Abstract
The ubiquitous pollution of the environment with microplastics, a diverse suite of contaminants, is of growing concern for science and currently receives considerable public, political, and academic attention. The potential impact of microplastics in the environment has prompted a great deal of research in recent years. Many diverse methods have been developed to answer different questions about microplastic pollution, from sources, transport, and fate in the environment, and about effects on humans and wildlife. These methods are often insufficiently described, making studies neither comparable nor reproducible. The proliferation of new microplastic investigations and cross-study syntheses to answer larger scale questions are hampered. This diverse group of 23 researchers think these issues can begin to be overcome through the adoption of a set of reporting guidelines. This collaboration was created using an open science framework that we detail for future use. Here, we suggest harmonized reporting guidelines for microplastic studies in environmental and laboratory settings through all steps of a typical study, including best practices for reporting materials, quality assurance/quality control, data, field sampling, sample preparation, microplastic identification, microplastic categorization, microplastic quantification, and considerations for toxicology studies. We developed three easy to use documents, a detailed document, a checklist, and a mind map, that can be used to reference the reporting guidelines quickly. We intend that these reporting guidelines support the annotation, dissemination, interpretation, reviewing, and synthesis of microplastic research. Through open access licensing (CC BY 4.0), these documents aim to increase the validity, reproducibility, and comparability of studies in this field for the benefit of the global community.
Keywords: Harmonization, standardization, plastic, microplastic, metadata, reproducibility, open science, methods, reporting guidelines, comparability
Introduction
The state of method reporting for investigations on microplastic pollution is currently at a turning point.1 As this new research field evolves, it is striving to establish a harmonized community approach to developing, applying, and reporting methodologies. Two of the main purposes for reporting scientific methods are to allow for their replication and enable data to be directly comparable among studies. For example, in the environmental sciences, data from studies might be compared during risk assessments, synthesized for meta-analyses, or used to inform policy creation and monitoring guidelines. Issues with reproducibility and comparability of both data and methods are common across all scientific fields,2–4 including microplastic research.1,5,6 Here, this diverse group of 23 microplastic researchers from around the world, present a proposed step towards addressing this issue for microplastics, first by capturing what is already in published literature, and then by prioritizing which types of information should be included in research to reach this goal. Our four aims are to (i) review key reproducibility and comparability problems and solutions for microplastic research; (ii) discuss the open science framework used to identify and prioritize key methodological parameters suggested here; (iii) develop reporting guidelines for researchers to use when reporting, comparing, and developing methods; and (iv) present our vision for future microplastic research.
The Reproducibility and Comparability Turning Point in Microplastics Research
It is well-known that microplastics have a ubiquitous presence in the environment,7–10 and the potential harm microplastics can cause to species across trophic levels has been recently reviewed.11,12 While there is mixed evidence for effects, a range of suborganismal, organismal, and population-level responses have been reported.6,11,13 These results have spurred substantial research activity, as evidenced by the continued exponential growth in the published literature on the topic of microplastics (Figure 1).
Figure 1.
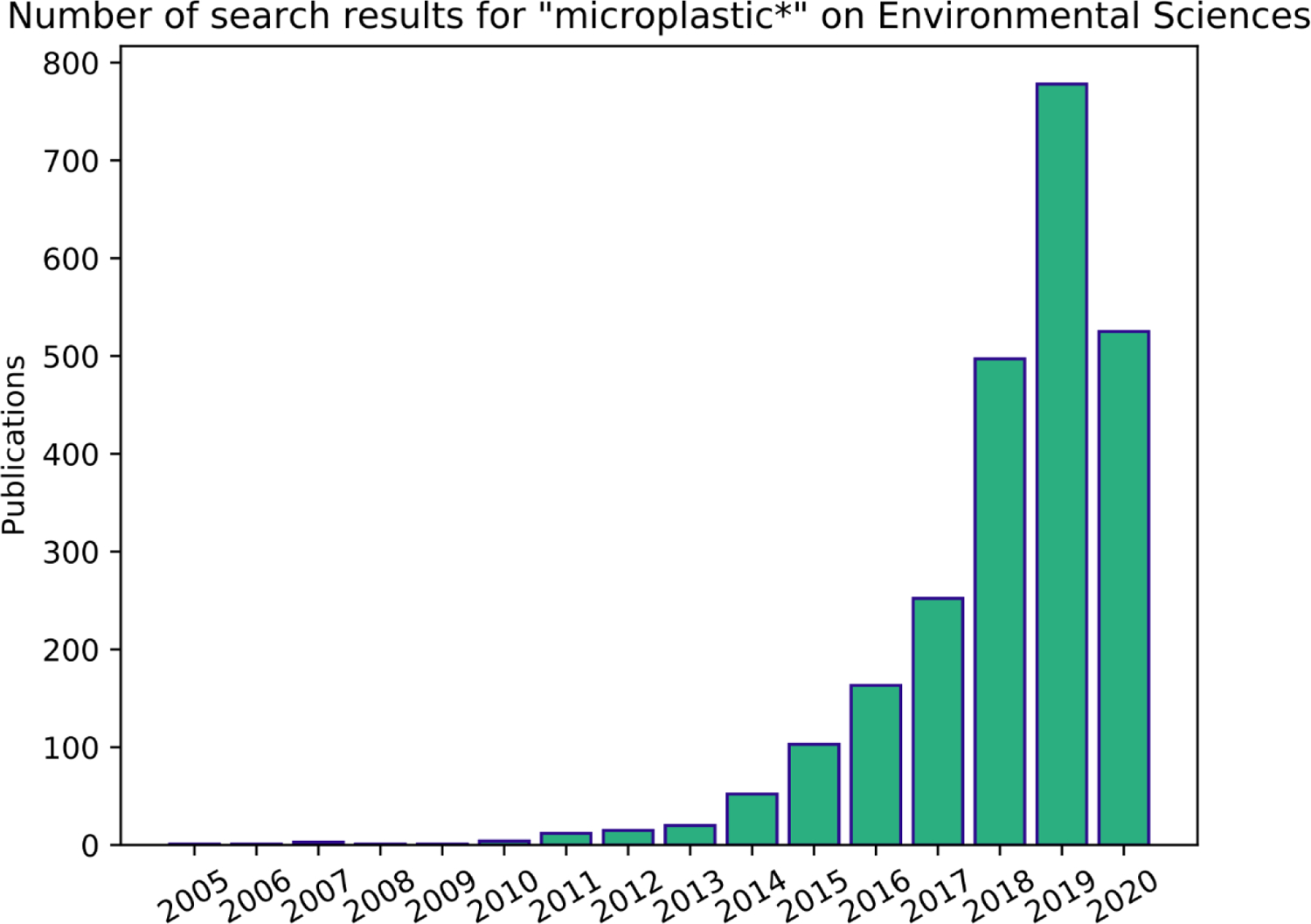
Data acquired from Scopus on 8 April 2020 using the search term “microplastic*” and querying the field of environmental sciences. Publications are annual sums. The figure was created using Python 3.6.9. The rapid expansion of research activities and the resulting data generated in the field of microplastics has resulted in a diverse suite of methods and non-standardized approaches to reporting sample collection, extraction, and analysis.1,14–21 Each method has its strengths and weaknesses, and there are continued efforts to optimize existing methods and develop new ones that may improve throughput, detection limit, and reproducibility. The development of new methods continues because currently there is no ‘catch-all’ combination of methods for sampling, extracting, analyzing, and reporting microplastics that is capable of accurately characterizing and quantifying all microplastics present in a sample.22,23 This is because microplastics are a diverse suite of contaminants that vary greatly in morphology, chemical properties, texture, color, density, and size.24 Moreover, environments and research goals are diverse and a universal solution is unable to capture this diversity, especially as research matures in this rapidly expanding field. With this in mind, methods should be chosen based on the scientific question and reported with enough detail to be comparable and reproducible.
Comparability between studies facilitates meta-analysis,25,26 which has been difficult for microplastics due to the diversity of methods employed and study details reported.17–21
Incomparability is caused by studies published without documenting the elements essential for translating units and metrics to others that are commonly used in the field. For example, studies that employ Raman spectroscopy might not be comparable to those that employ Fourier-transformed infrared (FT-IR) spectroscopy if neither describes their analysis and data transformation steps.18,27 Additionally, aquatic studies that use water volume grab sampling are not comparable to studies that use net sampling if the studies do not describe the mesh size used, depth of sample collection, or the sample volume.28 In another example, ingestion studies on the same species of animal are not comparable if they fail to mention which part of the gastrointestinal tract was analyzed (e.g., just the gizzard or the gizzard and proventriculus of birds).15,17 Moreover, a study using different chemical digestion methods to measure ingestion may be incomparable because some digestion procedures destroy certain plastics.29 Regardless of diverse methods and wherever possible, reporting raw - or less processed - data would allow reverse engineering and harmonization of some techniques. Still, raw data are seldom reported.16,30
Factors that cause incomparability can also hinder the reproducibility of research. Irreproducible research occurs, in part, when the elements that are critical for reproducing similar results are not elucidated. Reproducibility allows responsible decision-making and expansion of protocols. For example, software names should be reported when used because software often has proprietary algorithms and may not be reproducible unless the same software is used. In another example, if a study that employs organic matter digestion does not describe the chemical solution used, its manufacturer, and concentration used to digest the sample, the study cannot be reproduced.
Reporting guidelines provide a structured framework where method information critical to comparing and reproducing research can be referenced. There is a critical need for reporting guidelines in microplastic research as already initiated with the Minimum Information for Microplastic Studies (MIMS) concept for the study of microplastics in seafood,1 the minimum information for publication of infrared-related data,27 and other works assessing data quality in microplastic studies.31–33 The reporting guidelines we developed attempt to build on previous work and expand the scope to more methodological components in microplastic research. This study leverages the expertise of a diverse group of researchers from around the world to cover the breadth of the field. To be as transparent as possible, we elaborate on the reasons why each reporting guideline is necessary and provide examples for each. Other fields, like molecular biology,34 proteomics,35 and transcriptomics,36 already have highly successful examples of reporting guidelines that have been widely adopted by their field, and we hope this work serves a similar purpose in our field.
Methods
As a scientific community, we recognize that the need for reporting guidelines for microplastic methods is best addressed through a collaborative open science framework. With this goal in mind, the lead author sent out the following request on Twitter, and tagged several scientists in the microplastic community with a link to a collaborative document:
Frustrated with the reproducibility crisis in #microplastics research from poor method descriptions? Now is your chance to change that. I will publish this collaborative document OA [Open Access]. Add method considerations to this document and cite yourself in the Ack [Acknowledgements].
—Win Cowger, @Win_OpenData, 13 June 2019
The collaborative document was hosted open access on Google Drive and researchers were invited to provide input on the reporting guidelines for microplastic research methods. Over the subsequent week, 15 contributors edited the shared document directly. After one week, all initial contributors were invited to be coauthors, and additional coauthors were invited by word of mouth throughout the process using an open-door policy. Overall, there were 23 authors on this project and 26 other people acknowledged for their assistance. In a meeting of coauthors, the threshold for co-authorship was set at one full day of effort (self-defined and self-reported), while the threshold for acknowledgement was to review the document at least once. Authors contributed to this publication and the reporting guideline documents. The first author, Win Cowger, led the collaboration and the author order after the first author was randomized by agreement of all coauthors.
The reporting guidelines were identified by referencing standard operating procedures used by various authors and other peer-reviewed publications. All authors agreed not to use language that would imply an intent to standardize methodology or recommend specific methods over others; this was beyond the scope of the work. The task of the authors in developing the reporting guidelines was to outline what should be reported about a method when the method was used to make the method reproducible and comparable. To determine which guidelines were essential to add to the documents, each author was asked to fill out a Google Form survey where they designated each reporting category as required or not. The final reporting guidelines were formed by keeping only the guidelines that 51% or more of the authors agreed upon. During the review process, we received requests by reviewers to add additional reporting requirements. Where they were not already accounted for, we added them to the reporting guidelines and indicated those additions using an asterisk throughout the produced documents. The final reporting guidelines were packaged into three documents which have the same information summarized with specific user groups in mind: (i) thorough, a Detailed Document, (ii) quick and simple, a Checklist (Table I), and (iii) interactive, an online Mind Map (Figure 2).
Table I:
This is the Checklist of the reporting guidelines.
| Reporting Guidelines Checklist |
|---|
| Components to Report in All Procedures |
| Materials |
| All manufacturers of materials and instruments and their calibration37 |
| All software used and their calibration38 |
| Quality assurance/quality control |
| Error propagation |
| How instrumental, methodological, and/or statistical error was propagated39–41 |
| Replicates |
| Number of replicates42 |
| How replicates were nested within samples43 |
| Limit of detection |
| Quantitative detection threshold44 |
| Plastic morphology, size, color, and polymer limitations of method1,29,53,45–52 |
| Method of accounting for nondetects19,54 |
| Blank controls |
| Number of controls1,31 |
| Characteristics of plastics found in blanks with the same rigor as samples45 |
| Potential sources of contamination55 |
| Point of entry and exit to method55 |
| Positive controls |
| Morphology, size, color, and polymer type of positive controls1,31,56 |
| Positive control correction procedure31,56 |
| Point of entry and exit to method56 |
| Contamination mitigation |
| Clothing policies1,57 |
| Purification technique for reagents50,58 |
| Glassware cleaning techniques59 |
| Containment used (e.g. laminar flow cabinet/hoods, glove bags)1,50,60–62 |
| Data reporting |
| Share raw data and analysis code as often as possible18,22,38,63,64 |
| Field Sampling |
| Where (e.g., region) and when (e.g., date, time) the sample was collected19,65–70 |
| Size (e.g., m3, kg) and composition (e.g., sediment, water, biota) of the sample1,71 |
| Location at the site that sample was collected (e.g., 3 cm depth of surface sediment)72 |
| Sample device dimensions and deployment procedures14,31,73–75 |
| Environmental or infrastructure factors that may affect the interpretation of results75–81 |
| How samples are stored and transported1,82,83 |
| Sample Preparation |
| Homogenization |
| Homogenization technique84 |
| Splitting/subsetting |
| Sample splitting/subsetting technique75 |
| Drying |
| Sample drying temperature and time85 |
| Synthesized plastic |
| Synthesized plastic polymer, molecular characteristics, size, color, texture, and shape86,87 |
| Synthesized plastic synthesis technique86,88 |
| Fluorescent dye |
| Dye type, concentration, and solvent used89–91 |
| Dye application technique89 |
| Sieving strategy |
| Sieve mesh size84 |
| If the sample was wet or dry sieved84 |
| Density separation |
| Concentration, density, and composition (e.g. CaCl2, ZnCl) of solution82,92,93 |
| Time of separation94 |
| Device used61,94–98 |
| Digestion |
| Duration and temperature of digestion21,99,100 |
| Digestion solution composition21,56,100 |
| Ratio of digestion fluid to sample21,56,100,101 |
| Filtration |
| Filter composition, porosity, diameter50,102,103 |
| Microplastic Identification |
| Visual identification |
| Imaging settings |
| Image settings (e.g., contrast, gain, saturation, light intensity)18 |
| Magnification (e.g., scale bar, 50X objective)104 |
| Light microscopy |
| Magnification used during identification90 |
| Shapes, colors, textures, and reflectance, used to differentiate plastic104–106 |
| Fluorescence microscopy |
| Magnification used during identification90 |
| Fluorescence light wavelength, intensity, and exposure time to light source90,91,107 |
| Threshold intensity used to identify plastic107 |
| Scanning electron microscopy (SEM) |
| The coating used (e.g., metal type, water vapor)108 |
| Magnification used during identification108 |
| Textures used to differentiate plastic108 |
| Chemical identification |
| Pyrolysis gas chromatography mass spectrometry (py-GC/MS) |
| Pyrolysis reacting gases, temperature, duration49,109 |
| GC oven program, temperature, carrier gas, and column characteristics49,109 |
| MS ionization voltage, mass range, scanning frequency, temperature18,49 |
| py-GC/MS matching criteria (i.e., match threshold, linear retention indices (LRI), and Kovats index)49,110 |
| py-GC/MS quantification techniques109 |
| Raman spectroscopy |
| Acquisition parameters (i.e., laser wavelength, hole diameter, spectral resolution, laser intensity, number of accumulations, time of spectral acquisition)37,63,111–115 |
| Pre-processing parameters (i.e., spike filter, smoothing, baseline correction, data transformation)56,112,115,116 |
| Spectral matching parameters (i.e., spectral library source, range of spectral wavelengths used to match, match threshold, matching procedure)37,50,63,70,111–115,117 |
| Fourier-transform infrared spectroscopy (FT-IR) |
| Acquisition parameters (i.e., mode of spectra collection, accessories, crystal type, background recording, spectral range, spectral resolution, number of scans)63,64,103 |
| Pre-processing parameters (i.e. Fourier-transformation (FT) parameters, smoothing, baseline correction, data transformation)18 |
| Matching parameters (i.e., FT-IR spectral library source, match threshold, matching procedure, range of spectra used to match)38,50,64,112 |
| Differential scanning calorimetry (DSC) |
| Acquisition parameters (i.e., temperature, time, number of cycles)20 |
| Matching parameters (i.e., parameters assessed, reference library source, comparison technique)20 |
| Microplastic Categorization |
| Shape, size, texture, color, and polymer category definitions24,118,119 |
| Microplastic Quantification |
| Units (e.g., kg, count, mm)1,120 |
| Size dimensions (e.g., Feret minimum or maximum)18 |
| Quantification techniques18 |
| Toxicology Considerations |
| Dosed plastic age, polymer, size, color, and shapes121–130 |
| Animal husbandry131,132 |
| Exposure concentration, media, and time132–138 |
| Effects evaluation metrics (e.g., what markers were evaluated?)* |
| Biota metrics (e.g., which tissues were analyzed?)* |
Asterisks (*) indicate that the guideline was added as part of peer review; all other guidelines were voted on by a majority of the coauthors. The guidelines are grouped using bolded and indented labels. The guidelines are italicized and are the furthest indented for each group. Citations correspond to additional information related to the guideline and good examples of reporting.
Figure 2.
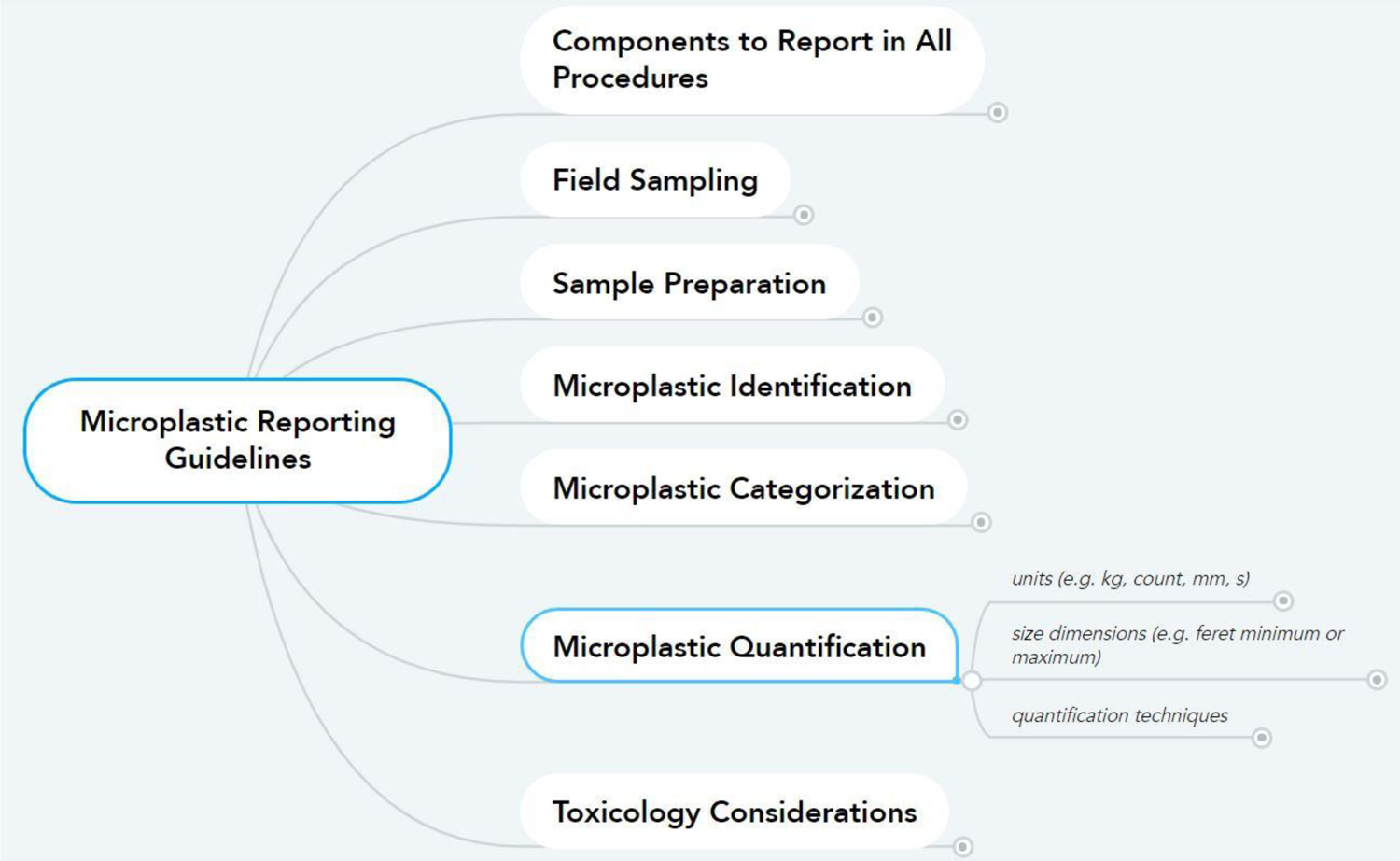
A screenshot of the Mind Map (LINK) showing the components and flow of reporting guidelines for microplastic studies. The first nodes branching off of “Microplastic Reporting Guidelines” are the general groups of the guidelines, subgroups follow in bold until the second to last nodes are the reporting guidelines (in italic) and the terminal node is the description of the guideline
The reporting guidelines were sent out to other colleagues in the field for an endorsement and critique designated as signatories in the acknowledgments. After the first week, we received 19 endorsements. The manuscript and supporting information were also subject to internal review at the National Institute of Standards and Technology (NIST) and single blind peer review from Applied Spectroscopy. In these ways, we attempted to receive as much feedback as we could to develop reporting guidelines that reflect the diverse group of experts and the broad scope of methods in microplastic research. This framework represents an example of a way that scientists in any field can develop robust collaborations by sharing ideas and learning from one another while developing useful reference documents, even if they have not met before.
Reporting Guideline Document Descriptions
The three documents we created of the reporting guidelines include a (i) Detailed Document, (ii) Checklist (Table I), and (iii) online Mind Map (Figure 2). Each document has the same information summarized with different users in mind. These documents are expected to be useful for scientists researching microplastics, peer reviewers asked to evaluate research, and users of the data. These documents outline what needs to be reported for common methods in microplastic research to be reproducible and comparable. The documents can also be used when developing methods internally to quickly identify the essential components of a method to calibrate and control in a lab. The Detailed Document can be used when every detail listed in the reporting guidelines are important to know. The Checklist can be used to quickly reference the reporting guidelines and check off the guidelines relevant to a specific study. The Mind Map is useful for those who prefer interactive information workflows and want to be able to quickly summarize and expand the reporting guidelines at any level of detail.
Any of these documents can be used to reference the report guidelines. All of the documents contain the same information reformatted and summarized. In the documents, the general method groups we define are: Materials, Quality Assurance/Quality Control (QA/QC), Data Reporting, Field Sampling, Sample Preparation, Identification, Categorization, and Toxicology Considerations. Subgroups describe specific method techniques within each group. Some of the groups may be used more than once in a study while some may not be used at all. It is important to note that these documents are templates and one need only consider the guidelines from the groups of methods relevant to a given study. When using the documents, first, assess which groups of methods apply to the study. Subgroups of methods are tab separated to indicate more detailed levels of grouping. Next, assess which of the subgroups apply. These can be highlighted or opened for easy reference. Where the most detailed subgroups apply, all italicized reporting guidelines must be defined, described, or discussed for that method to be reproducible and comparable. All reporting guidelines always apply to groups that do not have subgroups. Importantly, these reporting guidelines are not meant to completely define what should be reported but are a proposal for the minimum guidelines. Below we detail each document individually and outline a path forward for the documents to be updated.
Detailed Document
The Detailed Document (Supplemental Material 1; OSF) is the plain-text thorough version of the reporting guidelines containing the identical information, groups, and order to the Checklist and Mind Map described below. While this document is the primary result of this project, its length precludes including it in the main manuscript. The Detailed Document is meant for those who are new to the methods or want a detailed description and reference examples of the reporting guideline. This document may also be useful to those who find the Mind Map format to be challenging to navigate. The Detailed Document is easily printed for reference, which can be especially useful during the design stage of a study. The format of this document follows that the highest level of method grouping is in the largest text font and bolded. Subgroups of methods are in bold and identical font size but further indented if they are a subgroup of a subgroup. The essential elements to report are italicized and all the same font size. The explanation, reason, and examples for each essential element immediately follow the element and are light gray in color.
The Checklist
The Checklist (Supplemental Material 2; OSF; Table I) is meant for those already familiar with the methods and reasons for reporting outlined in the other documents. The format follows the Detailed Document but the explanation, reason, and examples for each reporting guideline are removed for quick reference and reading so that the elements can be checked off when reviewing or writing documents. Citations used in the Detailed document are added at the end of each guideline. The reporting guidelines are italicized and all the same font size as in the Detailed Document.
Mind Map
The Mind Map (Supplemental Material 3; LINK; OSF; Figure 2) was developed because we recognized a need to have many intermediate levels of detail between the detail provided by the Detailed Document and the Checklist. Interactive mind map documents allow the user to query to the level of detail they need quickly. This is meant for users who prefer spatially structured interactive information queries. The Mind Map was formatted using www.mindmeister.com, a free collaborative mind map creator that can reformat mind maps into tiered documents. The Mind Map is structured the same as the Detailed Document, where general method groups flow from the primary term “Microplastics Reporting Guidelines”. These general groups are further refined by subgroups of method types and instrument groups, where the terminal node of every branch leads to essential methodology elements (italicized) that should be reported. Each reporting guideline is described by an explanation, reasons to report, and/or examples from published microplastic literature.
Strategy for Updating the Reporting Guidelines
The field of microplastic research is rapidly evolving, and we expect that our documents, like most things in science, will need to be adapted, expanded, and revised. We recognize that as the field of microplastic pollution develops and grows, there will be new techniques and methods developed that will have reporting guidelines. We also acknowledge other methods are already useful to report that are not yet covered here. These documents are expected to be updated over time as new techniques are developed. That is why all documents are completely free and hold open access licenses (CC BY 4.0). The license allows for redistribution and adaptation with attribution to the original document. Additionally, we created an Open Science Framework project (OSF) for each document where researchers can reach out with suggestions and comments to update future editions of these documents. The authors will monitor the comments on the project and respond, as necessary. Future versions will be updated periodically on the OSF project site using version control. Additionally, we submitted this reporting guideline and others reported in the literature1,27 to the reporting guideline portal at https://fairsharing.org/. We hope that these documents and online forums are widely used for the benefit of the global community.
Our Vision of the Future of Research on Microplastics
We envision a future where research on microplastics is comparable, reproducible, and transparent. We aim for researchers in the field to be able to read a paper and use the methods for their work and/or use the data in a synthesis paper or meta-analysis. We aim for policymakers and managers to be able to review the literature and have the ability to compare data across sources, pathways, and geographies to inform the decision-making process. We envision a field where communication is clear amongst different stakeholders in the world of microplastics and where collaboration and research translation are made simpler. With our collaborative and open access framework, we aim to improve future work on microplastics and provide a framework for other emerging contaminants.
Supplementary Material
Acknowledgments
The authors would like to thank Justine Ammendolia, Sarah Nelms, Kristian Parton, Jessica Melvin, Matthew Cole, Shannon Tarby, and Alexander Turra for their helpful input throughout the writing and envisioning process. Additionally, we would like to thank those who endorsed the document that we developed: Greg Sambrook Smith, Claire Gwinnett, Dorthy Horn, Katie Allen, Jesse Vermaire, Garth Covernton, Francois Galgani, Pernilla Carlsson, Zacharias Steinmetz, Tanja Kögel, Louise Feld, Jakob Strand, Meredith Seeley, Bethanie Carney Almroth, Timothy Hoellein, Jessica Melvin, Katrin Wendt-Potthoff, Scott Coffin, and Susannah Bleakley.
We also thank our funders. W. Cowger was funded by the National Science Foundation Graduate Research Fellowship Program. A. Booth received funding from the Research Council of Norway through the Joint Programming Initiatives (JPI) Oceans project ‘PLASTOX: Direct and indirect ecotoxicological impacts of microplastics on marine organisms’ (grant agreement No. 257479) and the project ‘MICROFIBRE: Evaluating the fate, effects and mitigation measures for microplastic fiber pollution in aquatic environments’ (grant agreement No. 268404). C. Thaysen was funded by the Herbert W. Hoover Foundation. S. Primpke was funded by the German Federal Ministry of Education and Research (Project BASEMAN (JPI-Oceans) - Defining the baselines and standards for microplastics analyses in European waters; Federal Ministry of Education and Research (BMBF) grant 03F0734A). A. Dehaut is thankful to the French National Research Agency (ANR-15-CE34–0006-02), as part of the Nanoplastics project. He is also grateful to different bodies, as his contribution has been carried out thanks to the financial support of the European Union (ERDF), the French State, the French Region Hauts-de-France and Ifremer, in the framework of the project CPER MARCO 2015–2020. V. Vaz was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). M. Liboiron was funded by the Office of the Vice President Research, Memorial University; Social Sciences and Humanities Research Council; Northern Contaminants Program (NCP), and Memorial’s Undergraduate Career Experience Program (MUCEP) program. A. Gray was funded in part by the United States Department of Agriculture’s (USDA) National Institute of Food and Agriculture, Hatch and Multistate W4170 programs [project numbers CA-R-ENS-5120-H and CA-R-ENS-5189-RR]. S. Brander was funded by National Oceanic and Atmospheric Association grant #NA17NOS9990025, Oregon Agricultural Research Foundation, and NSF Growing Convergence Research grant #1935028. A. Sanchez was funded by the National Science Foundation Graduate Research Fellowships Program (No. DGE 1322106) and DC Water Blue Plains Advanced Wastewater Treatment Plant. H. Nel received funding from the Leverhulme Trust.
Footnotes
Disclaimer
Certain commercial equipment, instruments, or materials are identified in this paper to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
References
- 1.Dehaut A, Hermabessiere L, Duflos G. “Current Frontiers and Recommendations for the Study of Microplastics in Seafood”. TRaC, Trends Anal. Chem 2019. 116: 346–359. [Google Scholar]
- 2.Hutson M. “Artificial Intelligence Faces Reproducibility Crisis”. Science 2018. 359(6377): 725–726. [DOI] [PubMed] [Google Scholar]
- 3.Baker M. “Is There a Reproducibility Crisis? A Nature Survey Lifts the Lid on How Researchers View the Crisis Rocking Science and What They Think Will Help”. Nature 2016. 533(7604): 452–455. [DOI] [PubMed] [Google Scholar]
- 4.Peng R. “The Reproducibility Crisis in Science: A Statistical Counterattack”. Significance 2015. 12(3): 30–32. [Google Scholar]
- 5.Horton AA, Walton A, Spurgeon DJ, Lahive E, Svendsen C. “Microplastics in Freshwater and Terrestrial Environments: Evaluating the Current Understanding to Identify the Knowledge Gaps and Future Research Priorities”. Sci. Total Environ 2017. 586: 127–141. [DOI] [PubMed] [Google Scholar]
- 6.Rist S, Bloch Hartmann N. “Aquatic Ecotoxicity of Microplastics and Nanoplastics: Lessons Learned from Engineered Nanomaterials”. In: Wagner M, Lambert S, editors. The Handbook of Environmental Chemistry: Freshwater Microplastics: Emerging Environmental Contaminants? Heidelberg; New York: Springer, 2018. Chap. 2, Pp. 25–49. [Google Scholar]
- 7.Li J, Liu H, Chen JP. “Microplastics in Freshwater Systems: A Review on Occurrence, Environmental Effects, and Methods for Microplastics Detection”. Water Res 2018. 137: 362–374. [DOI] [PubMed] [Google Scholar]
- 8.Schwabl P, Liebmann B, Koppel S, Konigshofer P, Bucsics T, Trauner M, et al. “Assessment of Microplastic Concentrations in Human Stool-Preliminary Results of a Prospective Study”. United Eur. Gastroenterol J 2018. 6: A127. [Google Scholar]
- 9.Cox KD, Covernton GA, Davies HL, Dower JF, et al. “Human Consumption of Microplastics”. Environ. Sci. Technol 2019. 53(12): 7068–7074. [DOI] [PubMed] [Google Scholar]
- 10.Group of Experts on Scientific Aspects of Marine Environmental Protection (GESAMP). Guidlines for the Monitoring and Assessment of Plastic Litter in the Ocean 2019. http://www.gesamp.org/publications/guidelines-for-the-monitoring-and-assessment-of-plastic-litter-in-the-ocean [accessed 8 May 2020]. [Google Scholar]
- 11.Rochman CM, Browne MA, Underwood AJ, Van Franeker JA, et al. “The Ecological Impacts of Marine Debris: Unraveling the Demonstrated Evidence from What Is Perceived”. Ecology 2016. 97(2): 302–312. [DOI] [PubMed] [Google Scholar]
- 12.Barboza LGA, Cózar A, Gimenez BCG, Barros TL, Kershaw PJ, Guilhermino L. “Macroplastics Pollution in the Marine Environment”. World Seas: An Environmental Evaluation 2019. Pp. 305–328. [Google Scholar]
- 13.Foley CJ, Feiner ZS, Malinich TD, Höök TO. “A Meta-Analysis of the Effects of Exposure to Microplastics on Fish and Aquatic Invertebrates”. Sci. Total Environ 2018. 631–632: 550–559. [DOI] [PubMed] [Google Scholar]
- 14.Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M. “Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification”. Environ. Sci. Technol 2012. 46(6): 3060–3075. [DOI] [PubMed] [Google Scholar]
- 15.Provencher JF, Bond AL, Avery-Gomm S, Borrelle SB, et al. “Quantifying Ingested Debris in Marine Megafauna: A Review and Recommendations for Standardization”. Anal. Methods 2017. 9(9): 1454–1469. [Google Scholar]
- 16.Zhang S, Wang J, Liu X, Qu F, et al. “Microplastics in the Environment: A Review of Analytical Methods, Distribution, and Biological Effects”. TRaC, Trends Anal. Chem 2019. 111: 62–72. [Google Scholar]
- 17.Avery-Gomm S, Valliant M, Schacter CR, Robbins KF, et al. “A Study of Wrecked Dovekies (Alle Alle) in the Western North Atlantic Highlights the Importance of Using Standardized Methods to Quantify Plastic Ingestion”. Mar. Pollut. Bull 2016. 113(1–2): 75–80. [DOI] [PubMed] [Google Scholar]
- 18.Cowger W, Gray A, Christiansen SH, De Frond H, Deshpande AD, et al. “Critical Review of Processing and Classification Techniques for Images and Spectra in Microplastic Research”. Appl. Spectrosc [DOI] [PubMed] [Google Scholar]
- 19.Brander S. et al. “A Guide for Scientists Investigating the Occurrence of Microplastics Across Different Matrices”. Appl. Spectrosc. doi: 10.1177/0003702820945713. [DOI] [PubMed] [Google Scholar]
- 20.Primpke S. et al. “Critical Assessment of Analytical Methods for the Harmonized and Cost Efficient Analysis of Microplastics”. Appl. Spectrosc. doi: 10.1177/0003702820921465. [DOI] [PubMed] [Google Scholar]
- 21.Lusher A. et al. “Isolation and Extraction of Microplastics from Environmental Samples: An Evaluation of Practical Approaches and Recommendations for Further Harmonisation”. Appl. Spectrosc. doi: 10.1177/0003702820938993. [DOI] [PubMed] [Google Scholar]
- 22.Primpke S, Dias PA, Gerdts G. “Automated Identification and Quantification of Microfibres and Microplastics”. Anal. Methods 2019. 11(16): 2138–2147. [Google Scholar]
- 23.Zarfl C. “Promising Techniques and Open Challenges for Microplastic Identification and Quantification in Environmental Matrices”. Anal. Bioanal. Chem 2019. 411(17): 3743–3756. [DOI] [PubMed] [Google Scholar]
- 24.Rochman CM, Brookson C, Bikker J, Djuric N, et al. “Rethinking Microplastics as a Diverse Contaminant Suite”. Environ. Toxicol. Chem 2019. 38(4): 703–711. [DOI] [PubMed] [Google Scholar]
- 25.Schulz M, Van Loon W, Fleet DM, Baggelaar P, Van Der Meulen E. “OSPAR Standard Method and Software for Statistical Analysis of Beach Litter Data”. Mar. Pollut. Bull 2017. 122(1–2): 166–175. [DOI] [PubMed] [Google Scholar]
- 26.Everaert G, Van Cauwenberghe L, De Rijcke M, Koelmans AA, et al. “Risk Assessment of Microplastics in the Ocean: Modelling Approach and First Conclusions”. Environ. Pollut 2018. 242: 1930–1938. [DOI] [PubMed] [Google Scholar]
- 27.Andrade JM, Ferreiro B, López-Mahía P, Muniategui-Lorenzo S. “Standardization of the Minimum Information for Publication of Infrared-Related Data When Microplastics Are Characterized”. Mar. Pollut. Bull 2020. 154. 10.1016/J.Marpolbul.2020.111035. [DOI] [PubMed] [Google Scholar]
- 28.Barrows APW, Neumann CA, Berger ML, Shaw SD. “Grab: vs. Neuston Tow Net: A Microplastic Sampling Performance Comparison and Possible Advances in the Field”. Anal. Methods 2017. 9(9): 1446–1453. 10.1039/C6ay02387h. [DOI] [Google Scholar]
- 29.Enders K, Lenz R, Beer S, Stedmon CA. “Extraction of Microplastic from Biota: Recommended Acidic Digestion Destroys Common Plastic Polymers”. ICES J. Mar. Sci 2017. 74(1): 326–331. [Google Scholar]
- 30.Cowger W, Gray AB, Eriksen M, Moore C, Thiel M. “Evaluating Wastewater Effluent as a Source of Microplastics in Environmental Samples”. In: Karapanagioti HK, Kalavrouziotis IK, editors. Microplastics in Water and Wastewater London: IWA Publishing, 2019. Chap. 8, Pp. 109–131. [Google Scholar]
- 31.Hermsen E, Mintenig SM, Besseling E, Koelmans AA. “Quality Criteria for the Analysis of Microplastic in Biota Samples: A Critical Review”. Environ. Sci. Technol 2018. 52(18): 10230–10240. 10.1021/Acs.Est.8b01611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koelmans AA, Mohamed Nor NH, Hermsen E, Kooi M, et al. “Microplastics in Freshwaters and Drinking Water: Critical Review and Assessment of Data Quality”. Water Res 2019. 155: 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pahl S. Koelmans B. “A Scientific Perspective on Microplastics in Nature and Society”. Science Advice for Policy by European Academies (SAPEA) 2019. https://www.sapea.info/topics/microplastics/ [accessed 8 May 2020]. [Google Scholar]
- 34.Bustin SA, Benes V, Garson JA, Hellemans J, et al. “The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments”. Clin. Chem 2009. 55(4): 611–622. 10.1373/Clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 35.Taylor CF, Paton NW, Lilley KS, Binz P-A, et al. “The Minimum Information About a Proteomics Experiment (MIAPE)”. Nat. Biotechnol 2007. 25(8): 887–893. 10.1038/Nbt1329. [DOI] [PubMed] [Google Scholar]
- 36.Brazma A, Hingamp P, Quackenbush J, Sherlock G, et al. “Minimum Information About a Microarray Experiment (MIAME): Toward Standards for Microarray Data”. Nat. Genet 2001. 29(4): 365–371. 10.1038/Ng1201-365. [DOI] [PubMed] [Google Scholar]
- 37.Ossmann BE, Sarau G, Holtmannspötter H, Pischetsrieder M, et al. “Small-Sized Microplastics and Pigmented Particles in Bottled Mineral Water”. Water Res 2018. 141: 307–316. [DOI] [PubMed] [Google Scholar]
- 38.Primpke S, Lorenz C, Rascher-Friesenhausen R, Gerdts G. “An Automated Approach for Microplastics Analysis Using Focal Plane Array (FPA) FT-IR Microscopy and Image Analysis”. Anal. Methods 2017. 9(9): 1499–1511. [Google Scholar]
- 39.Hurley R, Woodward J, Rothwell JJ. “Microplastic Contamination of River Beds Significantly Reduced by Catchment-Wide Flooding”. Nat. Geosci 2018. 11(4): 251–257. [Google Scholar]
- 40.Kedzierski M, Villain J, Falcou-Préfol M, Kerros ME, et al. “Microplastics in Mediterranean Sea: A Protocol to Robustly Assess Contamination Characteristics”. PLoS One 2019. 14(2): E0212088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haave M, Lorenz C, Primpke S, Gerdts G. “Different Stories Told by Small and Large Microplastics in Sediment - First Report of Microplastic Concentrations in an Urban Recipient in Norway”. Mar. Pollut. Bull 2019. 141: 501–513. [DOI] [PubMed] [Google Scholar]
- 42.Cable RN, Beletsky D, Beletsky R, Wigginton K, et al. “Distribution and Modeled Transport of Plastic Pollution in the Great Lakes, the World’s Largest Freshwater Resource”. Front. Environ. Sci. Eng. China 2017. 5: 45. [Google Scholar]
- 43.Frias JPGL, Sobral P, Ferreira AM. “Organic Pollutants in Microplastics from Two Beaches of the Portuguese Coast”. Mar. Pollut. Bull 2010. 60(11): 1988–1992. [DOI] [PubMed] [Google Scholar]
- 44.Martin KM, Hasenmueller EA, White JR, Chambers LG, Conkle JL. “Sampling, Sorting, and Characterizing Microplastics in Aquatic Environments with High Suspended Sediment Loads and Large Floating Debris”. J. Vis. Exp 2018. 137. 10.3791/57969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mcneish RE, Kim LH, Barrett HA, Mason SA, et al. “Microplastic in Riverine Fish Is Connected to Species Traits”. Sci. Rep 2018. 8(1): 11639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liboiron M, Liboiron F, Wells E, Richárd N, et al. “Low Plastic Ingestion Rate in Atlantic Cod (Gadus Morhua) from Newfoundland Destined for Human Consumption Collected Through Citizen Science Methods”. Mar. Pollut. Bull 2016. 113(1–2): 428–437. [DOI] [PubMed] [Google Scholar]
- 47.Song YK, Hong SH, Jang M, Han GM, et al. “A Comparison of Microscopic and Spectroscopic Identification Methods for Analysis of Microplastics in Environmental Samples”. Mar. Pollut. Bull 2015. 93(1–2): 202–209. [DOI] [PubMed] [Google Scholar]
- 48.Nel HA, Dalu T, Wasserman RJ, Hean JW. “Colour and Size Influences Plastic Microbead Underestimation, Regardless of Sediment Grain Size”. Sci. Total Environ 2019. 655: 567–570. [DOI] [PubMed] [Google Scholar]
- 49.Hermabessiere L, Himber C, Boricaud B, Kazour M, et al. “Optimization, Performance, and Application of a Pyrolysis-GC/MS Method for the Identification of Microplastics”. Anal. Bioanal. Chem 2018. 410(25): 6663–6676. [DOI] [PubMed] [Google Scholar]
- 50.Lorenz C, Roscher L, Meyer MS, Hildebrandt L, et al. “Spatial Distribution of Microplastics in Sediments and Surface Waters of the Southern North Sea”. Environ. Pollut 2019. 252: 1719–1729. [DOI] [PubMed] [Google Scholar]
- 51.Devriese LI, Van Der Meulen MD, Maes T, Bekaert K, et al. “Microplastic Contamination in Brown Shrimp (Crangon crangon, Linnaeus 1758) from Coastal Waters of the Southern North Sea and Channel Area”. Mar. Pollut. Bull 2015. 98(1–2): 179–187. [DOI] [PubMed] [Google Scholar]
- 52.Vandermeersch G, Van Cauwenberghe L, Janssen CR, Marques A, et al. “A Critical View on Microplastic Quantification in Aquatic Organisms”. Environ. Res 2015. 143(Pt. B): 46–55. [DOI] [PubMed] [Google Scholar]
- 53.Hendrickson E, Minor EC, Schreiner K. “Microplastic Abundance and Composition in Western Lake Superior As Determined via Microscopy, Pyr-GC/MS, and FT-IR”. Environ. Sci. Technol 2018. 52(4): 1787–1796. [DOI] [PubMed] [Google Scholar]
- 54.Helsel DR. “Fabricating Data: How Substituting Values for Nondetects Can Ruin Results, and What Can Be Done About It”. Chemosphere 2006. 65(11): 2434–2439. [DOI] [PubMed] [Google Scholar]
- 55.Miller RZ, Watts AJR, Winslow BO, Galloway TS, Barrows APW. “Mountains to the Sea: River Study of Plastic and Non-Plastic Microfiber Pollution in the Northeast USA”. Mar. Pollut. Bull 2017. 124(1): 245–251. [DOI] [PubMed] [Google Scholar]
- 56.Dehaut A, Cassone A-L, Frère L, Hermabessiere L, et al. “Microplastics in Seafood: Benchmark Protocol for Their Extraction and Characterization”. Environ. Pollut 2016. 215: 223–233. [DOI] [PubMed] [Google Scholar]
- 57.Van Cauwenberghe L, Janssen CR. “Microplastics in Bivalves Cultured for Human Consumption”. Environ. Pollut 2014. 193: 65–70. [DOI] [PubMed] [Google Scholar]
- 58.De Witte B, Devriese L, Bekaert K, Hoffman S, et al. “Quality Assessment of the Blue Mussel (Mytilus edulis): Comparison Between Commercial and Wild Types”. Mar. Pollut. Bull 2014. 85(1): 146–155. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka K, Takada H. “Microplastic Fragments and Microbeads in Digestive Tracts of Planktivorous Fish from Urban Coastal Waters”. Sci. Rep 2016. 6: 34351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wesch C, Elert AM, Wörner M, Braun U, et al. “Assuring Quality in Microplastic Monitoring: About the Value of Clean-Air Devices as Essentials for Verified Data”. Sci. Rep 2017. 7(1): 5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Claessens M, Van Cauwenberghe L, Vandegehuchte MB, Janssen CR. “New Techniques for the Detection of Microplastics in Sediments and Field Collected Organisms”. Mar. Pollut. Bull 2013. 70(1–2): 227–233. [DOI] [PubMed] [Google Scholar]
- 62.Torre M, Digka N, Anastasopoulou A, Tsangaris C, Mytilineou C. “Anthropogenic Microfibres Pollution in Marine Biota. A New and Simple Methodology to Minimize Airborne Contamination”. Mar. Pollut. Bull 2016. 113(1–2): 55–61. [DOI] [PubMed] [Google Scholar]
- 63.Cabernard L, Roscher L, Lorenz C, Gerdts G, Primpke S. “Comparison of Raman and Fourier Transform Infrared Spectroscopy for the Quantification of Microplastics in the Aquatic Environment”. Environ. Sci. Technol 2018. 52(22): 13279–13288. [DOI] [PubMed] [Google Scholar]
- 64.Primpke S, Wirth M, Lorenz C, Gerdts G. “Reference Database Design for the Automated Analysis of Microplastic Samples Based on Fourier Transform Infrared (FT-IR) Spectroscopy”. Anal. Bioanal. Chem 2018. 410: 5131–5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bagaev A, Mizyuk A, Khatmullina L, Isachenko I, Chubarenko I. “Anthropogenic Fibres in the Baltic Sea Water Column: Field Data, Laboratory and Numerical Testing of Their Motion”. Sci. Total Environ 2017. 599–600: 560–571. [DOI] [PubMed] [Google Scholar]
- 66.Spear LB, Ainley DG, Ribic CA. “Incidence of Plastic in Seabirds from the Tropical Pacific, 1984−-1991: Relation with Distribution of Species, Sex, Age, Season, Year and Body Weight”. Mar. Environ. Res 1995. 40(2): 123–146. [Google Scholar]
- 67.Cheung PK, Cheung LTO, Fok L. “Seasonal Variation in the Abundance of Marine Plastic Debris in the Estuary of a Subtropical Macro-Scale Drainage Basin in South China”. Sci. Total Environ 2016. 562: 658–665. [DOI] [PubMed] [Google Scholar]
- 68.Lattin GL, Moore CJ, Zellers AF, Moore SL, Weisberg SB. “A Comparison of Neustonic Plastic and Zooplankton at Different Depths Near the Southern California Shore”. Mar. Pollut. Bull 2004. 49(4): 291–294. [DOI] [PubMed] [Google Scholar]
- 69.Browne MA, Galloway TS, Thompson RC. “Spatial Patterns of Plastic Debris along Estuarine Shorelines”. Environ. Sci. Technol 2010. 44(9): 3404–3409. [DOI] [PubMed] [Google Scholar]
- 70.Smith SDA, Markic A. “Estimates of Marine Debris Accumulation on Beaches Are Strongly Affected by the Temporal Scale of Sampling”. PLoS One 2013. 8(12): E83694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leslie HA, Brandsma SH, Van Velzen MJM, Vethaak AD. “Microplastics En Route: Field Measurements in the Dutch River Delta and Amsterdam Canals, Wastewater Treatment Plants, North Sea Sediments and Biota”. Environ. Int 2017. 101: 133–142. [DOI] [PubMed] [Google Scholar]
- 72.Willis KA, Eriksen R, Wilcox C, Hardesty BD. “Microplastic Distribution at Different Sediment Depths in an Urban Estuary”. Front. Mar. Sci 2017. 4: 419. [Google Scholar]
- 73.International Council for the Exploration of the Sea Working Group on Marin Litter (ICES WGML). “Interim Report of the Working Group on Marine Litter (WGML)” 2018. http://ices.dk/sites/pub/Publication%20Reports/Expert%20Group%20Report/HAPISG/2018/01%20WGML%20-%20Report%20of%20the%20Working%20Group%20on%20Marine%20Litter.pdf [accessed 8 May 2020]. [Google Scholar]
- 74.Covernton GA, Pearce CM, Gurney-Smith HJ, Chastain SG, et al. “Size and Shape Matter: A Preliminary Analysis of Microplastic Sampling Technique in Seawater Studies with Implications for Ecological Risk Assessment”. Sci. Total Environ 2019. 667: 124–132. [DOI] [PubMed] [Google Scholar]
- 75.Lusher AL, Burke A, O’Connor I, Officer R. “Microplastic Pollution in the Northeast Atlantic Ocean: Validated and Opportunistic Sampling”. Mar. Pollut. Bull 2014. 88(1–2): 325–333. 10.1016/J.Marpolbul.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 76.Reisser J, Slat B, Noble K, Du Plessis K, et al. “The Vertical Distribution of Buoyant Plastics at Sea: An Observational Study in the North Atlantic Gyre”. Biogeosci 2015. 12(4): 1249. [Google Scholar]
- 77.Enders K, Lenz R, Stedmon CA, Nielsen TG. “Abundance, Size, and Polymer Composition of Marine Microplastics ≥10 μm in the Atlantic Ocean and Their Modelled Vertical Distribution”. Mar. Pollut. Bull 2015. 100(1): 70–81. 10.1016/J.Marpolbul.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 78.Hardesty BD, Harari J, Isobe A, Lebreton L, et al. “Using Numerical Model Simulations to Improve the Understanding of Micro-Plastic Distribution and Pathways in the Marine Environment”. Front. Mar. Sci 2017. 4: 30. 10.3389/fmars.2017.00030 [DOI] [Google Scholar]
- 79.Iwasaki S, Isobe A, Kako S, Uchida K, Tokai T. “Fate of Microplastics and Mesoplastics Carried by Surface Currents and Wind Waves: A Numerical Model Approach in the Sea of Japan”. Mar. Pollut. Bull 2017. 121(1–2): 85–96. [DOI] [PubMed] [Google Scholar]
- 80.Kukulka T, Law KL, Proskurowski G. “Evidence for the Influence of Surface Heat Fluxes on Turbulent Mixing of Microplastic Marine Debris”. J. Phys. Ocean. Am. Meteorol. Soc 2016. 46(3): 809–815. [Google Scholar]
- 81.Frère L, Paul-Pont I, Rinnert E, Petton S, et al. “Influence of Environmental and Anthropogenic Factors on the Composition, Concentration and Spatial Distribution of Microplastics: A Case Study of the Bay of Brest (Brittany, France)”. Environ. Pollut 2017. 225: 211–222. [DOI] [PubMed] [Google Scholar]
- 82.Crichton EM, Noël M, Gies EA, Ross PS. “A Novel, Density-Independent, and FT-IR-Compatible Approach for the Rapid Extraction of Microplastics from Aquatic Sediments”. Anal. Methods 2017. 9(9): 1419–1428. [Google Scholar]
- 83.Courtene-Jones W, Quinn B, Murphy F, Gary SF, Narayanaswamy BE. “Optimisation of Enzymatic Digestion and Validation of Specimen Preservation Methods for the Analysis of Ingested Microplastics”. Anal. Methods 2017. 9(9): 1437–1445. [Google Scholar]
- 84.Wagner J, Wang Z-M, Ghosal S, Rochman C, et al. “Novel Method for the Extraction and Identification of Microplastics in Ocean Trawl and Fish Gut Matrices”. Anal. Methods 2017. 9(9): 1479–1490. [Google Scholar]
- 85.Dekiff JH, Remy D, Klasmeier J, Fries E. “Occurrence and Spatial Distribution of Microplastics in Sediments from Norderney”. Environ. Pollut 2014. 186: 248–256. [DOI] [PubMed] [Google Scholar]
- 86.Vicentini DS, Nogueira DJ, Melegari SP, Arl M, et al. “Toxicological Evaluation and Quantification of Ingested Metal-Core Nanoplastic by Daphnia Magna Through Fluorescence and Inductively Coupled Plasma-Mass Spectrometric Methods”. Environ. Toxicol. Chem 2019. 38(10): 2101–2110. [DOI] [PubMed] [Google Scholar]
- 87.Wang L, Zhang J, Hou S, Sun H. “A Simple Method for Quantifying Polycarbonate and Polyethylene Terephthalate Microplastics in Environmental Samples by Liquid Chromatography–Tandem Mass Spectrometry”. Environ. Sci. Technol. Lett 2017. 4(12): 530–534. 10.1021/Acs.Estlett.7b00454. [DOI] [Google Scholar]
- 88.Lu S, Qu R, Forcada J. “Preparation of Magnetic Polymeric Composite Nanoparticles by Seeded Emulsion Polymerization”. Mater. Lett 2009. 63(9–10): 770–772. 10.1016/J.Matlet.2008.12.045. [DOI] [Google Scholar]
- 89.Karakolis EG, Nguyen B, You JB, Rochman CM, Sinton D. “Fluorescent Dyes for Visualizing Microplastic Particles and Fibers in Laboratory-Based Studies”. Environ. Sci. Technol. Lett 2019. 6(6): 334–340. [Google Scholar]
- 90.Wiggin KJ, Holland EB. “Validation and Application of Cost and Time Effective Methods for the Detection of 3−-500 μm Sized Microplastics in the Urban Marine and Estuarine Environments Surrounding Long Beach, California”. Mar. Pollut. Bull 2019. 143: 152–162. [DOI] [PubMed] [Google Scholar]
- 91.Maes T, Jessop R, Wellner N, Haupt K, Mayes AG. “A Rapid-Screening Approach to Detect and Quantify Microplastics Based on Fluorescent Tagging with Nile Red”. Sci. Rep 2017. 7: 44501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mani T, Primpke S, Lorenz C, Gerdts G, Burkhardt-Holm P. “Microplastic Pollution in Benthic Midstream Sediments of the Rhine River”. Environ. Sci. Technol 2019. 53(10): 6053–6062. [DOI] [PubMed] [Google Scholar]
- 93.Ivleva NP, Wiesheu AC, Niessner R. “Microplastic in Aquatic Ecosystems”. Angew. Chem 2017. 56(7): 1720–1739. [DOI] [PubMed] [Google Scholar]
- 94.Imhof HK, Schmid J, Niessner R, Ivleva NP, Laforsch C. “A Novel, Highly Efficient Method for the Separation and Quantification of Plastic Particles in Sediments of Aquatic Environments: Novel Plastic Particle Separation Method”. Limnol. Ocean. Methods 2012. 10(7): 524–537. [Google Scholar]
- 95.Thompson RC, Olsen Y, Mitchell RP, Davis A, et al. “Lost at Sea: Where is All the Plastic?” Science 2004. 304(5672): 838. [DOI] [PubMed] [Google Scholar]
- 96.Wessel CC, Lockridge GR, Battiste D, Cebrian J. “Abundance and Characteristics of Microplastics in Beach Sediments: Insights into Microplastic Accumulation in Northern Gulf of Mexico Estuaries”. Mar. Pollut. Bull 2016. 109(1): 178–183. [DOI] [PubMed] [Google Scholar]
- 97.Masura J, Baker JE, Foster GD, Arthur C, Herring C. “Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments”. NOAA Marine Debris Program 2015. https://marinedebris.noaa.gov/sites/default/files/publications-files/noaa_microplastics_methods_manual.pdf [accessed 8 May 2020]. [Google Scholar]
- 98.Coppock RL, Cole M, Lindeque PK, Queirós AM, Galloway TS. “A Small-Scale, Portable Method for Extracting Microplastics from Marine Sediments”. Environ. Pollut 2017. 230: 829–837. [DOI] [PubMed] [Google Scholar]
- 99.Munno K, Helm PA, Jackson DA, Rochman C, Sims A. “Impacts of Temperature and Selected Chemical Digestion Methods on Microplastic Particles”. Environ. Toxicol. Chem 2018. 37(1): 91–98. [DOI] [PubMed] [Google Scholar]
- 100.Thiele CJ, Hudson MD, Russell AE. “Evaluation of Existing Methods to Extract Microplastics from Bivalve Tissue: Adapted KOH Digestion Protocol Improves Filtration at Single-Digit Pore Size”. Mar. Pollut. Bull 2019. 142: 384–393. [DOI] [PubMed] [Google Scholar]
- 101.Von Friesen LW, Granberg ME, Hassellöv M, Gabrielsen GW, Magnusson K. “An Efficient and Gentle Enzymatic Digestion Protocol for the Extraction of Microplastics from Bivalve Tissue”. Mar. Pollut. Bull 2019. 142: 129–134. [DOI] [PubMed] [Google Scholar]
- 102.Ossmann BE, Sarau G, Schmitt SW, Holtmannspötter H, et al. “Development of an Optimal Filter Substrate for the Identification of Small Microplastic Particles in Food by Micro-Raman Spectroscopy”. Anal. Bioanal. Chem 2017. 409(16): 4099–4109. [DOI] [PubMed] [Google Scholar]
- 103.Löder MGJ, Gerdts G. “Methodology Used for the Detection and Identification of Microplastics: A Critical Appraisal”. In: Bergmann M, Gutow L, Klages M, editors. Marine Anthropogenic Litter Switzerland: Springer International Publishing, 2015. Chap. 8, Pp. 201–227. [Google Scholar]
- 104.Fries E, Dekiff JH, Willmeyer J, Nuelle M-T, et al. “Identification of Polymer Types and Additives in Marine Microplastic Particles Using Pyrolysis-GC/MS and Scanning Electron Microscopy”. Environ. Sci. Process. Impacts 2013. 15(10): 1949–1956. [DOI] [PubMed] [Google Scholar]
- 105.Murray F, Cowie PR. “Plastic Contamination in the Decapod Crustacean Nephrops norvegicus (Linnaeus, 1758)”. Mar. Pollut. Bull 2011. 62(6): 1207–1217. [DOI] [PubMed] [Google Scholar]
- 106.Castañeda Rowshyra A., Avlijas Suncica, Simard M. Anouk, Ricciardia Anthony. “Microplastic Pollution Discovered in St. Lawrence River Sediments”. NRC Press 2014. 88(1–2): 5–6. [PubMed] [Google Scholar]
- 107.Erni-Cassola G, Gibson MI, Thompson RC, Christie-Oleza JA. “Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 μm) in Environmental Samples”. Environ. Sci. Technol 2017. 51(23): 13641–13648. [DOI] [PubMed] [Google Scholar]
- 108.Zbyszewski M, Corcoran PL, Hockin A. “Comparison of the Distribution and Degradation of Plastic Debris along Shorelines of the Great Lakes, North America”. J. Gt. Lakes Res 2014. 40(2): 288–299. [Google Scholar]
- 109.Fischer M, Scholz-Böttcher BM. “Simultaneous Trace Identification and Quantification of Common Types of Microplastics in Environmental Samples by Pyrolysis–Gas Chromatography–Mass Spectrometry”. Environ. Sci. Technol 2017. 51(9): 5052–5060. [DOI] [PubMed] [Google Scholar]
- 110.Van Den Dool H, Kratz P. Dec.. “A Generalization of the Retention Index System Including Linear Temperature Programmed Gas: Liquid Partition Chromatography”. J. Chromatogr. A 1963. 11: 463–471. [DOI] [PubMed] [Google Scholar]
- 111.Munno K, De Frond H, O’Donnell B, Rochman CM. “Increasing the Accessibility for Characterizing Microplastics: Introducing New Application-Based and Spectral Libraries of Plastic Particles (SLoPP and SLoPP-E)”. Anal. Chem 2020. 92(3): 2443–2451. [DOI] [PubMed] [Google Scholar]
- 112.Lenz R, Enders K, Stedmon CA, Mackenzie DMA, Nielsen TG. “A Critical Assessment of Visual Identification of Marine Microplastic Using Raman Spectroscopy for Analysis Improvement”. Mar. Pollut. Bull 2015. 100(1): 82–91. [DOI] [PubMed] [Google Scholar]
- 113.Karami A, Golieskardi A, Choo CK, Larat V, et al. “Microplastic and Mesoplastic Contamination in Canned Sardines and Sprats”. Sci. Total Environ 2018. 612: 1380–1386. [DOI] [PubMed] [Google Scholar]
- 114.Käppler A, Fischer D, Oberbeckmann S, Schernewski G, et al. “Analysis of Environmental Microplastics by Vibrational Microspectroscopy: FT-IR, Raman or Both?” Anal. Bioanal. Chem 2016. 408(29): 8377–8391. [DOI] [PubMed] [Google Scholar]
- 115.Collard F, Gilbert B, Eppe G, Parmentier E, Das K. “Detection of Anthropogenic Particles in Fish Stomachs: An Isolation Method Adapted to Identification by Raman Spectroscopy”. Arch. Environ. Contam. Toxicol 2015. 69(3): 331–339. [DOI] [PubMed] [Google Scholar]
- 116.Ghosal S, Chen M, Wagner J, Wang Z-M, Wall S. “Molecular Identification of Polymers and Anthropogenic Particles Extracted from Oceanic Water and Fish Stomach: A Raman Micro-Spectroscopy Study”. Environ. Pollut 2017. 33: 1113–1124. [DOI] [PubMed] [Google Scholar]
- 117.Karami A, Romano N, Galloway T, Hamzah H. “Virgin Microplastics Cause Toxicity and Modulate the Impacts of Phenanthrene on Biomarker Responses in African Catfish (Clarias gariepinus)”. Environ. Res 2016. 151: 58–70. [DOI] [PubMed] [Google Scholar]
- 118.Corcoran PL, Biesinger MC, Grifi M. “Plastics and Beaches: A Degrading Relationship”. Mar. Pollut. Bull 2009. 58(1): 80–84. [DOI] [PubMed] [Google Scholar]
- 119.Liboiron F, Ammendolia J, Saturno J, Melvin J, et al. “A Zero Percent Plastic Ingestion Rate by Silver Hake (Merluccius Bilinearis) from the South Coast of Newfoundland, Canada”. Mar. Pollut. Bull 2018. 131: 267–275. [DOI] [PubMed] [Google Scholar]
- 120.Simon M, van Alst N, Vollertsen J. “Quantification of Microplastic Mass and Removal Rates at Wastewater Treatment Plants Applying Focal Plane Array (FPA)-Based Fourier Transform Infrared (FT-IR) Imaging”. Water Res 2018. 142: 1–9. [DOI] [PubMed] [Google Scholar]
- 121.Walpitagama M, Carve M, Douek AM, Trestrail C, et al. “Additives Migrating from 3D-Printed Plastic Induce Developmental Toxicity and Neuro-Behavioural Alterations in Early Life Zebrafish (Danio rerio)”. Aquat. Toxicol 2019. 213: 105227. [DOI] [PubMed] [Google Scholar]
- 122.Liu F-F, Liu G-Z, Zhu Z-L, Wang S-C, Zhao F-F. “Interactions Between Microplastics and Phthalate Esters as Affected by Microplastics Characteristics and Solution Chemistry”. Chemosphere 2019. 214: 688–694. [DOI] [PubMed] [Google Scholar]
- 123.Hüffer T, Hofmann T. “Sorption of Non-Polar Organic Compounds by Micro-Sized Plastic Particles in Aqueous Solution”. Environ. Pollut 2016. 214: 194–201. [DOI] [PubMed] [Google Scholar]
- 124.Hartmann NB, Rist S, Bodin J, Jensen LH, et al. “Microplastics As Vectors for Environmental Contaminants: Exploring Sorption, Desorption, and Transfer to Biota”. Integr. Environ. Assess. Manag 2017. 13(3): 488–493. [DOI] [PubMed] [Google Scholar]
- 125.Jeong C-B, Won E-J, Kang H-M, Lee M-C, et al. “Microplastic Size-Dependent Toxicity, Oxidative Stress Induction, and P-JNK and P-P38 Activation in the Monogonont Rotifer (Brachionus koreanus)”. Environ. Sci. Technol 2016. 50(16): 8849–8857. [DOI] [PubMed] [Google Scholar]
- 126.Gray AD, Weinstein JE. “Size-and Shape-Dependent Effects of Microplastic Particles on Adult Daggerblade Grass Shrimp (Palaemonetes pugio)”. Environ. Toxicol. Chem 2017. 36(11): 3074–3080. [DOI] [PubMed] [Google Scholar]
- 127.Velzeboer I, Kwadijk CJAF, Koelmans AA. “Strong Sorption of PCBs to Nanoplastics, Microplastics, Carbon Nanotubes, and Fullerenes”. Environ. Sci. Technol 2014. 48(9): 4869–4876. [DOI] [PubMed] [Google Scholar]
- 128.Pascall MA, Zabik ME, Zabik MJ, Hernandez RJ. “Uptake of Polychlorinated Biphenyls (Pcbs) from an Aqueous Medium by Polyethylene, Polyvinyl Chloride, and Polystyrene Films”. J. Agric. Food Chem 2005. 53(1): 164–169. [DOI] [PubMed] [Google Scholar]
- 129.Rochman CM, Hoh E, Hentschel BT, Kaye S. “Long-Term Field Measurement of Sorption of Organic Contaminants to Five Types of Plastic Pellets: Implications for Plastic Marine Debris”. Environ. Sci. Technol 2013. 47(3): 1646–1654. [DOI] [PubMed] [Google Scholar]
- 130.Schuyler QA, Wilcox C, Townsend K, Hardesty BD, Marshall NJ. “Mistaken Identity? Visual Similarities of Marine Debris to Natural Prey Items of Sea Turtles”. BMC Ecol 2014. 14(14). 10.1186/1472-6785-14-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Devriese LI, De Witte B, Vethaak AD, Hostens K, Leslie HA. “Bioaccumulation of Pcbs from Microplastics in Norway Lobster (Nephrops norvegicus): An Experimental Study”. Chemosphere 2017. 186: 10–16. [DOI] [PubMed] [Google Scholar]
- 132.Athey SN, Albotra SD, Gordon CA, Monteleone B, et al. “Trophic Transfer of Microplastics in an Estuarine Food Chain and the Effects of a Sorbed Legacy Pollutant”. Limnol. Ocean 2020. 5(1): 154–162. [Google Scholar]
- 133.Wright SL, Rowe D, Thompson RC, Galloway TS. “Microplastic Ingestion Decreases Energy Reserves in Marine Worms”. Curr. Biol 2013. 23(23): R1031–R1033. [DOI] [PubMed] [Google Scholar]
- 134.Watts AJR, Urbina MA, Corr S, Lewis C, Galloway TS. “Ingestion of Plastic Microfibers by the Crab Carcinus Maenas and its Effect on Food Consumption and Energy Balance”. Environ. Sci. Technol 2015. 49(24): 14597–14604. [DOI] [PubMed] [Google Scholar]
- 135.Wu C, Zhang K, Huang X, Liu J. “Sorption of Pharmaceuticals and Personal Care Products to Polyethylene Debris”. Environ. Sci. Pollut. Res. Int 2016. 23(9): 8819–8826. [DOI] [PubMed] [Google Scholar]
- 136.Wu P, Cai Z, Jin H, Tang Y. “Adsorption Mechanisms of Five Bisphenol Analogues on PVC Microplastics”. Sci. Total Environ 2019. 650(Pt 1): 671–678. [DOI] [PubMed] [Google Scholar]
- 137.Key PB, Fulton MH, Scott GI, Layman SL, Wirth EF. “Lethal and Sublethal Effects of Malathion on Three Life Stages of the Grass Shrimp, Palaemonetes pugio”. Aquat. Toxicol 1998. 40(4): 311–322. [Google Scholar]
- 138.Rochman CM, Kurobe T, Flores I, Teh SJ. “Early Warning Signs of Endocrine Disruption in Adult Fish from the Ingestion of Polyethylene with and without Sorbed Chemical Pollutants from the Marine Environment”. Sci. Total Environ 2014. 493: 656–661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


