Abstract
The prevalence of obesity, type 2 diabetes mellitus, and metabolic syndromes is increasing globally. Minimally invasive metabobariatric (MB) endoscopic therapies are adjunct treatments that can potentially bridge the gap between surgical interventions and medical therapy. A growing number of MB techniques are becoming available, allowing for more personalized and patient-targeted treatment options for specific disease states. MB techniques are less invasive than surgery and can precisely target different parts of the gastrointestinal tract that may be responsible for the pathophysiology of obesity and metabolic syndromes such as type 2 diabetes mellitus. These alternatives should be selected on an individualized patient basis to balance the expected clinical outcomes and desired anatomical targets with the level of invasiveness and degree of acceptable risk. Each MB intervention presents great flexibility allowing for a tailored intervention and different levels of patient engagement. Patient awareness and motivation are essential to avoid therapy withdrawal and failure. Differences between MB procedures in terms of weight loss and metabolic benefit will be discussed in this review, along with the insights on clinical decision-making processes to evaluate the potential of further evolution and growth of bariatric and metabolic endoscopy.
INTRODUCTION
The gastrointestinal (GI) tract modulates nutrient absorption and glycemic control, releasing several important regulatory hormones (Figure 1) (1). Interestingly, different hormonal responses have been observed after common bariatric surgeries such as Roux-en-Y gastric bypass and sleeve gastrectomy. Roux-en-Y gastric bypass which bypasses the duodenum results in higher levels of glucagon-like peptide-1 (GLP-1), peptide YY (PYY), and fibroblast growth factor-19 (FGF-19) compared with sleeve gastrectomy, which demonstrates higher levels of gastrin (Figure 2) (2). Different hormonal responses support the role of the GI tract in glucose homeostasis and metabolic control. Metabolic changes after bariatric surgery persist over time, and they are not merely associated with weight loss (WL), suggesting additional weight-independent mechanisms of hormone regulation and glycemic control (3–6). Surgical interventions targeting the stomach and small intestine can have a significant and durable impact on WL and type 2 diabetes mellitus (T2DM).
Figure 1.
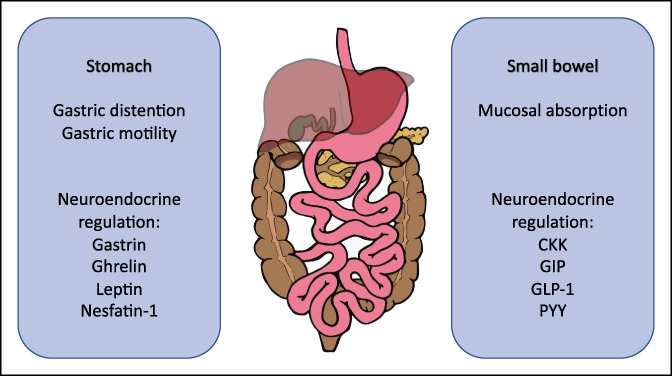
Mechanisms regulating food intake, nutrient absorption, and metabolic control originating from the gastrointestinal tract. The enteric nervous system, autonomic nervous system, and neuroendocrine cells can directly sense and regulate nutrients intake (i.e., orexigenic and anorexigenic hormones), as well as regulate nutrient metabolism with incretins (GLP-1 and GIP). GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; PYY, peptide YY.
Figure 2.
Effects of bariatric surgery (different hormonal changes after sleeve gastrectomy and Roux-en-Y gastric bypass). The BRAVE effects include bile flow changes, restriction of gastric size, anatomical gastrointestinal rearrangement, vagal manipulation, and enteric hormonal modulation. These changes induced by bariatric surgery are mimicked with endoscopic interventions.
Surgical interventions offer a greater WL but potentially have a greater impact on patients in terms of their invasive nature, associated postoperative morbidity, and adverse events (AEs). However, although bariatric surgical interventions are proven to be effective, only 1% of eligible patients undergo surgery (7). On the other hand, medical, lifestyle, and pharmacological therapies are largely available and cost-effective but might fail to treat all patients, and compliance remains indifferent (8,9). The advent of metabobariatric (MB) endoscopic interventions for bariatric and metabolic patients may bridge the treatment gap that exists between these therapeutic options, providing several minimally invasive interventions that could optimize treatment (Figure 3). These MB endoscopic procedures present different levels of efficacy, durability, risk, and AEs (Table 1) but are being increasingly explored to tackle the growing global pandemic of obesity and metabolic syndromes.
Figure 3.

Endoscopic interventions for obesity and dysmetabolic conditions. Endoscopic interventions: (a) duodenal mucosa resurfacing, (b) intragastric botox injection, (c) gas-filled intragastric balloon, (d) liquid-filled intragastric balloon, (e) transpyloric shuttle, (f) duodenal-jejunal bypass liner, (g) gastro-duodenal-jejunal bypass liner, (h) endoscopic sleeve gastroplasty, (i) Primary Obesity Surgery Endoluminal, (l) aspiration therapy, and (m) partial jejunal diversion.
Table 1.
Endoscopic intervention for WL and dysmetabolic conditions
| Name | Target | Availability and literature | Primary effect(s) | Metabolic effect(s) | Dwelling time | Advantage(s) | Disadvantage(s) | AE(s) |
| Intragastric balloon | Stomach | +++ | Early satiety Delayed gastric emptying Possible hormonal |
WL Hb1Ac reduction Improved liver tests |
6–12 mo | Easy and widely diffuse Long-term experience Several different models with different features |
Temporary effect with weight regain Patient compliance |
Accommodative symptoms, balloon migration, obstruction, perforation, and pancreatitis |
| Endoscopic gastroplasty | Stomach | ++ | Early satiety Delayed gastric motility Possible hormonal |
WL Improved glycemic control Improved lipid profile |
Semipermanent | Flexible procedure allowing for different suturing patterns Longer effect |
Complex procedure | Accommodative symptoms, perigastric collection, intragastric and extragastric bleeding, and hepatic abscess |
| Aspiration device | Stomach | + | Decreased caloric intake | WL HbA1c reduction |
12–24 mo | Patient-controlled procedure | Patient-dependent aspiration | Fistula, buried bumper and infection |
| Transpyloric shuttle | Stomach | + | Transient pyloric obstruction | TBC | 12 mo | Limited data | Limited data | |
| Botox injection | Stomach | + | Delayed gastric motility | TBC | N/A | Easy and widely diffuse minimally invasive | Temporary effect | |
| Duodenal bypass liner | Small bowel | ++ | Hormonal changes with restoration of incretin effect | WL HbA1c reduction Cardiometabolic marker improvement |
12 mo | Rapid improvement of glycemic control | Complex procedure with safety profile concerns | Hepatic abscess, acute pancreatitis, bleeding, and perforation |
| Duodenal mucosal resurfacing | Small bowel | + | TBC | HbA1c reduction with improved insulin sensitivity Liver improvement |
N/A | Limited data | Limited data | |
| Incisionless partial jejunal diversion | Small bowel | + | Enteric diversion | WL HbA1c reduction |
N/A | Limited data | Limited data |
AE, adverse event; HbA1c, glycated hemoglobin; N/A, not applicable; TBC, to be confirmed WL, weight loss.
Transmural and volume reduction therapies—procedures that result in high levels of total body WL
The following permanent or semipermanent treatments usually achieve high levels of total body WL (TBWL) involving all layers of the target organ, either creating an artificial transmural food passage or full-thickness plication. These may be justified when higher TBWL is aimed and when the alternative treatment with more invasive surgical intervention is less desirable.
Aspiration therapy
Aspiration devices reduce caloric intake by active aspiration of ingested food from the gastric cavity through an endoscopically placed percutaneous gastrostomy. AspireAssist (AA) (Aspire Bariatrics, Exton, PA) has been approved by the Food and Drug Administration (FDA) for treatment of class II and III obesity in patients failing conservative treatment. This device acts a long-term reversible treatment. Although active patient involvement has a possible negative impact on the quality of life, it allows for gradual therapy withdrawal. Thompson et al. (10) evaluated AA in a randomized controlled trial (RCT) on patients with a body mass index (BMI) of 35–55 kg/m2. A total of 74% of patients who were on AA (82/111 patients) completed 52 weeks of treatment. Per-protocol analysis showed a percentage of TBWL (%TBWL) of >10% in 70% of patients in the treatment group compared with 19% in the control group; the mean %TBWL was 14.2 ± 9.8% vs 4.9 ± 7.0%, respectively. The serious adverse event (SAE) rate was 3.6% and included mild peritonitis, severe abdominal pain, and prepyloric ulcers likely related to mucosal contact of the intragastric portion of the gastrostomy tube. Several non-SAEs were reported, including peristomal granulation tissue (45%), abdominal pain (37%), nausea and vomiting (17%), peristomal irritation (17%), and infection (13%). Compared with controls, AA achieved glycated hemoglobin (HbA1c) reduction (−0.36% relative to 5.7% P < 0.0001) (10). A total of 58 patients continued the treatment beyond 12 months, and 15 patients concluded 4-year treatment showing sustained WL with an 18.7% TBWL on a per-protocol analysis (11). Similarly, data from a multicenter European registry showed that aspiration therapy provides sustained WL with a safety profile similar to percutaneous endoscopic gastrostomy (12).
Endoscopic gastroplasty
Endoscopic gastroplasty alters the anatomy and physiology of the stomach by plicating the gastric walls and reducing the intragastric volume by up to 75%. The functional exclusion of part of the stomach results in a reduction of both gastric volume and motility (13). Two devices have been FDA-approved: the OverStitch (Apollo Endosurgery, Austin, TX) used for endoscopic sleeve gastroplasty (ESG) and Primary Obesity Surgery Endoluminal (POSE) (USGI Medical, San Clemente, CA) (14). Differences between these 2 techniques are shown in Figure 4. A meta-analysis published by Gys et al. (15) compared ESG vs POSE, where the percentage of excess WL (%EWL) was 68.3% vs 44.9% at 12 months. More recently, Lopez-Nava et al. (16) have modified of the POSE technique suturing the gastric body aiming to alter its motility (POSE-2). Their preliminary experience on 73 patients has shown a %TBWL of 15.7% at 6 months with no AEs. Further studies are needed to evaluate the efficacy of this technique compared with ESG and control groups.
Figure 4.

Endoscopic sleeve gastroplasty and POSE—illustration of different techniques and suturing patterns. On the left, endoscopic sleeve gastroplasty (a, b, c) suturing the antrum. Graus Morales et al. implemented a Z-pattern (c) instead of a triangular pattern (a, b). The Z-shaped running suture with multiple closer stitches aims to reduce suture tension and increase its durability (84). On the right, the POSE targeting the fundus (d), new suturing patterns targeting the antrum have been proposed by Lopez-Nava et al. POSE-2 (16) or distal-POSE by Jirapinyo et al. (85) (e). POSE, Primary Obesity Surgery Endoluminal.
A recent meta-analysis published by Khan et al. showed no significant WL difference between AA and endoscopic gastric plication (17). ESG has a significantly lower rate of AEs compared with laparoscopic sleeve (2.9% vs 11.8%, P = 0.001) and lower %TBWL at 12 months (17% vs 30.5%, P = 0.001) (18). The safety and efficacy of ESG were confirmed in 3 recent systematic reviews and meta-analyses, showing a %TBWL at 12 months between 16.1% and 16.5% with an SAE rate between 2.2% and 2.3% (19–21). WL at 24 months seems to be sustained with a %TBWL of 20.01% (21). Long-term data are promising, and abstract-based results presented by Hajifathalian et al. showed that 69% of the patients maintained a %TBWL ≥10% at 60 months (22). Their quadratic regression models a maximum WL at 24 months. After achieving minimum weight, patients regained 2.4 kg–14% (22).
Other cardiometabolic effects of gastric plication were reported by Brethauer et al. (23). Metabolic parameters, including mean systolic and diastolic blood pressure, mean triglyceride and fasting insulin values, decreased by 15.2 mm Hg (P = 0.0012) and 9.7 mm Hg (P = 0.0051), 27.9 mg/dL and 4.5μIU/mL, respectively. Sullivan et al. (24) published an RCT, including 332 patients with a significant decrease of both fasting glucose and low-density lipoprotein levels. Espinós et al. (25) showed improved glucose/insulin ratio, a postprandial increase of PYY, increased baseline ghrelin, and enhanced postprandial decrease. Interestingly, the gastric emptying time was longer at 2 months and returned to baseline at 6 months; this transient delay was associated with greater and sustained WL.
Partial jejunal diversion
This device consists of 2 self-assembled magnetic rings that create an ischemic fistulation apposing side-by-side proximal and distal small bowel (Figure 3m). First-in-human (FIH) study on 10 patients has shown no device-related SAE, 14.6% TBWL at 12 months, and HbA1c reduction of 1.9% and 1.0% in diabetic and prediabetic patients, respectively (26). Few other data are available, and this device is not FDA-approved at present.
Endoluminal implantable therapies—procedures that result in lower levels of TBWL
These techniques use temporary endoluminal implants that achieve lower levels of transient and short-lived TBWL. They are less invasive but not necessarily safer. They target either the stomach or proximal small intestine. Their effectiveness can be increased by repeating or combining them together (27–29).
Intragastric balloons
Intragastric balloons (IGBs) have been the most widespread minimally invasive endoscopic intervention for WL. Several IGBs are available, each presenting unique features (30) (Table 2). A retrospective study comparing IGB with ESG showed a significantly lower %TBWL at 6 months (15.0 vs 19.5%, respectively) and 12 months (13.9% vs 21.3%, respectively) with higher AE rates of 17% vs 5.2%, respectively (P = 0.048) (31). A recent meta-analysis confirmed that IGB might be clinically inferior to EGS in terms of WL and AEs (32).
Table 2.
Different types of intragastric balloons with their characteristics
 |
Most literature for liquid-filled balloons refers to Orbera (Apollo Endosurgery). Orbera achieved 11.27% TBWL (95% confidence interval, 8.17%–14.36%) at 12 months after implantation and a significant WL over controls (26.9% EWL; P < 0.01) as showed in the American Society for Gastrointestinal Endoscopy systematic review and meta-analysis, including 1,683 patients from 17 studies (33). Trang et al. reported a rate of nausea and vomiting of 63% and 55%, respectively, with the highest accommodative symptoms rate for Orbera compared with other IGBs (34).
Lower rates of explantation and intolerance can be obtained using gas-filled IGB, but this may compromise on WL (35). Indeed, in a recent meta-analysis, the gas-filled balloon does not achieve superior WL compared with lifestyle modification (36). Previously, Sullivan et al. conducted an RCT comparing Obalon, a swallowable gas-filled balloon and lifestyle therapy vs lifestyle therapy alone. Patients completing the treatment achieved a %TBWL of 7.1 ± 5.0% vs 3.6 ± 5.1% in the controls (P = 0.0085), and the SAE rate was 0.4%. At 48 weeks, 88.5% of the TWL was maintained (37). Over time, up to 3 balloons can be accommodated in the stomach to progressively reduce the volume of the gastric cavity.
Limited literature is available for other CE-marked IGBs that might present unique features. For instance, Elipse (Allurion, Natick, MA) is a swallowable liquid-filled balloon with a self-deflating valve mechanism with no endoscopy required; Spatz3 (Spatz, Fort Lauderdale, FL) is a liquid-filled balloon with adjustable volume and dwelling time up to 12 months. Retrospective studies and case series showed that down-volume adjustment might alleviate balloon intolerance, whereas up-volume adjustment may contrast the plateau effect facilitating additional WL (38–40) and provide a further delay of gastric emptying (41). A more recent, cross-sectional study showed that obese patients achieved greater WL with nonadjustable IGB, whereas overweight (BMI > 27) achieved greater WL with adjustable IGB (42). Additional prospective studies are needed to quantify the advantages of adjustable balloons in terms of WL and address safety concerns of catheter impaction as was seen in previous generations of devices (39,43).
In addition to WL, IGBs achieve other metabolic benefits. Popov et al. (44) published a large systematic review of 10 RCTs and 30 observational studies, including 5,668 patients, showing improvements in metabolic parameters that included fasting glucose, triglycerides, and diastolic blood pressure. In addition, Guedes et al. (45) reported a reduction of leptin, high-sensitivity C-reactive protein, glucose, insulin, and homeostatic model assessment of insulin resistance (shortly) with a concomitant increase of adiponectin/leptin ratio. Nguyen et al. (46) showed a significative reduction of alanine aminotransferase (ALT) and gamma-glutamyl transferase in obese patients with nonalcoholic steatohepatitis. A similar result was obtained by Folini et al. (47), showing liver steatosis reduction at magnetic resonance imaging. Bazerbachi et al. (48) reported a reduction of HbA1c (1.3% ± 0.5% P = 0.02), nonalcoholic fatty liver activity score, and liver fibrosis.
Transpyloric shuttle
The transpyloric shuttle (BAROnova) device consists of 2 silicon-based bulbs connected by a tether across the pylorus (Figure 3e). It intermittently obstructs the pylorus and delays the gastric emptying. Following initial safety concerns, it has been redesigned and is currently FDA-approved for a treatment period of 12 months (49). Marinos et al. (50) tested this device in an open-label trial of 20 patients assigned to 3- or 6-month treatment. The 6-month group achieved 14.5% TBWL. Overall, 10 gastric ulcers were noted; 2 required early device removal. More recently, a sham-controlled study was conducted by Rothstein et al. (51), where 270 patients were randomized in a 2:1 ratio to 12-month treatment or sham procedure. Abstract-based results showed 30.9% EWL in the treatment group vs 9.8% EWL in controls (P < 0.0001). Early device removal was required in 10.3% of patients, and the SAE rate was 2.5% (52).
Duodenal bypass liner
The duodenal bypass liners are impermeable sleeves interposed between the mucosa and GI lumen to impede nutrient digestion and absorption (Figure 3f–g). Currently, these devices are not FDA-approved. Most literature refers to EndoBarrier, a duodenal-jejunal bypass sleeve (DJBS) measuring 60 cm in length. During the placement, the self-expanding nitinol anchor is positioned into the duodenal bulb, and the impermeable fluorine polymer liner is deployed distally into the proximal jejunum. Similarly, ValenTx is a gastro-duodenal-jejunal bypass measuring 120 cm that is anchored to the gastroesophageal junction and extends to the jejunum. This second device requires laparoscopic assistance for its placement (53,54).
An American Society for Gastrointestinal Endoscopy pooled analysis of 4 RCTs has shown significant WL difference compared with the control group (9.4% EWL; 95% confidence interval, 8.26–10.65). Notably, the duration of these studies was 12 and 24 weeks, which were shorter than the recommended dwelling time of 12 months. This analysis also showed significant HbA1c reduction compared with controls (33). The availability of alternative and highly effective medical therapy might not justify its use; however, DJBS has shown potential in improving metabolic parameters.
After DJBS implantation, a rapid improvement of T2DM and glycemic control has been noted as well as hormonal changes (55,56). Muñoz et al. (57) suggest additional weight-independent mechanisms of glycemic control. Jirapinyo et al. (58) published a meta-analysis of 4 RCTs showing 1.3% HbA1c reduction, with a significant reduction by 0.9% compared with control. This reduction was sustained after 6 months. Pooled data from observational and RCTs showed a total WL of 18%, which remained significant after 1 year; GLP-1, PYY, and ghrelin increased, whereas glucose-dependent insulinotropic polypeptide decreased.
In addition, EndoBarrier improved liver enzymes (59,60) and liver elastography (61). van Nierop et al. (62) showed an increase of unconjugated bile acid and increase in GLP-1; similarly, Kaválková et al. (63) showed an increase of FGF-19 and bile acids with the restoration of postprandial peak of GLP-1 as well as an improved lipid and glucose regulation, and FGF-19 and GLP-1 can regulate lipid and glucose metabolism; upregulation of this pathway might contribute to restoring the incretin effect, which is downregulated in dysmetabolic conditions.
Moreover, EndoBarrier placement reduced triglyceride to high-density lipoprotein ratio (64) and serum low-density lipoprotein levels (65). More recently, Roehlen et al. (66)showed improvement of other cardiovascular markers, including high-sensitive C-reactive protein, lipoprotein-associated phospholipase A2, and small dense lipoprotein fraction. Studies attempting to prolong metabolic benefits by increasing the implantation duration registered higher AE rate. The authors of both studies recommended an implantation time of 12 months (67,68).
This device still has safety concerns that need to be addressed. Betzel et al. (69) published a systematic review including 1,057 patients, 33 of which had an SAE rate of 3.7%, including 11 hepatic abscesses, 8 GI hemorrhages, 4 esophageal perforations, and 3 acute pancreatitis). Surgery was required in 8 patients, but no deaths were reported. Despite these risks, Laubner et al. (70) showed possible positive risk-benefit ratio in favor of DJBL for T2DM treatment.
Endoluminal non–device-based therapies—lower AE profile
The need for safer procedures has led to the development of less invasive, nonimplantable, and deviceless techniques. These focus on manipulation of the mucosal environment of the stomach and small bowel to achieve sustained benefits on metabolic control that are so intricately linked to the hormonal signals that this part of the GI tract regulates.
Intragastric injection
Botulinum toxin A (BTA) injection can temporarily inhibit gastric peristalsis. Conflicting evidence has been published on the efficacy of this intervention. Initial studies showed a delay in gastric emptying with WL without AE (71–73). In 2015, a meta-analysis of 8 studies concluded that BTA is effective in the treatment of obesity (74). Other data showed no impact on BTA injection on WL (75,76). More recently, 2 meta-analyses have concluded that intragastric injection of BTA is not effective (36,77).
Duodenal mucosal resurfacing
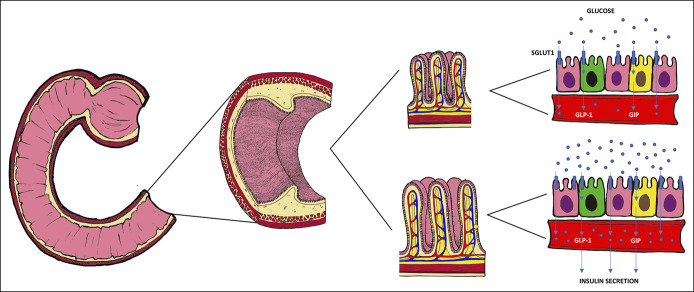
Duodenal mucosal resurfacing (DMR) is a novel procedure, which selectively ablates the duodenal mucosa and aims to revert mucosal changes present in T2DM (Figure 5).
Figure 5.
Mucosal alteration in dysmetabolic condition and westernized diet. Several mucosal alterations have been documented after chronic exposure to glucose and westernized diet (on the bottom) including increased barrier permeability, longer intestinal villi (86), increased expression of SGLUT1, as well as increased activity of sucrase and isomaltase (87,88). Mucosal changes involve L-cells (green) and K-cell (yellow) in terms of their number, ratio, and response to glucose stimulation. These cells regulate insulin secretion through incretins (glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide) (86,89–91).
Most literature refers to Revita (Fractyl Laboratories) (Figure 3a). This catheter creates a circumferential mucosal lift with submucosal injection followed by thermal mucosal hydroablation. Initial studies conducted on diabetic rats showed that duodenal mucosal abrasion reduces hyperglycemia. The same publication showed complete mucosal healing in a pig model treated with mucosal hydroablation after 6 weeks (78).
The FIH open-label trial evaluating safety and efficacy was conducted on a total of 39 patients; of which, 28 had long-segment ablation (average length = 9.3 cm), and 11 had short-segment ablation (average length = 3.4 cm). The pooled HbA1c reduction was 1.2% at 6 months (P < 0.001). The patients in the long-segment subgroup achieved greater HbA1c reduction compared with the short-segment group 2.5% vs 1.2% at 3 months (P < 0.05) and 1.4% vs 0.7% (P = 0.3) at 6 months. Within the long-segment group, patients with baseline HbA1c of 7.5%–10%, maintaining stable antidiabetic medications, achieved even greater HbA1c reduction (1.8% ± 0.5% at 6 months). Interestingly, HbA1c reduction was not significantly related to WL. Three patients experienced duodenal stenosis that was successfully treated with endoscopic balloon dilatation (79).
More recently, van Baar et al. (80) conducted a single-arm multicenter trial; in which, 9 of 46 patients were excluded from the final analysis because of technical failure. Per-protocol analysis on 36 patients showed 0.9% ± 0.2% HbA1c reduction (P < 0.001) and liver enzyme improvement (ALT from 40 ± 4U/L to 31 ± 2U/L). Changes were sustained and statistically significant up to 12 months. Once again, metabolic improvements were not related to WL. No DMR-related SAEs were reported. The same author published a pooled analysis of these 2 studies, showing a mean reduction of HbA1c of 1.1% at 6 months, with improvement in liver function tests, including ALT, AST, and fibrosis-4 index. Analysis of a subcohort of 14 patients showed additional metabolic effects at 3 months compared with baseline with improved mixed meal tolerance test and insulin sensitivity, improved lipid profile as well as a higher level of antioxidant and lower level of inflammatory molecules (81).
A multicentric RCT is currently evaluating comparative safety and efficacy. Abstract-based results showed a significant reduction of liver fat content measured with magnetic resonance proton density at 12 weeks as well as a significant reduction of HbA1c compared with the sham procedure (−0.6% [DMR] vs −0.3% [Sham]) at 24 weeks. Patients with baseline fasting plasma glucose ≥180 mg/dL achieved greater HbA1c reduction: −(1.2% [DMR] vs −0.3% [Sham] [P = 0.005]) (82).
The DiaGone (Digma, San Diego, CA) is a through-the-scope laser-based system for duodenal ablation. An FIH multicenter, open-label study is ongoing (NCT03390322).
Factors guiding clinical decision-making and role of multidisciplinary approach
MB procedural selection is a multifactorial decision process that should be performed on an individualized basis with a multidisciplinary approach taking into consideration several factors including fitness for surgery and anesthetic risk (i.e., comorbid or super obese patients), technical feasibility (i.e., frozen abdomen or anatomical variation), patient's preference and BMI, desired TBWL and metabolic endpoints, and reversibility of the intervention (i.e., younger patients). The willingness and appropriateness of implanting a device to support WL is another important factor to be evaluated. Those factors involved in the choice among surgical procedures and other MB endoscopic interventions are summarized in our proposed algorithm (Figure 6).
Figure 6.
Decision-making algorithm guiding the choice among different MB interventions. Several medical and nonmedical aspects (i.e., adherence to postprocedure MDT follow-ups (92)) will likely play a crucial role in determining the success of the intervention and have to be evaluated for each single patient individually. MB endoscopic procedures should be particularly considered for patients with body mass index between 30 and 40 kg/m2 who do not have other significant comorbidities. Those with a body mass index greater than this or with an obesity-related condition might be considered for surgery in the first instance because of the evidence of greater total body WL and resolution of comorbidities associated with obesity. Importantly, MB endoscopic procedures do not compromise the possibility of further bariatric surgery and, in most cases, are reversible and repeatable and can be used in high-risk patients with low risk of adverse events in expert hands (93). MB, metabobariatric; MDT, multidisciplinary team.
Among MB procedures, AA and endoscopic gastroplasty can ensure higher and sustained WL in a more consistent fashion. Those techniques are relatively newer and less widely available compared with IGBs. In particular, ESG and POSE require a higher level of technical skills and dedicated training to be performed, but adoption will grow with structured training and data. In addition, in those patients with a very high BMI who are not suitable for surgery, MB such as AA, IGBs, and endoscopic gastroplasty may also be used as a bridge to surgery or other endoscopic procedures that require a lower initial BMI.
Along with WL, other weight-independent benefits and indications for ESG (83), IGBs (44,48), and DMR are emerging including improved insulin sensitivity and improvement of liver pathology. Indeed, DMR and other techniques targeting the duodenum, once properly evaluated, could be introduced for DM2 and nonalcoholic fatty liver disease and favored for those patients with a lower BMI (80).
Implants may require a higher level of patient involvement, which may not be suitable for some patients. Of course, all techniques require patients' participation in postprocedure dietary changes and follow-up visits, but this may be particularly important for implantable devices. For these reasons, a careful evaluation of patient's personality, attitude, and approach toward their condition as well as other social factors such as time availability and access to the center has to be taken into account.
A multidisciplinary approach should consider all these different aspects. Bariatric surgeons and endoscopists can evaluate the technical aspects of the procedures. The involvement of an obesity physician is preferred, although a physician with an associated specialty such as diabetes with a particular focus on obesity can be an alternative. Furthermore, dietitians, an anesthetist with experience of bariatric procedures, a psychologist, and specialist nurses all play important roles in the various aspects of these decisions. This is particularly important when considering the long-term follow-up of these patients including quality of life and ongoing dietary changes. This approach should be followed at all stages in the patient's care including before and after procedure.
CONCLUSION
MB endoscopic interventions could represent an alternative to surgery for treatment of dysmetabolic conditions. RCTs are needed to evaluate their long-term efficacy and exclude confounders for metabolic parameter improvement. Those results will help to position those techniques in the decision-making algorithm. Multidisciplinary decision-making on a per-patient level is key to the success of these innovations.
CONFLICTS OF INTEREST
Guarantor of the article: Rehan J. Haidry MD.
Specific author contributions: A.T. and R.H. conceptualized and drafted the manuscript; C.M., S.A.A., L.B.L, and R.J.H revised critically for intellectual content. A.T., V.S., C.M., S.N., S.A.A., L.B.L., and R.J.H. provided intellectual contribution and were involved in the review of the final manuscript.
Financial support: None to report.
Potential competing interests: R.J.H. receives educational grants to support research infrastructure from Medtronic ltd. Cook endoscopy (fellowship support), Pentax Europe, C2 therapeutics, Beamline diagnostic, and Fractyl Ltd.
Contributor Information
Vinay Sehgal, Email: vinay.sehgal@nhs.net.
Cormac G. Magee, Email: cormac.magee@ucl.ac.uk.
S. Naik, Email: sarita.naik@nhs.net.
S.A. Alqahtani, Email: salqaht1@jhmi.edu.
L.B. Lovat, Email: L.lovat@ucl.ac.uk.
Rehan J. Haidry, Email: rehan.haidry@nhs.net.
REFERENCES
- 1.Monteiro MP, Batterham RL. The importance of the gastrointestinal tract in controlling food intake and regulating energy balance. Gastroenterology 2017;152(7):1707–e2. [DOI] [PubMed] [Google Scholar]
- 2.Pucci A, Batterham RL. Mechanisms underlying the weight loss effects of RYGB and SG: Similar, yet different. J Endocrinol Invest 2019;42(2):117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366(17):1577–85. [DOI] [PubMed] [Google Scholar]
- 4.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015;386(9997):964–73. [DOI] [PubMed] [Google Scholar]
- 5.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med 2017;376(7):641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashrafian H, Athanasiou T, Li JV, et al. Diabetes resolution and hyperinsulinaemia after metabolic Roux-en-Y gastric bypass. Obes Rev 2011;12(5):e257–72. [DOI] [PubMed] [Google Scholar]
- 7.English WJ, DeMaria EJ, Brethauer SA, et al. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis 2018;14(3):259–63. [DOI] [PubMed] [Google Scholar]
- 8.Pérez CM, Febo-Vázquez I, Guzmán M, et al. Are adults diagnosed with diabetes achieving the American Diabetes Association clinical practice recommendations?. P R Health Sci J 2012;31(1):18–23. [PMC free article] [PubMed] [Google Scholar]
- 9.Khunti K, Ceriello A, Cos X, et al. Achievement of guideline targets for blood pressure, lipid, and glycaemic control in type 2 diabetes: A meta-analysis. Diabetes Res Clin Pract 2018;137:137–48. [DOI] [PubMed] [Google Scholar]
- 10.Thompson CC, Abu Dayyeh BK, Kushner R, et al. Percutaneous gastrostomy device for the treatment of class II and class III obesity: Results of a randomized controlled trial. Am J Gastroenterol 2017;112(3):447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson CC, Abu Dayyeh BK, Kushnir V, et al. Aspiration therapy for the treatment of obesity: 4-year results of a multicenter randomized controlled trial. Surg Obes Relat Dis 2019;15(8):1348–54. [DOI] [PubMed] [Google Scholar]
- 12.Nyström M, Machytka E, Norén E, et al. Aspiration therapy as a tool to treat obesity: 1- to 4-year results in a 201-patient multi-center post-market European registry study. Obes Surg 2018;28(7):1860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu Dayyeh BK, Acosta A, Camilleri M, et al. Endoscopic sleeve gastroplasty alters gastric physiology and induces loss of body weight in obese individuals. Clin Gastroenterol Hepatol 2017;15(1):37–43.e1. [DOI] [PubMed] [Google Scholar]
- 14.McCarty TR, Thompson CC. The current state of bariatric endoscopy. Dig Endosc 2021;33(3):321–34. [DOI] [PubMed] [Google Scholar]
- 15.Gys B, Plaeke P, Lamme B, et al. Endoscopic gastric plication for morbid obesity: A systematic review and meta-analysis of published data over time. Obes Surg 2019;29(9):3021–9. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Nava G, Asokkumar R, Turro Arau R, et al. Modified primary obesity surgery endoluminal (POSE-2) procedure for the treatment of obesity. VideoGIE 2020;5(3):91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan Z, Khan MA, Hajifathalian K, et al. Efficacy of endoscopic interventions for the management of obesity: A meta-analysis to compare endoscopic sleeve gastroplasty, AspireAssist, and primary obesity surgery endolumenal. Obes Surg 2019;29(7):2287–98. [DOI] [PubMed] [Google Scholar]
- 18.Mohan BP, Asokkumar R, Khan SR, et al. Outcomes of endoscopic sleeve gastroplasty; how does it compare to laparoscopic sleeve gastrectomy? A systematic review and meta-analysis. Endosc Int Open 2020;8(4):E558–E65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedjoudje A, Abu Dayyeh BK, Cheskin LJ, et al. Efficacy and safety of endoscopic sleeve gastroplasty: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2020;18(5):1043–e4. [DOI] [PubMed] [Google Scholar]
- 20.de Miranda Neto AA, de Moura DTH, Ribeiro IB, et al. Efficacy and safety of endoscopic sleeve gastroplasty at mid term in the management of overweight and obese patients: A systematic review and meta-analysis. Obes Surg 2020;30(5):1971–87. [DOI] [PubMed] [Google Scholar]
- 21.Singh S, Hourneaux de Moura DT, Khan A, et al. Safety and efficacy of endoscopic sleeve gastroplasty worldwide for treatment of obesity: A systematic review and meta-analysis. Surg Obes Relat Dis 2020;16(2):340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajifathalian K, Ang B, Dawod QM, et al. 175 long-term follow up and outcomes after endoscopic sleeve gastroplasty for treatment of obesity (5 year data). Gastrointest Endosc 2019;89(6):AB58. [Google Scholar]
- 23.Brethauer SA, Chand B, Schauer PR, et al. Transoral gastric volume reduction as intervention for weight management: 12-month follow-up of TRIM trial. Surg Obes Relat Dis 2012;8(3):296–303. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan S, Swain JM, Woodman G, et al. Randomized sham-controlled trial evaluating efficacy and safety of endoscopic gastric plication for primary obesity: The ESSENTIAL trial. Obesity (Silver Spring) 2017;25(2):294–301. [DOI] [PubMed] [Google Scholar]
- 25.Espinós JC, Turró R, Moragas G, et al. Gastrointestinal physiological changes and their relationship to weight loss following the POSE procedure. Obes Surg 2016;26(5):1081–9. [DOI] [PubMed] [Google Scholar]
- 26.Machytka E, Bužga M, Zonca P, et al. Partial jejunal diversion using an incisionless magnetic anastomosis system: 1-year interim results in patients with obesity and diabetes. Gastrointest Endosc 2017;86(5):904–12. [DOI] [PubMed] [Google Scholar]
- 27.Koehestanie P, Betzel B, Aarts EO, et al. Is reimplantation of the duodenal-jejunal bypass liner feasible? Surg Obes Relat Dis 2015;11(5):1099–104. [DOI] [PubMed] [Google Scholar]
- 28.Leventi E, Gunthert SJ, Stier C, et al. Is early reimplantation of the duodenal-jejunal bypass liner viable? Obes Surg 2019;29(5):1690–3. [DOI] [PubMed] [Google Scholar]
- 29.Sartoretto A, Marinos G, Sui Z. Concurrent placements of a duodenal-jejunal bypass liner and an intragastric balloon among severely obese patients: A case series. ACG Case Rep J 2019;6:e00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bazerbachi F, Vargas EJ, Abu Dayyeh BK. Endoscopic bariatric therapy: A guide to the intragastric balloon. Am J Gastroenterol 2019;114(9):1421–31. [DOI] [PubMed] [Google Scholar]
- 31.Fayad L, Cheskin LJ, Adam A, et al. Endoscopic sleeve gastroplasty versus intragastric balloon insertion: Efficacy, durability, and safety. Endoscopy 2019;51(6):532–9. [DOI] [PubMed] [Google Scholar]
- 32.Singh S, de Moura DTH, Khan A, et al. Intragastric balloon versus endoscopic sleeve gastroplasty for the treatment of obesity: A systematic review and meta-analysis. Obes Surg 2020;30(8):3010–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abu Dayyeh BK, Kumar N, Edmundowicz SA, et al. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc 2015;82(3):425–38.e5. [DOI] [PubMed] [Google Scholar]
- 34.Trang J, Lee SS, Miller A, et al. Incidence of nausea and vomiting after intragastric balloon placement in bariatric patients—a systematic review and meta-analysis. Int J Surg 2018;57:22–9. [DOI] [PubMed] [Google Scholar]
- 35.Bazerbachi F, Haffar S, Sawas T, et al. Fluid-filled versus gas-filled intragastric balloons as obesity interventions: A Ne2rk meta-analysis of randomized trials. Obes Surg 2018;28(9):2617–25. [DOI] [PubMed] [Google Scholar]
- 36.Jung SH, Yoon JH, Choi HS, et al. Korean research group for endoscopic management of metabolic disorder and obesity. Comparative efficacy of bariatric endoscopic procedures in the treatment of morbid obesity: A systematic review and ne2rk meta-analysis. Endoscopy 2020;52(11):940–54. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan S, Swain J, Woodman G, et al. Randomized sham-controlled trial of the 6-month swallowable gas-filled intragastric balloon system for weight loss. Surg Obes Relat Dis 2018;14(12):1876–89. [DOI] [PubMed] [Google Scholar]
- 38.Usuy E, Brooks J. Response rates with the Spatz3 adjustable balloon. Obes Surg 2018;28(5):1271–6. [DOI] [PubMed] [Google Scholar]
- 39.Brooks J, Srivastava ED, Mathus-Vliegen EM. One-year adjustable intragastric balloons: Results in 73 consecutive patients in the U.K. Obes Surg 2014;24(5):813–9. [DOI] [PubMed] [Google Scholar]
- 40.Machytka E, Klvana P, Kornbluth A, et al. Adjustable intragastric balloons: A 12-month pilot trial in endoscopic weight loss management. Obes Surg 2011;21(10):1499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vargas EJ, Rizk M, Bazerbachi F, et al. Changes in gastric emptying with the Spatz3 adjustable intragastric balloon are associated with increased weight loss: A prospective study. Surg Obes Relat Dis 2018;14(11). [Google Scholar]
- 42.Schwaab ML, Usuy EN, Jr, Albuquerque MM, et al. Assessment of weight loss after non-adjustable and adjustable intragastric balloon use. Arq Gastroenterol 2020;57(1):13–8. [DOI] [PubMed] [Google Scholar]
- 43.Daniel F, Abou Fadel C, Houmani Z, et al. Spatz 3 adjustable intragastric balloon: Long-term safety concerns. Obes Surg 2016;26:159–60. [DOI] [PubMed] [Google Scholar]
- 44.Popov VB, Thompson CC, Kumar N, et al. Effect of intragastric balloons on liver enzymes: A systematic review and meta-analysis. Dig Dis Sci 2016;61(9):2477–87. [DOI] [PubMed] [Google Scholar]
- 45.Guedes MR, Fittipaldi-Fernandez RJ, Diestel CF, et al. Impact of intragastric balloon treatment on adipokines, cytokines, and metabolic profile in obese individuals. Obes Surg 2019;29(8):2600–8. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen V, Li J, Gan J, et al. Outcomes following serial intragastric balloon therapy for obesity and nonalcoholic fatty liver disease in a single centre. Can J Gastroenterol Hepatol 2017;2017:4697194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folini L, Veronelli A, Benetti A, et al. Liver steatosis (LS) evaluated through chemical-shift magnetic resonance imaging liver enzymes in morbid obesity; effect of weight loss obtained with intragastric balloon gastric banding. Acta Diabetol 2014;51(3):361–8.24085682 [Google Scholar]
- 48.Bazerbachi F, Vargas EJ, Rizk M, et al. Intragastric balloon placement induces significant metabolic and histologic improvement in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2021;19(1):146–54.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.FDA. TransPyloric Shuttle/TransPyloric Shuttle Delivery Device—P180024|FDA: @US_FDA (https://www.fda.gov/medical-devices/recently-approved-devices/transpyloric-shuttletranspyloric-shuttle-delivery-device-p180024). Accessed October 23, 2020. [Google Scholar]
- 50.Marinos G, Eliades C, Raman Muthusamy V, et al. Weight loss and improved quality of life with a nonsurgical endoscopic treatment for obesity: Clinical results from a 3- and 6-month study. Surg Obes Relat Dis 2014;10(5):929–34. [DOI] [PubMed] [Google Scholar]
- 51.clinicaltrials.gov. ENDObesity® II Study: TransPyloric Shuttle® System for Weight Loss—Study Results—ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/results/NCT02518685). Accessed October 23, 2020. [Google Scholar]
- 52.obesityweek2018. T-P-3277|Weight Reduction in Patients with Obesity Using the TransPyloric Shuttle (https://2018.obesityweek.com/abstract/weight-reduction-in-patients-with-obesity-using-the-transpyloric-shuttle-endobesity-ii-study/index.html). Accessed October 23, 2020. [Google Scholar]
- 53.Sandler BJ, Rumbaut R, Swain CP, et al. Human experience with an endoluminal, endoscopic, gastrojejunal bypass sleeve. Surg Endosc 2011;25(9):3028–33. [DOI] [PubMed] [Google Scholar]
- 54.Sandler BJ, Rumbaut R, Swain CP, et al. One-year human experience with a novel endoluminal, endoscopic gastric bypass sleeve for morbid obesity. Surg Endosc 2015;29(11):3298–303. [DOI] [PubMed] [Google Scholar]
- 55.de Jonge C, Rensen SS, Verdam FJ, et al. Endoscopic duodenal-jejunal bypass liner rapidly improves type 2 diabetes. Obes Surg 2013;23(9):1354–60. [DOI] [PubMed] [Google Scholar]
- 56.Cohen RV, Neto MG, Correa JL, et al. A pilot study of the duodenal-jejunal bypass liner in low body mass index type 2 diabetes. J Clin Endocrinol Metab 2013;98(2):E279–82. [DOI] [PubMed] [Google Scholar]
- 57.Muñoz R, Escalona A. Duodenal-jejunal bypass liner to treat type 2 diabetes mellitus in morbidly obese patients. Curr Cardiol Rep 2014;16(3):454. [DOI] [PubMed] [Google Scholar]
- 58.Jirapinyo P, Haas AV, Thompson CC. Effect of the duodenal-jejunal bypass liner on glycemic control in patients with type 2 diabetes with obesity: A meta-analysis with secondary analysis on weight loss and hormonal changes. Diabetes Care 2018;41(5):1106–15. [DOI] [PubMed] [Google Scholar]
- 59.Ryan PM, Hayward NE, Sless RT, et al. Effect of bariatric surgery on circulating FGF-19: A systematic review and meta-analysis. Obes Rev 2020;21(8):e13038. [DOI] [PubMed] [Google Scholar]
- 60.de Jonge C, Rensen SS, Koek GH, et al. Endoscopic duodenal-jejunal bypass liner rapidly improves plasma parameters of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2013;11(11):1517–20. [DOI] [PubMed] [Google Scholar]
- 61.Gollisch KS, Lindhorst A, Raddatz D. EndoBarrier gastrointestinal liner in type 2 diabetic patients improves liver fibrosis as assessed by liver elastography. Exp Clin Endocrinol Diabetes 2017;125(2):116–21. [DOI] [PubMed] [Google Scholar]
- 62.van Nierop FS, de Jonge C, Kulik W, et al. Duodenal-jejunal lining increases postprandial unconjugated bile acid responses and disrupts the bile acid-FXR-FGF19 axis in humans. Metabolism 2019;93:25–32. [DOI] [PubMed] [Google Scholar]
- 63.Kaválková P, Mráz M, Trachta P, et al. Endocrine effects of duodenal-jejunal exclusion in obese patients with type 2 diabetes mellitus. J Endocrinol 2016;231(1):11–22. [DOI] [PubMed] [Google Scholar]
- 64.de Moura EG, Orso IR, Martins BC, et al. Improvement of insulin resistance and reduction of cardiovascular risk among obese patients with type 2 diabetes with the duodenojejunal bypass liner. Obes Surg 2011;21(7):941–7. [DOI] [PubMed] [Google Scholar]
- 65.Stratmann B, Krepak Y, Schiffer E, et al. Beneficial metabolic effects of duodenal jejunal bypass liner for the treatment of adipose patients with type 2 diabetes mellitus: Analysis of responders and non-responders. Horm Metab Res 2016;48(10):630–7. [DOI] [PubMed] [Google Scholar]
- 66.Roehlen N, Laubner K, Bettinger D, et al. Duodenal-Jejunal bypass liner (DJBL) improves cardiovascular risk biomarkers and predicted 4-year risk of major CV events in patients with type 2 diabetes and metabolic syndrome. Obes Surg 2020;30(4):1200–10. [DOI] [PubMed] [Google Scholar]
- 67.Quezada N, Muñoz R, Morelli C, et al. Safety and efficacy of the endoscopic duodenal-jejunal bypass liner prototype in severe or morbidly obese subjects implanted for up to 3 years. Surg Endosc 2018;32(1):260–7. [DOI] [PubMed] [Google Scholar]
- 68.Betzel B, Cooiman MI, Aarts EO, et al. Clinical follow-up on weight loss, glycemic control, and safety aspects of 24 months of duodenal-jejunal bypass liner implantation. Surg Endosc 2020;34(1):209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Betzel B, Drenth JPH, Siersema PD. Adverse events of the duodenal-jejunal bypass liner: A systematic review. Obes Surg 2018;28(11):3669–77. [DOI] [PubMed] [Google Scholar]
- 70.Laubner K, Riedel N, Fink K, et al. Comparative efficacy and safety of the duodenal-jejunal bypass liner in obese patients with type 2 diabetes mellitus: A case control study. Diabetes Obes Metab 2018;20(8):1868–77. [DOI] [PubMed] [Google Scholar]
- 71.Foschi D, Corsi F, Lazzaroni M, et al. Treatment of morbid obesity by intraparietogastric administration of botulinum toxin: A randomized, double-blind, controlled study. Int J Obes (Lond) 2007;31(4):707–12. [DOI] [PubMed] [Google Scholar]
- 72.Foschi D, Lazzaroni M, Sangaletti O, et al. Effects of intramural administration of botulinum toxin A on gastric emptying and eating capacity in obese patients. Dig Liver Dis 2008;40(8):667–72. [DOI] [PubMed] [Google Scholar]
- 73.Topazian M, Camilleri M, De La Mora-Levy J, et al. Endoscopic ultrasound-guided gastric botulinum toxin injections in obese subjects: A pilot study. Obes Surg 2008;18(4):401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bang CS, Baik GH, Shin IS, et al. Effect of intragastric injection of botulinum toxin A for the treatment of obesity: A meta-analysis and meta-regression. Gastrointest Endosc 2015;81(5):1141–7. [DOI] [PubMed] [Google Scholar]
- 75.García-Compean D, Mendoza-Fuerte E, Martínez JA, et al. Endoscopic injection of botulinum toxin in the gastric antrum for the treatment of obesity. Results of a pilot study. Gastroenterologie clinique et biologique 2005;29(8-9):789–91. [DOI] [PubMed] [Google Scholar]
- 76.de Moura EG, Ribeiro IB, Frazão MSV, et al. EUS-guided intragastric injection of botulinum toxin A in the preoperative treatment of super-obese patients: A randomized clinical trial. Obes Surg 2019;29(1):32–9. [DOI] [PubMed] [Google Scholar]
- 77.Bustamante F, Brunaldi VO, Bernardo WM, et al. Obesity treatment with botulinum toxin-A is not effective: A systematic review and meta-analysis. Obes Surg 2017;27(10):2716–23. [DOI] [PubMed] [Google Scholar]
- 78.Haidry RJ, van Baar AC, Galvao Neto MP, et al. Duodenal mucosal resurfacing: Proof-of-concept, procedural development, and initial implementation in the clinical setting. Gastrointest Endosc 2019;90(4):673–e2. [DOI] [PubMed] [Google Scholar]
- 79.Rajagopalan H, Cherrington AD, Thompson CC, et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes: 6-Month interim analysis from the first-in-human proof-of-concept study. Diabetes Care 2016;39(12):2254–61. [DOI] [PubMed] [Google Scholar]
- 80.van Baar ACG, Holleman F, Crenier L, et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: One year results from the first international, open-label, prospective, multicentre study. Gut 2020;69(2):295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Baar ACG, Beuers U, Wong K, et al. Endoscopic duodenal mucosal resurfacing improves glycaemic and hepatic indices in type 2 diabetes: 6-month multicentre results. JHEP Rep 2019;1(6):429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fractyl (https://www.fractyl.com/wp-content/uploads/2019/11/FR-19001-07.R2.AASLD19.08Nov19_NoBuild_FINAL-Med-info.pdf). Accessed October 23, 2020. [Google Scholar]
- 83.Hajifathalian K, Mehta A, Ang B, et al. Improvement in insulin resistance and estimated hepatic steatosis and fibrosis after endoscopic sleeve gastroplasty. Gastrointest Endosc 2021;93(5):1110–18. [DOI] [PubMed] [Google Scholar]
- 84.Graus Morales J, Crespo Perez L, Marques A, et al. Modified endoscopic gastroplasty for the treatment of obesity. Surg Endosc 2018;32(9):3936–42. [DOI] [PubMed] [Google Scholar]
- 85.Jirapinyo P, Thompson CC. Endoscopic gastric body plication for the treatment of obesity: Technical success and safety of a novel technique (with video). Gastrointest Endosc 2020;91(6):1388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Baar ACG, Nieuwdorp M, Holleman F, et al. The duodenum harbors a broad untapped therapeutic potential. Gastroenterology 2018;154(4):773–7. [DOI] [PubMed] [Google Scholar]
- 87.Nguyen NQ, Debreceni TL, Bambrick JE, et al. Accelerated intestinal glucose absorption in morbidly obese humans: Relationship to glucose transporters, incretin hormones, and glycemia. J Clin Endocrinol Metab 2015;100(3):968–76. [DOI] [PubMed] [Google Scholar]
- 88.Adachi T, Mori C, Sakurai K, et al. Morphological changes and increased sucrase and isomaltase activity in small intestines of insulin-deficient and type 2 diabetic rats. Endocr J 2003;50(3):271–9. [DOI] [PubMed] [Google Scholar]
- 89.Theodorakis MJ, Carlson O, Michopoulos S, et al. Human duodenal enteroendocrine cells: Source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 2006;290(3):E550–9. [DOI] [PubMed] [Google Scholar]
- 90.Lee J, Cummings BP, Martin E, et al. Glucose sensing by gut endocrine cells and activation of the vagal afferent pathway is impaired in a rodent model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol 2012;302(6):R657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cherrington AD, Rajagopalan H, Maggs D, et al. Hydrothermal duodenal mucosal resurfacing: Role in the treatment of metabolic disease. Gastrointest Endosc Clin N Am 2017;27(2):299–311. [DOI] [PubMed] [Google Scholar]
- 92.Lopez-Nava G, Asokkumar R, Rull A, et al. Bariatric endoscopy procedure type or follow-up: What predicted success at 1 year in 962 obese patients? Endosc Int Open 2019;7(12):E1691–E1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lopez-Nava G, Laster J, Negi A, et al. Endoscopic gastroplasty: An effective solution in a high-risk patient with morbid obesity. Clin J Gastroenterol 2021;14(2):489–93. [DOI] [PubMed] [Google Scholar]