Abstract
Introduction
Due to the high demand for information regarding COVID-19 vaccination in people with epilepsy (PWE), we assessed the symptoms and seizure control of PWE following their COVID-19 vaccination.
Methods
All adult patients who were treated at our center were asked to report on their vaccination status and, if vaccinated, about their experiences following their first COVID-19 vaccination with regard to adverse effects and seizure control.
Results
Fifty-four PWE have already received their first vaccination against COVID-19 (27 female, 20% seizure free, 96<% on antiseizure medication) and were included in the study. Two-thirds tolerated the vaccines generally either very well or well. Thirty-three percent reported general vaccination adverse effects. The most frequently reported general adverse effects were, in descending order, headache, fatigue and fever, and shivering. With regard to epilepsy-related adverse effects, one patient reported increased seizure frequency one day after the first COVID-19 vaccination was administered, and one reported the occurrence of a new seizure type. None of the patients reported a status epilepticus or aggravation of preexisting adverse effects.
Conclusions
Our data suggest that vaccination against COVID-19 appears to be well tolerated in PWE, supporting the recommendation of vaccination to PWE.
Keywords: Epilepsy, COVID-19 pandemic, SARS-CoV-2, Vaccination, Adverse effect
1. Introduction
The Corona pandemic has led to dramatic changes in social and economic life worldwide. In addition to the high number of COVID-19-related dead, those who are seriously ill, and patients who are suffering from long/post COVID [1], [2], the general population is also affected by the way in which the dramatic pandemic measures have influenced psychosocial relations and daily living. All of these issues point toward the urgent need for vaccination. The development of vaccines against COVID-19 was a global effort that resulted in the first approval of vaccines in Europe at the end of 2020. Vaccines based on different technologies (e.g. vector-based or mRNA vaccines) were approved and are now available. The latter vaccines were approved in viral diseases for the first time. Because the amount of vaccine was limited, a vaccination plan was necessary in order to prioritize certain populations. In Germany, as in most other European countries, this plan gives the highest priority to vulnerable patients who are at high risk of lethal or serious course of disease, and those in assisted-living institutions along with their healthcare professionals. Epilepsy is not listed separately in these vaccination plans but, in special situations, people with epilepsy (PWE) can be upgraded to a higher priority [3].
Epilepsy is one of the most common neurological diseases and is associated with increased morbidity and mortality. Aspiration during seizures increases the risk of pneumonia and infections can worsen seizure symptoms in some PWE. In addition, patients with specific epilepsy syndromes who are taking immunosuppressive drugs (e.g., in tuberous sclerosis complex or autoimmune encephalitis) must be considered “at risk”. In addition, PWE suffer more often from comorbidities, which places them at an increased risk of a severe course of a SARS-CoV-2 infection [4]. An early analysis of COVID-19 admissions showed an association between epilepsy and age with a fatal outcome after contracting COVID-19 [5]. In contrast, larger studies did not find any evidence that a SARS-CoV-2 infection per se leads to a worse outcome in PWE than in the normal population [6] . Nevertheless, negative impact of COVID-19 pandemic on PWE in terms of health and health care is well described [7].
For now, having epilepsy is not a sufficient reason for obtaining the highest prioritization, at least in Germany. Knowledge concerning COVID-19 vaccination tolerance and complications in PWE is sparse leading to current recommendations for vaccination which are based more on the lack of evidence for complications and less on the evidence of lack of complications.
Therefore, we would like to share our clinical experience with other medical professionals by analyzing 54 consecutively followed up patients who were vaccinated against COVID-19 and provide important information for a good and safe care and treatment of PWE.
2. Material and methods
2.1. Patients und data acquisition
Our study criteria included all patients aged 18 years and older who were admitted to our ward or who visited our outpatient clinic between 19th March and 20th April 2021. Clinical data on COVID-19 vaccinations were obtained via a structured questionnaire. The questions were answered by the patients themselves; for mentally disabled or non-native German speakers, assistance was provided by the family members, caregivers, or the translators who accompanied them. All patients were given the opportunity to ask further questions. The survey was waived from ethical approval by the local medical ethics committee at the Medical Faculty of the Rheinische Friedrich-Wilhelms-University of Bonn.
The questionnaire was comprised of four questions on vaccination against COVID-19 and addressed the following: the type of vaccine and date of vaccination, epilepsy-related vaccination adverse effects (such as seizure frequency, seizure intensity, new seizures, and the effects on antiseizure medication (ASM) adverse effects), general vaccination adverse effects (multiple answers were possible), and tolerance. For latter a five-point scale (from 1 to 5) was used and scores were sorted into mild, moderate, and severe adverse effects. Cutoff was deliberately chosen from 3 for “moderate” and from 5 for “severe”.
The following demographic data were obtained: age, sex, age at onset of epilepsy, duration of epilepsy, epilepsy syndrome, seizure frequency, number of previously and currently prescribed ASMs, comorbidities, highest level of education, native German speaker, assisted-living institutions, and healthcare proxy. Seizure frequency was assessed on the basis of self-assessment using the Revised Seizure-based Outcome Classification System (Duke) with Analysis of Relationship to HRQOL (health-related quality of life) with three seizure categories (seizure free, ≤10 seizures per year, >10 seizures per year) according to Vickrey [8]. This classification system was chosen as it strongly reflects the close relation to quality of life.
2.2. Statistical analysis
The acquired data were analyzed using IBM SPSS Statistics (version 25). Stepwise logistic regression analysis was performed with adverse effects as dependent variable and age, sex, age at onset of epilepsy, duration of epilepsy, seizure frequency, number of actual ASMs, and psychiatric comorbidities as potential predictors (inclusion criterion p < 0.5, exclusion criterion <0.1). Results were considered statistically significant at a p-value of ≤0.05.
3. Results
In the above-mentioned period, 435 patients visited the Department of Epileptology, University Hospital of Bonn. Clinical data on COVID-19 vaccination status were available for 313 of them. Of those, 57 had already been vaccinated. At the time of the appointment, 25 patients were still awaiting vaccination and 18 of them agreed to be contacted later on. This could be carried out in 3 patients. Overall, information on 60 vaccinated patients was available, 54 of whom suffered from epilepsy. None of them had COVID-19 before. PWE characteristics are listed in Table 1 .
Table 1.
Patient characteristics (n = 54).
| Feature | |
|---|---|
| male/female | 27 (50%)/27 (50%) |
| age (years), mean ± SD/range | 47.9 ± 0.20.1/20–89 |
| age at onset of epilepsy (years), mean ± SD/range | 25.1 (±25.2)/1–89 |
| duration of epilepsy (years), mean ± SD/range* | 22.6 (±16.7)/0–68 |
| epilepsy syndrome | |
| focal | 40 (74%) |
| generalized | 6 (11%) |
| unknown | 8 (15%) |
| additional psychogenic seizures | 3 (6%) |
| seizure free | 11 (20%) |
| lower seizure frequency | 16 (30 %) |
| higher seizure frequency | 27 (50%) |
| medical therapy | |
| on anticonvulsant medication | 52 (96%) |
| exposition to ≥3 different anticonvulsants | 17 (32%) |
| number of actual anticonvulsants mean ± SD/range | 2.2(±1.4)/0–5 |
| education | |
| Education <10 years | 10 (19%) |
| Education >10 years | 11 (20%) |
| no education/school for mentally or physically handicapped persons | 15 (28%) |
| no information | 18 (33%) |
| Assisted-living institution | 7 (13%) |
| healthcare proxy | 21 (39%) |
| native German speaker | 45 (83%) |
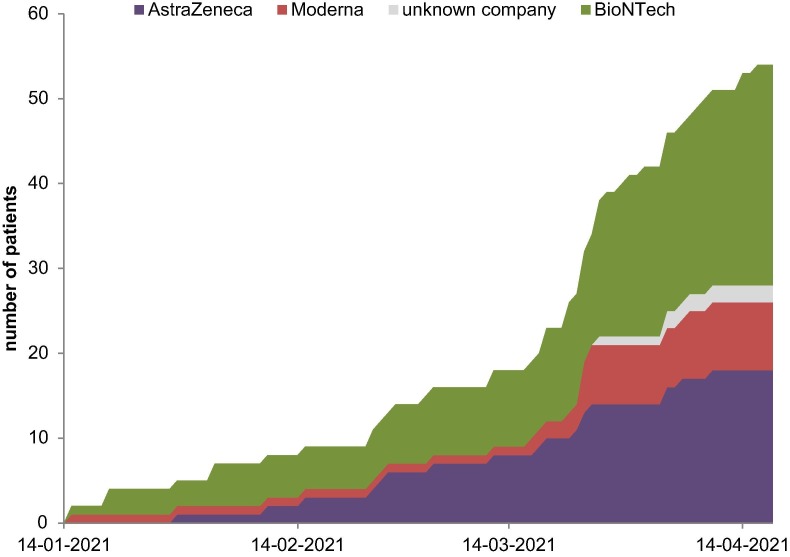
Altogether, 26 patients were administered vaccine from BioNTech, 8 patients were vaccinated with Moderna, and 18 with AstraZeneca. Two patients were unable to remember the name of the vaccine company (Fig. 1 ).
Fig. 1.
Course of first vaccination against SARS-CoV-2 with different vaccines (n = 54).
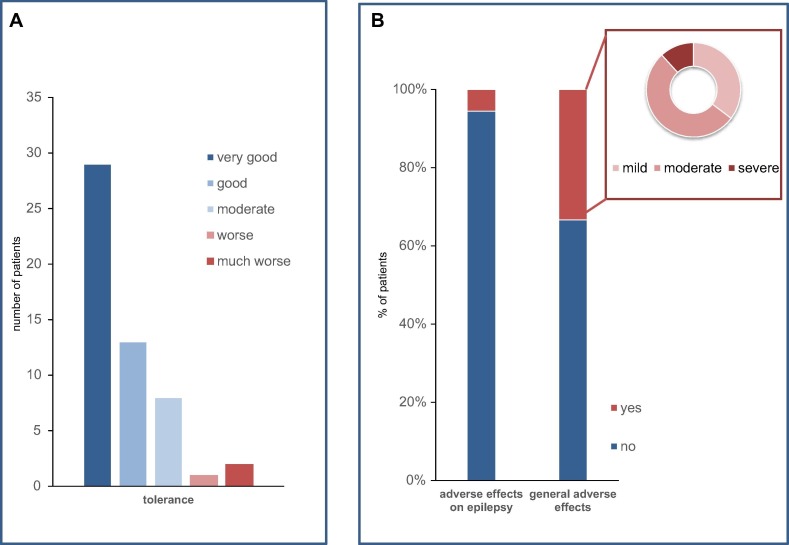
Rating of general tolerance can be seen in Fig. 2 . Eighteen patients (33%) reported having general adverse effects such as headache (17%), fatigue (15%), and fever/shivering (9%), as well as gastrointestinal symptoms (4%). Stepwise logistic regression analysis resulted in a prediction model relating an earlier onset of epilepsy, the presence of psychiatric comorbidities, female sex, and a lower number of ASMs to the statement of adverse vaccination effects (Chi2 for the model = 30.88, p < 0.000). The classification table of the prediction model indicates that 83.3% of the patients with adverse effects and 91.4% of those without were predicted correctly. With regard to epilepsy-related problems, one caregiver reported that one day after vaccination with BioNTech, a series of focal seizures with impaired awareness occurred (which is a regular occurrence for this patient), and did not cease after the usual lorazepam dosage was administered. The dosage had to be doubled in order to successfully stop seizures. Another patient reported to have had more seizures after being vaccinated with BioNTech, but as irregular ASM intake was reported as well, the reason for the reported increase in seizures remained unclear. Another patient reported that a new seizure type appeared four days after the COVID-19 vaccination with AstraZeneca. None of these patients narrated concomitant fever. None of all vaccinated patients reported a status epilepticus, more intense seizures, or aggravation of preexisting adverse effects (Fig. 2).
Fig. 2.
(A) Tolerance and (B) side effects of first COVID-19 vaccination in 54 PWE.
Data on the second COVID-19 vaccination were only available for six patients, three of whom reported the same tolerance level and adverse-effect profile as the first vaccination. One reported poor tolerance for the second vaccination (very good versus moderate), while one reported increased seizure intensity three days after the second vaccination with BioNTech.
4. Discussion
The demographic data reflect the population of our tertiary epilepsy center as shown in previous surveys [9], [10]. This was astonishing as a higher average age and a higher proportion of patients in assisted-living institutions would have been expected due to the mandatory vaccination plan. However, the vaccination schedule had to be adjusted several times over recent weeks, depending on the availability and limitations of licenses. This resulted in availability outside of the contracted schedule and might explain our results. The vaccination rate for our patients was as expected with respect to vaccination rate in the general population in Germany at that time [11].
Overall, tolerance of available COVID-19 vaccines was good in most cases with mild or moderate adverse effects, and in line with studies of the general population [12], [13], [14]. No adverse effects were reported that have not already been known before. The risk of general adverse effects is correlated to females, age, early onset of epilepsy, and psychiatric comorbidities. Unexpectedly higher number of ASMs was not correlated to general adverse effects. This could be explained by the fact that patients who take many ASMs do not necessarily perceive milder adverse effects or perhaps assign them less value and thus do not report them. However, statistical findings have to be interpreted with great caution as sample size is quite small; nevertheless, this can be an interesting trend which has to be followed up in larger cohorts.
Two patients reported of an increased seizure frequency, for one of whom relation to vaccination must be clearly questioned. A new seizure type occurred in another patient. The data must be evaluated with caution, since a causality between adverse effects and vaccination has not been proven and it may only be a timely coincidence.
Vaccinations are generally recommended in PWE; the risk of increased seizure frequency is low or modest [15], [16] and when weighing the benefits and risks of vaccination, the recommendations are unambiguously in favor of vaccination [17]. In fact, these recommendations are based on case series cohorts and retrospective analyses, mostly in children. In severe childhood encephalopathies such as Dravet-Syndrome, a vaccination-associated fever might increase seizure risk [18], but administering a fever prophylaxis simultaneously or prior to a vaccination enables these PWE to be immunized as well. Data on seizure risk for PWE with the new vaccines (vector-based or mRNA) are missing. Our data indicate that there is no unexpected increased risk of seizure exacerbation by COVID-19 vaccine based on new technologies.
We are aware of the limitations of our data and have interpreted them accordingly: The survey was retrospective, observational, and based on subjective measures which is inherent in most surveys. Furthermore, our data were collected in a specialized, tertiary epilepsy center representing a special group of PWE and not the general population of PWE. Of course, especially the results regarding the occurrence of adverse effects have to be considered with caution due to the small number of patients. Larger studies are needed to better evaluate the prevalence of adverse effects in PWE. In particular, the focus of future studies must also be on special patient groups, such as patients with Dravet-Syndrome or patients on immunosuppressive drugs. Nevertheless, these data give a first impression of the generally good tolerability of the COVID-19 vaccination in PWE and support recommendation in favor of vaccination against COVID-19 for broader patient groups.
5. Conclusion
Our data would appear to indicate that vaccinating against COVID-19 in PWE is safe and well tolerated, particularly with regard to epilepsy-related adverse effects. However, further studies in larger groups with sub-analyses covering different vaccines, with regard to first and second vaccinations (if needed) and efficacy of COVID-19 vaccination in PWE is needed. Therefore, future studies on above-mentioned topics are necessary to provide PWE and healthcare professionals with the information needed for COVID-19 vaccinations and for vaccines based on new technologies.
Funding
The research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Rass V., Beer R., Josef Schiefecker A., Kofler M., Lindner A., Mahlknecht P., et al. Neurological outcome and quality of life three months after COVID-19: a prospective observational cohort study. Eur J Neurol. 2021 doi: 10.1111/ene.14803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham E.L., Clark J.R., Orban Z.S., Lim P.H., Szymanski A.L., Taylor C., et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann Clin Transl Neurol. 2021;8 doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/C/Coronavirus/Verordnungen/Corona-ImpfV_BAnz_AT_11.03.2021_V1.pdf n.d.
- 4.Keezer M.R., Sisodiya S.M., Sander J.W. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016;15(1):106–115. doi: 10.1016/S1474-4422(15)00225-2. [DOI] [PubMed] [Google Scholar]
- 5.Cabezudo-García P., Ciano-Petersen N.L., Mena-Vázquez N., Pons-Pons G., Castro-Sánchez M.V., Serrano-Castro P.J. Incidence and case fatality rate of COVID-19 in patients with active epilepsy. Neurology. 2020;95(10):e1417–e1425. doi: 10.1212/WNL.0000000000010033. [DOI] [PubMed] [Google Scholar]
- 6.Asadi‐Pooya A.A., Emami A., Akbari A., Javanmardi F. COVID-19 presentations and outcome in patients with epilepsy. Acta Neurol Scand. 2021;143(6):624–628. doi: 10.1111/ane.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorpe J., Ashby S., Hallab A., Ding D., Andraus M., Dugan P., et al. Evaluating risk to people with epilepsy during the COVID-19 pandemic: preliminary findings from the COV-E study. Epilepsy Behav. 2021;115:107658. doi: 10.1016/j.yebeh.2020.107658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vickrey B.G., Hays R.D., Engel J., Spritzer K., Rogers W.H., Rausch R., et al. Outcome assessment for epilepsy surgery: The impact of measuring health-related quality of life. Ann Neurol. 1995;37(2):158–166. doi: 10.1002/ana.410370205. [DOI] [PubMed] [Google Scholar]
- 9.von Wrede R., Moskau-Hartmann S., Amarell N., Elger C.E., Helmstaedter C. Knowledge, expectations and fears of cannabis use of epilepsy patients at a tertiary epilepsy center. Epilepsy Behav. 2019;99:106458. doi: 10.1016/j.yebeh.2019.106458. [DOI] [PubMed] [Google Scholar]
- 10.von Wrede R., Meschede C., Brand F., Helmstaedter C. Levetiracetam, perampanel, and the issue of aggression: A self-report study. Epilepsy Behav. 2021;117:107806. doi: 10.1016/j.yebeh.2021.107806. [DOI] [PubMed] [Google Scholar]
- 11.https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/Impfquoten-Tab.html n.d.
- 12.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An mRNA Vaccine against SARS-CoV-2 - Preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet (London, England) 2020;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Top K.A., Righolt C.H., Hawken S., Donelle J., Pabla G., Brna P., et al. Adverse events following immunization among children with epilepsy: A self-controlled case series. Pediatr Infect Dis J. 2020;39(5):454–459. doi: 10.1097/INF.0000000000002553. [DOI] [PubMed] [Google Scholar]
- 16.Top K.A., Brna P., Ye L., Smith B. Risk of seizures after immunization in children with epilepsy: a risk interval analysis. BMC Pediatr. 2018;18:134. doi: 10.1186/s12887-018-1112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pruna D., Balestri P., Zamponi N., Grosso S., Gobbi G., Romeo A., et al. Epilepsy and vaccinations: Italian guidelines. Epilepsia. 2013;54:13–22. doi: 10.1111/epi.12306. [DOI] [PubMed] [Google Scholar]
- 18.Tro-Baumann B., von Spiczak S., Lotte J., Bast T., Haberlandt E., Sassen R., et al. A retrospective study of the relation between vaccination and occurrence of seizures in Dravet syndrome. Epilepsia. 2011;52(1):175–178. doi: 10.1111/j.1528-1167.2010.02885.x. [DOI] [PubMed] [Google Scholar]