Visual Abstract
Keywords: cardiovascular disease, chronic dialysis, chronic renal failure, electrolytes, end-stage renal disease, heart disease, peritoneal dialysis, renin angiotensin system, hemodialysis, mineralocorticoid receptor antagonists
Abstract
Background and objectives
Patients with kidney failure have a high risk of cardiovascular disease due to cardiac remodeling, left ventricular fibrosis, and hyperaldosteronism, all of which can be potentially mitigated by mineralocorticoid receptor antagonists. However, because of the fear of hyperkalemia, the use of mineralocorticoid receptor antagonists in patients with kidney failure is limited in current clinical practice, and few studies have investigated the efficacy and safety. Thus, we aimed to determine the benefits and side effects of mineralocorticoid receptor antagonists in patients with kidney failure treated with dialysis.
Design, setting, participants, & measurements
This is a systematic review and meta-analysis of randomized controlled trials published from 2005 to 2020 that compared the effect of mineralocorticoid receptor antagonists with either placebo or no treatment in patients with kidney failure. Two reviewers independently searched the PubMed, EMBASE, and Cochrane databases for all published studies, extracted data, assessed the risk of bias, and rated the quality of evidence. A meta-analysis was conducted on 14 eligible randomized controlled trials, and a total of 1309 patients were included.
Results
High-quality evidence suggested that mineralocorticoid receptor antagonists are associated with lower cardiovascular mortality (relative risk, 0.41; 95% confidence interval, 0.24 to 0.70; P=0.001) and all-cause mortality (relative risk, 0.44; 95% confidence interval, 0.30 to 0.66; P<0.001), and the risk of hyperkalemia was comparable with that of control group (relative risk, 1.12; 95% confidence interval, 0.91 to 1.36; P=0.29). However, no significant decrease in nonfatal cardiovascular events and stroke was observed, and there was no significant improvement in BP or cardiac performance parameters, including left ventricular ejection fraction and left ventricular mass index.
Conclusions
Our meta-analysis suggests that mineralocorticoid receptor antagonists might improve clinical outcomes of patients with kidney failure without significant increase in the risk of hyperkalemia.
Introduction
Patients with kidney failure have a high risk of cardiovascular disease. Being the most common cause of death, cardiovascular disease accounts for nearly half of all deaths among such patients (1). Despite recent advances in medicine, the overall mortality rate among patients with kidney failure remains as high as 136 per 1000 patient-years (1). With approximately 2.6 million patients with kidney failure receiving dialysis worldwide (2), the effects on health care are tremendous.
Cardiac remodeling, precipitated by atherosclerotic disease, hypertension, left ventricular hypertrophy (3), and an increase in circulating angiogenesis and nitric oxide inhibitors (4), is one of the most crucial mechanisms attributable to cardiovascular disease and mortality in patients with kidney failure (5), including ventricular dysfunction and fulminant arrhythmia (6). Moreover, kidney failure is a state of relative hyperaldosteronism, regardless of the presence of fluid overload, that may contribute to fibrosis, vascular smooth muscle proliferation, impaired vasodilation, and endothelial dysfunction (5), all of which are deleterious to cardiovascular function.
Mineralocorticoid receptor antagonists (MRAs), namely spironolactone and eplerenone, are direct aldosterone receptor antagonists, and they have been proven to ameliorate cardiac remodeling and, subsequently, reduce the risk of cardiovascular mortality and hospitalization in patients not on dialysis with reduced ejection fraction heart failure (7 –9). The effect of MRAs in patients with CKD had been demonstrated in one meta-analysis by reducing the risk of left ventricular mass development, all-cause mortality, and major adverse cardiovascular events (10). However, in patients with kidney failure, despite the potential effect of mitigating the adverse effect of both cardiac remodeling and hyperaldosteronism, MRAs are not frequently applied because of the fear of hyperkalemia. We found several prospective studies (11 –20) and a medium-sized meta-analysis (21) that focused on the efficacy of MRAs in the past decade. Many of these studies have reported clinical benefits of MRAs despite higher risk of hyperkalemia. Nevertheless, the results of these studies have been inconsistent due to small sample sizes and considerable heterogeneity between studies.
Therefore, in this study, we aimed to clarify the role of MRAs in patients with kidney failure receiving dialysis. We synthesized data from available randomized controlled trials (RCTs) to conduct a systemic review and meta-analysis.
Materials and Methods
This systematic review/meta-analysis was registered on the International Prospective Register of Systematic Reviews with registration number CRD42020222107, and it was done and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidance (22).
Search Strategy
A systematic review of the available published studies was conducted; PubMed, EMBASE, and Cochrane were searched, and only those studies published in English and before December 2020 were included. The following search terms were used: “mineralocorticoid receptor antagonists,” “MRA,” “aldosterone receptor blocker,” “aldosterone blocker,” “spironolactone,” “eplerenone,” “dialysis,” “hemodialysis,” “peritoneal dialysis,” “HD,” “PD,” “renal failure,” “kidney failure,” and “ESKD.” Details of search strategy are shown in Supplemental Table 1. Furthermore, we reviewed and screened the reference lists from all identified pertinent articles to further include potential and relevant studies that were overlooked during the database search.
Study Selection Criteria
The following criteria were used for study inclusion and selection: (1) study design: RCTs (crossover studies with a sufficient washout period were also allowed); (2) type of participants: adults (age ≥18 years) with kidney failure on hemodialysis or peritoneal dialysis; (3) intervention: MRAs, including spironolactone and eplerenone; (4) comparison: placebo or no treatment; and (5) primary outcomes: cardiovascular mortality and all-cause mortality; secondary outcomes: nonfatal cardiovascular events, stroke, BP changes, left ventricular mass index, left ventricular ejection fraction, hyperkalemia, and serum potassium level.
Data Extraction and Quality Assessment
Data on the study design, duration, patient characteristics, intervention characteristics (MRA type, dose, and duration), and outcomes were extracted independently by two authors (K.-T.C. and C.-C.K.) in parallel.
Quality assessment was also performed by two independent authors by using the revised tool for assessing risk of bias in randomized trials, developed by the Cochrane Collaboration (23), to evaluate all potentially relevant bias. Each study outcome was assigned as “low risk of bias,” “some concerns,” or “high risk of bias.”
Outcome Assessed
We compared the outcomes between the MRA-treated group and control groups. The primary outcomes were all-cause mortality and cardiovascular mortality, including death from acute myocardial infarction, heart failure, stroke, cardiovascular procedures, malignant arrhythmia, cardiovascular hemorrhages, and sudden cardiac death. Secondary outcomes were nonfatal cardiovascular events (including acute myocardial infarction, heart failure, and malignant arrhythmia), stroke, BP, left ventricular mass index, left ventricular ejection fraction, hyperkalemia, and serum potassium level.
Statistical Analyses
All analyses were performed using the Cochrane Collaboration Review Manager 5.3. The difference between therapeutic intervention and control groups was considered statistically significant when P was =0.05. Intention to treat analysis was used to assess primary outcomes to minimize type 1 error, whereas per-protocol analysis was used to assess secondary outcomes because of limited data availability.
The relative risks (RRs) of dichotomous data, mean differences of continuous data, and 95% confidence intervals (95% CIs) were separately calculated for each study, and a summary RR estimate was calculated using the Mantel–Haenszel method. Mean differences were pooled using the inverse variance method. All of the meta-analyses were done in the random effects model (DerSimonian and Laird). I 2 and Q statistics were used to estimate the degree of variation across studies caused by heterogeneity rather than chance, and I 2 values <40% were considered less heterogeneous.
Funnel plots according to cardiovascular and all-cause mortality were generated to assess possible publication bias. Finally, level of evidence assessment was conducted using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method (24).
Results
Study Selection
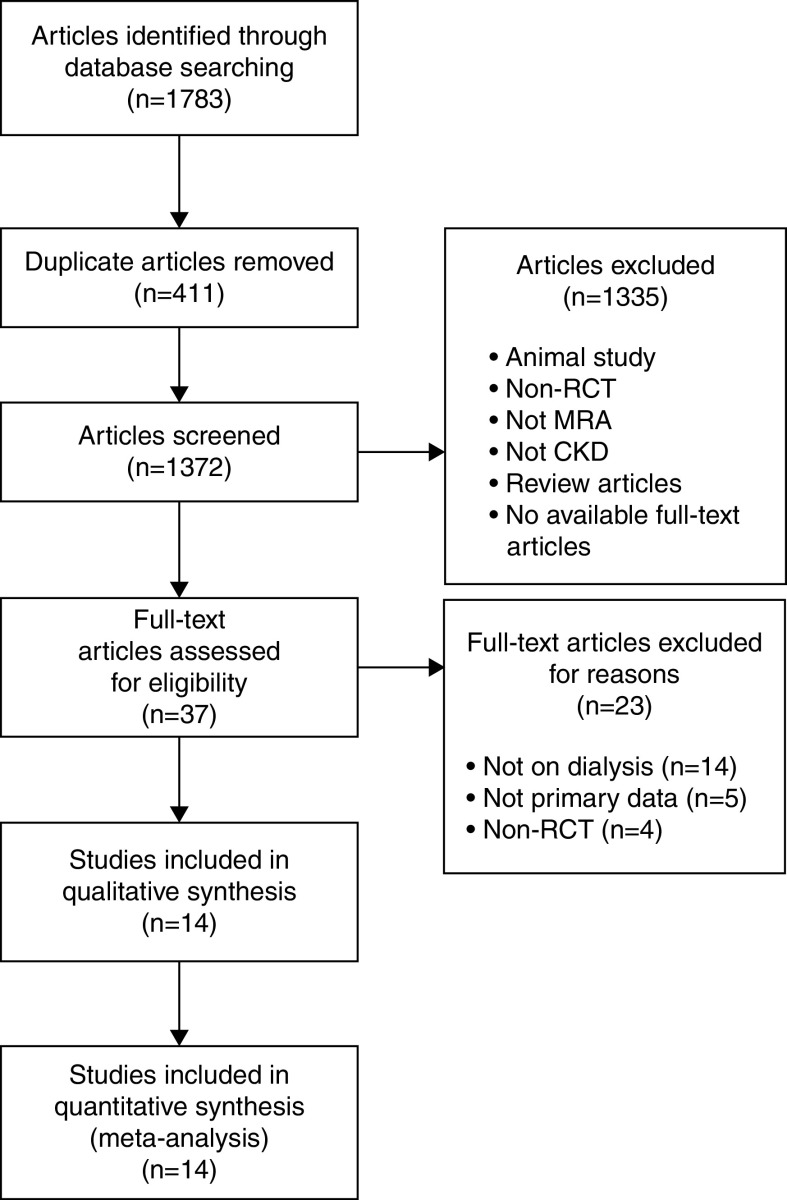
A total of 1783 potentially relevant articles were retrieved, among which 411 were duplicates. After reviewing the titles and abstracts, 1335 articles were excluded. Consequently, 37 articles were screened for eligibility after full-text reading, 23 of which were excluded for various reasons, including 14 articles that focused on patients with nondialysis CKD, five articles that did not provide primary data, and four articles that were not RCTs. Finally, 14 articles in total fulfilled all of the inclusion criteria and were included in the qualitative and quantitative synthesis (Figure 1).
Figure 1.
Flow chart of the literature search. Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart of the study search and review process. MRA, mineralocorticoid receptor antagonist; RCT, randomized controlled trial.
Study Characteristics
The characteristics of the included trials are summarized in Table 1. All of the articles were RCTs published in English from 2005 to 2020, and a total of 1309 patients were included. Among them, 12 adopted a parallel design (11 –20,25,26), whereas the other two used a crossover design (27,28). The chosen MRA was spironolactone in 13 trials (11 –14,16 –20,25 –28), and eplerenone was used in one trial (15). The study populations ranged from eight to 309 patients. The follow-up duration varied from 7 weeks to 36 months. The mean age of the study population ranged from 53 to 70 years, and the patients were predominantly men in most of the studies. Ten studies included patients undergoing hemodialysis (11 –13,15,16,18 –20,25,27), two included patients receiving peritoneal dialysis (17,28), and the other two included both populations (14,26). Patients with heart failure were excluded in four of the trials (11,14,25,27), three included only patients with heart failure (18 –20), and the other seven had no limitations regarding the comorbidity of heart failure (12,13,15 –17,26,28).
Table 1.
Characteristics of studies included in a meta-analysis of mineralocorticoid receptor antagonists in patients with kidney failure treated with dialysis
| Study | Population | Intervention | Comparison | Sample Size, n | Mean Age, yr | Dialysis Vintage, yr | Heart Failure, % | Follow-Up | Complete Follow-Up, n (%) | Reported Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Charytan et al. (13) | HD | Spironolactone 12.5 and 25 and 50 mg once daily | Placebo | 129 | I: 54.6 C: 55.5 | 3.4 | I:14 C:20 | 9 mo | I: 67 (86) C: 48 (94) | Cardiovascular mortality; all-cause mortality; nonfatal cardiovascular events; stroke; hyperkalemia |
| Ziaee et al. (11) | HD | Spironolactone 25 mg TIW | No treatment | 48 | I: 69.2±13.5 C: 67.5±10 | NA | Excluded | 9 mo | I: 22 (92) C: 21 (88) | All-cause mortality; left ventricular mass index; left ventricular ejection fraction; BP |
| Hammer et al. (12) | HD | Spironolactone 50 mg once daily | Placebo | 97 | I: 60.6±13.1 C: 59.9±13.4 | NA | I: 6 C: 2 | 40 wk | I: 44 (88) C: 41 (87) | Cardiovascular mortality; all-cause mortality; left ventricular mass index; BP; hyperkalemia |
| Lin et al. (14) | HD/PD | Spironolactone 25 mg once daily | Placebo | 253 | I: 70.3±10.9 C: 70.6±8.4 | 3.5 | Excluded | 24 mo | I: 100 (80) C: 98 (77) | Cardiovascular mortality; all-cause mortality; left ventricular mass index; left ventricular ejection fraction |
| Feniman-De-Stefano et al. (25) | HD | Spironolactone 12.5 or 25 mg once daily | Placebo | 19 | I: 52.3±9.2 C: 56.3±10.9 | 3.1 | Excluded | 6 mo | I: 8 (80) C: 9 (100) | Left ventricular mass index; BP |
| Yongsiri et al. (28)* | PD | Spironolactone 25 mg once daily | Placebo | 24 | 52.4±12.6 | 2 | NA | 12 wk | All: 20 (83) | Hyperkalemia |
| Walsh et al. (15) | HD | Eplerenone 50 mg once daily + down titration | Placebo | 154 | I: 62.1±14.6 C: 63.1±13.7 | 3 | I: 10 C: 8 | 13 wk | I: 75 (97) C: 71 (92) | Cardiovascular mortality; all-cause mortality; nonfatal cardiovascular events; hyperkalemia |
| Ito et al. (17) | PD | Spironolactone 25 mg once daily | No treatment | 158 | I: 57.5±12.3 C: 55.7±14.4 | 0.6 | NA | 24 mo | I: 50 (64) C: 58 (73) | All-cause mortality; nonfatal cardiovascular events; stroke; left ventricular mass index; left ventricular ejection fraction; hyperkalemia |
| Matsumoto et al. (16) | HD | Spironolactone 25 mg once daily | No treatment | 309 | I: 67.4±12.3 C: 67.7±11.2 | 9.5 | NA | 36 mo | I: 112 (71) C: 128 (84) | Cardiovascular mortality; all-cause mortality; nonfatal cardiovascular events; stroke |
| Ni et al. (26) | HD/PD | Spironolactone 25 mg once daily | Placebo | 76 | I: 55.7±12.3 C: 54.9±14.2 | NA | NA | 12 wk | I: 36 (90) C: 34 (94) | BP |
| Taheri et al. (18) | HD | Spironolactone 25 mg QOD | Placebo | 18 | I: 50.7±17.4 C: 57.2±13.1 | 1.4 | I: 100, C: 100 NYHA approximately class 3–4 | 6 mo | I: 7 (78) C: 9 (100) | Cardiovascular mortality; all-cause mortality; stroke; left ventricular ejection fraction; hyperkalemia |
| Vukusich et al. (19) | HD | Spironolactone 50 mg TIW | Placebo | 53 | I: 60.1±5.2 C: 55.6±3.6 | 8.3 | I: 100, C: 100 NYHA approximately class 1–3 | 24 mo | I: 30 (91) C: 23 (70) | All-cause mortality; hyperkalemia |
| Taheri et al. (20) | HD | Spironolactone 25 mg TIW | Placebo | 16 | I: 59.5±6.5 C: 56.8±9.3 | NA | I: 100, C: 100 NYHA approximately class 3–4 | 6 mo | I: 8 (100) C: 8 (100) | All-cause mortality; cardiovascular mortality; left ventricular ejection fraction |
| Gross et al. (27)* | HD | Spironolactone 50 mg twice a day | Placebo | 8 | 53±10 | 1.7 | Excluded | 7 wk | All: 8 (100) | BP |
HD, hemodialysis; I, intervention group; C, control group; TIW, three times weekly; NA, not applicable; PD, peritoneal dialysis; QOD, every other day; NYHA, New York Heart Association; *, crossover studies.
Risk of Bias and Quality Assessment
The overall study quality was moderate to high, with some potential for bias. Risk of bias was assessed on the basis of several domains, including allocation bias, performance bias, attrition bias, detection bias, and reporting bias, and the result of assessment is presented in Table 2 using version 2 of the Cochrane risk of bias tool.
Table 2.
Risk of bias assessment conducted using the Cochrane risk of bias tool of the included studies
| Author | Allocation Bias | Performance Bias | Attrition Bias | Detection Bias | Reporting Bias |
|---|---|---|---|---|---|
| Charytan et al. (13) | Low | Some concern | Low | Some concern | Low |
| Ziaee et al. (11) | Some concern | Some concern | Low | Low | Low |
| Hammer et al. (12) | Some concern | Low | Low | Some concern | Low |
| Lin et al. (14) | Low | Some concern | Some concern | Low | Low |
| Feniman-De-Stefano et al. (25) | Some concern | Low | Low | Some concern | Low |
| Yongsiri et al. (28) | Some concern | Some concern | Low | Low | Low |
| Walsh et al. (15) | Low | Low | Low | Low | Low |
| Ito et al. (17) | Low | Low | Low | Low | Low |
| Matsumoto et al. (16) | Some concern | Some concern | Low | Low | Low |
| Ni et al. (26) | Some concern | Low | Low | Some concern | Some concern |
| Taheri et al. (18) | Some concern | High | Low | Low | Some concern |
| Vukusich et al. (19) | Some concern | Low | Some concern | Low | Low |
| Taheri et al. (20) | Some concern | Some concern | Some concern | Low | Some concern |
| Gross et al. (27) | Some concern | Low | Low | High | Some concern |
Most studies did not disclose adequate details regarding sequence generation or allocation concealment. The use of other treatments, namely BP control and comedication use, was not reported clearly in some studies, thus indicating “high risk” and “some concern” in the performance bias domain. Regarding reporting bias, some studies did not report cardiac performance outcomes. In addition, the different dosages and durations of MRA treatments in the individual studies caused clinical heterogeneity between studies.
Primary Outcomes: Cardiovascular Mortality and All-Cause Mortality
Cardiovascular mortality and all-cause mortality were reported in seven (12 –16,18,20) and ten (11 –20) of the trials, respectively. Pooling data revealed a significant reduction in both cardiovascular mortality (RR, 0.41; 95% CI, 0.24 to 0.70; P=0.001) and all-cause mortality (RR, 0.44; 95% CI, 0.30 to 0.66; P<0.001) in patients receiving MRAs compared with the control group (Figure 2). Low heterogeneity was observed for both cardiovascular mortality (I 2=0%; P=0.78) and all-cause mortality (I 2=0%; P=0.75), and inspection of both of the funnel plots suggested no strong evidence of publication bias (Figure 3). No obvious relationship between treatment duration or dosage of MRA and RR reduction was observed.
Figure 2.

Forest plots of the included randomized controlled trials. Risk ratios for (A) cardiovascular mortality and (B) all-cause mortality among patients receiving MRAs compared with placebo. 95% CI, 95 confidence interval; df, degrees of freedom; M-H, Mantel–Haenszel.
Figure 3.
Funnel plots of the included randomized controlled trials. Risk ratios (RRs) for (A) cardiovascular mortality and (B) all-cause mortality among patients receiving MRAs compared with placebo.
On the basis of the GRADE assessment, the certainties of the abovementioned findings were concluded to be high, and the details of the GRADE assessment are shown in Supplemental Table 2.
Secondary Outcomes: Nonfatal Cardiovascular Events and Stroke
Nonfatal cardiovascular events were reported in four (15 –18) of the trials, in which 27 events were included, and the pooled RR was 0.51 (95% CI, 0.15 to 1.67; P=0.27). Moreover, four (13,16 –18) of the trials reported the incidence of stroke. Twenty-eight events were recorded, and pooled RR was 0.57 (95% CI, 0.27 to 1.21; P=0.14) (Figure 4).
Figure 4.

Forest plots of the included randomized controlled trials. Risk ratios for (A) nonfatal cardiovascular events and (B) stroke among patients receiving MRAs compared with placebo. 95% CI, 95% confidence interval; df, degrees of freedom; M-H, Mantel–Haenszel.
Blood Pressure, Left Ventricular Mass Index, and Left Ventricular Ejection Fraction
In the studies reporting the measurements, most of the studies provided only the pre- and post-treatment results but not the details of their interval change. Therefore, on the basis of insufficient data, we could not perform a meta-analysis without creating further bias. Instead, we performed forest plots to present the trend of BP, left ventricular mass index, and left ventricular ejection fraction at baseline and post-treatment between MRAs and control groups (Supplemental Figures 1–3), but for other measurements, only a systemic review was performed.
BP was reported in ten trials (11 –13,15,16,19,25 –28). Of these, only two (26,27) reported significant BP reduction in MRA-treated groups, whereas the other eight reported neutral effects on BP.
Left ventricular mass index was reported in four trials (12 –14,25). Among them, two reported significant improvements in MRA-treated groups (14,25), whereas the other two (12,13) reported nonsignificant results.
Left ventricular ejection fraction was reported in eight of the included trials (11 –14,17,18,20,25). Significant improvements in the MRA-treated groups were observed in five studies (11,14,17,18,20), neutral effects were found in two (12,25), and one study reported significant improvements in the control group (13).
Hyperkalemia and Serum Potassium Level
The incidence of hyperkalemia was reported in 11 of the 14 trials (12 –20,26,28), whereas the other three trials (11,25,27) only reported the changes in serum potassium level. Among the 11 studies, only seven (12,13,15,17 –19,28) were included in the final meta-analysis of the risk of hyperkalemia; three of the studies (16,20,26) were excluded for lack of a clear cut point of hyperkalemia, and one (14) was excluded for only reporting the incidence of hyperkalemia in the MRA-treated group but not in the control group. The definitions of hyperkalemia varied across studies; it was defined as potassium level of >5.5 in three (18,19,28), >6.0 in another four (12,13,15,17), and >6.5 in three (12,13,15). Thus, we conducted a forest plot with subgroups according to the cutoff values (Figure 5). Pooling data analysis revealed a nonsignificant risk increment in hyperkalemia (RR, 1.12; 95% CI, 0.91 to 1.36; P=0.29), and the overall between-study heterogeneity was low (I 2=0%; P=0.65). Moreover, no obvious subgroup difference was observed (I 2=0%; P=0.48).
Figure 5.

Forest plots of the included randomized controlled trials for hyperkalemia. This figure showsrisk ratios for overall hyperkalemia and subgroups according to the serum potassium threshold used to define hyperkalemia. 95% CI, 95% confidence interval; df, degrees of freedom; M-H, Mantel–Haenszel.
Of the included trials, 13 compared the mean change in pretreatment and post-treatment serum potassium levels between the MRAs and control groups (11 –15,17 –20,25 –28). Among them, eight trials (11 –13,18,25 –28) concluded that the changes in serum potassium levels were comparable in the two groups, and four trials (14,17,19,20) reported a significant increase in serum potassium levels compared with the control group. The last study reported mixed data (15); a significant increase in potassium levels could be observed only in patients treated with 50 mg eplerenone daily but not in those treated with lower doses.
Discussion
Our systemic review and meta-analysis revealed a high level of evidence that MRAs are associated with lower cardiovascular and all-cause mortality in patients with kidney failure treated with dialysis without significant increment in the risk of hyperkalemia. However, it should be noted that both of the mortality data were heavily influenced by just two studies of modest size, despite consistent results and large effect size. Furthermore, we failed to demonstrate a significant risk reduction of nonfatal cardiovascular events and stroke with the use of MRAs, and because of insufficient data, a meta-analysis of the cardiac function, including left ventricular mass index and left ventricular ejection fraction, could not be performed. As such, the pathogenesis and clinical benefits of treating patients with kidney failure with MRAs remain insufficient, and further studies are warranted to better support or refute the findings of our meta-analysis.
For cardiovascular mortality, the RR reduction in our meta-analysis was 59% among those treated with MRAs; surprisingly, this result is higher than that observed among patients not on dialysis with heart failure and reduced ejection fraction, in whom the use of MRAs was better validated by large RCTs and meta-analyses (7,9,29,30). It is possible that some of the benefit of MRAs actually resulted from treating heart failure because the inclusion, exclusion, and criteria for diagnosing heart failure varied considerably among studies. Nonetheless, the bias may be modest because the proportion of patients diagnosed with heart failure was small in our study.
Furthermore, we observed a 56% RR reduction of all-cause mortality in MRA-treated groups, which was comparable with that of cardiovascular mortality. It is possible that some of the effects on all-cause mortality were achieved through other mechanisms other than cardiovascular protection, which is what the majority of studies focused on. However, no such conclusion could be drawn with these results, so further research on other organ systems should be sought to better clarify the benefits of MRAs on patients with kidney failure.
One potential source of bias is that the results of our meta-analysis were primarily driven by two trials (Lin et al. [14] and Matsumoto et al. [16]) because of much larger total observed events. There are three reasons for this predominance in size for the two trials. First of all, the two trials have much larger sample sizes than others, resulting in more event numbers. The mean age for the two trials was greater than most of the rest, so the expected mortality rates are also higher. Finally, the follow-up periods for the two trials were longer, which may contribute to the larger event numbers as well. However, the heterogeneities between studies were low in the primary outcomes, namely cardiovascular and all-cause mortality, despite diverse inclusion criteria, and inspection of the funnel plots revealed no significant publication bias. Thus, it is less likely that the results we find possess major deviation from the true effects.
Nonetheless, we did not identify a risk reduction of nonfatal cardiovascular events and stroke. One limitation we observed was that the number of studies reporting either of the outcomes was much smaller than those of cardiovascular and all-cause mortality, and thus, the total patient numbers were also significantly smaller. Despite some positive trends favoring the use of MRAs that were found in the included trials, no conclusion can be drawn until further data are provided.
To better support the clinical benefits observed in our study, an improvement in the cardiac performance parameters, including left ventricular ejection fraction and left ventricular mass index, is paramount. However, the development of left ventricular hypertrophy is multifactorial (31) and includes anemia, BP control, and interdialytic weight gain; therefore, MRAs may play only a modest role in left ventricular hypertrophy among patients with kidney failure. In addition, no meta-analytic results could be generated due to limitations from the nature of the provided data, thus making the effect of MRAs on the cardiac performance indices inconclusive.
Regarding the risk of hyperkalemia, no significant difference was observed between the MRAs and control groups. This result contradicts that of a previous meta-analysis (21) that described a significantly higher risk of hyperkalemia in MRA-treated patients. However, after incorporating more recent trials (12,13), the risk of hyperkalemia was negated. One of the most important and fundamental reasons for this result is that in patients with kidney failure, most of the potassium is excreted through dialysis instead of urine (26,32). Therefore, blockage of the renin-angiotensin-aldosterone system is unlikely to result in hyperkalemia through the inhibition of excretion through the kidneys alone. As a result, we infer that MRAs have a lower effect on serum potassium concentrations in patients on dialysis than in those with CKD without the need for dialysis, and this effect may be even lower if the patient is anuric.
Some studies have recognized the colon as an important organ for potassium excretion in patients with a reduced GFR (33 –35). However, the number of related studies is extremely low, and notable heterogeneity was identified. An animal study suggested that colonic potassium secretion can be increased with upregulation with angiotensin II receptors in CKD rats (36), but the role of aldosterone in colonic potassium homeostasis is not proven. Consequently, whether MRAs cause a decline in colonic potassium secretion and, additionally, hyperkalemia in patients with kidney failure remain uncertain.
Our meta-analysis has several strengths. It is by far the largest meta-analysis on the use of MRAs in patients with kidney failure, and we demonstrated a high level of evidence that the risk of cardiovascular and all-cause mortality might be reduced by more than half while having comparable risk of hyperkalemia with control groups. However, there are still several drawbacks in our study. First of all, we failed to demonstrate improvement in nonfatal cardiovascular events, stroke, and cardiac performance parameters. Second, the dosages of MRAs and follow-up durations varied considerably among the studies, resulting in possible discrepancy in clinical benefits or side effects. Finally, most of the included studies were not designed to examine hard outcomes, and the follow-up durations were too short for hard outcomes to occur. Therefore, uncertainty still remains, and future larger trials are required to support or refute our findings before MRAs are incorporated into current treatment guidelines.
In summary, our meta-analysis suggested that MRAs might improve clinical outcomes in patients with kidney failure treated with dialysis without significant increase in the risk of hyperkalemia. However, it is still prudent to monitor patients' serum potassium levels more closely while taking these medications.
Disclosures
W.-C. Chang, T.-C. Fang, and C.-C. Kao report employment with Taipei Medical University. K.-T. Chen reports employment with Mackay Memorial Hospital, Taipei, Taiwan. M.-S. Wu reports employment with ShuangHo Hospital, Taipei Medical University and receiving honoraria from Astellas, AZ, Baxter, and Roche. All remaining authors have nothing to disclose.
Funding
The study was supported by Ministry of Science and Technology grant 107-2314-B-038-019-MY3 and Taipei Medical University grant 109TMU-TMUH-22.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Mineralocorticoid Receptor Antagonists and Cardiovascular Health with Kidney Failure,” on pages 843–845.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.15841020/-/DCSupplemental.
Supplemental Table 1. Primary search strategy in PubMed.
Supplemental Table 2. Level of evidence assessment for (A) cardiovascular mortality and (B) all-cause mortality among patients receiving mineralocorticoid receptor antagonist compared with placebo using GRACE analysis.
Supplemental Figure 1. Forest plots of mean difference in BP before and after treatment between mineralocorticoid receptor antagonists and placebo.
Supplemental Figure 2. Forest plots of mean difference in left ventricular mass index before and after treatment between mineralocorticoid receptor antagonists and placebo.
Supplemental Figure 3. Forest plots of mean difference in left ventricular ejection fraction before and after treatment between mineralocorticoid receptor antagonists and placebo.
References
- 1.United States Renal Data System : Previous ADRs. Available at: https://www.usrds.org/annual-data-report/previous-adrs/
- 2.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V: Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 385: 1975–1982, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Harnett JD, Foley RN, Kent GM, Barre PE, Murray D, Parfrey PS: Congestive heart failure in dialysis patients: Prevalence, incidence, prognosis and risk factors. Kidney Int 47: 884–890, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Charytan DM, Padera R, Helfand AM, Zeisberg M, Xu X, Liu X, Himmelfarb J, Cinelli A, Kalluri R, Zeisberg EM: Increased concentration of circulating angiogenesis and nitric oxide inhibitors induces endothelial to mesenchymal transition and myocardial fibrosis in patients with chronic kidney disease. Int J Cardiol 176: 99–109, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyubarova R, Gosmanova EO: Mineralocorticoid receptor blockade in end-stage renal disease. Curr Hypertens Rep 19: 40, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Azevedo PS, Polegato BF, Minicucci MF, Paiva SA, Zornoff LA: Cardiac remodeling: Concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq Bras Cardiol 106: 62–69, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS-HF Study Group: Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 364: 11–21, 2011. 21073363 [Google Scholar]
- 8.The RALES Investigators: Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). Am J Cardiol 78: 902–907, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J; Randomized Aldactone Evaluation Study Investigators: The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341: 709–717, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Lu R, Zhang Y, Zhu X, Fan Z, Zhu S, Cui M, Zhang Y, Tang F: Effects of mineralocorticoid receptor antagonists on left ventricular mass in chronic kidney disease patients: A systematic review and meta-analysis. Int Urol Nephrol 48: 1499–1509, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Ziaee SAR, Karvandi M, Ziaee NS, Ghozloujeh ZG, Shahrbaf MA, Roshan A: Effects of spironolactone on cardiovascular complications in hemodialysis patients of taleghani hospital during the period of 2016-2017: A randomized double-blind controlled clinical trial. Iranian Heart J 20: 45–52, 2019 [Google Scholar]
- 12.Hammer F, Malzahn U, Donhauser J, Betz C, Schneider MP, Grupp C, Pollak N, Störk S, Wanner C, Krane V; MiREnDa Study Group: A randomized controlled trial of the effect of spironolactone on left ventricular mass in hemodialysis patients. Kidney Int 95: 983–991, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Charytan DM, Himmelfarb J, Ikizler TA, Raj DS, Hsu JY, Landis JR, Anderson AH, Hung AM, Mehrotra R, Sharma S, Weiner DE, Williams M, DiCarli M, Skali H, Kimmel PL, Kliger AS, Dember LM; Hemodialysis Novel Therapies Consortium: Safety and cardiovascular efficacy of spironolactone in dialysis-dependent ESRD (SPin-D): A randomized, placebo-controlled, multiple dosage trial. Kidney Int 95: 973–982, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin C, Zhang Q, Zhang H, Lin A: Long-term effects of low-dose spironolactone on chronic dialysis patients: A randomized placebo-controlled study. J Clin Hypertens (Greenwich) 18: 121–128, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh M, Manns B, Garg AX, Bueti J, Rabbat C, Smyth A, Tyrwhitt J, Bosch J, Gao P, Devereaux PJ, Wald R: The safety of eplerenone in hemodialysis patients: A noninferiority randomized controlled trial. Clin J Am Soc Nephrol 10: 1602–1608, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto Y, Mori Y, Kageyama S, Arihara K, Sugiyama T, Ohmura H, Yakushigawa T, Sugiyama H, Shimada Y, Nojima Y, Shio N: Spironolactone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol 63: 528–536, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Ito Y, Mizuno M, Suzuki Y, Tamai H, Hiramatsu T, Ohashi H, Ito I, Kasuga H, Horie M, Maruyama S, Yuzawa Y, Matsubara T, Matsuo S; Nagoya Spiro Study Group: Long-term effects of spironolactone in peritoneal dialysis patients. J Am Soc Nephrol 25: 1094–1102, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taheri S, Mortazavi M, Pourmoghadas A, Seyrafian S, Alipour Z, Karimi S: A prospective double-blind randomized placebo-controlled clinical trial to evaluate the safety and efficacy of spironolactone in patients with advanced congestive heart failure on continuous ambulatory peritoneal dialysis. Saudi J Kidney Dis Transpl 23: 507–512, 2012 [PubMed] [Google Scholar]
- 19.Vukusich A, Kunstmann S, Varela C, Gainza D, Bravo S, Sepulveda D, Cavada G, Michea L, Marusic ET: A randomized, double-blind, placebo-controlled trial of spironolactone on carotid intima-media thickness in nondiabetic hemodialysis patients. Clin J Am Soc Nephrol 5: 1380–1387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taheri S, Mortazavi M, Shahidi S, Pourmoghadas A, Garakyaraghi M, Seirafian S, Eshaghian A, Ghassami M: Spironolactone in chronic hemodialysis patients improves cardiac function. Saudi J Kidney Dis Transpl 20: 392–397, 2009 [PubMed] [Google Scholar]
- 21.Quach K, Lvtvyn L, Baigent C, Bueti J, Garg AX, Hawley C, Haynes R, Manns B, Perkovic V, Rabbat CG, Wald R, Walsh M: The safety and efficacy of mineralocorticoid receptor antagonists in patients who require dialysis: A systematic review and meta-analysis. Am J Kidney Dis 68: 591–598, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 6: e1000097, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT: RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366: l4898, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ: GRADE guidelines. 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64: 383–394, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Feniman-De-Stefano GM, Zanati-Basan SG, De Stefano LM, Xavier PS, Castro AD, Caramori JC, Barretti P, Franco RJ, Martin LC: Spironolactone is secure and reduces left ventricular hypertrophy in hemodialysis patients. Ther Adv Cardiovasc Dis 9: 158–167, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Ni X, Zhang J, Zhang P, Wu F, Xia M, Ying G, Chen J: Effects of spironolactone on dialysis patients with refractory hypertension: A randomized controlled study. J Clin Hypertens (Greenwich) 16: 658–663, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross E, Rothstein M, Dombek S, Juknis HI: Effect of spironolactone on blood pressure and the renin-angiotensin-aldosterone system in oligo-anuric hemodialysis patients. Am J Kidney Dis 46: 94–101, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Yongsiri S, Thammakumpee J, Prongnamchai S, Tengpraettanakorn P, Chueansuwan R, Tangjaturonrasme S, Dinchuthai P: Randomized, double-blind, placebo-controlled trial of spironolactone for hypokalemia in continuous ambulatory peritoneal dialysis patients. Ther Apher Dial 19: 81–86, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Pitt B, White H, Nicolau J, Martinez F, Gheorghiade M, Aschermann M, van Veldhuisen DJ, Zannad F, Krum H, Mukherjee R, Vincent J; EPHESUS Investigators: Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol 46: 425–431, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Ezekowitz JA, McAlister FA: Aldosterone blockade and left ventricular dysfunction: A systematic review of randomized clinical trials. Eur Heart J 30: 469–477, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Di Lullo L, Gorini A, Russo D, Santoboni A, Ronco C: Left ventricular hypertrophy in chronic kidney disease patients: From pathophysiology to treatment. Cardiorenal Med 5: 254–266, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han SW, Won YW, Yi JH, Kim HJ: No impact of hyperkalaemia with renin-angiotensin system blockades in maintenance haemodialysis patients. Nephrol Dial Transplant 22: 1150–1155, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Martin RS, Panese S, Virginillo M, Gimenez M, Litardo M, Arrizurieta E, Hayslett JP: Increased secretion of potassium in the rectum of humans with chronic renal failure. Am J Kidney Dis 8: 105–110, 1986 [DOI] [PubMed] [Google Scholar]
- 34.Batlle D, Boobés K, Manjee KG: The colon as the potassium target: Entering the colonic age of hyperkalemia treatment? EBioMedicine 2: 1562–1563, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandle GI, Gaiger E, Tapster S, Goodship TH: Enhanced rectal potassium secretion in chronic renal insufficiency: Evidence for large intestinal potassium adaptation in man. Clin Sci (Lond) 71: 393–401, 1986 [DOI] [PubMed] [Google Scholar]
- 36.Hatch M, Freel RW, Vaziri ND: Local upregulation of colonic angiotensin II receptors enhances potassium excretion in chronic renal failure. Am J Physiol 274: F275–F282, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.