Abstract
Autosomal-recessive (AR) nonsyndromic hearing impairment (NSHI) displays a high degree of genetic heterogeneity with >100 genes identified. Recently, TMEM132E, which is highly expressed in inner hair cells, was suggested as a novel ARNSHI gene for DFNB99. A missense variant c.1259G>A: p.(Arg420Gln) in TMEM132E was identified that segregated with ARNSHI in a single Chinese family with two affected members. In the present study, a family of Pakistani origin with prelingual profound sensorineural hearing impairment displaying AR mode of inheritance was investigated via exome and Sanger sequencing. Compound heterozygous variants c.382G>T: p.(Ala128Ser) and c.2204C>T: p.(Pro735Leu) in TMEM132E were observed in affected but not in unaffected family members. TMEM132E variants identified in this and the previously reported ARNSHI family are located in the extracellular domain. In conclusion, we present a second ARNSHI family with TMEM132E variants which strengthens the evidence of the involvement of this gene in the etiology of ARNSHI.
Subject terms: Medical genomics, Next-generation sequencing
Introduction
Hearing impairment (HI) is a heterogeneous disorder that occurs at all ages with varying severity, affecting 1 in 500 newborns and >360 million people worldwide [1]. To date, more than 100 autosomal recessive nonsyndromic hearing impairment (NSHI) genes have been identified. Due to extreme locus heterogeneity and the very low frequency of many ARNSHI variants often candidate HI genes have only been observed in a single family [1]. Identification of additional families is important to confirm their role in disease pathogenesis and to improve genotype–phenotype correlation.
The TMEM132 family contains five genes TMEM132A, TMEM132B, TMEM132C, TMEM132D, and TMEM132E. The role of the TMEM132 gene family remains poorly understood, however a few studies have reported their involvement in NSHI, panic disorders, and cancer [2].
TMEM132E (MIM 616178) is located within the DFNB99 locus on chromosome 17q12. This gene contains ten exons and encodes the TMEM132E protein which is highly expressed in the inner ear and other tissues including brain, kidney, lung, liver, spleen, heart, small intestine, colon, thymus, and stomach. In 2015, TMEM132E was suggested as a causative gene for ARNSHI [3, 4]. The main function of the TMEM132E protein is to connect the extracellular medium with the intracellular actin cytoskeleton [2].
To date only one TMEM132E variant has been suggested to be associated with ARNSHI [4]. We describe a family with TMEM132E compound heterozygous variants that segregate with ARNSHI, adding support of the involvement of this gene in HI etiology.
Methods
Clinical and molecular evaluation
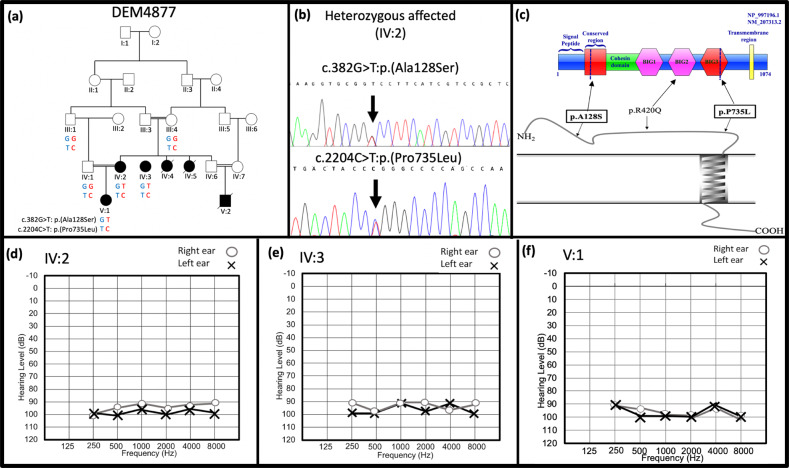
This study was approved by the Institutional Review Boards of the Quaid-i-Azam University and the Baylor College of Medicine and Affiliated Hospitals. Consanguineous family, DEM4877, with ARNSHI was ascertained from a rural area of Sindh Province of Pakistan. Written informed consent was obtained from all participating members of DEM4877 and peripheral blood samples were collected from six family members: IV:2, IV:3, and V:1 who are affected with sensorineural HI and III:1, III:4, and IV:1 who are unaffected (Fig. 1a). Extraction of genomic DNA was performed using a Phenol–chloroform method [5]. Hearing impaired family members underwent pure tone air and bone conduction audiometry at a local government hospital (Fig. 1d–f and Supplementary Fig. 1). Tandem gait and Romberg tests were also performed to exclude vestibular dysfunction.
Fig. 1.
Pedigree diagram, sequence chromatogram, and audiograms of family DEM4877. a DEM4877 pedigree drawings and segregations results for TMEM132E variants c.382G>T: p.(Ala128Ser) and c.2204C>T: p.(Pro735Leu). Squares represent males and circles females; filled symbols denote hearing impaired individuals and clear symbols unaffected family members. Double lines indicate consanguineous marriages. b Sequencing chromatogram of TMEM132E compound heterozygous variants c.382G>T: p.(Ala128Ser) (upper panel) and c.2204C>T: p.(Pro735Leu) (lower panel) of affected individual IV:2 from family DEM4877. c The domain architecture of the TMEM132 protein family (predicted by Sanchez-Pulido and Chris P. Ponting, 2018) (upper panel) and predicted transmembrane helices in TMEM132E (adapted from the result of TMHMM 2.0 analysis) (lower panel) showing the positions of previously reported one missense p.(Arg420Gln) variant mapped on BIG2 domain, a novel variants p.(Ala128Ser) located in the conserved region (CR) and another variant p.(Pro735Leu) lies in the BIG3 domain. The previously reported and new ARNSHI variants located in the extracellular region of TMEM132E. Variants in the box were identified in this study. d–f Displays the pure tone audiograms (bone conduction audiometry can be found in Supplementary Fig. 1) for affected individuals d IV:2, e IV:3, and f V:1, respectively
Initial screening of GJB2, SLC26A4, HGF, and CIB2
Prior to exome sequencing, the entire coding region of GJB2, which is a common cause of HI, was screened by Sanger sequencing. Additional common ARHI variants in the Pakistani population were also screened via Sanger sequencing: i.e., intronic HGF variants (c.482+1986_1988delTGA and c.482+1991_2000delGATGATGAAA), missense variants p.(Phe91Ser), and p.(Cys99Trp) within CIB2 and SLC26A4 missense variants p.(Gln446Arg) and p.(Val239Asp) [6–8].
Exome sequencing and bioinformatics analysis
Exome sequencing was performed using a DNA sample from affected DEM4877 pedigree member IV:3. For the preparation of exome libraries, the SureSelect Human All Exon V6 kit was used, that target 60.46 Mb of coding sequences in the human genome (~20,000 protein coding genes), which is 99% of the protein coding regions of the National Center for Biotechnology Information‘s reference sequence (RefSeq) database [9], the consensus coding sequence (CCDS) [10] project and GENCODE [11]. Sequencing was performed using 100 bp paired-end on a HiSeq2500/4000 instrument (Illumina Inc, San Diego, CA, USA). The mean sequencing depth for targeted regions is 64.22×. Reads were aligned to the Human genome (hg19/GRC37) using the Burrows–Wheeler algorithm and duplicates were removed with Picard (GATKIndelRealigner). Single-nucleotides variants and small insertions/deletions (Indels) were called using GATK as described previously [12]. Conservation and damaging effects of the variants were evaluated in silico using annotation tools incorporated in dbNSFP and ANNOVAR [13, 14]. Filtering was performed to analyze the exome sequence data. Frameshift, in-frame indels, missense with a Combined Annotation-Dependent Depletion (CADD) C-score >15, start/stop altering, nonsense, and splice-site variants with a minor allele frequency <0.005 in every Genome Aggregation Database (gnomAD) [15] population were retained. Segregation of the identified variants in the family was validated by Sanger sequencing using the BigDye terminator v3.1 on an ABI 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA) (Fig. 1b). Primers surrounding region of interest were designed using primer3 software [16].
Three-dimensional modeling
The transmembrane helical structure was predicted using TMHMM server v. 2.0 [17]. The three-dimensional structure of TMEM132E was built using the I-TASSER server based on ab initio/threading method [18]. For evaluation of stereochemical quality of protein structure, the PROCHECK program was used [19].
Results
Clinical description
Pure tone air and bone conduction audiometry diagnosed bilateral profound sensorineural HI in all DEM4877 affected pedigree members IV:2, IV:3, and V:1 (Fig. 1d–f and Supplementary Fig. 1). HI is prelingual and most likely congenital, since it was detected before the age of one in all affected family members. The hearing impaired family members were physically examined, neither gross vestibular dysfunction nor any episodes of vertigo were observed.
Whole exome and Sanger sequencing
GJB2 as well as selected variants in CIB2, HGF, and SLC26A4 were excluded as the underlying cause of HI in family DEM4877. Exome sequencing was performed using a DNA sample from individual IV:3. After variant filtering to identify homozygous and potentially compound heterozygous rare variants, four homozygous variants in ERICH3, DNAH11, SNAPC4, and RIMBP2 and potential compound heterozygous variants in PHLDB1 and TMEM132E were observed and tested for segregation (Supplementary Table 1), No rare homozygous variants were found in the previously reported HI genes. Sanger sequencing confirmed the segregation of the compound heterozygous variants c.382G>T: p.(Ala128Ser) and c.2204C>T: p.(Pro735Leu) in TMEM132E (ENSG00000181291) (Fig. 1b). The compound variants in PHLDB1 as well as homozygous variants in ERICH3, DNAH11, SNAPC4, and RIMBP2 did not segregate with ARNSHI.
In silico analysis
Both TMEM132E variants c.382G>T: p.(Ala128Ser) and c.2204C>T: p.(Pro735Leu) have high CADD C-scores of 24.3 and 23, respectively and are also predicted to be damaging by various bioinformatic tools (Supplementary Table 1). The variants are both rare in gnomAD (Supplementary table 1) with no homozygous variants observed.
Three-dimensional modeling
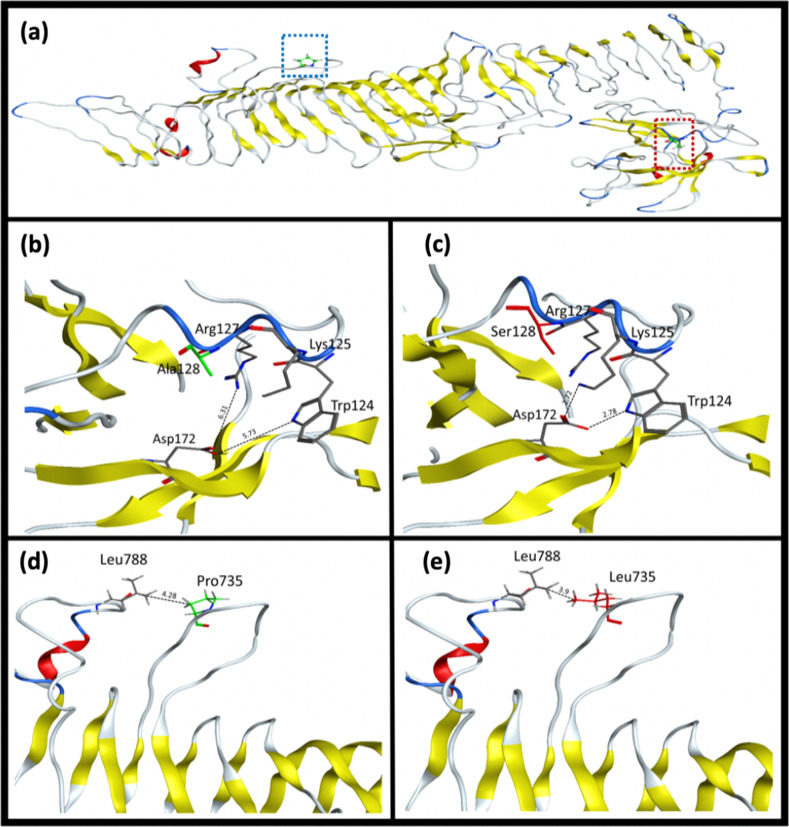
TMHMM analysis demonstrated that both identified variants lie in the extracellular domain of the TMEM132E protein (Fig. 1c). In addition, we carried out comparative modeling to determine the structural difference between the wild type and mutant TMEM132E structure. Both the p.Ala128 and p.Pro735 positions lie in the loop region of the extracellular surface region of the protein. Residue p.Ala128 is not involved in any interaction with nearby residues, while p.Pro735 shows a hydrophobic interaction with a nearby p.Leu788 residue. In the case of the p.Ala128Ser variant, the insertion of polar charged side chain of serine residue establishes a strong interaction network with the nearby residues while the p.Pro735Leu variant shows local conformational changes in nearby residues (Fig. 2a–e).
Fig. 2.
Predicted three-dimensional structure of TMEM132E. a Ball and stick model representation of p.Ala128 and p.Pro735 residues were highlighted by red and blue square respectively while b–e showing the interaction pattern of amino acid residues. b p.Ala128 (wild type), c p.Ser128 (mutant), d p.Pro735 (wild type) and e p.Leu735 (mutant)
Discussion
In this study, we investigated three family members (IV:2, IV:3, and V:1) with prelingual profound HI and three unaffected members (II:1, II:4, and IV:1) of a consanguineous family. Neither vestibular dysfunction nor any other abnormality was observed in the affected individuals, suggesting these patients present with NSHI. Analysis of exome sequence data obtained from hearing impaired family member IV:3 revealed potentially compound heterozygous variants c.382G>T: p.(Ala128Ser) and c.2204C>T: p.(Pro735Leu) in TMEM132E. Sanger sequencing of the remaining family members confirmed the compound heterozygous status and demonstrated that these variants segregate with HI in DEM4877. To date, only one homozygous missense variant c.1259G>A: p.(Arg420Gln) in TMEM132E has been reported in a Chinese family segregating ARNSHI, suggesting involvement of the gene in HI [2, 4]. Through three-dimensional modeling of TMEM132E, we show that the variants p.(Ala128Ser) and p.(Pro735Leu) which segregate with ARNSHI in family DEM4877 cause local conformational changes due to the insertion of a new interaction network (Fig. 2b–e). TMHMM analysis revealed that all three amino acid residues altered in ARNSHI, p.Ala128, p.Arg420, and p.Pro735, are located in the extracellular region of TMEM132E protein. The extracellular portions of TMEM132 proteins contain three important regions i.e., a conserved region (CR), a cohesion domain (Ch) and three bacterial immunoglobulin-like (BIG) domains BIG1, BIG2, and BIG3 [2] (Fig. 1c). The variants p.(Ala128Ser) and p.(Pro735Leu) identified in the present study are located in the CR and the BIG3 domain respectively, while the previously identified variant p.(Arg420Gln) lies in the extracellular BIG2 domain of TMEM132E protein [2] (Fig. 1c). Due to the location of all three variants, the extracellular region of TMEM132E is likely critically important for proper function of the protein.
The TMEM132E gene is highly expressed in the brain and involved in neuronal function. Furthermore, mouse model studies show that Tmem132e is highly expressed in the inner hair cells of the cochlea, apical, and basal region of outer hair-cell cytoplasm but not in the hair bundle, in mice organ of Corti [4]. Knockdown of its ortholog (tmem132e) in Danio rerio affects the mechanotransduction of hair cells [4].
In conclusion, we identified compound heterozygous variants c.382G>T: p.(Ala128Ser) and c.2204C>T: p.(Pro735Leu) in TMEM132E that segregates with HI in a consanguineous Pakistani family. The previously reported TMEM132E variant in a Chinese family and newly identified compound heterozygous variants in Pakistani family suggesting that this gene could be involved in HI in both east and south Asian populations. This finding provides additional evidence for the involvement of TMEM132E in ARNSHI and expands the spectrum of variants involved in HI in the Pakistani population.
Web resources
ANNOVAR, http://annovar.openbioinformatics.org/
Burrows–Wheeler Aligner, http://bio-bwa.sourceforge.net/
Combined Annotation-Dependent Depletion (CADD), https://cadd.gs.washington.edu/
dbSNP, https://www.ncbi.nlm.nih.gov/projects/SNP/
Exome Aggregation Consortium (ExAC), http://exac.broadinstitute.org/
gnomAD, http://gnomad.broadinstitute.org/
Genome Analysis Toolkit (GATK), https://software.broadinstitute.org/gatk/
Genomic Evolutionary Rate Profiling (GERP), http://mendel.stanford.edu/SidowLab/downloads/gerp/
Hereditary hearing loss, https://hereditaryhearingloss.org/
MutationTaster, http://www.mutationtaster.org/
Online Mendelian Inheritance of Man (OMIM), https://www.omim.org/
PhastCons and PhyloP, http://compgen.cshl.edu/phast/
Picard, https://broadinstitute.github.io/picard/
PyMol, https://pymol.org/
eXome-Hidden Markov Model (XHMM), https://atgu.mgh.harvard.edu/xhmm/
Uniport, http://www.uniprot.org/
UCSF Chimera, http://www.rbvi.ucsf.edu/chimera
Supplementary information
Acknowledgements
We thank the family members for participating in this study. This work was supported by the Higher Education Commission of Pakistan (to WA) and National Institutes of Health (NIH)-National Institute of Deafness and other Disorders grants R01 DC011651 and R01 DC003594 (to SML).
Funding
This work was supported by the Higher Education Commission of Pakistan (to WA) and National Institutes of Health (NIH)-National Institute of Deafness and other Communication Disorders grants R01 DC011651 and R01 DC003594 (to SML).
Author contributions
KL drafted the manuscript and performed data analysis. KL, SH, and IS performed data analysis. KL and AA performed the laboratory experiments. MB, RHA, MU, and SN collected the samples and analyzed the clinical data while protein modeling was performed by AN. WA, IS, and SML edited the manuscript and all authors revised and approved the final manuscript. Funds for the work were obtained by SML.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
Members of the DEM4877 family provided written informed consents for the proposed study.
Footnotes
The original online version of this article was revised: The article “Further evidence of involvement of TMEM132E in autosomal recessive nonsyndromic hearing impairment”, written by Khurram Liaqat, et al., was originally published electronically on the publisher’s internet portal on 28 October 2019 without open access. With the author’ decision to opt for Open Choice the copyright of the article changed on 11 June 2021 to © Author(s) 2019.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/16/2021
A Correction to this paper has been published: 10.1038/s10038-021-00951-9
Supplementary information
The online version of this article (10.1038/s10038-019-0691-4) contains supplementary material, which is available to authorized users.
References
- 1.Morton CC, Nance WE. Newborn hearing screening — a silent revolution. N Engl J Med. 2006;354:2151–64. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Pulido L, Ponting CP. TMEM132: an ancient architecture of cohesin and immunoglobulin domains define a new family of neural adhesion molecules. Bioinformatics. 2018;34:721–4. doi: 10.1093/bioinformatics/btx689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng L, Gong Y, Liu Q, Chen B, Guo C, Li J, et al. Gene mapping of a nonsyndromic hearing impairmint family. Chin J Med Genet. 2003;20:89–93. [PubMed] [Google Scholar]
- 4.Li J, Zhao X, Xin Q, Shan S, Jiang B, Jin Y, et al. Whole-exome sequencing identifies a variant in TMEM132E causing autosomal-recessive nonsyndromic hearing loss DFNB99. Hum Mutat. 2015;36:98–105. doi: 10.1002/humu.22712. [DOI] [PubMed] [Google Scholar]
- 5.Green MR, Sambrook J. Isolation of high-molecular-weight DNA using organic solvents. Cold Spring Harb Protoc. 2017;2017:pdb.prot093450. doi: 10.1101/pdb.prot093450. [DOI] [PubMed] [Google Scholar]
- 6.Schultz JM, Khan SN, Ahmed ZM, Riazuddin S, Waryah AM, Chhatre D, et al. Noncoding mutations of HGF are associated with nonsyndromic hearing loss, DFNB39. Am J Hum Genet. 2009;85:25–39. doi: 10.1016/j.ajhg.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riazuddin S, Belyantseva IA, Giese A, Lee K, Indzhykulian AA, Nandamuri SP, et al. Mutations in CIB2, a calcium and integrin binding protein, cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat Genet. 2012;44:1265–71. doi: 10.1038/ng.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahzad M, Sivakumaran TA, Qaiser TA, Schultz JM, Hussain Z, Flanagan M, et al. Genetic analysis through OtoSeq of pakistani families segregating prelingual hearing loss. Otolaryngol-Head Neck Surg. 2013;149:478–87. doi: 10.1177/0194599813493075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Leary NA, Wright MW, Brister JR, Ciufo S, Haddad D, McVeigh R, et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016;44:D733–45. doi: 10.1093/nar/gkv1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pujar S, O’Leary NA, Farrell CM, Loveland JE, Mudge JM, Wallin C, et al. Consensus coding sequence (CCDS) database: a standardized set of human and mouse protein-coding regions supported by expert curation. Nucleic Acids Res. 2018;46:D221–8. doi: 10.1093/nar/gkx1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frankish A, Diekhans M, Ferreira A-M, Johnson R, Jungreis I, Loveland J, et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019;47:D766–73. doi: 10.1093/nar/gky955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liaqat K, Schrauwen I, Raza SI, Lee K, Hussain S, Chakchouk I, et al. Identification of CACNA1D variants associated with sinoatrial node dysfunction and deafness in additional Pakistani families reveals a clinical significance. J Hum Genet. 2019;64:153–60. doi: 10.1038/s10038-018-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Jian X, Boerwinkle E. dbNSFP: a lightweight database of human nonsynonymous snps and their functional predictions. Hum Mutat. 2011;32:894–9. doi: 10.1002/humu.21517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics. 2007;23:1289–91. doi: 10.1093/bioinformatics/btm091. [DOI] [PubMed] [Google Scholar]
- 17.Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden markov model: application to complete genomes11Edited by F. Cohen. J Mol Biol. 2001;305:567–80. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr. 1993;26:283–91. doi: 10.1107/S0021889892009944. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.