Abstract
Background. Real-world evidence can be a valuable tool when clinical trial data are incomplete or uncertain. Bevacizumab was adopted as first-line therapy for metastatic colorectal cancer (mCRC) based on significant survival improvements in initial clinical trials; however, survival benefit diminished in subsequent analyses. Consequently, there is uncertainty surrounding the cost-effectiveness of bevacizumab therapy achieved in practice. Objective. To assess real-world cost-effectiveness of first-line bevacizumab with irinotecan-based chemotherapy versus irinotecan-based chemotherapy alone for mCRC in British Columbia (BC), Saskatchewan, and Ontario, Canada. Methods. Using provincial cancer registries and linked administrative databases, we identified mCRC patients who initiated publicly funded irinotecan-based chemotherapy, with or without bevacizumab, in 2000 to 2015. We compared bevacizumab-treated patients to historical controls (treated before bevacizumab funding) and contemporaneous controls (receiving chemotherapy without bevacizumab), using inverse-probability-of-treatment weighting with propensity scores to balance baseline covariates. We calculated incremental cost-effectiveness ratios (ICER) using 5-year cost and survival adjusted for censoring, with bootstrapping to characterize uncertainty. We also conducted one-way sensitivity analysis for key drivers of cost-effectiveness. Results. The cohorts included 12,112 (Ontario), 1,161 (Saskatchewan), and 2,977 (BC) patients. Bevacizumab significantly increased treatment costs, with mean ICERs between $78,000 and $84,000/LYG (life-year gained) in the contemporaneous comparisons and $75,000 and $101,000/LYG in the historical comparisons. Reducing the cost of bevacizumab by 50% brought ICERs in all comparisons below $61,000/LYG. Limitations. Residual confounding in observational data may bias results, while the use of original list prices overestimates current bevacizumab cost. Conclusion. The addition of bevacizumab to irinotecan-based chemotherapy extended survival for mCRC patients but at significant cost. At original list prices bevacizumab can only be considered cost-effective with certainty at a willingness-to-pay threshold over $100,000/LYG, but price reductions or discounts have a significant impact on cost-effectiveness.
Keywords: colorectal cancer, cost-effectiveness, linked health data, oncology, real-world evidence
Introduction
Drug funding decisions are typically informed by evidence from randomized controlled trials (RCTs), but there are often unresolved questions about effectiveness in routine clinical practice. Real-world evidence (RWE) is a valuable tool for situations where there is residual uncertainty about the comparative effectiveness, safety, or cost-effectiveness of a health technology or drug. 1 RWE is derived from data collected outside the setting of RCTs, such as registries, administrative data, surveys, or health records. 2 A key strength of RWE generated from population-based administrative data is that it can account for the entire unselected patient populations, including older patients, patients with comorbid conditions, or patients treated outside major centers, who are often excluded from RCTs. 3 RWE can also incorporate real-world health care provider or policy factors, such as treatment patterns, that are strictly controlled in RCTs, and can include data that are not usually collected in RCTs, such as long-term health system resource use and cost. 2 However, the greater external validity of RWE comes at the expense of potential bias arising from nonrandomized data. RWE provides the means to better understand the actual effectiveness and cost-effectiveness of funded therapies achieved in practice, but robust RWE requires high-quality data and appropriate observational study design and analytical methods.
An example of this evidentiary uncertainty is the case of bevacizumab for metastatic colorectal cancer (mCRC). Initial clinical trials of bevacizumab in combination with chemotherapy demonstrated improvements in median overall survival of up to 7.7 months. 4 On the basis of these results, bevacizumab quickly became standard first-line therapy for advanced colorectal cancer. However, a subsequent meta-analysis of seven clinical trials reported an improvement in median overall survival of only 2.6 months. 5 While the improvement in survival remained statistically significant, the magnitude of benefit was considerably smaller than initial expectations and may not be clinically significant.
This reduction in anticipated survival benefit also affects the anticipated cost-effectiveness of bevacizumab. Economic models developed with initial clinical trial evidence generated incremental cost-effectiveness ratios of over £60,000/QALY (quality-adjusted life-year; CAD$135,000/QALY)6,7 and 668,000 Norwegian Krone (NOK)/life-year gained (LYG; CAD$122,000/LYG)7,8 for bevacizumab in combination with irinotecan, and over £100,000/QALY (CAD$187,000/QALY)9,10 and 549,000 NOK/LYG (CAD$101,000/LYG) 8 for bevacizumab with oxaliplatin. These models use short-term survival estimates from clinical trials, combined with cost estimates from secondary sources or assumptions, to project costs and survival over patients’ lifetimes. Decision models are a valuable tool for economic evaluation and make the most use of the evidence available at the time they are built; however, they rely on extrapolation beyond the data observed in trials and synthesis of multiple data sources, making results sensitive to the assumptions underlying the model and the quality of the inputs used. 11 With the considerable uncertainty around the actual survival benefits achieved with bevacizumab, there is also uncertainty around its cost-effectiveness in real-world practice. Furthermore, biosimilar bevacizumab was introduced in late 2019, 12 presenting an opportunity to significantly reduce treatment costs. Understanding the cost-effectiveness of bevacizumab attained in practice can provide decision makers with evidence of the real-world value of treatment, to inform price negotiations for biosimilars. 13
The objective of our study was to conduct a real-world evaluation of the cost-effectiveness of bevacizumab in combination with irinotecan-based chemotherapy for mCRC and to demonstrate the feasibility of generating high-quality RWE using population-based administrative data in three Canadian provinces.
Methods
Study Design
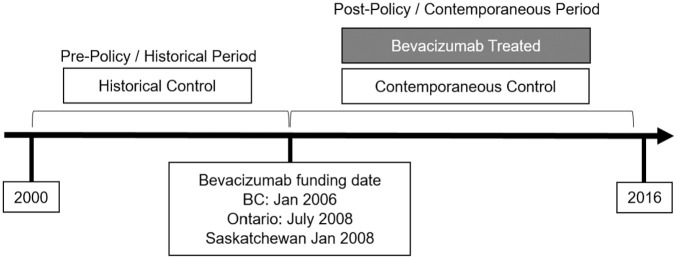
We used a historical cohort study design to evaluate the cost-effectiveness of irinotecan-based chemotherapy with bevacizumab or irinotecan-based chemotherapy alone as first-line therapy for mCRC. The study was conducted in parallel in three Canadian provinces: British Columbia (BC), Saskatchewan, and Ontario. Our intervention of interest was the use of bevacizumab with irinotecan-based chemotherapy, compared to irinotecan-based chemotherapy alone. Each patient’s date of treatment initiation was used as their index date for this study. Patients who initiated therapy with bevacizumab after each province’s funding date (policy change) were assigned to the bevacizumab treatment group (Figure 1). Bevacizumab was funded effective January 1, 2006, in BC; January 1, 2008, in Saskatchewan; and July 1, 2008, in Ontario. Patients who initiated chemotherapy without bevacizumab were assigned to one of two control groups: those with index dates before the policy change were included in the historical comparison group, and those with index dates after the policy change were included in the contemporaneous control group. This study design allows us to capture the cost-effectiveness of bevacizumab achieved in standard clinical practice, without the restrictive protocols of a clinical trial, or the parametric assumptions of a decision model. Survival with mCRC is relatively short, and bevacizumab has been standard practice for a sufficiently long period of time that most patients in our mCRC cohort have complete (lifetime) cost and survival data over the study period.
Figure 1.
Study design
Cohort Definition
The study cohort included all adult patients (≥18 years old at diagnosis) with a registry-confirmed diagnosis of colorectal cancer (ICD-O-3 codes C18-C20), who initiated irinotecan-based chemotherapy between January 1, 2000, and March 31, 2015 (BC and Ontario), or January 1, 2003, and December 31, 2015 (Saskatchewan). Patients were excluded from the cohort if they had a missing ICD-O-3 diagnosis code; missing age, sex, or provincial health insurance number; if their registered cancer diagnosis date was more than 60 days after initiating treatment; if they received bevacizumab before the policy change; or if they initiated irinotecan before the policy change, and subsequently added bevacizumab when it became available. We did not restrict patients based on specific irinotecan treatment protocols; however, FOLFIRI (irinotecan with leucovorin and fluorouracil) is the standard of care in all three provinces.
Data Sources
Cohorts were created separately in each of the three provinces using common definitions. Cohorts were defined using the BC Cancer Registry and BC Provincial Systemic Therapy Program data 14 in BC, the Saskatchewan Cancer Registry and Saskatchewan Cancer Agency Pharmacy Oncology database in Saskatchewan, and the Ontario Cancer Registry and the New Drug Funding Program database in Ontario. Cohorts in each province were linked to their respective administrative databases using unique patient identifiers (Table 1).15–19
Table 1.
Data Sources in British Columbia, Saskatchewan, and Ontario
| British Columbia | Saskatchewan | Ontario | |
|---|---|---|---|
| Patient identifier | BC Personal Health Number | Saskatchewan Health Service Number | Ontario Health Insurance Plan Number |
| Cohort characteristics | |||
| Patient demographics | Consolidation file, Population Data BC | Saskatchewan Cancer Registry | Ontario Cancer Registry, Registered Persons Database |
| Disease characteristics | BC Cancer Registry | Saskatchewan Cancer Registry | Ontario Cancer Registry |
| Treatment history | BC Provincial Systemic Therapy Program BC Cancer Radiotherapy database CIHI DAD |
SCA Pharmacy Oncology database SCA Radiation Oncology database CIHI DAD |
New Drug Funding Program Ontario Drug Benefit database Activity Level Reporting database CIHI DAD |
| Survival | |||
| Death records | Deaths file, BC Vital Statistics | Saskatchewan Vital Statistics | Registered Persons Database |
| Cost | |||
| Systemic therapy | BC Provincial Systemic Therapy Program | SCA Pharmacy Oncology database | New Drug Funding Program |
| Hospitalization | CIHI DAD | CIHI DAD | CIHI DAD |
| Physician Services | Medical Services Plan Payment Information file | Fee-for-service physician claims, SK MOH | Ontario Health Insurance Plan database |
| Other | BC Cancer Radiotherapy database BC PharmaNet database |
SCA Radiotherapy database | CIHI NACRS Ontario Drug Benefit database Activity Level Reporting Complex Continuing Care Reporting System Home Care Database |
ALR, activity-level reporting; CIHI DAD, Canadian Institutes for Health Information Discharge Abstract Database; NACRS, National Ambulatory Care Reporting System; NDFP, New Drug Funding Program; SCA, Saskatchewan Cancer Agency.
Patient Characteristics
Baseline demographic and clinical characteristics were obtained from the administrative data, and included age at index date, sex, health region (province-specific), neighborhood income quintile, rurality, colorectal cancer type (ICD-O-3 code C18, C19, or C20), index year, days from diagnosis to index date, comorbidity (Charlson comorbidity score and number of Johns Hopkins Adjusted Clinical Groups [ACG] 20 excluding cancer, where available), and indicators for other prior cancer diagnosis, prior adjuvant chemotherapy (oxaliplatin, capecitabine, or fluorouracil, where available), prior radiation treatment, and prior colorectal surgery. Cohort characteristics were summarized with descriptive statistics, stratified by policy group (pre- or post-policy implementation) and treatment group (chemotherapy with or without bevacizumab). Differences between groups were examined using chi-squared tests for categorical variables, Cochran-Armitage trend test for ordinal variables, and one-way ANOVA for continuous variables.
Propensity Score Weighting
Propensity score-based methods were used to balance baseline cohort characteristics. Propensity score is the probability of being in the treatment group of interest, given a set of observed variables. Balancing propensity scores between groups can serve to balance baseline characteristics, to reduce selection bias and confounding in observational studies. 21 Propensity scores were estimated separately for the contemporaneous and historical comparisons, using multivariable logistic regression adjusting for baseline variables described above. The scores were used for propensity score matching (PSM), where cases are matched to controls with similar propensity score value, and inverse probability of treatment weighting (IPTW), where individuals are weighted based on their propensity score to create a synthetic sample where baseline covariates are independent of treatment group. 21 PSM was carried out with a caliper width of 0.2 standard deviations, sampled with replacement and matched 1:1 between treated and control groups. For the contemporaneous comparison, patients were also hard-matched on index year. For IPTW, weights were calculated as the inverse of the propensity score. Weights above five were truncated to reduce the influence of outliers. Following matching or weighting, we assessed the balance of baseline variables in the contemporaneous and historical comparisons using standardized differences. All analyses were conducted in both the PSM and IPTW cohorts to verify that our choice of adjustment method did not influence results. However, the two sets of results have slightly different interpretations: IPTW provides the average treatment effect in the cohort, while PSM provides the average treatment effect among patients in the treatment group. 22 Results for the IPTW cohort are presented in this article; results from PSM are available in the appendix.
Survival and Cost Outcomes
Survival and cost for this study were calculated using a 5-year time horizon. Our preliminary analysis indicated that by 5 years after starting first-line treatment, Kaplan-Meier survival curves in most comparison groups had flattened and converged, with survival rates <15% (Appendix Figure 1). Restricting the time horizon may introduce bias by underestimating survival benefit, especially for the Ontario analysis, where a small survival benefit remained after 5 years. In BC and Saskatchewan, very few patients in the cohort were observed for more than 5 years, and after 5 years there was minimal difference in survival between treatment groups.
Patient survival was calculated as the total time from index date to death or censoring. Patients were censored at 5 years of follow-up, at the end of observation (December 31, 2015), or if they were no longer registered for provincial health insurance. Survival was expressed in years.
Total cost was calculated at the patient level, using the most complete administrative data available in each province. Components of total costs varied from province to province, but all provinces included the following: hospitalization and surgery costs, calculated using the Canadian Institute for Health Information (CIHI) Resource Intensity Weight (RIW) method 23 ; cost of physician services, calculated from provincial fee-for-service claims databases; and systemic therapy costs, calculated from pharmacy dispensing records (BC and Saskatchewan) or drug program data (Ontario). In all three provinces, systemic therapy costs were calculated based on price when the drug was dispensed. Additionally, costs of radiotherapy were included in BC and Saskatchewan, costs of outpatient prescription drugs were included in BC and Ontario, and costs of emergency department visits, complex continuing care, home care, and long-term care were included in Ontario. Due to changes in data collection over time, Ontario was also able to include the costs of ambulatory cancer care services in the contemporaneous comparison. Costs were calculated from the perspective of the public payer, and expressed in 2019 Canadian dollars. 24
We conducted inverse probability weighting (IPW) of 5-year cost and survival to adjust for censoring. In IPW, cost and survival values for patients under observation are re-weighted to account for patients who have been censored earlier in the study. 25 IPW costs and survival were calculated for each comparison group using Kaplan-Meier survival estimates for each 30-day interval over the 5-year follow-up period. Costs and survival time were discounted at 1.5% per year. 26
Cost-Effectiveness Analysis
Our primary endpoint for this cost-effectiveness analysis was the incremental cost-effectiveness ratio (ICER), calculated as
where ΔC is the change in total cost and ΔE is the change in total survival time between patients receiving irinotecan with bevacizumab (C1 and E1), versus irinotecan alone (C0 and E0). 27 The ICER represents the cost associated with each additional year of life from treatment, and is expressed as dollars per life-year gained ($/LYG). ICERs were calculated for both the historical and contemporaneous comparisons. We estimated mean and 95% confidence intervals for cost, survival, and ICER values using nonparametric bootstrapping with 1,000 samples. 28 In each bootstrap iteration in the IPTW cohort, propensity weights were recalculated to maintain covariate balance. In the PSM cohort, pairs were sampled together. The distribution of ICERs from bootstrapping is summarized in cost-effectiveness acceptability curves (CEACs).
The secondary endpoint of interest was the incremental net monetary benefit (NMB). NMB is calculated at the patient level as
where λ is the willingness-to-pay in $/LYG, is total patient costs, and is survival time. 27 The incremental NMB of a treatment is estimated using the following model:
where α is the intercept, ti is the treatment indicator, Xi represents a vector of covariates, and the regression coefficient for treatment, β1, is the incremental NMB of treatment. Positive incremental NMB values (incremental NMB > 0) indicate an intervention is cost effective at the specified threshold. 29 We estimated incremental NMB at λ=$50,000/LYG and λ=$100,000/LYG using two models: Model 1, a simple linear regression with treatment indicator only; and Model 2, multivariable linear regression, adjusted for subsequent treatments, with indicators for second-line therapy, third-line therapy, liver resection, lung resection, and colorectal surgery. We conducted exploratory analysis to identify potential heterogeneity in NMB across patient or disease characteristics, but found no association.
Sensitivity Analysis
We conducted sensitivity analysis to assess the impact of alternative assumptions on our results, including discount rates of 0% and 3%, 26 and limiting the costing scope to include only the resource categories with complete data common to all three provinces (hospitalization, fee-for-service physician claims, and drug costs for cancer systemic therapy). To explore the potential impact of cost reductions associated with the introduction of biosimilar bevacizumab, we included scenarios in sensitivity analysis where we reduced the cost of bevacizumab by 25% and 50%. Last, we incorporated utility weights to calculate quality-adjusted life-years (QALYs) and the cost-utility ratio ($/QALY). A limitation of administrative data analysis is that individual-level utility data were not available. To calculate QALYs, we divided patients’ survival time into two health states, time on first-line treatment and post-treatment survival, and applied utility weights from the NICE evaluation of first-line bevacizumab across all relevant patients. 6 Time on treatment was defined as the time from index date to the end of first-line therapy (defined as 30 days after last dispensing record for first-line therapy), and post-treatment survival was defined as the time from the end of first-line therapy to death or censoring. Utility weights for time on treatment were sampled from a beta distribution with a mean of 0.80 and SD 0.08, and utility for posttreatment was applied as a disutility (subtracted from the sampled time-on-treatment value) with a mean of 0.20 and SD 0.02. Utility values were sampled once per person per bootstrap iteration.
All analysis was conducted in SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Cohort Characteristics
The study cohorts consisted of 2,977 patients in BC, 1,161 patients in Saskatchewan, and 12,112 patients in Ontario, with 1,568 (BC), 502 (Saskatchewan), and 4,914 (Ontario) patients receiving bevacizumab after the drug was funded. The characteristics of the cohorts are summarized in Table 2. The average age of the cohorts was between 63 and 64 years, over 60% of patients were male, and the majority had colon cancer. In all provinces, patients receiving first-line bevacizumab were significantly younger, and less likely to have had a prior cancer diagnosis than contemporaneous patients receiving chemotherapy alone. In the BC and Ontario cohorts, the bevacizumab treatment group also had significantly lower comorbidity scores and a shorter time between diagnosis and first-line treatment than the contemporaneous comparison group. The characteristics of the cohort after IPTW are summarized in the appendix. Balance (defined as standardized difference ≤0.10) was achieved in all baseline covariates, with the exception of age in the BC contemporaneous comparison (weighted mean [SD] of 65.50 [17.89] years in the chemotherapy group and 63.82 [13.55] years in the bevacizumab group, standard difference of 0.11).
Table 2.
Baseline Cohort Characteristics by Time Period (Before or After Bevacizumab Funding Policy) and by Treatment Group, in British Columbia, Saskatchewan, and Ontario
| British Columbia | Saskatchewan | Ontario | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Policy | Post-Policy | Pre-Policy | Post-Policy | Pre-Policy | Post-Policy | |||||
| Chemo | Chemo + Bevacizumab | Chemo | Chemo + Bevacizumab | Chemo | Chemo + Bevacizumab | |||||
| (N = 601) | (N = 808) | (N = 1,568) | (N = 402) | (N = 239) | (N = 502) | (N = 5,886) | (N = 1,312) | (N = 4,914) | ||
| Age in years | Mean ± SD | 61.8 ± 11.1 | 67.8 ± 11.0 | 62.5 ± 10.9* | 64.8 ± 11.0 | 66.5 ± 11.9 | 62.9 ± 11.3* | 62.9 ± 10.9 | 65.8 ± 11.3* | 62.5 ± 11.1* |
| Median (IQR) | 63 (55–70) | 69 (60–76) | 64 (56–70) | 65.7 (57–73) | 68.8 (56–76) | 63.5 (56–72)* | 64 (56–71) | 67 (59–74)* | 63 (55–71)* | |
| Sex, n (%) | Female | 220 (36.6) | 326 (40.4) | 641 (40.9) | 137 (34.1) | 88 (36.8) | 203 (39.0) | 2,293 (39.0) | 524 (39.9) | 1,964 (40.0%) |
| Male | 381 (63.4) | 482 (59.7) | 927 (59.1) | 265 (65.9) | 151 (63.2) | 317 (61.0) | 3,593 (61.0) | 788 (60.1) | 2,950 (60.0%) | |
| Income quintile, n (%) | 1 (lowest) | 121 (20.1) | 175 (21.7) | 311 (19.8) | 74 (18.4) | 44 (18.4) | 101 (19.4) | 1,004 (17.1) | 238 (18.1) | 832 (16.9%) |
| 2 | 106 (17.6) | 167 (20.7) | 306 (19.5) | 93 (23.1) | 58 (24.3) | 96 (18.5) | 1,226 (20.8) | 245 (18.7) | 997 (20.3%) | |
| 3 | 126 (21.0) | 150 (18.6) | 316 (20.2) | 85 (21.1) | 34 (14.2) | 109 (21.0) | 1,175 (20.0) | 285 (21.7) | 1,015 (20.7%) | |
| 4 | 125 (20.8) | 163 (20.2) | 325 (20.7) | 78 (19.4) | 55 (23.0) | 111 (21.3) | 1,228 (20.9) | 253 (19.3) | 1,036 (21.1%) | |
| 5 (highest) | 123 (20.5) | 153 (18.9) | 310 (19.8) | 69 (17.2) | 45 (18.8) | 97 (18.7) | 1,218 (20.7) | 279 (21.3) | 1,015 (20.7%) | |
| Rurality, n (%) | Urban | 531 (88.4) | 675 (83.5) | 1,367 (87.2) | 212 (52.7) | 123 (51.5) | 293 (56.3) | |||
| Rural | 70 (11.7) | 133 (16.5) | 201 (12.8) † | 190 (47.3) | 116 (48.5) | 227 (43.7) | 887 (15.1) | 216 (16.5) | 747 (15.2%) | |
| Colorectal cancer type, n (%) | C18 | 374 (62.2) | 515 (63.7) | 1,008 (64.3) | 243 (60.4) | 155 (64.9) | 322 (61.9) | 3,754 (63.8)* | 889 (67.8) | 3,205 (65.2%) |
| C19 | 55 (9.2) | 55 (6.8) | 102 (6.5) | 52 (12.9) | 9 (3.8) | 44 (8.5) | 800 (13.6)* | 129 (9.8) | 561 (11.4%) | |
| C20 | 172 (28.6) | 238 (29.5) | 458 (29.2) | 107 (26.6) | 75 (31.4) | 154 (29.6) | 1,332 (22.6)* | 294 (22.4) | 1,148 (23.4%) | |
| Other prior cancer diagnosis, n (%) | 64 (10.7%) | 121 (15.0) | 178 (11.4) † | 58 (14.4) | 45 (18.8) | 65 (12.5) † | 328 (5.6)* | 132 (10.1)* | 409 (8.3)* | |
| Days from diagnosis to treatment | Mean ± SD | 347 ± 383 | 554 ± 648 | 469 ± 654 † | 544 ± 756 | 442 ± 628 | 471 ± 790 | 566 ± 923 | 607 ± 1,001* | 538 ± 861* |
| Median (IQR) | 175 (70–517) | 304 (88–805) | 132 (57–672.5) | 207.5 (85–737) | 189 (59–598) | 111 (64–608) | 193 (65–710) | 218 (59–769) | 162 (62–719) | |
| Total ADGs | Mean ± SD | 8.94 ± 3.40 | 9.91 ± 3.41 | 9.05 ± 3.32* | Not available | 8.40 ± 3.10 | 9.07 ± 3.25* | 8.27 ± 3.17* | ||
| Charlson’s score | Mean ± SD | 0.14 ± 0.56 | 0.27 ± 0.72 | 0.14 ± 0.45* | 0.40 ± 0.86 | 0.58 ± 1.04 | 0.33 ± 0.78 | 0.25 ± 0.65* | 0.46 ± 0.95* | 0.25 ± 0.66* |
| Prior chemotherapy, n (%) | Capecitabine | 62 (10.3) | 293 (36.3) | 359 (22.9)* | 24 (6.0) | 65 (27.2) | 73 (14.0) | Not available | 183 (13.9) | 340 (6.9) |
| Fluorouracil | 231 (38.4) | 193 (23.9) | 371 (23.7) | 84 (20.9) | 40 (16.7) | 71 (13.7) | 838 (63.9) | 2,879 (58.6) | ||
| Oxaliplatin | 51 (8.5) | 113 (14.0) | 316 (20.2)* | <5 (0.5) | 47 (19.7) | 101 (19.4) | 269 (20.5) | 1,301 (26.48) | ||
| Prior rectal radiotherapy, n (%) | 69 (11.5%) | 86 (10.6) | 165 (10.5) | 20 (5.0) | 32 (13.4) | 62 (11.9) | 32 (2.44) | 142 (2.89) | ||
| Prior colorectal surgery, n (%) | 481 (80.0%) | 632 (78.2) | 1,265 (80.7) | 348 (86.6) | 188 (78.7) | 375 (72.1) | 4,467 (75.9)* | 956 (72.9)* | 3,948 (80.3)* | |
IQR, interquartile range.
P < 0.05, *P < 0.001.
Cost
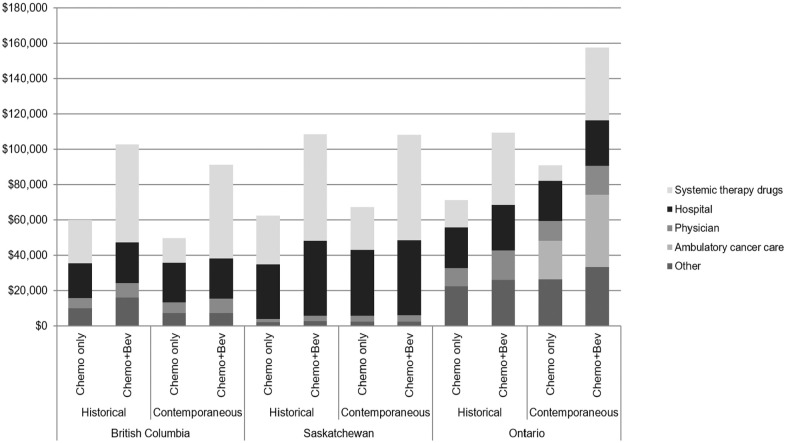
Average 5-year costs ranged from $60,100 to $71,300 in the historical comparison group, $49,700 to $90,800 in the contemporaneous comparison group, and $91,300 to $157,400 in the bevacizumab treatment group (Figure 2). In all provinces, the largest component of the total cost in the bevacizumab-treated group was systemic therapy drug costs. In all but one of the comparison groups, hospital costs were the largest component of total cost.
Figure 2.
Five-year costs of treatment in British Columbia (BC), Saskatchewan, and Ontario. Costs expressed as 2019 Canadian dollars. Other costs include radiotherapy treatment (BC and Saskatchewan), outpatient prescription drugs (BC and Ontario), and long-term care, complex continuing care, home care, and emergency department visits (Ontario only). Ambulatory cancer clinic costs were only available for Ontario contemporaneous comparison.
Incremental Cost-Effectiveness Ratios
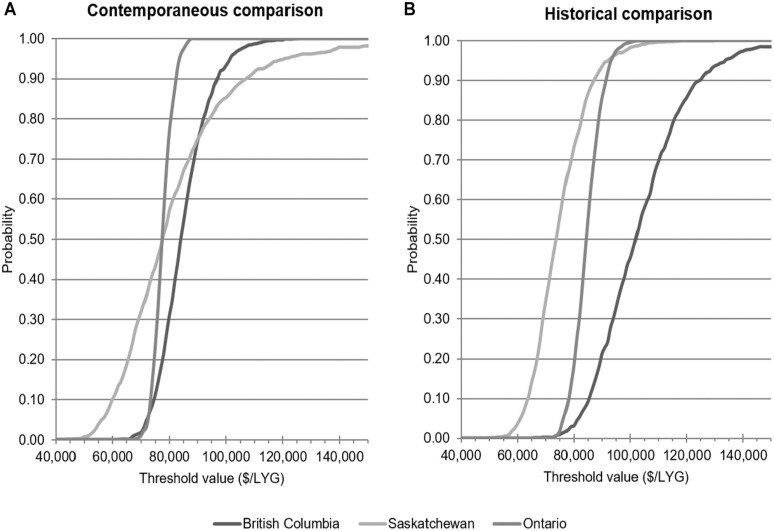
The results of cost-effectiveness analysis are summarized in Table 3. Average incremental costs for the addition of bevacizumab to first-line irinotecan chemotherapy were similar in BC and Saskatchewan, for both the contemporaneous and historical comparisons after IPTW weighting, were around $41,000 to $47,000. Incremental costs were higher in Ontario for the contemporaneous comparison, and lower for the historical comparison. The addition of bevacizumab produced a mean survival improvement of around 0.4 to 0.6 LYG in all comparisons, except for the contemporaneous comparison in Ontario, where the mean effectiveness was 0.83 LYG (95% confidence interval [CI]: 0.75–0.92). Incremental cost-effectiveness ratios for the historical comparison ranged from a low of $74,882/LYG (95% CI: 58,954–98,230) in Saskatchewan, to a high of $101,181/LYG (95% CI: 78,653–140,349) in BC. The range of ICERs for the contemporaneous comparison was narrower, between roughly $78,000 and $84,000/LYG. At a threshold of $50,000/LYG, there was a very low probability (<1%) that bevacizumab is cost-effective in any of the comparisons (Figure 3). At a threshold value of $100,000/LYG, there was a high probability (>85%) of bevacizumab being cost-effective according to the contemporaneous comparisons, but the results of the historical comparisons were mixed.
Table 3.
Incremental Cost-Effectiveness of Bevacizumab Plus Irinotecan-Based Chemotherapy Versus Irinotecan-Based Chemotherapy Alone, in British Columbia, Saskatchewan, and Ontario a
| Province | Comparison | Incremental Cost | Incremental Effectiveness (Life-Years Gained [LYG]) | Incremental Cost-Effectiveness Ratio ($/LYG) | |||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||
| British Columbia | Contemporaneous | $46,841 | 41,817–51,661 | 0.56 | 0.43–0.69 | $84,243 | 70,806–105,820 |
| Historical | $42,535 | 37,345–47,573 | 0.42 | 0.29–0.57 | $101,181 | 78,653–140,349 | |
| Saskatchewan | Contemporaneous | $41,212 | 29,460–52,549 | 0.53 | 0.28–0.78 | $82,002 | 53,739–138,793 |
| Historical | $46,023 | 37,326–54,789 | 0.62 | 0.43–0.83 | $74,882 | 58,954–98,230 | |
| Ontario | Contemporaneous | $64,674 | 59,491–70,163 | 0.83 | 0.75–0.92 | $77,660 | 71,523–85,125 |
| Historical | $38,541 | 35,855–41,083 | 0.46 | 0.40–0.52 | $84,609 | 75,287–95,228 | |
CI, confidence interval; LYG, life-year gained.
Inverse-probability-weighted estimates with 5-year follow-up; 2019 Canadian dollars; discount rate for cost and life-years of 1.5% per year.
Figure 3.
Cost-effectiveness acceptability curve for bevacizumab plus irinotecan-based chemotherapy versus irinotecan-based chemotherapy alone in contemporaneous control group (panel A) or historical control group (panel B), in British Columbia, Saskatchewan, and Ontario.
Incremental Net Monetary Benefit
The unadjusted NMB regression (Model 1 in Table 4) indicated that only the incremental NMB values from the contemporaneous comparisons in Ontario, and the historical comparisons in Ontario and Saskatchewan, were significantly greater than 0 at a threshold of $100,000/LYG. Adjusting for subsequent treatment (Model 2) decreased the incremental NMB in all comparisons with one exception where only the contemporaneous comparison in Ontario remains positive and statistically significant, with an incremental NMB of $16,870 (95% CI: 11,024–22,716). The results for Saskatchewan (from both contemporaneous and historical comparisons) and BC (from contemporaneous comparison) showed positive incremental NMB but the findings were not significant.
Table 4.
Incremental Net Monetary Benefit of Bevacizumab Plus Irinotecan-Based Chemotherapy, Versus Chemotherapy Alone, at a Willingness-to-Pay of $100,000 per Life-Year Gained
| Province | Comparison | Model 1 a | Model 2 b | ||
|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||
| British Columbia | Contemporaneous | $8,728 | −1,134, 18,590 | $3,785 | −5,500, 13,071 |
| Historical | −$976 | −11,435, 9,482 | −$7,620 | −17,386, 2,145 | |
| Saskatchewan | Contemporaneous | $11,715 | −8,944, 32,375 | $11,369 | −8,146, 30,867 |
| Historical | $16,264 | 750, 31,769* | $11,166 | −3,693, 26,025 | |
| Ontario | Contemporaneous | $23,004 | 17,011, 28,997* | $16,870 | 11,024, 22,716* |
| Historical | $9,185 | 3,566, 14,804* | −$1,456 | −7,090, 4,178 | |
Model 1: Bevacizumab treatment only.
Model 2: Adjusted for subsequent treatment, with indicators for second-line therapy, third-line therapy, liver resection, lung resection, or colorectal surgery.
P < 0.05.
Sensitivity Analysis
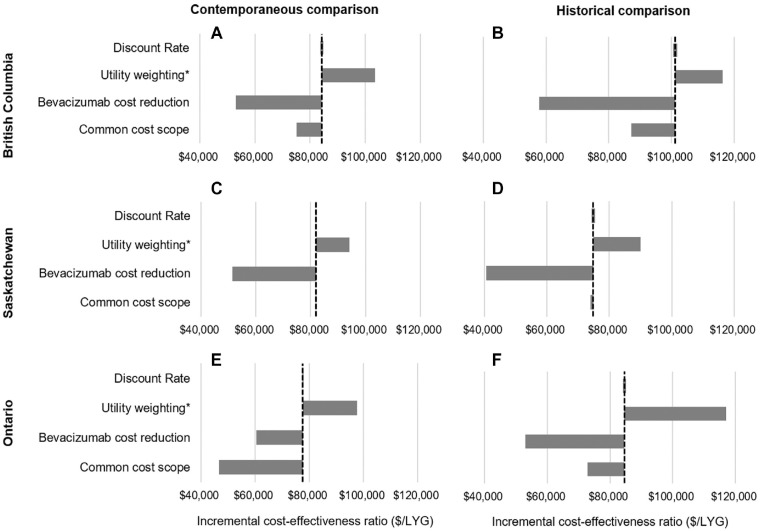
Adjusting for quality of life results in incremental QALY estimates of 0.45 to 0.66 for the contemporaneous comparison and 0.33 to 0.52 for the historical comparison (Appendix Tables 4 and 5), resulting in ICERs of $90,000 to $117,100/QALY (Figure 4). Reducing the costs of bevacizumab by up to 50% substantially reduces the incremental costs of treatment, bringing the ICER to under $61,000/LYG in all comparisons. Last, limiting the scope of the included costs to only the elements in common for all three provinces (hospitalization, systemic therapy, and fee-for-service physician claims) reduces the ICERs in the Ontario analysis to $46,696/LYG (95% CI: 42,566–51,664) in the contemporaneous comparison and $72,748/LYG (95% CI: 64,644–81,714) in the historical comparison. The ICER is not sensitive to the choice of discount rate.
Figure 4.
Results of sensitivity analysis for cost-effectiveness of bevacizumab in British Columbia (panels A and B), Saskatchewan (panels C and D), and Ontario (panels E and F), in contemporaneous and historical comparisons, respectively. Vertical dashed line indicates results of base case analysis. Discount rate was varied from 0% to 3%. Utility weights applied were a mean of 0.8 for time on first-line treatment and mean of 0.6 for posttreatment survival. Bevacizumab costs were reduced by 50%. Common cost scope was limited to hospitalization, systemic therapy drug cost, and physician claims. *Incremental cost-effectiveness ratio for utility weighted results are expressed in dollars per quality-adjusted life-year ($/QALY).
Discussion
In this study, we compared the cost-effectiveness of irinotecan-based chemotherapy in combination with bevacizumab to irinotecan-based chemotherapy alone in patients with advanced colorectal cancer, using real-world data from BC, Saskatchewan, and Ontario. While bevacizumab extended survival by about half a year in most comparisons, this benefit came at a significant cost. The mean incremental cost between patients receiving bevacizumab with irinotecan chemotherapy, compared to those receiving irinotecan chemotherapy alone, was at least $40,000. The majority of this incremental cost was made up by the cost of systemic therapy. Using the contemporaneous comparison group, we found that mean ICERs for bevacizumab treatment were below $100,000/LYG in all provinces. Using a historical comparison group, bevacizumab was cost-effective at $100,000/LYG in Saskatchewan and Ontario. At historical list prices, bevacizumab with irinotecan-based chemotherapy can only be considered cost-effective with a high degree of certainty if payers’ willingness-to-pay threshold is over $100,000/LYG. However, we also found that these results are highly sensitive to the cost of bevacizumab. A reduction in bevacizumab costs of 25% or 50%, discounts achievable with biosimilars, brought ICERs to under $80,000/LYG and $61,000/LYG, respectively.
Published cost-effectiveness analyses of bevacizumab with irinotecan-based chemotherapy provide wide-ranging results. The economic evaluation conducted as part of the UK National Institute for Health and Care Excellence (NICE) review used clinical trial data to construct a health economic model to simulate overall survival, health-related quality of life, and resource use. The study reported incremental costs of £19,361 (roughly CAD$43,000) 7 for the addition of bevacizumab to irinotecan-based chemotherapy, giving an ICER of £46,853/LYG (CAD$103,000/LYG) or £62,857/QALY (CAD$139,000/QALY). 6 Despite the difference in method and setting, both the incremental cost and ICER values from the original NICE evaluation are similar to our findings in this analysis. Other decision models of bevacizumab with irinotecan have provided similar estimates of 668,000 NOK/LYG (CAD$122,000/LYG), 8 ¥11.9M/LYG (CAD$123,500/LYG), 30 and CAD$139,000/QALY. 31 Lee and colleagues reported an ICER of 41.2M won per LYG (CAD$39,000/LYG), 32 but the survival projected by the model (1.2 LYG) is considerably longer than other results in the literature 33 and our findings here. A previous study by researchers in BC used preliminary observational data to build a health economic model following the funding of bevacizumab with either irinotecan- or oxaliplatin-based chemotherapy. The analysis compared pre- and post-policy periods, and reported incremental costs of only $3,792 per patient, and an ICER of $15,617/LYG or $62,469/QALY, 34 much lower than the values reported here. The earlier study was limited by short follow-up time after the policy change, and reflected the initial funding policy restricting bevacizumab use to 6 months. Observational studies of bevacizumab with irinotecan have also reported mixed results; Ruiz-Millo and colleagues reported increased costs of €12,700 per patient (CAD$17,300), with no significant improvement in progression-free survival in a small hospital setting, 35 while Shankaran and colleagues reported an ICER of US$75,303 (CAD$98,700) using linked SEER-Medicare data. 36
The secondary objective of our study was to demonstrate the feasibility of generating high-quality RWE using Canadian administrative data. An important finding of our study is that incremental costs and ICER estimates were generally similar between all three provinces, despite minor differences in funding policies, health system structure, and data availability. This concordance strengthens our conclusions and suggests that our findings may be generalizable to other Canadian provinces. Among the three provinces included in this study, Saskatchewan and BC have the most similar systemic therapy program structure and data structure. In both BC and Saskatchewan, all systemic therapy is provided or funded through their respective centralized cancer agency programs (with central databases of dispensing records), while in Ontario systemic therapy is funded through a combination of cancer agency programs, public prescription drug insurance, and hospital budgets. 37 However, the scope of the cost calculation in BC and Saskatchewan was narrower than the cost calculation in Ontario due to limitations in available data; Ontario was able to conduct a comprehensive cost calculation, including emergency department care, ambulatory cancer care, continuing care, and home care. BC and Saskatchewan were limited to calculating fee-for-service physician costs, while Ontario was able to include cost of physician services delivered through alternative funding arrangements. We found that the incremental costs of bevacizumab were within a very narrow range in BC and Saskatchewan for both the contemporaneous and historical comparisons ($39,500–$44,200), while the costs reported in Ontario were higher, as expected. In sensitivity analysis, we restricted the scope of the cost calculation to only common elements and found large reduction in the ICER from the Ontario contemporaneous comparison. In the original comparison, ambulatory cancer care costs (Figure 2) contributed significantly to the incremental costs of bevacizumab treatment in Ontario, but we were unable to capture these costs in the other provinces. This finding reveals a considerable gap in the cost data in BC and Saskatchewan that should be addressed in future RWE research, and suggests that both the average and incremental costs reported for these provinces are underestimated.
We observed that results from the contemporaneous comparisons were very similar between provinces, while the results of the historical comparisons were more variable. There are strengths and weaknesses to both comparison groups, and consequently both sets of results should be interpreted carefully. There is likely to be residual confounding in the historical comparisons, arising from changes in policy and practice over time. For example, in BC and Ontario, bevacizumab was initially approved for 6 months of treatment, with extensions reviewed on a case-by-case basis; bevacizumab became available for use until progression in early 2008 in BC and in late 2009 in Ontario. 38 Concurrently, generic irinotecan became available during the study period, decreasing systemic therapy costs in the post-policy period, independent of bevacizumab treatment. Cetuximab and panitumumab also became available in all provinces as third-line therapy in 2009, likely increasing systemic therapy costs and survival in later years of the study. We found that adjusting for subsequent lines of treatment attenuates incremental NMB, suggesting that some of the observed survival benefit can be attributed to later treatment. In the contemporaneous analysis, results may be biased by the highly selected nature of the comparison group. Bevacizumab became standard first-line therapy in the post-policy period, and patients who did not receive bevacizumab were significantly older, had higher comorbidity scores, and were more likely to have a previous cancer diagnosis than patients who received bevacizumab. We used propensity score methods to create comparison groups that were balanced on baseline covariates to adjust for potential confounding. The IPTW results described above are very similar to the propensity-score matched results presented in the appendix, indicating that the choice of balancing method did not influence our findings. However, with both IPTW and PSM, there are additional unmeasured or unobserved patient or provider factors contributing to residual confounding in the analysis. We were limited to covariates available in the administrative data, and some patient sociodemographic and disease characteristics, such as educational attainment, Eastern Cooperative Oncology Group (ECOG) performance status, and extent of metastatic disease, could not be included. By using both historical and contemporaneous comparison groups, we are able to provide two slightly different perspectives from which to address the research question.
Unlike model-based economic evaluation or cost-effectiveness analysis conducted alongside clinical trials, using real-world observational data allows us to evaluate the cost-effectiveness of bevacizumab as it is used in practice. We chose this study design specifically to understand the effect of bevacizumab on the entire unselected patient population and to demonstrate the strengths and limitations of RWE in this setting; however, conducting secondary analysis of administrative data in this context is subject to its inherent limitations. First, our systemic therapy costs are overestimated, which has the effect of overestimating our ICER values. Our analysis does not account for negotiated price discounts or rebates obtained by the cancer agencies, due to the confidential nature of these agreements. Bevacizumab is also now available as a biosimilar, and the list prices used in this analysis do not reflect current prices. Our sensitivity analysis suggests that a reduction in bevacizumab costs of 50% could bring the ICER below $61,000/LYG. No Canadian jurisdictions use an explicit willingness-to-pay threshold, but this value falls well below previously funded cancer therapies. 39 We also found that the survival benefit observed in this study is larger than the benefit reported in clinical trials, suggesting there may be residual confounding in our analysis, or that there is a difference between RCT and real-world populations. Moreover, our choice of 5-year time horizon may underestimate survival. Overestimating drug costs and underestimating survival could bias our ICER estimates upwards, and the overall magnitude of the effect on our cost-effectiveness results is unknown. A small proportion of patients survived beyond 5 years, and that survival time does not contribute to our cost-effectiveness estimates. In Ontario, we observed small survival differences up to 8 years after treatment in favor of bevacizumab, and survival appeared to have converged in the other provinces. While there was insufficient sample size in the smaller provinces to extend the observation time, given that survival curves had converged or nearly converged, effects on incremental life years would likely be minimal. Model-based analysis typically requires extrapolating short-term survival estimates from clinical trials, and is sensitive to assumptions about the underlying hazard function and the magnitude and duration of benefit. A strength of using nonparametric survival estimates in this analysis is that they do not require these assumptions; however, we are limited by the follow-up time available in the data.
The primary endpoint was cost-effectiveness reported in units of dollars per LYG because we did not have real-world utility data for our patient cohorts, but Canadian economic evaluation guidelines recommend cost-utility analysis, with ICERs expressed as cost per QALY, as the reference case. 26 In model-based analysis, average utility is estimated by applying utility weights collected from clinical trial or population samples to modeled survival estimates. In our sensitivity analysis, we similarly applied utility weights to survival estimates from the real-world data for each patient, and found QALY gains of 0.45 to 0.66 in the contemporaneous comparison and 0.33 to 0.52 in the historical comparison. However, in the absence of individual-level, longitudinal utility data, this approach requires a number of simplifying assumptions. We were unable to identify disease progression from the data, and were limited to defining two health states according to stoppage of treatment. We applied randomly sampled utility weights, independent of treatment group, based on the utilities from the original NICE evaluation of bevacizumab. 6 Our analysis of safety and toxicity of bevacizumab has indicated that patients receiving bevacizumab experienced more adverse events, but lower adverse event rates over time, than patients receiving irinotecan chemotherapy alone. 40 The implications for overall health-related quality of life are not clear. There has been a push for routine collection of patient-reported outcomes in Canada, including utility and quality of life metrics, but such practice is not yet widespread.41,42 Collecting high-quality utility data on a population level and further development of methods to incorporate utility weights into observational studies would considerably strengthen future RWE.
Finally, due to limitations in the data in BC and Saskatchewan, we were unable to evaluate bevacizumab in combination with oxaliplatin-based chemotherapy for first-line therapy of mCRC. Oxaliplatin with bevacizumab is also indicated as adjuvant therapy for patients with resectable primary cancer, and as pseudoadjuvant therapy for patients with resectable liver metastases. We were unable to accurately distinguish adjuvant and pseudoadjuvant indications from first-line therapy in the systemic therapy dispensing records in BC and Saskatchewan, and consequently limited the scope of our analysis to patients receiving first-line irinotecan chemotherapy. Preliminary analysis indicated that a large majority (over 85%) of patients received first-line irinotecan versus oxaliplatin. Published cost-effectiveness estimates suggest that bevacizumab in combination with first-line oxaliplatin may be less cost-effective than bevacizumab in combination with irinotecan, with ICERs of £105,000/QALY (approximately CAD$166,300/QALY) in the UK NICE evaluation, 9 549,000 NOK/LYG (CAD$101,000/LYG) in the Norwegian evaluation, 8 US$571,240/QALY (CAD$630,000/QALY) in a US model, 43 and over US$277,400/QALY (CAD$354,700/QALY) in an international comparison. 44
Conclusion
Bevacizumab improves survival for patients with advanced colorectal cancer, but at historical list prices this improvement came at a considerable cost. We found that the cost-effectiveness of bevacizumab in combination with irinotecan ranged from $78,000 to $84,000/LYG relative to a contemporaneous comparison group and $75,000 to $101,000/LYG relative to a historical comparison group from the perspective of public payer. However, the recent availability of biosimilar bevacizumab may considerably improve the cost-effectiveness of treatment achieved in current practice. In sensitivity analysis, reducing the cost of bevacizumab by 50% reduced the ICER to $51,000 to $61,000/LYG in the contemporaneous comparison and $41,000 to $58,000/LYG in the historical comparison. We used the best available real-world data in our analysis in order to demonstrate the feasibility of generating RWE, and found that our results are consistent across three Canadian provinces, using two different comparison groups and propensity score methods. As practice and policies change, it is important to reevaluate cost-effectiveness to continuously provide accurate and relevant evidence. High-quality RWE has the potential to inform resource re-allocation decisions, helping health care systems to maximize health benefit with the available resources.
Supplemental Material
Supplemental material, sj-docx-1-mpp-10.1177_23814683211021060 for Real-World Cost-Effectiveness of Bevacizumab With First-Line Combination Chemotherapy in Patients With Metastatic Colorectal Cancer: Population-Based Retrospective Cohort Studies in Three Canadian Provinces by Reka E. Pataky, Jaclyn Beca, David Tran, Wei Fang Dai, Erind Dvorani, Wanrudee Isaranuwatchai, Stuart Peacock, Riaz Alvi, Winson Y. Cheung, Craig C. Earle, Scott Gavura and Kelvin K. W. Chan in MDM Policy & Practice
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support for this study was provided in part by a grant from the Canadian Partnership Against Cancer. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. The following author is employed by the sponsor: Dr. Craig Earle. The Canadian Centre for Applied Research in Cancer Control is funded by the Canadian Cancer Society. All inferences, opinions, and conclusions drawn in this publication are those of the authors, and do not reflect the opinions or policies of the data steward(s).
ORCID iDs: Reka E. Pataky  https://orcid.org/0000-0003-0159-582X
https://orcid.org/0000-0003-0159-582X
Jaclyn Beca  https://orcid.org/0000-0001-8858-9566
https://orcid.org/0000-0001-8858-9566
Kelvin K. W. Chan  https://orcid.org/0000-0002-2501-3057
https://orcid.org/0000-0002-2501-3057
Supplemental Material: Supplementary material for this article is available on the Medical Decision Making Policy & Practice website at https://journals.sagepub.com/home/mpp.
Contributor Information
Reka E. Pataky, BC Cancer, Vancouver, British Columbia, Canada; Canadian Centre for Applied Research in Cancer Control, Vancouver, British Columbia and Toronto, Ontario, Canada.
Jaclyn Beca, Canadian Centre for Applied Research in Cancer Control, Vancouver, British Columbia and Toronto, Ontario, Canada; Cancer Care Ontario, Toronto, Ontario, Canada.
David Tran, Saskatchewan Cancer Agency, Saskatoon, Saskatchewan, Canada.
Wei Fang Dai, Canadian Centre for Applied Research in Cancer Control, Vancouver, British Columbia and Toronto, Ontario, Canada; Cancer Care Ontario, Toronto, Ontario, Canada.
Erind Dvorani, ICES, Toronto, Ontario, Canada.
Wanrudee Isaranuwatchai, Canadian Centre for Applied Research in Cancer Control, Vancouver, British Columbia and Toronto, Ontario, Canada; St. Michael’s Hospital, Toronto, Ontario, Canada.
Stuart Peacock, BC Cancer, Vancouver, British Columbia, Canada; Canadian Centre for Applied Research in Cancer Control, Vancouver, British Columbia and Toronto, Ontario, Canada; Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada.
Riaz Alvi, Saskatchewan Cancer Agency, Saskatoon, Saskatchewan, Canada.
Winson Y. Cheung, Cancer Care Alberta, Calgary, Alberta, Canada
Craig C. Earle, ICES, Toronto, Ontario, Canada Canadian Partnership Against Cancer, Toronto, Ontario, Canada; Sunnybrook Odette Cancer Centre, Toronto, Ontario, Canada.
Scott Gavura, Cancer Care Ontario, Toronto, Ontario, Canada.
Kelvin K. W. Chan, Canadian Centre for Applied Research in Cancer Control, Vancouver, British Columbia and Toronto, Ontario, Canada Cancer Care Ontario, Toronto, Ontario, Canada; Sunnybrook Odette Cancer Centre, Toronto, Ontario, Canada.
References
- 1. Chan KKW, Nam S, Evans B, et al. Developing a framework to incorporate real-world evidence in cancer drug funding decisions: the Canadian Realworld Evidence for Value of Cancer Drugs (CanREValue) collaboration. BMJ Open. 2020;2020:e032884. doi: 10.1136/bmjopen-2019-032884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garrison LP, Jr, Neumann PJ, Erickson P, Marshall D, Mullins CD. Using real-world data for coverage and payment decisions: the ISPOR Real-World Data Task Force Report. Value Health. 2007;10:326–35. doi: 10.1111/j.1524-4733.2007.00186.x [DOI] [PubMed] [Google Scholar]
- 3. Batra A, Cheung WY. Role of real-world evidence in informing cancer care: lessons from colorectal cancer. Curr Oncol. 2019;26(suppl 1):S53–S56. doi: 10.3747/co.26.5625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–5. doi: 10.1200/jco.2003.10.066 [DOI] [PubMed] [Google Scholar]
- 5. Hurwitz HI, Tebbutt NC, Kabbinavar F, et al. Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials. Oncologist. 2013;18:1004–12. doi: 10.1634/theoncologist.2013-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tappenden P, Jones R, Paisley S, Carroll C. The cost-effectiveness of bevacizumab in the first-line treatment of metastatic colorectal cancer in England and Wales. Eur J Cancer. 2007;43:2487–94. doi: 10.1016/j.ejca.2007.08.017 [DOI] [PubMed] [Google Scholar]
- 7. Bank of Canada. Historical noon and closing rates [cited February 25, 2020]. Available from: https://www.bankofcanada.ca/rates/exchange/legacy-noon-and-closing-rates/
- 8. Aaserud M, Kristiansen IS, Neilson AR, et al. Health economic evaluation of bevacizumab for metastatic colorectal cancer [cited May 13, 2021]. Available from: https://www.fhi.no/en/publ/2009-and-older/health-economic-evaluation-of-bevacizumab-for-metastatic-colorectal-cancer/ [PubMed]
- 9. Whyte S, Pandor A, Stevenson M. Bevacizumab for metastatic colorectal cancer. Pharmacoeconomics. 2012;30:1119–32. doi: 10.2165/11597210-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 10. National Institute for Health and Clinical Excellence. Bevacizumab in combination with oxaliplatin and either fluorouracil plus folinic acid or capecitabine for the treatment of metastatic colorectal cancer [cited May 13, 2021]. Available from: https://www.nice.org.uk/guidance/ta212/documents/colorectal-cancer-metastatic-bevacizumab-final-appraisal-determination3
- 11. Buxton MJ, Drummond MF, Van Hout BA, et al. Modelling in economic evaluation: an unavoidable fact of life. Health Econ. 1997;6:217–27. doi: [DOI] [PubMed] [Google Scholar]
- 12. BC Cancer. Biosimilar drugs [cited December 22, 2020]. Available from: http://www.bccancer.bc.ca/health-professionals/clinical-resources/biosimilar-drugs
- 13. Pan-Canadian Pharmaceutical Alliance. Pan-Canadian oncology biosimilars Initiative [cited March 2019]. Available from: https://www.pcpacanada.ca/biologics-biosimilars
- 14. BC Cancer Registry Data. BC Cancer [publisher]. Data Extract. BC Cancer (2017).
- 15. British Columbia Ministry of Health [creator]: Medical Services Plan (MSP) Payment Information File. Population Data BC [publisher]. Data Extract. MOH (2017).
- 16. Canadian Institute for Health Information [creator]. Discharge Abstract Database (Hospital Separations). Population Data BC/Ministry of Health; 2017. [Google Scholar]
- 17. British Columbia Ministry of Health [creator]. Consolidation File (MSP Registration & Premium Billing). Population Data BC [publisher]. Data Extract. MOH (2017). [Google Scholar]
- 18. British Columbia Ministry of Health [creator]. PharmaNet. Population Data BC [publisher], Data Stewardship Committee (2018). [Google Scholar]
- 19. BC Vital Statistics Agency [creator]. Vital Statistics Deaths. Population Data BC [publisher]. BC Vital Statistics Agency (2017)
- 20. Johns Hopkins. The Johns Hopkins ACG® System [cited May 13, 2021]. Available from: https://www.hopkinsacg.org/
- 21. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25:1–21. doi: 10.1214/09-STS313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Canadian Institute for Health Information. The cost of hospital stays: why costs vary [cited May 13, 2021]. Available from: https://secure.cihi.ca/free_products/2008hospcosts_report_e.pdf
- 24. Statistics Canada. Table 18-10-0005-01. Consumer price index, annual average, not seasonally adjusted [cited November 18, 2020]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000501
- 25. Willan AR, Briggs AH. Chapter 3: Parameter estimation for censored data. In: Statistical Analysis of Cost-Effectiveness Data. John Wiley; 2006. [Google Scholar]
- 26. CADTH Evidence Driven. Guidelines for the Economic Evaluation of Health Technologies: Canada [cited May 13, 2021]. https://www.cadth.ca/about-cadth/how-we-do-it/methods-and-guidelines/guidelines-for-the-economic-evaluation-of-health-technologies-canada
- 27. Drummond MF. Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press; 2005. [Google Scholar]
- 28. Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ. 1997;6:327–40. [DOI] [PubMed] [Google Scholar]
- 29. Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making. 1998;18(2 Suppl):S68–S80. doi: 10.1177/0272989x98018002s09 [DOI] [PubMed] [Google Scholar]
- 30. Shiroiwa T, Fukuda T, Tsutani K. Cost-effectiveness analysis of bevacizumab combined with chemotherapy for the treatment of metastatic colorectal cancer in Japan. Clin Ther. 2007;29:2256–67. doi: 10.1016/j.clinthera.2007.10.013 [DOI] [PubMed] [Google Scholar]
- 31. Lawrence D, Maschio M, Leahy KJ, Yunger S, Easaw JC, Weinstein MC. Economic analysis of bevacizumab, cetuximab, and panitumumab with fluoropyrimidine-based chemotherapy in the first-line treatment of KRAS wild-type metastatic colorectal cancer (mCRC). J Med Econ. 2013;16:1387–98. doi: 10.3111/13696998.2013.852097 [DOI] [PubMed] [Google Scholar]
- 32. Lee EK, Revil C, Ngoh CA, et al. Clinical and cost effectiveness of bevacizumab + FOLFIRI combination versus FOLFIRI alone as first-line treatment of metastatic colorectal cancer in South Korea. Clin Ther. 2012;34:1408–19. doi: 10.1016/j.clinthera.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 33. Lange A, Prenzler A, Frank M, Kirstein M, Vogel A, Von Der Schulenburg JM. A systematic review of cost-effectiveness of monoclonal antibodies for metastatic colorectal cancer. Eur J Cancer. 2014;50:40–9. doi: 10.1016/j.ejca.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 34. Hedden L, Kennecke H, Villa D, et al. Incremental cost-effectiveness of the pre- and post-bevacizumab eras of metastatic colorectal cancer therapy in British Columbia, Canada. Eur J Cancer. 2012;48:1969–76. doi: 10.1016/j.ejca.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 35. Ruiz-Millo O, Albert-Mari A, Sendra-Garcia A, Jimenez-Torres NV. Comparative cost-effectiveness of bevacizumab-irinotecan-fluorouracil versus irinotecan-fluorouracil in first-line metastatic colorectal cancer. J Oncol Pharm Pract. 2014;20:341–50. doi: 10.1177/1078155213508437 [DOI] [PubMed] [Google Scholar]
- 36. Shankaran V, Mummy D, Koepl L, et al. Survival and lifetime costs associated with first-line bevacizumab use in older patients with metastatic colorectal cancer. Oncologist. 2014;19:892–9. doi: 10.1634/theoncologist.2013-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. CanREValue Collaboration. Mapping Canadian provincial data assets to conduct real-world studies on cancer drugs [cited May 13, 2021]. Available from: https://cc-arcc.ca/wp-content/uploads/2020/04/The-CanREValue-Data-WG-Interim-Report-Revision_Final_v1.pdf
- 38. Ontario’s Watchdog. A vast injustice [cited May 13, 2021]. Available from: https://www.ombudsman.on.ca/resources/reports-and-case-summaries/reports-on-investigations/2009/a-vast-injustice
- 39. Skedgel C, Wranik D, Hu M. The relative importance of clinical, economic, patient values and feasibility criteria in cancer drug reimbursement in Canada: a revealed preferences analysis of recommendations of the Pan-Canadian Oncology drug review 2011–2017. Pharmacoeconomics. 2018;36:467–75. doi: 10.1007/s40273-018-0610-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beca J. Real-World Safety of Bevacizumab with First-Line Combination Chemotherapy in Patients with Metastatic Colorectal Cancer (MCRC): Population-Based Retrospective Cohort Studies in Three Provinces. [Conference presentation] 2019 CADTH Symposium, Edmonton, Canada. [Google Scholar]
- 41. McGrail K, Bryan S, Davis J. Let’s all go to the PROM: the case for routine patient-reported outcome measurement in Canadian healthcare. Healthc Pap. 2011;11:8–18. doi: 10.12927/hcpap.2012.22697 [DOI] [PubMed] [Google Scholar]
- 42. Canadian Institute for Health Information. Patient-reported outcome measures (PROMs) [cited June 3, 2021]. Available from: https://www.cihi.ca/proms
- 43. Goldstein DA, Chen Q, Ayer T, et al. First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: a united states–based cost-effectiveness analysis. J Clin Oncol. 2015;33:1112–8. doi: 10.1200/jco.2014.58.4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goldstein DA, Chen Q, Ayer T, et al. Bevacizumab for metastatic colorectal cancer: a global cost-effectiveness analysis. Oncologist. 2017;22:694–9. doi: 10.1634/theoncologist.2016-0455 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mpp-10.1177_23814683211021060 for Real-World Cost-Effectiveness of Bevacizumab With First-Line Combination Chemotherapy in Patients With Metastatic Colorectal Cancer: Population-Based Retrospective Cohort Studies in Three Canadian Provinces by Reka E. Pataky, Jaclyn Beca, David Tran, Wei Fang Dai, Erind Dvorani, Wanrudee Isaranuwatchai, Stuart Peacock, Riaz Alvi, Winson Y. Cheung, Craig C. Earle, Scott Gavura and Kelvin K. W. Chan in MDM Policy & Practice