INTRODUCTION:
Hepatic encephalopathy (HE) is a major complication of transjugular intrahepatic portosystemic shunt (TIPS) creation. This study was aimed to determine whether underdilated TIPS with 8-mm polytetrafluoroethylene-covered stents could reduce the risk of HE and liver damage yet maintain clinical and hemodynamic efficacy.
METHODS:
This retrospective case-controlled study included 134 patients treated with TIPS from March 2017 to November 2019. All the TIPS procedures were created using 8-mm covered stents, and according to the diameter of expansion balloon catheters, the patients were divided into 2 groups, an underdilated group (6-mm balloon catheter, n = 73) and a control group (8-mm balloon catheter, n = 61).
RESULTS:
The Kaplan-Meier analysis indicated that the cumulative incidence of overt HE in the underdilated group was significantly lower than that in the control group (11.0% vs 29.5%, log rank P = 0.007), but no statistical differences were found toward variceal rebleeding, shunt dysfunction, and survival between groups. In multivariate analysis, the independent risk factors for overt HE were identified as age (hazard ratio [HR] = 1.036, 95% confidence interval [CI] = 1.003–1.069, P = 0.032), Child-Pugh score (HR = 1.519, 95% CI = 1.212–1.905, P < 0.001), and group assignment (HR = 0.291, 95% CI = 0.125–0.674, P = 0.004).
DISCUSSION:
Underdilated TIPS with 8-mm polytetrafluoroethylene-covered stents could reduce the risk of HE and liver function impairment compared with completely dilated TIPS, but not increase the risk of variceal rebleeding, shunt dysfunction, and death.
INTRODUCTION
Transjugular intrahepatic portosystemic shunt (TIPS) is currently used to treat some complications of portal hypertension (1,2). Since the Freiburg TIPS project was started in 1988 (3,4), it has gradually developed and spread worldwide (5,6). Notably, with the use of polytetrafluoroethylene (PTFE)-covered stents, the incidence of shunt dysfunction has been significantly reduced (7). TIPS can not only effectively control variceal bleeding and refractory ascites but also play a significant role on portal vein thrombosis as shown in some research studies (8,9).
However, hepatic encephalopathy (HE) is one of the major complications of the TIPS procedure, and its incidence rate remains around 30% despite the widely application of covered stents (5,10). There are many risk factors for post-TIPS HE including history of HE, older age, higher Child-Pugh score, and lower serum sodium. Overall, it is caused by 2 main mechanisms: portal blood diversion and reduced hepatic metabolic capacity (11,12). After TIPS placement, the portal blood diversion from liver to systemic circulation and the reduction in residual liver perfusion would lead to a high risk of HE and liver dysfunction.
The volume of portal blood diversion is variable and dependent on the TIPS diameter, so the occurrence of HE is somewhat related to the TIPS diameter (12). Many researchers now recommend the small diameter (8-mm) stent to replace the large diameter (10-mm) stent for TIPS creation, and some randomized controlled trials confirmed that the 8-mm stent could indeed reduce the incidence of HE after TIPS implantation (13,14). Nevertheless, TIPS diameter should not be too small, for example, the 6-mm stent not only fails to reach the target post-TIPS portal pressure gradient (PPG) but also causes a high risk of shunt dysfunction (15).
However, completely dilated TIPS (8-mm PTFE-covered stent completely dilated by 8-mm balloon) may cause excessive shunting, resulting in unnecessary risk of HE or liver function damage. Thus, underdilated TIPS (8-mm PTFE-covered stent partially dilated by 6-mm balloon) seems to be a reliable alternative.
We hypothesized that underdilated TIPS could reduce the incidence of post-TIPS HE and liver damage while ensuring the target of reducing PPG. Overall, this study was aimed to confirm our conjecture by comparing the clinical outcomes between underdilated and completely dilated TIPS.
MATERIALS AND METHODS
This retrospective case-controlled study was conducted according to the Declaration of Helsinki. The protocol was approved by the institutional review board, and the requirement for informed consent was waived.
Patients and study design
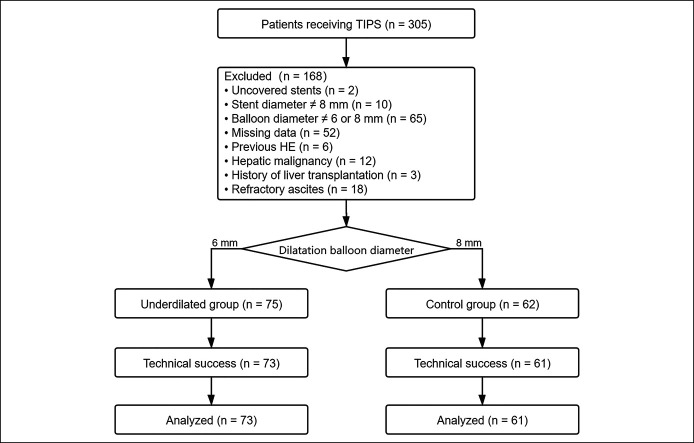
From March 2017 to November 2019, 305 consecutive patients with portal hypertension underwent TIPS creation in our institution. The indications for TIPS included uncontrollable or recurrent variceal bleeding, which were diagnosed by clinical symptoms, laboratory tests, endoscopy, and computer tomography (CT) examinations. Given that the number of patients with refractory ascites was too few to perform meaningful statistics between groups, none were included. The exclusion criteria were as follows: (i) HE within 3 months before TIPS; (ii) TIPS creation with uncovered stents; (iii) stent diameter ≠ 8 mm or dilating balloon diameter ≠ 6 or 8 mm; (iv) hepatic malignancy; (v) portal vein occlusion or portal cavernoma; (vi) history of liver transplantation; and (vii) refractory ascites.
Finally, 171 patients were excluded, and a total of 134 patients were included in this study. They were then divided into 2 groups according to the diameters of expansion balloon catheters. In the underdilated group, 73 patients received TIPS created by 8-mm covered stents and 6-mm balloon catheter, and in the control group, 61 patients received TIPS created by 8-mm covered stents and 8-mm balloon catheter (Figure 1).
Figure 1.
Study design and flowchart. HE, hepatic encephalopathy; TIPS, transjugular intrahepatic portosystemic shunt.
TIPS procedure
The TIPS procedure has been detailed described previously (16,17). After local anesthesia with lidocaine, catheterization of the right or middle hepatic vein was performed through the right internal jugular vein with a transjugular liver access set (RUPS-100; Cook Inc, Bloomington, IN). Under the fluoroscopic guidance, one of the branches of portal vein was punctured through the hepatic vein, and the portogram was obtained subsequently. After the parenchymal tract was preballoon dilated by a 6-mm balloon catheter (Bard, Karlsruhe, Germany), an 8-mm bare metal stent (E-Luminexx or Lifestent; Bard Inc, Tempe, AZ) combined with an 8-mm ePTFE-covered stent (Fluency; Bard Inc. or Viabahn; Gore Inc.) was implanted between the hepatic vein and portal vein; the length of the covered stent inside the portal vein was less than 1 cm. In underdilated and control groups, stents were, respectively, dilated using 6- and 8-mm balloon catheters. PPG was measured in every patient before and after TIPS insertion. Current guidelines recommend that post-TIPS PPG should be reduced below 12 mm Hg, and a PPG reduction of more than 50% has been suggested as an alternative target (18). Stent diameter was measured through poststenting venogram immediately after TIPS placement and through thin-slice CT image 3 months after TIPS placement.
There are 3 medical teams in our center. In our team, we used to perform completely dilated TIPS using an 8-mm balloon catheter. However, after reading latest literatures and combined with our lessons learned, we assumed underdilated TIPS using 6 mm might be more appropriate, and thus, we adopted this method from then on. As a result, in the 2 groups of cases that we collected, the subjects with 6-mm balloon dilatation were mainly from our team; the patients with 8-mm balloon dilatation were partly from earlier patients in our team and partly from the other 2 medical teams in our center. It is important to note that, during the TIPS procedure, same steps were used in each team according to the standard processes (16).
Follow-up and clinical outcomes
After the procedure, no patients received pharmacologic prophylaxis for HE. All the patients were followed up with clinical evaluation, biochemical tests, Doppler ultrasonography, and contrast-enhanced CT examination at 1 month, 3 months, and every 6 months thereafter, which was mainly conducted through outpatient or inpatient reviews, telephone interviews, and other forms. The Child-Pugh, Model for End-Stage Liver Disease (MELD), MELD-Na, and albumin-bilirubin (ALBI) scores were calculated at each follow-up.
The primary outcome of this study was the incidence and severity of HE, and secondary outcomes included liver function, the incidence of variceal rebleeding, shunt dysfunction, and survival.
Variceal rebleeding was defined as a single episode of clinically significant rebleeding (recurrent melena or hematemesis resulting in hospital admission, blood transfusion, 3 g drop in hemoglobin, or death) from portal hypertensive sources (19). According to the West Haven criteria, HE is evaluated and divided into 4 grades. Grade 2 and above is defined as overt HE (OHE). Patients diagnosed with mild HE (grade 1 or 2) were immediately ordered to be hospitalized for treatment using lactulose. Patients diagnosed with severe HE (grade 3 or 4) or recurrent HE (≥2 episodes of OHE within 6 months) were considered for shunt reduction (20). Shunt dysfunction was suspected based on Doppler ultrasonography (maximum shunt flow velocity of ≤50 or ≥250 cm/s) or on clinical findings (variceal bleeding and recurrent ascites). If shunt dysfunction was suspected, venography through the transjugular route was used to confirm it. Once positive, TIPS revision was advised, which included thrombus aspiration, balloon angioplasty, or stent implantation within the existing stent (19,21).
Statistical analyses
All continuous variables were expressed as mean ± SD, and differences were compared using the paired or unpaired t test. Categorical variables were expressed as number (%), and differences were compared using the corrected χ2 test or Fisher exact test; comparisons across ordinal data were made using the Wilcoxon rank-sum test. Kaplan-Meier curves were used to illustrate the cumulative incidence of OHE, variceal rebleeding, shunt dysfunction, and survival, and differences were tested using the log-rank test. Multivariate Cox proportional-hazards analysis was used for identifying independent prognostic factors; the hazard ratio (HR) and 95% confidence interval (CI) were calculated and reported. Data processing and analyses were performed by using IBM SPSS statistics version 22.0 (IBM, Chicago, IL), and we used GraphPad Prism version 8 for drawing figures.
RESULTS
Patient characteristics
Main clinical and biological baseline characteristics in underdilated and control groups are shown in Table 1, and the 2 groups were comparable in terms of age; sex; etiology; indications for TIPS; laboratory parameters; Child-Pugh, MELD, MELD-Na, and ALBI scores; pre-TIPS PPG; the presence of ascites; portal vein thrombosis; and spontaneous portosystemic shunts. The median follow-up time was 20.5 months (range, 12–42 months).
Table 1.
Baseline characteristics of patients
| Variables | Underdilated group (n = 73) | Control group (n = 61) | P values |
| Age, yr | 55.0 ± 11.2 | 53.2 ± 10.6 | 0.246 |
| Sex, male | 41 (56.2) | 42 (68.9) | 0.155 |
| Etiology | 0.691 | ||
| Hepatitis B virus | 35 (47.9) | 32 (52.5) | |
| Hepatitis C virus | 9 (12.3) | 7 (11.5) | |
| Alcohol misuse | 7 (9.6) | 2 (3.3) | |
| Schistosome | 10 (13.7) | 8 (13.1) | |
| Others | 12 (16.4) | 12 (19.7) | |
| Indications for TIPS | 0.138 | ||
| Acute variceal bleeding | 33 (45.2) | 21 (34.4) | |
| Secondary prophylaxis of variceal bleeding | 40 (54.8) | 40 (65.6) | |
| Laboratory parameters | |||
| TB, mg/dL | 1.36 ± 1.02 | 1.58 ± 1.28 | 0.272 |
| Albumin, g/L | 30.2 ± 6.0 | 31.0 ± 7.0 | 0.522 |
| Alanine aminotransferase, U/L | 35.3 ± 36.0 | 38.1 ± 38.2 | 0.661 |
| Aspartate aminotransferase, U/L | 43.6 ± 40.7 | 49.2 ± 48.2 | 0.474 |
| Creatinine, mg/dL | 0.74 ± 0.27 | 0.75 ± 0.19 | 0.825 |
| Prothrombin time, seconds | 16.4 ± 2.7 | 17.0 ± 2.9 | 0.226 |
| International normalized ratio | 1.35 ± 0.28 | 1.41 ± 0.32 | 0.272 |
| Hemoglobin, g/L | 76.4 ± 17.2 | 79.0 ± 14.3 | 0.362 |
| Platelet count, 109/L | 110.5 ± 93.2 | 98.2 ± 87.1 | 0.436 |
| Child-Pugh score | 7.6 ± 1.6 | 7.7 ± 1.8 | 0.889 |
| Child-Pugh class | 0.159 | ||
| A | 18 (24.7) | 21 (34.4) | |
| B | 49 (67.1) | 31 (50.8) | |
| C | 6 (8.2) | 9 (14.8) | |
| MELD score | 11.1 ± 3.2 | 11.6 ± 3.9 | 0.362 |
| MELD-Na score | 12.4 ± 3.1 | 12.9 ± 4.8 | 0.490 |
| ALBI score | −1.72 ± 0.56 | −1.75 ± 0.67 | 0.822 |
| Ascites | 0.976 | ||
| Nonascites | 16 (18.6) | 12 (18.2) | |
| Slight ascites | 33 (38.4) | 24 (36.3) | |
| Moderate or severe ascites | 37 (43.0) | 30 (45.5) | |
| Portal vein thrombosis | 31 (42.5) | 20 (32.8) | 0.286 |
| Spontaneous portosystemic shunts | 23 (26.7) | 13 (15.2) | 0.241 |
| Pre-TIPS PPG, mm Hg | 26.1 ± 5.4 | 26.9 ± 5.4 | 0.404 |
Data are presented as mean ± SD or number of patients (%) where appropriate.
ALBI, albumin-bilirubin; MELD, Model for End-Stage Liver Disease; PPG, portal pressure gradient; TIPS, transjugular intrahepatic portosystemic shunt.
According to different medical teams, there were 103, 17, and 14 patients included in this study from team 1, team 2, and team 3, respectively. In team 1, 63 patients belonged to the underdilated group and 40 belonged to the control group. In team 2, 4 patients belonged to the underdilated group and 17 belonged to the control group. In team 3, 6 patients belonged to the underdilated group and 8 belonged to the control group. As shown in Supplementary Table 1 (see Supplementary Digital Content 2, http://links.lww.com/CTG/A643), we compared the baseline characteristics between the 3 medical teams, and no significant differences were found.
Stent diameter and PPG
TIPS was successfully created in all patients, with no immediate procedural-related complications such as hemobilia, intrahepatic bleeding, or hemoperitoneum identified within 24 hours after TIPS placement.
In the underdilated group, the mean stent diameter significantly expanded from 6.0 ± 0.5 mm (immediately after TIPS) to 8.0 ± 0.4 mm (3 months after TIPS), P < 0.001. But in the control group, the mean stent diameter was 7.9 ± 0.3 mm and 8.0 ± 0.3 mm, respectively, immediately and 3 months after TIPS, with no statistical difference (P = 0.870) (Figure 2).
Figure 2.
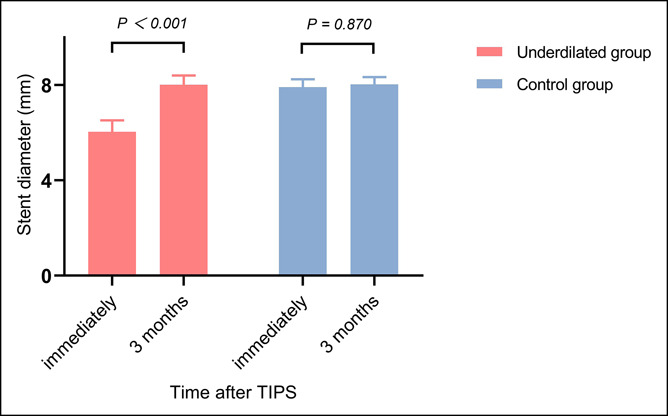
Changes in stent diameter: Mean stent diameter of 2 groups immediately and 3 months after TIPS. TIPS, transjugular intrahepatic portosystemic shunt.
The average PPG significantly decreased immediately after TIPS placement in underdilated and control groups, with no between-group difference (26.1 ± 5.4 to 11.0 ± 3.8 mm Hg vs 26.9 ± 5.4 to 10.6 ± 2.7 mm Hg, P = 0.194). In this study cohort, the PPG of 51 (69.9%) patients in the underdilated group and 46 (75.4%) in the control group fell below 12 mm Hg (P = 0.562). Despite remaining 37 patients with post-TIPS PPG still over 12 mm Hg, the PPG decrease rates of them all exceeded 50% (Figure 3).
Figure 3.
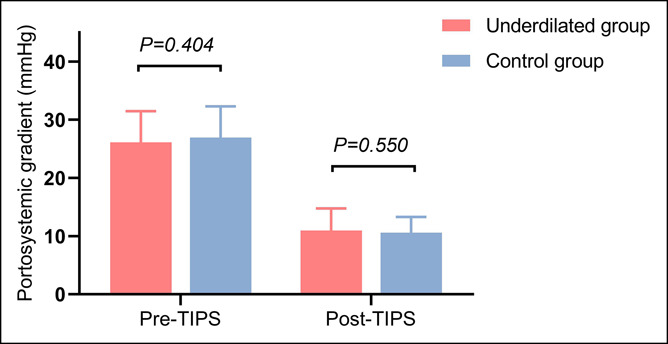
Comparison of pre- and post-TIPS PPG measurements between the underdilated and control groups. PPG, portal pressure gradient; TIPS, transjugular intrahepatic portosystemic shunt.
Clinical outcomes
Hepatic encephalopathy
During the follow-up period, 11 (15.1%) patients developed HE in the underdilated group, and among them, 3, 5, 2, and 1 patients were assessed as grade 1, 2, 3, and 4, respectively. However, 23 (37.7%) patients developed HE in the control group. Five, 10, 6, and 2 patients were assessed as grade 1, 2, 3, and 4, respectively. Of all 34 cases with HE, 30 (88.2%) occurred within 3 months of TIPS implantation. Patients diagnosed with grade 1 or 2 HE were immediately ordered to be hospitalized for treatment using lactulose and diet intervention, and the symptoms of them disappeared subsequently. Patients diagnosed with grade 3 or 4 or refractory HE were treated with shunt reduction, and the symptoms disappeared but 1 of them developed stent thrombosis.
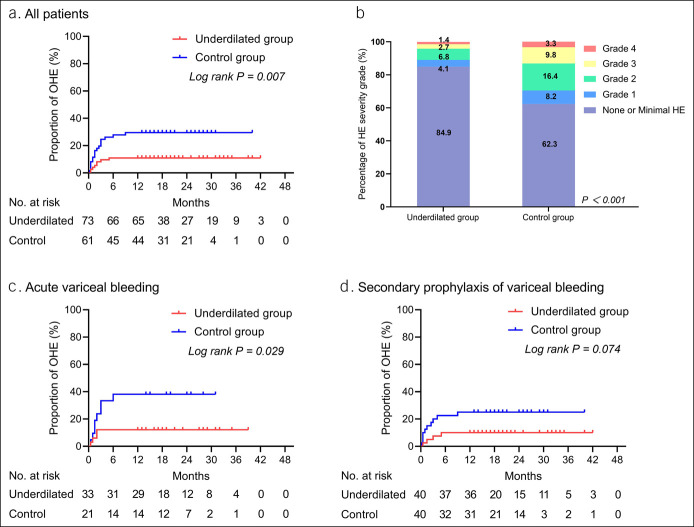
Kaplan-Meier analysis revealed that the cumulative incidence of OHE in the underdilated group was significantly lower than that in the control group (11.0% vs 29.5%, HR = 0.339, 95% CI = 0.156–0.736, log rank P = 0.007) (Figure 4A), and the degree of HE was more severe in the control group than that in the underdilated group (P < 0.001) (Figure 4B). Multivariate analysis showed that the independent risk factors for OHE were age (HR = 1.036, 95% CI = 1.003–1.069, P = 0.032), Child-Pugh score (HR = 1.519, 95% CI = 1.212–1.905, P < 0.001), and group assignment (HR = 0.291, 95% CI = 0.125–0.674, P = 0.004) (Table 2).
Figure 4.
Kaplan-Meier curve of OHE and percentage bar chart of HE grade: (a) Cumulative incidence of OHE observed in all patients. (b) The percentage of the most severe HE grade was compared with the Mann-Whitney U test and was significantly different in the 2 groups. (c) Cumulative incidence of OHE observed in patients with acute variceal bleeding. (d) Cumulative incidence of OHE observed in patients with secondary prophylaxis of variceal bleeding. HE, hepatic encephalopathy; OHE, overt HE.
Table 2.
Univariate and multivariate analysis of factors associated with post-TIPS OHE
| Univariate analysis | Multivariate analysis | |||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age, y (1-U increment) | 1.035 | 1.007–1.070 | 0.044 | 1.036 | 1.003–1.069 | 0.032 |
| Gender (male vs. female) | 0.664 | 0.278–1.587 | 0.357 | — | — | |
| Child-Pugh score (1-U increment) | 1.492 | 1.200–1.855 | <0.001 | 1.519 | 1.212–1.905 | <0.001 |
| MELD score (1-U increment) | 1.136 | 1.054–1.224 | 0.001 | — | ||
| Group (underdilated vs. control) | 0.339 | 0.147–0.779 | 0.011 | 0.291 | 0.125–0.674 | 0.004 |
MELD, Model for End-Stage Liver Disease; OHE, overt hepatic encephalopathy; TIPS, transjugular intrahepatic portosystemic shunt.
Subgroup analysis was performed because of the different indications for TIPS. In patients with acute variceal bleeding, the cumulative incidence of OHE in underdilated and control groups was 12.1% and 38.1%, respectively (HR = 0.290, 95% CI = 0.090–0.938, log rank P = 0.029). But in patients with secondary prophylaxis of variceal bleeding, the cumulative incidence of OHE in 2 groups was 10.0% and 25.0%, respectively (HR = 0.366, 95% CI = 0.128–1.043, log rank P = 0.074) (Figure 4C, D).
In addition, the cumulative incidence of HE in different medical teams was shown in Supplementary Figure 1A (see Supplementary Digital Content 1, http://links.lww.com/CTG/A642).
Variceal rebleeding, shunt dysfunction, and survival
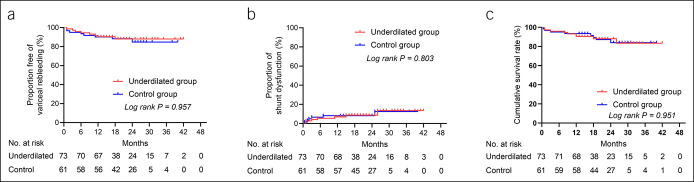
During the follow-up period, 7 (9.6%) and 6 (9.8%) cases developed variceal rebleeding in underdilated and control groups, respectively (log rank P = 0.957). Shunt dysfunction was identified in 8 (11.0%) patients in the underdilated group and 8 (13.1%) in the control group (log rank P = 0.803). After TIPS revision, the restoration of stent patency was achieved in all the above patients. As for survival, 9 (12.3%) deaths were recorded in the underdilated group (3 died of liver failure, 3 of variceal rebleeding, 2 of septic shock, and 1 of unknown cause). And, there were 8 (6.1%) deaths in the control group (5 died of liver failure, 2 of heart failure, and 1 of variceal rebleeding).
There were no significant statistical differences between the 2 groups toward the cumulative incidence of variceal rebleeding (log rank P = 0.957), shunt dysfunction (log rank P = 0.803), and survival (log rank P = 0.951), no matter which subgroup of different indications for TIPS (Figure 5).
Figure 5.
Kaplan-Meier curves of variceal rebleeding (a), shunt dysfunction (b), and survival (c). There were no significant statistical differences between underdilated and control groups.
In addition, the cumulative incidence of HE in different medical teams was shown in Supplementary Figure 1B, C, and D (see Supplementary Digital Content 1, http://links.lww.com/CTG/A642).
Liver function
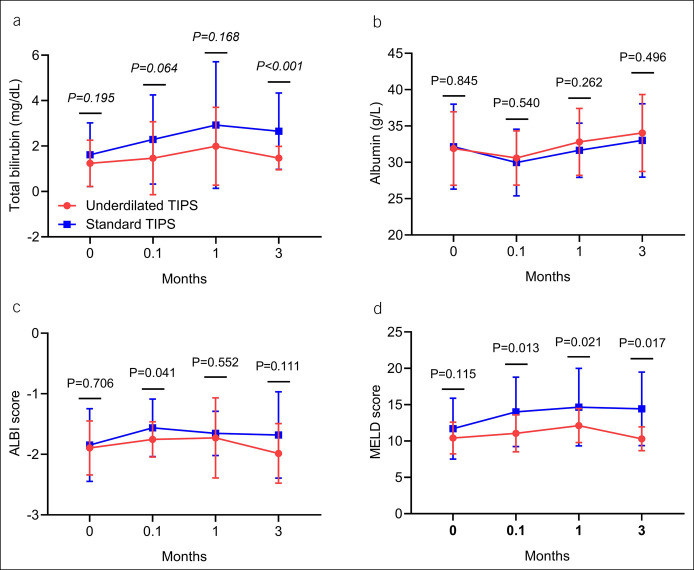
The baseline laboratory parameters were comparable between the 2 groups. Total bilirubin (TB) value, ALBI score, and MELD score showed a trend of increasing first and then decreasing in both groups. Initially, there was no significant difference in the mean TB value and the MELD score between the 2 groups, but over time, at 3 months after TIPS implantation, the mean TB value and the MELD score were significantly lower in the underdilated group than those in the control group. As for the albumin value, it first decreased and then increased in both groups with no significant differences (Figure 6).
Figure 6.
Line chart of total bilirubin (a), albumin (b), ALBI score (c), and MELD score (d) in underdilated and control groups was compared with the Student t test. ALBI, albumin-bilirubin; MELD, Model for End-Stage Liver Disease; TIPS, transjugular intrahepatic portosystemic shunt.
DISCUSSION
It is very important to select a proper shunt diameter during the TIPS procedure. On the one hand, it needs to be large enough to achieve the target post-TIPS PPG. On the other hand, it should not be too large. Otherwise, it may lead to shunt-related complications, which mainly include HE and deterioration of liver function (22,23). The diameter of the stent that should be used for TIPS creation is still a matter of research; some previous studies demonstrated that an 8-mm stent seemed to be more appropriate than a 10-mm stent for TIPS creation (14,24).
According to the Italian consensus conference, underdilation TIPS is recommended to balance the reduction of PPG and the occurrence of HE resulted from excessive shunting (25). Trebicka et al. (26) found that 10-mm stents with or without underdilation showed a similar reduction of PPG. The same results were observed in our study; no significant difference of PPG reduction was found between underdilated and completely dilated TIPS, and both of them could reach the target post-TIPS PPG (below 12 mm Hg or a reduction of at least 50%). Meanwhile, Kaplan-Meier analysis showed that underdilated TIPS significantly reduced the incidence of OHE compared with completely dilated TIPS, and the degree of HE was more severe in the control group than that in the underdilated group. Multivariate analysis showed that group assignment was a significant risk factor for the development of OHE, which suggested that 8-mm stents with underdilation could not only achieve satisfactory PPG reduction but also reduce the risk of developing OHE.
These results were similar to a previous prospective, nonrandomized study (27), which compared the clinical outcomes of TIPS creation with 10-mm stents underdilated by smaller-diameter (7 or 6 mm) and larger-diameter balloon (8 mm or more). But in another retrospective single-arm study (28), 28 patients received underdilated TIPS with 8-mm stents, and the incidence rate of post-TIPS HE was 31.8%, which showed no improvement compared with previously published studies elsewhere. Also, several studies have shown that passive expansion of underdilated stent grafts occurs within a short postprocedure time frame approximating weeks to months (29,30). The study of Pieper et al. (31) demonstrated that if 10-mm stents were underdilated to 80% of their nominal diameter, the stents would self-expand to approximately 88% of the nominal diameter 1 week after TIPS creation and to 94% after 6 weeks. Based on this, they came to the conclusion that underdilated TIPS could not provide any clinical benefits (32,33).
However, our present findings confirmed the ability of underdilated TIPS to reduce the incidence of HE, which may be due to the fact that HE was particularly frequent during the first few months after TIPS placement and less common thereafter (11). And, we found that most patients (88.2%) developed HE within 3 months of TIPS implantation. That is, during the peak of HE occurrence, the underdilated stent is still in the process of passive expansion, and with smaller diameter than nominal, which is similar to shunt reduction, the risk of HE is rather lower than completely dilated TIPS. As the brain gradually adapts to the neurotoxic environment and the hepatic arterial compensatory flow increases gradually, the individual has already passed the high-risk period of HE when the stent completely expand to the nominal diameter. All in all, underdilated TIPS could ensure that the stent diameter is minimized during the high-risk period of HE, thereby minimizing the risk of HE.
In subgroup analysis, when the indication was acute variceal bleeding, the effect of underdilated TIPS to reduce HE seemed to be more pronounced. Because of the deficient blood volume and related inadequate liver perfusion in patients with acute variceal bleeding, their hepatic metabolic capacity decreased and HE was more likely to occur. Thus, it is more urgent to choose underdilated TIPS for such patients.
A further important finding was the fewer liver damages of underdilated TIPS. In our results, the TB value and the MELD score increased initially after TIPS creation, but they returned to baseline levels quickly over time in underdilated TIPS, but this was not the case in the control group. Therefore, we have reason to believe that underdilated TIPS could reduce the risk of liver function impairment in contrast to completely dilated TIPS.
Other clinical outcomes including variceal rebleeding and survival were similar in underdilated and control groups; this could correlate with the same PPG reduction between the 2 groups. The study of Rössle et al. (34) showed a probability of rebleeding of 18%, 7%, and 1% for a reduction of the index pressure gradient by 0%, 25%–50%, and >50%, respectively. In our study, post-PPG in 72.4% of the patients fell below 12 mm Hg in those who received underdilated TIPS, and all remaining patients had a reduction of >50%. The above results proved that using 6-mm balloon to expand an 8-mm stent could reach the target of reducing PPG and controlling rebleeding.
During the underdilated TIPS procedure, the shunt diameter was initially only 6 mm, which may be related to shunt dysfunction, so special attention should be paid to stent patency. In the study of Madoff et al. (35), shunt reduction was performed in 6 patients to treat refractory HE by reducing shunt diameters to 6 mm, and 2 patients (33%) had thrombosis development afterward. But in our study, no significant difference was found in shunt dysfunction between underdilated and control groups, which may be due to the passive expansion of underdilated stent grafts.
In addition, several limitations were present in this study. First, as a single center study, there may be some selection bias. Second, bare metal stent/Fluency- or Viabahn-covered stent combination rather than the Viatorr-covered stent was used because only the former was available in China. But some researchers confirmed that double stents could be considered to be an alternative choice when the availability of the Viatorr stent was limited (36,37). Finally, although the data were derived from 3 different medical teams, during the TIPS procedure, same steps were used in each team, and good matching of a number of pretreatment characteristics was shown in the results.
In conclusion, underdilated TIPS with 8-mm PTFE-covered stents could reduce the risk of HE and liver function impairment compared with completely dilated TIPS, but not increase the risk of variceal rebleeding, shunt dysfunction, and death.
CONFLICTS OF INTEREST
Guarantor of the article: Bin Xiong, PhD.
Specific author contributions: Jiacheng Liu, MD, and Jinqiang Ma, MS, contributed equally to this article. B.X., J.L., and J.M. conceived and designed the project. J.M., J.L., C.Z., and C.Y. collected the data. J.L., S.H., and Q.S. analyzed and interpreted the data. J.L. and J.M. drafted the manuscript. B.X. revised the manuscript. All authors read and approved the final manuscript.
Financial support: This work was supported by the National Natural Science Foundation of China (grant number: 81873917).
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Complete dilation of 8-mm covered stents was usually used for transjugular intrahepatic portosystemic shunt (TIPS) creation, but the risk of post-TIPS complications such as hepatic encephalopathy and liver function impairment was relatively high.
WHAT IS NEW HERE
✓ This study is the first to apply underdilation of 8-mm covered stents in transjugular intrahepatic portosystemic shunt creation.
TRANSLATIONAL IMPACT
✓ Underdilated TIPS with 8-mm polytetrafluoroethylene-covered stents could reduce the incidence and severity of HE and the risk of liver function impairment compared with completely dilated TIPS.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A642 and http://links.lww.com/CTG/A643
Contributor Information
Jiacheng Liu, Email: jiacheng6jc@163.com.
Jinqiang Ma, Email: 18942931650@163.com.
Chen Zhou, Email: zhouchenjr@126.com.
Chongtu Yang, Email: Henrys1011@163.com.
Songjiang Huang, Email: hsjhzkjdx@163.com.
Qin Shi, Email: shiq1010@126.com.
REFERENCES
- 1.Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017;65(1):310–35. [DOI] [PubMed] [Google Scholar]
- 2.de Franchis R. Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63(3):743–52. [DOI] [PubMed] [Google Scholar]
- 3.Rössle M, Richter GM, Nöldge G, et al. New non-operative treatment for variceal haemorrhage. Lancet 1989;2(8655):153. [DOI] [PubMed] [Google Scholar]
- 4.Rössle M. TIPS: 25 years later. J Hepatol 2013;59(5):1081–93. [DOI] [PubMed] [Google Scholar]
- 5.Bosch J, García-Pagán JC. Prevention of variceal rebleeding. Lancet 2003;361(9361):952–4. [DOI] [PubMed] [Google Scholar]
- 6.García-Pagán JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010;362(25):2370–9. [DOI] [PubMed] [Google Scholar]
- 7.Bureau C, Garcia-Pagan JC, Otal P, et al. Improved clinical outcome using polytetrafluoroethylene-coated stents for tips: Results of a randomized study. Gastroenterology 2004;126(2):469–75. [DOI] [PubMed] [Google Scholar]
- 8.Lv Y, Qi X, He C, et al. Covered TIPS versus endoscopic band ligation plus propranolol for the prevention of variceal rebleeding in cirrhotic patients with portal vein thrombosis: A randomised controlled trial. Gut 2018;67(12):2156. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Zhou C, Shi Q, et al. Exploration of interventional therapy strategy for portal vein occlusion: A case series study. Eur J Gastroenterol Hepatol 2020;32(4):507–16. [DOI] [PubMed] [Google Scholar]
- 10.Riggio O, Merlli M, Pedretti G, et al. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Incidence and risk factors. Dig Dis Sci 1996;41(3):578–584. [DOI] [PubMed] [Google Scholar]
- 11.Riggio O, Nardelli S, Moscucci F, et al. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Clin Liver Dis 2012;16(1):133–46. [DOI] [PubMed] [Google Scholar]
- 12.Bai M, Qi X, Yang Z, et al. Predictors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients: A systematic review. J Gastroenterol Hepatol 2011;26(6):943–51. [DOI] [PubMed] [Google Scholar]
- 13.Riggio O, Ridola L, Angeloni S, et al. Clinical efficacy of transjugular intrahepatic portosystemic shunt created with covered stents with different diameters: Results of a randomized controlled trial. J Hepatol 2010;53(2):267–72. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, Lv Y, Bai M, et al. Eight millimetre covered TIPS does not compromise shunt function but reduces hepatic encephalopathy in preventing variceal rebleeding. J Hepatol 2017;67(3):508–16. [DOI] [PubMed] [Google Scholar]
- 15.Cura M, Cura A, Suri R, et al. Causes of TIPS dysfunction. AJR Am J Roentgenol 2008;191(6):1751–7. [DOI] [PubMed] [Google Scholar]
- 16.Cejna M, Peck-Radosavljevic M, Thurnher SA, et al. Creation of transjugular intrahepatic portosystemic shunts with stent-grafts: Initial experiences with a polytetrafluoroethylene-covered nitinol endoprosthesis. Radiology 2001;221(2):437–46. [DOI] [PubMed] [Google Scholar]
- 17.Madoff DC, Gaba RC, Weber CN, et al. Portal venous interventions: State of the art. Radiology 2016;278(2):333–53. [DOI] [PubMed] [Google Scholar]
- 18.Boyer TD, Haskal ZJ. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 2005;41(2):386–400. [DOI] [PubMed] [Google Scholar]
- 19.de Franchis R, Faculty BV. Revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53(4):762‐768. [DOI] [PubMed] [Google Scholar]
- 20.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60(2):715–35. [DOI] [PubMed] [Google Scholar]
- 21.Abraldes JG, Gilabert R, Turnes J, et al. Utility of color Doppler ultrasonography predicting tips dysfunction. Am J Gastroenterol 2005;100(12):2696–701. [DOI] [PubMed] [Google Scholar]
- 22.Casado M, Bosch J, García-Pagán JC, et al. Clinical events after transjugular intrahepatic portosystemic shunt: Correlation with hemodynamic findings. Gastroenterology 1998;114(6):1296–1303. [DOI] [PubMed] [Google Scholar]
- 23.Bettinger D, Schultheiss M, Boettler T, et al. Procedural and shunt-related complications and mortality of the transjugular intrahepatic portosystemic shunt (TIPSS). Aliment Pharmacol Ther 2016;44(10):1051–61. [DOI] [PubMed] [Google Scholar]
- 24.Luo X, Wang X, Zhu Y, et al. Clinical efficacy of transjugular intrahepatic portosystemic shunt created with expanded polytetrafluoroethylene-covered stent-grafts: 8-mm versus 10-mm. Cardiovasc Intervent Radiol 2019;42(5):737–743. [DOI] [PubMed] [Google Scholar]
- 25.Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: Techniques, indications, contraindications. Dig Liver Dis 2017;49(2):121–137. [DOI] [PubMed] [Google Scholar]
- 26.Trebicka J, Bastgen D, Byrtus J, et al. Smaller-diameter covered transjugular intrahepatic portosystemic shunt stents are associated with increased survival. Clin Gastroenterol Hepatol 2019;17(13):2793–9. [DOI] [PubMed] [Google Scholar]
- 27.Schepis F, Vizzutti F, Garcia-Tsao G, et al. Under-dilated TIPS associate with efficacy and reduced encephalopathy in a prospective, non-randomized study of patients with cirrhosis. Clin Gastroenterol Hepatol 2018;16(7):1153–62.e7. [DOI] [PubMed] [Google Scholar]
- 28.Cui J, Smolinski SE, Liu F, et al. Incrementally expandable transjugular intrahepatic portosystemic shunts: Single-center experience. Am J Roentgenology 2017;210(2):438–46. [DOI] [PubMed] [Google Scholar]
- 29.Pieper CC, Sprinkart AM, Nadal J, et al. Postinterventional passive expansion of partially dilated transjugular intrahepatic portosystemic shunt stents. J Vasc Interv Radiol 2015;26(3):388‐394. [DOI] [PubMed] [Google Scholar]
- 30.Borghol S, Perarnau JM, Pucheux J, et al. Short- and long-term evolution of the endoluminal diameter of underdilated stents in transjugular intrahepatic portosystemic shunt. Diagn Interv Imaging 2016;97(11):1103–1107. [DOI] [PubMed] [Google Scholar]
- 31.Pieper CC, Jansen C, Meyer C, et al. Prospective evaluation of passive expansion of partially dilated transjugular intrahepatic portosystemic shunt stent grafts-A three-dimensional sonography study. J Vasc Interv Radiol 2017;28(1):117–125. [DOI] [PubMed] [Google Scholar]
- 32.Mollaiyan A, Bettinger D, Rössle M. The underdilation of nitinol stents at TIPS implantation: Solution or illusion? Eur J Radiol 2017;89:123–128. [DOI] [PubMed] [Google Scholar]
- 33.Gaba RC, Parvinian A, Minocha J, et al. Should transjugular intrahepatic portosystemic shunt stent grafts be underdilated? J Vasc Interv Radiol 2015;26(3):382–387. [DOI] [PubMed] [Google Scholar]
- 34.Rössle M, Siegerstetter V, Olschewski M, et al. How much reduction in portal pressure is necessary to prevent variceal rebleeding? A longitudinal study in 225 patients with transjugular intrahepatic portosystemic shunts. Am J Gastroenterol 2001;96(12):3379–3383. [DOI] [PubMed] [Google Scholar]
- 35.Madoff DC, Perez-Young IV, Wallace MJ, et al. Management of TIPS-related refractory hepatic encephalopathy with reduced Wallgraft endoprostheses. J Vasc Interv Radiol 2003;14(3):369–374. [DOI] [PubMed] [Google Scholar]
- 36.Saad WEA, Darwish WM, Davies MG, et al. Stent-grafts for transjugular intrahepatic portosystemic shunt creation: Specialized TIPS stent-graft versus generic stent-graft/bare stent combination[J]. J Vasc Interv Radiol 2010;21(10):1512–20. [DOI] [PubMed] [Google Scholar]
- 37.Qi XS, Bai M, Yang ZP, et al. Selection of a TIPS stent for management of portal hypertension in liver cirrhosis: An evidence-based review. World J Gastroenterol 2014;20(21):6470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.