Visual Abstract
Keywords: ADPKD, epidemiology and outcomes, obesity, polycystic kidney disease, overweight, disease progression
Abstract
Background and objectives
On the basis of earlier observations, we evaluated the association between overweight and obesity and rapid progression of autosomal dominant polycystic kidney disease in participants in the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes (TEMPO) 3:4 trial. More importantly, we also determined whether efficacy of tolvaptan was attenuated in individuals with baseline overweight or obesity.
Design, setting, participants, & measurements
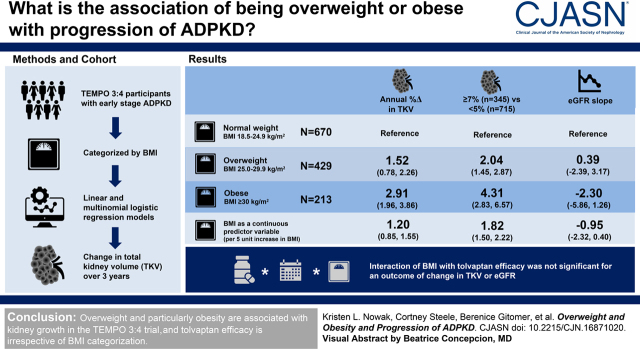
A total of 1312 study participants with relatively early-stage autosomal dominant polycystic kidney disease (mean eGFR 78±22 ml/min per 1.73 m2) who were at high risk of rapid progression were categorized by body mass index (BMI; calculated using nonkidney weight) as normal weight (18.5–24.9 kg/m2; n=670), overweight (25.0–29.9 kg/m2; n=429), or obese (≥30 kg/m2; n=213). Linear and multinomial logistic regression models were used to determine the association of baseline overweight and obesity with change in total kidney volume (TKV) over the 3-year study period.
Results
In fully adjusted models, higher BMI was associated with greater annual percent change in TKV (difference of 1.20 [95% confidence interval (95% CI), 0.85 to 1.55] per five-unit higher BMI). Overweight and obesity were associated with higher odds of annual percent change in TKV of ≥7% versus <5% (overweight: odds ratio, 2.04 [95% CI, 1.45 to 2.87]; obese: odds ratio, 4.31 [95% CI, 2.83 to 6.57] versus normal weight). eGFR decline did not differ according to BMI (fully adjusted difference in decline of −0.95 [95% CI, −2.32 to 0.40] ml/min per 1.73 m2 per year per five-unit higher BMI). The three-way interaction (treatment×time×BMI group) was not statistically significant in linear mixed models with an outcome of TKV (log-transformed estimated coefficient comparing the treatment effect for overweight versus normal weight: 0.56% [95% CI, −0.70% to 1.84%] per year; P=0.38; obese versus normal weight: 0.07% [95% CI, −1.47% to 1.63%] per year; P=0.93) or eGFR (estimated coefficient comparing overweight versus normal weight: −0.07 [95% CI, −0.95 to 0.82] ml/min per 1.73 m2 per year; P=0.88; obese versus normal weight: 0.22 [95% CI, −0.93 to 1.36] ml/min per 1.73 m2 per year; P=0.71).
Conclusions
Overweight and particularly obesity are strongly and independently associated with kidney growth, but not eGFR slope, in the TEMPO 3:4 trial, and tolvaptan efficacy is irrespective of BMI categorization.
Clinical Trial registry name and registration number:
Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes (TEMPO) 3:4, NCT00428948
Introduction
There are notable overlapping features and pathways among obesity, metabolism, and autosomal dominant polycystic kidney disease (ADPKD) (1). Studies in mouse models of ADPKD have suggested that the disease is characterized by defective glucose metabolism and metabolic reprogramming (2,3). Additionally, in several mouse models of ADPKD, mild-to-moderate food restriction slows disease progression (4,5), indicating that energy status may significantly influence ADPKD progression. However, surprisingly, the association of overweight and obesity with progression in patients with ADPKD was only recently evaluated by our group (6).
Using data from the Halt Progression of Polycystic Kidney Disease (HALT-PKD) Study A, a randomized, double-blind, placebo-controlled study in nondiabetic patients with early-stage ADPKD (Clinicaltrials.gov identifier NCT00283686) (7), we demonstrated that both overweight and obesity were strongly and independently associated with rate of progression in early-stage ADPKD (6). To extend this observation in another cohort, we sought to evaluate the association of overweight and obesity with progression in participants in the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes (TEMPO) 3:4 trial, which has a large sample size of participants with relatively early-stage ADPKD at high risk of fast progression (Clinicaltrials.gov identifier NCT00428948) (8).
Obesity can affect drug metabolism and elimination (9). Additionally, higher copeptin levels, a marker of circulating vasopressin concentrations (10), are directly associated with abdominal obesity (11). Hence, a key objective of this analysis was to determine whether efficacy of the vasopressin V2 receptor antagonist tolvaptan, which was dosed in TEMPO 3:4 irrespective of body mass, was attenuated in individuals with baseline overweight or obesity classification.
Materials and Methods
Study Design
The design of the TEMPO 3:4 trial has been described in detail previously (8,12). Briefly, the study was a phase 3, multicenter, randomized, double-blind, placebo-controlled, 3-year trial. A total of 1445 participants aged 18–50 years with ADPKD, a total kidney volume (TKV) of at least 750 ml (13 –15), and an estimated creatinine clearance of ≥60 ml/min (Cockcroft-Gault) (16) were randomized 2:1 to either the V2 receptor antagonist tolvaptan (highest tolerable of three twice-daily dose regimens) or placebo. Eligible participants were enrolled worldwide across 129 clinical sites from January 2007 through January 2009. The institutional review board or ethics committee at each site approved the protocol and all participants provided written informed consent.
The primary outcome was annual rate of change in TKV. Secondary end points included a composite of time to clinical progression (worsening kidney function, kidney pain, hypertension, and albuminuria) and rate of kidney function decline. Of the 1445 participants in the trial, three were missing data for body mass index (BMI), 51 were excluded because of a BMI classification of underweight (see below), and 79 were missing baseline or on-treatment postbaseline TKV or eGFR measurements, leaving 1312 participants for the current analysis.
Study Variables
An adjusted body weight was calculated by subtracting out kidney weight, assuming a tissue density equal to that of water (1 g/cm3) (17), thus removing the contribution of kidney size to BMI classification (6). Because liver volume was not assessed in the TEMPO 3:4 trial, it was not possible to additionally adjust BMI for liver weight. BMI was then calculated using baseline-adjusted body weight (in kilograms) divided by baseline height (in meters squared), and rounded to the nearest tenth. BMI throughout the text refers to this adjusted BMI. Using their BMI value, participants were categorized as normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), or obese (≥30 kg/m2), according to the National Heart, Lung, and Blood Institute criteria (18). A total of 51 participants had a BMI <18.5 kg/m2 (i.e., underweight) and were excluded from analyses, consistent with our prior analysis in the HALT-PKD study (6), because underweight individuals may differ physiologically from those of normal weight. Standardized magnetic resonance imaging scans of the kidneys were performed at baseline and months 12, 24, and 36 (±2 weeks), and within 2 weeks of early withdrawal for those not completing the study (8,12). The magnetic resonance imaging acquisition protocol has been described in detail previously (12). All images were sent to a central reading facility for quality control and blinded quantification of TKV (12).
Blood and urine samples were collected every 4 months. Serum creatinine level was measured centrally using the isotope dilution mass spectrometry-traceable Roche enzymatic method, and was reported to two decimal places (19). eGFR was calculated at each time point using the CKD Epidemiology Collaboration equation (19). Plasma copeptin levels, a marker of plasma vasopressin, were measured as a post hoc analysis in 1280 of the TEMPO 3:4 trial participants included in the current analysis, using an automated immunofluorescence assay (20).
Confounders related to BMI and the dependent variables were selected a priori as potential covariates, and all were measured at baseline. Demographics and clinical characteristics were assessed previously in the TEMPO 3:4 trial. Mutation analysis was performed previously on participants with an available sample for DNA analysis, which was limited to those who enrolled in the open-label extension trial TEMPO 4:4 (21), and were categorized as PKD1 or PKD2 mutations (22).
Statistical Analyses
The association of overweight or obesity with change in TKV was assessed using linear regression and multinomial logistic regression models. Participants were divided into three categories according to BMI (normal weight, overweight, and obese), with the normal weight category serving as the reference group for all analyses. In the linear regression models, the dependent variable was annual percent change in TKV calculated from the first and last available on-treatment measurements, as done in our previous analysis of HALT-PKD Study A data (6). The number of participants with TKV measurements at 12, 24, and 36 months was 1228, 1146, and 1039, respectively. We tested for a statistical interaction between BMI and sex and race/ethnicity as predictors of annual percent change in TKV. There were no significant interaction terms between BMI categories and either sex or race/ethnicity, thus stratified analyses were not performed.
In the multinomial logistic regression models, the outcome was three categories of annual TKV growth (<5%, 5%–7%, and ≥7%), also consistent with our previous analysis of HALT-PKD Study A data (6). Odds ratios were calculated with the <5% annual TKV growth, with the normal weight group serving as the reference. We additionally compared ≥5% versus <5% annual TKV growth.
In both approaches, the initial model was unadjusted, and multivariable adjusted models were performed to include age, sex, and race/ethnicity (model 1); plus randomization group, systolic BP, and serum glucose (model 2); plus baseline eGFR (CKD Epidemiology Collaboration equation) and urinary microalbumin (model 3). Plasma copeptin and mutation class were added separately to model 3, for those with the information available (n=1159 and n=724, respectively), to determine their effect as a potential mediator and confounder, respectively. We additionally considered BMI as a continuous predictor variable. As a sensitivity analysis, we also evaluated achieving a final TKV on-treatment >1500 ml as a clinically meaningful end point (13). The same covariates were included in these models as described previously.
Linear regression models were also used to evaluate the association of BMI categories with eGFR slope, which was obtained by fitting a linear regression model to all eGFR measurements from an individual participant, beginning with the end of the study drug dose escalation (titration) period (12). The number of participants with eGFR measurements at month 4, 8, 12, 16, 20, 24, 28, 32, and 36 was 1278, 1235, 1213, 1180, 1161, 1135, 1124, 1110, and 1086, respectively. The initial model was unadjusted, and multivariable adjusted models were performed using the same covariates as in the TKV analysis. There were no significant interaction terms between BMI categories and either sex or race/ethnicity, thus stratified analyses were not performed.
For the sensitivity analyses, we performed linear mixed-model analysis incorporating all available measurements of TKV and eGFR as continuous end points with BMI category as the predictor variable. TKV was log10-transformed because of a skewed distribution, and an unstructured variance covariance matrix was used. The same covariates were included as described above. The mixed-model repeated measures analyses were performed to compare BMI category by time point.
To determine whether tolvaptan treatment had a differential effect on annual percent change in TKV or annual change in eGFR according to baseline BMI, we tested the three-way interaction between treatment, time, and BMI group in a linear mixed-model analysis for each end point. We also performed a stratified analysis to compare the treatment effect of tolvaptan versus placebo on annual percent change in TKV or annual change in eGFR, according to baseline BMI category.
In all analyses, baseline characteristics were summarized by BMI categories and presented as mean±SD or median (interquartile range [IQR]) for continuous variables, and n (%) for categorical variables. In the spline plot (association of BMI with annual percent change in TKV), knots were set at 25 and 30 kg/m2. Comparisons across BMI categories were made using a chi-squared test for categorical data and ANOVA for continuous variables.
Two-tailed values of P<0.05 were considered statistically significant for all analyses. All statistical analyses were performed using SAS Enterprise Guide version 7.1.
Results
Participant Characteristics at Baseline
Among the 1312 participants included in this analysis, mean age was 39±7 years, 85% (n=1108) were White, and annual percent change in TKV was 4.2±6.2% per year. Mean BMI at baseline was 25.7±4.7 kg/m2. The median baseline TKV was 1478 (interquartile range, 1073–2034) ml. Individuals with a higher BMI were more likely to be male and White; have higher systolic BP, serum glucose, and plasma copeptin; and have a larger kidney volume and lower eGFR at baseline (Table 1). The characteristics of participants included in the current analysis (TKV or eGFR end point) were similar to the original TEMPO 3:4 study cohort (Supplemental Table 1).
Table 1.
Baseline characteristics of study participants according to body mass index category
| Variable | Normal Weight, BMI=18.5–24.9 kg/m2, n=670 | Overweight, BMI=25–29.9 kg/m2, n=429 | Obese, BMI≥30 kg/m2, n=213 |
|---|---|---|---|
| Age, yr | 39±7 | 39±7 | 39±7 |
| Sex, n (%), male | 298 (45%) | 282 (66%) | 110 (52%) |
| Race, n (%), White | 535 (80%) | 372 (87%) | 201 (94%) |
| Randomization group, % tolvaptan | 428 (64%) | 293 (68%) | 136 (64%) |
| BMI, kg/m2 | 22.3±1.7 | 27.1±1.5 | 34.0±3.7 |
| CKD-EPI eGFR, ml/min per 1.73 m2 | 80±21 | 76±21 | 74±23 |
| Systolic BP, mm Hg | 127±13 | 130±13 | 132±14 |
| Urinary microalbumin, mg/dl | 2.2 (1.1–4.9) | 2.3 (1.1–5.3) | 1.9 (1.0–5.2) |
| Serum glucose, mg/dl | 91±14 | 95±13 | 98±16 |
| TKV, ml | 1374 (1025–1882) | 1557 (1139–2177) | 1642 (1169–2167) |
| Plasma copeptin, pmol/L a | 5.5 (3.5–9.8) | 6.9 (4.1–11.3) | 8.2 (4.7–13.7) |
| Mutation class, n (%) | |||
| PKD1 | 326 (49%) | 206 (48%) | 103 (48%) |
| PKD2 | 35 (5%) | 33 (8%) | 21 (10%) |
| Missing | 309 (46%) | 190 (44%) | 89 (42%) |
Data are displayed as mean±SD, n (%), or median (interquartile range). BMI is calculated from body weight adjusted to remove the contribution of the kidneys to total weight. BMI, body mass index; CKD-EPI, CKD Epidemiology Collaboration equation; TKV, total kidney volume.
Plasma copeptin data are missing in 153 participants.
Relationship between Overweight or Obesity and Change in Total Kidney Volume
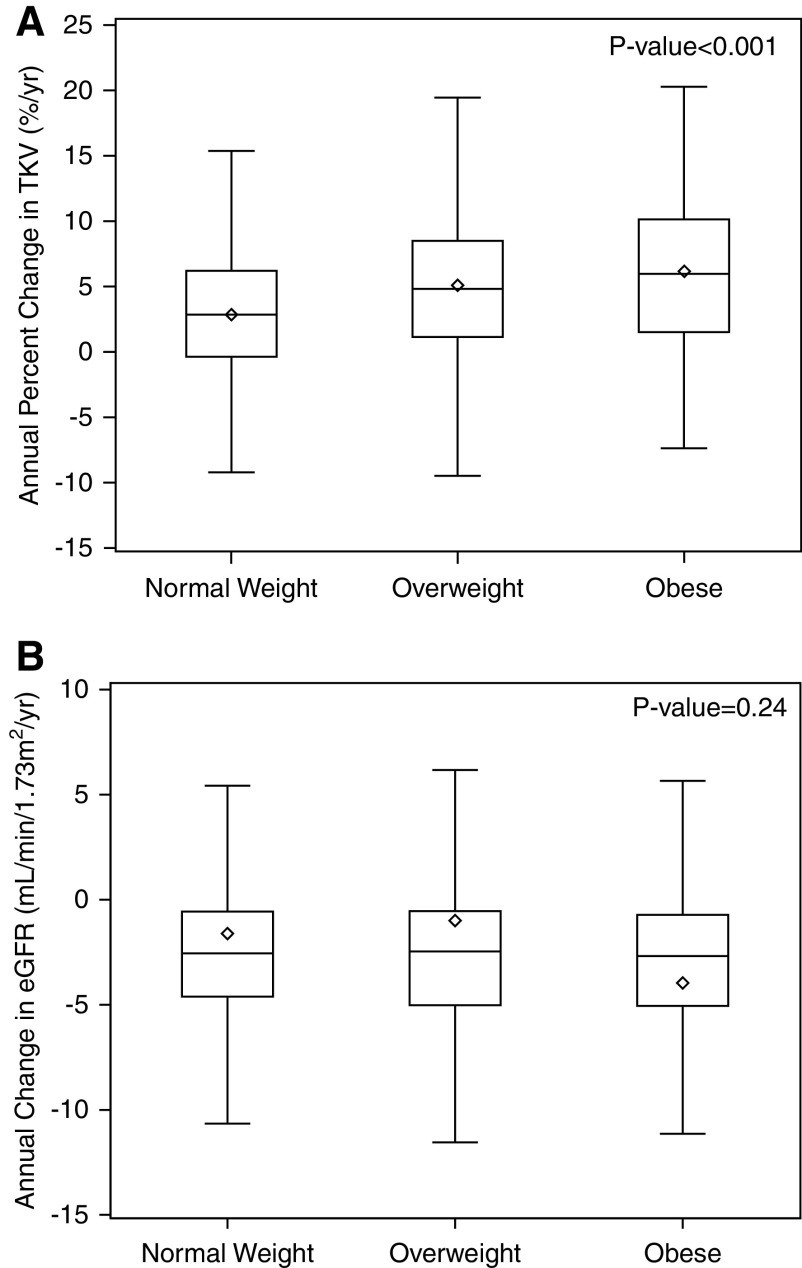
The annual percent change in TKV increased with higher BMI categories (normal weight: 2.85% [IQR, −0.40% to 6.23%] per year; overweight: 4.82% [IQR, 1.14% to 8.47%] per year; obese: 5.97% [IQR, 1.51% to 10.15%] per year; P<0.01; Figure 1A), whereas annual absolute change in eGFR did not differ across BMI categories (Figure 1B). A spline plot examining the functional form of the association between BMI and TKV is shown in Supplemental Figure 1. In both unadjusted and adjusted analyses, overweight and obesity were associated with a greater rate of change in TKV compared with the normal weight group (Table 2). A similar association was observed when BMI was considered as a continuous variable. Results were similar when either plasma copeptin or genotype were added to the fully adjusted model (both models: 1.10; 95% confidence interval [95% CI], 0.71 to 1.49; difference in annual percent change in TKV per five-unit higher BMI).
Figure 1.
Annual percent change in total kidney volume and eGFR according to baseline body mass index categories. Annual percent change in total kidney volume (TKV) is greater with overweight or obesity compared with normal weight (A), whereas annual change in eGFR does not differ (B). The bottom and top edges of the boxes indicate the interquartile range, the line represents the median, the diamond represents the mean, and the whiskers are drawn from the box to the most extreme point that is ≤1.5 times the interquartile range.
Table 2.
Associations of body mass index categories with difference in annual percent change in total kidney volume and annual change in eGFR
| Model | Normal Weight, BMI=18.5–24.9 kg/m2 | Overweight, BMI=25–29.9 kg/m2 | Obese, BMI≥30 kg/m2 | BMI as a Continuous Predictor Variable, per Five-Unit Higher BMI |
|---|---|---|---|---|
| Annual percent change in TKV | 2.85% [−0.40% to 6.23%] | 4.82% [1.14% to 8.47%] | 5.97% [1.51% to 10.15%] | |
| Unadjusted | 0 (Reference) | 2.20 [1.44 to 2.95] | 3.27 [2.31 to 4.23] | 1.40 [1.05 to 1.75] |
| Model 1 | 0 (Reference) | 1.73 [0.99 to 2.48] | 3.20 [2.27 to 4.14] | 1.30 [0.95 to 1.67] |
| Model 2 | 0 (Reference) | 1.83 [1.10 to 2.55] | 3.17 [2.24 to 4.09] | 1.35 [1.00 to 1.70] |
| Model 3 | 0 (Reference) | 1.52 [0.78 to 2.26] | 2.91 [1.96 to 3.86] | 1.20 [0.85 to 1.55] |
| eGFR slope, ml/min per 1.73 m 2 per yr | −2.55 [−4.61 to −0.56] | −2.46 [−5.02 to −0.54] | −2.68 [−5.05 to −0.72] | |
| Unadjusted | 0 (Reference) | 0.62 [−1.96 to 3.20] | −2.35 [−5.64 to 0.93] | −0.85 [−2.10 to 0.35] |
| Model 1 | 0 (Reference) | 0.77 [−1.88 to 3.40] | −2.41 [−5.73 to 0.92] | −0.85 [−2.10 to 0.40] |
| Model 2 | 0 (Reference) | 0.67 [−2.00 to 3.34] | −2.35 [−5.74 to 1.05] | −0.90 [−2.20 to 0.35] |
| Model 3 | 0 (Reference) | 0.39 [−2.39 to 3.17] | −2.30 [−5.86 to 1.26] | −0.95 [−2.32 to 0.40] |
Reference group (normal BMI) has a value of zero (annual percent change in TKV or eGFR slope). Values are presented as median (interquartile range) for annual percent change in TKV and eGFR slope values or estimate (95% confidence interval) for all models. The unadjusted model was n=1230 for TKV end point and n=1312 for eGFR end point. Model 1 is adjusted for age, sex, and race/ethnicity (n=1230 for TKV end point and n=1312 for eGFR end point). Model 2 is adjusted for model 1 plus randomization group, systolic BP, and serum glucose (n=1230 for TKV end point and n=1312 for eGFR end point). Model 3 is adjusted for model 2 plus baseline eGFR (CKD Epidemiology Collaboration equation) and urinary microalbumin (n=1168 for TKV end point and n=1247 for eGFR end point. BMI, body mass index; TKV, total kidney volume.
In the fully adjusted, multinomial logistic regression model, compared with the normal weight group, the overweight and the obese groups had a 2.04 (95% CI, 1.45 to 2.87) and 4.31 (95% CI, 2.83 to 6.57) greater odds of progressing at a rate of ≥7% per year compared with <5% per year TKV growth, respectively, with a similar association with BMI as a continuous variable (Table 3). Estimates were similar when adding plasma copeptin (overweight: 2.05 [95% CI, 1.42 to 2.97]; obese: 3.92 [95% CI, 2.49 to 6.15]) or genotype (overweight: 1.79 [95% CI, 1.14 to 2.82]; obese: 4.28 [95% CI, 2.47 to 7.39]; odds of ≥7% per year compared with <5% per year TKV growth) to the fully adjusted model.
Table 3.
Associations of body mass index categories with categories of annual percent change in total kidney volume
| End Point, Annual Percent Change in TKV | Model | 5%–7% (n=170) versus <5% (n=715) | ≥7% (n=345) versus <5% (n=715) | ≥5% (n=515) versus <5% (n=715) |
|---|---|---|---|---|
| Normal weight, BMI=18.5–24.9 kg/m2 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |
| Overweight, BMI=25–29.9 kg/m2 | Unadjusted | 1.19 [0.82 to 1.73] | 2.35 [1.74 to 3.16] | 1.84 [1.42 to 2.37] |
| Model 1 | 1.10 [0.75 to 1.61] | 2.08 [1.52 to 2.84] | 1.63 [1.25 to 2.14] | |
| Model 2 | 1.19 [0.80 to 1.76] | 2.29 [1.65 to 3.19] | 1.78 [1.34 to 2.36] | |
| Model 3 | 1.07 [0.71 to 1.61] | 2.04 [1.45 to 2.87] | 1.59 [1.19 to 2.13] | |
| Obese, BMI≥30 kg/m2 | Unadjusted | 1.14 [0.68 to 1.92] | 3.80 [2.65 to 5.43] | 2.62 [1.89 to 3.63] |
| Model 1 | 1.14 [0.68 to 1.94] | 4.13 [2.83 to 6.02] | 2.71 [1.93 to 3.80] | |
| Model 2 | 1.27 [0.74 to 2.19] | 4.62 [3.08 to 6.93] | 2.98 [2.08 to 4.27] | |
| Model 3 | 1.27 [0.72 to 2.22] | 4.31 [2.83 to 6.57] | 2.84 [1.95 to 4.13] | |
| BMI as a continuous predictor variable, per five-unit higher BMI | Unadjusted | 1.16 [0.97 to 1.14] | 1.74 [1.53 to 2.01] | 1.57 [1.42 to 1.73] |
| Model 1 | 1.16 [0.96 to 1.41] | 1.83 [1.54 to 2.08] | 1.57 [1.42 to 1.73] | |
| Model 2 | 1.22 [1.00 to 1.49] | 1.92 [1.57 to 2.33] | 1.65 [1.49 to 1.82] | |
| Model 3 | 1.22 [1.00 to 1.49] | 1.82 [1.50 to 2.22] | 1.57 [1.29 to 1.91] |
Reference group is an odds ratio of 1. Values are presented as odds ratios (95% confidence intervals). The unadjusted model was n=1230. Model 1 was adjusted for age, sex, and race/ethnicity (n=1230). Model 2 was adjusted for model 1 plus randomization group, systolic BP, and serum glucose (n=1230). Model 3 was adjusted for model 2 plus baseline eGFR (CKD Epidemiology Collaboration equation) and urinary microalbumin (n=1168 [n=680 for <5%, n=158 for 5%–7%, and n=330 for ≥7% annual percent change in total kidney volume]). TKV, total kidney volume; BMI, body mass index.
For the sensitivity analysis, we also considered a final TKV end point of >1500 ml as clinically meaningful. In the fully adjusted model, the odds of reaching this end point was significantly greater in both the obese group (odds ratio, 1.86; 95% CI, 1.26 to 2.75) and the overweight group (odds ratio, 1.36; 95% CI, 1.01 to 1.83) compared with the normal weight group.
Finally, in a sensitivity analysis (a linear mixed model incorporating all available time points where TKV was measured), there was a significant BMI×time interaction (P<0.01 for both overweight and obese), consistent with the results of the primary analysis. Compared with the normal weight group using mixed-model repeated measures analysis, percent change in TKV from baseline was greater in the overweight group by 2.23% (95% CI, 0.89% to 3.58%) at month 12, 3.58% (95% CI, 1.73% to 5.43%) at month 24, and 5.87% (95% CI, 3.39% to 8.35%) at month 36. Percent change in TKV from baseline was greater in the obese group by 3.07% (95% CI, 1.34% to 4.79%) at month 12, 5.94% (95% CI, 3.58% to 8.31%) at month 24, and 9.08% (95% CI, 5.91% to 12.26%) at month 36.
Relationship between Overweight and Obesity and eGFR Slope
Annual decline in eGFR (post-titration) was −2.55 (IQR, −4.82 to −0.59) ml/min per 1.73 m2 per year. The annual change in the reciprocal of serum creatinine level (1/serum creatinine) and annual percent change in eGFR according to BMI category were −2.5 (IQR, −4.9 to 0) [mg/ml]−1/yr and −3.1 [IQR, −6.5 to 0.2] %/yr for normal weight; −2.2 [IQR, −5.2 to 0] [mg/ml]−1/yr and −3.6 [IQR, −7.5 to 0.3] %/yr for overweight; and −2.4 [IQR, −5.3 to −0.2] [mg/ml]−1/yr and −3.8 [IQR, −8.6 to −0.4] %/yr for obese (P=0.33 and P=0.04, respectively). In the fully adjusted linear regression model incorporating all available postdrug-titration measurements, decline in eGFR did not differ according to BMI group or with BMI as a continuous variable (Table 2). Results remained nonsignificant when plasma copeptin or genotype were added to the fully adjusted model (copeptin: −1.10 [95% CI, −2.57 to 0.37]; genotype: −0.10 [95% CI, −0.39 to 0.19]; both difference in annual change in eGFR per five-unit higher BMI).
In a sensitivity analysis (a linear mixed-effect model incorporating all available time points where eGFR was measured), the BMI group×time interaction was not statistically significant (P=0.09 for the overweight group; P=0.18 for the obese group). Compared with the normal weight group, using mixed-model repeated measures analysis, change in eGFR from baseline did not differ in the overweight group (−1.46 [95% CI, −3.19 to 0.28] ml/min per 1.73 m2 at month 32 and −1.72 [95% CI, −3.56 to 0.11] ml/min per 1.73 m2 at month 36) or obese group (−1.73 [95% CI, −3.94 to 0.48] ml/min per 1.73 m2 at month 32 and −1.03 [95% CI, −3.38 to 1.31] ml/min per 1.73 m2 at month 36).
The Interaction of Overweight or Obesity with Tolvaptan Efficacy
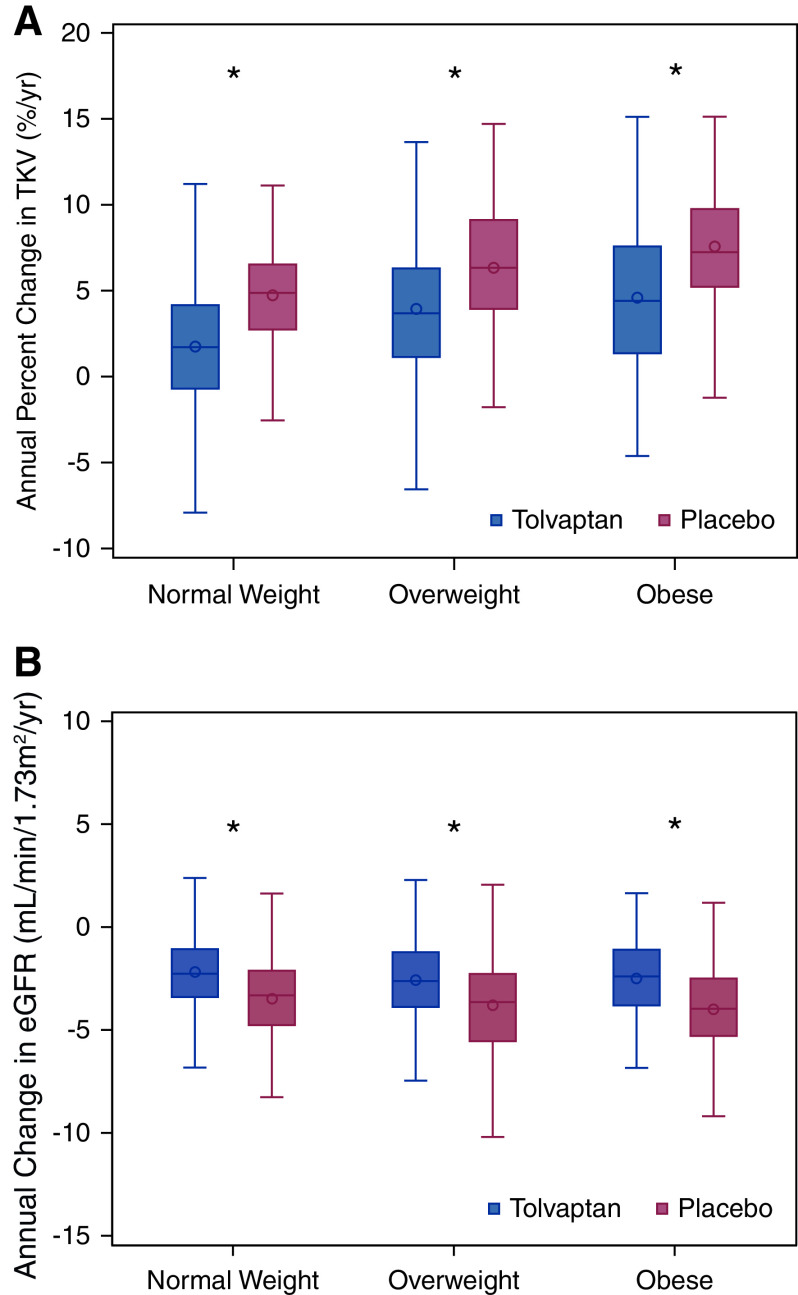
The three-way interaction among treatment×time×BMI group was not statistically significant in linear mixed models with either an outcome of TKV (log-transformed estimated coefficient comparing the treatment difference of overweight to normal weight: 0.56% [95% CI, −0.70% to 1.84%] per year; P=0.38; comparing the treatment difference of obese to normal weight: 0.07% [95% CI, −1.47% to 1.63%] per year; P=0.93) or eGFR (estimated coefficient comparing the treatment difference of overweight to normal weight: −0.07 [95% CI, −0.95 to 0.82] ml/min per 1.73 m2 per year; P=0.88; comparing the treatment difference of obese to normal weight: 0.22 [95% CI, −0.93 to 1.36] ml/min per 1.73 m2 per year; P=0.71). The effect of tolvaptan on both change in TKV and eGFR over the study duration, stratified by BMI group, is shown in Figure 2 and Supplemental Table 2. Tolvaptan had comparable efficacy irrespective of baseline BMI classification.
Figure 2.
Efficacy of tolvaptan is similar across categories of baseline body mass index category. Effect of tolvaptan (versus placebo) on (A) annual change in TKV and (B) annual change in eGFR as calculated across the entire study duration was similar in individuals with overweight and obesity compared with normal weight categorization at baseline. The bottom and top edges of the boxes indicate the interquartile range, the line represents the median, the circle represents the mean, and the whiskers are drawn from the box to the most extreme point that is ≤1.5 times the interquartile range. *P<0.01 versus placebo.
Discussion
We have confirmed our earlier observation from the HALT-PKD Study A that overweight and obesity are strongly associated with a faster rate of kidney growth, as measured by annual change in percent TKV, in early-stage ADPKD. In participants with relatively early-stage ADPKD in the TEMPO 3:4 trial who were at high risk of progression, overweight or obesity was associated with approximately 2.5 times greater adjusted odds of progressing an annual rate of change in TKV of ≥7% compared with <5% compared with normal weight individuals. The annual percent increase in TKV in obese individuals was nearly double that of individuals of normal weight. As in our prior analysis, this association is not a consequence of larger baseline kidney size, as an adjusted BMI was calculated to remove the contribution of the kidneys to overall body weight. Additionally, baseline TKV was included in all fully adjusted models.
Unlike in our prior analysis in the HALT-PKD Study A, there was no association of BMI with decline in eGFR in the TEMPO 3:4 study cohort. This may be because of several reasons. The association of BMI with kidney function decline was weaker than the association with change in height-adjusted TKV is HALT-PKD Study A, consistent with the fact that HALT-PKD Study A enrolled a cohort with early-stage disease, with relatively preserved kidney function. Participants in the TEMPO 3:4 trial also had relatively early-stage ADPKD. Additionally, the TEMPO 3:4 study cohort had a slightly lower baseline BMI and a slightly lesser prevalence of obesity than the HALT-PKD Study A cohort (7), possibly driven in part by a greater frequency of Asian participants. Finally, the TEMPO 3:4 trial was a shorter duration (3 years) than the HALT-PKD study (5 years), which may have limited the ability to observe group differences in decline in kidney function.
We extended our previous analysis by also examining the influence of overweight and obesity on the efficacy of tolvaptan. In contrast to our hypothesis that a greater BMI would decrease the efficacy of tolvaptan, annual percent change in TKV and decline in eGFR were significantly different with tolvaptan as compared with placebo in all three BMI groups. Additionally, the three-way interaction among BMI, time, and randomization group was not statistically significant. We conclude that the efficacy of tolvaptan is independent of BMI.
Common mechanistic pathways are shared among obesity, metabolism, and ADPKD. Overnutrition and obesity can activate the mTOR complex 1 and its downstream target S6 kinase (S6K) via PI3K/Akt, stimulate IGF, and suppress AMP-activated protein kinase (AMPK) (23 –25). In contrast, caloric restriction represses mTOR via AMPK activation in the presence of low glucose, high AMP/ATP ratios, and decreased amino acids (26,27). The mTOR/S6K pathway is also critical to ADPKD progression, promoting cystic epithelial hyperproliferation (28,29). Likewise, AMPK activity regulates secretion of cyst fluid in ADPKD (30,31), in addition to mTOR signaling (32,33). Notably, mild-to-moderate food restriction slows ADPKD progression in multiple rodent models of disease, mediated in part by suppression of mTOR and activation AMPK signaling (4,5). Finally, we observed no appreciable change in the association of BMI with kidney growth when adjusting for plasma copeptin level, suggesting that copeptin is not a major mediating factor.
The major strength of our study is that we extended our prior observation from the HALT-PKD Study A in a large cohort of participants from the TEMPO 3:4 trial and determined that there was no effect of overweight and obesity on efficacy of tolvaptan, which is a clinically important question given recent US Food and Drug Administration approval. Three years of longitudinal data were available, with covariates well-characterized as part of the clinical trial. There are several notable limitations. These results are associative rather than causal, and there may still be residual confounding in our analyses. Liver volume was not assessed in the TEMPO 3:4 trial, thus we could adjust our BMI calculations only for the contribution of kidney and not liver weight. Additionally, genotype, which was assessed as part of the TEMPO 4:4 trial, was available in only approximately half of this cohort, and data on smoking status and physical activity were not collected in TEMPO 3:4. Although clinically ubiquitous, BMI has inherent limitations and does not necessarily reflect adiposity. Finally, there were too few participants in the underweight BMI category to include this group in an appropriately powered analysis.
Our findings provide mounting support that overweight and obesity are important modifiable factors influencing rate of progression in patients with ADPKD. It remains unknown if weight loss may slow progression in this population. A current, ongoing clinical trial will provide initial insight into this question (Clinicaltrials.gov identifier NCT03342742). Tolvaptan had similar efficacy regardless of baseline BMI. A compelling future direction is to evaluate whether tolvaptan and weight loss may have additive effects in individuals with ADPKD who are overweight or obese.
Disclosures
M.B. Chonchol reports employment with University of Colorado Denver; consultancy agreements with Amgen, Corvidia Therapeutics, Otsuka Pharmaceutical, Reata, Tricidia, and Vifor; research funding from Corvidia Therapeutics, Otsuka Pharmaceutical, Reata, and Sanofi; honoraria from Amgen, Corvidia Therapeutics, Reata, Tricidia, and Vifor; and serving as a Deputy Editor of CJASN. B. Gitomer reports employment with University of Colorado Division of Renal Diseases and Hypertension; and serving on the editorial board of CJASN and as a PKD Foundation member of the Scientific Advisory Council. K. L. Nowak reports employment with University of Colorado Anschutz Medical Campus; research funding from Corvidia Therapeutics, Otsuka Pharmaceutical Development & Commercialization (data analysis), and Verdure Sciences; and interacting with the PKD Foundation. J. Ouyang and W. Wang report employment with Otsuka Pharmaceutical Development & Commercialization. The remaining author has nothing to disclose.
Funding
K.L. Nowak is supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants K01 DK103678 and R03 DK103678, PKD Foundation grant 241G20a, Baltimore PKD Research and Clinical Core Center Pilot and Feasibility Program grant P30DK090868, and the Zell Family Foundation (funding to the University of Colorado). C. Steele is supported by NIDDK grant 5T32DK007135-46.
Supplementary Material
Acknowledgments
This was an investigator-initiated study completed in collaboration with Otsuka Pharmaceutical Development & Commercialization. The statistical analysis plan was developed by the University of Colorado Anschutz Medical Campus investigators, and the manuscript was also written by the investigators. Otsuka Biostatistics provided feedback on the statistical analysis plan, completed the subsequent programming and analyses, and reviewed the manuscript. Data validation was outsourced to WuXi AppTec.
The funding agencies had no direct role in the conduct of the study; the collection, management, analyses, and interpretation of the data; or preparation and approval of the manuscript.
Because Dr. Michel B. Chonchol is a Deputy Editor of CJASN, he was not involved in the peer review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “To Add Weight to Overweight,” on pages 850–852.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.16871020/-/DCSupplemental.
Supplemental Table 1. Baseline characteristics of study participants from the TEMPO 3:4 trial, according to body mass index category.
Supplemental Table 2. The interaction of overweight or obesity with tolvaptan efficacy.
Supplemental Figure 1. Spline plot of the association of body mass index with annual percent change in total kidney volume.
References
- 1.Nowak KL, Hopp K: Metabolic reprogramming in autosomal dominant polycystic kidney disease: Evidence and therapeutic potential. Clin J Am Soc Nephrol 15: 577–584, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe I, Chiaravalli M, Mannella V, Ulisse V, Quilici G, Pema M, Song XW, Xu H, Mari S, Qian F, Pei Y, Musco G, Boletta A: Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med 19: 488–493, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riwanto M, Kapoor S, Rodriguez D, Edenhofer I, Segerer S, Wüthrich RP: Inhibition of aerobic glycolysis attenuates disease progression in polycystic kidney disease. PLoS One 11: e0146654, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warner G, Hein KZ, Nin V, Edwards M, Chini CC, Hopp K, Harris PC, Torres VE, Chini EN: Food restriction ameliorates the development of polycystic kidney disease. J Am Soc Nephrol 27: 1437–1447, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kipp KR, Rezaei M, Lin L, Dewey EC, Weimbs T: A mild reduction of food intake slows disease progression in an orthologous mouse model of polycystic kidney disease. Am J Physiol Renal Physiol 310: F726–F731, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nowak KL, You Z, Gitomer B, Brosnahan G, Torres VE, Chapman AB, Perrone RD, Steinman TI, Abebe KZ, Rahbari-Oskoui FF, Yu ASL, Harris PC, Bae KT, Hogan M, Miskulin D, Chonchol M: Overweight and obesity are predictors of progression in early autosomal dominant polycystic kidney disease. J Am Soc Nephrol 29: 571–578, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrier RW, Abebe KZ, Perrone RD, Torres VE, Braun WE, Steinman TI, Winklhofer FT, Brosnahan G, Czarnecki PG, Hogan MC, Miskulin DC, Rahbari-Oskoui FF, Grantham JJ, Harris PC, Flessner MF, Bae KT, Moore CG, Chapman AB; HALT-PKD Trial Investigators: Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med 371: 2255–2266, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS; TEMPO 3:4 Trial Investigators: Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 23121377 [Google Scholar]
- 9.Brill MJ, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CA: Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet 51: 277–304, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Lewandowski KC, Brabant G: Potential clinical utility of copeptin (C-terminal provasopressin) measurements in clinical medicine. Exp Clin Endocrinol Diabetes 124: 173–177, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Enhörning S, Bankir L, Bouby N, Struck J, Hedblad B, Persson M, Morgenthaler NG, Nilsson PM, Melander O: Copeptin, a marker of vasopressin, in abdominal obesity, diabetes and microalbuminuria: The prospective Malmö Diet and Cancer Study cardiovascular cohort. Int J Obes 37: 598–603, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Torres VE, Meijer E, Bae KT, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang JJ, Czerwiec FS: Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes) 3-4 study. Am J Kidney Dis 57: 692–699, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP; CRISP Investigators: Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ: Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 479–486, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM: Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343: 824–827, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 17.Wallace DP, Hou YP, Huang ZL, Nivens E, Savinkova L, Yamaguchi T, Bilgen M: Tracking kidney volume in mice with polycystic kidney disease by magnetic resonance imaging. Kidney Int 73: 778–781, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults–the evidence report. National Institutes of Health [published correction appears in Obes Res 6: 464, 1998]. Obes Res 6[Supp 2]: 51S–209S, 1998 [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration: Using standardized serum creatinine values in the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate [published correction appears in Ann Intern Med 149: 519, 2008]. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Gansevoort RT, van Gastel MDA, Chapman AB, Blais JD, Czerwiec FS, Higashihara E, Lee J, Ouyang J, Perrone RD, Stade K, Torres VE, Devuyst O; TEMPO 3:4 Investigators: Plasma copeptin levels predict disease progression and tolvaptan efficacy in autosomal dominant polycystic kidney disease. Kidney Int 96: 159–169, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Dandurand A, Ouyang J, Czerwiec FS, Blais JD; TEMPO 4:4 Trial Investigators: Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: The TEMPO 4:4 trial. Nephrol Dial Transplant 33: 477–489, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornec-Le Gall E, Blais JD, Irazabal MV, Devuyst O, Gansevoort RT, Perrone RD, Chapman AB, Czerwiec FS, Ouyang J, Heyer CM, Senum SR, Le Meur Y, Torres VE, Harris PC: Can we further enrich autosomal dominant polycystic kidney disease clinical trials for rapidly progressive patients? Application of the PROPKD score in the TEMPO trial. Nephrol Dial Transplant 33: 645–652, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J: Multiple signal pathways in obesity-associated cancer. Obes Rev 12: 1063–1070, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Moore T, Beltran L, Carbajal S, Strom S, Traag J, Hursting SD, DiGiovanni J: Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res (Phila) 1: 65–76, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Dann SG, Selvaraj A, Thomas G: mTOR complex1-S6K1 signaling: At the crossroads of obesity, diabetes and cancer. Trends Mol Med 13: 252–259, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Cantó C, Auwerx J: Calorie restriction: Is AMPK a key sensor and effector? Physiology (Bethesda) 26: 214–224, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia G, Aroor AR, Martinez-Lemus LA, Sowers JR: Overnutrition, mTOR signaling, and cardiovascular diseases. Am J Physiol Regul Integr Comp Physiol 307: R1198–R1206, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibraghimov-Beskrovnaya O, Natoli TA: mTOR signaling in polycystic kidney disease. Trends Mol Med 17: 625–633, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Laplante M, Sabatini DM: mTOR signaling in growth control and disease. Cell 149: 274–293, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK: Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest 105: 1711–1721, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King JD Jr, Fitch AC, Lee JK, McCane JE, Mak DO, Foskett JK, Hallows KR: AMP-activated protein kinase phosphorylation of the R domain inhibits PKA stimulation of CFTR. Am J Physiol Cell Physiol 297: C94–C101, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ: AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall MN: mTOR-what does it do? Transplant Proc 40[Suppl]: S5–S8, 2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.