.J.), and F30HL137255 (to M.E.M.).
ORCID iDs: Divya P. Menon https://orcid.org/0000-0002-2990-1748
Krishna C. Penumatsa https://orcid.org/0000-0003-2962-7758
Ioana R. Preston https://orcid.org/0000-0002-1378-7362
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Sakao S, Tatsumi K, Voelkel NF. Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir Res 2009; 10: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrell NW, Yang X, Upton PD, et al. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation 2001; 104: 790–79. [DOI] [PubMed] [Google Scholar]
- 3.Stenmark KR, Yeager ME, El Kasmi KC, et al. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol 2013; 75: 23–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassoun PM, Mouthon L, Barbera JA, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 2009; 54: S10–S19. [DOI] [PubMed] [Google Scholar]
- 5.Sachdev A, Villarraga HR, Frantz RP, et al. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest 2011; 139: 1299–1309. [DOI] [PubMed] [Google Scholar]
- 6.Arriza JL, Weinberger C, Cerelli G, et al. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 1987; 237: 268–275. [DOI] [PubMed] [Google Scholar]
- 7.Rogerson FM, Fuller PJ. Mineralocorticoid action. Steroids 2000; 65: 61–73. [DOI] [PubMed] [Google Scholar]
- 8.DuPont JJ, Jaffe IZ. 30 years of the mineralocorticoid receptor: the role of the mineralocorticoid receptor in the vasculature. J Endocrinol 2017; 234: T67–T82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caprio M, Newfell BG, la Sala A, et al. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res 2008; 102: 1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res 2005; 96: 643–650. [DOI] [PubMed] [Google Scholar]
- 11.Pruthi D, McCurley A, Aronovitz M, et al. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vasc Biol 2014; 34: 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koenig JB, Jaffe IZ. Direct role for smooth muscle cell mineralocorticoid receptors in vascular remodeling: novel mechanisms and clinical implications. Curr Hypertens Rep 2014; 16: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galmiche G, Pizard A, Gueret A, et al. Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension 2014; 63: 520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCurley A, Pires PW, Bender SB, et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med 2012; 18: 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffe IZ, Newfell BG, Aronovitz M, et al. Placental growth factor mediates aldosterone-dependent vascular injury in mice. J Clin Invest 2010; 120: 3891–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davel AP, Anwar IJ, Jaffe IZ. The endothelial mineralocorticoid receptor: mediator of the switch from vascular health to disease. Curr Opin Nephrol Hypertens 2017; 26: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davel AP, Jaffe IZ, Tostes RC, et al. New roles of aldosterone and mineralocorticoid receptors in cardiovascular disease: translational and sex-specific effects. Am J Physiol Heart Circ Physiol 2018; 315: H989–H999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia G, Habibi J, Aroor AR, et al. Endothelial mineralocorticoid receptor mediates diet-induced aortic stiffness in females. Circ Res 2016; 118: 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maron BA, Leopold JA. The role of the renin-angiotensin-aldosterone system in the pathobiology of pulmonary arterial hypertension (2013 Grover Conference series) . Pulm Circ 2014; 4: 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maron BA, Opotowsky AR, Landzberg MJ, et al. Plasma aldosterone levels are elevated in patients with pulmonary arterial hypertension in the absence of left ventricular heart failure: a pilot study. Eur J Heart Fail 2013; 15: 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maron BA, Zhang YY, White K, et al. Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation 2012; 126: 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preston IR, Sagliani KD, Warburton RR, et al. Mineralocorticoid receptor antagonism attenuates experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2013; 304: L678– L688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller KB, Bender SB, Hong K, et al. Endothelial mineralocorticoid receptors differentially contribute to coronary and mesenteric vascular function without modulating blood pressure. Hypertension 2015; 66: 988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SK, McCurley AT, DuPont JJ, et al. Smooth muscle cell-mineralocorticoid receptor as a mediator of cardiovascular stiffness with aging. Hypertension 2018; 71: 609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DuPont JJ, Kim SK, Kenney RM, et al. Sex differences in the time course and mechanisms of vascular and cardiac aging in mice: role of the smooth muscle cell mineralocorticoid receptor. Am J Physiol Heart Circ Physiol 2021; 320: H169–H180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alva JA, Zovein AC, Monvoisin A, et al. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn 2006; 235: 759–767. [DOI] [PubMed] [Google Scholar]
- 27.Benetos A, Lacolley P, Safar ME. Prevention of aortic fibrosis by spironolactone in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol 1997; 17: 1152–1156. [DOI] [PubMed] [Google Scholar]
- 28.Sakurabayashi-Kitade S, Aoka Y, Nagashima H, et al. Aldosterone blockade by Spironolactone improves the hypertensive vascular hypertrophy and remodeling in angiotensin II overproducing transgenic mice. Atherosclerosis 2009; 206: 54–60. [DOI] [PubMed] [Google Scholar]
- 29.Nicolls MR, Mizuno S, Taraseviciene-Stewart L, et al. New models of pulmonary hypertension based on VEGF receptor blockade-induced endothelial cell apoptosis. Pulm Circ 2012; 2: 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preston IR, Hill NS, Gambardella LS, et al. Synergistic effects of ANP and sildenafil on cGMP levels and amelioration of acute hypoxic pulmonary hypertension. Exp Biol Med (Maywood). 2004; 229: 920–925. [DOI] [PubMed] [Google Scholar]
- 31.Chintalgattu V, Ai D, Langley RR, et al. Cardiomyocyte PDGFR-beta signaling is an essential component of the mouse cardiac response to load-induced stress. J Clin Invest 2010; 120: 472–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf CM, Moskowitz IP, Arno S, et al. Somatic events modify hypertrophic cardiomyopathy pathology and link hypertrophy to arrhythmia. Proc Natl Acad Sci U S A 2005; 102: 18123–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCurley A, Jaffe IZ. Mineralocorticoid receptors in vascular function and disease. Mol Cell Endocrinol 2012; 350: 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi GM, Jia LX, Li YL, et al. Adiponectin suppresses angiotensin II-induced inflammation and cardiac fibrosis through activation of macrophage autophagy. Endocrinology 2014; 155: 2254–2265. [DOI] [PubMed] [Google Scholar]
- 35.McCurley A, McGraw A, Pruthi D, et al. Smooth muscle cell mineralocorticoid receptors: role in vascular function and contribution to cardiovascular disease. Pflugers Arch 2013; 465: 1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salvador AM, Moss ME, Aronovitz M, et al. Endothelial mineralocorticoid receptor contributes to systolic dysfunction induced by pressure overload without modulating cardiac hypertrophy or inflammation. Physiol Rep 2017; 5: e13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moss ME, Lu Q, Iyer SL, et al. Endothelial mineralocorticoid receptors contribute to vascular inflammation in atherosclerosis in a sex-specific manner. Arterioscler Thromb Vasc Biol 2019; 39: 1588–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lax A, Sanchez-Mas J, Asensio-Lopez MC, et al. Mineralocorticoid receptor antagonists modulate galectin-3 and interleukin-33/ST2 signaling in left ventricular systolic dysfunction after acute myocardial infarction. JACC Heart Fail 2015; 3: 50–58. [DOI] [PubMed] [Google Scholar]
- 39.Dinh QN, Young MJ, Evans MA, et al. Aldosterone-induced oxidative stress and inflammation in the brain are mediated by the endothelial cell mineralocorticoid receptor. Brain Res 2016; 1637: 146–153. [DOI] [PubMed] [Google Scholar]
- 40.Barrera-Chimal J, Estrela GR, Lechner SM, et al. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int 2018; 93: 1344–1355. [DOI] [PubMed] [Google Scholar]
- 41.Bender SB, DeMarco VG, Padilla J, et al. Mineralocorticoid receptor antagonism treats obesity-associated cardiac diastolic dysfunction. Hypertension 2015; 65: 1082–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bender SB, McGraw AP, Jaffe IZ, et al. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes 2013; 62: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lieber GB, Fernandez X, Mingo GG, et al. Mineralocorticoid receptor antagonists attenuate pulmonary inflammation and bleomycin-evoked fibrosis in rodent models. Eur J Pharmacol 2013; 718: 290–298. [DOI] [PubMed] [Google Scholar]
- 44.Leier CV, Bambach D, Nelson S, et al. Captopril in primary pulmonary hypertension. Circulation 1983; 67: 155–161. [DOI] [PubMed] [Google Scholar]
- 45.Ikram H, Maslowski AH, Nicholls MG, et al. Haemodynamic and hormonal effects of captopril in primary pulmonary hypertension. Br Heart J 1982; 48: 541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calvier L, Legchenko E, Grimm L, et al. Galectin-3 and aldosterone as potential tandem biomarkers in pulmonary arterial hypertension. Heart 2016; 102: 390–396. [DOI] [PubMed] [Google Scholar]
- 47.Bogaard HJ, Abe K, Vonk Noordegraaf A, et al. The right ventricle under pressure. Chest 2009; 135: 794–804. [DOI] [PubMed] [Google Scholar]
- 48.Jia G, Habibi J, DeMarco VG, et al. Endothelial mineralocorticoid receptor deletion prevents diet-induced cardiac diastolic dysfunction in females. Hypertension 2015; 66: 1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miwa K, Igawa A, Inoue H. Soluble E-selectin, ICAM-1 and VCAM-1 levels in systemic and coronary circulation in patients with variant angina. Cardiovasc Res 1997; 36: 37–44. [DOI] [PubMed] [Google Scholar]
- 50.Salvador AM, Nevers T, Velazquez F, et al. Intercellular adhesion molecule 1 regulates left ventricular leukocyte infiltration, cardiac remodeling, and function in pressure overload-induced heart failure. J Am Heart Assoc 2016; 5: e003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diah S, Zhang GX, Nagai Y, et al. Aldosterone induces myofibroblastic transdifferentiation and collagen gene expression through the Rho-kinase dependent signaling pathway in rat mesangial cells. Exp Cell Res 2008; 314: 3654–36. [DOI] [PubMed] [Google Scholar]
- 52.Nagai Y, Miyata K, Sun GP, et al. Aldosterone stimulates collagen gene expression and synthesis via activation of ERK1/2 in rat renal fibroblasts. Hypertension 2005; 46: 1039–1045. [DOI] [PubMed] [Google Scholar]
- 53.Rickard AJ, Morgan J, Chrissobolis S, et al. Endothelial cell mineralocorticoid receptors regulate deoxycorticosterone/salt-mediated cardiac remodeling and vascular reactivity but not blood pressure. Hypertension 2014; 63: 1033–1040. [DOI] [PubMed] [Google Scholar]
- 54.Samokhin AO, Stephens T, Wertheim BM, et al . NEDD9 targets COL3A1 to promote endothelial fibrosis and pulmonary arterial hypertension. Sci Transl Med 2018; 10: eaap7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCurley A, McGraw A, Pruthi D, et al. Smooth muscle cell mineralocorticoid receptors: role in vascular function and contribution to cardiovascular disease. Pflugers Arch 2013; 465: 1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGraw AP, Bagley J, Chen WS, et al . Aldosterone increases early atherosclerosis and promotes plaque inflammation through a placental growth factor-dependent mechanism. J Am Heart Assoc 2013; 2: e000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moss ME, DuPont JJ, Iyer SL, et al. No significant role for smooth muscle cell mineralocorticoid receptors in atherosclerosis in the apolipoprotein-E knockout mouse model. Front Cardiovasc Med 2018; 5: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frieler RA, Ray JJ, Meng H, et al . Myeloid mineralocorticoid receptor during experimental ischemic stroke: effects of model and sex. J Am Heart Assoc 2012; 1: e002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun JY, Li C, Shen ZX, et al. Mineralocorticoid receptor deficiency in macrophages inhibits neointimal hyperplasia and suppresses macrophage inflammation through SGK1-AP1/NF-kappaB pathways. Arterioscler Thromb Vasc Biol 2016; 36: 874–885. [DOI] [PubMed] [Google Scholar]
- 60.Usher MG, Duan SZ, Ivaschenko CY, et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest 2010; 120: 3350–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen ZX, Chen XQ, Sun XN, et al. Mineralocorticoid receptor deficiency in macrophages inhibits atherosclerosis by affecting foam cell formation and efferocytosis. J Biol Chem 2017; 292: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jia G, Habibi J, Aroor AR, et al. Endothelial mineralocorticoid receptor mediates diet-induced aortic stiffness in females. Circ Res 2016; 118: 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davel AP, Lu Q, Moss ME, et al . Sex-specific mechanisms of resistance vessel endothelial dysfunction induced by cardiometabolic risk factors. J Am Heart Assoc 2018; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Austin ED, Lahm T, West J, et al. Gender, sex hormones and pulmonary hypertension. Pulm Circ 2013; 3: 294–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mathai SC, Hassoun PM, Puhan MA, et al. Sex differences in response to tadalafil in pulmonary arterial hypertension. Chest 2015; 147: 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spironolactone for Pulmonary Arterial Hypertension. NCT01712620. 2012. http://clinicaltrials.gov/ct2/show/NCT01712620?term=spironolactone+AND+%22pulmonary+hypertension%22&rank=3
- 67.Good ME, Chiu YH, Poon IKH, et al. Pannexin 1 channels as an unexpected new target of the anti-hypertensive drug spironolactone. Circ Res 2018; 122: 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elinoff JM, Chen LY, Dougherty EJ, et al. Spironolactone-induced degradation of the TFIIH core complex XPB subunit suppresses NF-kappaB and AP-1 signalling. Cardiovasc Res 2018; 114: 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Capelli I, Gasperoni L, Ruggeri M, et al. New mineralocorticoid receptor antagonists: update on their use in chronic kidney disease and heart failure. J Nephrol 2020; 33: 37–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_20458940211025240 for Vascular cell-specific roles of mineralocorticoid receptors in pulmonary hypertension by Divya P. Menon, Guanming Qi, Seung K. Kim, M. Elizabeth Moss, Krishna C. Penumatsa, Rod R. Warburton, Deniz Toksoz, Jamie Wilson, Nicholas S. Hill, Iris Z. Jaffe and Ioana R. Preston: On behalf of THALES Registry Investigators in Pulmonary Circulation