Abstract
Background:
Glucocorticoid (GC) pulse therapy is used for multiple sclerosis (MS) relapse treatment; however, GC resistance is a common problem. Considering that GC dosing is individual with several response-influencing factors, establishing a predictive model, which supports clinicians to estimate the maximum GC dose above which no additional therapeutic value can be expected presents a huge clinical need.
Method:
We established two, independent retrospective cohorts of MS patients. The first was an explorative cohort for model generation, while the second was established for its validation. Using the explorative cohort, a multivariate regression analysis with the GC dose used as the dependent variable and serum vitamin D (25D) concentration, sex, age, EDSS, contrast enhancement on cranial magnetic resonance imaging (MRI), immune therapy, and the involvement of the optic nerve as independent variables was established.
Results:
In the explorative cohort, 113 MS patients were included. 25-hydroxyvitamin D (25D) serum concentration and the presence of optic neuritis were independent predictors of the GC dose needed to treat MS relapses [(25D): −25.95 (95% confidence interval (CI)): −47.40 to −4.49; p = 0.018; optic neuritis: 2040.51 (95% CI: 584.64–3496.36), p = 0.006]. Validation of the multivariate linear regression model was performed within a second cohort. Here, the predicted GC dose did not differ significantly from the dose administered in clinical routine (mean difference: −843.54; 95% CI: −2078.08–391.00; n = 30, p = 0.173).
Conclusion:
Our model could predict the GC dose given in clinical, routine MS relapse care, above which clinicians estimate no further benefit. Further studies should validate and improve our algorithm to help the implementation of predictive models in GC dosing.
Keywords: multiple sclerosis, relapse treatment, steroid dose, vitamin D
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease affecting the central nervous system (CNS). It typically begins in young adulthood, mostly with a relapsing disease course. 1 Relapses represent focal autoimmune inflammatory CNS lesions that cause the acute occurrence of new neurological symptoms or the worsening of pre-existing deficits lasting for longer than 24 h. 2 The most established treatment option for MS relapse is a pulse therapy with systemic glucocorticoids (GC) due to their pleiotropic immunological effects.3–5 Although in our hospital, we stick to the guidelines of the German Neurology Society (Deutsche Gesellschaft für Neurologie, DGN 6 ) for the GC administration, we need to adapt this scheme from case-to-case. This is particularly important because GC doses needed to achieve sufficient symptom control differ between each patient. The DGN suggests a 3–5 day course of intravenous methylprednisolone (or equivalent) 1000 mg/day. 6 This course may be repeated if symptom control is insufficient. Nevertheless, in patients who do not respond to high dosages of GC, plasma exchange may be needed for relapse treatment. 3
The biomolecular understanding of the mechanisms underlying GC resistance has already been deepened. For example, the significance of an altered level of glucocorticoid receptor (GR) complex 7 is appreciated as an important factor. In addition, the role of vitamin D (VD) in dexamethasone-induced binding of the GR to GC response elements, 8 and the inhibition of the mechanistic target of rapamycin (mTOR) pathway, which generally regulates cell metabolism, growth, proliferation, survival, and which appears to also be relevant for GC signaling, as it upregulates the GR as shown by our group in human and murine T cells. This leads to increased GC-induced effects, which have previously been described.9,10 However, no predictive clinical model exists that can be used to determine the individual dose of GC needed for relapse treatment until either sufficient symptom control is achieved, or a further escalation of the GC dose is not appropriate due to GC resistance. Therefore, the aim of this study is to establish such a predictive model, based only on the clinical data available at the time point of relapse, in order to calculate the GC dosages needed for relapse treatment.
Methods
Patient cohort
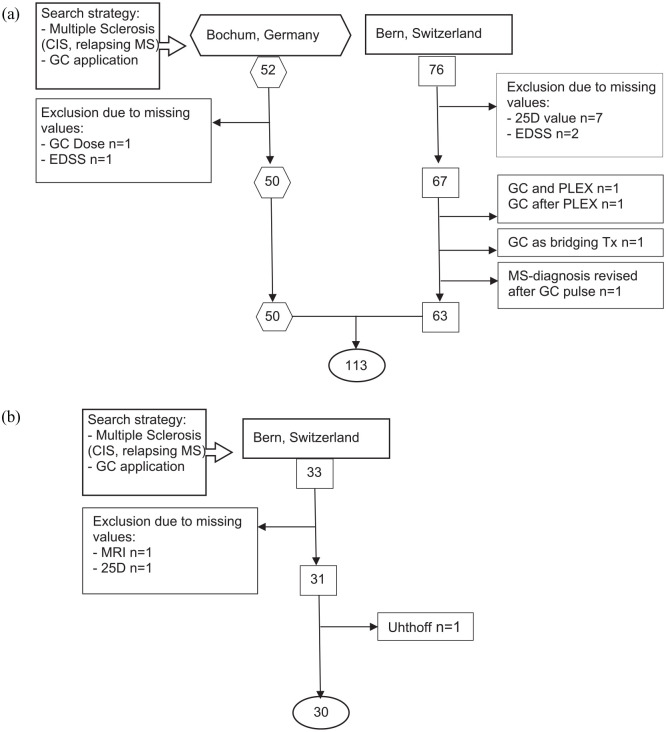
We conducted a retrospective bicentric study and established two independent cohorts. The first was an explorative cohort, while the second was a validation cohort. For description of patient identification and cohort definition, please refer to Figure 1.
Figure 1.
Flowchart of the cohort formation process. We screened outpatient wards. (a) For the explorative cohort in Bochum from March 2013 until August 2015 (employment period of co-author at the Hospital of Bochum) and in Bern from 2014 (beginning of specialized neuroimmunological consultation) until March 2019. (b) For the validation cohort in Bern from March 2019 until December 2019. Screening terms were as listed above. We included any type of MS except progressive forms. Only 1 secondary progressive MS patient was included in Bern, because he had a clear relapse including MRI-enhancing lesions. By adding GC application, we restricted all found MS patients to those with acute relapse symptoms. If any needed value (GC dose, MRI, EDSS, 25D) could not be retrieved, patients were excluded from the analysis.
25D, 25-hydroxy-vitamin D; CIS, clinically isolated syndrome; EDSS, expanded disability status scale; GC, glucocorticoids; MS, multiple sclerosis; PLEX, plasma exchange; Tx, therapy.
Evaluated data
The following eight variables were extracted from medical records: (I) The serum level of 25D: only values dating no more than 3 months prior or after the relapse were included. (II) Sex. (III) Age at the time of relapse. (IV) Expanded Disability Status Scale (EDSS) score at the time of relapse. If no clear EDSS number was written in medical reports, we recalculated it only if every needed clinical data was described accurately at the time of relapse. If some information (for example visual acuity or information on bowel and bladder function) was missing, the EDSS value of this patient was determined as missing. (V) The presence of any gadolinium-enhancing lesion on post gadolinium T1 magnetic resonance imaging (MRI). We analyzed cerebral and/or spinal MRIs to look for gadolinium uptake and considered contrast enhancement positive, if, on either scan, one or more lesions were found to uptake gadolinium. (VI) MS treatment: treatment running at the time of relapse was considered. A patient was considered untreated whenever the last treatment was stopped for a longer time than the wash-out period for each specific medication. 11 The different immunotherapies were divided into the following: no treatment, first-line therapies (interferons, glatiramer acetate, dimethyl fumarate, teriflunomide), and second-line therapies (fingolimod, natalizumab, rituximab) according to the European Medicines Agency. (VII) The presence of an optic neuritis. Optic neuritis was considered present if clinical reports mentioned according symptoms. The main parameter assessed to evaluate optic neuritis was visual acuity. (VIII) The dose of glucocorticoids used. We summed up the total amount of GC administered in milligrams. The main GC used was methyl-prednisolone. If dexamethasone was administered, we added the amount as methyl-prednisolone equivalent dose by multiplying the dexamethasone in milligrams by 4.
Study endpoints
The study endpoint was to develop a regression model to predict the GC dose used for relapse treatment. Treatment stop with GC could have two reasons: recovery of relapse symptoms [defined as sufficient symptom control without any left symptoms affecting the daily life (n = 60 patients with a decrease of EDSS and 10 with a stable EDSS)] or poor steroid response [defined as therapy escalation to plasmapheresis (n = 43 received PLEX (two, despite a decreased EDSS due to a devastating disability of the relapse, and eight with an increased EDSS after steroids and 33 with no EDSS change)].
Statistics
We used IBM SPSS statistics 25 (Armonk, USA) to analyze the data. Comparative statistics were used to compare categorical and continuous variables between both data sets. For the prediction of GC dose, a multivariate regression analysis with GC dose used as the dependent variable and 25D level, sex, age, EDSS, contrast enhancement, immune therapy, and the involvement of optic nerve as independent variables was performed. Afterwards, this model was used to calculate the GC dose of the validation cohort. To do so, we retrieved the clinical data of all above-mentioned independent variables in our validation cohort. By inserting those in the formula of the regression analysis retrieved within the explorative cohort, we were able to calculate the supposedly ‘optimal’ GC dosage for each patient. This dose was then subtracted from the GC each patient received in the real setting, in order to assess whether our model could predict GC dose in a separate and independent cohort.
Ethics statement
This study was performed under the ethics votes KEK-BE 2017-01369 for Bern, Switzerland and 4801-13 for Ruhr-University Bochum, Germany, and followed the principles of the Declaration of Helsinki.
Results
The basic characteristics of the explorative and the validation cohorts are presented in Table 1. Most variables were equally-distributed between the cohorts, except for EDSS and 25D serum concentrations; these were both higher in the explorative cohort compared with the validation cohort (Table 1).
Table 1.
Baseline characteristics.
| Variable | Basis cohort | Validation cohort | p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean, CI 95%, (LL/UL) | Minimum value/maximum value | Absolute numbers | Percentage | n | Mean, CI 95%, (LL/UL) | Minimum value/maximum value | Absolute numbers | Percentage | n | ||
| Age (years) | 35.92 (33.91/37.93) | 17.52/59.75 | 113 | 34.21 (30.05/38.37) | 17.84/60.90 | 30 | 0.49 | ||||
| EDSS before therapy | 3.42 (3.10/3.73) | 0.00 * /9.00 | 2.18 (1.96/2.41) | 1.00/3.50 | <0.001 | ||||||
| 25D (nmol/l) | 47.15 (41.39/52.92) | 7.77/156.00 | 53.29 (45.18/61.40) | 24.9/113.00 | 0.04 | ||||||
| GC dose (mg) | 5749.12 (5109.40/6388.83) | 480.00/19000.00 | 5042.67 (3896.64/6188.70) | 2500/16000.00 | 0.31 | ||||||
| Sex | |||||||||||
| Male | 33 | 29.2 | 113 | 10 | 33.3 | 30 | 0.66 | ||||
| Female | 80 | 70.8 | 20 | 66.7 | |||||||
| Presence of optic neuritis | |||||||||||
| Any patients | 24 | 21.2 | 113 | 6 | 20 | 30 | 0.09 | ||||
| Immunotherapy | |||||||||||
| No immunotherapy | 77 | 68.1 | 113 | 26 | 86.7 | 30 | 0.35 | ||||
| INF | 10 | 8.8 | 0 | 0 | |||||||
| GLAT | 6 | 5.3 | 0 | 0 | |||||||
| DMF | 7 | 6.2 | 1 | 3.3 | |||||||
| FNG | 7 | 6.2 | 3 | 10 | |||||||
| TERIF | 1 | 0.9 | 0 | 0 | |||||||
| NTZ | 3 | 2.7 | 0 | 0 | |||||||
| RTX | 2 | 1.8 | 0 | 0 | |||||||
| Presence of Gd enhancing lesions | |||||||||||
| Any MRI with Gd enhancing lesion | 89 | 78.8 | 113 | 22 | 73.3 | 30 | 0.53 | ||||
| Spinal MRI with Gd enhancing lesion | 22 | 26.8 | 82 | 10 | 41.7 | 24 | 0.16 | ||||
Painful dysesthesia has no effect on EDSS.
25D, 25-hydroxy-vitamin D; CI, confidence interval; DMF, dimethyl fumarate; EDSS, expanded disability status scale; FNG, fingolimod; GC, glucocorticoids; Gd, gadolinium; GLAT, glatiramer acetate; INF, interferon; LL, lower limit; MRI, magnetic resonance imaging; n, patient number; NTZ, natalizumab; RTX, rituximab; TERIF, teriflunomid; UL, upper limit.
Receiver operating characteristic (ROC) curve analysis revealed that the highest sensitivity and specificity, to distinguish between patients experiencing sufficient symptom control through steroid treatment versus those needing escalation treatment, was at a GC dose of 6100 mg, with a specificity of 60.5% and a sensitivity of 75.5% (data not shown).
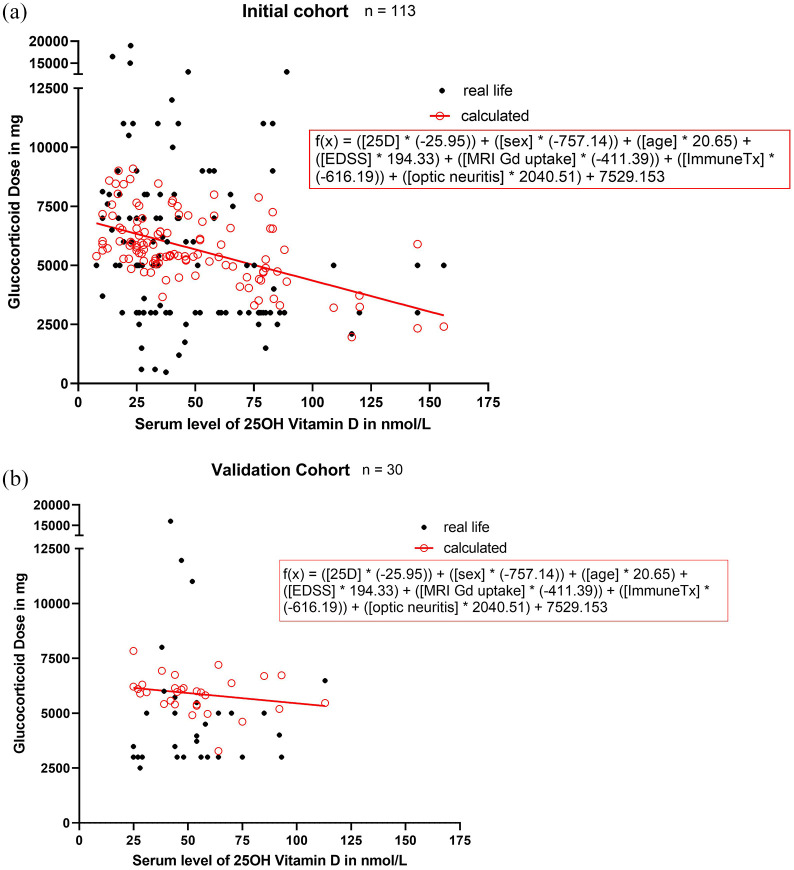
With the regression analysis performed as described under methods, we found that 25D serum concentration and the presence of optic neuritis were independent predictors of the GC dose needed to treat the present MS relapse [regression coefficient of 25D serum concentration: −25.95 (95% CI: −47.40 to −4.49), p = 0.018; regression coefficient of optic neuritis: 2040.5 (95% CI: 584.64–3496.36), p = 0.006] [Figure 2(a) and (b)].
Figure 2.
The glucocorticoid dose of every patient plotted against respective vitamin D serum level. (a) In the explorative cohort. (b) In the validation cohort. Vitamin D was found to be an independent predictor for the GC dose administered in a linear regression analysis (regression coefficient of vitamin D: −25.95 (95% CI: −47.40 to −4.49), p = 0.018), Nagelkerks R2 = 0.17.
25D, 25-hydroxy-vitamin D; CI, confidence interval; EDSS, expanded disability status scale; GC, glucocorticoids; ImmuneTx, immunotherapy.
In the clinical context, this signifies that the higher the VD serum level of a patient, the smaller the volume GC needed to treat relapse. However, if optic neuritis was present in a relapsed patient, higher GC dosages were applied. Furthermore, the following formula was retrieved by multivariate linear regression analysis:
In a second step, the validation cohort was used to proof the relevance of our statistical model by comparing the used GC dose with the one calculated with the above-described model. It demonstrated that our model predicted the cumulative GC dose in the validation cohort (mean difference: −843.54; 95% CI: −2078.08–391.00; p = 0.173) (Figure 3). Practically applied, this would signify that by taking selected clinical patient aspects and inserting these into our formula, we could calculate a supposedly optimal-limit of GC individually for every patient. It is important to highlight that our study does not argue for the safe treatment option of ultra-high dosages of vitamin D exceeding the limit of a normal range, as this is known to be associated with possibly-severe side effects.
Figure 3.
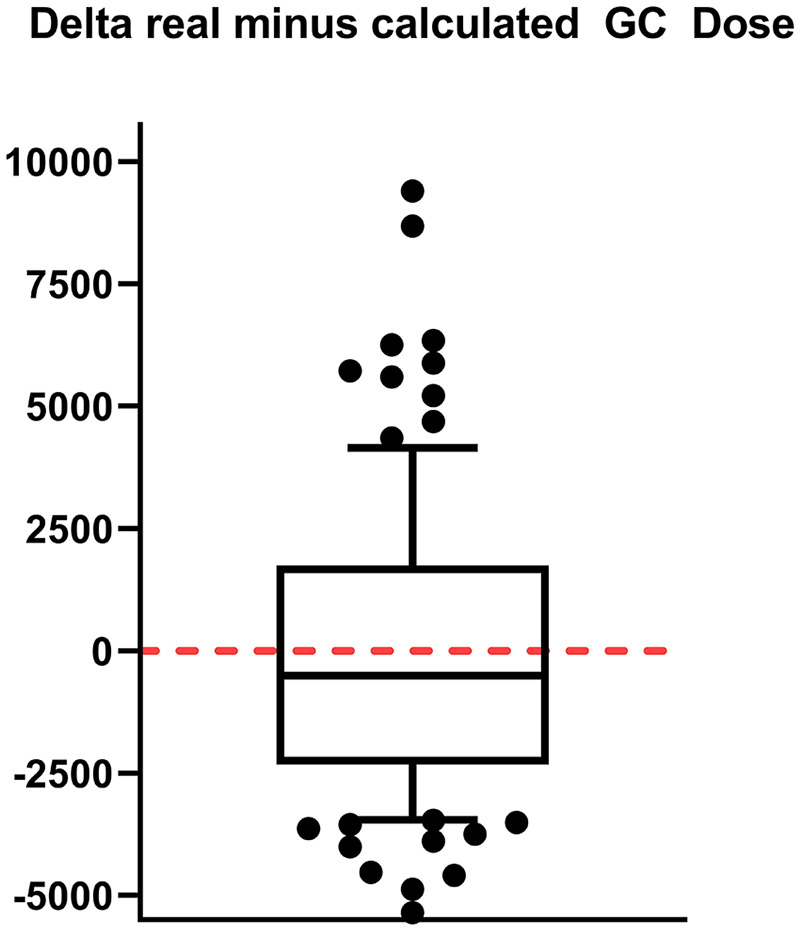
For this boxplot, we took the validation cohort and subtracted from each patient the glucocorticoid dose (in mg) administered in real life from the one we calculated applying the regression analysis established in the explorative cohort. The median is shown as bar of the boxplot. The high values are rare cases with prolonged relapse symptoms, where our steroid dosing regimen was administered repeatedly (e.g. after a 7 day course of steroid administration the symptoms did not improve, but GC had been well tolerated, we would decide to administer steroids for another 5–7 days). There was no significant difference between the dosages [one sample t-test (test value 0), p = 0.173; box: median, 25–75 percentile; whisker: 10–90 percentile].
GC, glucocorticoids.
Conclusion
Our study aims to identify a predictive model for the cumulative GC dose needed for the treatment of MS relapses. As previously demonstrated by our group and others8,10, 25D serum concentration was negatively associated with GC dose, highlighting VD as a risk factor for poor GC response. Furthermore, the presence of an optic neuritis was also independently-associated with the need for higher doses of GC. The available data on the effectiveness of intravenous or oral GC on optic neuritis points towards restricted benefit, with only a positive effect on the rate of return to normal visual acuity but not on the pooled risk-ratio of normal visual acuity, normal contrast sensitivity, and normal visual fields. 12 This might point towards underlying, as yet unknown, pathomechanisms in optic neuritis, making GC administration less effective than in other MS relapse symptoms. However, the same study showed a faster recovery with intravenous steroids compared with oral or no treatment. Therefore, our findings of higher dosages used for ON treatment might be the result of a more aggressive treatment because of the disabling visual symptom. Finally, the developed model could be validated using a second independent validation cohort, as the calculated dose did not differ significantly from the dose given in clinical settings.
The limitations of our study will be detailed. When analyzing the administrated GC dose, we investigated physicians’ decisions. Therefore, as centers included are both European German speaking centers (Bochum, Germany and Bern, Switzerland) an international validation should follow. For the regression analysis, we included clinical aspects that are, in our opinion, most relevant and can be retrieved easily at the time point of relapse. However, this might not comprise all relevant clinical aspects, which could be a limitation of our study. Including a baseline EDSS, rather than the EDSS at the time point of relapse would be interesting. We lacked this information for many patients, since, at time of inclusion, many received their initial diagnosis of MS while others presented for the first time in our department, having been treated at other facilities prior to the study. A further limitation is the retrospective design of the two cohorts and the small patient number of the validation cohort in comparison to the explorative cohort.
Considering that GC dosing appears to be individual with a range used in our cohort from 480 to 19,000 mg, with several factors influencing GC response, including the presence of an optic neuritis or VD serum concentration, establishing patient specific models, which support clinicians to estimate the GC dosage from which no more therapeutic added value, as suggested by our retrospective data analysis, might be expected has an obvious clinical need. Further studies should validate and further develop our algorithm to improve the prediction of clinically-needed GC dose.
Footnotes
Conflict of interest statement: J Gili-Kovács reports no disclosures.
R Hoepner has received research and travel grants from Roche, Novartis, and Biogen Idec and speaker honoraria from Biogen, Novartis, Merck, Celgene, and Almirall, not related to this work. He reports no conflicts of interest related to this manuscript.
A Salmen received speaker honoraria and/or travel compensation for activities with Almirall Hermal GmbH, Biogen, Merck, Novartis, Roche, and Sanofi Genzyme, and research support by the Swiss MS Society, not related to this work.
M Bagnoud reports no disclosures.
R Gold received research support, and speaker’s honoraria from Bayer Schering, Biogen, Celgene, Chugai, Eisai, Janssen Pharmaceuticals, Merck Serono, Nikkiso Pharma, Novartis, Roche, Sandoz, Sanofi-Genzyme, and TEVA. They received consulting honoraria from ZLB Behring, Baxter, Novartis, and Talecris. They have personal stock options in Bayer, Merck, and Roche.
A Chan has served on advisory boards for, and received funding for travel or speaker honoraria from, Actelion-Janssen, Almirall, Bayer, Biogen, Celgene, Sanofi-Genzyme, Merck, Novartis, Roche, and Teva, all for hospital research funds; and research support from Biogen, Genzyme, and UCB. A. Chan is associate editor of the European Journal of Neurology and serves on the editorial board for Clinical and Translational Neuroscience and as topic editor for the Journal of International Medical Research.
M Briner received travel grants from Merck, Biogen, and Genzyme.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Gili-Kovács J.  https://orcid.org/0000-0002-2134-1416
https://orcid.org/0000-0002-2134-1416
Hoepner R.  https://orcid.org/0000-0002-0115-7021
https://orcid.org/0000-0002-0115-7021
Salmen A.  https://orcid.org/0000-0002-4751-299X
https://orcid.org/0000-0002-4751-299X
Data availability statement: Raw data were generated at University Hospital Inselspital Bern and St Josef-Hospital/Ruhr-University Bochum. Derived data supporting the findings of this study are available from the corresponding author J.G. on request.
Contributor Information
Judit Gili-Kovács, Department of Neurology, University Hospital Bern, Inselspital, Freiburgstrasse 18, Bern, 3010, Switzerland.
Robert Hoepner, Department of Neurology, University Hospital Bern, Inselspital, Bern, Switzerland.
Anke Salmen, Department of Neurology, University Hospital Bern, Inselspital, Bern, Switzerland.
Maud Bagnoud, Department of Neurology, University Hospital Bern, Inselspital, Bern, Switzerland; Graduate School for Cellular and Biomedical Sciences, University of Bern, Bern, Switzerland.
Ralf Gold, Department of Neurology, St Josef-Hospital/Ruhr-University Bochum, Bochum, Germany.
Andrew Chan, Department of Neurology, University Hospital Bern, Inselspital, Bern, Switzerland.
Myriam Briner, Department of Neurology, University Hospital Bern, Inselspital, Bern, Switzerland.
References
- 1. Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med 2000; 343: 938–952. [DOI] [PubMed] [Google Scholar]
- 2. Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology 2003; 61: 1528–1532. [DOI] [PubMed] [Google Scholar]
- 3. Berkovich R. Treatment of acute relapses in multiple sclerosis. Neurotherapeutics 2013; 10: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luhder F, Reichardt HM. Traditional concepts and future avenues of glucocorticoid action in experimental autoimmune encephalomyelitis and multiple sclerosis therapy. Crit Rev Immunol 2009; 29: 255–273. [DOI] [PubMed] [Google Scholar]
- 5. Schmidt J, Gold R, Schonrock L, et al. T-cell apoptosis in situ in experimental autoimmune encephalomyelitis following methylprednisolone pulse therapy. Brain 2000; 123: 1431–1441. [DOI] [PubMed] [Google Scholar]
- 6. DGN. Diagnostik und therapie der multiplen sklerose, https://dgn.org/wp-content/uploads/2013/01/ll08kap_034.pdf (2008, accessed 4 March 2021).
- 7. Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet 2009; 373: 1905–1917. [DOI] [PubMed] [Google Scholar]
- 8. Zhang Y, Leung DY, Goleva E. Anti-inflammatory and corticosteroid-enhancing actions of vitamin D in monocytes of patients with steroid-resistant and those with steroid-sensitive asthma. J Allergy Clin Immunol 2014; 133: 1744–752.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 2017; 168: 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoepner R, Bagnoud M, Pistor M, et al. Vitamin D increases glucocorticoid efficacy via inhibition of mTORC1 in experimental models of multiple sclerosis. Acta Neuropathol 2019; 138: 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kompetenznetz-Multiple-Sklerose. Qualitätshandbuch MS/NMOSD, https://www.kompetenznetz-multiplesklerose.de/wp-content/uploads/2020/03/KKNMS_Qualitätshandbuch-MSNMOSD_202001_webfrei-1.pdf (2020, accessed 4 March 2021).
- 12. Gal RL, Vedula SS, Beck R. Corticosteroids for treating optic neuritis. Cochrane Database Syst Rev 2015; 2015: CD001430. [DOI] [PMC free article] [PubMed] [Google Scholar]