Abstract
Purpose
To determine whether time-to-intubation was associated with higher ICU mortality in patients with COVID-19 on mechanical ventilation due to respiratory insufficiency.
Materials and methods
We conducted an observational, prospective, single-center study of patients with confirmed SARS-CoV-2 infection hospitalized with moderate to severe ARDS, connected to mechanical ventilation in the ICU between March 17 and July 31, 2020. We examined their general and clinical characteristics. Time-to-intubation was the time from hospital admission to endotracheal intubation.
Results
We included 183 consecutive patients; 28% were female, and median age was 62 years old. Eighty-eight patients (48%) were intubated before 48 h (early) and ninety-five (52%) after 48 h (late). Patients intubated early had similar admission PaO2/FiO2 ratio (123 vs 99; p = 0.179) but were younger (59 vs 64; p = 0.013) and had higher body mass index (30 vs 28; p = 0.006) compared to patients intubated late. Mortality was higher in patients intubated late (18% versus 43%), with admission PaO2/FiO2 ratio < 100 mmHg (OR 5.2; p = 0.011), of older age (OR 1.1; p = 0.001), and with previous use of ACE inhibitors (OR 4.8; p = 0.026).
Conclusions
In COVID-19 patients, late intubation, Pafi <100, older age, and previous ACE inhibitors use were associated with increased ICU mortality.
Keywords: SARS-CoV-2, COVID-19, ARDS, Mechanical ventilation
Abbreviations: ARDS, Acute respiratory distress syndrome; ICU, Intensive care unit; WOB, work of breathing; HFNC, high-flow nasal cannula; MV, Mechanical ventilation; P-SILI, self-inflicted lung injury by the patient; RT-PCR, reverse transcriptase polymerase chain reaction; Crs, Respiratory system compliance; PEEP, Positive end-expiratory pressure; ECMO, extracorporeal membrane oxygenation; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; ACE inhibitors, angiotensin converting enzyme inhibitors
1. Background
Pneumonia associated with SARS-CoV-2 (COVID-19) may evolve to acute respiratory distress syndrome (ARDS), which is associated with high mortality risk [[1], [2], [3], [4]]. Many patients are admitted with pneumonia and arterial hypoxemia without evidence of dyspnea, increased work of breathing, or impending fatigue [[5], [6], [7]]. Long-accepted clinical practice and expert consensus support prompt intubation of patients with severe hypoxemia [8,9]. However, due to the high demand for intensive care unit (ICU) beds during the pandemic [6], some authors have advocated for a conservative approach, promoting high-flow nasal cannula (HFNC) [10], or non-invasive ventilation [11] while the patient is in an awake prone position [[10], [11], [12]].
Apart from the well-known risks of mechanical ventilation (MV), an infection [13], and ventilator-induced lung injury [14,15], among others, the potential shortage of ICU resources related to the massive demand for MV during the pandemic has added stress and uncertainties to clinicians managing these acute patients [16]. Furthermore, many of these patients exhibited precarious condition, worsening after an initial improvement and eventually required intubation at some time. It is not clear whether time-to-intubation directly associates with increased mortality in COVID-19 hypoxemic patients [17].
A recent study showed that neither time from ICU admission to intubation nor HFNC use were associated with increased mortality [18] in a time frame of 8 h. However, different criteria may influence the moment of admission to the ICU, ranging from the initial clinical impression despite poor oxygenation to bed availability. Unlike ICU admission, hospital admission is objectively based on hypoxemia with diverse manifestations of dyspnea or increased work of breathing (WOB) in COVID-19 patients.
We hypothesized that later intubation was associated with worse outcomes than early intubation in patients with confirmed SARS-CoV-2 infection admitted to the hospital with respiratory insufficiency.
Our main objective was to determine whether the time-to-intubation in hospitalized COVID-19 patients was associated with outcomes. To address this issue, we analyzed a prospectively collected database of mechanically ventilated COVID-19 patients treated in our ICU during the peak months of the pandemic.
2. Materials and methods
This prospective observational study was carried out at the Clinical Hospital of the UC-CHRISTUS Health Network in Santiago, Chile. Patients with laboratory-confirmed SARS-CoV-2 infection and moderate to severe ARDS [19] were consecutively included between March 17 and July 31, 2020. Admission pathways comprised the emergency department and basic ward. The Institutional Ethics Committee approved this project (Research Ethics Committee N° 200,504,004, Faculty of Medicine, Pontificia Universidad Católica de Chile) and waived the need for informed consent.
Our university hospital has a 32-bed both medical-and-surgical ICU with extracorporeal membrane oxygenation (ECMO) capability. Due to the COVID-19 pandemic, the hospital ICU capacity was surged, incorporating up to 56 beds from other reconverted units, as needed. Intensivists and ICU-trained nurses were deployed to these expanded ICUs to ensure a similar standard of care.
Laboratory confirmation of SARS-CoV-2 was defined as a positive real-time reverse transcriptase-polymerase chain reaction (RT-PCR) result of nasal and pharyngeal swabs.
Respiratory failure management protocol. All patients undergo an initial respiratory failure management protocol that included HFNC and awake prone positioning when tolerated. Orotracheal intubation and connection to MV were performed if the patient had increased work of breathing (WOB) and other conditions. The decision to intubate was by attending physicians, and MV started in the volume-control ventilation mode according to local management protocol (See details on Additional file 1A and 1B).
Data Collection. Data were recorded prospectively by the research team in an electronic worksheet during the patient's stay in the ICU. Data were collected and managed using the REDCap electronic data capture tools hosted at Pontificia Universidad Católica de Chile. Clinical data included sex, age, weight, height, medical comorbidities, days since the start of symptoms, laboratory parameters, and PaO2/FiO2 ratio by the time of hospital admission. APACHE II, SOFA and Call scores were calculated within 24 h of ICU admission [[20], [21], [22]] Subsequently, clinical, laboratory, and ventilatory parameters were recorded from the start of invasive MV and included respiratory support mode, positive end-expiratory pressure (PEEP) level, arterial blood gases, PaO2/FiO2 ratio, pH, and respiratory system compliance (Crs).
Outcomes. Time of intubation was defined as the time from hospital admission to endotracheal intubation. The primary outcome was ICU death. Secondary outcomes included duration of MV, ICU and hospital length of stay, and mortality on day 28, and on discharge from the ICU. According to ROC curve analyses from our data, the time of intubation was classified as early (<48 h) or late (≥48 h).
Statistical Analysis. For variables with non-normal distribution, non-parametric tests were used. Accordingly, descriptive statistics are shown as medians [interquartile range 25–75] or percentages (%). Mann-Whitney U, Kruskal Wallis, chi-square, and Fisher's exact, were used when appropriate. We performed univariate analyses between primary outcome and clinical and laboratory variables. Those with a univariate p-value of 0.1 were later included in the multivariate analysis plus other clinically relevant ones. Logistic models were fitted to test individual and interaction variables. A multivariable fractional polynomial regression was fitted, including admission PaO2/FiO2 ratio, time to intubation, age, d-dimer, and ACE inhibitors usage. In addition, we categorized PaO2/FiO2 ratio, time to intubation, and age after generating ROC curves to provide more manageable parameters for making timely clinical decisions when facing acute patients with respiratory insufficiency due to COVID-19.
Data were analyzed using Stata 16 (StataCorp, College Station, TX. USA) and IBM SPSS Statistics for Mac, Version 27 (Armonk, NY) statistical packages. A two-tailed p-value of <0.05 was considered statistically significant.
3. Results
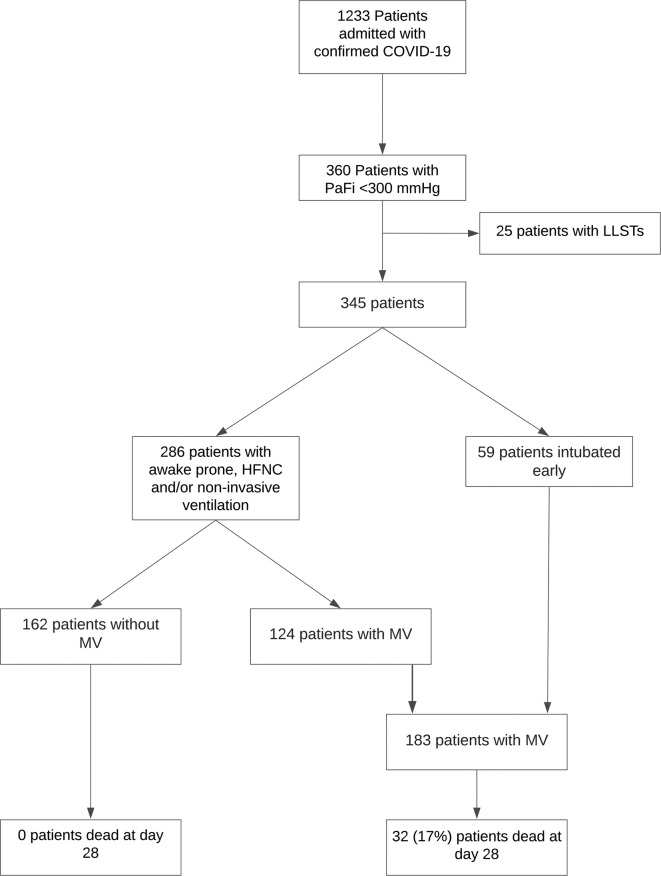
During the study period, 1233 patients with confirmed COVID-19 were admitted, of which 360 evolved with PaO2/FiO2 ratio < 300 mmHg. Of these patients, 25 (7%) underwent a limitation on life-support interventions. Fifty-nine (16.4%) patients were intubated upon admission to the ICU, while 286 (79.4%) underwent an HFNC, non-invasive ventilation, and prone trial, of which 162 (45%) patients did not require MV during hospitalization (Additional file 2). Thus, 183 patients confirmed COVID-19 required invasive ventilatory support and were included in the study (Fig. 1 ).
Fig. 1.
Flowchart of COVID-19 patients enrolled in the study.
Demographic and clinical characteristics of patients are shown in Table 1 according to time to intubation. Overall, 132 patients (78%) were men, their median age was 62 years (54–70), and 138 patients (75%) had one or more comorbidities being hypertension (48%), diabetes (33%), and other cardiovascular diseases (8.2%) the most common. Patients intubated late were older than those intubated early, and their body mass index (BMI) was lower. SOFA score was higher, and CALL score lower in patients intubated early. Thoracic CT scan showed a predominance of ground-glass opacities, with no difference between groups. Several days of symptoms before hospital admission was not associated with the outcome (Table 1). Both groups were similar in the use of prone positioning.
Table 1.
General and clinical characteristics of patients with severe COVID-19 respiratory failure according to timing from admission to orotracheal intubation.
| <48 h to OI (early) | >48 h to OI (late) | P-value | |
|---|---|---|---|
| Number (%) | 88 (48) | 95 (52) | |
| Age (years) | 59 [53–66] | 64 [55–71] | 0.013 |
| Male n (%) | 62 (71) | 70 (74) | 0.626 |
| Comorbidities | |||
| Diabetes mellitus n (%) | 27 (31) | 34 (36) | 0.464 |
| Arterial hypertension n (%) | 41 (47) | 46 (48) | 0.688 |
| BMI (kg/m2) | 30 [29–33] | 28 [26–31] | 0.006 |
| Days of symptoms before admission | 7 [4–8] | 7 [5–10] | 0.124 |
| APACHE score | 12 [8–15] | 13 [8–20] | 0.354 |
| SOFA score | 6 [4–8] | 4 [2–8] | 0.014 |
| CALL score | 10 [8–12] | 11 [10–12] | 0.021 |
| Laboratory values | |||
| Ferritin (ng/mL) | 1449 [850–1934] | 1415 [909–2472] | 0.764 |
| D-dimer (ng/mL) | 2565 [1074–4796] | 1701 [1074–4653] | 0.194 |
| Lymphocytes (103/μL) | 650 [470–1020] | 580 [435–880] | 0.199 |
| PaO2/FiO2 at hospital admission | 123 [82–166] | 99 [77–158] | 0.179 |
| CT scan n (%) | |||
| Normal | 1 (1.75) | 3 (4.17) | 0.422 |
| Predominance of ground-glass opacities | 44 (77.19) | 56 (77.78) | |
| Predominance of consolidations | 1 (1.75) | 5 (6.94) | |
| Ground-glass opacities and consolidations | 10 (17.54) | 7 (9.72) | |
| Mainly consolidations with lung architecture distortion | 1 (1.75) | 1 (1.39) | |
Categorical variables are expressed as frequency and percentage (%) and continuous variables as median and interquartile range 25–75 [IQR].
Abbreviations: OI orotracheal intubation; BMI body mass index; COPD chronic obstructive pulmonary disease; APACHE II Acute Physiology and Chronic Health Evaluation II; SOFA sequential organ failure assessment.
Regarding dexamethasone use [23], 38% of our patients received this drug; 34% in the early and 41% in the late group. Pulmonary embolism (PE), renal replacement therapy (RRT), and tracheostomy were similar between groups. Patients intubated after 48 h had an ICU stay 7-days longer, hospital stay 5-days longer, and more than double higher mortality compared to those intubated before 48 h after hospital admission. ECMO requirements in both groups were similar (Table 2 ). The use of awake proning, HNFC, and non-invasive ventilation is detailed in Additional file 2.
Table 2.
Interventions and outcomes by time from ICU admission to intubation.
| <48 h to OI | >48 h to OI | P-value | |
|---|---|---|---|
| Number (%) | 88 (48) | 95 (52) | |
| Dexamethasone | 30 (34) | 39 (41) | 0.332 |
| Prone position (%) | 62 (71%) | 68 (72%) | 0.872 |
| Tracheostomy (%) | 18 (21%) | 27 (29%) | 0.196 |
| RRT (%) | 12 (14%) | 12 (13%) | 0.841 |
| ECMO | 4 (5) | 7 (7) | 0.422 |
| Pulmonary thromboembolism | 20 (23%) | 15 (16%) | 0.233 |
| ICU LOS (days) | 15 [9–23] | 23 [12–39] | 0.003 |
| MV days | 13 [8–25] | 16 [9–33] | 0.131 |
| Ventilator-free days | 15 [3−20] | 12 [0–19] | 0.196 |
| Hospital LOS (days) | 31 [17–45] | 36 [24–62] | 0.031 |
| 28-day mortality (%) | 11 (13%) | 21 (22%) | 0.087 |
| ICU mortality (%) | 16 (18%) | 43 (43%) | <0.001 |
Categorical variables are expressed as frequency and percentage (%) and continuous variables as median and interquartile range 25.75 [IQR].
Abbreviations: ICU intensive care unit; MV mechanical ventilation; RRT renal replacement therapy; ECMO extracorporeal membrane oxygenation support; LOS length of stay.
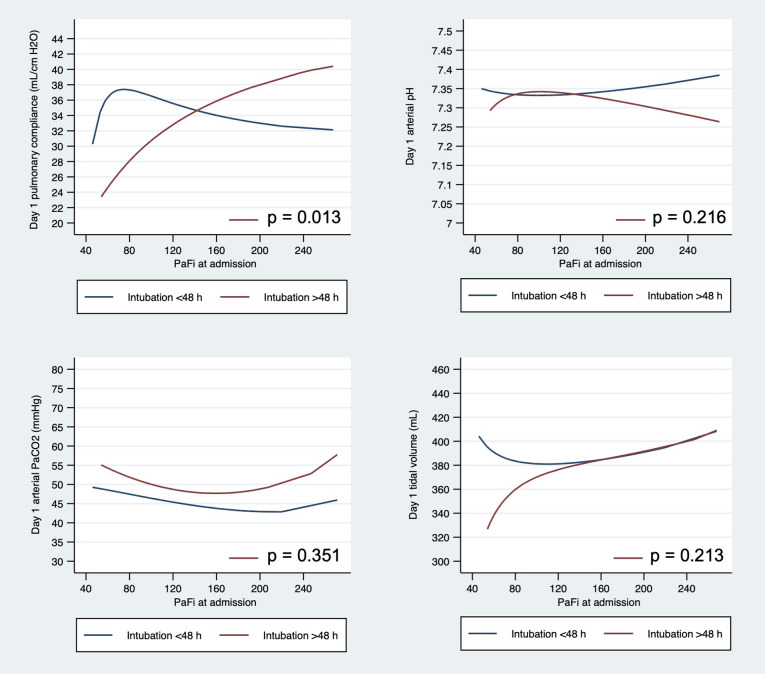
In addition, patients intubated late presented lower pulmonary compliance significantly at day one of intubation compared to those intubated early (p = 0.013). Of note, pulmonary compliance lowered progressively as PaO2/FiO2 ratio lowered, but arterial pH, pCO2 and tidal volume did not show significant differences (Additional file 3). Patients with severe ARDS intubated late exhibited lower compliance and higher driving pressure on the first MV day compared to patients intubated early, while pH and height-adjusted tidal volume were similar (Table 3 ).
Table 3.
Mechanical ventilation variables according to PaO2/FiO2 and time to intubation.
| Admission PaO2/FiO2 < 100 mmHg |
Admission PaO2/FiO2 > 100 mmHg |
|||||
|---|---|---|---|---|---|---|
| <48 h to OI | >48 h to OI | P-value | <48 h to OI | >48 h to OI | P-value | |
| Number (%) | 32 (17) | 48 (26) | 56 (31) | 47 (26) | ||
| Respiratory rate | 26 [23−30] | 28 [24–30] | 0.332 | 26 [24–28] | 28 [24–30] | 0.557 |
| Tidal volume (ml) | 390 [370–400] | 345 [303–400] | 0.090 | 366 [329–434] | 390 [355–422] | 0.628 |
| PEEP (cmH2O) | 10 [8–10] | 8 [6–10] | 0.010 | 10 [8–10] | 8 [7–10] | 0.382 |
| P plateau (cmH2O) | 19 [18–23] | 21 [20–24] | 0.191 | 20 [20−23] | 21 [19–23] | 0.592 |
| Crs (ml/cmH20) | 32 [30–39] | 23 [21−33] | 0.003 | 31 [26–39] | 32 [27–41] | 0.700 |
| Driving pressure (cmH2O) | 12 [9–12] | 14 [12–16] | 0.041 | 12 [10–14] | 11 [10−13] | 0.699 |
| PaO2/FiO2 ratio (mmHg) | 134 [89–176] | 123 [100–150] | <0.001 | 178 [132−202] | 117 [109–155] | 0.233 |
| Arterial pH | 7.36 [7.28–7.44] | 7.36 [7.26–7.40] | 0.946 | 7.39 [7.28–7.41] | 7.32 [7.24–7.42] | 0.238 |
| PaCO2 (mmHg) | 49 [38–50] | 46 [41–61] | 0.365 | 46 [38–50] | 53 [43–58] | 0.020 |
Categorical variables are expressed as frequency and percentage (%) and continuous variables as median and interquartile range 25.75 [IQR].
Abbreviations: PEEP positive end-expiratory pressure; Crs respiratory system compliance.
Multivariate logistic regression showed that PaO2/FiO2 ratio at admission (OR 0.52 [0.31–0.86], p = 0.01), time to intubation (OR 1.01 [1.00–1.01], p = 0.02), age (OR 1.01 [1.00–1.01], p < 0.001), and angiotensin converting enzyme inhibitors (ACE) inhibitors use (OR 12.37 [2.28–67.09], p = 0.004) were significantly associated with mortality. D-dimer, tested in the same model, did not reach statistical significance (p = 0.077). Other variables, as LDH and lymphocytes count at admission, tested in different models did not reach statistical significance. However, the recent developed CALL score, which incorporated LDH, age, lymphocytes count, and comorbidities, reached statistical significance in a different multivariate model that excluded age and ACE inhibitors use to avoid overfitting (OR 1.57 [1.16–2.11], p = 0.005).
We generated ROC curves for PaO2/FiO2 ratio, time to intubation and age to explore potential practical cutoffs for facilitating clinical decisions in the acute setting (Additional file 4). Optimal cutpoints for PaO2/FiO2 ratio, time to intubation and age were 100, 48 h and 60 years, respectively. Accordingly, we generated four subgroups that were tested in a logistic regression model. Patients presenting with PaO2/FiO2 ratio < 100 mmHg and intubated >48 h after hospital admission showed a statistically significant association with mortality in the ICU (OR 5.20 [1.46–18.46], p = 0.011) compared to the other three groups (Fig. 2 ).
Fig. 2.
Kaplan-Meier survival curve according to the timing of intubation and PaO2/FiO2 ratio.
4. Discussion
Our main finding is that among hospitalized patients with COVID-19 with respiratory insufficiency, intubation after 48 h of hospital admission and PaO2/FiO2 ratio on admission <100 mmHg was associated with increased mortality. In addition, older age and previous use of ACE inhibitors were also associated with increased mortality.
We cannot establish a valid reason for this clinical course. All patients not intubated at admission were given an awake prone trial, combined with HFNC and careful monitoring, and intubation was not delayed in any patient when indicated. As all patients were hypoxemic, the PaO2/FiO2 ratio was never considered alone as the sole criterion for intubation. This concept has been referred to as happy hypoxemia and has been widely discussed recently [[5], [6], [7],24]. In our patients, an increase in the WOB or a subtle clinical deterioration, characterized by the appearance of initial signs of fatigue or physical discomforts such as delirium, restlessness, or disorientation, prompted the clinicians to consider intubation. This explains why some patients lasted a long time with severe hypoxemia before being intubated while others underwent the procedure much earlier. There were patients with PaO2/FiO2 ratio < 100 who did not require intubation and were uneventfully discharged to a lower-care unit, and they all survived.
The different clinical courses between patients intubated earlier or late may have been determined by a natural evolution of the disease unveiling a phenotype with a more rapid progression of lung damage and, possibly, patient-specific factors as patients intubated late were older and had a lower BMI than their counterparts. The spontaneous ventilatory efforts could have determined another contributing factor during prolonged periods, and that would be capable of inducing the progression of lung damage, what we knewn as patient self-inflicted lung injury (P-SILI) [[25], [26], [27], [28]] if present, cannot be credited or ruled-out as a relevant mechanism with the available information this would not provide justification for liberal use of endotracheal intubation [29,30]. Nevertheless, the pulmonary mechanics we reported were just the initial values after intubation. Other ventilatory strategies employed after that were not recorded for this study.
The fact that almost half of the total number of patients with PaO2/FiO2 ratio < 300 mmHg admitted to our center did not require MV confirmed that the use of awake prone or HFNC might helpful in COVID-19 patients with respiratory failure, as have been published [10,12,30]. Most of our more severe hypoxemic patients underwent a trial of awake prone or HFNC to manage respiratory failure. This strategy may have helped many of them to avoid intubation and its potential complications without increasing mortality [30,34], especially in a pandemic situation.
The timing of intubation in patients with COVID-19 has been the subject of intense debate. While some advocate for early intubation, others claim for a more conservative approach, trying noninvasive methods (NIV, HFNC, and prone) [29] to prevent intubation and connection to MV [11,33]. Our findings differ from the results reported by recent studies that address the impact of time from ICU admission to intubation on the outcome [18,[35], [36], [37]]. In the Hernandez-Romieu study [18], the median from hospital to ICU admission was 1.0 days. We can speculate that the short period between hospital admission and ICU admission could have effectively limited the appearance of different clues of disease progression and lung damage after a long time of spontaneous or assisted ventilation in hypoxemic COVID-19 patients.
On the other hand, a recent review [37] that included about 9000 patients did not show statistically detectable differences in mortality between patients undergoing early versus late intubation, suggesting that intubation time may not affect mortality and morbidity of critically ill patients with COVID-19. These results might justify a wait-and-see approach, which may lead to fewer intubations. However, a limitation is a significant variability regarding the definition de early and late intubation and the use of random times based on previous studies, unlike our results that consider the cutoffs to establish optimal cutpoints.
Our study has several limitations. First, it is a single-center cohort study in a tertiary academic hospital, not reflecting necessarily the reality of other hospitals in our country, in which prioritization and triage of MV were even more demanding. Second, we followed a ventilatory management protocol that included HFNC trial, awake proning position, prolonged proning cycles, and ultra-protective ventilation, among other interventions. Notwithstanding that this protocol has a physiological and clinical rationale and is evidence-supported, it could differ from other centers' algorithms, hindering the external validity of our results. Moreover, although we did not have critical personnel shortages, there could have been protocol violations due to highly stressed periods or the weekends, when ICU personnel becomes even more reduced. In these cases, patient care could have been affected, and clinical deterioration overlooked. Nevertheless, in spite of that, ultimately, the decision to intubate depended on the attending clinician. Finally, as we previously mentioned, our results are only hypothesis-generating but provide relevant suggestions to guide future decision-making in the clinical management of hypoxemic COVID-19 patients.
5. Conclusion
In conclusion, we found that hospitalized patients with COVID-19 admitted with a PaO2/FiO2 ratio < 100 mmHg and intubated 48 h after hospital admission had increased mortality. Other identifiable risk factors on admission, such as older age and the use of ACE inhibitors, may increase the risk associated with late intubation. Further studies are required to confirm our findings and establish the best time for intubation in COVID-19 patients admitted with moderate to severe ARDS, the impact of adjuvant therapies, and the ventilatory approach.
Respiratory failure management protocol (figure and text). Description of data: Fig. A. Respiratory failure management protocol. B. Mechanical ventilation protocol.
Ventilatory support according to ARDS severity.
Supplementary material 3.
Differences on arterial pCO2, arterial pH, tidal volume and pulmonary compliance according to time to intubation < or ≥ 48 h. Description of data: Differences on (A) pulmonary compliance, (B) arterial pH, © arterial pCO2 and (D) tidal volume in COVID-19 mechanically ventilated patients according to time to intubation < or ≥ 48 h.
Details on the generation of cutoffs for PaO2/FiO2 ratio, time to intubation and age.
Ethics approval
The Institutional Ethics Committee approved this project (Research Ethics Committee N° 200,504,004, Faculty of Medicine, Pontificia Universidad Católica de Chile), and waived the need for informed consent.
Consent for publication
Not applicable.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
None.
Authors' contributions
MV and RC are guarantors of the entire manuscript; MV, RC, EK, GH and GB.
designed the study; MV, EK, BL, PB, ER, AN, IR, MA, EE, LR, GH and RC collected and analyzed all the data. All the authors helped in the data interpretation and the manuscript draft. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
None.
References
- 1.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA – J Am Med Assoc. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botta M., Tsonas A.M., Pillay J., Boers L.S., Algera A.G., Bos L.D.J., et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir Med. 2020;19:1–10. doi: 10.1016/S2213-2600(20)30459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y., Lu X., Li Y., Chen H., Chen T., Su N., et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201:1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sauld S., Caridi-Scheible M., Blum J.M., Robichaux C. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020;48 doi: 10.1097/CCM.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhont S., Derom E., Van Braeckel E., Depuydt P., Lambrecht B.N. The pathophysiology of “happy” hypoxemia in COVID-19. Respir Res. 2020;21:1–9. doi: 10.1186/s12931-020-01462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobin M.J., Laghi F., Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202:356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jounieaux V., Rodenstein D.O., Mahjoub Y. On happy hypoxia and on sadly ignored “acute vascular distress syndrome” in patients with COVID-19. Am J Respir Crit Care Med. 2020;202:1598–1599. doi: 10.1164/rccm.202006-2521le. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiumello D., Brioni M. Severe hypoxemia: which strategy to choose. Crit Care. 2016;20:1–9. doi: 10.1186/s13054-016-1304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brochard L., Slutsky A., Pesenti A. 2017. Critical care perspective mechanical ventilation to minimize progression of lung injury in acute respiratory failure. [DOI] [PubMed] [Google Scholar]
- 10.Rochwerg B., Einav S., Chaudhuri D., Mancebo J., Mauri T., Helviz Y., et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020;46:2226–2237. doi: 10.1007/s00134-020-06312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raoof S., Nava S., Carpati C., Hill N.S. High-flow, noninvasive ventilation and awake (nonintubation) Proning in patients with coronavirus disease 2019 with respiratory failure. Chest. 2020;158:1992–2002. doi: 10.1016/j.chest.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weatherald J., Solverson K., Zuege D.J., Loroff N., Fiest K.M., Parhar K.K.S. Awake prone positioning for COVID-19 hypoxemic respiratory failure: a rapid review. J Crit Care. 2021;61:63–70. doi: 10.1016/j.jcrc.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi H., Uchino S., Takinami M., Uezono S. 2017. The impact of ventilator-associated events in critically Ill subjects with prolonged mechanical ventilation. [DOI] [PubMed] [Google Scholar]
- 14.Finfer S.R., Vincent J.-L., Slutsky A.S., Ranieri V.M. Critical care medicine ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2162. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 15.Gattinoni L., Marini J.J., Collino F., Maiolo G., Rapetti F., Tonetti T., et al. 2017. The future of mechanical ventilation: lessons from the present and the past. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Megan R., Valerie G., Ashish J. Vol. 41. 2020. Critical supply shortages-the need for ventilators and personal protective equipment during the Covid-19 pandemic; pp. 1489–1491. [DOI] [PubMed] [Google Scholar]
- 17.Kangelaris K.N., Ware L.B., Wang C.Y., Janz D.R., Zhuo H., Matthay M.A., et al. Timing of intubation and clinical outcomes in adults with acute respiratory distress syndrome. Crit Care Med. 2016;44:120–129. doi: 10.1097/CCM.0000000000001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Romieu A.C., Adelman M.W., Hockstein M.A., Robichaux C.J., Edwards J.A., Fazio J.C., et al. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: A single-center cohort study. Crit Care Med. 2020:E1045–E1053. doi: 10.1097/CCM.0000000000004600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA - J Am Med Assoc. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 20.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Vincent J.-L., Moreno R., Takala J., Willatts S., De Mendona A., Bruining H., et al. vol. 22. Springer-Verlag; 1996. The SOFA (Sepsis.related Organ Failure Assessment) score to describe organ dysfunction/failure. [DOI] [PubMed] [Google Scholar]
- 22.Ji D., Zhang D., Xu J., Chen Z., Yang T., Zhao P., et al. 2020. Prediction for progression risk in patients with COVID-19 pneumonia: The CALL score. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;8:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breville G., Accorroni A., Allali G., Adler D. Pathophysiology of COVID-19 related happy hypoxemia. Revue Med Suisse. 2021;17:831–834. [PubMed] [Google Scholar]
- 25.Cruces P., Retamal J., Hurtado D.E., Erranz B., Iturrieta P., González C., et al. 2020. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tobin M.J., Laghi F., Jubran A. P-SILI is not justification for intubation of COVID-19 patients. Ann Intensive Care. 2020;10:105. doi: 10.1186/s13613-020-00724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida T., Amato M.B.P., Kavanagh B.P., Fujino Y. Impact of spontaneous breathing during mechanical ventilation in acute respiratory distress syndrome. Curr Opin Crit Care. 2019;25:192–198. doi: 10.1097/MCC.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 28.Neetz B., Flohr T., Herth F.J.F., Müller M.M. Patient self-inflicted lung injury (P-SILI): From pathophysiology to clinical evaluation with differentiated management. Med Klin Intensivmed Notfallmed. 2021 doi: 10.1007/s00063-021-00823-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tobin M.J., Laghi F., Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care. 2020;10:78. doi: 10.1186/s13613-020-00692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demoule A., Baron A.V., Darmon M., Beurton A., Géri G., Voiriot G., et al. High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202:1039–1042. doi: 10.1164/rccm.202005-2007LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tobin M.J. Basing respiratory management of COVID-19 on physiological principles. Am J Respir Crit Care Med. 2020;201:1319–1336. doi: 10.1164/rccm.202004-1076ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chosidow S., Plantefève G., Fraissé M., Mentec H., Cally R., Contou D. Non-intubated COVID-19 patients despite high levels of supplemental oxygen. Critical Care (London, England) 2021;25:170. doi: 10.1186/s13054-021-03599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y.H., Choi K.-J., Choi S.H., Lee S.Y., Kim K.C., Kim E.J., et al. Clinical significance of timing of intubation in critically ill patients with COVID-19: a multi-center retrospective study. J Clin Med. 2020;9:2847. doi: 10.3390/jcm9092847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Q., Shen J., Chen L., Li S., Zhang W., Jiang C., et al. Timing of invasive mechanic ventilation in critically ill patients with coronavirus disease 2019. J Trauma Acute Care Surg. 2020;89:1092–1098. doi: 10.1097/TA.0000000000002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papoutsi E., Giannakoulis V.G., Xourgia E., Routsi C., Kotanidou A., Siempos I.I. 2020. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: A systematic review and meta-analysis of non-randomized cohort studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Respiratory failure management protocol (figure and text). Description of data: Fig. A. Respiratory failure management protocol. B. Mechanical ventilation protocol.
Ventilatory support according to ARDS severity.
Details on the generation of cutoffs for PaO2/FiO2 ratio, time to intubation and age.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.