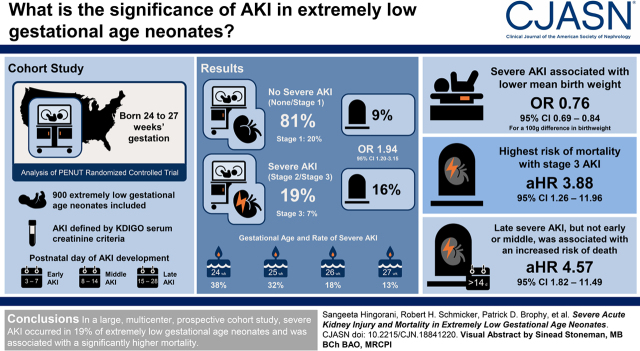
Visual Abstract
Keywords: acute kidney injury, pediatric nephrology, mortality, neonatal, gestational age
Abstract
Background and objectives
AKI is associated with poor short- and long-term outcomes. Questions remain about the frequency and timing of AKI, and whether AKI is a cause of death in extremely low gestational age neonates.
Design, setting, participants, & measurements
The Recombinant Erythropoietin for Protection of Infant Kidney Disease Study examines the kidney outcomes of extremely low gestational age neonates enrolled in the Preterm Epo Neuroprotection study, a randomized, placebo-controlled trial of recombinant human erythropoietin. We included 900 of 941 patients enrolled in Preterm Epo Neuroprotection. Baseline characteristics were compared by primary exposure (severe AKI versus none/stage 1 AKI) using unadjusted logistic regression models. Cox regression models estimated the relationship between severe AKI and death after adjustment for potential confounders. Time-dependent AKI was modeled as a binary outcome and a categorical variable by stage of AKI. We fit Cox models using time-dependent AKI status lagged by <7 days before death. Landmark analyses examined the relationship of death with development of severe AKI.
Results
Severe AKI occurred in 168 of 900 (19%, 95% confidence interval, 17% to 20%) neonates, and stage 3 AKI occurred in 60 (7%, 95% confidence interval, 5% to 8%). Stage 3 AKI occurring 7 days before death (hazard ratio, 3.88; 95% confidence interval, 1.26 to 11.96), intraventricular hemorrhage (hazard ratio, 2.01; 95% confidence interval, 1.01 to 3.99) and sepsis (hazard ratio, 2.85; 95% confidence interval, 1.12 to 7.22) were all independently associated with death. Severe AKI occurring 7 days before death (hazard ratio, 2.21; 95% confidence interval, 0.92 to 5.26) was associated with death but not statistically significant. In a landmark analysis, after adjusting for potential confounders, late (after day 14 and before day 28) severe AKI was strongly associated with higher hazard of death (hazard ratio, 4.57; 95% confidence interval, 1.82 to 11.5).
Conclusions
Severe AKI occurs frequently in extremely low gestational age neonates. Stage 3 AKI is associated with mortality, and this association is present 7 days before death.
Introduction
Approximately 15 million babies are born prematurely worldwide each year (1). In the United States, 40,000 neonates are born at less than 28 weeks of gestation annually. Premature infants are at increased risk for AKI and its consequences during their neonatal intensive care unit (NICU) course.
The nephrology and critical care communities have shown that AKI is associated with poor short- and long-term outcomes, in critically ill children (2–4) and adults (5–9). Although investigations of neonatal AKI are less common, approximately 18%–60% of extremely low gestational age neonates (10–12) and very low birthweight neonates (<1500 gm) develop AKI within the first 2 weeks of life (10–14). The variation in prevalence is likely due to differing AKI definitions, the postnatal day chosen for baseline serum creatinine, and variation in the number and timing of serum creatinine measurements. In addition, whether AKI contributes to death or merely a reflection of the severity of illness of the premature neonate and a consequence of impending death has not been evaluated.
To improve our understanding of the association between AKI and mortality, we evaluated unique high-quality data captured as part of a prospective, multi center trial in extremely low gestational age neonates. First, we evaluated the association between developing early (days 3–7), middle (days 8–14), and late (days 15 to discharge or 44 weeks) AKI and subsequent death in extremely low gestational age neonates. Second, we evaluated the independent association between severe AKI and death after controlling for numerous potential confounders. Finally, we characterized associations between mortality and AKI assessed daily for 7 days before the at-risk day to elucidate whether AKI preceded mortality by enough time to be a potential contributing cause of death.
Materials and Methods
The Preterm Epo Neuroprotection (PENUT) Trial is a phase 3 randomized, placebo-controlled, double-masked study of recombinant human erythropoietin neuroprotection in an extremely low gestational age neonate (ELGAN) population. The study design has been described elsewhere (15, 16). Briefly, patients were eligible if born between 24–0/7 and 27–6/7 weeks’ gestation at a PENUT study site and <24 hours of age. Patients were excluded on the basis of known major life-threatening anomalies, chromosomal anomalies, disseminated intravascular coagulopathy, twin-twin transfusion such that one twin was not eligible due to polycythemia or hydrops, hematocrit >65%, hydrops fetalis, or known congenital infection. Additional protocol details are published elsewhere (15, 16). Parental consent was obtained prenatally or postnatally, as permitted by each institutional review board. The Recombinant Erythropoietin for Protection of Infant Renal Disease Study is a National Institute of Diabetes and Digestive and Kidney Diseases–funded ancillary study (R01DK103608) to the PENUT trial (U01NS077955 and U01NS077953).
Participants
The participants were neonates enrolled in PENUT, with additional exclusion of those who died within the first 3 days of life (n=13) and those without any serum creatinine measures (n=23).
Ascertainment of AKI Status
We have reported detailed analysis of the prevalence of neonatal AKI in this cohort (17). Serum-creatinine measurements collected as part of standard clinical care at each site were used for AKI determination. Each NICU measured serum creatinine according to their institutional guidelines, using the local laboratory methodology available (11 Jaffe, eight enzymatic). Daily AKI stages were determined by the serum creatinine–based Kidney Diseases Improving Global Outcomes definition; stage 1 is defined as a 1.5–1.9× increase or an increase of 0.3 within 48 hours from baseline creatinine, stage 2 is 2.0–2.9× increase, and stage 3 is ≥3× baseline or serum creatinine ≥2.5 mg/dl. For AKI determination, baseline was the lowest serum creatinine at any point before the date of diagnosis of AKI stage. Individual creatinine measurements had to be >0.5 to have its ratio against baseline considered in the AKI calculation. To account for creatinine levels on postnatal days 0–2 that may represent that of the mother, we began the AKI calculation on postnatal day 3. We defined severe AKI as stage 2 or 3. To further characterize the timing of AKI, we defined early AKI as AKI that occurred between days 3 and 7, middle if between days 8 and 14, and late that occurred after day 15–28. Urine output was not used to define AKI.
Baseline characteristics were compared by severe AKI status (maximum stage 2 or 3 versus none or stage 1). Predefined serious adverse events recorded per the PENUT protocol included severe necrotizing enterocolitis defined as either stage 2b or 3, severe intraventricular hemorrhage defined as grade III or IV hemorrhage, and sepsis defined as culture-proven and requiring BP support shock or new respiratory support.
Statistical Analyses
Baseline characteristics were compared by the primary exposure (severe AKI versus none/stage 1 AKI occurring anytime during follow-up) using logistic regression models. Our analysis focuses on the maximum AKI stage observed; for each day, t, our predictor of interest is the maximum of AKI stages achieved over the time period from day 3 through day t, and can be viewed as characterizing the worst AKI stage experienced in the past. Unadjusted estimates of the relationship between time-dependent severe AKI and risk of death were calculated in two ways: first via crude incidence calculations where the number of deaths per AKI stage is divided by the total time (person-years) in each AKI stage, and second via Cox regression with AKI history as a time-varying covariate. We used multivariable Cox regression with AKI stage as a time-varying covariate, mother as a clustering variable to account for sibship, and adjusted for the following baseline characteristics: gestational age in weeks (24–27); sex; maternal race and ethnicity; size for gestational age (small, appropriate, large); Apgar score at 5 minutes; intubation (yes, no), chest compressions (yes, no) and epinephrine (yes, no) given in delivery room. We also included necrotizing enterocolitis, intraventricular hemorrhage, and sepsis as time-varying covariates during the NICU course and before the development of AKI. These variables were chosen a priori as potential confounders. We estimated the regression models for our primary exposure (severe AKI versus none or stage 1) and our secondary exposure for categorical AKI (none versus 1, versus 2, versus 3).
To assess the potential association between the timing (early, middle, late) of AKI development and mortality, we first used Kaplan-Meier methods. To summarize survival using Kaplan-Meier methods, we performed a series of landmark analyses (18) to characterize death the rates after days 7, 14, and 28. For each landmark time, we included only subjects that survived until the specified day. Each landmark time classifies subjects into fixed groups on the basis of their AKI history before the landmark time, and then looks prospectively at the time until death for these fixed groups. Landmark analysis uses a fixed “baseline” covariate status with the baseline time updated using longitudinal information before each updated baseline or landmark time. Subjects were classified as none/stage 1 AKI if the neonate did not have a severe AKI classification before the landmark time. In addition, for day 14 and 28 analyses, we focus on recent severe AKI occurring during days 8–14 and 15–28, respectively, and exclude subjects with severe AKI before the start of each landmark period. For this analysis, we limited timing of AKI up to postnatal day 28.
To examine potential predictive effects of an increase in AKI stage, we fit multiple Cox regression models using AKI status lagged by 1–7 days to ensure change in AKI status preceded mortality by enough time to be a potential cause rather than a consequence of death. We assessed associations between AKI history measured through each day t, and risk of mortality 3 days later (day t+3), or in a parallel analysis on mortality 7 days later (day t+7), using survival analysis with lagged time-dependent covariates. These analyses ensure AKI transitions precede mortality by enough time to minimize the potential for reverse causality. Our primary model conservatively includes a 7-day lag, which represents the scenario where AKI stage at any given day is used to predict risk of mortality 7 days in the future. Such a lagged model ensures the participant’s AKI occurred days before death rather than including in the association severe AKI that develops on the same day of death, which we presume is not due to the AKI but rather part of the process of death. Analyses were done evaluating stages of AKI as a binary variable (severe AKI versus none/stage 1 AKI) and a categorical variable with multiple levels (none, stage 1, stage 2, and stage 3). In addition, we evaluated lag times of 0, -1, -2, -3, -4, -5, -6, and -7 days before death.
Before removing the 23 neonates who were not able to be classified into an AKI stage (given they did not have any serum creatinine data available), we performed sensitivity analyses (data not shown) and found the results of our analyses did not change when they were included as having none/stage 1 AKI.
We did not adjust for treatment arm in this study, given previously published studies in this cohort demonstrating that recombinant human erythropoietin did not affect mortality, neurocognitive outcomes at 2 years, or AKI (16, 26).
Analyses were performed using R v3.5.1 (R Core Stats Team, 2008).
Results
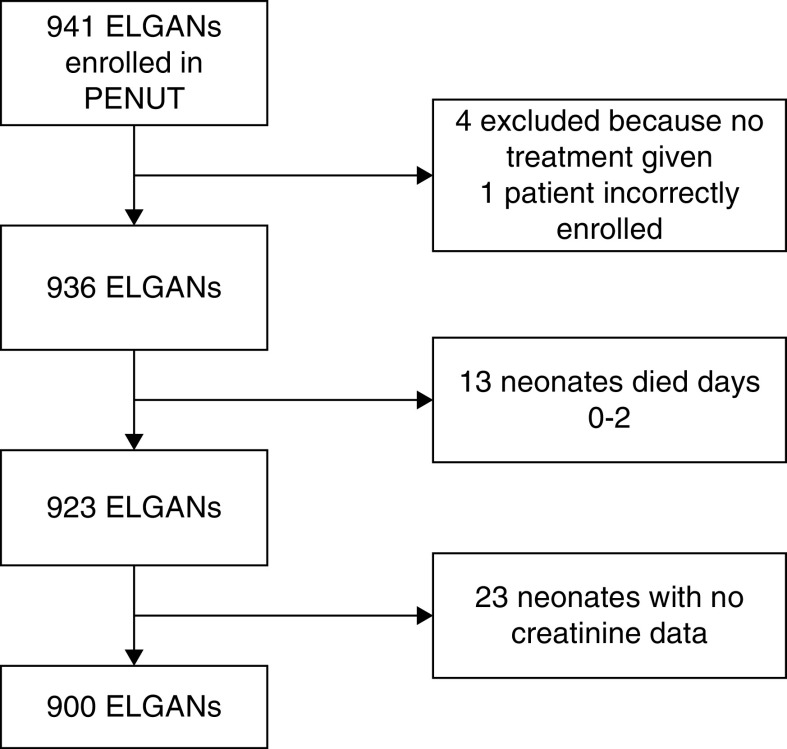
Of the 941 neonates enrolled in the PENUT Trial, 900 patients were included in this analysis, as shown in the Consort diagram (Figure 1). We did not have final hospital discharge outcome data on two babies, and two were withdrawn from the study before hospital discharge. These four babies were included as censored data in our time to death analysis.
Figure 1.
Consort diagram of study cohort. ELGAN, extremely low gestational age neonate.
Demographic and clinical characteristics are shown in Table 1 by severe AKI at any time versus none/stage 1 AKI. Approximately 52% of the cohort was male. Severe AKI developed in 168 neonates (19%, 95% confidence interval [95% CI], 17% to 20%). Severe AKI was more common in more premature infants (24 weeks, 38%; 25 weeks, 32%; 26 weeks, 18%; and 27 weeks, 13%; odds ratio [OR], 0.65; 95% CI, 0.55 to 0.76). Mean birthweight was lower in neonates who developed severe AKI (725.1 g), compared with those with none/stage 1 AKI (814.6 g), OR, 0.76, 95% CI, 0.69 to 0.84 for a 100-U difference. Approximately 15% of neonates with severe AKI were small for gestational age compared with only 8% among those who were not small for gestational age. Maternal characteristics were similar between those with versus without severe AKI. Neonates with severe AKI were more often intubated (91% versus 79%, OR, 2.74; 95% CI, 1.52 to 4.93), had necrotizing enterocolitis (any stage; 23% versus 8%; OR, 3.33; 95% CI, 2.13 to 5.21), or had a patent ductus arteriosus (55% versus 40%, OR, 1.84; 95% CI, 1.30 to 2.59).
Table 1.
Demographic and clinical characteristics for extremely low gestational age neonates with and without AKI (any time up to discharge)
| Characteristics | No Severe AKI (None/Stage 1) |
Severe AKI (Stages 2–3) |
Total |
|---|---|---|---|
| N | 732 | 168 | 900 |
| Male, n (%) | 370 (51) | 95 (57) | 465 (52) |
| GA, n (%) | |||
| 24 wk | 162 (22) | 63 (38) | 225 (25) |
| 25 wk | 183 (25) | 53 (32) | 236 (26) |
| 26 wk | 186 (25) | 30 (18) | 216 (24) |
| 27 wk | 201 (27) | 22 (13) | 223 (25) |
| Birth weight (g), mean (SD) | 815 (185) | 725 (176.3) | 798 (186.6) |
| Size for GA, n (%) | |||
| Small | 55 (8) | 25 (15) | 80 (9) |
| Average | 593 (81) | 127 (76) | 720 (80) |
| Large | 84 (11) | 16 (9) | 100 (11) |
| 1-min Apgar, median (IQR) | 4 (2–6) | 4 (2–6) | 4 (2–6) |
| 5-min Apgar, median (IQR) | 7 (5–8) | 6 (4–7) | 7 (5–8) |
| Maternal characteristics, n (%) | |||
| Multiple gestations | 187 (26) | 46 (27) | 233 (26) |
| Diabetes | 40 (6) | 6 (4) | 46 (5) |
| Hypertension | 53 (7) | 16 (10) | 69 (8) |
| Steroids for fetal maturation | 659 (90) | 151 (90) | 810 (90) |
| Pre-eclampsia | 113 (15) | 23 (14) | 136 (15) |
| Maternal race | |||
| American Indian/Native Alaskan | 13 (2) | 3 (2) | 16 (2) |
| Asian | 27 (4) | 2 (1) | 29 (3) |
| Black | 195 (27) | 42 (25) | 237 (26) |
| Native Hawaiian or other Pacific Islander | 7 (1) | 2 (1) | 9 (1) |
| White | 472 (65) | 113 (67) | 585 (65) |
| Unknown | 2 (0.3) | 0 (0) | 2 (0.2) |
| Not reported | 20 (3) | 6 (4) | 26 (3) |
| Maternal ethnicity | |||
| Hispanic or Latino | 153 (21) | 40 (24) | 193 (21) |
| Not Hispanic or Latino | 571 (78) | 126 (75) | 697 (77) |
| Unknown | 3 (0.4) | 0 (0) | 3 (0.3) |
| Not reported | 5 (0.7) | 2 (1) | 7 (0.8) |
| Infant characteristic, n (%) | |||
| Neonatal intubation | 577 (79) | 153 (91) | 730 (81) |
| Necrotizing enterocolitis | 59 (8) | 38 (23) | 97 (11) |
| Patent ductus arteriosus | 290 (40) | 92 (55) | 382 (42) |
| Resuscitation drugs given | 25 (3) | 7 (4) | 32 (4) |
| Epinephrine | 23 (3) | 5 (3) | 28 (3) |
| Chest compressions | 60 (8) | 12 (7) | 72 (8) |
GA, gestational age; IQR, interquartile range.
Supplemental Table 1 shows mortality rates by highest AKI status. In total, 183 extremely low gestational age neonates (20%) developed AKI stage 1, 108 (12%) developed stage 2 AKI, and 60 (7%) developed stage 3 AKI. Approximately 16% of extremely low gestational age neonates with severe AKI died compared with 9% of neonates with none/stage 1 AKI (OR, 1.94; 95% CI, 1.20 to 3.15). Approximately 10% of neonates with stage 1 or 2 AKI and 25% of those with stage 3 AKI died compared with 8% of neonates without AKI (OR, 1.43; 95% CI, 1.15 to 1.77).
Cox Regression Models for AKI and Death with 7-Day Lag
Unadjusted and adjusted hazard ratios (HRs) for the association between time-dependent AKI status 7 days before death are shown in Table 2. This analysis excludes 35 extremely low gestational age neonates who died on days 3–9.
Table 2.
Multivariable associations of AKI modeled as both a categorical and binary predictor and mortality in extremely low gestational age neonates: Time varying AKI with 7-d lag
| Demographic and Clinical Characteristics | Total Deaths | Total Children | % Died | Total Person-Yr | Mortality Per Person-Yr | Crudea Hazard Ratio (95% Confidence Interval) | Adjusteda,b Hazard Ratio (95% Confidence Interval) | |
|---|---|---|---|---|---|---|---|---|
| Categorical AKI Model | Binary AKI Model | |||||||
| Max AKI stage | ||||||||
| Stage 0 | 29 | – | – | 168.7 | 0.17 | Reference | Reference | – |
| Stage 1 | 11 | – | – | 53.1 | 0.21 | 1.57 (0.72 to 3.42) | 1.47 (0.62 to 3.48) | – |
| Stage 2 | 8 | – | – | 23.6 | 0.34 | 3.90 (1.64 to 9.29) | 1.88 (0.66 to 5.38) | – |
| Stage 3 | 6 | – | – | 12 | 0.5 | 4.88 (1.85 to 12.92) | 3.88 (1.26 to 11.96) | – |
| Severe AKI | ||||||||
| No | 40 | – | – | 221.8 | 0.18 | Reference | Reference | |
| Yes | 14 | – | – | 35.6 | 0.39 | 3.66 (1.84 to 7.27) | – | 2.21 (0.92 to 5.26) |
| GA | ||||||||
| 24 wk | 31 | 225 | 14% | – | – | Reference | Reference | Reference |
| 25 wk | 29 | 236 | 12% | – | – | 0.85 (0.51 to 1.42) | 1.33 (0.59 to 2.99) | 1.37 (0.62 to 3.02) |
| 26 wk | 18 | 216 | 8% | – | – | 0.61 (0.34 to 1.09) | 1.35 (0.60 to 3.03) | 1.35 (0.59 to 3.09) |
| 27 wk | 11 | 223 | 5% | – | – | 0.38 (0.19 to 0.76) | 1.18 (0.47 to 2.95) | 1.17 (0.45 to 3.00) |
| Sex | ||||||||
| Female | 39 | 435 | 9.0% | – | – | Reference | Reference | Reference |
| Male | 50 | 465 | 11% | – | – | 1.17 (0.77 to 1.78) | 0.85 (0.46 to 1.57) | 0.83 (0.45 to 1.52) |
| Size for GA | ||||||||
| Average | 67 | 720 | 9% | – | – | Reference | Reference | Reference |
| Small | 15 | 80 | 19% | – | – | 1.77 (1.00 to 3.13) | 1.96 (0.94 to 4.10) | 1.92 (0.91 to 4.04) |
| Large | 7 | 100 | 7.0% | – | – | 0.80 (0.37 to 1.74) | 1.38 (0.59 to 3.23) | 1.38 (0.59 to 3.24) |
| Apgar 5-min score | 89 | 897 | 10% | – | – | 0.78 (0.71 to 0.85) | 0.88 (0.74 to 1.03) | 0.87 (0.74 to 1.02) |
| Intubation | ||||||||
| No | 7 | 170 | 4.1% | – | – | Reference | Reference | Reference |
| Yes | 82 | 730 | 11% | – | – | 3.30 (1.44 to 7.56) | 2.58 (0.71 to 9.36) | 2.44 (0.70 to 8.49) |
| Chest compressions | ||||||||
| No | 73 | 828 | 9% | – | – | Reference | Reference | Reference |
| Yes | 16 | 72 | 22% | – | – | 2.78 (1.62 to 4.78) | 0.81 (0.24 to 2.71) | 0.77 (0.23 to 2.60) |
| Epinephrine given | ||||||||
| No | 81 | 872 | 9% | – | – | Reference | Reference | Reference |
| Yes | 8 | 28 | 29% | – | – | 3.13 (1.51 to 6.50) | 1.32 (0.27 to 6.51) | 1.34 (0.27 to 6.67) |
| Necrotizing enterocolitis SAE | ||||||||
| No | 62 | 836 | 7% | – | – | Reference | Reference | Reference |
| Yes | 27 | 64 | 42% | – | – | 5.18 (3.26 to 8.22) | 2.94 (0.94 to 9.22) | 2.87 (0.90 to 9.12) |
| Intraventricular hemorrhage SAE | ||||||||
| No | 59 | 778 | 8% | – | – | Reference | Reference | Reference |
| Yes | 30 | 122 | 25% | – | – | 3.71 (2.38 to 5.79) | 2.01 (1.01 to 3.99) | 1.81 (0.91 to 3.60) |
| Sepsis SAE | ||||||||
| No | 64 | 824 | 8% | – | – | Reference | Reference | Reference |
| Yes | 25 | 76 | 33% | – | – | 4.21 (2.64 to 6.72) | 2.85 (1.12 to 7.22) | 2.63 (1.00 to 6.89) |
| Maternal race | ||||||||
| White | 63 | 582 | 11% | – | – | Reference | Reference | Reference |
| Black | 23 | 237 | 10% | – | – | 0.82 (0.51 to 1.33) | 1.01 (0.50 to 2.03) | 1.01 (0.50 to 2.02) |
| Other | 3 | 81 | 4% | – | – | 0.31 (0.10 to 0.98) | 0.15 (0.02 to 1.15) | 0.15 (0.02 to 1.16) |
| Maternal ethnicity | ||||||||
| Hispanic | 23 | 193 | 12% | – | – | Reference | Reference | Reference |
| Not Hispanic | 66 | 697 | 10% | – | – | 0.75 (0.46 to 1.20) | 0.62 (0.30 to 1.27) | 0.60 (0.31 to 1.17) |
| Unknown | 0 | 3 | 0.0% | – | – | – | – | – |
| Not reported | 0 | 7 | 0.0% | – | – | – | – | – |
This analysis excluded 35 children that died on d 3–9. Total number of deaths in this analysis is 54. Percentages in the fourth column represent death rate for variables that are unchanging. These rates include all children who died after d 2 (n=91). Mortality rate in column five is similar in meaning to column four, but is given in terms of deaths per total person yr because AKI stage varies. AKI stage represents the maximum stage up to 44 wk adjusted age or hospital discharge. GA, gestational age; SAE, serious adverse events.
Clustered by mother to account for multiple births.
Cox model with AKI stage as a time-varying covariate; adjusted for all covariates in this table. Not adjusted for PENUT treatment arm.
After adjusting for covariates, stage 3 AKI was associated with a higher risk of mortality (HR, 3.88; 95% CI, 1.26 to 12.0) compared with no AKI. The adjusted hazard of death associated with stage 3 AKI was independent of necrotizing enterocolitis, intraventricular hemorrhage, and sepsis, and higher in magnitude to the risk associated with necrotizing enterocolitis (HR, 2.94; 95% CI, 0.94 to 9.22), intraventricular hemorrhage (HR, 2.01; 95% CI, 1.01 to 3.99), and sepsis (HR, 2.85; 95% CI, 1.12 to 7.22).
When AKI was analyzed as a binary variable, severe AKI was associated with an adjusted higher hazard of death (HR, 2.21; 95% CI, 0.92 to 5.26) 7 days before death, although this did not reach statistical significance.
Cox Regression Models for AKI and Death with 3-Day Lag
To evaluate the effect of the choice of exposure lag, we also considered a 3-day lag for AKI and the association with death (Supplemental Table 2). This analysis excludes 20 extremely low gestational age neonates who died on days 3–5. In the categorical AKI model, after controlling for potential confounders, stage 3 AKI (adjusted HR, 4.17; 95% CI, 1.56 to 11.1) was associated with a higher hazard of death compared with no AKI. Using the binary AKI model, severe AKI was associated with a higher hazard of death (adjusted HR, 2.19; 95% CI, 1.00 to 4.77) compared with none/stage 1 AKI. In the adjusted models, being small for gestational age (SGA) was also associated with an approximately two-fold higher hazard of death when AKI was modeled as either a categorical or binary variable. In this model, intraventricular hemorrhage was no longer associated with mortality (Supplemental Table 2).
Mortality was not associated with gestational age, maternal race or ethnicity, 5-minute Apgar score, or need for intubation, chest compressions, or epinephrine in either the 7- or 3-day lag analyses (Table 2 and Supplemental Table 2).
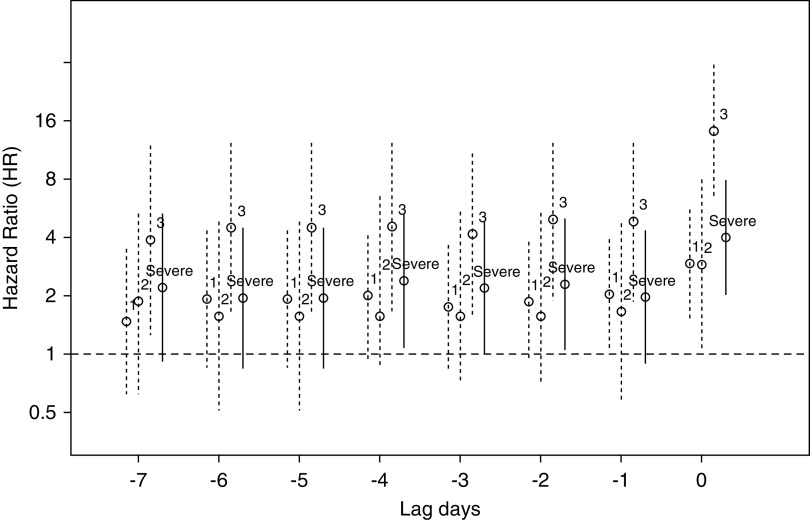
Although our primary focus is on AKI as a time-varying predictor anchored at 7 days, we also explored the full range of lagged values using AKI measured at day t-L for lags L=0,1,2…7. Figure 2 displays adjusted HRs for both categorical AKI and binary severe AKI as a function of the lag value L=0,…7. The adjusted HR for mortality with severe AKI was between 2.2 and 3.2 for 1–6 days before death.
Figure 2.
Hazard ratio of mortality (y axis) associated with development of AKI by stage and by severe AKI before day of death (day 0; x axis).
Landmark Analysis
Table 3 describes estimates from the landmark analysis of AKI and death across the three timeframes (early, middle, late). After adjusting for gestational age, sex, maternal race, ethnicity, 5-minute Apgar, need for chest compressions, intubation, epinephrine use, necrotizing enterocolitis, intraventricular hemorrhage, and sepsis, late severe AKI is associated with a four-fold adjusted higher hazard of death (HR, 4.57; 95% CI, 1.82 to 11.5). We did not find a statistically significant association between early or middle AKI and death (HR, 2.36; 95% CI, 0.47 to 1.70 and HR, 0.69; 95% CI, 0.11 to 4.19; respectively). We dropped necrotizing enterocolitis from the model given the low frequency in the early timeframes and no deaths after day 14.
Table 3.
Multivariable associations of in-hospital mortality in extremely low gestational age neonates in Recombinant Erythropoietin for Protection of Infant Kidney Disease: Severe AKIa at various timepoints
| Timing of AKI | Deaths After Time Period | Total | % Deaths After Time Period | Crudeb Hazard Ratio (95% Confidence Interval) | Adjustedb,c,d Hazard Ratio (95% Confidence Interval) |
|---|---|---|---|---|---|
| Early severe AKIe | |||||
| No | 56 | 849 | 7% | Reference | Reference |
| Yes | 3 | 20 | 15% | 2.70 (0.84 to 8.66) | 2.36 (0.47 to 1.70) |
| Middle severe AKIf | |||||
| No | 39 | 797 | 5% | Reference | Reference |
| Yes | 2 | 37 | 5% | 1.24 (0.30 to 5.17) | 0.69 (0.11 to 4.19) |
| Late severe AKIg | |||||
| No | 21 | 730 | 3% | Reference | Reference |
| Yes | 6 | 53 | 11% | 4.08 (1.61 to 10.3) | 4.57 (1.82 to 11.49) |
GA, gestational age; SGA, small for gestational age.
0-d lag for AKI stage.
Clustered by mother to account for multiple births.
Cox model adjusted for GA, sex, SGA, 5-min Apgar, intubation, epinephrine, chest compressions, necrotizing enterocolitis, sepsis, and intraventricular hemorrhage.
Covariates estimates not shown.
This excludes 30 children who died and one child who was discharged alive on d 3–7.
This excludes an additional 15 children who died on d 8–14 and 20 children with severe AKI during d 3–7.
This excludes an additional 12 children who died and two children who were withdrawn from the study on d 15–28, and 37 children with severe AKI during d 8–14.
Discussion
In this large, multi center, prospective cohort study, we report that 19% of extremely low gestational age neonates had at least one episode of severe AKI and 7% had stage 3 AKI. Those with stage 3 AKI have an almost four-fold increased hazard of death, and those with severe AKI have a 2.2-fold increased hazard of death. This association is independent of important confounders and is present up to a week before death. We found that being small for gestational age was associated with an increased risk of mortality, independent of birthweight and gestational age. We did not find an association with maternal race or ethnicity and outcomes in this study. The associations between severe AKI and mortality were strongest when AKI occurred after the first 2 weeks of life, compared with earlier time points. Additionally, the effect of AKI on mortality is similar to that of necrotizing enterocolitis and intraventricular hemorrhage, yet kidney function is not routinely monitored during the NICU stay.
To our knowledge, our lag time analysis is novel and demonstrates that AKI precedes death and likely is a contributor to death rather than an innocent consequence of multiorgan failure and impending death. This observation supports the concept initially proposed over 20 years ago in adults that patients are dying from, and not just with, AKI (19). Our findings suggest that severe AKI after day 14 up to day 28 affects death in extremely low gestational age neonates to a similar extent as necrotizing enterocolitis. In total, 50% of the deaths occurred after day 14, which supports the importance of ongoing monitoring of kidney function beyond the first few weeks of life.
This study adds to the literature that suggests AKI is independently associated with mortality in low birthweight and/or premature babies. The rates of AKI in our study differ from previous studies, likely due to different patient populations, serum creatinine surveillance practices, and timeframe in which AKI is defined. In addition, we did not use creatinine values on day 0, 1, or 2 of life as these likely represent maternal creatinine values. A prospective single-center study of 229 very low birthweight neonates reported a similar AKI incidence of 18%; however, 42% of neonates with AKI died compared with only 5% without AKI. In adjusted models, the association between AKI and mortality was no longer significant, although the HR was similar to what we report (11). Recent studies of the Assessment of Worldwide Acute Kidney Epidemiology in Neonates cohort found a 48% incidence of AKI in neonates <29 weeks gestation (20, 21). In this cohort, 29% had late AKI (>7 days); both early and late AKI were associated with an increased risk of death (22, 23). In a retrospective matched case-control study of 472 extremely low gestational age neonates (<1000 g), after matching for time period, birth weight (±10%), and gestational age (±1 week), the investigators found neonates with AKI had higher mortality (33 of 46, 70%, versus 10 of 46, 22%; P<0.001) (12). A single-center study of all babies admitted to the NICU over 1 year, revealed a 54% AKI incidence, with a five-fold increased risk of death in neonates <32 weeks gestation with AKI (14). This study also used urine output criteria to define AKI.
Although most studies confirm the association between AKI and mortality, a retrospective cohort study of 266 premature (<32 weeks gestation) and very low birthweight (<1500 g) neonates found no such association (13). The incidence of AKI in this study was higher than we report, with 65% of 22–25-week extremely low gestational age neonates developing AKI, and 25% of neonates born at a gestational age of 26–28 weeks. The difference in this study may be attributed to their patient population because they combined all neonates (22–32 weeks gestation), whereas we include only those neonates 24 to <28 weeks gestation.
There are a few limitations of this study; first, we acknowledge that not every infant had a serum creatinine value measured every day. We relied on serum creatinine values drawn for clinical indications at each of the sites, and the frequency of serum creatinine measurements differed by site (centers that measured serum creatinine more often will have higher rates of AKI). Thus, we acknowledge we may have misclassified participants on their AKI status, but this would likely lead to an underestimation of the frequency of AKI. We did not include urine output in the definition of AKI given the tremendous variability across sites in how urine output was captured. Given this, we were not confident we could accurately estimate urine output in this cohort. We do not know the cause of death for the study participants. The Clinical Risk Index for Babies (CRIB)-II and Score for Neonatal Acute Physiology with Perinatal Extension (SNAPPE)-II neonatal severity scoring systems are used to predict mortality in neonates within the first hour and first 12 hours of admission, respectively (4, 24). We were not able to adjust for these scores in our analyses because many of participants did not have all of the parameters. However, many elements in these scoring systems are independently factored into the regression analyses in this study.
In conclusion, stage 3 AKI and severe AKI are independently associated with death, and this association is present for <7 days before death. This study highlights the importance of recognition and monitoring for severe AKI, especially after day 14, given the frequency and effect of AKI on mortality. We encourage a more systematic way to document urine output in future AKI studies. Potential measures to improve care and outcomes for neonates with AKI include first early recognition, followed by limitation of nephrotoxic medications, monitoring of fluid status, fluid given and urine output to avoid volume overload, and a systematic approach to uncover and address the etiology of AKI (25). However, studies that improve our understanding of which neonates and at what intervals surveillance for AKI should be done, along with interventions to mitigate the consequences, are greatly needed.
Disclosures
D. Askenazi reports employment with University of Alabama at Birmingham; consultancy agreements with the AKI Foundation, Baxter, BioPorto, CHF Solutions, and Medtronic; and reports receiving research funding from Baxter Renal Products and CHF Solutions. P. Brophy reports employment with University of Rochester; reports consultancy agreements with Zeta Biolongevity; reports having an ownership interest in Zeta Biolongevity; reports receiving honoraria from American Board Medical Specialties; reports serving as a scientific advisor or member of American Board of Medical Specialties; and reports receiving royalties from UpToDate. P. Heagerty reports employment with University of Washington; and reports serving as a member of a data safety monitoring board for Verily and as a member of Board of Directors of Cancer Research and Biostatistics. S. Goldstein reports employment with Cincinnati Children’s Hospital Medical Center; reports consultancy agreements with Akebia, Baxter Healthcare, Bayer, BioPorto Inc., CHF Solutions, Fresenius, Kaneka Inc., La Jolla Pharmaceuticals, MediBeacon, Medtronic, Otsuka, Reata, and Renibus; reports having an ownership interest in MediBeacon; reports receiving research funding from Baxter Healthcare, BioPorto, and CHF Solutions; reports receiving honoraria from Baxter Healthcare and Fresenius; reports having patents and inventions with Vigilanz; reports serving as a scientific advisor or member of MediBeacon; and speakers bureau for Baxter Healthcare and Fresenius. R. Schmicker reports employment with University of Washington Center for Biomedical Statistics. S. Hingorani reports employment with Seattle Children’s Hospital/University of Washington and consultancy agreements with Omeros. S. Juul reports employment with University of Washington and serving on the Editorial Board of Neonatology.
Funding
The Recombinant Erythropoietin for Protection of Infant Renal Disease Study is an ancillary study funded by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK103608, and was designed to look at kidney outcomes in patients enrolled in the PENUT Trial, which is funded by NIH NINDS grants U01 NS077953 and U01 NS077955.
Supplementary Material
Data Sharing Statement
De‐identified individual participant data will be made available through the National Institute of Neurological Disorders (NINDS) Data Archive: https://www.ninds.nih.gov/Current‐Research/Research‐Funded‐NINDS/Clinical‐Research/Archived‐Clinical‐Research‐Datasets. The data will be de‐identified and a limited access dataset will be available after May 2021 through a request form on that page. Data dictionaries, in addition to study protocol, the statistical analysis plan, and the informed consent form will be included. The data will be made available upon publication of all PENUT Trial–related manuscripts to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal. NINDS archived clinical research dataset repository, https://www.ninds.nih.gov/Current‐Research/Research‐Funded‐NINDS/Clinical‐Research/Archived‐Clinical‐Research‐Datasets.
Clinical Trial registry name and registration number: The ClinicalTrials.gov identifier is NCT01378273. All authors have nothing to disclose.
The list of nonauthor contributors is extensive and has been provided in Supplemental Summary 1.
Published online ahead of print. Publication date available at www.cjasn.org.
See related Patient Voice, “Depressive Symptoms and Rapid Kidney Function Decline,” on page 839.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.18841220/-/DCSupplemental.
Supplemental Figure 1. Consort diagram of ELGANs included in the AKI analyses for this study.
Supplemental Table 1. AKI status by mortality status at time of discharge.
Supplemental Table 2. Multivariable associations of AKI modeled as both a categorical and binary predictor and mortality in extremely low gestational age neonates: Time varying AKI with 3-day lag.
References
- 1.Walani SR: Global burden of preterm birth. Int J Gynecol Obstet 150: 31–22, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL: Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71: 1028–1035, 2007. 10.1038/sj.ki.5002231 [DOI] [PubMed] [Google Scholar]
- 3.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL: Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol 3: 948–954, 2008. 10.2215/CJN.05431207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manktelow BN, Draper ES, Field DJ: Predicting neonatal mortality among very preterm infants: A comparison of three versions of the CRIB score. Arch Dis Child Fetal Neonatal Ed 95: F9–F13, 2010. 10.1136/adc.2008.148015 [DOI] [PubMed] [Google Scholar]
- 5.Ricci Z, Cruz D, Ronco C: The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int 73: 538–546, 2008. 10.1038/sj.ki.5002743 [DOI] [PubMed] [Google Scholar]
- 6.Cuhaci B: More data on epidemiology and outcome of acute kidney injury with AKIN criteria: Benefits of standardized definitions, AKIN and RIFLE classifications. Crit Care Med 37: 2659–2661, 2009. 10.1097/CCM.0b013e3181ad76c2 [DOI] [PubMed] [Google Scholar]
- 7.Uchino S: Outcome prediction for patients with acute kidney injury. Nephron Clin Pract 109: c217–c223, 2008. 10.1159/000142931 [DOI] [PubMed] [Google Scholar]
- 8.Macedo E, Castro I, Yu L, Abdulkader RR, Vieira Jr. JM: Impact of mild acute kidney injury (AKI) on outcome after open repair of aortic aneurysms. Ren Fail 30: 287–296, 2008. 10.1080/08860220701857522 [DOI] [PubMed] [Google Scholar]
- 9.Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committee: Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care 11: R68, 2007. 10.1186/cc5949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Askenazi DJ, Griffin R, McGwin G, Carlo W, Ambalavanan N: Acute kidney injury is independently associated with mortality in very low birthweight infants: A matched case-control analysis. Pediatr Nephrol 24: 991–997, 2009. 10.1007/s00467-009-1133-x [DOI] [PubMed] [Google Scholar]
- 11.Koralkar R, Ambalavanan N, Levitan EB, McGwin G, Goldstein S, Askenazi D: Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res 69: 354–358, 2011. 10.1203/PDR.0b013e31820b95ca [DOI] [PubMed] [Google Scholar]
- 12.Viswanathan S, Manyam B, Azhibekov T, Mhanna MJ: Risk factors associated with acute kidney injury in extremely low birth weight (ELBW) infants. Pediatr Nephrol 27: 303–311, 2012. 10.1007/s00467-011-1977-8 [DOI] [PubMed] [Google Scholar]
- 13.Mian AN, Guillet R, Ruck L, Wang H, Schwartz GJ: Acute kidney injury in premature, very low-birth-weight infants. J Pediatr Intensive Care 5: 69–78, 2016. 10.1055/s-0035-1564797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shalaby MA, Sawan ZA, Nawawi E, Alsaedi S, Al-Wassia H, Kari JA: Incidence, risk factors, and outcome of neonatal acute kidney injury: A prospective cohort study. Pediatr Nephrol 33: 1617–1624, 2018. 10.1007/s00467-018-3966-7 [DOI] [PubMed] [Google Scholar]
- 15.Juul SE, Mayock DE, Comstock BA, Heagerty PJ: Neuroprotective potential of erythropoietin in neonates; design of a randomized trial. Matern Health Neonatol Perinatol 1: 27, 2015. 10.1186/s40748-015-0028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juul SE, Comstock BA, Wadhawan R, Mayock DE, Courtney SE, Robinson T, Ahmad KA, Bendel-Stenzel E, Baserga M, LaGamma EF, Downey LC, Rao R, Fahim N, Lampland A, Frantz Iii ID, Khan JY, Weiss M, Gilmore MM, Ohls RK, Srinivasan N, Perez JE, McKay V, Vu PT, Lowe J, Kuban K, O’Shea TM, Hartman AL, Heagerty PJ; PENUT Trial Consortium: A randomized trial of erythropoietin for neuroprotection in preterm infants. N Engl J Med 382: 233–243, 2020. 10.1056/NEJMoa1907423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Askenazi DJ, Heagerty PJ, Schmicker RH, Griffin R, Brophy P, Juul SE, Mayock DE, Goldstein SL, Hingorani S; PENUT Trial Consortium: Prevalence of acute kidney injury (AKI) in extremely low gestational age neonates (ELGAN). Pediatr Nephrol 35: 1737–1748, 2020. 10.1007/s00467-020-04563-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Houwelingen JC, Putter H: Dynamic Prediction in Clinical Survival Analysis, Boca Raton, FL, CRC Press, 2012 [Google Scholar]
- 19.Kellum JA, Angus DC: Patients are dying of acute renal failure. Crit Care Med 30: 2156–2157, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Askenazi D, Abitbol C, Boohaker L, Griffin R, Raina R, Dower J, Davis TK, Ray PE, Perazzo S, DeFreitas M, Milner L, Ambalavanan N, Cole FS, Rademacher E, Zappitelli M, Mhanna M; Neonatal Kidney Collaborative: Optimizing the AKI definition during first postnatal week using Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) cohort. Pediatr Res 85: 329–338, 2019. 10.1038/s41390-018-0249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, Chishti AS, Woroniecki R, Mammen C, Swanson JR, Sridhar S, Wong CS, Kupferman JC, Griffin RL, Askenazi DJ; Neonatal Kidney Collaborative (NKC): Incidence and outcomes of neonatal acute kidney injury (AWAKEN): A multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1: 184–194, 2017. 10.1016/S2352-4642(17)30069-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlton JR, Boohaker L, Askenazi D, Brophy PD, D’Angio C, Fuloria M, Gien J, Griffin R, Hingorani S, Ingraham S, Mian A, Ohls RK, Rastogi S, Rhee CJ, Revenis M, Sarkar S, Smith A, Starr M, Kent AL; Neonatal Kidney Collaborative: Incidence and risk factors of early onset neonatal AKI. Clin J Am Soc Nephrol 14: 184–195, 2019. 10.2215/CJN.03670318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlton JR, Boohaker L, Askenazi D, Brophy PD, Fuloria M, Gien J, Griffin R, Hingorani S, Ingraham S, Mian A, Ohls RK, Rastogi S, Rhee CJ, Revenis M, Sarkar S, Starr M, Kent AL; Neonatal Kidney Collaborative (NKC): Late onset neonatal acute kidney injury: Results from the AWAKEN study. Pediatr Res 85: 339–348, 2019. 10.1038/s41390-018-0255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson DK, Corcoran JD, Escobar GJ, Lee SK: SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr 138: 92–100, 2001. 10.1067/mpd.2001.109608 [DOI] [PubMed] [Google Scholar]
- 25.Harer MW, Selewski DT, Kashani K, Basu RK, Gist KM, Jetton JG, Sutherland SM, Zappitelli M, Goldstein SL, Mottes TA, Askenazi DJ: Improving the quality of neonatal acute kidney injury care: Neonatal-specific response to the 22nd Acute Disease Quality Initiative (ADQI) conference. J Perinatol 41: 185–195, 2021. 10.1038/s41372-020-00810-z [DOI] [PubMed] [Google Scholar]
- 26.Askenazi DJ, Heagerty P J, Schmicker RH, Brophy P, Juul SE, Goldstein SL, Hingorani S, on behalf of the PENUT Trial Consortium: The impact of erythropoietin on short- and long-term kidney-related outcomes in neonates of extremely low gestational age: Results of a multicenter, double-blind, placebo-controlled randomized clinical trial. J Pediatr 232: 65–72 e67, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.