Abstract
Objective
To determine whether CSF interleukin 8 (IL-8) concentration can help to distinguish Guillain-Barré syndrome (GBS) from chronic inflammatory demyelinating polyneuropathy (CIDP) at the initial stage of the disease.
Methods
We performed retrospective immunoassay of IL-8 in CSF, collected at the University Hospitals of Geneva between 2010 and 2018, from patients diagnosed with GBS (n = 45) and with CIDP (n = 30) according to the Brighton and European Federation of Neurological Societies/Peripheral Nerve Society criteria by a physician blinded to biological results.
Results
CSF IL-8 was higher in GBS (median: 83.9 pg/mL) than in CIDP (41.0 pg/mL) (p < 0.001). Receiver operating characteristic analyses indicated that the optimal IL-8 cutoff was 70 pg/mL. Above this value, patients were more likely to present GBS than CIDP (specificity 96.7%, sensitivity 64.4%, positive predictive value [PPV] 96.7%, and negative predictive value [NPV] 64.4%). Among GBS subcategories, IL-8 was higher in acute inflammatory demyelinating polyneuropathy (AIDP, median: 101.8 pg/mL) than in other GBS variants (median: 53.7 pg/mL). In addition, with CSF IL-8 above 70 pg/mL, patients were more likely to present AIDP than acute-onset CIDP (p < 0.001; specificity 100%, sensitivity 78.8%, PPV 100%, and NPV 46.2%) or other CIDP with nonacute presentation (p < 0.0001; specificity 95.8%, sensitivity 78.8%, PPV 96.3%, and NPV 76.7%).
Conclusion
CSF IL-8 levels can help to differentiate AIDP variant of GBS from CIDP, including acute-onset CIDP, with high specificity and PPV. This may improve early and appropriate treatment.
Classification of Evidence
This study provides Class II evidence that CSF IL-8 levels accurately distinguish patients with GBS from those with CIDP.
Guillain-Barré syndrome (GBS) and chronic inflammatory demyelinating polyneuropathy (CIDP) are heterogeneous neuropathic diseases caused by an immune-mediated inflammatory process, with different temporal evolution.1,2 In GBS, symptoms develop over 4 weeks before reaching a steady state,1 whereas CIDP has continuous clinical worsening for more than 8 weeks.2,3 Although CIDP usually has progressive onset, a study reported that 16% of patients with CIDP have acute symptoms with a nadir within 8 weeks from the onset of disease, followed by a chronic course. These patients were defined as acute-onset CIDP.4
Both GBS and CIDP diagnostic strategies rely on clinical, electroneuromyography (ENMG), and CSF findings, namely albuminocytologic dissociation.1-3 Although CIDP differs from GBS in its time course, disease duration, prognosis, and steroid responsiveness, no specific biomarker exists in both blood and CSF to distinguish these 2 diseases.5 Nevertheless, 2 cytokine screening–based studies—with a limited number of patients—have shown a trend of CSF interleukin 8 (IL-8) elevation in GBS more than in CIDP.6,7 IL-8 is a proinflammatory chemokine primarily secreted by circulating monocytes and local macrophages. It plays a crucial role in the inflammatory cascade by inducing chemotaxis gradient, migration, and oxidative burst.8
In practice, although GBS and CIDP are usually distinguishable in most of the cases, overlapping clinical and paraclinical features may occur, especially when CIDP begins with acute onset, which can mimic GBS at early stages.4 This study aims to evaluate the potential use of CSF IL-8 as a biomarker to help to differentiate GBS and CIDP at disease onset.
Methods
This study aims to determine whether CSF IL-8 concentration can help to distinguish GBS from CIDP at the initial stage of the disease. It provides Class II evidence that CSF IL-8 levels accurately distinguish patients with GBS from those with CIDP. The protocol was approved by the Geneva University Hospitals (HUG) Medical Ethical board (authorization #2019-01210), and the requirement for patient consent was waived.
Patients
This study is based on a cohort of 75 patients diagnosed with GBS (n = 45) or CIDP (n = 30) between 2010 and 2018 and followed in the Neurology Clinics of the HUG. Diagnoses were established according to Brighton9 and European Federation of Neurological Societies/Peripheral Nerve Society criteria3 by a physician blinded to biological results (A.M.L.). Baseline demographic, clinical, and biological patients' characteristics are summarized in table e-1, links.lww.com/NXI/A505. Disease duration was defined as the time between symptom onset and lumbar puncture. Exclusion criteria were age <18 years, active systemic autoimmune/tumoral diseases at the time of lumbar puncture, no ENMG, and follow-up <1 year.
Determination of IL-8 Levels in the CSF
IL-8 was measured using commercially available multiplex bead immunoassays (Fluorokine MAP Multiplex Human Cytokine Panel, R&D Systems).
Statistical Analysis
Because of the asymmetric data distribution of CSF IL-8 levels, we applied nonparametric tests for statistical analysis. We used the Wilcoxon-Mann-Whitney test for the distribution analysis for the head-to-head comparisons. A p value <0.05 was considered statistically significant. We used receiver operating characteristic (ROC) curve analysis to determine the power (sensitivity and specificity) of CSF IL-8 as a diagnostic marker for GBS.
Data Availability
The corresponding author is in possession of detailed methods and results of this study, which are available on request.
Results
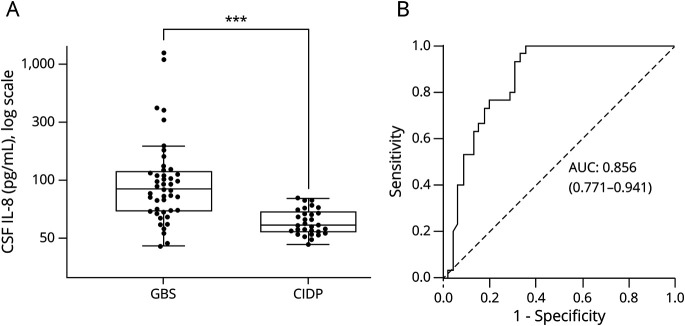
The median CSF IL-8 concentration was 83.9 pg/mL in the GBS group (n = 45; interquartile range [IQR] 64.4 [118.9–54.5]) compared with 41.0 pg/mL in the CIDP group (n = 30; IQR 17.3 [53.2–35.9]) (p value <0.001, figure 1A). ROC curves, to determine IL-8 diagnostic accuracy for GBS, indicated that the IL-8 area under the curve was of 0.86 (95% CI: 0.77–0.94) (p < 0.001, figure 1B). Using the Youden index, the optimal cutoff value was set at 70 pg/mL, beyond which IL-8 was considered as positive. This cutoff yields a specificity of 96.7% and a sensitivity of 64.4%, with a positive predictive value (PPV) of 96.7% and a negative predictive value (NPV) of 64.4%, for the diagnosis of GBS instead of CIDP. Additional serum and CSF analyses are summarized in table 1. Both groups showed similar levels of CSF white blood cells, abnormal protein levels, and albumin quotient.
Figure 1. Distribution of CSF IL-8 Levels and ROC Curve.
(A) Distribution of CSF IL-8 levels between patients with GBS and CIDP. Box-and-whisker plot (median, first and third quartiles, and range) of the distribution of CSF IL-8 levels between GBS (n = 45) and CIDP (n = 30) patient groups. Compared with CIDP (median: 41.0 pg/mL; IQR 17.3 [53.2–35.9]), CSF IL-8 was significantly increased in GBS (median: 83.9 pg/mL; IQR 64.4 [118.9–54.5]) (p < 0.001). The CSF IL-8 levels of all individual patients were measured using multiplex bead immunoassays. (B) IL-8 ROC curves. The ROC curve was calculated for optimal cutoff values. The AUC was calculated at 0.856 (0.771–0.941). The optimal cutoff value of CSF IL-8 to discriminate GBS from CIDP was set at 70 pg/mL for a sensitivity of 64.4% and a specificity of 96.7%. AUC = area under the curve; CIDP = chronic inflammatory demyelinating polyneuropathy; GBS = Guillain-Barré syndrome; IL-8 = interleukin 8; ROC = receiver operating characteristic. ***p < 0.001.
Table 1.
IL-8 Results and Other Biological Characteristics of Patients With GBS and CIDP (n = 75)
We next performed a subanalysis to test the different GBS vs CIDP subgroups (figure 2). Most of the IL-8–positive patients with GBS were diagnosed with acute inflammatory demyelinating polyneuropathy (AIDP) (n = 33; median: 101.8 pg/mL, IQR 74.2 [146.6–72.4]). Other GBS variants, including acute motor axonal neuropathy/acute motor and sensitive axonal neuropathy/MFS, showed lower IL-8 levels (n = 12; median: 53.7 pg/mL, IQR 27.4 [70.5–43.1]). Above the cutoff value of 70 pg/mL, patients were more likely to present AIDP than other GBS phenotypes (p < 0.001; specificity 75.0%, sensitivity 78.8%, PPV 89.7%, and NPV 56.3%) or CIDP (p < 0.0001; specificity 96.7%, sensitivity 78.8%, PPV 96.3%, and NPV 80.5%). With this cutoff value, IL-8 was positive in 78.8% of patients with AIDP (26/33) against 3.3% (1/30) in patients with CIDP.
Figure 2. Distribution of CSF IL-8 Levels in Patient Subgroups.
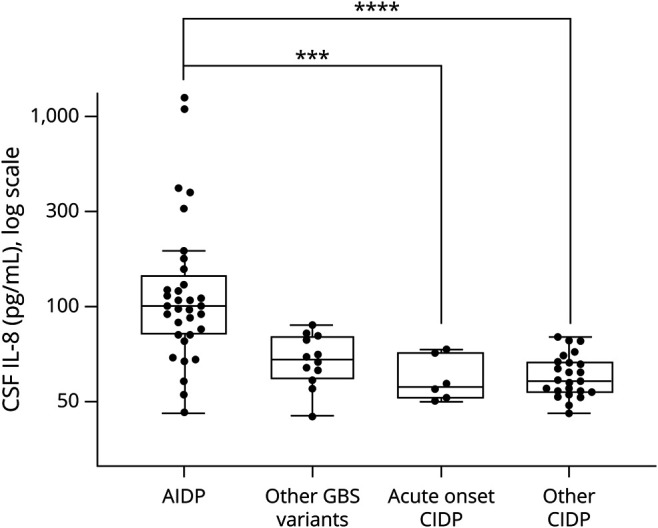
Box-and-whisker plot (median, first and third quartiles, and range) of the distribution of CSF IL-8 levels in patient subgroups. Among GBS subcategories, CSF IL-8 concentration was higher in AIDP (n = 33; median: 101.8 pg/mL, IQR 74.2 [146.6–72.4]) as opposed to axonal GBS phenotypes (AMAN and AMSAN) or MFS (n = 12; median: 53.7 pg/mL, IQR 27.4 [70.5–43.1]) (p < 0.001). Compared with acute-onset CIDP (n = 6; median: 38.9 pg/mL, IQR 25.2 [58.7–33.5]) and other CIDP (n = 24, median: 41.6 pg/mL; IQR 15.8 [52.1–36.3]), CSF IL-8 was significantly increased in AIDP (respectively p < 0.001 and p < 0.0001). AIDP = acute inflammatory demyelinating polyneuropathy; CIDP = chronic inflammatory demyelinating polyneuropathy; GBS = Guillain-Barré syndrome; IQR = interquartile range; IL-8 = interleukin 8. ***p < 0.001, ****p < 0.0001.
Among the 75 patients studied, 6 (8%) were diagnosed as GBS at symptom onset, based on clinical presentation and ENMG results. These patients were finally relabeled as acute-onset CIDP because of a steadily progressive evolution over 8 weeks and further ENMG results compatible with CIDP criteria.3 These patients had a lumbar puncture at symptom onset with IL-8 results <70 pg/mL (n = 6; median: 38.9 pg/mL, IQR 25.2 [58.7–33.5]). Among other CIDP without acute presentation (n = 24), IL-8 was negative except for 1 patient (70.7 pg/mL; median: 41.6 pg/mL; IQR 15.8 [52.1–36.3]). Above the cutoff value of 70 pg/mL, patients were more likely to present AIDP than acute-onset CIDP (p < 0.001; specificity 100%, sensitivity 78.8%, PPV 100%, and NPV 46.2%) or other CIDP (p < 0.0001; specificity 95.8%, sensitivity 78.8%, PPV 96.3%, and NPV 76.7%). Finally, IL-8 concentrations among GBS variants were compared with typical CIDP and atypical CIDP (European Federation of Neurological Societies/Peripheral Nerve Society criteria3) (figure e-1, links.lww.com/NXI/A504).
Discussion
This retrospective study shows that the CSF IL-8 concentration, when measured at the time of the initial diagnostic workup, is significantly increased in GBS when compared with CIDP. Our results suggest that a measurement of IL-8 with a cutoff of >70 pg/mL yields a PPV of 96% to differentiate patients with GBS from those with CIDP.
Among GBS subgroups, patients with AIDP had the most elevated IL-8 levels. AIDP and CIDP both sharing similar ENMG parameters, their distinction can be difficult at the acute phase. In this sense, CSF IL-8 appears to be a useful biomarker to differentiate AIDP from CIDP at the acute phase, with a median of 101.8 pg/mL in the AIDP group instead of 41.0 pg/mL in the CIDP group. However, it is important to emphasize that no subject had active systemic autoimmune/tumoral disease.
These findings have direct clinical implications because acute-onset CIDP may mimic GBS at early stages. In our cohort, 6 patients (8%) were misdiagnosed at disease onset as having GBS instead of CIDP.4 The diagnostic adjustment of acute-onset CIDP was made based on the clinical evolution and repeated ENMG. Of interest, all patients had retrospectively negative CSF IL-8. Despite the low number of patients with acute CIDP analyzed, the IL-8 difference with AIDP remains highly significant (p < 0.001). Nevertheless, these results will have to be confirmed with other studies before CSF IL-8 is validated as a routine test.
Improving the initial diagnosis of GBS vs CIDP may allow to better tailor the medical treatment and to prevent disease progression. IVIg and plasmapheresis are indicated in both diseases, but steroids are effective only for CIDP2 and may worsen GBS outcome.1 In nonacute CIDP, clinical examination and evolution in time as well as ENMG are sufficient to rule out GBS or AIDP. Thus, CSF IL-8 testing could be performed during the acute phase of investigation to permit a more rapid discriminatory diagnosis in combination with the clinical and paraclinical parameters. Cytokines, such as IL-8, can be easily measured in most university hospital laboratories and in some private laboratories with a routine test.
Glossary
- CIDP
chronic inflammatory demyelinating polyneuropathy
- GBS
Guillain-Barré syndrome
- HUG
Geneva University Hospitals
- IL-8
interleukin 8
- IQR
interquartile range
- NPV
negative predictive value
- PPV
positive predictive value
- ROC
receiver operating characteristic
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Yuki N, Hartung HP. Guillain-Barré syndrome. N Engl J Med. 2012;366(24):2294-2304. [DOI] [PubMed] [Google Scholar]
- 2.Dalakas MC. Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat Rev Neurol. 2011;7(9):507-517. [DOI] [PubMed] [Google Scholar]
- 3.Van den Bergh PY, Hadden RD, Bouche P, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society–first revision. Eur J Neurol. 2010;17(3):356-363. [DOI] [PubMed] [Google Scholar]
- 4.Ruts L, Drenthen J, Jacobs BC, van Doorn PA. Distinguishing acute-onset CIDP from fluctuating Guillain-Barre syndrome: a prospective study. Neurology. 2010;74(21):1680-1686. [DOI] [PubMed] [Google Scholar]
- 5.Illes Z, Blaabjerg M. Cerebrospinal fluid findings in Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathies. Handb Clin Neurol. 2017;146:125-138. [DOI] [PubMed] [Google Scholar]
- 6.Sainaghi PP, Collimedaglia L, Alciato F, et al. The expression pattern of inflammatory mediators in cerebrospinal fluid differentiates Guillain-Barré syndrome from chronic inflammatory demyelinating polyneuropathy. Cytokine. 2010;51(12):138-143. [DOI] [PubMed] [Google Scholar]
- 7.Breville G, Lascano AM, Roux-Lombard P, Lalive PH. IL-8 as a potential biomarker in Guillain-Barre Syndrome. Eur Cytokine Netw. 2019;30(4):130-134. [DOI] [PubMed] [Google Scholar]
- 8.Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics. 2017;7(6):1543-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fokke C, Van Den Berg B, Drenthen J, Walgaard C, Van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain. 2014;137(pt 1):33-43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author is in possession of detailed methods and results of this study, which are available on request.