INTRODUCTION
The epidermal growth factor receptor (EGFR) T790M mutation has been well studied in lung cancer as a somatically acquired mutation that is predictive of resistance to first- and second-generation EGFR tyrosine kinase inhibitors but sensitive to the third-generation kinase inhibitor osimertinib.1-3 Rarely, EGFR T790M has been identified as a germline variant associated with hereditary lung cancer.4,5 Only a limited number of germline EGFR T790M carriers have been described, mostly white female nonsmokers diagnosed with lung cancer.6-9 The occurrence of lung cancer among germline EGFR T790M carriers is proposed to be higher in women potentially because of estrogen-EGFR pathway interactions.8,10 Overall, the estimated risk of developing lung cancer among nonsmoking EGFR T790M carriers is 31%, compared with a 0.2% risk in a general population of nonsmokers and an approximately 23% risk in a general population of smokers.6,11 However, the sparse clinical data among non–lung cancer cohorts could falsely elevate the estimated hereditary lung cancer risk as a result of ascertainment bias.
Two newly identified, unrelated families harboring germline EGFR T790M are presented. In addition, a clinical laboratory database consisting of > 65,000 patients who underwent germline hereditary cancer risk assessment, not enriched for lung cancer, was data mined to determine the incidence of EGFR T790M and associated patient demographics. A greater understanding of the clinical characteristics associated with germline EGFR T790M may allow for continued development of lung cancer screening strategies and surveillance plans.
CASE REPORTS
Kindred A
A never-smoker 43-year-old white woman with a family history of breast cancer (Fig 1) was incidentally diagnosed with atypical adenomatous lung hyperplasia and followed by surveillance. Radiographic changes prompted bilateral lung biopsies with pathology consistent for lung adenocarcinoma. Pretreatment somatic molecular analysis identified EGFR T790M (61% allele frequency) and EGFR L858R mutations (42% allele frequency). Shortly after the proband was diagnosed with lung cancer, her maternal cousin was diagnosed with breast cancer at age 47 years and underwent a germline hereditary cancer risk panel test (Invitae, San Francisco, CA). A deleterious PALB2 mutation was reported and likely contributed to the family’s breast cancer history.
FIG 1.
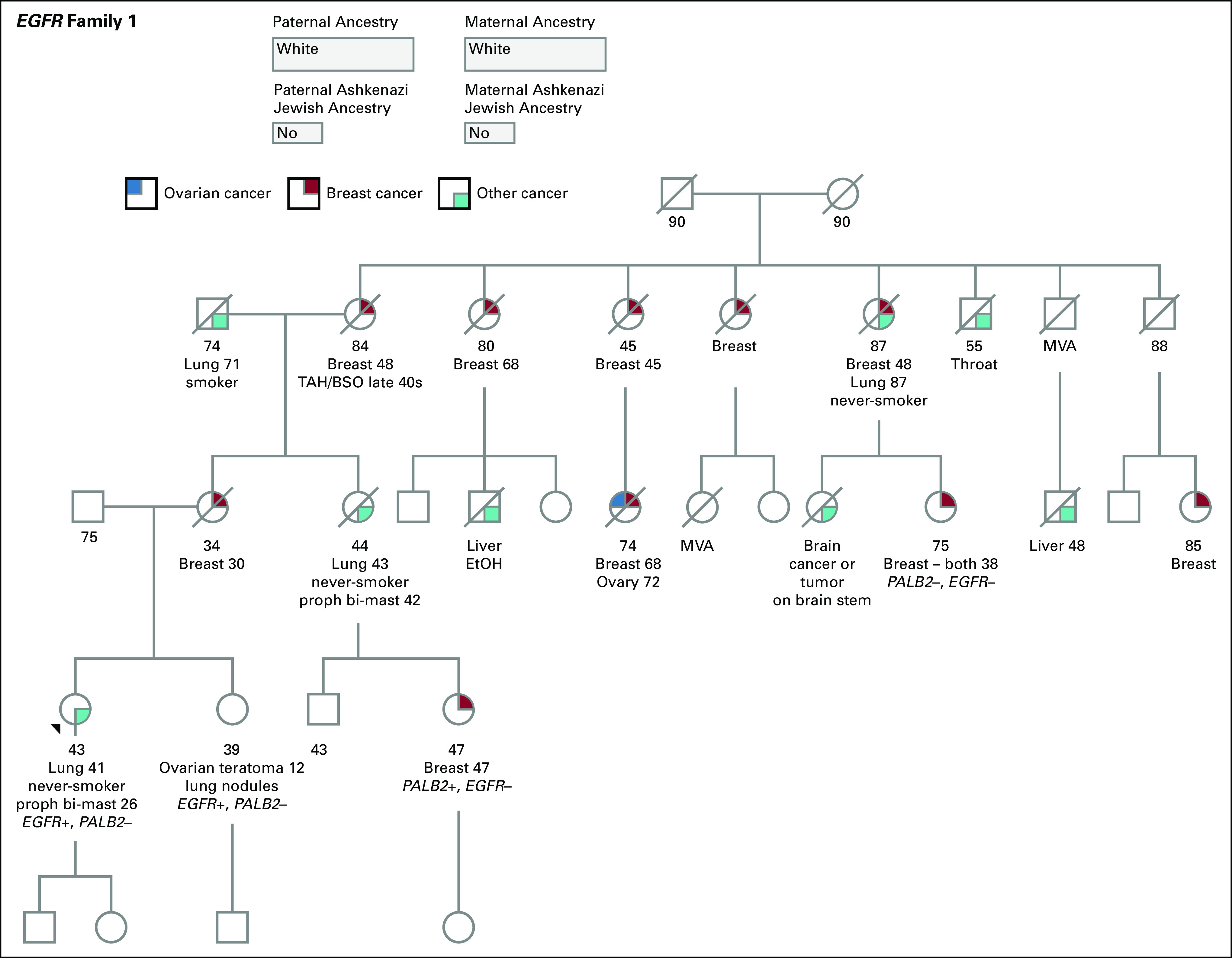
Pedigree of kindred A. The black arrow identifies the proband. BSO, bilateral salpingo-oophorectomy; EtOH, ethanol; MVA, motor vehicle accident; proph bi-mast, prophylactic bilateral mastectomy; TAH, total abdominal hysterectomy.
The discovery of the PALB2 mutation in a relative prompted the proband and her 39-year-old sister to undergo genetic testing (Invitae) for hereditary cancer risk assessment. No PALB2 mutations were observed, but unexpectedly, both individuals were heterozygous for germline EGFR T790M. Evaluation for a family history of lung cancer revealed that the proband’s sister, who is a never-smoker, was diagnosed with bilateral lower lobe lung nodules by computed tomography (CT) at age 38 years. Furthermore, a never-smoker maternal aunt had been diagnosed with metastatic lung cancer at age 43 years, and the maternal grandfather, who was a smoker, had been diagnosed with lung cancer at age 71 years (Fig 1). The proband and her sister were referred to the INHERIT EGFR study (ClinicalTrials.gov identifier: NCT01754025), with a recommendation that the proband’s sister undergo annual low-dose lung CT screening. Because no increased pediatric lung cancer risk has been described to date, it was recommended that the children of the proband and the proband’s sister have EGFR genetic testing in early adulthood.
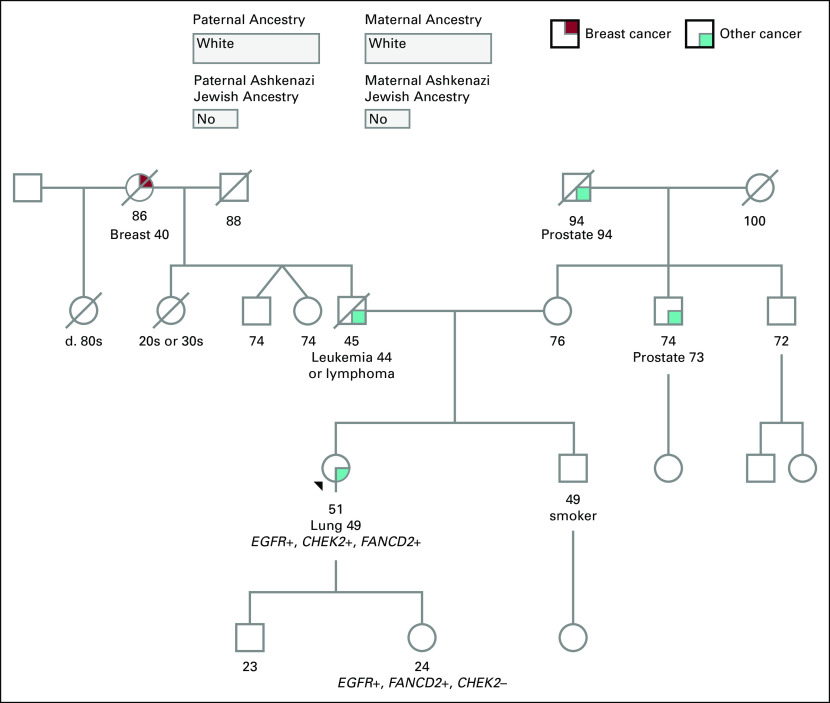
Kindred B
A never-smoker 49-year-old white woman was diagnosed with poorly differentiated metastatic lung adenocarcinoma. Pretreatment somatic molecular analysis (Foundation Medicine, Cambridge, MA) reported several EGFR alterations, including EGFR amplification (copy number of 10.0), EGFR T790M (57.8% allele frequency), and EGFR L861Q (40.8% allele frequency). A CHEK2 T367fs*15 (64% allele frequency) nonsense mutation and FANCD2 loss of exons 2-18 were also reported. Additional molecular analysis was performed to determine whether the reported mutations were somatic or germline alterations. Germline testing (Invitae) confirmed heterozygosity for EGFR T790M, CHEK2 T367fs*15, and FANCD2 deletion of exons 2-18. On the basis of the test results, the proband’s 24-year-old daughter had germline testing performed and was found to have heterozygous EGFR T790M and FANCD2 alterations (Fig 2). For the EGFR T790M variant, a recommendation was given to consider low-dose CT surveillance in the future.
FIG 2.
Pedigree of kindred B. Although the proband and her daughter are carriers of EGFR T790M, there was not a family history of lung cancer. The black arrow identifies the proband.
CLINICAL COHORT ANALYSIS
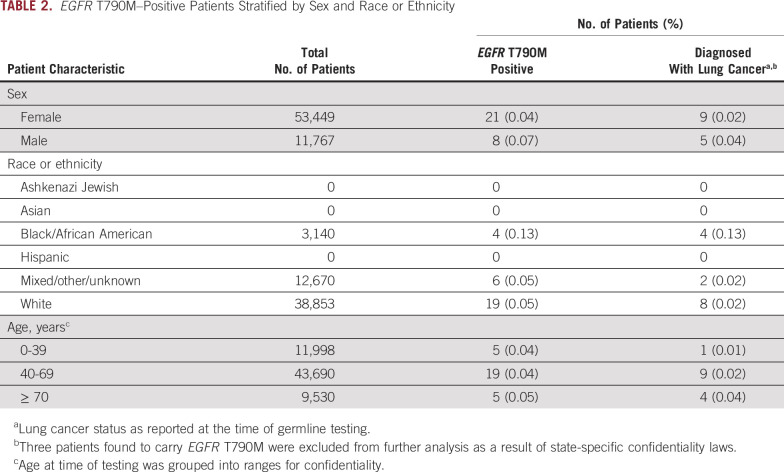
Deidentified clinical data from a cohort of 65,218 individuals undergoing germline hereditary cancer risk assessment (Invitae) were analyzed for the incidence of EGFR T790M (methods described in Data Supplement). Although single-gene EGFR T790M germline testing was available, the clinical cohort consisted of individuals who were tested using an 83-gene cancer panel that included EGFR T790M. There was an overrepresentation of females (82.0%) and whites (59.6%) in the clinical cohort (Table 1). Age at time of germline testing was grouped into ranges to ensure confidentiality, with most patients having molecular analysis performed in the age range of 40-69 years (median, 54 years). A total of 29 individuals (0.04%) were found to be heterozygous carriers of EGFR T790M within this population of individuals undergoing hereditary cancer testing. There was no difference in incidence among males and females (P = .2; Table 2). Stratifying by race, 4 African Americans (0.13%), 19 whites (0.05%), and 6 individuals of mixed or unknown race (0.05%) were EGFR T790M carriers. The rate of incidence trended higher among African Americans, although it was not significant when compared with whites (P = .09) likely because of small sample sizes.
TABLE 1.
Invitae Clinical Cohort Demographic and Testing Characteristics
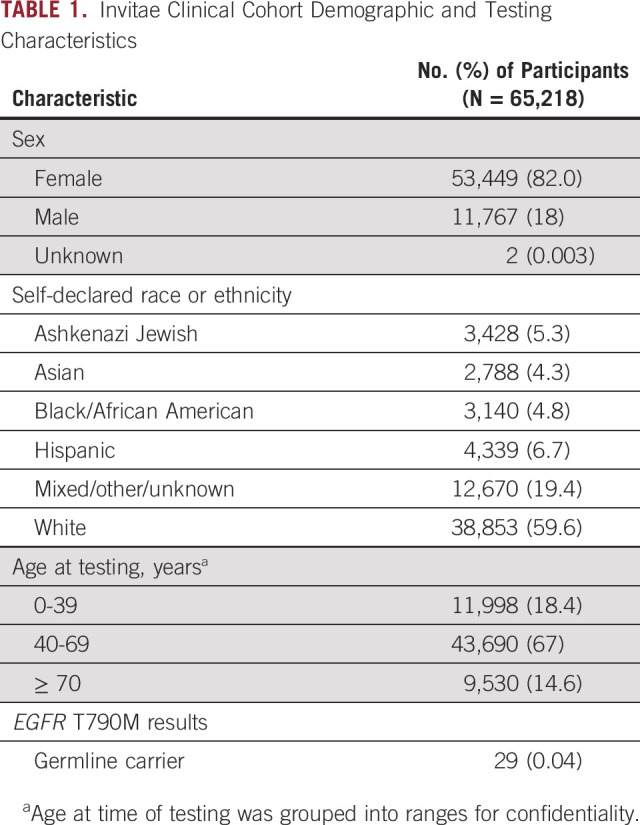
TABLE 2.
EGFR T790M–Positive Patients Stratified by Sex and Race or Ethnicity
Cancer history was available for 26 individuals positive for EGFR T790M, with 14 (53.8%) having a history of lung cancer at time of genetic testing and 2 additional individuals having lung nodules (Data Supplement). The 26 individuals were found among 20 families. Thirteen families had knowledge of an EGFR T790M variant discovered by somatic testing. On the basis of reported personal and/or family history, the remaining 7 families likely had genetic testing performed because of concerns regarding breast cancer risk. The mean age at lung cancer diagnosis was 56 years (range, 37-75 years; Data Supplement). No differences in rate of lung cancer diagnosis were observed among sex (P = .2) or age groups (P = .2; Table 2). The African American patients in the mutation-positive cohort were also impacted, with all 4 African Americans harboring EGFR T790M diagnosed with lung cancer.
DISCUSSION
A review of the literature found 14 studies describing histories of 38 patients with germline EGFR T790M.4-9,12-19 Consistent with our case reports, 88.5% of individuals identified from the literature review for which race or ethnicity was reported were white. Sex was reported for 32 individuals, with 62.5% being female. Of the individuals with lung cancer for whom sex was reported, 71% were women. Taken together, prior studies consisting largely of familial lung cancer or lung cancer cohorts suggest that EGFR T790M hereditary lung cancer syndrome is more prominent among white females.
We analyzed a clinical database for incidence of EGFR T790M that consisted of > 65,000 patients, not enriched for lung cancer, who underwent germline hereditary cancer testing. Only 29 individuals were EGFR T790M carriers, consistent with prior studies suggesting that germline EGFR T790M is rare. The occurrence of EGFR T790M trended higher in African Americans. This observation is consistent with population studies showing EGFR T790M allele frequency of 0.02% in an African population and 0.004% in a European population.20 Further epidemiologic studies are needed in African American populations, because confirmation would have important implications for screening strategies across patient groups in the United States.
Prior studies of EGFR T790M carriers suggest lung cancer development is observed in women of younger age. In the clinical cohort, development of lung cancer among those positive for EGFR T790M was comparable among men and women. Of the 22 patients identified in a literature review with age at lung cancer diagnosis provided, the median age was 57 years (range, 29-81 years). Consistent with historical data, the median age of cancer diagnosis in the clinical cohort was 56 years, ranging from 37-75 years. Taken together, the age at lung cancer diagnosis appears to be significantly younger for EGFR T790M carriers when compared with a median age of 70 years at diagnosis for lung and bronchus cancer reported by SEER21; however, this finding may be a result of a testing bias toward individuals diagnosed at unusually young ages with lung cancer. Younger age of lung cancer onset should be considered when planning surveillance of unaffected mutation carriers.
Analysis of the clinical cohort had limitations, including a lack of race and sex diversity, unknown smoking status, small sample size as a result of the rarity of the mutation, and unknown testing indication for all individuals. Diagnosis of lung nodules or lung cancer after genetic testing was also not available for the clinical cohort. Penetrance could not be calculated because of incomplete family histories and limited genetic information among family members. Prevalence of germline EGFR T790M among a general population could not be inferred because our clinical cohort was enriched for those with personal and family histories suspicious for a hereditary cancer syndrome. These limitations do not diminish our findings that germline EGFR T790M and development of lung cancer are observed across races and ethnicities, sexes, and ages.
For both families described, EGFR T790M was initially discovered by somatic testing. The unexpected result of EGFR T790M in the pretreatment setting only prompted germline confirmation in the second family and was incidentally confirmed in the first family. A lack of germline EGFR T790M confirmation could prevent the consideration of screening and surveillance plans for family members that could potentially reduce the incidence of incurable metastatic lung cancer. Although there are currently no national surveillance recommendations for unaffected EGFR mutation carriers, previous studies have suggested implementing low-dose lung CT based on an observed reduction in lung cancer–associated mortality rates among smokers who underwent low-dose CT surveillance.6,7,22 Both probands had lung cancer with activating somatic EGFR mutations. Among those with germline EGFR T790M who develop lung cancer, prior studies suggest the majority of individuals also have activating EGFR somatic mutations that potentiate cancer development.8
In the clinical cohort, > 60% of individuals with EGFR T790M were documented to have lung cancer or lung nodules. Because germline EGFR T790M can be identified incidentally and is clinically meaningful, individuals undergoing hereditary cancer risk assessment should be given the option to select a gene panel that includes EGFR. For individuals with a suspicious family history of lung cancer, EGFR testing should be strongly considered. Overall, our results support previous recommendations5,15 that all individuals carrying somatic EGFR T790M mutations in the pretreatment setting or with high allele fraction on plasma cell-free DNA testing3 be offered germline testing regardless of sex, ethnicity or race, or age.
ACKNOWLEDGMENT
We thank the patients described in the case reports for allowing their characteristics to be shared. The authors would also like to acknowledge the genetic testing laboratory Invitae for sharing de-identified patient and family history of their EGFR mutation-carrier cohort.
Footnotes
This work was supported in part by National Cancer Institute Cancer Center Support Grant No. P30 CA076292.
AUTHOR CONTRIBUTIONS
Conception and design: Darcy K. Berry, Scott T. Michalski, Howard L. McLeod, J. Kevin Hicks
Financial support: Howard L. McLeod
Administrative support: Howard L. McLeod
Provision of study material or patients: Benjamin C. Creelan
Collection and assembly of data: Darcy K. Berry, Scott T. Michalski, Hio Chung Kang, Shan Yang, Benjamin C. Creelan, Howard L. McLeod, J. Kevin Hicks
Data analysis and interpretation: Darcy K. Berry, Xia Wang, Scott T. Michalski, Shan Yang, Benjamin C. Creelan, Howard L. McLeod, J. Kevin Hicks
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Scott T. Michalski
Employment: Invitae
Stock and Other Ownership Interests: Invitae
Travel, Accommodations, Expenses: Invitae
Hio Chung Kang
Employment: Invitae, Guardant Health (I)
Stock and Other Ownership Interests: Invitae, Guardant Health (I)
Travel, Accommodations, Expenses: Invitae, Guardant Health (I)
Shan Yang
Employment: Invitae
Stock and Other Ownership Interests: Invitae
Travel, Accommodations, Expenses: Invitae
Benjamin C. Creelan
Consulting or Advisory Role: Boehringer Ingelheim, AbbVie, KSQ Therapeutics, BerGenBio, AstraZeneca/MedImmune, ARMO BioSciences, E.R. Squibb Sons, Gilead Sciences, GlaxoSmithKline
Speakers' Bureau: AstraZeneca/MedImmune, ARIAD, Genentech, Takeda, Foundation Medicine
Research Funding: Boehringer Ingelheim (Inst), Bristol-Myers Squibb (Inst), Iovance Biotherapeutics (Inst), Prometheus Laboratories (Inst), NeoGenomics Laboratories (Inst), Biodesix (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Howard L. McLeod
Leadership: Cancer Genetics
Stock and Other Ownership Interests: Cancer Genetics, Interpares Biomedicine
Honoraria: Genentech, Illumina
Consulting or Advisory Role: Gentris, Cancer Genetics, Saladax Biomedical, National Institutes of Health/National Cancer Institute, Admera Health, eviCore Healthcare, Pharmazam, Viecure
Speakers' Bureau: Genentech
Other Relationship: Northwestern University, Xiangya Hospital
J. Kevin Hicks
Consulting or Advisory Role: Quest Diagnostics, 23andMe, Novartis
Research Funding: OneOme
No other potential conflicts of interest were reported.
REFERENCES
- 1.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain PLoS Med: 2e73, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer N Engl J Med: 378113–125, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Oxnard GR, Thress KS, Alden RS, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer J Clin Oncol: 343375–3382, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell DW, Gore I, Okimoto RA, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR Nat Genet: 371315–1316, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Alden RS, Odegaard JI, et al. Discrimination of germline EGFR T790M mutations in plasma cell-free DNA allows study of prevalence across 31,414 cancer patients Clin Cancer Res: 237351–7359, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gazdar A, Robinson L, Oliver D, et al. Hereditary lung cancer syndrome targets never smokers with germline EGFR gene T790M mutations J Thorac Oncol: 9456–463, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu HA, Arcila ME, Harlan Fleischut M, et al. Germline EGFR T790M mutation found in multiple members of a familial cohort J Thorac Oncol: 9554–558, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lou Y, Pecot CV, Tran HT, et al. Germline mutation of T790M and dual/multiple EGFR mutations in patients with lung adenocarcinoma Clin Lung Cancer: 17e5–e11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parry EM, Gable DL, Stanley SE, et al. Germline mutations in DNA repair genes in lung adenocarcinoma J Thorac Oncol: 121673–1678, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stabile LP, Lyker JS, Gubish CT, et al. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects Cancer Res: 651459–1470, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Crispo A, Brennan P, Jöckel KH, et al. The cumulative risk of lung cancer among current, ex- and never-smokers in European men Br J Cancer: 911280–1286, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu S, Yu Y, Li Z, et al. Brief report: EGFR and ERBB2 germline mutations in Chinese lung cancer patients and their roles in genetic susceptibility to cancer J Thorac Oncol: 14732–736, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Prudkin L, Tang X, Wistuba II.Germ-line and somatic presentations of the EGFR T790M mutation in lung cancer J Thorac Oncol: 4139–141, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tibaldi C, Giovannetti E, Vasile E, et al. Inherited germline T790M mutation and somatic epidermal growth factor receptor mutations in non-small cell lung cancer patients J Thorac Oncol: 6395–396, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Oxnard GR, Miller VA, Robson ME, et al. Screening for germline EGFR T790M mutations through lung cancer genotyping J Thorac Oncol: 71049–1052, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Walsh MF, Wu G, et al. Germline mutations in predisposition genes in pediatric cancer N Engl J Med: 3732336–2346, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sukari A, Nagasaka M, Wakeling E.EGFR-mutant non-small cell lung cancer in the era of precision medicine: Importance of germline EGFR T790M testing J Natl Compr Canc Netw: 151188–1192, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Ancevski Hunter K, Friedland DM, Villaruz LC, et al. First-line osimertinib in patients with treatment-naive somatic or germline EGFR T790M-mutant metastatic NSCLC J Thorac Oncol: 13e3–e5, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Girard N, Lou E, Azzoli CG, et al. Analysis of genetic variants in never-smokers with lung cancer facilitated by an Internet-based blood collection protocol: A preliminary report Clin Cancer Res: 16755–763, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karczewski K, Francioli L, Tiao G, et al. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. bioRxiv. 10.1101/531210, 2019. [Google Scholar]
- 21.National Cancer Institute: Cancer Stat Facts: Lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html
- 22.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening N Engl J Med: 365395–409, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]