Abstract
Introduction
Screening for coronavirus disease 2019 (COVID-19) exposure, coupled with engaged decision making to prioritize cancer treatment in parallel with reducing risk of exposure and infection, is crucial in the management of COVID-19 during cancer treatment. After two reported case studies of imaging findings during daily computed tomography (CT)-based image-guided radiotherapy (RT) scans, a call for submission of anonymized case reports was published with the objective of rapidly determining if there was a correlation between the onset of new pulmonary infiltrates found during RT and COVID-19. We hereby report the results of the aggregate analysis.
Methods
Data of deidentified case reports for patients who developed biochemically confirmed COVID-19 during RT were submitted through an online portal. Information requested included a patient’s sex, age, cancer diagnosis and treatment, and COVID-19 diagnosis and outcome. Coplanar CT-based imaging was requested to reveal the presence or absence of ground-glass opacities or infiltrates.
Results
A total of seven reports were submitted from Turkey, Spain, Belgium, Egypt, and the United States. Results and imaging from the patients reported by Suppli et al. and McGinnis et al. were included for a total of nine patients for analysis. All patients were confirmed COVID-19 positive using polymerase chain reaction-based methods or nasopharyngeal swabs. Of the nine patients analyzed, abnormalities consistent with ground-glass opacities or infiltrates were observed in eight patients.
Conclusions
This is the largest case series revealing the potential use of CT-based image guidance during RT as a tool for identifying patients who need further workup for COVID-19. Considerations for reviewing image guidance for new pulmonary infiltrates and immediate COVID-19 testing in patients who develop new infiltrates even without COVID-19 symptoms are strongly encouraged.
Keywords: COVID-19, Computed tomography, Radiotherapy, Ground-glass opacities
Introduction
In the previous year, the coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 has spread to all corners of the globe. By mid-May 2021, global tracking through Johns Hopkins University estimates more than 165 million cases worldwide, including approximately 33 million cases in the United States, 25 million cases in India, 15 million cases in Brazil, and more than 1 million cases in 25 other countries.1 Approximately 3.4 million deaths have already been attributable to COVID-19, and the COVID-19 pandemic is accelerating as third waves across multiple countries through early 2021. Although COVID-19 is most often associated with severe acute respiratory syndrome, it can affect a wide spectrum of organ systems with substantial effects on the lungs, heart, gastrointestinal tract, kidneys, circulatory system, immune system, and even the brain.2 , 3 Cancer treatment has been disrupted by the COVID-19 pandemic including delays in screening that may ultimately lead to substantial changes in survival patterns owing to delays in starting or pauses of active treatment and patient reticence to address medical issues for fear of contracting COVID-19.4 , 5 Addressing COVID-19 during cancer treatment requires close coordination across health systems to accurately screen for COVID-19 exposure and infection, coupled with engaged decision making to prioritize cancer treatment in parallel with reducing risk of exposure.6 Furthermore, patients with cancer who develop COVID-19 require careful consideration of the urgency to treat cancer while balancing the need to manage COVID-19 and risking exposure to other patients with cancer.
Screening for COVID-19 in the general population is based on assessing exposure, symptoms, and testing using polymerase chain reaction (PCR) or serologic antibody-based assays.7 People suspected of infection can receive further testing including radiologic thoracic imaging to evaluate for the presence of pulmonary infiltrates. A recent Cochrane review across 34 studies suggested a pooled sensitivity of 89.9% and specificity of 61.1% for computed tomography (CT)-based imaging.8 Nevertheless, authors noted a high or unclear risk of bias. In April 2020, two separate groups reported case studies of imaging findings during daily CT-based image-guided radiotherapy (RT) scans.9 , 10 A subsequent call for submission of anonymized case reports was published to try and ascertain if similar findings were observed during RT.11 Results of submissions are reported in the subsequent texts.
Methods
A call for aggregation of deidentified case reports for patients who developed biochemically confirmed COVID-19 during RT was published in April 2020.11 The primary objective was to determine whether there was a correlation between the onset of new pulmonary infiltrates found during RT and a diagnosis of COVID-19. Submitting groups would send anonymized data through an online link. Data requested included a patient’s sex, age, cancer diagnosis, histologic evaluation, cancer stage, chemotherapy agent, planned RT dose, completed RT dose, method of COVID-19 diagnosis, date of COVID-19 diagnosis relative to the start of RT, date of COVID-19 symptoms relative to the start of RT, any COVID-19 symptoms, and data related to patient care and outcome. Reporting for outcome was at the discretion of the submitting contributors as a free text field. Coplanar CT-based imaging was requested to reveal the presence or absence of ground-glass opacities or infiltrates. Because of the expected variability of imaging quality among contributors and different institutional practices for daily image verification, there were no stipulations regarding select imaging and contributors were allowed to submit representative images at their own discretion. Furthermore, because deidentified data were reported such that subjects could not be identified, this study is exempt as supported by the Code of Federal Regulations section 45, part 46.
Results
A total of seven reports were submitted through the online portal representing cases from Turkey, Spain, Belgium, Egypt, and the United States. The case of patient one was subsequently published12 and included in this report with author’s permission under the Creative Commons Attribution License. Results and imaging from the patients reported by Suppli et al.9 and McGinnis et al.10 were included for a total of nine patients for analysis (Table 1 ). The nine patients presented included four females and one patient with metastatic disease. All patients were confirmed COVID-19 positive using PCR-based methods or nasopharyngeal swabs. All nine patients were diagnosed with lung cancer, including four with squamous cell carcinoma and five with adenocarcinoma.
Table 1.
Patient Characteristics
| Patient | Sex | Age | Cancer Diagnosis | Histologic Classification | Cancer Stage | Method of COVID-19 Diagnosis |
|---|---|---|---|---|---|---|
| 1 (Kirakli et al.12) | M | 61 | Lung | AC | T4N2M1 | PCR |
| 2 | M | 72 | Lung | SCC | T4N2M0 | NPS |
| 3 | M | 65 | Lung | SCC | T4N0M0 | PCR |
| 4 | F | 74 | Lung | AC | T3N2M0 | PCR |
| 5 | M | 66 | Lung | AC | TxN3M0 | NPS |
| 6 | F | 63 | Lung | SCC | T4N0M0 | PCR |
| 7 | F | 77 | Lung | SCC | T1cN2M0 | NPS |
| 8 (Suppli et al.9) | M | 74 | Lung | AC | T3N2M0 | PCR |
| 9 (McGinnis et al.10) | F | 63 | Lung | AC | Recurrent | PCR |
AC, adenocarcinoma; COVID-19, coronavirus disease 2019; F, female; M, male; NPS, nasopharyngeal swab; PCR, polymerase chain reaction; SCC, squamous cell carcinoma.
The cancer treatment characteristics and COVID-19 outcomes are reported in Table 2 . Chemotherapy was used as a part of cancer treatment for seven patients, and one patient was on a trial for immunotherapy in combination with stereotactic ablative RT. The planned course of RT was completed by three patients, with the remaining patients having treatment interrupted or discontinued. Symptoms were noted in six patients with fever the most common symptom present in all six patients. Hospitalization occurred in seven patients, including one patient who was hospitalized without symptoms as part of standard local policy. As of the date of data submission, deaths were reported in two patients.
Table 2.
Cancer Treatment and COVID-19 Outcomes
| Patient | Planned RT Dose (Gy) | RT Dose Completed | Chemotherapy | COVID-19 Symptoms | Hospitalized | Overall Patient Outcome |
|---|---|---|---|---|---|---|
| 1 (Kirakli et al.12) | 20 | 0 | None | None | Yes | CT findings before starting RT, asymptomatic but hospitalized owing to local policy, symptoms 48 h after CT simulation, discharged from hospital after 20 d |
| 2 | 60 | 60 | Carboplatin, paclitaxel | Fever, cough | Yes | Completed RT as inpatient, chemotherapy held after COVID-19 diagnosis, discharged from hospital after 2 consecutive negative tests |
| 3 | 60 | 46 | Carboplatin, paclitaxel | None | Yes | Admitted for neutropenia and esophagitis (not COVID-19), oncology treatment resumed as an inpatient, additional COVID-19 exposure while hospitalized, death from COVID-19 |
| 4 | 55 | 50 | Cisplatin, pemetrexed | Fever | Yes | Recovered |
| 5 | 66 | 24 | Cisplatin, pemetrexed | Fever, cough, loss of smell/taste | Yes | RT interrupted, 4 wk hospitalization with severe illness at the time of submission |
| 6 | 60 | 38 | Cisplatin, etoposide | Fever | No | Self-isolating at home |
| 7 | 60 | 60 | Carboplatin, etoposide | Fever, cough | Yes | Completed RT 4 d before COVID-19 diagnosis, discharged to rehabilitation facility after 5 d, 2 negative tests 1 mo after |
| 8 (Suppli et al.9) | 60 | 18 | Platinum doublet | Fever, cough, fatigue, myalgia | Yes | Death from respiratory failure 6 d after symptoms |
| 9 (McGinnis et al.10) | 50 | 50 | None | None | No | Asymptomatic, completed SABR |
COVID-19, coronavirus disease 2019; CT, computed tomography; RT, radiotherapy; SABR, stereotactic ablative radiotherapy.
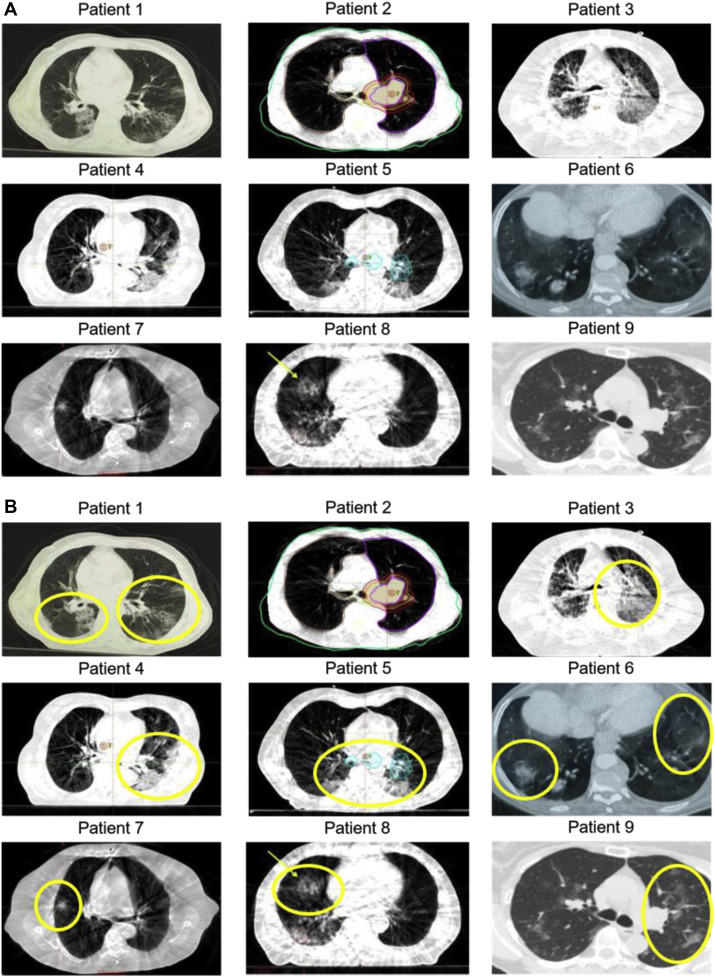
Figure 1 reveals CT findings submitted as screenshots for each patient. Images submitted by contributors were analyzed, and it was decided to present representative axial images for consistency across all patients (Fig. 1 A). Of nine sets of patient images, abnormalities consistent with ground-glass opacities or infiltrates were observed in eight patients (Fig. 1 B). Only one patient (patient 2) had no observable opacities or infiltrates on submitted images.
Figure 1.
Cross-sectional imaging of patients. Representative axial images are displayed. (A) Submitted axial images are displayed for each patient. Images submitted were not altered for enhancement but formatted for size and fit within the figure. (B) Areas with ground-glass opacities or infiltrates are highlighted in yellow for each patient. Patient 2 had no observed infiltrates or ground-glass opacities on any submitted images. Patient 1 (Kirakli et al.12); patient 8 (Suppli et al.9); patient 9.10
Discussion
Initial reports suggest that patients with lung cancer who develop COVID-19 have observable infiltrates or ground-glass opacities that can be detected during CT-based image guidance for RT. Data do not support guidance on how to manage patients or whether RT procedures should be disrupted. Furthermore, data are insufficient to provide guidance on any COVID-19 outcomes. Nevertheless, data do support the conclusion that new ground-glass opacities or pulmonary infiltrates observed during CT-based image guidance warrant further diagnostic evaluation for COVID-19.
Screening patients during RT setup procedures has been discussed as a potentially useful tool for detecting COVID-19.13 In a letter to the editor, analysis of temperature screening yielded a comparable detection rate as compared with CT screening at the time of RT setup, both approximately 0.5%.14 A typical course of conventional RT could consist of 2 to 3 weeks for consultation and RT setup and 5 to 7 weeks for RT treatment. This results in 7 to 10 weeks of potential exposure time for a typical patient within a RT department. Patients can be exposed before or during treatment, and a single point-of-care assessment is likely to misrepresent risk in the entirety of treatment. For patients receiving daily or weekly CT-based image guidance, this would enable screening both at the time of RT setup with a CT simulation and repeated assessments for the 5 to 7 weeks of treatment. Monitoring CT scans for changes suggesting ground-glass opacities or infiltrates may not add any time or burden to treatment staff but would require additional time for people responsible for image review. This could represent an added time burden for physicians. Nevertheless, the reward for this added time could be an increased detection of COVID-19 exposure and a secondary system for alerting clinicians and patients for dedicated COVID-19 screening. Increased early detection is anticipated to reduce overall exposure within an oncology clinic and ultimately reduce impact on effective cancer treatment.
The objective of this aggregation of cases was to rapidly determine if there was a correlation between the onset of new pulmonary infiltrates found during RT and COVID-19.11 The nine cases presented suggest that there is a correlation and support consideration of further COVID-19 screening and workup for any patients exhibiting new infiltrates during the pandemic. There are several limitations to any interpretation beyond this conclusion. There were no data collected on patients who did not have COVID-19, thus limiting the ability to estimate any sensitivity or specificity for new pulmonary infiltrates. Though most patients exhibited symptoms, there are likely many COVID-19–positive patients who were asymptomatic and who were never screened for COVID-19. It was recognized early that responses were anticipated to be multinational and that submission of CT-based images used for RT would have a wide range of institution-specific technical capability and standard practice procedures. For this reason, the request for images was liberal and did not require specific image quality or anatomical representation. Although this limits the quality of interpretation, the observation that eight of nine patients had new observable pulmonary infiltrates across a spectrum of technical capability and practice strengthens the potential use of CT-based image guidance as a potential screening tool for COVID-19. It is also important to note that method of COVID-19 detection was described as “nasopharyngeal swabs” in three reports. Although PCR was the likely method, it is possible that alternative tests, such as rapid antigens using nasopharyngeal swabs, were used, and potential variations in accuracy among different tests were not available.
To the authors’ knowledge, this is the largest case series revealing the potential use of CT-based image guidance during RT as a tool for identifying patients who need further workup for COVID-19. It is anticipated that collecting a large structured data set providing strong estimates of sensitivity, specificity, and with well-defined imaging criteria would take a moderately long time and limit access to information that could immediately benefit patients during the ongoing pandemic. Two recommendations arise from these cases. First, radiation oncologists and RT departments should strongly consider reviewing image guidance for new pulmonary infiltrates and consider immediate COVID-19 testing in patients who develop new infiltrates even without COVID-19 symptoms. Second, substantially more data are needed to determine the sensitivity and specificity of these findings and the temporal nature. Although highly effective COVID-19 vaccines are available at the time of completing this report, there is substantial variability in vaccine distribution and uptake across different countries, and it may take years before a substantial number of individuals are vaccinated globally to achieve herd immunity. It is ancitipated that COVID-19 infection may become endemic or seasonal, akin to the common influenzas. Thus, these findings are anticipated to remain relevant for several years.
CRediT Authorship Contribution Statement
Graham W. Warren: Conceptualization, Methodology, Formal analysis, Writing-original draft preparation, reviewing, and editing, Visualization, Supervision
Vun-Sin Lim: Methodology, Data curation, Project administration, Editing
Mudit Chowdhary, Gaurav Marwaha, Osama Mostafa Abd Elbadee, Esra Korkmaz Kirakli, Charlotte Billiet, Alexandra Giraldo Marin, Monica Ramos, Morten Hiul Suppli, Gwendolyn J. McGinnis: Investigation, Resources
Alex A. Adjei: Conceptualization, Methodology, Writing-original draft preparation, Supervision
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Johns Hopkins University (JHU) COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) https://coronavirus.jhu.edu/map.html
- 2.Wadman M., Couzin-Frankel J., Kaiser J., Matacic C. How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from the brain to toes. Science. https://www.sciencemag.org/news/2020/04/how-does-coronavirus-kill-clinicians-trace-ferocious-rampage-through-body-brain-toes [DOI] [PubMed]
- 3.Gupta A., Madhavan M.V., Sehgal K., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 4.Sharpless N.E. COVID-19 and cancer. Science. 2020;368:1290. doi: 10.1126/science.abd3377. [DOI] [PubMed] [Google Scholar]
- 5.Bakouny Z., Hawley J.E., Choueiri T.K., et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell. 2020;38:629–646. doi: 10.1016/j.ccell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dingemans A.C., Soo R.A., Jazieh A.R., et al. Treatment guidance for patients with lung cancer during the coronavirus 2019 pandemic. J Thorac Oncol. 2020;15:1119–1136. doi: 10.1016/j.jtho.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinnes J., Deeks J.J., Adriano A., et al. Cochrane COVID-19 Diagnostic Test Accuracy Group. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020;8:CD013705. doi: 10.1002/14651858.CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Islam N., Salameh J.P., Leeflang M.M., et al. Cochrane COVID-19 Diagnostic Test Accuracy Group. Thoracic imaging tests for the diagnosis of COVID-19. Cochrane Database Syst Rev. 2020;(11):CD013639. doi: 10.1002/14651858.CD013639.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Suppli M.H., Riisgaard de Blanck S., Elgaard T., Josipovic M., Pøhl M. Early appearance of coronavirus disease 2019 associated pulmonary infiltrates during daily radiotherapy imaging for lung cancer. J Thorac Oncol. 2020;15:1081–1084. doi: 10.1016/j.jtho.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGinnis G.J., Ning M.S., Nitsch P.L., et al. Rapid detection of asymptomatic coronavirus disease 2019 by computed tomography image guidance for stereotactic ablative radiotherapy. J Thorac Oncol. 2020;15:1085–1087. doi: 10.1016/j.jtho.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren G.W., Adjei A.A. A call for rapid submission of data for aggregate review: can daily radiotherapy imaging be used as a potential screen for coronavirus disease 2019? J Thorac Oncol. 2020;15:876–877. doi: 10.1016/j.jtho.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirakli E.K., Erdem S., Yılmaz H. RT. Imaging as an unintended COVID-19 diagnostic tool: can be used for screening before thoracic RT? Clin Case Rep Int. 2021;5:1210. [Google Scholar]
- 13.Vitullo A., De Santis M.C., Marchianò A., Valdagni R., Lozza L. The simulation-CT: radiotherapy’s useful tool in the race against COVID-19 pandemic. A serendipity approach. Radiother Oncol. 2020;147:151–152. doi: 10.1016/j.radonc.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nevens D., Billiet C., Weytjens R., et al. The use of simulation-CTs as a coronavirus disease 2019 screening tool during the severe acute respiratory syndrome coronavirus 2 pandemic. Radiother Oncol. 2020;151:17–19. doi: 10.1016/j.radonc.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]