Abstract
Digital Pathology (also referred to as Telepathology and Whole Slide Imaging) is the process of producing high resolution digital images from tissue sections on glass slides. These glass slides are normally examined under a microscope by a pathologist as part of the diagnostic process. The emergence of digital pathology now means that digital images are stored on secure servers and can be viewed on computer monitors; enabling pathologists to work remotely and to collaborate with other colleagues when second opinions are needed. The implementation of digital pathology into clinical practice has many potential benefits. Although this has been long recognised, its adoption as a diagnostic tool remains low and pathologists’ projections about its future deployment are cautious. Notable early digital pathology adopters have led the way. The challenge now is to scale-up digital pathology beyond the relatively few large networks and centres of excellence. Many other areas of healthcare have accumulated experience about optimising approaches to digital health/healthcare technology deployment and sustainability. This has been done in a multi-disciplinary context and has applied theoretical/conceptual frameworks. Thus far there has been little use of similar frameworks in the planning of digital pathology deployment in clinical practice. In this essay, I will explore the scope of digital pathology implementation approaches that have been deployed in clinical practice and examine what can be learned from the wider healthcare experience of adopting, scaling-up and sustaining innovative healthcare solutions.
Keywords: Digital pathology, telepathology, whole slide imaging, implementation, theoretical frameworks, pathology, diagnostics
Introduction
Digital Pathology (also referred to as Telepathology and Whole Slide Imaging) is the process of producing high resolution digital images from tissue sections on glass slides which are normally examined under a microscope. The digital images are stored on secure servers; pathologists can view them on computer monitors and are able to work and to collaborate remotely with other colleagues when second opinions are needed. 1 Its implementation into clinical practice is reported to have many potential benefits, including improved efficiencies and cost-savings.2,3 However, the widespread adoption of digital pathology remains low 4 and projections for it deployment as a primary diagnostic tool are modest. 5 The scaling up of digital pathology deployment is necessary so that the challenges of increasing demand 6 and reducing workforce capacity 7 in pathology services and workforce,8,9 respectively, can be addressed. This is not only important to achieve the maximum benefits of digital pathology; it is also of fundamental importance for the delivery of equitable healthcare to all patients.
Pathology does not exist in isolation, with many associated areas of health and care spearheading innovations in digital health (telemedicine, telehealth, mHealth, eHealth).10,11 This wider healthcare landscape will need to be taken into consideration as histopathology looks to develop its own diagnostic services. This is highlighted in Eric Topol’s recent review of the impact of technology in the wider healthcare context. 12 The report, commissioned by Health Education England, invites the reader to think about new models of care to improve diagnoses and treatment and to provide clinicians with the “gift of time”: a reference to Artificial Intelligence (AI). A more recent editorial highlights the promise of AI to reduce health disparities for under-served patients and in so-doing help develop better race equality in the USA. 13 On an international level, the World Health Organisation identifies digital health technologies as a means of strengthening health systems and supporting patients to live healthier lives.14,15 These are reminders that pathology services exist in a wider medical (-omics, big data) and social (behavioural, environmental) and international context. Furthermore, widespread and sustainable implementation of digital pathology across the healthcare system is central to the emerging role for AI as an assistive diagnostic tool in pathology and its potential to improve patient outcomes.16,17
It is recognised that the implementation of digital health technologies comes with a number of challenges. 18 The track record of digital health programmes is poor and so a great deal of experience has been gained in the area of e-Health implementation.19–22 It is increasingly apparent that effective implementation requires consideration of a diverse set of issues that are distinct from the technology in question. 23 The interplay of the technology with stakeholders, environment, policy makers and stakeholder expectations produce, it would seem, a combination of challenges that need to be accommodated to ensure adoption and sustainability of a technology solution in healthcare. This will equally apply to the implementation of digital pathology in clinical practice, and any subsequent developments to incorporate AI into the diagnostic workflow. It is evident that in order to realise the potential of AI it is necessary first to ensure that digitialisation of the pathology workflow is effective, that it is spread beyond a small number of centres and that its implementation into clinical practice is sustained.
In this essay I will explore the scope of digital pathology implementation approaches that have been deployed in clinical practice and compare this to the experience of those deploying digital health solutions. The essay is structured in three parts. The first part describes the scope of digital pathology implementation into clinical practice: this includes case studies and scoping investigations which describe the experience and impact of digital pathology deployment in individual pathology departments and larger diagnostic pathology networks. The second part discusses the digital pathology literature that has applied theoretical/conceptual frameworks to evaluate and/or plan clinical implementation programmes. In the third part, I will focus on how digital pathology programmes can learn from the telehealth literature to develop scalable and sustainable implementation processes in diagnostic practice.
Scope of digital pathology implementation literature
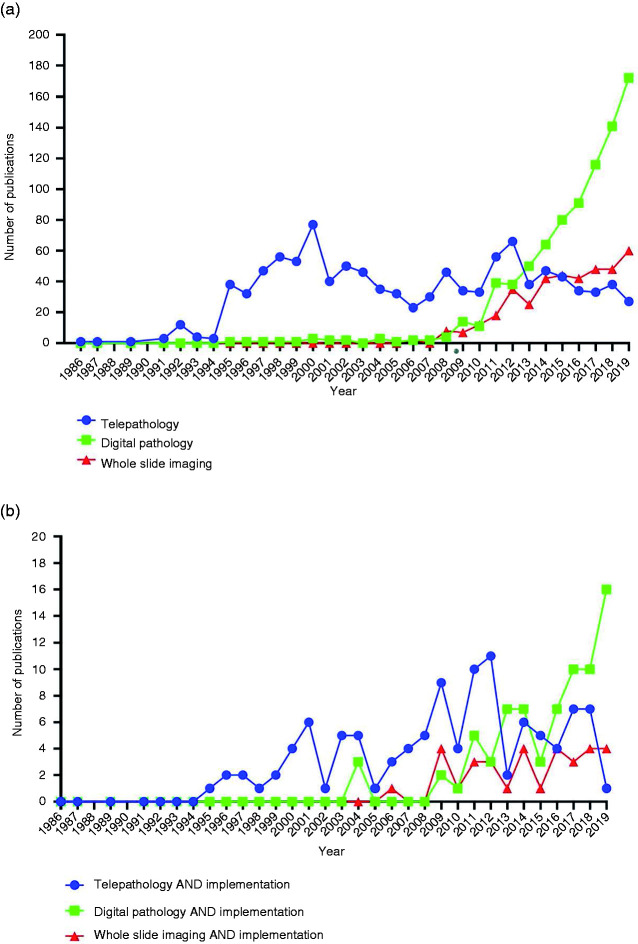
There has been an increasing interest in the ability to provide remote diagnostics since the earliest descriptions of telepathology.24,25 A literature search for the most used terms: “Digital Pathology”, “Telepathology” and “Whole Slide Imaging”, between 1986–2019, shows a steep rise in publications from 2012, and also demonstrates the change in preference for terminology to “Digital Pathology”, Figure 1(a). In the rest of this essay, I will therefore use the term “digital pathology” as an umbrella term to cover all three terms, bearing in mind that whilst these terms are used interchangeably some authors regard WSI as the process of creating a replica of a glass slide and telepathology as the service component of digital pathology. 26 The number of studies that focus on implementation of digital pathology into clinical practice is comparatively low (10.4% of studies); with an apparent increase since 2015, Figure 1(b).
Figure 1.
Results of a PubMed literature search reveals the number of publications containing search items in the title / abstract for each year in period 1986 – 2019 (a) all publications, (b) publications also including terms “implementation”.
The majority of digital pathology implementation publications are technical papers (30%) covering areas such as Artificial Intelligence (AI)/Machine Learning (ML); image navigation/management systems; image analysis; colour standardization; archiving platforms; Augmented Reality (AR). A further 14% of studies can be grouped under evaluation/pilot/validation/concordance studies and also include surveys of pathologists’ attitude to digital pathology. The same proportion of articles are case studies describing the experience of single/networked pathology services which take either a whole service or a single-use approach (e.g. immunohistochemistry, frozen section) to the deployment of digital pathology in diagnostic practice. The remaining literature includes general telehealth/telemedicine publications that refer tangentially to digital pathology (11%), reviews (9%); digital pathology practical guides/scoping documents (7%); digital pathology as a platform for international collaboration/consultation (5%); digital pathology uses in education and training (5%) and research (4%). Only two publications (1.2%) report on a theoretical framework as a planning tool for the deployment of diagnostic digital pathology.
Case studies and scoping documents in digital pathology implementation
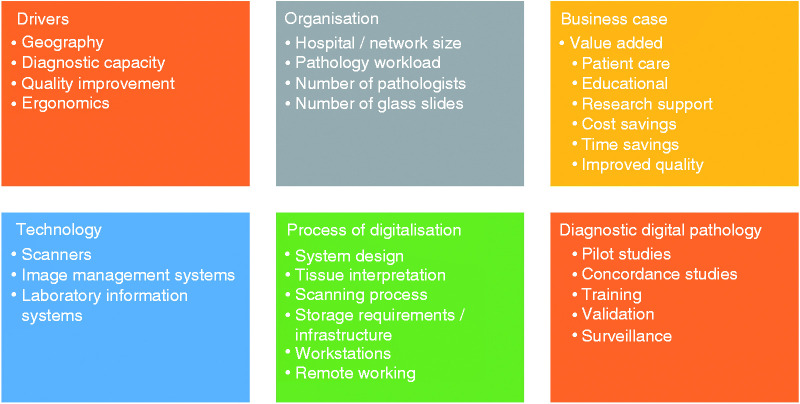
Case studies and scoping investigations from around the world, and in different healthcare systems, provide a wealth of information about the drivers for digital pathology deployment,27,28 guidance on its operational needs 29 and effective business case planning. 30 These are predominately from the perspective of the pathologist. Nevertheless, they play an important role in raising awareness of digital pathology’s technical requirements: hardware (scanners) and software (image management systems).31–33 Importantly, the case studies provide an overview of the process of digitalisation4,34,35 and a possible blueprint for its deployment in clinical practice,36,37 Figure 2. However, there is ambiguity about the numbers of pathologists who are reporting digitally. Indeed, in the case studies identified only Retamero et al.’s study provides a definitive statement about uptake: “Within 2 weeks from going live, all of the pathologists at GUH were using digital pathology for primary diagnosis and the analog workflows were suspended”. 35 In the studies that do provide the number of pathologists in individual departments/networks, the proportion of pathologists that are using digital pathology in diagnostic practice is unclear (I have calculated this to be in the range 5–28%) as is the sustainability and spread of adoption.
Figure 2.
Issues highlighted by digital pathology case studies, scoping documents and buiness case benchmarking publications.
The implementation literature does address human factors through the perspective of early adopters 38 and stakeholder attitudes towards digital pathology.5,39,40 Such studies are important as they highlight potential barriers to adoption of digital pathology, and so should enable a more user-centred approach to implementation. However, feedback is predominately sought from pathologists and/or pathology trainees, with insufficient attention being paid to biomedical scientists/histotechnologists and wider pathology stakeholders including surgeons, oncologists and patients. A holistic perspective of digital pathology which takes us beyond the pathology laboratory would, I believe, better inform policy makers and contribute to the development of, currently lacking, national strategies for its deployment. 41 A recent digital pathology focus group of UK-based pathologists and biomedical scientists highlighted this absence of a co-ordinated national approach as a potential barrier to adoption of digital pathology. 42
Healthcare context
It would be reasonable to assume that in a tax-funded healthcare system, such as the National Health Service (NHS) in the UK, that it would be straightforward to adopt digital pathology at scale. Recent policy strategy 43 has indeed directed funding to support the deployment of digital pathology in three centres of excellence across the UK.44–46 However, despite positive policy developments widespread and connected national deployment of digital pathology remains challenging, and national data about its diagnostic rollout is hard to find. The complexity of the NHS (e.g. commissioning of services) and its fragmentation are highlighted as significant factors in limiting adoption and scale-up of innovations. 47 Of course, the COVID-19 pandemic has had an impact on the use of digital technology in the NHS, resulting in a rapid adoption of technology and major changes in the way some services are delivered. This has been most noticeable in how patients have been accessing healthcare, and it is too early to know what the impact this has had on clinician and patient experience and if the changes that have occurred will be sustained beyond pandemic restrictions. 48 The transition to digital pathology in those departments that already had some technology infrastructure has certainly accelerated in the UK during the pandemic (personal observation) and some institutions have reported that, whilst implementation challenges persist, their existing digital pathology infrastructure has provided evidence that this technology can contribute to improved service resilience. 49
The challenges of providing diagnostic pathology services in Low- and Middle-Income Countries (LMIC) are recognised, and range from the provision of appropriately trained staff (pathologists, technologists, IT specialists) to the availability of fit-for-purpose laboratory infrastructure. 50 There have been a number of international digital pathology initiatives going back to the early 1990s 51 – driven by unmet diagnostic needs and modelled on consultation/second opinion platforms as part of broad international collaborations. However, more recent literature continues to highlight the challenges of deploying digital pathology in resource poor countries such as continued workforce challenges and a lack of the appropriate infrastructure due to economic constraints.52–54
Theoretical framework analysis in digital pathology
Theoretical frameworks have been applied predominately to evaluate the efficacy of digital pathology implementation programmes; with few publications proposing prospective frameworks for the planning of digital pathology implementation. In their scoping review Meyer and Pare identified studies that applied theoretical frameworks to retrospectively investigate the impact and challenges of digital pathology implementation. 55 Delone and McLean’s Information System Success Model 56 has been used to assess the success of digital pathology deployment from a technology perspective: reliability and accessibility, monitor quality, systems integration, technical support. 57 A Technology Acceptance Model 58 has been applied to describe digital pathology adoption in an investigation of a telepathology platform in microbiology. 59 The challenges of digital pathology implementation have been investigated using Knowledge Barriers Theory which looked at domains of knowledge that prevent individuals from adopting technology. 60 A value-added approach was applied by Isaacs et al. to provide a cost-benefit analysis of digital pathology benefits; exploring the factors that can support the development of an effective business case for the deployment of this technology. 30
Alami et al. evaluated arguably one of the world’s largest digital pathology networks in Canada (Eastern Quebec Telepathology Network, EQTN) using a Utilisation-focused Evaluation from the perspective of the network’s stakeholders and partners. 61 Setting off with the premise that digital pathology would improve recruitment and retention of pathologists, this holistic evaluation approach identified that in fact it improved the recruitment and retention of surgeons. They also identified that the relationships developed between pathologists, technologists (Biomedical Scientist) and surgeons are “decisive in the success or failure of telepathology”. Indeed, the significant changes in the technologist’s role embodied across the network raised issues of training and accreditation and ultimately of status that will need to be addressed if the project is to expand. A further fundamental, although unsurprising, finding is that “the practice of pathology is inseparable from the practices of other clinicians, especially surgeons”. The authors correctly identify that successful implementation of telepathology requires its alignment with the wider national strategies for telehealth/telemedicine. Whilst such retrospective evaluation frameworks are necessary for a better understanding of the impact of digital pathology and the challenges of adoption, they are not in themselves sufficient for planning effective, sustained and scaled-up use of digital pathology in diagnostic practice.
Ho et al. apply a prospective framework to qualitatively assess the overall context into which digital pathology is to be deployed – a so-called contextual enquiry.62,63 This is a qualitative method of analysis, but in contrast to other methods, such as interviews, focus group sessions, surveys, it aims to capture “unarticulated knowledge”: details of routine work that are so familiar to the user that they have become “habitual and so invisible”. 64 This framework has been used by Ho et al. to understand how best to design digital pathology systems in a way to promote their adoption into clinical practice in an academic centre 62 and in the pathology departments of the US Air Force Medical Service (AFMS). 63
A contextual enquiry has two components/phases: observation followed by interpretation; the latter comprising a number of different analyses. The observations are carried out by researchers who observe and interview pathologists over two sessions: an initial session lasting between 2 and 3 h and a follow-up session of 1–2 h duration. The approach is one of “master-apprentice” where the pathologist (the user/”master”) teaches the observers (“apprentices”) about how they do their job. A number of models are then used to describe in detail how the pathologist’s work is carried out, namely: flow; sequence; artefact; cultural; physical, Table 1.
Table 1.
Components of contextual enquiry analysis, derived from Ho et al. 62
| Observations | “master-apprentice” model – users teach observers about how they do their job | ||
| Interpretations | Affinity diagram | Describes how pathologist does their job and the interactions they have. | |
| Models | Flow | Of physical artefacts, data & communications | |
| Sequence | Of main work tasks; including triggers for each work task | ||
| Artefact | Physical objects needed to complete work | ||
| Cultural | Policies, values, relationships | ||
| Physical | Layout of work environment | ||
The data collected in this way provide a consolidated view of how pathologists work: leading to the creation of an “affinity diagram” which captures the pathologist’s interactions with other staff in the diagnostic workflow (e.g. clinicians, pathology trainees, laboratory staff, administrative/clerical staff) and any necessary interaction with the “bureaucracy” of handling diagnostic samples in a medical laboratory (e.g. electronic patient records, laboratory information systems). The observational component determined that pathologists “feel at home with microscope and glass slide” and that pathologists had individual approaches to the way they completed their work; indicating that digital pathology systems will need to offer a similar user experience. Of particular interest is the cultural model the authors apply which depicts the multiple social influences on an academic centre pathologist: from an overarching theme of “delivering accurate and timely diagnoses” to the interplay between the different sets of values (e.g. patient care focus), policies (e.g. documentation and maintenance of standards), information systems (e.g. as enablers and potential obstacles to effective working) and clinical interactions that are part of a pathologist’s professional profile and their working environment. 62 In the US AFMS study, the cultural model captures additional influences on the AFMS pathologists. The AFMS workforce planning is characterized by a high turnover of pathologists and histotechnologists (biomedical scientists) where enlisted staff are assigned to laboratories for short periods of time, and who would move into civilian practice once military service commitment was met (4–5 years, in accordance with military sponsorship of medical training). Also uncovered was the impact of an imbalance between pathology experience across different AFMS centres: subspecialists being located at the larger centres, with more junior pathologists at peripheral pathology departments. 63
Both of the contextual enquiry studies were able to examine the pathology workflow and assess the needs of respective pathology services, in order to best support the deployment of diagnostic digital pathology networks. The importance of “change management” is highlighted in both, with an emphasis on a focus on people and their concerns during the introduction of change. Furthermore, the studies recognize that whilst general implementation challenges will operate, there will also be more specific challenges according to the nature of the pathology service under examination. In the academic centre study, 62 the authors put forward twelve concepts which they identify as key in the effective deployment of a diagnostic digital pathology service, Table 2.
Table 2.
Contextual enquiry – findings and potential impact on digital pathology service design, derived from Ho et al. 62
| Findings | Potential impact on design of digital pathology service |
|---|---|
| Pathologists value familiarity associated with use of a microscope in diagnostic practice | Digital pathology systems should aim to provide• similar experience to using a microscope• advantages that outweigh perceived digital deficits |
| Concerns around quality of digital vs optical (microscope) images | Importanct of digital image quality and standards |
| Importance to the pathologist of the information they are able to acquire from the slide tray – prior to case examination under the microscope | Functionality of slide tray should not be overlooked |
| Pathologists value being able to have a view of their daily workload so that they can plan/prioritise workload | Facility to enable pathologists to plan their day efficiently |
| Orientation of tissue specimens on slide is important | Facility to re-orientate digital sections as appropriate. |
| Pathologists approach the diagnosis of cases in an indiviual manner, e.g. examine slides in a particular order | Digital pathology system should be customisable to individual requirements |
| Diagnostic process involves reference to external resources | Consider how digital pathology system can interface with external information sources to streamline diagnostic process |
| Pathologists valued use of “working draft” report to organise their thoughts, document and track workflow before a final report is produced | Consider reproducing such a process as part of the Laboratory Information System (LIS) |
| Pathologists expect that their cases will be reviewed by other pathologists | Facility to support the making of annotations and particular diagnostic interpretations |
| Diagnosis is a multi-faceted process | Importance of integration of digital imaging & LIS |
| Ensuring correct diagnosis for their patients is important to pathologists | Appropriate validation of diagnostic digital pathology |
| Important for pathologists to develop and maintain “trusting relationships” with their clinicians | Digital pathology systems should not impact negatively on clinical relationships |
In the US AFMS study, the contextual enquiry identified that the deployment of digital pathology would address unique circumstances operating in a military hospital service: namely, better support of more junior pathologists and improved workload distribution amongst pathologists more generally. 63 The contextual enquiry therefore provides information about how digital pathology can deliver unmet service needs, but it also highlights areas of pathologist working practice that a new technology would need to match and/or better to optimize its adoption by the end users.
The contextual enquiry framework emphasises the importance of having a clear understanding of user and system needs to inform the design of a product: in this case the deployment of digital pathology in clinical practice. 65 Importantly, the contextual enquiry identifies the social and professional influences on a pathologist; factors that will have an impact on pathologists’ readiness to adopt digital pathology. To date there has been no follow-up studies to demonstrate the outcome of this approach in planning successful digital pathology implementation, and so the efficacy of contextual enquiry in digital pathology remains untested. However, the contextual enquiry framework has been used successfully to understand and improve an informatics intervention for pharmacy dispensary workflow, 66 and it is applied in a wide range of telehealth/telemedicine implementation planning. 67 One criticism of Ho et al.s’ contextual enquiry studies is that they are too pathologist centric. Whilst there is an argument to understand the needs of the perceived primary user (the pathologist), this overlooks the necessary contribution of histotechnologists and the needs of pathology’s stakeholders (surgeons, oncologists, patients) and expectations of the wider societal context.
Learning from telehealth
In the main, digital pathology implementation in clinical practice has been a story of individual early adopters, evaluation and validation studies with notable larger networks such as the EQTN 61 and more recently in the UK the three digital pathology/AI centres of excellence.44–46 The diffusion of diagnostic digital pathology across the wider pathology landscape has been slow. This is not unusual in healthcare technology terms. van Limburg et al. have identified some structural obstacles to adoption of innovation in healthcare, including financial and legislation constraints, the fragmented deployment of innovation and the framing of implementation strategies too much around engineering-driven solutions. 68 Furthermore, even when telehealth solutions are adopted; the use of the technology is not always sustained.69,70 Greenhalgh et al. have proposed the “Nonadoption, Abandonment, Scale-up, Spread and Sustainability (NASSS)” framework to be applied to health technology innovations to support improved implementation. 71 The NASSS framework is organised around seven domains that guide consideration of the overall context into which technology is to be deployed: the patient; the nature of technology; the value proposition to technology developer and patient; the adopters across the whole pathway; the organisation (including its capacity to innovate and its technology readiness); the wider system (including policy, regulatory and professional requirements) – all of this is set within a recognition that the implementation will necessarily adapt over time. The framework is proposed for patient facing technologies/innovations, but I suggest that it can be adapted to improve the scalability of digital pathology in clinical practice. I believe that a similar analysis can be used to better understand and provide solutions for the challenges in deploying digital pathology internationally, including in LMICs. The importance of a holisitic approach to healthcare technology deployment has been advanced to scale-up digital health in LMICs – also emphasising the importance of considering human factors, the healthcare and extrinsic ecosystems in addition to technology-specific factors. 72
The NASSS’ holistic overview of implementation is very much context embedded and the milestones proposed for each of the domains drive participants to consider not only the technology and the pathologists but also the wider healthcare team, organisation and society that are also its potential beneficiaries. Thus far the societal impact of digital pathology adoption has not been quantified, and the challenges of funding its rollout are articulated mainly in terms of efficiency gains and cost-savings. 73 The operational advantages of digital pathology rollout leading to improved patient care/safety have also been advanced30,73 but these are mainly defined in the context of the technology and the pathology service rather than the societal benefit achieved.
The NASSS framework brings together elements of Contextual Enquiry with elements of the frameworks that have been applied retrospectively to evaluate the impact of digital pathology in practice. Its application would also set digital pathology implementation into a much wider and realistic context; breaking down the conventional approach to date that digital pathology is the domain of pathology departments alone. The NASSS framework also brings into consideration input from developers (industry) and aims to understand the readiness of healthcare organisations to innovate and implement the necessary changes to pathology services. Importantly, it also seeks the participation of patients; something that has not received sufficient attention in the deployment of digital pathology to date and so hampered an understanding of its societal impact. This type of holistic consideration which includes pathology’s many stakeholders will be central to the vision of deploying technology effectively to improve diagnostic services for patients. It will undoubtedly also be important for any future introduction of AI into the diagnostic workflow.
Conclusions
An increasing interest in digital pathology has been notable over the past decade, enhanced more recently by the potentially revolutionary benefits that have been promulgated for AI tools in pathology. Several examples of implementation of digital pathology in clinical practice are reported by early adopters and champions of the technology. The process for 100% digitalisation of glass slides, including its challenges and impacts, has been eloquently detailed by early adopters. However, widespread adoption remains low. We are still at a relatively early stage of diagnostic application of digital pathology; with implementation being the subject of only 10% of digital pathology/telepathology/whole slide imaging publications identified in this essay. This is surprising considering the often-stated benefits of digital pathology and the significant passage of time since the first forays into remote transmission of pathology images in the late 1960’s.
Whilst the deployment of digital pathology in clinical practice has been led by a relatively few early adopters, the challenge now is to understand how digital pathology can be scaled-up beyond these notable large networks and centres of excellence. This requires that we have a much better understanding of human, organisational and systems factors as they relate to technology adoption, and we need to do this both in the context of the immediate users in pathology departments as well as pathology’s stakeholders (clinical and non-clinical). The field of diagnostic digital pathology needs to look more widely at the telehealth/telemedicine experience of the challenges of adopting, scaling-up and sustaining innovative healthcare solutions. I would recommend adapting the NASSS framework for the planning, deployment and monitoring of digital pathology deployment in clinical practice. This will facilitate effective redesign of the diagnostic histopathology workflow; which in itself will prepare the ground for eventual deployment of AI tools in diagnostic practice.
Footnotes
Acknowledgement: This essay is part of the author’s ongoing work into development of effective implementation strategies for digital pathology in clinical practice.
Contributorship: Sole contributor.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor: Dr Samar Betmouni.
Peer review: DJ Hartman, University of Pittsburgh School of Medicine has reviewed this manuscript.
ORCID iD: Samar Betmouni https://orcid.org/0000-0002-4947-0658
References
- 1.Parwani A. Next generation diagnostic pathology: use of digital pathology and artificial intelligence tools to augment a pathological diagnosis. Diagn Pathol 2019; 14: 138–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanna MG, Reuter VE, Samboy J, et al. Implementation of digital pathology offers clinical and operational increase in efficiency and cost savings. Arch Pathol Lab Med 2019; 143: 1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baidoshvili A, Bucur A, van Leeuwen J, et al. Evaluating the benefits of digital pathology implementation: time savings in laboratory logistics. Histopathology 2018; 73: 784–794. [DOI] [PubMed] [Google Scholar]
- 4.Stathonikos N, Nguyen TQ, Spoto CP, et al. Being fully digital: perspective of a Dutch academic pathology laboratory. Histopathology 2019; 75: 621–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams BJ, Lee J, Oien KA, et al. Digital pathology access and usage in the UK: results from a national survey on behalf of the National Cancer Research Institute’s CM-Path initiative. J Clin Pathol 2018; 71: 463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Research UK. Testing times to come? An evaluation of pathology capacity across the UK, https://cancerresearchuk.org/sites/default/files/testing_times_to_come_nov_16_cruk.pdf (2016, accessed 24 January 2020).
- 7.Royal College of Pathologists. Histopathology workforce survey. RCPath, https://rcpath.org/profession/workforce-planning/our-workforce-research/histopathology-workforce-survey-2018.html (2018, accessed 14 May 2021).
- 8.Sayed S, Lukande R, Fleming KA. Providing pathology support in low-income countries. J Glob Oncol 2015; 1: 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metter DM, Colgan TJ, Leung ST, et al. Trends in the US and Canadian pathologist workforces from 2007 to 2017. JAMA Netw Open 2019; 2: e194337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith AC. Telemedicine: challenges and opportunities. Expert Rev Med Devices 2007; 4: 5–7. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar R. New beginnings. Lancet Digit Health 2020; 2: e1. [DOI] [PubMed] [Google Scholar]
- 12.Topol E. The Topol review: preparing the healthcare workfroce to deliver the digital future, https://topol.hee.nhs.uk/the-topol-review/ (2018, accessed 14 May 2021).
- 13. Can artificial intelligence help create racial equality in the USA? Lancet Digit Health 2021; 3: e135. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organisation. WHO Global Strategy on Digital Health 2020–2025, https://cdn.who.int/media/docs/default-source/documents/gs4dhdaa2a9f352b0445b afbc79ca799dce4d_02adc66d-800b-4eb5-82d4-f0bc778a5 a2c.pdf?sfvrsn=f112ede5_68 (2019, accessed 14 May 2021).
- 15.Mariano B. Towards a global strategy on digital health. Bull World Health Organ 2020; 98: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niazi MKK, Parwani AV, Gurcan MN. Digital pathology and artificial intelligence. Lancet Oncol 2019; 20: e253–e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colling R, Pitman H, Oien K, et al.; CM-Path AI in Histopathology Working Group. Artificial intelligence in digital pathology: a roadmap to routine use in clinical practice. J Pathol 2019; 249: 143–150. [DOI] [PubMed] [Google Scholar]
- 18.Kostkova P. Grand challenges in digital health. Front Public Health 2015; 3: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross J, Stevenson F, Lau R, et al. Exploring the challenges of implementing e-health: a protocol for an update of a systematic review of reviews. BMJ Open 2015; 5: e006773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross J, Stevenson F, Lau R, et al. Factors that influence the implementation of e-health: a systematic review of systematic reviews (an update). Implement Sci 2016; 11: 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christie HL, Bartels SL, Boots LMM, et al. A systematic review on the implementation of eHealth interventions for informal caregivers of people with dementia. Internet Interv 2018; 13: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varsi C, Nes LS, Kristjansdottir OB, et al. Implementation strategies to enhance the implementation of eHealth programs for patients with chronic illnesses: realist systematic review. J Med Internet Res 2019; 21: e14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broens TH, Huis in't Veld RM, Vollenbroek-Hutten MM, et al. Determinants of successful telemedicine implementations: a literature study. J Telemed Telecare 2007; 13: 303–309. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein RS, Holcomb MJ, Krupinski EA. Invention and early history of telepathology (1985–2000). J Pathol Inform 2019; 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan KJ, Weinstein RS, Pantanowitz L. Telepathology. In: Pantanowitz L, Tuthill JM, Balis UJ. (eds) Pathology informatics: theory and practice. Chicago, IL: ASCP Press, 2012, pp.257–282. [Google Scholar]
- 26.Kaplan JK, Rao LKF. (eds). Digital pathology. Cham, Switzerland: Springer, 2016. [Google Scholar]
- 27.Chen J, Jiao Y, Lu C, et al. A nationwide telepathology consultation and quality control program in China: implementation and result analysis. Diagn Pathol 2014; 9: S2–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorstenson S, Molin J, Lundstrom C. Implementation of large-scale routine diagnostics using whole slide imaging in Sweden: digital pathology experiences 2006–2013. J Pathol Inform 2014; 5: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartman DJ, Pantanowitz L, McHugh JS, et al. Enterprise implementation of digital pathology: feasibility, challenges, and opportunities. J Digit Imaging 2017; 30: 555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isaacs M, Lennerz JK, Yates S, et al. Implementation of whole slide imaging in surgical pathology: a value added approach. J Pathol Inform 2011; 2: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraggetta F, Garozzo S, Zannoni GF, et al. Routine digital pathology workflow: the Catania experience. J Pathol Inform 2017; 8: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunn BE, Choi H, Almagro UA, et al. Combined robotic and nonrobotic telepathology as an integral service component of a geographically dispersed laboratory network. Hum Pathol 2001; 32: 1300–1303. [DOI] [PubMed] [Google Scholar]
- 33.Dunn BE, Choi H, Almagro UA, et al. Telepathology networking in VISN-12 of the Veterans health administration. Telemed J E Health 2000; 6: 349–354. [DOI] [PubMed] [Google Scholar]
- 34.Stathonikos N, Veta M, Huisman A, et al. Going fully digital: perspective of a Dutch academic pathology lab. J Pathol Inform 2013; 4: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Retamero JA, Aneiros-Fernandez J, Del Moral RG. Complete digital pathology for routine histopathology diagnosis in a multicenter hospital network. Arch Pathol Lab Med 2020; 144: 221–228. [DOI] [PubMed] [Google Scholar]
- 36.Zarella MD, Bowman D, Aeffner F, et al. A practical guide to whole slide imaging: a white paper from the digital pathology association. Arch Pathol Lab Med 2019; 143: 222–234. [DOI] [PubMed] [Google Scholar]
- 37.Baidoshvili A, Stathonikos N, Freling G, et al. Validation of a whole-slide image-based teleconsultation network. Histopathology 2018; 73: 777–783. [DOI] [PubMed] [Google Scholar]
- 38.Evans AJ, Salama ME, Henricks WH, et al. Implementation of whole slide imaging for clinical purposes: issues to consider from the perspective of early adopters. Arch Pathol Lab Med 2017; 141: 944–959. [DOI] [PubMed] [Google Scholar]
- 39.Browning L, Colling R, Rittscher J, et al. Implementation of digital pathology into diagnostic practice: perceptions and opinions of histopathology trainees and implications for training. J Clin Pathol 2020; 73: 223–227. [DOI] [PubMed] [Google Scholar]
- 40.Gozali E, Safdari R, Sadeghi M, et al. Preconceived stakeholders’ attitude toward telepathology: implications for successful implementation. J Pathol Inform 2017; 8: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Betmouni S. Digital pathology: a diagnostic revolution. In: Specialised commissioning.United Kingdom: MGP Ltd, 2018, pp.23–28.
- 42.Turnquist C, Roberts-Gant S, Hemsworth H, et al. On the edge of a digital pathology transformation: views from a cellular pathology laboratory focus group. J Pathol Inform 2019; 10: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell J. Life Sciences Industrial Strategy – a report to the Government from the life sciences sector, https://gov.uk/government/publications/life-sciences-industrial-strategy (2017, accessed 14 May 2021).
- 44.Northern Pathology Imaging Co-operative – NPIC, https://virtualpathology.leeds.ac.uk/npic/ (accessed 3 February 2020).
- 45.Industrial Centre for Artifical Intelligence Research – iCAIRD, https://icaird.com/ (accessed 3 February 2020).
- 46.PathLAKE – Computational Pathology Excellence, https://pathlake.org/ (accessed 3 February 2020).
- 47.Asthana S, Jones R, Sheaff R. Why does the NHS struggle to adopt eHealth innovations? A review of macro, meso and micro factors. BMC Health Serv Res 2019; 19: 984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutchings R. The impact of COVID-19 on the use of digital technology in the NHS. London, UK: Nuffield Trust, 2020, https://nuffieldtrust.org.uk/files/2020-08/the-impact-of-covid-19-on-the-use-of-digital-technology-in-the-nhs-web-2.pdf (accessed 14 May 2021). [Google Scholar]
- 49.Browning L, Fryer E, Roskell D, et al. Role of digital pathology in diagnostic histopathology in the response to COVID-19: results from a survey of experience in a UK tertiary referral hospital. J Clin Pathol 2021; 74: 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fleming KA, Naidoo M, Wilson M, et al. An essential pathology package for low- and middle-income countries. Am J Clin Pathol 2017; 147: 15–32. [DOI] [PubMed] [Google Scholar]
- 51.Park S, Parwani AV, Aller RD, et al. The history of pathology informatics: a global perspective. J Pathol Inform 2013; 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sankaye S, Kachewar S. Telepathology for effective healthcare in developing nations. Australas Med J 2011; 4: 592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sareen R. Telepathology in developing countries – the cloud with silver lining. Health Care Curr Rev 2017; 5: 1–3. [Google Scholar]
- 54.Orah N, Rotimi O. Telepathology in low resource African settings. Front Public Health 2019; 7: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyer J, Pare G. Telepathology impacts and implementation challenges: a scoping review. Arch Pathol Lab Med 2015; 139: 1550–1557. [DOI] [PubMed] [Google Scholar]
- 56.DeLone WH, McLean ER. Measuring e-commerce success: applying the DeLone & McLean information systems success model. Int J Electronic Commerce 2004; 9: 31–47. [Google Scholar]
- 57.Trudel MC, Pare G, Tetu B, et al. The effects of a regional telepathology project: a study protocol. BMC Health Serv Res 2012; 12: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taherdoost H. A review of technology acceptance and adoption models and theories. Procedia Manuf 2018; 22: 960–967. [Google Scholar]
- 59.Djamasbi S, Fruhling AL, Loiacono ET. The influence of affect, attitude and usefulness in the acceptance of telemedicine systems. J Info Technol Theory Appl 2009; 10. [Google Scholar]
- 60.Tanriverdi H, Iacono CS. Diffusion of telemedicine: a knowledge barrier perspective. Telemed J 1999; 5: 223–244. [DOI] [PubMed] [Google Scholar]
- 61.Alami H, Fortin J-P, Gagnon M-P, et al. The challenges of a complex and innovative telehealth project: a qualitative evaluation of the Eastern Quebec telepathology network. Int J Health Policy Manag 2018; 7: 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ho J, Aridor O, Parwani AV. Use of contextual inquiry to understand anatomic pathology workflow: implications for digital pathology adoption. J Pathol Inform 2012; 3: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ho J, Aridor O, Glinski DW, et al. Needs and workflow assessment prior to implementation of a digital pathology infrastructure for the US air force medical service. J Pathol Inform 2013; 4: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wixon D, Holtzblatt K, Knox S. (eds). Contextual design: an emergent view of system design. In: Conference on human factors in computing systems, 1990, https://dl.acm.org/doi/10.1145/97243.97304 (accessed 14 May 2021).
- 65.Holtzblatt K, Beyer H. Principles of contextual inquiry. In: Contextual design: design for life. 2nd ed. Cambridge, MA: Morgan Kaufman, 2017, pp.43–80. https://www.elsevier.com/books/contextual-design/holtzblatt/978-0-12-800894-2
- 66.Fisher AM, Herbert MI, Douglas GP. Understanding the dispensary workflow at the Birmingham free clinic: a proposed framework for an informatics intervention. BMC Health Serv Res 2016; 16: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Healion D, O’Dowd E, Russell S. The development of a methodology for contextual user research in healthcare design projects. Amsterdam, the Netherlands: IOS Press, 2018, http://ebooks.iospress.nl/publication/50567 (accessed 14 May 2021). [PubMed]
- 68.van Limburg M, van Gemert-Pijnen JE, Nijland N, et al. Why business modeling is crucial in the development of eHealth technologies. J Med Internet Res 2011; 13: e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gorst SL, Armitage CJ, Brownsell S, et al. Home telehealth uptake and continued use among heart failure and chronic obstructive pulmonary disease patients: a systematic review. Ann Behav Med 2014; 48: 323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zanaboni P, Wootton R. Adoption of routine telemedicine in norwegian hospitals: progress over 5 years. BMC Health Serv Res 2016; 16: 496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greenhalgh T, Wherton J, Papoutsi C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res 2017; 19: e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Labrique AB, Wadhwani C, Williams KA, et al. Best practices in scaling digital health in low and middle income countries. Global Health 2018; 14: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams BJ, Bottoms D, Treanor D. Future-proofing pathology: the case for clinical adoption of digital pathology. J Clin Pathol 2017; 70: 1010–1018. [DOI] [PubMed] [Google Scholar]