See related articles p 5, p 26, and p 317
We report a case of a 17-year-old male adolescent with a recurrence of acute myocarditis 4 months after an initial episode of acute myocarditis negative for severe acute respiratory syndrome coronavirus 2 and 48 hours after receiving his second dose of the Pfizer-BioNTech coronavirus disease 2019 messenger ribonucleic acid vaccine.
The Pfizer-BioNTech coronavirus disease 2019 (COVID-19) messenger ribonucleic acid (mRNA) vaccine has an efficacy of 95% at preventing severe COVID-19, with a low reported incidence of serious adverse events during phase 2/3 global clinical trials.1 On December 11, 2020, the US Food and Drug Administration (FDA) issued an Emergency Use Authorization for the Pfizer-BioNTech vaccine for the prevention of COVID-19 in persons 16 years of age and older. On May 10, 2021, the FDA issued an Emergency Use Authorization for the Pfizer-BioNTech vaccine for persons 12-16 years of age.2 We describe a case of an adolescent male patient who had recurrence of acute myocarditis temporally associated with receipt of a second dose of the Pfizer-BioNTech mRNA COVID-19 vaccine.
Case Presentation
A 17-year-old male adolescent with no significant medical history came to medical attention in January 2021 with chest pain consistent with myocarditis. Serum troponin level peaked at 5.06 ng/mL (normal <0.04 ng/mL). Cardiac magnetic resonance imaging (cMRI) showed 2 small areas of delayed gadolinium enhancement of the left ventricular myocardium (Figure 1 ). Electrocardiogram (EKG) showed diffuse ST-segment changes. Respiratory viral pathogen panel polymerase chain reaction (PCR) testing including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as well as SARS-CoV-2 nucleocapsid IgG and IgA was negative. He received supportive care and was discharged after 6 days of hospitalization with normal cardiac testing, including serum troponin, EKG, and echocardiogram at 2-week follow-up. On April 15, 2021 he received his first dose of the Pfizer-BioNTech COVID-19 mRNA vaccine without any side effects noted.
Figure 1.
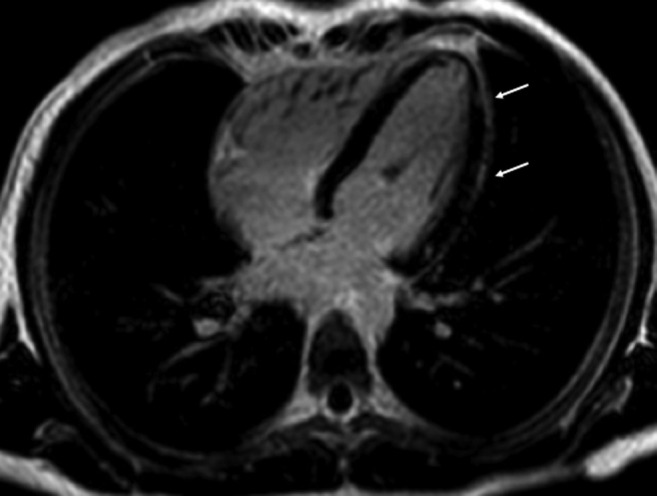
cMRI horizontal long-axis view showing late gadolinium enhancement of the mid-lateral and apical regions (annotated by arrows) during the first episode of myocarditis.
One day after receiving the second dose of the Pfizer-BioNTech COVID-19 mRNA vaccine on May 7, 2021, he developed fever (Tmax 101.3°F) and body aches that responded to acetaminophen. The following day, he developed sudden onset of severe, burning left-sided chest pain that radiated to the left shoulder and the upper left arm. He reported that the chest pain worsened with exertion and movement and was similar to that experienced during his previous episode of myocarditis. He denied any other symptoms.
The patient presented to our emergency department hemodynamically stable; however, EKG revealed diffuse ST-segment elevations. Initial laboratory test results showed a serum troponin of 2.3 ng/mL, C-reactive protein of 29 mg/L (normal 0-5 mg/L), and erythrocyte sedimentation rate of 5 mm/h (normal <15 mm/h). Respiratory pathogen panel PCR testing including SARS-CoV-2 was negative. SAR-CoV-2 nucleocapsid IgG and IgA were negative and SARS-CoV-2 IgM antibody for the spike protein was positive, consistent with recent immunization. Rheumatologic testing including antinuclear antibody test and rheumatoid factor was negative.
He was admitted to the pediatric intensive care unit for telemetry and observation. Initial echocardiogram revealed normal biventricular systolic and diastolic function and no evidence of regional wall motion abnormalities or an effusion. Telemetry revealed occasional isolated premature ventricular contractions. Troponin peaked at 51.37 ng/mL. cMRI showed low normal left ventricular ejection fraction (53%), trivial pericardial effusion, and sub-epicardial late gadolinium in the same distribution as seen in the previous episode but with interval increased enhancement (Figure 2 ). His chest pain improved with nonsteroidal anti-inflammatory drug treatment and supportive care, and he was discharged after 6 days of hospitalization. At the time of discharge, his troponin was 0.17 ng/mL and EKG findings were normal. At an outpatient cardiology visit 3 days after discharge, the patient’s cardiac evaluation, including EKG and echocardiogram, was normal.
Figure 2.
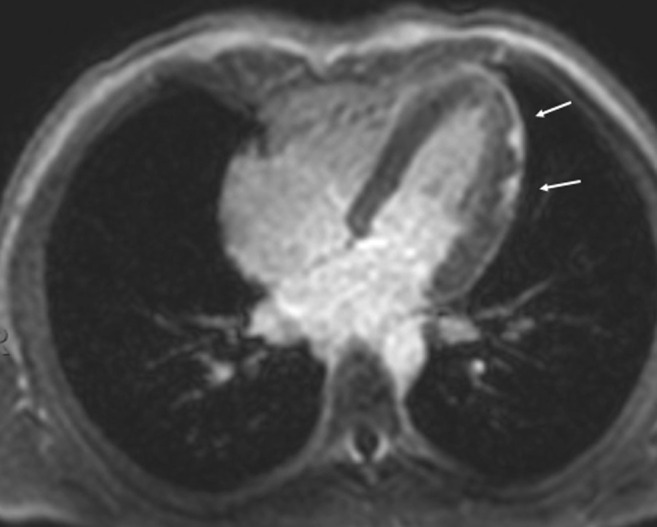
cMRI horizontal long-axis view showing late gadolinium enhancement in the same distribution as that seen in Figure 1 (annotated by arrows), with interval increased late gadolinium enhancement during the second episode.
Discussion
Since initiation of mRNA COVID-19 vaccinations around the world, most side effects of the vaccine have been described as mild and include fatigue, headache, chills, fever, and muscle pain.1 In a randomized control trial of the BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech vaccine) there were 4 serious adverse events: shoulder injury related to vaccine administration, right axillary lymphadenopathy, paroxysmal ventricular arrhythmia, and right leg paresthesia. There were 2 deaths in the BNT162b2 group, both from ischemic heart disease. There were 4 deaths in the placebo group, 2 from unknown causes, 1 from myocardial infarction, and 1 from a hemorrhagic stroke. None of these deaths was thought to be attributable to either vaccine or placebo.1
The first reported case of myocarditis temporally related to mRNA vaccine occurred in a 39-year-old male patient who developed chest pain 21 days after administration the BNT162b2 mRNA vaccine. A cMRI confirmed findings of myocarditis, and patient had a full and uneventful recovery after this illness.3 A Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices COVID-19 Vaccine Safety Technical report issued on May 17, 2021, noted a few reports of myocarditis after the mRNA COVID-19 vaccine to the Vaccine Adverse Event Reporting System, a self-reporting database of the CDC. The cases were predominantly in adolescents and young adults, more often in male than female patients, more often following dose #2 than dose #1, and typically within 4 days after vaccination. These cases appeared mild. CDC officials recommended collaboration between infectious diseases, cardiology, and rheumatology specialists to guide diagnosis and management of myocarditis.4
A case series from Israel described 7 adolescent male patients who presented within 72 hours of the Pfizer-BioNTech COVID-19 mRNA with chest pain. Six patients experienced symptoms after the second dose, and 1 developed symptoms after the first dose. These patients exhibited cardiac injury, as evidenced by cardiac biomarker elevations, EKG changes, or echocardiographic abnormalities. All cases were mild and did not require any cardiovascular or respiratory support. All cases resolved with supportive measures.5 Marshall et al report 7 cases of myocarditis in healthy male adolescents in the US who presented within 4 days of the dose of the Pfizer-BioNTech COVID-19 mRNA vaccine. All patients had chest pain, elevated serum troponins, EKG abnormalities, and cMRI findings consistent with myocarditis. All of these patients were PCR negative for SARS-CoV-2, and none of them met criteria for multisystem inflammatory syndrome in children related to COVID-19. Three patients were treated with nonsteroidal anti-inflammatory drugs only, and 4 received intravenous immunoglobulin and corticosteroids. None were critically ill, and all improved rapidly.6
Recurrent myocarditis is a rare entity in the general population, with most cases described in adults and only 2 reports in children. The first was an 8-year-old female patient who had 3 episodes of acute myocarditis. During the third episode, she was found to be H1N1 positive by nasopharyngeal PCR test. She died on the second day of her third hospitalization after a bradycardic cardiac arrest. The other case was a 14-year-old male patient with evidence of myocarditis by endomyocardial biopsy and cMRI. PCR testing of an endomyocardial specimen revealed Parvovirus B 19 on second episode.7 Viral triggers have been postulated to be responsible for some recurrence of myocarditis.7 There has been 1 recent case of immune-mediated (virus-negative) lymphocytic myocarditis with a fulminant relapse in a 40-year-old woman. The episodes of fulminant myocarditis occurred 5 years apart. Serologic testing during the second episode showed evidence of past SARS-CoV-2 infection.8
Our patient had his first episode of idiopathic acute myocarditis 4 months before recurrence. This recurrent episode of myocarditis may have been triggered by the vaccine administration through an autoinflammatory or autoimmune phenomenon. Cardiac MRI during both illnesses demonstrated enhancement after gadolinium administration in the same distribution during both episodes. This case highlights the possibility that the COVID-19 mRNA vaccine may trigger recurrent acute myocarditis in children and adolescents who have had a previous episode of acute myocarditis.
The FDA has recently approved this vaccine in children 12 years and older2 and recommends that clinicians be aware of the potential for myocarditis to occur following COVID-19 mRNA vaccination.4
Acknowledgments
We thank Dr Puneet Bhatla for his review of the cMRI imaging for this report.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Polak F., Thomas S., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2021:1–13. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration Coronavirus (COVID-19) update: FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in adolescents in another important action in fight against pandemic. FDA news release. May 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use
- 3.García JB, Ortega PP, Antonio Bonilla Fernández J, León AC, Burgos LR, Dorta EC. Miocarditis aguda tras administración de vacuna BNT162b2 contra la COVID-19 [Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19]. Rev Esp Cardiol [in Spanish]. doi: 10.1016/j.recesp.2021.03.009 [DOI] [PMC free article] [PubMed]
- 4.Centers for Disease Control and Prevention COVID-19 VaST Work Group technical report—May 17, 2021. Advisory Committee on Immunization Practices (ACIP) May 2021. https://www.cdc.gov/vaccines/acip/work-groups-vast/technical-report-2021-05-17.html
- 5.Snapiri O, Rosenberg Danziger C, Shirman N, Weissbach A, Lowenthal A, Ayalon I, et al. Transient cardiac injury in adolescents receiving the BNT162b2 mRNA COVID-19 vaccine. Pediatr Infect Dis J. doi: 10.1097/INF.0000000000003235 [DOI] [PMC free article] [PubMed]
- 6.Marshall M., Ferguson I.D., Lewis P., Jaggi P., Gagliardo C., Collins J.S., et al. Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021 Jun 4 doi: 10.1542/peds.2021-052478. e2021052478. [DOI] [PubMed] [Google Scholar]
- 7.Floyd A., Lal A., Molina K., Puchalski M., Miller D., May L. When lightning strikes twice in pediatrics: case report and review of recurrent myocarditis. Pediatrics. 2018;141(3):e20164096. doi: 10.1542/peds.2016-4096. [DOI] [PubMed] [Google Scholar]
- 8.Caraffa R., Marcolongo R., Bottio T., Rizzo S., Bifulco O., Onofrio A., et al. Recurrent autoimmune myocarditis in a young woman during the coronavirus disease 2019 pandemic. Esc Hear Fail. 2021;8:756–760. doi: 10.1002/ehf2.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]


