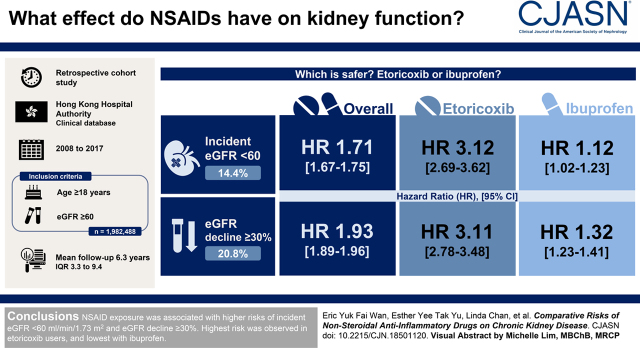
Visual Abstract
Keywords: chronic renal disease, chronic kidney disease, anti-inflammatory agents, non-steroidal, NSAID, etoricoxib ibuprofen
Abstract
Background and objectives
There have been doubts about the association between nonsteroidal anti-inflammatory drug use and worsening kidney function, and whether there is a difference between risks of individual nonsteroidal anti-inflammatory drugs is presently unclear. Therefore, this study aimed to evaluate the association between nonsteroidal anti-inflammatory drug exposure and the risk of incident eGFR <60 ml/min per 1.73 m2 and compare the risks between nonsteroidal anti-inflammatory drug subtypes in the Chinese population.
Design, setting, participants, & measurements
From 2008 to 2017, a total of 1,982,488 subjects aged 18 years or older with baseline eGFR ≥60 ml/min per 1.73 m2 were enrolled in this retrospective cohort study. Multivariable Cox proportional hazards regression adjusted for each patient’s baseline characteristics was adopted to examine the association between nonsteroidal anti-inflammatory drug and incident eGFR <60 ml/min per 1.73 m2 or eGFR decline ≥30% with reference to baseline.
Results
After a median follow-up duration of 6.3 (interquartile range, 3.3–9.4) years, 271,848 cases (14%) of incident eGFR <60 ml/min per 1.73 m2 and 388,386 (21%) events of eGFR decline ≥30% were recorded. After adjusting for each patient’s baseline characteristics, nonsteroidal anti-inflammatory drug treatment was shown to be associated with a significantly higher risk of incident eGFR <60 ml/min per 1.73 m2 (hazard ratio, 1.71; 95% confidence interval, 1.67 to 1.75) and eGFR decline ≥30% (hazard ratio, 1.93; 95% confidence interval, 1.89 to 1.96) when compared with no nonsteroidal anti-inflammatory drug, with etoricoxib exhibiting the highest risk of eGFR<60 ml/min per 1.73 m2 (hazard ratio, 3.12; 95% confidence interval, 2.69 to 3.62) and eGFR decline ≥30% (hazard ratio, 3.11; 95% confidence interval, 2.78 to 3.48) and ibuprofen displaying the lowest risk of eGFR<60 ml/min per 1.73 m2 (hazard ratio, 1.12; 95% confidence interval, 1.02 to 1.23) and eGFR decline ≥30% (hazard ratio, 1.32; 95% confidence interval, 1.23 to 1.41).
Conclusions
Nonsteroidal anti-inflammatory drug exposure was associated with higher risks of incident eGFR <60 ml/min per 1.73 m2 and eGFR decline ≥30%. Highest risk was observed in etoricoxib users, and lowest risk was with ibuprofen.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2021_04_28_CJN18501120.mp3
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been one of the most commonly prescribed drugs in many countries, including the United States, for the treatment of pain and inflammation (1). Nonetheless, association between the exposure of NSAIDs and incident CKD has been questioned since the 1950s (2). The risk of developing CKD in relation to chronic use of NSAIDs remains inadequately explored.
Several meta-analyses summarized the association between NSAID exposure and risk of AKI (3,4), but no unequivocal conclusion about NSAID use and the development of CKD has been drawn, possibly because of its insidious nature. Previous studies that investigated CKD risk were conducted in heterogenous populations, and they defined NSAID use and kidney outcome differently (5,6). Also, patterns of NSAID use were not recorded in the majority of the case-control studies comparing patients with CKD and controls (5). More importantly, current studies have scarcely compared the different NSAID subtypes. Selective cyclooxygenase-2 (COX-2) inhibitors have been traditionally regarded as less preferable in patients with high cardiovascular risk after the withdrawal of rofecoxib from the market due to an almost doubled risk of myocardial infarction (7). However, celecoxib, one of the selective COX-2 inhibitors, was shown to be noninferior to naproxen and ibuprofen with respect to cardiovascular safety in the Prospective Randomized Evaluation of Celecoxib Integrated Safety versus Ibuprofen or Naproxen (PRECISION) trial in 2016 (8), thus triggering doubts about real-world outcomes of using COX-2 inhibitors versus nonselective NSAIDs. Whether COX-2 inhibitors as a group differ from traditional NSAIDs in terms of CKD risk is presently unclear, and there is no consensus on specific NSAID recommendations for patients with CKD because of conflicting evidence (9). Current evidence of CKD risk regarding chronic NSAID exposure in individuals with normal kidney function has mainly focused on the White population in the United States and Europe. Because Asian individuals might be more susceptible to kidney failure when compared with White individuals (10,11), the effect of NSAID use on kidney outcomes in Asian populations, especially Chinese, is of great interest.
Hence, the aim of this study was to examine the association between NSAID use and worsening kidney function. CKD risks contributed by individual NSAIDs were critically compared. In this study, kidney risk was measured by the incidence of eGFR<60 ml/min per 1.73 m2, eGFR decline ≥30%, and their composite using a population-based electronic health database in Hong Kong, which is a comprehensive dataset representative of the southern Chinese.
Materials and Methods
Study Design
This was a retrospective cohort study. All individuals with eGFR≥60 ml/min per 1.73 m2 between January 1, 2008 and December 31, 2017 were identified from the Hong Kong Hospital Authority’s clinical database. The Hospital Authority is the statutory administrative body that manages the public health care sector in Hong Kong. More than 20 million attendances at 43 public hospitals, 49 specialist outpatient clinics, and 73 primary care clinics under the Hospital Authority were recorded in the year 2018–2019 (12). All information, including patients’ characteristics and outcome events, was extracted from the electronic health database of the Hospital Authority’s Clinical Management System. Clinicians and related health care professionals in the Hospital Authority were well trained to record clinical information and patient demographics, including patients’ diagnoses, prescriptions, laboratory tests and results, emergency department visits, hospitalizations, and specialist and general outpatient clinic visits in the Clinical Management System. Validity and coding accuracy of the database have previously been evaluated by high-quality population-based epidemiologic studies (13,14). The baseline eGFR for each patient within the subject inclusion period was determined at the date of the first dispensing record of NSAID or the first attendance record of any clinical service under the Hospital Authority if an NSAID was never prescribed in the subject inclusion period. Each patient was followed until the incidence of outcome events, death, or the last visit before December 31, 2018, whichever occurred first.
Drug Exposure
Nine types of oral NSAIDs, namely celecoxib, etoricoxib, diclofenac, ibuprofen, indomethacin, mefenamic acid, naproxen, piroxicam, and sulindac, were included in this study. All frequencies of use, including scheduled and as needed use, were included. Previous studies reported that the incidence of NSAID-associated adverse events would significantly increase after 4 consecutive weeks of treatment (15). Hence, NSAID treatment in this study was defined as prescription of NSAIDs for a minimum of 28 d/mo to avoid random effect due to short-term or one-off NSAID treatment. NSAID exposure was considered a time-varying variable in order to take into account the dynamic change of NSAID treatment status in each patient during follow-up.
Study Outcome
The primary outcomes of this study were incident eGFR <60 ml/min per 1.73 m2, eGFR decline ≥30% when compared with baseline value, and their composite. eGFR was calculated using the abbreviated Modification of Diet in Renal Disease study formula recalibrated for Chinese: eGFR in milliliters per minute per 1.73 m2=186×[(serum creatinine in micromoles per liter)×0.011]−1.154×(age)−0.203×(0.742 if a woman) ×1.233, whereas 1.233 is the adjusted coefficient for the Chinese population. The serum creatinine (Jaffe kinetic method) was measured with a Dimension AR system (Dade Behring, Deerfield, IL) or equivalent model. One single eGFR value was used in defining the primary outcome in the main analysis. The main analysis was then repeated by only including patients who had two consecutive eGFR values with at least 2 months in between and both satisfying the outcome criteria in one of the sensitivity analyses.
Ethical Approval
Ethical approval for this study was granted by the institutional review board of the Hong Kong Hospital Authority.
Baseline Characteristics as Confounders
Baseline characteristics consisted of age; sex; smoking status; body mass index; systolic BP; diastolic BP; fasting blood glucose; LDL cholesterol; eGFR; comorbidities; and the use of antihypertensive drugs, antidiabetic drugs, lipid-lowering agents, and aspirin (16). All laboratory assays were performed in laboratories accredited by the College of American Pathologists, the Hong Kong Accreditation Service, or the National Association of Testing Authorities, Australia.
Statistical Analyses
Missing data for baseline characteristics were treated by multiple imputation with the chained equation method. Each missing value was imputed five times on the basis of all baseline characteristics and all outcomes including incident eGFR <60 ml/min per 1.73 m2 and ≥30% decline, hence generating five different datasets that were applied in the same analysis. The results were then pooled according to the Rubin rule (17).
Patients were categorized into ten groups, including one nonuser group and nine NSAID user groups, on the basis of their NSAIDs prescription at baseline. Before conducting the analysis, fine stratification weights were applied to minimize potential confounding bias and selection bias among different treatment groups (18). This method was an extension of propensity score matching that combines propensity score stratification with the weighting technique. The propensity scores were used to create fine stratums on the basis of a fixed width of probability. This approach can avoid extreme weights when exposure prevalence is low and propensity score distribution is skewed (18). The fine stratification weights were conducted by using the “MMWS” package in Stata with the 50 quantile categories of propensity score for each stratum (19).
Descriptive statistics were used to summarize baseline characteristics. The incident rates for each outcome and their corresponding 95% confidence intervals (95% CIs) were calculated on the basis of Poisson distribution. Multivariable Cox proportional hazard regression adjusted for each patient’s baseline characteristics, as shown in the baseline characteristics section, was used to evaluate the association between NSAID exposure and the risk of outcome events. Considering the dynamic nature of NSAID treatment status in each patient during follow-up, NSAID exposure was treated as a time-varying covariate in this model. To ensure robustness of the results, eight sensitivity analyses were conducted in this study. The minimum duration of NSAID treatment to be included in the analysis was reduced from 28 days to 7, 14, and 21 days in the first three sensitivity analyses, respectively. After that, a complete patient analysis was performed to avoid biases caused by imputation inaccuracy. In the next two sensitivity analyses, patients with follow-up duration <1 year were excluded to minimize reverse causality, and the main analysis was repeated without using fine stratification weights. In the seventh sensitivity analysis, only patients who had two consecutive eGFR values with at least 2 months in between, with both satisfying the outcome criteria, were included. Lastly, an additional analysis was performed to examine patients who had both incident eGFR <60 ml/min per 1.73 m2 and eGFR decline ≥30%.
In the subgroup analyses, differential associations between NSAID treatment and outcomes were explored. Patients were divided into subgroups by sex (women and men), age (<65 and ≥65 years), the Charlson comorbidity index (20) (less than three and greater than or equal to three), and the use of antihypertensive drugs (no and yes). Statistical significance of interactions between the NSAID treatment group and each subgroup was examined.
Two-tailed tests with P value significance level of 0.05 were adopted by this study. The statistical analysis was executed in Stata version 15.1 (College Station, TX).
Results
A total of 1,982,488 subjects were included in this study. As shown in Supplemental Table 1, most baseline characteristics had a percentage of data completion of above 50%, except for body mass index (38%). The total number of complete cases was 577,889. After excluding unmatched individuals in fine stratification weights, 1,889,692 were included in the analysis. Weighted baseline characteristics for each NSAID treatment group are summarized in Table 1. The average age of participants was 55±17 years old, and 47% of them were men. Eight percent of the participants had received NSAID treatment. Among these people, diclofenac (58%) was the most frequently prescribed NSAID, followed by naproxen (19%) and ibuprofen (10%). Unweighted baseline characteristics of subjects are listed in Supplemental Table 2.
Table 1.
Baseline characteristics of 1,982,488 subjects aged 18 years or older with baseline eGFR ≥60 ml/min per 1.73 m2 in different drug groups after weighting
| Characteristics | No Nonsteroidal Anti-Inflammatory Drug, n=1,734,701 | Nonsteroidal Anti-Inflammatory Drug, n=154,991 | Celecoxib, n=1274 | Etoricoxib, n=1030 | Diclofenac, n=90,829 | Ibuprofen, n=15,169 | Indomethacin, n=5199 | Mefenamic Acid, n=10,697 | Naproxen, n=28,966 | Piroxicam, n=1308 | Sulindac, n=519 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | 811,803 (47%) | 76,236 (49%) | 657 (52%) | 487 (47%) | 44,983 (50%) | 7486 (49%) | 2299 (44%) | 5157 (48%) | 14,221 (49%) | 719 (55%) | 230 (44%) |
| Age, yr | 55 (17) | 55 (16) | 55 (16) | 56 (16) | 55 (15) | 55 (16) | 55 (16) | 54 (17) | 55 (16) | 55 (14) | 56 (15) |
| Current smoker | 51,890 (3%) | 4813 (3%) | 29 (2%) | 26 (3%) | 2858 (3%) | 452 (3%) | 160 (3%) | 333 (3%) | 899 (3%) | 36 (3%) | 19 (4%) |
| Systolic BP, mm Hg | 132 (20) | 131 (20) | 130 (20) | 133 (21) | 131 (20) | 131 (20) | 132 (20) | 131 (20) | 131 (20) | 131 (20) | 131 (20) |
| Diastolic BP, mm Hg | 76 (12) | 76 (12) | 76 (12) | 77 (13) | 76 (12) | 76 (12) | 76 (12) | 76 (12) | 76 (12) | 76 (12) | 76 (12) |
| Fasting glucose, mg/dl | 102 (26) | 103 (27) | 101 (25) | 103 (29) | 103 (27) | 103 (28) | 103 (28) | 103 (29) | 103 (27) | 104 (28) | 103 (36) |
| BMI, kg/m2 | 24.5 (4.0) | 24.7 (4.0) | 24.4 (4.4) | 24.6 (4.4) | 24.7 (4.0) | 24.7 (4.0) | 24.8 (4.0) | 24.7 (4.2) | 24.7 (4.0) | 25.2 (3.9) | 24.4 (4.1) |
| LDL cholesterol, mg/dl | 117 (35) | 117 (35) | 116 (36) | 115 (34) | 117 (34) | 117 (35) | 117 (35) | 116 (36) | 117 (35) | 118 (35) | 118 (37) |
| eGFR, ml/min per 1.73 m2 | 113 (28) | 114 (27) | 116 (27) | 115 (26) | 114 (27) | 114 (28) | 115 (30) | 114 (27) | 114 (27) | 114 (27) | 115 (29) |
| Charlson comorbidity index | 2.4 (1.9) | 2.4 (1.8) | 2.4 (1.8) | 2.5 (1.9) | 2.4 (1.8) | 2.5 (1.8) | 2.5 (1.9) | 2.4 (1.9) | 2.4 (1.8) | 2.5 (1.7) | 2.6 (1.8) |
| Coronary heart disease | 80,730 (5%) | 7351 (5%) | 62 (5%) | 59 (6%) | 4261 (5%) | 704 (5%) | 227 (4%) | 564 (5%) | 1366 (5%) | 76 (6%) | 31 (6%) |
| Heart failure | 24,404 (1%) | 2162 (1%) | 16 (1%) | 13 (1%) | 1314 (1%) | 198 (1%) | 69 (1%) | 128 (1%) | 394 (1%) | 23 (2%) | 9 (2%) |
| Diabetes mellitus | 205,218 (12%) | 19,186 (12%) | 134 (11%) | 123 (12%) | 11,077 (12%) | 1856 (12%) | 659 (13%) | 1475 (14%) | 3605 (12%) | 190 (15%) | 64 (12%) |
| Atrial fibrillation | 31,443 (2%) | 2847 (2%) | 22 (2%) | 13 (1%) | 1744 (2%) | 267 (2%) | 94 (2%) | 137 (1%) | 534 (2%) | 26 (2%) | 11 (2%) |
| Peripheral vascular disease | 3928 (0.2%) | 549 (0.4%) | 1 (0.1%) | 5 (0.5%) | 316 (0.3%) | 46 (0.3%) | 21 (0.4%) | 55 (0.5%) | 93 (0.3%) | 10 (0.8%) | 1 (0.2%) |
| Stroke | 85,152 (5%) | 8133 (5%) | 54 (4%) | 64 (6%) | 4809 (5%) | 763 (5%) | 275 (5%) | 539 (5%) | 1522 (5%) | 84 (6%) | 23 (4%) |
| Amputation | 1725 (0.1%) | 176 (0.1%) | 3 (0.3%) | 4 (0.4%) | 92 (0.1%) | 19 (0.1%) | 4 (0.1%) | 15 (0.1%) | 36 (0.1%) | 3 (0.2%) | 0 (0%) |
| Dementia | 14,142 (0.8%) | 587 (0.4%) | 5 (0.4%) | 6 (0.6%) | 287 (0.3%) | 82 (0.5%) | 15 (0.3%) | 68 (0.6%) | 120 (0.4%) | 2 (0.2%) | 0 (0%) |
| Lung disease | 55,372 (3%) | 4989 (3%) | 37 (3%) | 33 (3%) | 2931 (3%) | 488 (3%) | 155 (3%) | 337 (3%) | 936 (3%) | 55 (4%) | 18 (3%) |
| Connective tissue disease | 4131 (0.2%) | 516 (0.3%) | 3 (0.2%) | 3 (0.3%) | 272 (0.3%) | 50 (0.3%) | 9 (0.2%) | 31 (0.3%) | 139 (0.5%) | 6 (0.4%) | 2 (0.4%) |
| Peptic ulcer | 38,341 (2%) | 3516 (2%) | 37 (3%) | 22 (2%) | 2049 (2%) | 329 (2%) | 123 (2%) | 253 (2%) | 649 (2%) | 45 (3%) | 10 (2%) |
| Liver disease | 41,312 (2%) | 3640 (2%) | 23 (2%) | 37 (4%) | 2158 (2%) | 351 (2%) | 116 (2%) | 251 (2%) | 664 (2%) | 25 (2%) | 15 (3%) |
| Hemiplegia | 7510 (0.4%) | 742 (0.5%) | 2 (0.1%) | 2 (0.2%) | 444 (0.5%) | 73 (0.5%) | 31 (0.6%) | 38 (0.4%) | 151 (0.5%) | 0 (0%) | 2 (0.4%) |
| Leukemia | 2244 (0.1%) | 112 (0.1%) | 2 (0.1%) | 0 (0%) | 43 (0%) | 26 (0.2%) | 6 (0.1%) | 15 (0.1%) | 20 (0.1%) | 0 (0%) | 0 (0%) |
| Malignant lymphoma | 2706 (0.2%) | 175 (0.1%) | 1 (0.1%) | 1 (0.1%) | 87 (0.1%) | 27 (0.2%) | 6 (0.1%) | 18 (0.2%) | 33 (0.1%) | 1 (0.1%) | 0 (0%) |
| Cancer | 95,455 (6%) | 8791 (6%) | 80 (6%) | 53 (5%) | 5004 (6%) | 830 (5%) | 300 (6%) | 864 (8%) | 1545 (5%) | 79 (6%) | 33 (6%) |
| Use of antidiabetic drugs | 207,087 (12%) | 19,217 (12%) | 136 (11%) | 125 (12%) | 11,090 (12%) | 1858 (12%) | 639 (12%) | 1536 (14%) | 3596 (12%) | 178 (14%) | 55 (11%) |
| Use of lipid-lowering agents | 184,958 (11%) | 16,640 (11%) | 121 (9%) | 104 (10%) | 9441 (10%) | 1634 (11%) | 555 (11%) | 1491 (14%) | 3076 (11%) | 157 (12%) | 55 (11%) |
| Use of antihypertensive drugs | 625,677 (36%) | 56,366 (36%) | 427 (33%) | 396 (38%) | 32,717 (36%) | 5455 (36%) | 1838 (35%) | 4421 (41%) | 10,409 (36%) | 508 (39%) | 186 (36%) |
| Use of aspirin | 178,623 (10%) | 16,147 (10%) | 127 (10%) | 143 (14%) | 9378 (10%) | 1617 (11%) | 526 (10%) | 1220 (11%) | 2933 (10%) | 148 (11%) | 55 (11%) |
All parameters are expressed in either number (percentage) or mean (SD). BMI, body mass index.
After a median follow-up duration of 6.3 years (interquartile range, 3.3–9.4; 12,606,178 person-years) (the follow-up by NSAID treatment groups is shown in Supplemental Table 3), 271,848 cases (14%) of incident eGFR <60 ml/min per 1.73 m2, 388,386 events (21%) of eGFR decline ≥30%, and 419,506 cases (23%) of their composite among all participants were recorded. The incident rates of incident eGFR <60 ml/min per 1.73 m2 were 22.8 (95% CI, 22.7 to 22.9) and 33.0 (95% CI, 32.3 to 33.8) cases per 1000 person-years in nonusers and NSAID users, respectively. Regarding eGFR decline ≥30%, the incident rates were 33.4 (95% CI, 33.3 to 33.6) and 62.1 (95% CI, 61.0 to 63.2) cases per 1000 person-years in nonusers and NSAID users, respectively. As for the composite of incident eGFR <60 ml/min per 1.73 m2 and eGFR decline ≥30%, the incidence rates were 36.8 (95% CI, 36.6 to 36.9) and 68.0 (95% CI, 66.9 to 69.1) cases per 1000 person-years in nonusers and NSAID users, respectively (Table 2). Figure 1 illustrates the results of Cox proportional hazard regression for the association between NSAID treatment and the outcomes. Our findings indicated that any NSAID treatment was associated with a significantly higher risk of incident eGFR <60 ml/min per 1.73 m2 (hazard ratio [HR], 1.71; 95% CI, 1.67 to 1.75), eGFR decline ≥30% (HR, 1.93; 95% CI, 1.89 to 1.96), and their composite (HR, 1.88; 95% CI, 1.85 to 1.91).
Table 2.
Incidence of eGFR<60 ml/min per 1.73 m2 and eGFR decline ≥30% according to nonsteroidal anti-inflammatory drug use
| NSAID Use | Subject | eGFR<60 ml/min per 1.73 m2 | eGFR Decline ≥30% | Composite a | |||
|---|---|---|---|---|---|---|---|
| Event | Incidence Rate b | Event | Incidence Rate b | Event | Incidence Rate b | ||
| No NSAIDs | 1,734,701 | 263,112 | 22.8 (22.7 to 22.9) | 373,051 | 33.4 (33.3 to 33.6) | 402,932 | 36.8 (36.6 to 36.9) |
| Any NSAIDs | 154,991 | 8736 | 33.0 (32.3 to 33.8) | 15,335 | 62.1 (61.0 to 63.2) | 16,574 | 68.0 (66.9 to 69.1) |
| Celecoxib | 1274 | 213 | 32.2 (27.1 to 38.5) | 375 | 63.8 (56.4 to 72.4) | 392 | 67.8 (60.2 to 76.7) |
| Etoricoxib | 1030 | 183 | 45.0 (38.7 to 52.6) | 322 | 88.8 (79.0 to 100.0) | 335 | 93.5 (83.6 to 104.8) |
| Diclofenac | 90,829 | 5925 | 34.1 (33.2 to 35.0) | 10,487 | 64.7 (63.4 to 66.0) | 11,267 | 70.4 (69.0 to 71.8) |
| Ibuprofen | 15,169 | 465 | 24.8 (22.5 to 27.5) | 817 | 45.9 (42.6 to 49.5) | 930 | 52.9 (49.4 to 56.7) |
| Indomethacin | 5199 | 320 | 33.5 (30.0 to 37.6) | 505 | 56.5 (51.5 to 62.2) | 577 | 65.8 (60.3 to 71.9) |
| Mefenamic acid | 10,697 | 144 | 16.7 (11.8 to 24.4) | 284 | 34.0 (27.4 to 42.6) | 306 | 37.0 (30.1 to 46.1) |
| Naproxen | 28,966 | 1361 | 34.5 (32.6 to 36.5) | 2343 | 63.5 (60.8 to 66.3) | 2541 | 69.7 (66.9 to 72.7) |
| Piroxicam | 1308 | 78 | 33.4 (25.3 to 45.1) | 124 | 56.7 (44.7 to 72.8) | 138 | 63.9 (50.6 to 81.9) |
| Sulindac | 519 | 47 | 35.8 (27.3 to 47.9) | 78 | 63.7 (50.1 to 82.2) | 90 | 76.4 (61.1 to 96.7) |
NSAID, nonsteroidal anti-inflammatory drug.
Composite is defined by either incident eGFR <60 ml/min per 1.73 m2 or eGFR decline ≥30%.
Incidence rates (cases per 1000 person-year) with 95% confidence intervals are on the basis of Poisson distribution.
Figure 1.
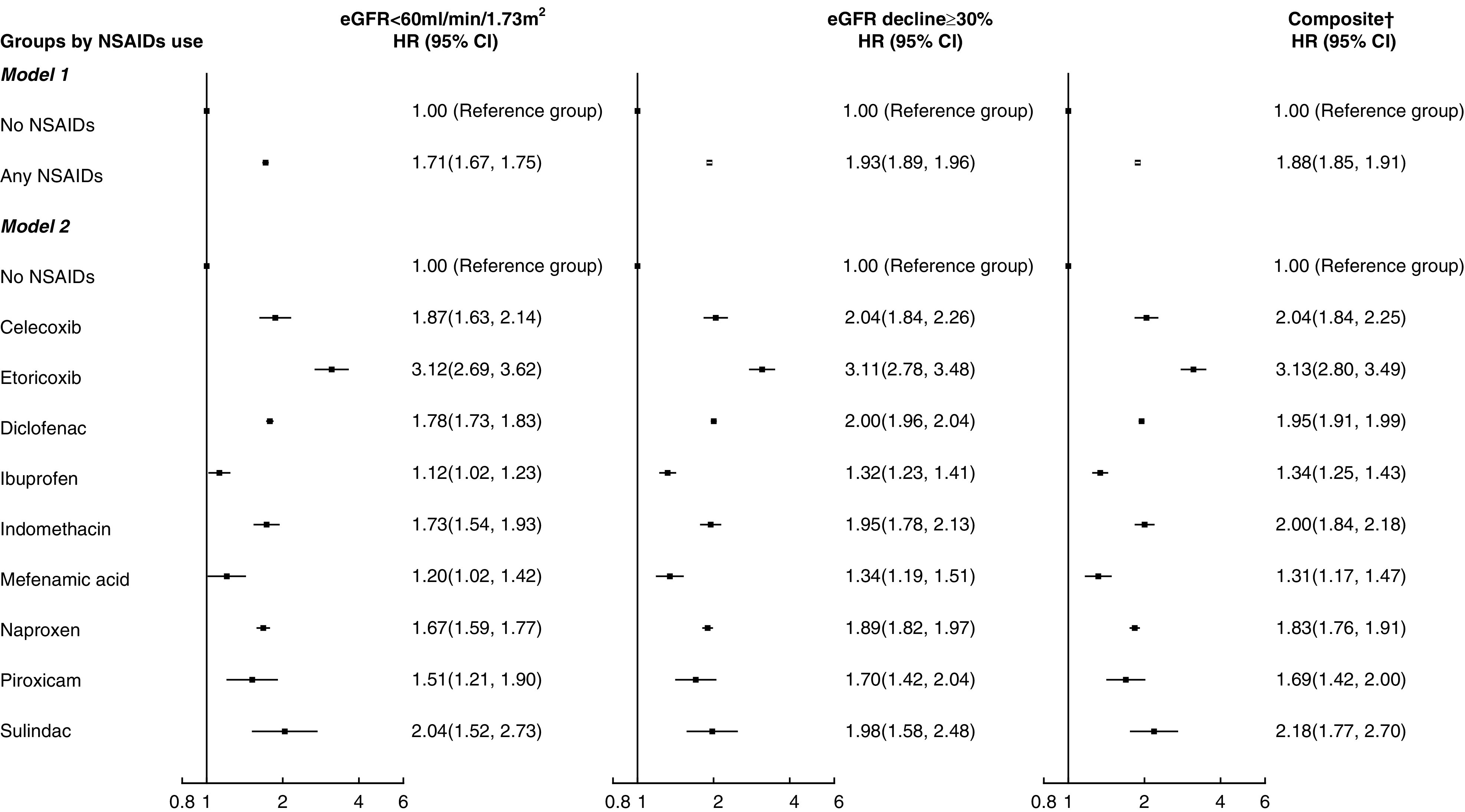
Associations of nonsteroidal anti-inflammatory drug (NSAID) use with incident eGFR <60 ml/min per 1.73 m 2 and eGFR decline ≥30%. Hazard ratio (HR) was adjusted by age; sex; smoking status; body mass index; systolic BP; diastolic BP; fasting glucose; LDL cholesterol; eGFR; the usage of antihypertensive drugs, antidiabetic drugs, lipid-lowering agents, and aspirin; and all of the comorbidities listed in Table 1 at baseline. 95% CI, 95% confidence interval. †Composite is defined by either incident eGFR <60 ml/min per 1.73 m2 or eGFR decline ≥30%.
Among all of the NSAID subtypes, ibuprofen was found to be associated with the lowest risks of incident eGFR <60 ml/min per 1.73 m2 (HR, 1.12; 95% CI, 1.02 to 1.23), eGFR decline ≥30% (HR, 1.31; 95% CI, 1.22 to 1.41), and their composite (HR, 1.34; 95% CI, 1.25 to 1.43), whereas etoricoxib had significantly higher risks of incident eGFR <60 ml/min per 1.73 m2 (HR, 3.12; 95% CI, 2.69 to 3.62), eGFR decline ≥30% (HR, 3.11; 95% CI, 2.78 to 3.48), and their composite (HR, 3.13; 95% CI, 2.80 to 3.49) in comparison with other NSAIDs. Eight sensitivity analyses were performed by including NSAID treatment of shorter duration, reducing from 28 days to (1) 7 days (Supplemental Figure 1), (2) 14 days (Supplemental Figure 2), and (3) 21 days (Supplemental Figure 3); the inclusion of (4) patients with no missing baseline characteristics only (Supplemental Figure 4) and (5) patients with >1 year of follow-up only (Supplemental Figure 5); and repeating the analysis (6) without fine stratification weights (Supplemental Figure 6), (7) by defining outcome with two consecutive eGFR values with at least 2 months in between while both satisfying the criteria (Supplemental Figure 7), and (8) to examine patients who had both incident eGFR <60 ml/min per 1.73 m2 and eGFR decline ≥30% (Supplemental Figure 8). Similar results from the sensitivity analyses further confirmed the findings of our main analysis.
As shown in Supplemental Figure 9, the subgroup analyses showed that NSAID treatment interacted with all subgroups (sex, age, Charlson comorbidity index, and use of antihypertensive drugs) on the risk of incident eGFR <60 ml/min per 1.73 m2, eGFR decline ≥30%, and their composite. In general, the effect of NSAID treatment on kidney outcomes was similar between men and women, but it was attenuated in older individuals, those who took antihypertensive drugs, and those with a higher Charlson comorbidity index.
Discussion
This large-scale study adds value to the presently available evidence for NSAID-associated CKD risk in a Chinese population with normal eGFR. All studied NSAIDs were found to be significantly associated with elevated risks of incident eGFR <60 ml/min per 1.73 m2 and eGFR decline ≥30%. Ibuprofen seemed to have the lowest risk of eGFR<60 ml/min per 1.73 m2, and the risk was substantially lower than that of etoricoxib. Although NSAIDs should be reserved for situations when benefits outweigh risks, this study remains a retrospective observation, and further study regarding causality is warranted.
Past studies in the early 2000s failed to demonstrate an association between NSAID use and CKD, with most of them having limited sample sizes and on the basis of self-reported drug use (21 –23), whereas this study attempted to minimize differences in variables among large-sized groups. In this study, associations between NSAID exposure and risk of kidney outcomes were shown, which was in agreement with the majority of the more recent cohort studies (24 –27). On the other hand, our findings contradicted a prior clinical study that reported that 6-month exposure to high-dose celecoxib had no effect on GFR in 44 elderly patients with prostate carcinoma (28), and another Swiss study that demonstrated no significant difference in kidney function between NSAID users and nonusers in 4101 patients with rheumatoid arthritis and baseline eGFR >30 ml/min (29). The difference in our findings could be attributed to their highly disease-specific population, in which the effect of NSAIDs could be obscured by other comorbidities. This study included a substantially larger number of subjects without kidney function impairment who also had longer follow-up periods. Together with a greater number of outcome events recorded, all of these suggested a much greater power of this study to identify the association between NSAID use and eGFR<60 ml/min per 1.73 m2. Moreover, the Swiss study (29) compared between the two groups by mean absolute eGFR change per year instead of percentage change or rate of incident CKD. Therefore, direct comparison between our findings may not be appropriate.
In this study, etoricoxib, which is a COX-2 selective inhibitor, was shown to be associated with the highest risk of adverse kidney outcomes, followed by other nonselective NSAIDs and celecoxib, with ibuprofen displaying the lowest risk. To a certain extent, this finding aligned with results from a systematic review that reported association between NSAID use and kidney risks for rofecoxib, celecoxib, naproxen, diclofenac, and indomethacin but not for meloxicam and ibuprofen (30). Ibuprofen appeared to be safer among the list of NSAIDs in both studies, although they did not reveal a statistically higher risk for ibuprofen, possibly because only studies that involved meloxicam were taken into consideration; this, in turn, restricted the scope of their analysis, and kidney risks were only reported as secondary outcomes in most of their included studies. In contrast, two large clinical trials, namely the PRECISION trial and the Celecoxib Long-Term Arthritis Safety Study (CLASS), that were not included in the above systematic review revealed different findings; they reported that patients taking celecoxib were less susceptible to kidney events when compared with those taking ibuprofen (8,31). Discrepancy between our findings could be ascribed to the difference in defining kidney events. The incidence of acute kidney failure and the persistence of elevated serum creatinine for ≥24 hours were considered in the PRECISION trial, whereas occurrence of peripheral edema, increased creatinine level, and hypertension during the 6-month treatment period were regarded as kidney adverse events in CLASS. On the contrary, acute events were not taken into account in this study. Whether short-term increases in creatinine and acute kidney events translate into risk of developing CKD remained unclear. Furthermore, Asians only constituted a minority in their trials. Therefore, direct comparison between this study and previous randomized clinical trials should be interpreted with caution.
Chronic kidney effects of NSAIDs have rarely been compared critically. Some studies may have inappropriately assumed a difference between COX-2 selective inhibitors and other NSAIDs, hence reporting HRs for each group, whereas individual agents in the same group displayed highly divergent effect sizes (32,33). In this study, etoricoxib was shown to be associated with the highest CKD risk. To the best of our knowledge, there were only three studies that clearly indicated etoricoxib in their analyses (25,26,34), but the risk of CKD for etoricoxib alone was not reported, thus making direct comparison impossible. Because there is insufficient evidence for head-to-head comparison between the NSAIDs, further investigations are important to enhance our knowledge of the comparative risks between the NSAIDs.
Unlike the COX-1 enzyme, COX-2 was initially believed to be an “induced” isoform and did not take part in constitutive functions. However, previous clinical studies illustrated that COX-2 inhibitors produced similar hemodynamic effects in the kidney when compared with nonselective NSAIDs (35,36), hence confirming the role of constitutive COX-2 in kidney homeostasis and the renin-angiotensin system (37). By inhibiting both COX-1 and COX-2 enzymes in the kidneys, NSAIDs suppress the formation of protective prostaglandins, leading to reduced kidney perfusion and sodium retention (38), which may affect kidney function in the long run.
Why NSAIDs seem heterogenous in increasing CKD risks remained controversial. Difference in COX selectivity has been one of the proposed mechanisms (37). Regarding relative COX-2 versus COX-1 selectivity, etoricoxib and celecoxib came first, followed by diclofenac and sulindac. Naproxen and indomethacin were more COX-1 selective, whereas piroxicam and ibuprofen were comparatively more nonselective (39). Interestingly, our study results somewhat mimicked this trend, with nonselective NSAIDs exhibiting the lowest risk when compared with the more COX-1 or -2 selective NSAIDs. An in vivo experiment in mice demonstrated opposite effects of COX-1 and -2 on kidney homeostasis, with COX-2 inhibition reducing kidney medullary blood flow and urine flow (40). Previous studies have also added that COX-1 inhibition may potentially attenuate the adverse effect of COX-2 inhibition in the kidney (41), therefore justifying why nonselective NSAIDs may do less harm to the kidney when compared with COX-2 inhibitors. This study revealed that etoricoxib, which was the most COX-2 selective among the studied drugs, had a significantly higher risk, but the risk associated with celecoxib use was comparable with other NSAIDs except for ibuprofen. The class effect of COX-2 inhibitors was not conspicuous in this study, which echoes the findings of a meta-analysis of randomized trials (42).
However, some authors hypothesized that kidney outcomes of NSAIDs were not dependent on COX-2 selectivity (43). Instead, physiochemical properties that determine drug distribution in the kidney relative to plasma concentration play a more important role, rendering celecoxib more nephrotoxic than meloxicam, despite both of them being COX-2 selective (43,44). Nonetheless, this study was unable to validate this hypothesis because meloxicam was not included. Instead of being two distinct drug moieties, COX-2 selective inhibitors and other NSAIDs have different positions along the continuous scale of COX selectivity, in addition to their different potencies and pharmacokinetics properties (42). Some investigators also suspected that differences in half-lives (34) and polymorphic expression of enzymes metabolizing NSAIDs (45) were possible causes. All of these could have contributed to the observed differences, although the exact mechanism is yet to be fully elucidated and experimental data on various NSAIDs are still lacking.
The effect of NSAID was subject to age, use of antihypertensive drugs, and comorbidities. In this study, the adverse effect of NSAID was more prominent in younger (<65 years old) and relatively healthier patients who had Charlson comorbidity index less than three when compared with the older and more ill counterparts. The effect of NSAID use in older patients could be masked by their frailty and other concurrent diseases. Therefore, this possibly explains why the effect of NSAID exposure was apparently smaller in those patients. Furthermore, this study illustrated a difference in the risk of kidney function decline between those who were on antihypertensive drugs versus those who were not. NSAIDs raise BP via inhibiting synthesis of prostaglandin E2 and prostaglandin I2, which in turn, disrupt vascular tone regulation and reduce natriuresis (46). Previous studies proposed that patients treated with antihypertensives were more prone to the BP-increasing effect of NSAIDs in contrast to normotensive individuals because NSAIDs could potentially reverse the effect of antihypertensives in addition to their intrinsic effect of elevating BP (47,48). Antihypertensive drugs acting on renin-angiotensin system were shown to be most affected because NSAIDs inhibit prostaglandin synthesis, which in turn, interferes with the effect of bradykinin and angiotensin II (48,49). Although hypertension is a known risk factor for both CKD and kidney failure (50), whether higher CKD risk was solely contributed by the drug-drug interaction between NSAIDs and antihypertensives is presently unknown.
This study had several strengths. First, this was the first large-scale cohort study that compared the risk of CKD of different NSAIDs among the Chinese population. Second, NSAID exposure was captured by the clinical database instead of self-reporting, hence minimizing recall bias. Advanced statistical methods were adopted to evaluate NSAID exposure as a time-varying variable.
Several limitations were present in this study. First, this retrospective cohort study did not provide information about causality despite displaying an association between NSAID use and kidney outcomes. Second, our database from the Hospital Authority did not cover all NSAIDs available in Hong Kong due to formulary restrictions. Third, over-the-counter NSAID use was not captured in this study, and patients’ drug adherence was not taken into account, which could vary among NSAID users. Fourth, although HRs have been adjusted by patients’ history of peptic ulcer disease, the pattern of physicians prescribing COX-2 selective inhibitors to patients who were at greater risk of gastrointestinal complications might still be a potential confounder. Moreover, inpatient and outpatient eGFR values were not distinguished in this study. An additional sensitivity analysis that defines primary outcome as having two consecutive eGFR values below 60 ml/min per 1.73 m2 with at least 2 months in between was performed to reduce the likelihood of misclassifying patients with AKI as patients with CKD. Furthermore, multiple NSAID use during the same period was recorded in a small number of patients. The drug that was prescribed for a shorter duration in the overlapping period was omitted. Lastly, information on drug dosing and indication (i.e., which type of pain) was not available in this study.
In conclusion, there was an association between NSAID exposure and elevated risks of incident eGFR <60 ml/min per 1.73 m2 and eGFR decline ≥30%. Further study is warranted to confirm the heightened risk observed in etoricoxib users. Ibuprofen appeared to be a safer choice when NSAID use is deemed necessary.
Disclosures
E.W.Y. Chan reports employment with The University of Hong Kong; receiving research funding from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Janssen, the Narcotics Division of the Security Bureau of the Hong Kong Special Administrative Region (HKSAR) Government, the National Health and Medical Research Council (Australia), the National Natural Science Foundation of China, Pfizer, Research Fund Secretariat of the Food and Health Bureau (Health and Medical Research Fund, HKSAR), Research Grants Council (RGC; HKSAR), RGA, Takeda, and Wellcome Trust; and receiving honoraria from The Hospital Authority, Hong Kong. C.L.K. Lam reports employment with The University of Hong Kong and receiving honoraria from Oxford University Press (Family Medicine Journal). C.L.K. Lam also reports serving as the director of the board of directors of Blue Care (BVI) JV Limited, an associate editor for Family Practice (Oxford University Press), a member of the Grant Review Board of the Health and Medical Research Fund, Food and Health Bureau, the Government of the HKSAR, and an international advisor to Malaysian Family Physician Journal. A.H.Y. Mok, E.Y.F. Wan, and E.Y.T. Yu report employment with The University of Hong Kong. I.C.K. Wong reports employment with The University of Hong Kong; received research funding outside the submitted work from Amgen, Bayer, Bristol-Myers Squibb, the European Commission, GSK, the Hong Kong RGC, Janssen, the National Health and Medical Research Council in Australia, the National Institute for Health Research in England, Novartis, and Pfizer; and received speaker fees from Janssen and Medice in the previous 3 years. All remaining authors have nothing to disclose.
Funding
This study is funded by University of Hong Kong Start-up Fund (Reference no. 006027001).
Supplementary Material
Acknowledgments
The authors acknowledge Hong Kong Hospital Authority for the contributions of data extraction. The computations were performed using research computing facilities offered by Information Technology Services, the University of Hong Kong.
No funding organization had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript.
Prof. C.L.K. Lam, Dr. E.Y.F. Wan, and Dr. E.Y.T. Yu contributed to the study design and acquisition of data; Prof. C.L.K. Lam, Dr. E.Y.F. Wan, and Dr. E.Y.T. Yu researched the data; Prof. C.L.K. Lam, Dr. E.Y.F. Wan, and Mr. Y. Wang contributed to the statistical analysis; all authors contributed to the interpretation of the results; Prof. C.L.K. Lam, Ms. A.H.Y. Mok, Dr. E.Y.F. Wan, and Mr. Y. Wang wrote the manuscript; all authors edited the manuscript; and Dr. E.Y.F. Wan is the guarantor of this work, had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.18501120/-/DCSupplemental.
Supplemental Table 1. Percentage of data completion of the baseline characteristics in studied patients.
Supplemental Table 2. Baseline characteristics of 1,982,488 subjects aged 18 years or older with baseline eGFR ≥60 ml/min per 1.73 m2 in different drug groups before weighting.
Supplemental Table 3. Follow-up period by NSAID treatment groups.
Supplemental Figure 1. Associations of NSAID use with incident eGFR <60 ml/min per 1.73 m2 and eGFR decline ≥30% with NSAID usage defined by at least 7 consecutive days.
Supplemental Figure 2. Associations of NSAID use with incident eGFR <60 ml/min per 1.73 m2 and eGFR decline ≥30% with NSAID usage defined by at least 14 consecutive days.
Supplemental Figure 3. Associations of NSAID use with incident eGFR <60 ml/min per 1.73 m2 and eGFR decline ≥30% with NSAID usage defined by at least 21 consecutive days.
Supplemental Figure 4. Associations of NSAID use with incident eGFR <60 ml/min per 1.73 m2 and eGFR decline ≥30% in complete case analysis.
Supplemental Figure 5. Associations of NSAID use with incident eGFR <60 ml/min per 1.73 m2 and eGFR decline ≥30% for patients with at least 1-year follow-up.
Supplemental Figure 6. Associations of NSAID use with incident eGFR <60 ml/min per 1.73 m2 and eGFR decline ≥30% without weighting.
Supplemental Figure 7. Associations of NSAID use with incident eGFR <60 ml/min per 1.73 m2 and eGFR decline ≥30% using two consecutive eGFR values with at least 2 months in between to define outcome.
Supplemental Figure 8. Associations of NSAID use with the risk of both incident eGFR <60 ml/min per 1.73 m2 and eGFR decline ≥30%.
Supplemental Figure 9. Association between NSAIDs usage and eGFR<60 ml/min per 1.73 m2, eGFR decline ≥30%, and the composite outcome within subgroups.
References
- 1.Green GA: Understanding NSAIDs: From aspirin to COX-2. Clin Cornerstone 3: 50–60, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Murray TG, Stolley PD, Anthony JC, Schinnar R, Hepler-Smith E, Jeffreys JL: Epidemiologic study of regular analgesic use and end-stage renal disease. Arch Intern Med 143: 1687–1693, 1983 [PubMed] [Google Scholar]
- 3.Zhang X, Donnan PT, Bell S, Guthrie B: Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: Systematic review and meta-analysis. BMC Nephrol 18: 256, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ungprasert P, Cheungpasitporn W, Crowson CS, Matteson EL: Individual non-steroidal anti-inflammatory drugs and risk of acute kidney injury: A systematic review and meta-analysis of observational studies. Eur J Intern Med 26: 285–291, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Yaxley J, Litfin T: Non-steroidal anti-inflammatories and the development of analgesic nephropathy: A systematic review. Ren Fail 38: 1328–1334, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Nderitu P, Doos L, Jones PW, Davies SJ, Kadam UT: Non-steroidal anti-inflammatory drugs and chronic kidney disease progression: A systematic review. Fam Pract 30: 247–255, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Sibbald B: Rofecoxib (Vioxx) voluntarily withdrawn from market. CMAJ 171: 1027–1028, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nissen SE, Yeomans ND, Solomon DH, Lüscher TF, Libby P, Husni ME, Graham DY, Borer JS, Wisniewski LM, Wolski KE, Wang Q, Menon V, Ruschitzka F, Gaffney M, Beckerman B, Berger MF, Bao W, Lincoff AM; PRECISION Trial Investigators: Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med 375: 2519–2529, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Szeto C-C, Sugano K, Wang J-G, Fujimoto K, Whittle S, Modi GK, Chen CH, Park JB, Tam LS, Vareesangthip K, Tsoi KKF, Chan FKL: Non-steroidal anti-inflammatory drug (NSAID) therapy in patients with hypertension, cardiovascular, renal or gastrointestinal comorbidities: Joint APAGE/APLAR/APSDE/APSH/APSN/PoA recommendations. Gut 69: 617–629, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Hall YN, Hsu C-Y, Iribarren C, Darbinian J, McCulloch CE, Go AS: The conundrum of increased burden of end-stage renal disease in Asians. Kidney Int 68: 2310–2316, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV: Ethnic disparities in diabetic complications in an insured population. JAMA 287: 2519–2527, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Hong Kong Hospital Authority : Hong Kong Hospital Authority Statistical Report 2018–2019
- 13.Chan EW, Lau WCY, Leung WK, Mok MTC, He Y, Tong TSM, Wong ICK: Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: A population-based study. Gastroenterology 149: 586–595.e3, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Wong AYS, Root A, Douglas IJ, Chui CSL, Chan EW, Ghebremichael-Weldeselassie Y, Siu C-W, Smeeth L, Wong ICK: Cardiovascular outcomes associated with use of clarithromycin: Population based study. BMJ 352: h6926, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Osani MC, Vaysbrot EE, Zhou M, McAlindon TE, Bannuru RR: Duration of symptom relief and early trajectory of adverse events for oral nonsteroidal antiinflammatory drugs in knee osteoarthritis: A systematic review and meta-analysis. Arthritis Care Res (Hoboken) 72: 641–651, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Y-C, Zuo L, Chen J-H, Luo Q, Yu X-Q, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY: Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 17: 2937–2944, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Rubin DB: Multiple Imputation for Nonresponse in Surveys, New York, John Wiley & Sons, 2004 [Google Scholar]
- 18.Desai RJ, Rothman KJ, Bateman BT, Hernandez-Diaz S, Huybrechts KF: A propensity-score-based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology 28: 249–257, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong G: Marginal mean weighting through stratification: Adjustment for selection bias in multilevel data. J Educ Behav Stat 35: 499–531, 2010 [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Kurth T, Glynn RJ, Walker AM, Rexrode KM, Buring JE, Stampfer MJ, Hennekens CH, Gaziano JM: Analgesic use and change in kidney function in apparently healthy men. Am J Kidney Dis 42: 234–244, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Rexrode KM, Buring JE, Glynn RJ, Stampfer MJ, Youngman LD, Gaziano JM: Analgesic use and renal function in men. JAMA 286: 315–321, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Ibáñez L, Morlans M, Vidal X, Martínez MJ, Laporte J-R: Case-control study of regular analgesic and nonsteroidal anti-inflammatory use and end-stage renal disease. Kidney Int 67: 2393–2398, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Gooch K, Culleton BF, Manns BJ, Zhang J, Alfonso H, Tonelli M, Frank C, Klarenbach S, Hemmelgarn BR: NSAID use and progression of chronic kidney disease. Am J Med 120: 280.e1–280.e7, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Hsu C-C, Wang H, Hsu Y-H, Chuang S-Y, Huang Y-W, Chang Y-K, Liu JS, Hsiung CA, Tsai HJ: Use of nonsteroidal anti-inflammatory drugs and risk of chronic kidney disease in subjects with hypertension: Nationwide longitudinal cohort study. Hypertension 66: 524–533, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Tsai HJ, Hsu YH, Huang YW, Chang YK, Liu JS, Hsu CC: Use of non-steroidal anti-inflammatory drugs and risk of chronic kidney disease in people with type 2 diabetes mellitus, a nationwide longitudinal cohort study. Diabet Med 32: 382–390, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Nelson DA, Marks ES, Deuster PA, O’Connor FG, Kurina LM: Association of nonsteroidal anti-inflammatory drug prescriptions with kidney disease among active young and middle-aged adults. JAMA Netw Open 2: e187896, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benson P, Yudd M, Sims D, Chang V, Srinivas S, Kasimis B: Renal effects of high-dose celecoxib in elderly men with stage D2 prostate carcinoma. Clin Nephrol 78: 376–381, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Möller B, Pruijm M, Adler S, Scherer A, Villiger PM, Finckh A; Swiss Clinical Quality Management in Rheumatic Diseases (SCQM) Foundation, CH-8048 Zurich, Switzerland: Chronic NSAID use and long-term decline of renal function in a prospective rheumatoid arthritis cohort study. Ann Rheum Dis 74: 718–723, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Asghar W, Jamali F: The effect of COX-2-selective meloxicam on the myocardial, vascular and renal risks: A systematic review. Inflammopharmacology 23: 1–16, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, Geis GS: Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: The CLASS study: A randomized controlled trial. Celecoxib long-term arthritis safety study. JAMA 284: 1247–1255, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Chang Y-K, Liu J-S, Hsu Y-H, Tarng D-C, Hsu C-C: Increased risk of end-stage renal disease (ESRD) requiring chronic dialysis is associated with use of nonsteroidal anti-inflammatory drugs (NSAIDs): Nationwide case-crossover study. Medicine (Baltimore) 94: e1362, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo HW, Tsai SS, Tiao MM, Liu YC, Lee IM, Yang CY: Analgesic use and the risk for progression of chronic kidney disease. Pharmacoepidemiol Drug Saf 19: 745–751, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Ingrasciotta Y, Sultana J, Giorgianni F, Fontana A, Santangelo A, Tari DU, Santoro D, Arcoraci V, Perrotta M, Ibanez L, Trifirò G: Association of individual non-steroidal anti-inflammatory drugs and chronic kidney disease: A population-based case control study. PLoS One 10: e0122899, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swan SK, Rudy DW, Lasseter KC, Ryan CF, Buechel KL, Lambrecht LJ, Pinto MB, Dilzer SC, Obrda O, Sundblad KJ, Gumbs CP, Ebel DL, Quan H, Larson PJ, Schwartz JI, Musliner TA, Gertz BJ, Brater DC, Yao SL: Effect of cyclooxygenase-2 inhibition on renal function in elderly persons receiving a low-salt diet. A randomized, controlled trial. Ann Intern Med 133: 1–9, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Curtis SP, Ng J, Yu Q, Shingo S, Bergman G, McCormick CL, Reicin AS: Renal effects of etoricoxib and comparator nonsteroidal anti-inflammatory drugs in controlled clinical trials. Clin Ther 26: 70–83, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Hörl WH: Nonsteroidal anti-inflammatory drugs and the kidney. Pharmaceuticals (Basel) 3: 2291–2321, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucas GNC, Leitão ACC, Alencar RL, Xavier RMF, Daher EF, Silva Junior GBD: Pathophysiological aspects of nephropathy caused by non-steroidal anti-inflammatory drugs. J Bras Nefrol 41: 124–130, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt M, Lamberts M, Olsen A-MS, Fosbøll E, Niessner A, Tamargo J, Rosano G, Agewall S, Kaski JC, Kjeldsen K, Lewis BS, Torp-Pedersen C: Cardiovascular safety of non-aspirin non-steroidal anti-inflammatory drugs: Review and position paper by the working group for Cardiovascular Pharmacotherapy of the European Society of Cardiology. Eur Heart J 37: 1015–1023, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Qi Z, Hao C-M, Langenbach RI, Breyer RM, Redha R, Morrow JD, Breyer MD: Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest 110: 61–69, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.García Rodríguez LA, Tacconelli S, Patrignani P: Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti-inflammatory drugs in the general population. J Am Coll Cardiol 52: 1628–1636, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Ding EL, Song Y: Adverse effects of cyclooxygenase 2 inhibitors on renal and arrhythmia events: Meta-analysis of randomized trials. JAMA 296: 1619–1632, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Harirforoosh S, Aghazadeh-Habashi A, Jamali F: Extent of renal effect of cyclo-oxygenase-2-selective inhibitors is pharmacokinetic dependent. Clin Exp Pharmacol Physiol 33: 917–924, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Harirforoosh S, Asghar W, Jamali F: Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J Pharm Sci 16: 821–847, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Harirforoosh S, Jamali F: Renal adverse effects of nonsteroidal anti-inflammatory drugs. Expert Opin Drug Saf 8: 669–681, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Sudano I, Flammer AJ, Roas S, Enseleit F, Noll G, Ruschitzka F: Nonsteroidal antiinflammatory drugs, acetaminophen, and hypertension. Curr Hypertens Rep 14: 304–309, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Cheng HF, Harris RC: Renal effects of non-steroidal anti-inflammatory drugs and selective cyclooxygenase-2 inhibitors. Curr Pharm Des 11: 1795–1804, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Khatchadourian ZD, Moreno-Hay I, de Leeuw R: Nonsteroidal anti-inflammatory drugs and antihypertensives: How do they relate? Oral Surg Oral Med Oral Pathol Oral Radiol 117: 697–703, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Moore N, Pollack C, Butkerait P: Adverse drug reactions and drug-drug interactions with over-the-counter NSAIDs. Ther Clin Risk Manag 11: 1061–1075, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kazancioğlu R: Risk factors for chronic kidney disease: An update. Kidney Int Suppl (2011) 3: 368–371, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.