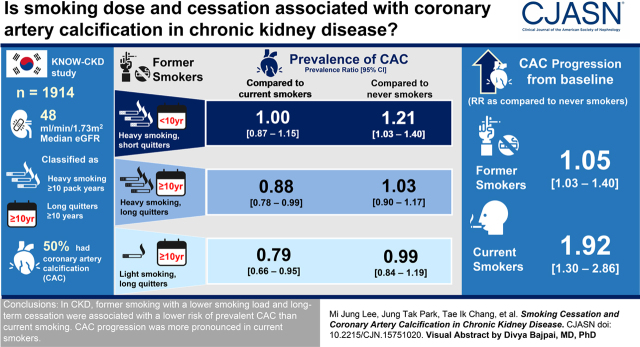
Visual Abstract
Keywords: cardiovascular disease, chronic kidney disease, coronary calcification, coronary artery disease, smoking cessation, smoking
Abstract
Background and objectives
Smoking is associated with vascular calcification and a higher risk of cardiovascular disease. In this study, we investigated the association of smoking dose and cessation with coronary artery calcification (CAC) in patients with CKD.
Design, setting, participants, & measurements
From a nationwide, prospective cohort of Korean patients with CKD, 1914 participants were included. Prevalent CAC was defined as an Agatston score >0, using computed tomography. CAC progression was defined as ≥30%/yr increase in Agatston score at the 4-year follow-up examination in patients with baseline CAC.
Results
Prevalent CAC was observed in 952 (50%) patients. Compared with never smokers, former smokers had a similar prevalence ratio for CAC, but current smokers had a 1.25-fold higher prevalence ratio (95% confidence interval [95% CI], 1.10 to 1.42). Among former smokers, a lower smoking load of <10 pack-years (prevalence ratio, 0.77; 95% CI, 0.65 to 0.90) and longer duration of smoking cessation (prevalence ratio for 10 to <20 years, 0.85; 95% CI, 0.73 to 0.98: prevalence ratio for ≥20 years, 0.83; 95% CI, 0.73 to 0.96) were associated with lower risk of prevalent CAC compared with current smoking. The prevalence ratios did not differ between never smoking and long-term cessation. However, short-term cessation with heavy smoking load was associated with a higher risk of prevalent CAC (prevalence ratio, 1.21; 95% CI, 1.03 to 1.40) compared with never smoking. CAC progression was observed in 111 (33%) patients with baseline CAC. Compared with never smokers, former smokers showed a similar risk of CAC progression, but current smokers had a higher risk (relative risk, 1.92; 95% CI, 1.30 to 2.86).
Conclusions
In CKD, former smoking with a lower smoking load and long-term cessation were associated with a lower risk of prevalent CAC than current smoking. CAC progression was more pronounced in current smokers.
Introduction
Smoking cessation is strongly recommended to alleviate cardiovascular risk (1). In several prospective studies, longer smoking cessation duration was consistently associated with a lower cardiovascular risk (2 –4). Former smokers who had stopped smoking over 5 years had a lower cardiovascular risk compared with current smokers (2 –4). However, former smokers required a more prolonged smoking cessation, from at least 5−10 years (4,5) to 20−30 years (3), to have comparable risk to never smokers, indicating the importance of smoking prevention and early smoking cessation.
Current clinical guidelines in the kidney disease field recommend smoking cessation as a lifestyle modification (6). However, these guidelines are mostly derived from epidemiologic observational studies involving participants without CKD. To date, studies on the association between smoking and cardiovascular disease in patients with CKD have yielded conflicting results (7 –10). In a previous study of patients with CKD who did not undergo dialysis or kidney transplantation, current smokers had a higher risk of atherosclerotic and nonatherosclerotic events compared with never smokers (10). Former smokers also showed higher vascular event rates than never smokers (10). In contrast, a meta-analysis showed that current smoking was associated with higher risk of all-cause mortality in patients on dialysis; however, the risk for cardiovascular events was nonsignificant (8). Because of limited information regarding smoking status (former or current) and lack of data on smoking dose and cessation duration, the association between smoking cessation and cardiovascular disease has not been fully investigated in the CKD population. On the basis of evidence in the general population (2 –4), we hypothesized that cardiovascular risk may vary depending on cumulative dose or smoking cessation in patients with CKD.
Coronary artery calcification (CAC) is significantly associated with the development of cardiovascular disease, myocardial infarction, and heart failure in patients with CKD (11). Furthermore, CAC is the most significant atherosclerosis measure for assessing cardiovascular disease risk in patients with CKD who do not have a previous history of cardiovascular disease (12). Prediction performance of CAC was greater than other measures, such as carotid intima thickness and ankle-brachial index (12). In this regard, CAC can represent a subclinical cardiovascular disease in patients with CKD. Therefore, in this study, CAC was considered a surrogate for subclinical cardiovascular disease, and the association between CAC and smoking was investigated in a nationwide, prospective cohort of patients with CKD in the Republic of Korea.
Materials and Methods
Study Design and Participants
Participants from the Korean Cohort Study for Outcome in Patients with CKD (KNOW-CKD) between 2011 and 2016 were included in this study. The KNOW-CKD is an ongoing, nationwide, prospective cohort study that includes patients with CKD stages 1–5 who had not undergone dialysis or kidney transplantation in the Republic of Korea (ClinicalTrials.gov identifier: NCT01630486). The detailed design and methods have been previously described (13). Among 2238 initial participants, 125 patients who had a history of coronary artery disease or peripheral vascular disease at study enrollment were excluded. Coronary artery disease was defined as a history of myocardial infarction, or angina requiring percutaneous coronary intervention or coronary artery bypass graft. Peripheral vascular disease was defined as a history of ischemic limb loss and/or ulceration requiring peripheral revascularization procedure. An additional 199 patients were excluded because of missing data regarding smoking status, CAC, or baseline laboratory variables. Consequently, 1914 participants were included in the analysis of the association between smoking status and CAC. Furthermore, to test the association of smoking status with CAC progression, we assessed 755 participants who had a follow-up CAC examination at year 4 (Supplemental Figure 1). This study was performed in accordance with the Declaration of Helsinki, and the study protocol was approved by the institutional review board of each participating clinical center. We obtained informed written consent from all participants.
Data Collection
At study enrollment, baseline demographics and clinical data, including comorbidities, cause of CKD, economic status, educational level, and medications, were collected by a well-trained research coordinator. An 8-hour fasting blood and the second voided or random urine were used to measure biochemical variables. Details of data collection and definitions of baseline data are available in Supplemental Appendix 1. All data were managed using an electronic database system (PhactaX, Seoul, Korea).
Assessment of Smoking History
Smoking history was obtained at study enrollment using a structured questionnaire under supervision of a research coordinator. Participants were categorized into never, former, and current smokers. Smoking load was expressed in pack-years by multiplying the number of packs per day by duration in years in former and current smokers. In former smokers, years from quitting smoking to study enrollment were calculated.
Assessment of the Presence and Progression of Coronary Artery Calcification
The presence of CAC was evaluated using electrocardiography-gated, multislice computed tomography (CT) scanning of the thorax at study enrollment. Repeated scanning was planned at 4 years after the baseline examination. The CAC score was determined using Agatston units (AUs) on a digital radiologic workstation (14). At baseline, the presence of CAC was defined as an AU score >0, in accordance with previous studies (15 –18). Among 755 participants who had an available follow-up CAC score, changes in CAC score were calculated using two methods: annualized percentage change (%/yr) (19 –22) and annualized absolute CAC score change (AU/yr) (18,23). Because baseline CAC score was closely associated with CAC progression (17,18,23), the relative risk of CAC progression was evaluated separately in patients with and without baseline CAC. In patients without baseline CAC, CAC progression was defined as any increase in CAC score at the 4-year follow-up (17,18). In patients with baseline CAC, CAC progression was defined as a change in CAC score of ≥30%/yr.
Statistical Analyses
Statistical analyses were performed using Stata version 15.1 (StataCorp LLC, College Station, TX) and R (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org). Continuous variables were expressed as the mean±SD or median (interquartile range [IQR]). Categorical variables were expressed as number (percentage). Baseline characteristics were compared on the basis of smoking status using an ANOVA with Bonferroni or Kruskal-Wallis test for continuous variables and a chi-squared test for categorical variables. The prevalence ratios for CAC and relative risks for CAC progression according to smoking status were determined by Poisson regression, with robust variance estimation. Multivariable models were constructed, including traditional cardiovascular risk factors and the significant variables in unadjusted analysis (P<0.05): age, sex, diabetes mellitus, economic status, educational level, body mass index, systolic BP, statin use, calcium-phosphate products, eGFR, and urinary protein-to-creatinine ratio. To examine the association of smoking dose and cessation with CAC prevalence, former smokers were divided into three groups on the basis of pack-years (group 1, 0.1 to <10 pack-years; group 2, 10 to <20 pack-years; group 3, ≥20 pack-years) and cessation duration (group 1, 0.5 to <10 years; group 2, 10 to <20 years; group 3, ≥20 years; in 495 former smokers who had available data of cessation duration). The CAC prevalence in former smokers was evaluated by comparing never smokers and current smokers. Furthermore, former smokers were categorized using a 2×2 design on the basis of a combination of smoking dose and cessation duration. Using a smoking dose of 10 pack-years (0.1 to <10 pack-years as light smoking; ≥10 pack-years as heavy smoking) and cessation duration of 10 years (0.5 to <10 years as short-term cessation; ≥10 years as long-term cessation), former smokers were divided into four groups: light smoking/short-term quitter, heavy smoking/short-term quitter, light smoking/long-term quitter, and heavy smoking/long-term quitter. The results of crosscategorization analysis were further tested in patients with CKD stages 3–5, and also tested using different CAC cutoff values (CAC score >10 or CAC score >100 AU) as sensitivity analyses. The relative risk of CAC progression according to smoking status was determined in patients with and without baseline CAC, respectively. In patients with baseline CAC, natural log-transformed baseline CAC score was added in a multivariable model. Different definitions of CAC progression (ΔCAC score ≥100 AU/yr and ΔCAC score ≥15%/yr) were used for sensitivity analysis (17 –25). P<0.05 was considered statistically significant.
Results
Baseline Characteristics
Baseline characteristics categorized by smoking status are presented in Table 1. The median age of participants was 54 years, and 1141 (60%) patients were men. The median baseline eGFR was 48 ml/min per 1.73 m2, and 1201 (63%) patients had proteinuria >0.3 g/g. Among participants, 990 (52%) were never smokers. There were 592 (31%) former and 332 (17%) current smokers with a median smoking dose of 15 (IQR, 7–30) and 22 (IQR, 11–35) pack-years, respectively.
Table 1.
Baseline characteristics of study participants according to smoking status
| Variables | All, N=1914 | Never Smoker, n=990 | Former Smoker, n=592 | Current Smoker, n=332 |
|---|---|---|---|---|
| Age, median [IQR], yr | 54 [44−63] | 54 [45−63] | 57 [46−64] | 52 [41−59] |
| Men, n (%) | 1141 (60) | 280 (28) | 554 (94) | 307 (93) |
| Diabetes mellitus, n (%) | 588 (31) | 268 (27) | 201 (34) | 119 (36) |
| Cause of CKD, n (%) | ||||
| Diabetic kidney disease | 435 (23) | 192 (19) | 148 (25) | 95 (29) |
| Hypertensive | 368 (19) | 162 (16) | 141 (24) | 65 (20) |
| GN | 638 (33) | 358 (36) | 186 (31) | 94 (28) |
| Polycystic kidney disease | 340 (18) | 201 (20) | 80 (14) | 59 (18) |
| Interstitial/unspecified | 133 (7) | 77 (8) | 37 (6) | 19 (6) |
| Economic status, n (%) | ||||
| High (>4500 $/mo) | 437 (23) | 212 (21) | 151 (26) | 74 (22) |
| Middle (1500–4500 $/mo) | 1058 (55) | 577 (58) | 311 (53) | 170 (51) |
| Low (<1500 $/mo) | 419 (22) | 201 (20) | 130 (22) | 88 (27) |
| Educational level, n (%) | ||||
| ≤6 yr | 230 (12) | 156 (16) | 51 (9) | 23 (7) |
| 7–12 yr | 900 (47) | 481 (49) | 265 (45) | 154 (47) |
| ≥13 yr | 784 (41) | 353 (36) | 276 (47) | 155 (47) |
| BMI, mean (SD), kg/m2 | 24.5 (3.4) | 24.3 (3.6) | 24.7 (3.0) | 25.0 (3.6) |
| Systolic BP, mean (SD), mm Hg | 128 (16) | 127 (16) | 128 (17) | 130 (17) |
| Medications, n (%) | ||||
| RAAS blocker | 1635 (85) | 828 (84) | 513 (87) | 294 (89) |
| Lipid-lowering agents | 944 (49) | 502 (51) | 275 (47) | 167 (50) |
| Calcium-based phosphate binders | 168 (9) | 113 (11) | 38 (6) | 14 (4) |
| eGFR, median [IQR], ml/min per 1.73 m2 | 48 [29−76] | 48 [30−81] | 45 [27−68] | 50 [30−78] |
| eGFR categories, n (%) | ||||
| ≥90 ml/min per 1.73 m2 | 244 (13) | 151 (15) | 48 (8) | 45 (14) |
| 60–89 ml/min per 1.73 m2 | 371 (19) | 194 (20) | 112 (19) | 65 (20) |
| 30–59 ml/min per 1.73 m2 | 715 (40) | 367 (35) | 255 (39) | 134 (40) |
| 15–29 ml/min per 1.73 m2 | 412 (22) | 211 (21) | 139 (24) | 72 (22) |
| <15 ml/min per 1.73 m2 | 119 (6) | 65 (7) | 38 (6) | 16 (5) |
| UPCR, median [IQR], g/g | 0.52 [0.15–1.54] | 0.48 [0.13–1.25] | 0.60 [0.18–1.68] | 0.56 [0.20–2.05] |
| Hemoglobin, mean (SD), g/dl | 13 (2) | 12 (2) | 13 (2) | 14 (2) |
| Glucose, mean (SD), mg/dl | 110 (39) | 107 (35) | 108 (34) | 120 (53) |
| Albumin, mean (SD), g/dl | 4.2 (0.4) | 4.2 (0.4) | 4.2 (0.4) | 4.2 (0.5) |
| Triglyceride, median [IQR], mg/dl | 132 [91−194] | 123 [88−183] | 129 [87−193] | 157 [113−233] |
| Total cholesterol, mean (SD), mg/dl | 175 (39) | 179 (38) | 169 (36) | 176 (42) |
| LDL cholesterol, mean (SD), mg/dl | 98 (32) | 100 (32) | 95 (30) | 99 (35) |
| HDL cholesterol, mean (SD), mg/dl | 50 (16) | 53 (16) | 47 (14) | 46 (15) |
| Calcium-phosphate products, mean (SD), mg2/dl2 | 34 (6) | 35 (6) | 33 (6) | 32 (6) |
| Intact PTH, median [IQR], pg/ml | 51 [33−83] | 52 [33−81] | 53 [34−87] | 47 [31−79] |
IQR, interquartile range; BMI, body mass index; RAAS, renin-angiotensin-aldosterone system; UPCR, urinary protein-to-creatinine ratio; PTH, parathyroid hormone.
Prevalence of Coronary Artery Calcification according to Smoking Status
CAC was observed in 952 (50%) patients. In patients with CAC, the median CAC score was 67 AU (IQR, 12–280 AU). The prevalence rate for CAC was the lowest (42%) in never smokers, and the highest (59%) in current smokers (Table 2). The median CAC score of each group was 0 AU in never smokers (IQR, 0–35 AU), 4 AU in former smokers (IQR, 0–122 AU), and 8 AU in current smokers (IQR, 0–105 AU). Current smokers had a higher prevalence of CAC compared with never smokers (prevalence ratio, 1.25; 95% confidence interval [95% CI], 1.10 to 1.42; P<0.001). We further tested this association by sex because there were remarkably more smokers in men than in women (75% versus 8%). The results showed consistently higher prevalence of CAC among current smokers in both men and women (Supplemental Table 1).
Table 2.
Associations of smoking status with prevalent coronary artery calcification
| Smoking Status | No. of Events (%) | Crude | Fully Adjusteda | ||
|---|---|---|---|---|---|
| Prevalence Ratio (95% CI) | P Value | Prevalence Ratio (95% CI) | P Value | ||
| Never smoker | 411/990 (42) | 1.00 | 1.00 | ||
| Former smoker | 346/592 (58) | 1.41 (1.27 to 1.56) | <0.001 | 1.09 (0.98 to 1.22) | 0.1 |
| Current smoker | 195/332 (59) | 1.41 (1.26 to 1.59) | <0.001 | 1.25 (1.10 to 1.42) | <0.001 |
95% CI, 95% confidence interval.
Model was adjusted for age, sex, diabetes mellitus, economic status, educational level, body mass index, systolic BP, statin use, calcium-phosphate products, eGFR, and urinary protein-to-creatinine ratio.
Association of Smoking Dose and Cessation Duration with Coronary Artery Calcification Prevalence
Former smokers were divided according to pack-years and cessation duration (Supplemental Table 2), and the prevalence ratio of CAC was assessed by comparison with never and current smokers (Table 3). A significant difference was not observed in the prevalence of CAC according to smoking dose, compared with never smokers. When current smokers were the reference, former light smokers with smoking load <10 pack-years had a lower prevalence of CAC (prevalence ratio, 0.77; 95% CI, 0.65 to 0.90). Former smokers with short-term cessation <10 years had a higher prevalence of CAC than never smokers (Table 3). In the analysis with current smokers as the reference, former smokers who had quit smoking for ≥10 years showed a lower prevalence of CAC.
Table 3.
Associations of smoking cessation duration and cumulative smoking dose with prevalent coronary artery calcification in former smokers
| Smoking History | No. of Events (%) | Former versus Never Smoker | Former versus Current Smoker | ||
|---|---|---|---|---|---|
| Prevalence Ratio (95% CI) a | P Value | Prevalence Ratio (95% CI) a | P Value | ||
| Smoking dose | |||||
| Never smoker | 411/990 (42) | 1.00 | − | ||
| Former smoker | 346/592 (58) | ||||
| Group 1 (0.1 to <10 pack-yr) | 785/190 (41) | 0.98 (0.83 to 1.15) | 0.81 | 0.77 (0.65 to 0.90) | 0.001 |
| Group 2 (10 to <20 pack-yr) | 75/136 (55) | 1.08 (0.92 to 1.28) | 0.40 | 0.88 (0.76 to 1.03) | 0.13 |
| Group 3 (≥20 pack-yr) | 193/266 (73) | 1.13 (1.00 to 1.27) | 0.05 | 0.95 (0.85 to 1.06) | 0.40 |
| Current smoker | 195/332 (59) | − | 1.00 | ||
| Duration of smoking cessation | |||||
| Never smoker | 411/990 (42) | 1.00 | − | ||
| Former smoker b | 284/495 (57) | ||||
| Group 1 (0.5 to <10 yr) | 105/190 (55) | 1.19 (1.02 to 1.39) | 0.02 | 0.96 (0.33 to 1.10) | 0.56 |
| Group 2 (10 to <20 yr) | 98/183 (54) | 1.05 (0.90 to 1.22) | 0.54 | 0.85 (0.73 to 0.98) | 0.02 |
| Group 3 (≥20 yr) | 81/122 (66) | 0.99 (0.86 to 1.14) | 0.9 | 0.83 (0.73 to 0.96) | 0.01 |
| Current smoker | 195/332 (59) | − | 1.00 | ||
95% CI, 95% confidence interval.
Model was adjusted for age, sex, diabetes mellitus, economic status, educational level, body mass index, systolic BP, statin use, calcium-phosphate products, eGFR, and urinary protein-to-creatinine ratio.
Because of data availability for cessation duration, only 495 former smokers were analyzed.
Prevalence of Coronary Artery Calcification according to the Combination of Smoking Dose and Cessation Duration
Former smokers were further categorized according to combination of smoking dose and cessation duration (Supplemental Table 3). Compared with never smokers, the prevalence of CAC was not different in long-term quitters (≥10 years of cessation), regardless of smoking dose. However, former heavy smokers with short-term cessation had a 1.21-fold (95% CI, 1.03 to 1.40) higher prevalence of CAC (Figure 1A). In comparison with current smokers, long-term quitters with cessation duration ≥10 years showed a significantly lower CAC prevalence, irrespective of smoking load (prevalence ratio, 0.79; 95% CI, 0.66 to 0.95 for light smokers; prevalence ratio, 0.88; 95% CI, 0.78 to 0.99 for heavy smokers; Figure 1B).
Figure 1.
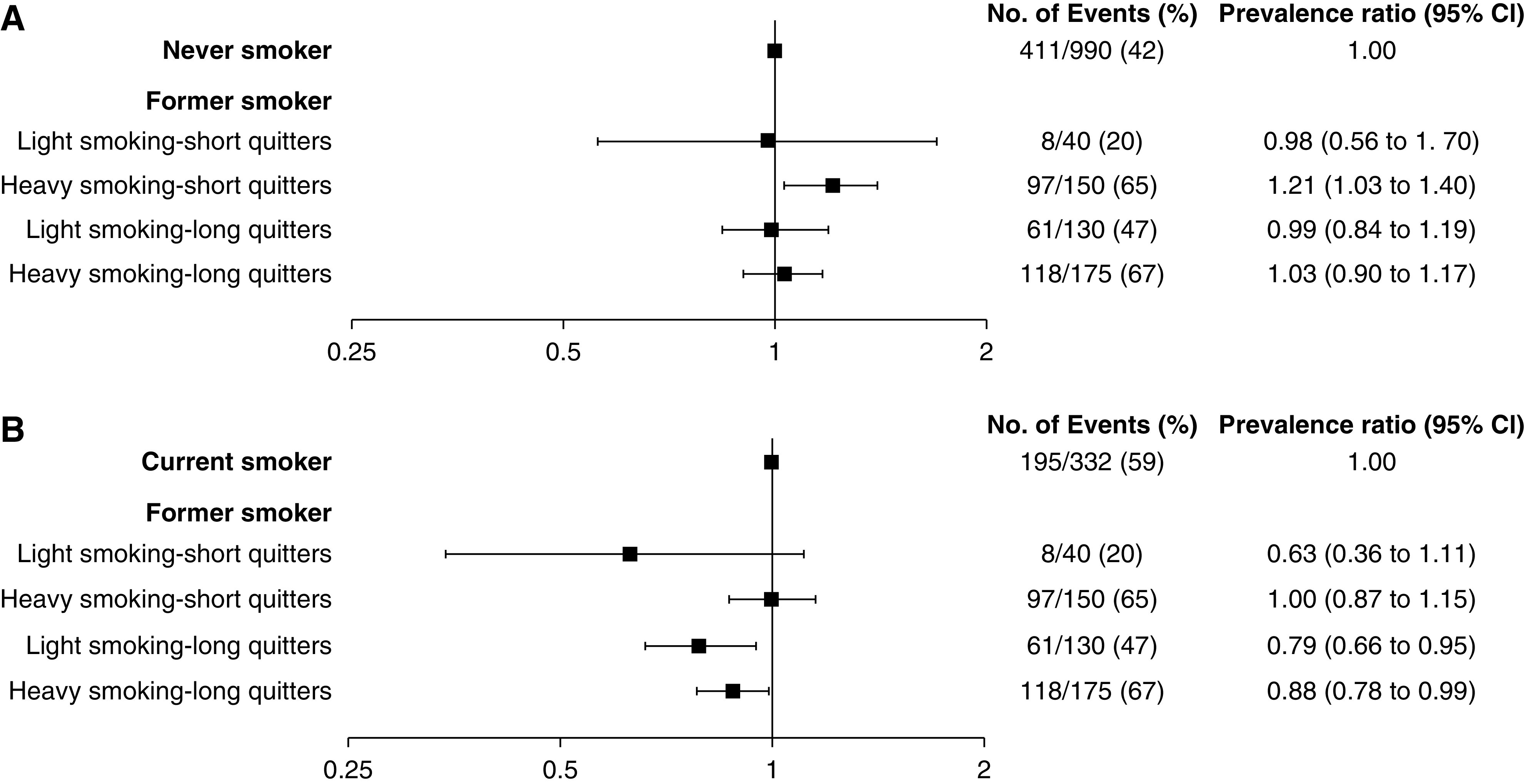
Combined associations of duration of smoking cessation and cumulative dose of smoking exposure with prevalent CAC. (A) Former smokers compared with never smokers. (B) Former smokers compared with current smokers. According to 10 pack-years of smoking dose, former smokers were categorized into light (<10 pack-years) and heavy (≥10 pack-years) smokers. According to 10 years of cessation duration, former smokers were categorized into short-term (<10 years) and long-term (≥10 years) quitters. The model was adjusted for age, sex, diabetes mellitus, economic status, educational level, body mass index, systolic BP, statin use, calcium-phosphate products, eGFR, and urinary protein-to-creatinine ratio. 95% CI, 95% confidence interval; CAC, coronary artery calcification.
Sensitivity Analysis
In a subgroup of 1229 patients with CKD stages 3–5, the lower prevalence of CAC in long-term quitters compared with current smokers remained significant independent of smoking dose (Supplemental Table 4). Additional analyses with different CAC cutoff values (CAC score >10) consistently showed the lower prevalence of CAC in former smokers with a long-term cessation compared with current smokers. However, when a cutoff of CAC score >100 AU was used, only heavy smoking/long-term quitters showed a significantly lower prevalence (Supplemental Table 5).
Relative Risk of Coronary Artery Calcification Progression according to Smoking Status
Among 755 participants who underwent follow-up CT at year 4, 337 (45%) participants had CAC at baseline (Table 4). Differences in baseline characteristics between those with and without follow-up CT are detailed in Supplemental Table 6. Baseline CAC score was positively associated with changes in CAC score (r=0.74; P<0.001; Supplemental Figure 2). In patients without baseline CAC, 332 (79%) patients remained free of CAC during follow-up. The median change in CAC score was significantly higher in patients with baseline CAC than in those without baseline CAC (61 versus 0 AU; P<0.001; Supplemental Figure 3). Therefore, CAC progression was assessed separately according to the presence of baseline CAC (Table 4). Incident CAC was observed in 86 (21%) patients without CAC at baseline. In these patients, current smoking was not significantly associated with incident CAC (Table 4). CAC progression (annualized percentage change in CAC score of ≥30%) was observed in 111 (33%) patients who had CAC at baseline. Current smokers had a higher relative risk for CAC progression in these patients (relative risk, 1.92; 95% CI, 1.30 to 2.86) (Table 4). In the analyses using additional cutoffs, higher relative risk for CAC progression remained significant when using the definition of ΔCAC score ≥15%/yr (Supplemental Table 7).
Table 4.
Associations of smoking and coronary artery calcification progression in patients with and without baseline coronary artery calcification
| Smoking Status | Patients without Baseline CAC, n=418 a | Patients with Baseline CAC, n=337 b | ||||
|---|---|---|---|---|---|---|
| No. of Events (%) | Relative Risk (95% CI) c | P Value | No. of Events (%) | Relative Risk (95% CI) d | P Value | |
| Never smoker | 51/248 (21) | 1.00 | 44/142 (31) | 1.00 | ||
| Former smoker | 22/113 (20) | 1.10 (0.65 to 1.88) | 0.35 | 37/135 (27) | 1.05 (0.70 to 1.58) | 0.81 |
| Current smoker | 13/57 (23) | 1.53 (0.83 to 2.82) | 0.18 | 30/60 (50) | 1.92 (1.30 to 2.86) | 0.001 |
CAC, coronary artery calcification; 95% CI, 95% confidence interval.
In patients without baseline CAC, CAC progression was defined as any increase in CAC score at follow-up examination.
In patients with baseline CAC, CAC progression was defined as annualized percentage change in CAC score of ≥30%.
Model was adjusted for age, sex, diabetes mellitus, economic status, educational level, body mass index, systolic BP, statin use, calcium-phosphate products, eGFR, and urinary protein-to-creatinine ratio.
Model was adjusted for age, sex, diabetes mellitus, economic status, educational level, body mass index, systolic BP, statin use, calcium-phosphate products, eGFR, urinary protein-to-creatinine ratio, and natural log-transformed baseline CAC score.
Discussion
In our study, current smokers had a higher prevalence of CAC and a higher relative risk for CAC progression in patients with CKD. In former smokers, cumulative smoking load <10 pack-years and cessation duration >10 years were significantly associated with lower prevalence of CAC compared with current smokers. In particular, former smokers with long-term cessation duration had a similar prevalence of CAC to never smokers, and about 20% lower prevalence than current smokers, irrespective of smoking load. However, former heavy smokers with short-term cessation duration <10 years showed a higher prevalence of CAC compared with never smokers.
The association between smoking and cardiovascular disease in patients with CKD has been investigated in only a few studies (7 –10). In a meta-analysis of patients on dialysis, although heterogeneity existed, active smoking was associated with higher all-cause mortality compared with nonsmoking, but not with cardiovascular events (8). In contrast, the post hoc Study of Heart and Renal Protection (SHARP) showed that the risk for both atherosclerotic and nonatherosclerotic events was higher in current smokers than in never smokers (10). In accordance with these findings, our study also showed that current smokers were more likely to have CAC than never smokers. However, whether quitting smoking can prevent further calcification is currently unknown (7,10). Former smokers had a similar cardiovascular risk compared with nonsmokers in patients on incident dialysis (7). Conversely, in the SHARP study, former smokers had a higher risk of vascular events (10). However, the effects of smoking dose or cessation duration were not evaluated in those studies. In our study, patients with light smoking load (<10 pack-years) and cessation >10 years showed a lower prevalence of CAC compared with current smokers. Notably, the lower prevalence of CAC in long-term quitters was consistent in both light and heavy smokers, and not affected by advanced CKD and different CAC cutoff values. Furthermore, CAC prevalence in long-term quitters did not differ from nonsmokers. These findings suggest a possible benefit of early and long-term smoking cessation for reducing cardiovascular risk. Therefore, prompt smoking cessation and continuous monitoring for avoiding resmoking are encouraged in both current and former smokers with CKD.
Former heavy smokers with smoking cessation <10 years had a 1.21-fold higher prevalence ratio compared with never smokers. Although the study design, participants, and outcomes are different, this result is in accordance with previous studies involving people without CKD (3 –5). Among the Framingham Heart Study participants, former heavy smoking was associated with higher risk of incident cardiovascular disease compared with never smoking after 5 years of cessation (4). In the Atherosclerotic Cardiovascular Disease Risk Estimator Plus, the risk for former smokers was considered identical to never smokers after 5 years of cessation (26). However, we surmised that 5-year smoking cessation may underestimate the prevalence in patients with CKD, especially in patients with a heavy smoking load. Therefore, detailed profiling of smoking history is clinically relevant for cardiovascular risk stratification in patients with CKD.
The association between current smoking and CAC progression was also investigated in our study. According to previous studies (17,18), smoking was not a significant risk factor for CAC progression in patients with CKD. In the Multi-Ethnic Study of Atherosclerosis, only male sex and diabetes were significantly associated with CAC progression (17). Smoking was also not associated with CAC progression in the Chronic Renal Insufficiency Cohort (CRIC) study (18). In our study, the association differed depending on the definition of CAC progression. When CAC progression was defined as annualized percentage change in CAC score of ≥30%, the progression events occurred in 111 (33%) patients with baseline CAC, and current smokers had a 1.92-fold greater relative risk for CAC progression compared with never smokers. However, only 34 (10%) patients experienced increases in ΔCAC score ≥100 AU/yr, and smoking was not associated with CAC progression when using this cutoff value. Hypothetically, the small number of participants and events limits the significant association of smoking with CAC progression. Compared with the CRIC cohort, the quantity of baseline CAC score and the proportion of CAC progression were lower in our cohort (18). Exclusion of patients with previous history of cardiovascular disease at baseline, and a lower proportion of patients with diabetes and obese patients, might explain the lower quantity of CAC score or CAC progression rate in our study. Another explanation could be the lower cardiovascular risk burden observed in East Asian populations compared with Western populations (27,28). Therefore, relatively short-term assessment of CAC might be insufficient to observe significant changes in CAC. The KNOW-CKD study is ongoing, and the investigators are encouraging patients to continue the study visits for follow-up measurements, including CAC score. Future studies with more participants may be helpful to clarify this issue.
This study had several limitations. First, smoking status was determined at study enrollment and was not assessed during follow-up. The confounding effect of resuming or quitting smoking was not excluded in the analysis of CAC progression. Second, we assessed smoking history using a self-reporting questionnaire. Thus, recall bias cannot be excluded, especially in quitting duration. In this study, the proportion of smokers (former and current) was significantly higher in men than in women (75% versus 8%; P<0.001). There has been evidence that light and intermittent smoking is common in women and young adulthood (29). The prevalence of light and intermittent smoking could be underestimated because they tend to report as nonsmokers (30). This might contribute to the lower proportion of smokers in women in our study. However, although the proportion of smokers was low in women, a subgroup analysis showed a consistently higher prevalence of CAC in current smokers in both men and women. In the Republic of Korea, electronic cigarettes are becoming increasingly popular, but such information was not surveyed in this study. Third, the effects of smoking dose and cessation duration on CAC progression could not be completely evaluated because of the small number of participants who had follow-up CAC scores. This can result in a lack of statistical power and underestimate the effects of smoking. Furthermore, although follow-up measurements are performed according to a prespecified schedule of 4 years after the baseline evaluation, selection bias can exist between patients with and without follow-up CT scans. The prevalence rate of baseline CAC was not different between patients with and without follow-up CT scans. However, patients without follow-up measurements were more likely to be current smokers and diabetic. Because this study is ongoing, more in-depth investigation is required to delineate this issue. Fourth, because we did not perform repeated CT scans at baseline, we could not calculate repeatability limits for CAC. Previous studies defined CAC progression as a difference in CAC score surpassing baseline repeatability limits in patients with baseline CAC, which means that the difference exceeds measurement error (18,31,32). Nevertheless, because of the lack of information on repeatability limits in our own study, we cannot totally exclude the possibility that changes in CAC might be affected by measurement process rather than progression of atherosclerosis. Fifth, although CAC was a surrogate of subclinical cardiovascular disease, and CAC progression was investigated in this study, we could not clarify the effects of smoking cessation on the development of adverse cardiovascular events or mortality. Because randomized controlled trials are not feasible to address the direct preventive effects of smoking cessation on cardiovascular outcomes, further long-term prospective observation is needed. Finally, all study participants were Korean patients with CKD who had not undergone dialysis or kidney transplantation. Therefore, our results cannot be generalized to other populations. Notwithstanding these limitations, a detailed smoking history, including smoking load and cessation duration, can provide specific information on elaborate cardiovascular risk stratification in former smokers who have received less attention in clinical practice.
In conclusion, current smokers had a higher CAC prevalence and a higher relative risk for CAC progression in Korean patients with CKD. Smoking cessation >10 years indicated a significantly lower prevalence of CAC compared with current smoking, regardless of smoking dose. However, former heavy smokers with short-term smoking cessation had a higher CAC prevalence than never smokers. These findings suggest that regular education and counseling for maintaining cessation is needed in former heavy smokers with short-term cessation duration.
Disclosures
S.H. Han reports serving as a subeditor of Nephrology and serving as a scientific advisor or member of the Korean Society of Nephrology. K.-H. Oh reports receiving research funding from Fresenius Medical Care, Korea. All remaining authors have nothing to disclose.
Funding
This study was supported by a research program of the Korea Centers for Disease Control and Prevention (grants 2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, 2016E3300200, 2016E3300201, 2016E3300202, 2019E320100, and 2019E320101).
Supplementary Material
Acknowledgments
The institutional review board of each participating clinical center approved the study protocol as follows: Seoul National University Hospital (1104-089-359), Seoul National University Bundang Hospital (B-1106/129-008), Yonsei University Severance Hospital (4-2011-0163), Kangbuk Samsung Medical Center (2011-01-076), Seoul St. Mary’s Hospital (KC11OIMI0441), Gil Hospital (GIRBA2553), Eulji General Hospital (201105-01), Chonnam National University Hospital (CNUH-2011-092), and Pusan Paik Hospital (11-091).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.15751020/-/DCSupplemental.
Supplemental Appendix 1. Supplemental methods.
Supplemental Table 1. The prevalence of CAC according to smoking status between men and women.
Supplemental Table 2. Categorization of former smokers according to smoking load and cessation duration.
Supplemental Table 3. Prevalence rate for CAC in four combination groups, according to smoking dose and cessation duration, in 495 former smokers.
Supplemental Table 4. The prevalence of CAC for former smokers among participants with CKD stages 3−5.
Supplemental Table 5. The prevalence of CAC for former smokers compared with current smokers, using different CAC cutoff values.
Supplemental Table 6. Comparison of baseline characteristics in patients with and without follow-up CAC measurements.
Supplemental Table 7. Associations of smoking with CAC progression, using different definitions in patients with baseline CAC.
Supplemental Figure 1. Study participants.
Supplemental Figure 2. Correlation of baseline CAC score and CAC score changes.
Supplemental Figure 3. Comparison of CAC score changes between patients with and without baseline CAC.
References
- 1.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr., Virani SS, Williams KA Sr., Yeboah J, Ziaeian B: 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 140: e596–e646, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mons U, Müezzinler A, Gellert C, Schöttker B, Abnet CC, Bobak M, de Groot L, Freedman ND, Jansen E, Kee F, Kromhout D, Kuulasmaa K, Laatikainen T, O’Doherty MG, Bueno-de-Mesquita B, Orfanos P, Peters A, van der Schouw YT, Wilsgaard T, Wolk A, Trichopoulou A, Boffetta P, Brenner H; CHANCES Consortium : Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES Consortium. BMJ 350: h1551, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding N, Sang Y, Chen J, Ballew SH, Kalbaugh CA, Salameh MJ, Blaha MJ, Allison M, Heiss G, Selvin E, Coresh J, Matsushita K: Cigarette smoking, smoking cessation, and long-term risk of 3 major atherosclerotic diseases. J Am Coll Cardiol 74: 498–507, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duncan MS, Freiberg MS, Greevy RA Jr, Kundu S, Vasan RS, Tindle HA: Association of smoking cessation with subsequent risk of cardiovascular disease. JAMA 322: 642–650, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee: Heart disease and stroke statistics-2018 update: A report from the American Heart Association [published correction appears in Circulation 137: e493, 2018 10.1161/CIR.0000000000000573]. Circulation 137: e67–e492, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Chapter 4: Other complications of CKD: CVD, medication dosage, patient safety, infections, hospitalizations, and caveats for investigating complications of CKD. Kidney Int Suppl (2011) 3:91–111, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley RN, Herzog CA, Collins AJ: Smoking and cardiovascular outcomes in dialysis patients: The United States Renal Data System Wave 2 study. Kidney Int 63: 1462–1467, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Liebman SE, Lamontagne SP, Huang LS, Messing S, Bushinsky DA: Smoking in dialysis patients: A systematic review and meta-analysis of mortality and cardiovascular morbidity. Am J Kidney Dis 58: 257–265, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mc Causland FR, Brunelli SM, Waikar SS: Association of smoking with cardiovascular and infection-related morbidity and mortality in chronic hemodialysis. Clin J Am Soc Nephrol 7: 1827–1835, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staplin N, Haynes R, Herrington WG, Reith C, Cass A, Fellström B, Jiang L, Kasiske BL, Krane V, Levin A, Walker R, Wanner C, Wheeler DC, Landray MJ, Baigent C, Emberson J; SHARP Collaborative Group: Smoking and adverse outcomes in patients with CKD: The Study of Heart and Renal Protection (SHARP). Am J Kidney Dis 68: 371–380, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Budoff MJ, Reilly MP, Yang W, Rosas SE, Rahman M, Zhang X, Roy JA, Lustigova E, Nessel L, Ford V, Raj D, Porter AC, Soliman EZ, Wright JT Jr, Wolf M, He J; CRIC Investigators: Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol 2: 635–643, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsushita K, Sang Y, Ballew SH, Shlipak M, Katz R, Rosas SE, Peralta CA, Woodward M, Kramer HJ, Jacobs DR, Sarnak MJ, Coresh J: Subclinical atherosclerosis measures for cardiovascular prediction in CKD. J Am Soc Nephrol 26: 439–447, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh KH, Park SK, Park HC, Chin HJ, Chae DW, Choi KH, Han SH, Yoo TH, Lee K, Kim YS, Chung W, Hwang YH, Kim SW, Kim YH, Kang SW, Park BJ, Lee J, Ahn C; Representing KNOW-CKD Study Group: KNOW-CKD (KoreaN cohort study for Outcome in patients With Chronic Kidney Disease): Design and methods. BMC Nephrol 15: 80, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R: Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15: 827–832, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Cheng YJ, Church TS, Kimball TE, Nichaman MZ, Levine BD, McGuire DK, Blair SN: Comparison of coronary artery calcium detected by electron beam tomography in patients with to those without symptomatic coronary heart disease. Am J Cardiol 92: 498–503, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Loria CM, Liu K, Lewis CE, Hulley SB, Sidney S, Schreiner PJ, Williams OD, Bild DE, Detrano R: Early adult risk factor levels and subsequent coronary artery calcification: The CARDIA study. J Am Coll Cardiol 49: 2013–2020, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kestenbaum BR, Adeney KL, de Boer IH, Ix JH, Shlipak MG, Siscovick DS: Incidence and progression of coronary calcification in chronic kidney disease: The Multi-Ethnic Study of Atherosclerosis. Kidney Int 76: 991–998, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bundy JD, Chen J, Yang W, Budoff M, Go AS, Grunwald JE, Kallem RR, Post WS, Reilly MP, Ricardo AC, Rosas SE, Zhang X, He J; CRIC Study Investigators: Risk factors for progression of coronary artery calcification in patients with chronic kidney disease: The CRIC study. Atherosclerosis 271: 53–60, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raggi P, Callister TQ, Shaw LJ: Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arterioscler Thromb Vasc Biol 24: 1272–1277, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P: Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 68: 1815–1824, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Qunibi W, Moustafa M, Muenz LR, He DY, Kessler PD, Diaz-Buxo JA, Budoff M; CARE-2 Investigators: A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: The Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis 51: 952–965, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi Y, Hamano T, Obi Y, Monden C, Oka T, Yamaguchi S, Matsui I, Hashimoto N, Matsumoto A, Shimada K, Takabatake Y, Takahashi A, Kaimori JY, Moriyama T, Yamamoto R, Horio M, Yamamoto K, Sugimoto K, Rakugi H, Isaka Y: A randomized trial of magnesium oxide and oral carbon adsorbent for coronary artery calcification in predialysis CKD. J Am Soc Nephrol 30: 1073–1085, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrotra R, Budoff M, Hokanson JE, Ipp E, Takasu J, Adler S: Progression of coronary artery calcification in diabetics with and without chronic kidney disease. Kidney Int 68: 1258–1266, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, Detrano RC, Bild DE, Guerci AD, Liu K, Shea S, Szklo M, Post W, Lima J, Bertoni A, Wong ND: Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 61: 1231–1239, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bundy JD, Cai X, Scialla JJ, Dobre MA, Chen J, Hsu CY, Leonard MB, Go AS, Rao PS, Lash JP, Townsend RR, Feldman HI, de Boer IH, Block GA, Wolf M, Smith ER, Pasch A, Isakova T; CRIC Study Investigators: Serum calcification propensity and coronary artery calcification among patients with CKD: The CRIC (Chronic Renal Insufficiency Cohort) study. Am J Kidney Dis 73: 806–814, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd-Jones DM, Huffman MD, Karmali KN, Sanghavi DM, Wright JS, Pelser C, Gulati M, Masoudi FA, Goff DC Jr: Estimating longitudinal risks and benefits from cardiovascular preventive therapies among Medicare patients: The Million Hearts Longitudinal ASCVD Risk Assessment Tool: A special report from the American Heart Association and American College of Cardiology. Circulation 135: e793–e813, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee C, Park KH, Joo YS, Nam KH, Chang TI, Kang EW, Lee J, Oh YK, Jung JY, Ahn C, Lee KB, Park JT, Yoo TH, Kang SW, Han SH: Low high-sensitivity C-reactive protein level in Korean patients with chronic kidney disease and its predictive significance for cardiovascular events, mortality, and adverse kidney outcomes: Results from KNOW-CKD. J Am Heart Assoc 9: e017980, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka K, Watanabe T, Takeuchi A, Ohashi Y, Nitta K, Akizawa T, Matsuo S, Imai E, Makino H, Hishida A; CKD-JAC Investigators: Cardiovascular events and death in Japanese patients with chronic kidney disease. Kidney Int 91: 227–234, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Amos A, Greaves L, Nichter M, Bloch M: Women and tobacco: A call for including gender in tobacco control research, policy and practice. Tob Control 21: 236–243, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Schane RE, Ling PM, Glantz SA: Health effects of light and intermittent smoking: A review. Circulation 121: 1518–1522, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hokanson JE, MacKenzie T, Kinney G, Snell-Bergeon JK, Dabelea D, Ehrlich J, Eckel RH, Rewers M: Evaluating changes in coronary artery calcium: An analytic method that accounts for interscan variability. AJR Am J Roentgenol 182: 1327–1332, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Chung H, McClelland RL, Katz R, Carr JJ, Budoff MJ: Repeatability limits for measurement of coronary artery calcified plaque with cardiac CT in the Multi-Ethnic Study of Atherosclerosis. AJR Am J Roentgenol 190: W87–W92, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.