Abstract
Host characteristics, such as sex and age, are closely associated with the structure and function of gut microbiota; however, less is known about the effects of age and sex on the gut microbiota of nonhuman primates, and therefore, our knowledge of interindividual variability in host gut microbiota is limited. In this study, 153 fecal samples from rhesus macaques (Macaca mulatta) were analyzed using high‐throughput 16S rRNA sequencing in order to explore associations between age and sex of the host and their gut microbiota. The results indicated that female macaques had higher alpha diversity and a more unique gut microbiota than did males. The proportion of Proteobacteria, Tenericutes, Cyanobacteria, unclassified bacteria, and Verrucomicrobia was higher in females than that in males. We also found that adults of both sexes had a higher alpha diversity, a higher proportion of norank Ruminococcaceae, Oscillospira, norank Lachnospiraceae, norank Clostridiales, and Succinivibrio, and a lower proportion of Enterococcus than immatures. Functional analyses revealed that the richness of metabolic pathways was higher in females than males and in adults compared with immatures. These results could be attributed to differences in the nutritional requirements and hormone levels of macaques of different sex and age classes. We conclude that variation in the gut microbiota of different sex and age classes of rhesus macaques may be linked to age‐ and sex‐specific differences in nutrient requirements and hormone levels. These results highlight the importance of host age and sex on the structure and function of the gut microbiota and the need to consider physiological traits when conducting studies on the gut microbiota.
Keywords: age difference, gut microbiota, Macaca mulatta, sex difference
Sex and age were associated with differences in the diversity and relative abundance of gut microbiota in rhesus macaques. These differences could be linked to age‐ and sex‐specific differences in nutrient requirements and hormone levels, highlighting the effects of age and sex on the structure and function of the gut microbiota, as well as the need to consider physiological traits when conducting gut microbiota studies.

1. INTRODUCTION
Interindividual differences in sex and age strongly affect host gut microbiota (Derrien et al., 2019; Koren et al., 2012; Markle et al., 2013; Peng et al., 2020; Reveles et al., 2019; Vujkovic‐Cvijin et al., 2020). Numerous studies have focused on the effect of diet on gut microbiota. These studies indicate that diet can rapidly alter the composition of a host's gut microbiota (David et al., 2014; De Filippo et al., 2010). Different diets provide a variety of nutritional substrates to the intestinal microbiota, allowing its growth and reproduction (David et al., 2014; Ley et al., 2008). However, the importance of sex and age on hosts' gut microbiota has generally been overlooked (Amato et al., 2014; Kashtanova et al., 2016). There are studies that indicate that females tend to increase their consumption of nutrient‐rich foods during pregnancy and lactation (Koch et al., 2017), and mammalian diets tend to change from breastmilk to complex foods during the growth process (Kashtanova et al., 2016). Therefore, individuals of different age and sex classes are likely to exhibit differences in their gut microbiota (Byrd et al., 2020).
Sex differences in mammalian behavior and physiology are extremely common, which may further cause changes in their gut microbiota (Dunbar et al., 2009; Koch et al., 2017; Koren et al., 2012; O'Mara & Hickey, 2014). Previous studies have demonstrated that gut microbiota diversity in females is higher than in males, which could be associated with sex‐based differences in diet (Amato et al., 2014; de la Cuesta‐Zuluaga et al., 2019). In Verreaux's sifakas (Propithecus verreauxi), for example, females were found to devote more time to feeding and increased the intake of macronutrients than males (Koch et al., 2017). In ring‐tailed lemurs (Lemur catta), females altered their diet in order to compensation for higher nutritional and/or energetic demands caused by reproduction (Dunbar et al., 2009; Koch et al., 2017; Li et al., 2014; O'Mara & Hickey, 2014).
Based on these sex‐based differences in diet, the mammalian gut microbiota might be subjected to different selective pressures, further promoting the formation of dimorphism in gut microbiota (David et al., 2014). Conversely, although males and females living in the same social group generally share the same dietary pattern, their microbiota may be affected by differences in the timing and production of sex steroids (Markle et al., 2013; Peng et al., 2020; vom Steeg & Klein, 2017; Yurkovetskiy et al., 2013). Researchers have confirmed that gut microbiota can regulate or metabolize testosterone and estrogen and employ sex steroids for growth and survival (Baker et al., 2017; vom Steeg & Klein, 2017). For instance, rodent disease models indicated that the gut microbiota of males was more susceptible to the development of metabolic disorders than that of females (Peng et al., 2020). This occurred because the males were exposed to a high‐fat diet (Peng et al., 2020). However, the testosterone levels of female recipient increase and metabolic changes when the gut microbiota of adult males are transferred to the intestines of immature females, which results in the prevalence of islet inflammation decreased significantly (Markle et al., 2013). These differences were attributed to sex steroids (Markle et al., 2013; Peng et al., 2020). Thus, considering both nutritional requirements and hormonal differences, the interaction between sex and gut microbiota needs to be carefully studied (vom Steeg & Klein, 2017).
Age is also associated with the gut microbiota of mammals (Byrd et al., 2020; Hasegawa et al., 2018). This has been documented in humans (de la Cuesta‐Zuluaga et al., 2019) and nonhuman primates, such as wild western lowland gorillas (Gorilla gorilla gorilla) (Pafčo et al., 2019), black howler monkeys (Alouatta pigra) (Amato et al., 2014), and Tibetan macaques (Macaca thibetana) (Sun et al., 2018). In general, the diversity of the gut microbiota increases with the host age and stabilizes after reaching an adult‐like microecosystem and digestive ability (Derrien et al., 2019; Kashtanova et al., 2016; But see Reese et al., 2021). This trend may be related to the complexity of dietary composition at different growth stages. Dietary complexity in adults is higher than that in juveniles and infants who have limited mobility or hunting skills (Schiel et al., 2010). In addition, different age classes are differentially affected by hormones, especially during puberty (Vandenberg et al., 2012). Therefore, studies of the gut microbiota need to consider the effects of age on digestive ecology (Byrd et al., 2020; Hasegawa et al., 2018).
Rhesus macaques (Macaca mulatta) are frequently used as animal models to explore interactions between the gut microbiota and diseases (Cui et al., 2019). Previous studies have shown that the dominant genus in the gut microbiota of rhesus macaques was Prevotella, followed by norank Ruminococcaceae, and norank Clostridiaceae (Chen et al., 2020a). These gut microorganisms are specialists in digesting cellulose (De Filippo et al., 2010; Koeck et al., 2014; La Reau & Suen, 2018). In Tibetan macaques, the abundance of Ruminococcaceae increased following the increase in cellulose and lignin content in their diet (Zhao et al., 2018). In addition to diet, factors that influence the gut microbiota of rhesus macaque include interspecific differences in physiology (Chen et al., 2020b; Cui et al., 2019) and environment (Chen et al., 2020a; Zhao et al., 2018). While age and sex are known to influence gut microbiota composition (Byrd et al., 2020; de la Cuesta‐Zuluaga et al., 2019; Hasegawa et al., 2018), more research is needed on this relationship in nonhuman primates. Particularly for species used as animal models, disregarding sex and/or age information may exacerbate the risk of obtaining false positives in gut microbiota studies of human disease (Vujkovic‐Cvijin et al., 2020). For example, although Streptococcus gordonii may be associated with increased risk of infective endocarditis (Douglas et al., 1993), a recent cohort study suggests that it is also strongly related to age and may even serve as a gut microbial marker for predicting host age (Zhang et al., 2021). Therefore, we explored associations between sex and age and the gut microbiota of rhesus macaques. In this study, 153 fecal samples from one group of semi‐provisioned rhesus macaques were collected and differences in the composition, abundance, diversity, and function of the gut microbiota were analyzed by sex and age. We tested the following predictions:
Considering the higher nutritional and metabolic requirements caused by reproduction (Amato et al., 2014; Dunbar et al., 2009), adult females are expected to have higher diversity and richness in their gut microbiota than adult males;
Considering the higher dietary complexity in adults (Schiel et al., 2010), Ruminococcaceae and Clostridiaceae, which are typically associated with fiber metabolism, are expected to be in higher abundance in adult rhesus macaques than in immatures.
2. METHODS
2.1. Study site and subjects
This study was conducted in the Guangxi Longhu Mountain Nature Reserve in Long'an County, Nanning, Guangxi, China (22°56′–23°00′N, 107°27′–107°41′E). This natural reserve is a scenic spot, covered by karst landforms with an altitude of approximately 300–500 m above sea level and dominated by subtropical mountain monsoon rain forest (Wang et al., 1996). The study area has a tropical monsoon climate, with an annual mean temperature of 21.8°C, and an annual mean rainfall of 1,500 mm (Wang et al., 1996).
The study subjects were members of a group comprising approximately 400 rhesus macaques. Their diet was comprised of cooked corn provided by the park managers twice daily (10 a.m. and 3 p.m.) and peanuts provided by visitors. During nonprovisioned time or when visitors were not present, they mainly relied on natural food resources, such as leaves and fruits (Chen et al., 2020a).
2.2. Sample collection
We collected fecal samples of rhesus macaques using sterile gloves and used sterilized bamboo sticks to remove the outer layer of feces that had been in contact with the ground. The inner part of the stool was placed into a sterile centrifuge tube and put into a dry ice portable case to be sent to the laboratory for storage at −80°C until DNA was extracted. In total, 153 rhesus macaque fecal samples were collected (Table 1).
TABLE 1.
Host information on 153 fecal samples of rhesus macaques
| Month | No. of samples | Sex | Age | ||
|---|---|---|---|---|---|
| Male | Female | Adult | Immature | ||
| October 2018 | 7 | 2 | 2 | 5 | 2 |
| November 2018 | 1 | 1 | 1 | ||
| December 2018 | 6 | 1 | 3 | 6 | |
| January 2019 | 6 | 4 | 2 | ||
| February 2019 | 13 | 4 | 3 | 3 | 10 |
| March 2019 | 12 | 3 | 9 | 10 | 2 |
| April 2019 | 11 | 6 | 5 | 7 | 4 |
| May. 2019 | 12 | 8 | 4 | 5 | 7 |
| June 2019 | 12 | 3 | 9 | 10 | 2 |
| July 2019 | 12 | 4 | 7 | 7 | 5 |
| August 2019 | 13 | 5 | 7 | 10 | 3 |
| September 2019 | 12 | 8 | 4 | 7 | 5 |
| October 2019 | 12 | 3 | 9 | 12 | |
| November 2019 | 12 | 12 | 10 | 2 | |
| December 2019 | 12 | 2 | 10 | 12 | |
| Total | 153 | 49 | 85 | 109 | 44 |
All samples were categorized by sex (male or female) and age (adult or immature). A total of 109 samples were adult (≥ 5 years old), and 44 samples were immature (< 5 years old). Sex was identified in 134 samples and unidentified in 19 samples. Of the 134 identified samples, 49 were males and 85 were females. Samples for which sex was not determined were excluded from subsequent analysis.
2.3. DNA extraction, 16S rRNA gene amplification, and sequence processing
The total DNA of gut microbiota from fecal samples was extracted using the E.Z.N.A. Soil DNA Kit (Omega Bio‐Tek) following the manufacturer's protocol; next, the concentration of the extracted DNA was tested and purified using a NanoDrop 2000 ultraviolet‐visible light spectrophotometer (Thermo Fisher Scientific, Inc), followed by 1% agarose gel electrophoresis. Bacterial primers 338F (5′‐ACTCCTACGGGAGGCAGCAG‐3′) and 806R (5′‐GGACTACHVGGGTWTCTAAT‐3′) targeting the V3–V4 hypervariable region of the 16S rRNA gene were used for polymerase chain reaction (PCR) amplification. The PCR products were extracted from a 2% agarose gel and purified further with the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences) and quantified using the QuantiFluor™‐ST (Promega) following the manufacturer's protocol. Purified amplicons were sequenced on an Illumina MiSeq platform at Majorbio Bio‐Pharm Technology Co. Ltd.
The 16S rRNA raw reads obtained from MiSeq sequencing were filtered to obtain high‐quality sequences using FLASH (version 1.2.11; https://ccb.jhu.edu/software/FLASH/index.shtml) as follows: (a) We set up a 50 bp window. Next, the sequence with an average quality score of < 20 from the sliding window was truncated, and any sequence of < 50 bp during quality control was removed; (b) barcodes were matched exactly. The barcode and primer sequences at both ends of the sequence were used to distinguish samples to obtain effective sequences. The direction of sequences was corrected, and the ambiguous bases were discarded. The maximum mismatch number of barcodes and primers was zero and two, respectively; (c) sequences were stitched. Sequences with overlap of > 10 bp were merged according to overlapping relationship of paired‐end reads. The sequences that could not be stitched were removed (Bokulich et al., 2013; Caporaso et al., 2010).
2.4. Bioinformatics analysis
The high‐quality sequences were clustered into operational taxonomic units (OTUs) with a 97% similarity using UPARSE (version 7.0.1090; http://drive5.com/uparse) (Edgar, 2013), and the chimeric sequences were removed using UCHIME.37 (Edgar et al., 2011). Species taxonomic analysis of OTU was performed by the ribosomal database program classifier Bayesian algorithm (version 2.11; https://sourceforge.net/projects/rdp‐classifier/) and aligned to the Greengenes 16S bacteria database (version 13.5), with a confidence threshold of 80%. Good's coverage scores were calculated to determine species coverage using the Mothur software (version 1.30.1; http://www.mothur.org/wiki/Schloss_SOP#Alpha_diversity) (Schloss et al., 2009). Rarefaction curves were generated using the R statistical software for each sample to assess sequencing depth. The relative abundance of community composition was represented by mean ± standard deviation (SD). The differences in relative abundance within sex and age categories were analyzed using the Wilcoxon rank‐sum test. A false discovery rate was used to correct p‐values. Linear discriminant analysis (LDA) and effect size (LEfSe) (http://huttenhower.sph.harvard.edu/galaxy/) were conducted to further detect taxa (from phylum to genus) with differential abundance by sex and age categories (LDA score ≥3; a significance of p < 0.05 was determined using the nonparametric factorial Kruskal–Wallis sum‐rank test).
Alpha diversity was assessed using two diversity indices (Shannon and Simpson) and two richness indices (Ace and Chao), calculated using Mothur software, and visualized in R (version 4.0.4) (R Core Team, 2021). A lower Simpson index suggested a higher alpha diversity. We examined the effect of interaction between age and sex on alpha diversity using lmer and anova functions in lme4 package of R (Bates et al., 2015). We set alpha diversity as a response variable and age, sex, and age–sex interaction as fixed factors and include sampling time as a random factor. For the purpose of improving linearity, the Shannon index, ACE, and Chao values were log10(X)‐transformed and the Simpson index was logit(X)‐transformed (Li et al., 2020; Warton & Hui, 2011). We ran likelihood tests comparing general linear mixed models with and without the age–sex interaction. The results suggested a significant influence of age–sex interaction on the alpha diversity when the p‐value was <0.05. Our results showed that there were no significant effects of age/sex interaction on the alpha diversity (Shannon index: χ 2 = 0.234, df = 2, p = 0.890; Simpson: χ 2 = 0.429, df = 2, p = 0.807; Ace: χ 2 = 1.349, df = 2, p = 0.510; Chao: χ 2 = 1.497, df = 2, p = 0.473). Thus, the Wilcoxon rank‐sum test was used to analyze alpha diversity differences between sex and age groups. To assess differences in community composition and structure, principal coordinate analysis (PCoA) and permutational multivariate analysis of variance (PERMANOVA) were performed based on the Bray–Curtis dissimilarity matrix calculated by QIIME (version 1.9.1; http://qiime.org/install/index.html).
The functional profiles from the 16S rRNA data were predicted using the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States 2 program (PICRUSt2) (version 2.1.3‐b; http://picrust.github.io/picrust/) to better understand the bacterial functional profiles associated with sex and age. The Nearest Sequenced Taxon Index (NSTI) was calculated for evaluating accuracy of functional profiles, with a lower value indicating a higher prediction accuracy (Langille et al., 2013). The mean NSTI for all samples was 0.11 ± 0.03, which was lower than the mean NSTI of mammals (0.14 ± 0.06) (Langille et al., 2013). Predictive functional pathways were annotated using the Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.genome.ad.jp/kegg/) at level 1 to level 3 KEGG orthology groups (KOs). Mann–Whitney U test was used to examine differences in metabolic pathways in level 3 between two groups (male vs. female; adult vs. immature). Significant differences were set at p < 0.05.
3. RESULTS
3.1. Summary of sequencing data
After quality filtering and removal of the chimera, a total of 7,691,681 effective reads were obtained from 153 fecal samples, with an average of 50,272 ± 9,265 effective reads, an average length of 61,847 ± 8,521 effective tags, and an average length of 416 ± 6 bp. The Good's coverage estimates of the 153 samples ranged from 99.12% to 99.61% (mean ± SD = 99.28% ± 0.11%), suggesting that almost all bacterial taxa in each sample were identified. The end of the rarefaction curve tended to be an asymptote, indicating that the sample size in this study was sufficient (Figure 1). After subsampling all samples to an equal sequencing depth (21,561 reads per sample) and clustering, 1,770 OTUs at 97% sequence similarity were obtained and classified into 26 phyla, 59 classes, 103 orders, 180 families, and 298 genera.
FIGURE 1.

Rarefaction curves, coverage, and results of Shannon, Simpson, Ace, and Chao tests
3.2. Differences in the composition and abundance of gut microbiota
Based on all samples, the three phyla with the highest relative abundance were Firmicutes (59.31% ± 16.76%), Bacteroidetes (31.11% ± 16.31%), and Proteobacteria (3.85% ± 4.81%). The top three genera with the highest realative abundance were Prevotella (24.98% ± 15.98%), norank Ruminococcaceae (8.84% ± 4.65%), and Lactobacillus (4.75% ± 8.93%) (Figure 2).
FIGURE 2.

Gut microbiota composition at the level of phylum and genus
At the phylum level, considering both sex and age, the three dominating phyla were Firmicutes, Bacteroidetes, and Proteobacteria (see Table A1). At the genus level, Prevotella had the highest relative abundance in both sex and age classes, followed by norank Ruminococcaceae, Lactobacillus, and norank Clostridiaceae (Tables A2, A3 and A2, A3).
In analyzing the samples by sex, 22 bacterial phyla were detected in the male group and 24 phyla were detected in the female group. At the phylum level, Synergistetes and WS6 were found only in females. Among the top 15 phyla based on relative abundance, the proportions of Proteobacteria, Tenericutes, Cyanobacteria, unclassified bacteria, and Verrucomicrobia were higher in females (Figure 3a). At the genus level, male rhesus macaques had 17 unique genera and females had 40 unique genera. No significant differences were observed between males and females in the top 15 genera (Table A2).
FIGURE 3.
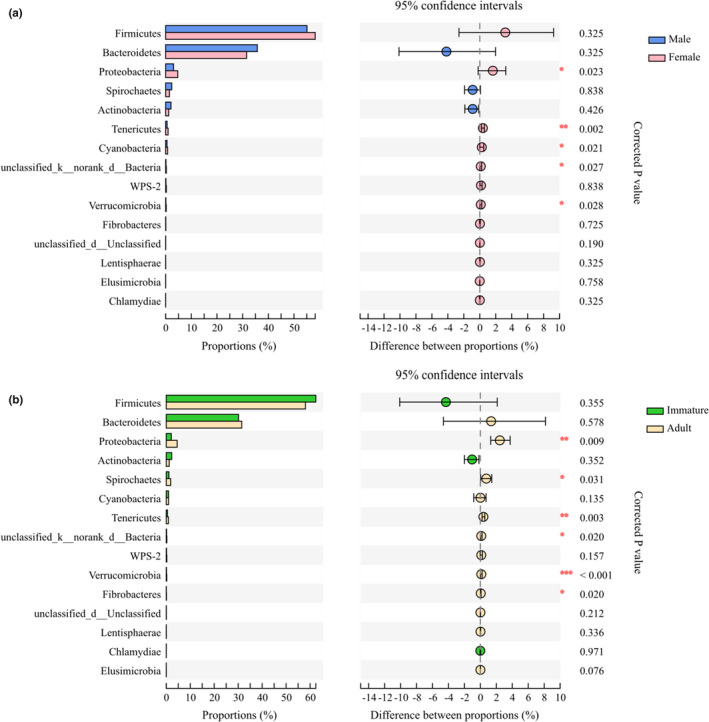
The gut microbiota composition and difference at the phylum level in sex (a) and age (b). The p‐value is represented by asterisks. Significant difference 0.01 < p < 0.05 is marked as “*”, 0.001 < p < 0.01 is marked as “**”, and p < 0.001 is marked as “***”
In evaluating age‐based differences, 23 bacterial phyla were detected in the immature group and 26 phyla were detected in adults. Synergistetes, WS6, and norank Bacteroidetes were exclusively found in the adult group. Among the top 15 phyla, the proportion of Proteobacteria, Spirochaetes, Tenericutes, unclassified bacteria, Verrucomicrobia, and Fibrobacteres were higher in adults than that in immatures (Figure 3b). At the genus level, immatures had 36 unique genera and adults had 31 unique genera. Among the top 15 genera, adults had a higher proportion of norank Ruminococcaceae, Oscillospira, norank Lachnospiraceae, norank Clostridiales, and Succinivibrio, and a lower proportion of Enterococcus than did immatures (Table A3).
LEfSe analysis showed that 28 gut bacterial taxa were significantly different between males and females, of which four taxa were from the male group and 24 were from the female group. The class Clostridia, the order Clostridiales, and the family Succinivibrionaceae were the major taxa contributing to sex differences (Figure 4a). In the age category, 50 gut bacterial taxa were significantly different between adults and immatures, of which 35 were from the adult group and 15 taxa were from the immature group. The class Bacilli, the order Lactobacillales, the family Enterococcaceae, and the family Ruminococcaceae were the most important taxa contributing to age differences (Figure 4b).
FIGURE 4.

The LEfSe of gut microbiota abundance of semi‐provision rhesus macaques in sex (a) and age (b). Cladogram showing the relationship among taxa (from the inner to outer rings, phylum, class, order, family, and genus). The bar plot showing the different taxa with a LDA score of > 3
3.3. Difference in diversity of gut microbiota
Alpha diversity indices were significantly different among sex–age classes. Females had higher Shannon, Ace, and Chao indices and a lower Simpson index than did males. The adult group had higher Shannon, Ace, and Chao indices and a lower Simpson index than did immatures (Figure 5 and Table 2). PCoA based on Bray–Curtis distances showed a significant difference in the community structure of the gut microbiota among sex–age classes (sex: R 2 = 0.0133, p = 0.031; age: R 2 = 0.0279, p = 0.001; Figure 6).
FIGURE 5.

Alpha diversity difference in gut microbiota within sex (a) and age (b). The p‐value is represented by asterisks. Significant difference 0.01 < p < 0.05 is marked as “*”, 0.001 < p < 0.01 is marked as “**”, and p < 0.001 is marked as “***”
TABLE 2.
Alpha diversity of the gut microbiota in rhesus macaques
| Estimators | Male | Female | p‐value (sex) | Adult | Immature | p‐value (age) |
|---|---|---|---|---|---|---|
| Shannon | 4.439 ± 0.507 | 4.703 ± 0.473 | 0.002 | 4.608 ± 0.605 | 4.176 ± 0.682 | <0.001 |
| Simpson | 0.044 ± 0.035 | 0.032 ± 0.019 | 0.012 | 0.038 ± 0.035 | 0.061 ± 0.068 | <0.001 |
| Ace | 743.510 ± 112.402 | 816.678 ± 115.477 | <0.001 | 798.619 ± 118.813 | 698.859 ± 143.445 | <0.001 |
| Chao | 758.083 ± 112.963 | 831.674 ± 118.627 | <0.001 | 810.343 ± 126.667 | 704.223 ± 154.085 | <0.001 |
FIGURE 6.

Beta diversity difference in gut microbiota within sex (a) and age (b). The ellipses represent 95% confidence intervals in multivariate space. The dotted lines represent the distance of every sample to the group's centroid
3.4. Functional profiles of gut microbiota predicted by PICRUSt2
Pathways associated with phenylalanine, tyrosine, and tryptophan biosynthesis; porphyrin and chlorophyll metabolism; lysine biosynthesis; glyoxylate and dicarboxylate metabolism; bacterial secretion system; flagellar assembly; and thiamine metabolism were significantly richer in females than in males, whereas no metabolic pathways in level 3 were found with greater abundance in males than in females (Figure 7). Based on age, pathways associated with amino sugar and nucleotide sugar metabolism, glycolysis/gluconeogenesis, and starch and sucrose metabolism were significantly richer in immatures compared with adults. However, significant enrichment in biosynthesis of amino acids, glycine, serine and threonine metabolism, carbon fixation pathways in prokaryotes was detected in adults (Figure 7).
FIGURE 7.

Differences in the functional profiles in pathway level 3 of the gut microbiota in sex and age. The p‐value is represented by asterisks. Significant difference 0.01 < p < 0.05 is marked as “*”, 0.001 < p < 0.01 is marked as “**”, and p < 0.001 is marked as “***”
4. DISCUSSION
In this study, the gut microbiota diversity, abundance, and function of rhesus macaques inhabiting limestone forest differed by sex and age. Specifically, female rhesus macaques had higher gut microbiota diversity and more biosynthesis and metabolism pathways than male. In addition, adult rhesus macaques had a higher diversity of gut microbiota, richer norank Ruminococcaceae and norank Clostridiales, and more functional metabolic pathways than immatures. These differences could be associated with sex‐ and age‐specific differences in diet and/or differences in hormone production.
4.1. Effects of sex on gut microbiota
Our results showed that female rhesus macaques had higher alpha diversity and richness of their gut microbiota than males. These results were consistent with our prediction 1. Similar results have been found in several primates species, including Verreaux's sifakas (P. verreauxi) (Koch et al., 2017), western lowland gorillas (G. gorilla gorilla) (Pafčo et al., 2019), and humans (Sinha et al., 2019). Considering the requirement for higher nutritional and/or energetic demands caused by reproduction (Dunbar et al., 2009; Koch et al., 2017; Li et al., 2014; O'Mara & Hickey, 2014), female primates might be expected to have higher dietary diversity, based on a behavioral strategy to supplement nutritional needs (Dunbar et al., 2009; Li et al., 2014). Sex differences in feeding behavior would result in distinct dietary substrates for gut microbiota and exert different selective pressures on the gut microecosystem, facilitating the acquisition or evolution of new microbial communities and changes in digestive efficiency and nutrient absorption in the host (David et al., 2014). Higher species diversity in a microecosystem indicates increased functional diversity and redundancy (Moya & Ferrer, 2016). Thus, increased gut microbial diversity in females suggests that the gut microbiota in females may have more diverse functions, facilitating digestion and metabolism. This is supported by differences in functional profiles, with enriched biosynthesis and metabolic pathways in the female gut microbiota compared with males (Figure 7).
In addition, it is noteworthy that the abundance of bacteria belonging to the phylum Proteobacteria in female rhesus macaques was higher than that in males, which could reflect physiological differences. Most bacteria belonging to the phylum Proteobacteria have been shown to induce inflammation in humans (Mukhopadhya et al., 2012). Females appear to be more susceptible to pathogenic bacteria while improving their ability to absorb nutrients and energy, as documented in black howler monkeys (A. pigra) (Amato et al., 2014) and humans (Koren et al., 2012). Interestingly, androgens play a role in protecting host health (Yurkovetskiy et al., 2013). When gut microbiota was transplanted from male mice into female mice, females showed a reduction in islet inflammation and autoantibody production as testosterone levels increased (Markle et al., 2013). Therefore, sex differences in the diversity and composition of gut microbiota in rhesus monkeys might have been driven, in part, by differences in sex steroid hormone production.
4.2. Effects of age on gut microbiota
Adult rhesus macaques showed higher alpha diversity of their gut microbiota and a higher abundance of norank Ruminococcaceae and norank Clostridiales, compared to immatures, which supports prediction 2. These differences might be associated with age‐related differences in diet. In general, the digestive system and gut microbiota develop with age (Derrien et al., 2019). In addition, the dietary composition of adults is more complex and more indigestible than that of immatures who are limited by mobility or hunting skills (Kashtanova et al., 2016; Schiel et al., 2010). Therefore, the increased gut microbiota diversity in adult rhesus macaques might correspond to their relatively higher dietary diversity. Higher gut microbiota diversity might provide adults with higher functional redundancy, contributing to a more stable gut microecosystem than that in immatures (Lozupone et al., 2012; Tian et al., 2020). In this regard, taxa such as Ruminococcaceae and Clostridiales have been considered specialists in the degradation of complex plant materials (Koeck et al., 2014; La Reau & Suen, 2018). A higher abundance of Ruminococcaceae and Clostridiales in adult rhesus macaques also suggests that the macaques mainly relied on these two microbial taxa to enhance their digestion of complex cellulose. Additionally, fiber‐enriched diets also are associated with a reduction in the Firmicutes/Bacteroidetes ratio (De Filippo et al., 2010). Despite their relatively simple dietary structure, immature macaques had a higher ratio of Firmicutes/Bacteroidetes than adults, which may be attributed to the need for increased nutrient absorption during their growth and development. Vulnerable groups such as immatures and females may increase the nutritional harvest from their gut microbiota to compensate for growth and reproduction (Amato et al., 2014). Furthermore, the abundance of Proteobacteria was higher in adult rhesus macaques. Similarly, geriatric marmosets had higher Proteobacteria abundance than younger individuals, which was assumed to be related to the decline in immune function with age (Reveles et al., 2019). Thus, both nutritional requirements and age‐related physiological factors influence gut microbiota composition.
However, it must be acknowledged that the effects of the sex–age class on the community structure of gut microbiota were relatively weak. Overall, sex and age only explained 1.33% and 2.79% of the variation in community structure of the gut microbiota of rhesus macaques, respectively (Figure 6). This implies that other underlying determinants also influence the structure of the gut microbiota. This study preliminarily quantified the effects of sex–age class on the gut microbiota of rhesus macaques. Future studies should focus on the comprehensive effects of multiple factors, such as host genetics, sex–age class, dietary composition, and climate on the gut microbiota, offering a more comprehensive analysis of the association between the host and their symbiotic gut microbiota.
There are several limitations to our study. We have documented a general pattern of differences in the gut microbiota of limestone forest‐dwelling rhesus macaques based on sex and age class. Our results must be viewed with caution given the unequal sample sizes for each sex and age class. Moreover, due to the difficulty in following the macaques across an entire day and collecting fecal samples in the karst forest, the effects of sex and age on the gut microbiota of macaques require more controlled studies. In addition, we did not accurately estimate the effects of provisioned foods from park managers and visitors on their gut microbiota. Considering the fact that the sex and age effects might be overshadowed by other factors, additional studies should focus on the interactive influences of multiple factors such as morphology and anatomy and diet on the gut microbiota. This would provide a more comprehensive basis for understanding the evolutionary significance of the macaque gut microbiota.
In summary, sex and age were associated with differences in the diversity and relative abundance of gut microbiota in rhesus macaques. Female rhesus macaques had a higher diversity of gut microbiota and more biosynthesis and metabolism pathways than male individuals. Adult rhesus macaques had a higher diversity of gut microbiota, richer norank Ruminococcaceae and norank Clostridiales, and more functional metabolic pathways than immatures. These differences could be linked to age‐ and sex‐specific differences in nutrient requirements and hormone levels, highlighting the effects of age and sex on the structure and function of the gut microbiota, as well as the need to consider physiological traits when conducting gut microbiota studies.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Yuhui Li: Formal analysis (equal); Investigation (equal); Writing‐original draft (lead); Writing‐review & editing (supporting). Ting Chen: Formal analysis (equal); Investigation (equal). Youbang Li: Funding acquisition (supporting); Writing‐review & editing (equal). Yin Tang: Investigation (equal); Project administration (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Zhonghao Huang: Conceptualization (lead); Formal analysis (equal); Funding acquisition (lead); Methodology (lead); Project administration (equal); Supervision (lead); Writing‐review & editing (equal).
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Nos. 31960106, 31960104, 31660616) and the Guangxi Natural Science Foundation, China (2018GXNSFAA281029). We would like to thank the Guangxi Longhu Mountain Nature Reserve for permitting us to collect samples in the study site. We also thank Dr. Gang Hu from Nanning Normal University for help in fecal samples collecting and conserving. Finally, we are very grateful to Professor Paul A. Garber from Department of Anthropology, Program in Ecology, Evolution, and Conservation Biology, University of Illinois, USA, for his help in language editing and valuable comments on earlier manuscript versions.
APPENDIX A.
TABLE A1.
Differences in the relative abundance of gut microbiota (at the phylum level) among sex and age classes in rhesus macaques
| Species name | Female‐Mean (%) | Female‐SD (%) | Male‐Mean (%) | Male‐SD (%) | Corrected p‐value | Adult‐Mean (%) | Adult‐SD (%) | Immature‐Mean (%) | Immature‐SD (%) | Corrected p value |
|---|---|---|---|---|---|---|---|---|---|---|
| p__Firmicutes | 58.29 | 14.02 | 55.09 | 18.47 | 0.325 | 58.07 | 15.87 | 62.39 | 18.62 | 0.355 |
| p__Bacteroidetes | 31.55 | 13.29 | 35.73 | 18.39 | 0.325 | 31.50 | 15.20 | 30.15 | 18.95 | 0.578 |
| p__Proteobacteria | 4.72 | 4.92 | 3.08 | 5.01 | 0.023 | 4.55 | 5.33 | 2.12 | 2.46 | 0.009 |
| p__Actinobacteria | 1.19 | 1.15 | 2.08 | 2.84 | 0.426 | 1.25 | 1.12 | 2.28 | 2.99 | 0.352 |
| p__Spirochaetes | 1.49 | 1.92 | 2.38 | 3.21 | 0.838 | 1.85 | 2.61 | 1.11 | 1.58 | 0.031 |
| p__Cyanobacteria | 0.78 | 0.80 | 0.52 | 0.69 | 0.021 | 0.98 | 1.96 | 0.97 | 2.35 | 0.135 |
| p__Tenericutes | 0.95 | 0.66 | 0.56 | 0.43 | 0.002 | 0.90 | 0.67 | 0.52 | 0.40 | 0.003 |
| p__unclassified_k__norank_d__Bacteria | 0.28 | 0.41 | 0.15 | 0.23 | 0.027 | 0.25 | 0.38 | 0.13 | 0.20 | 0.020 |
| p__WPS‐2 | 0.29 | 0.57 | 0.14 | 0.18 | 0.838 | 0.24 | 0.48 | 0.12 | 0.35 | 0.157 |
| p__Verrucomicrobia | 0.24 | 0.45 | 0.12 | 0.23 | 0.028 | 0.21 | 0.40 | 0.09 | 0.26 | <0.001 |
| p__Fibrobacteres | 0.09 | 0.16 | 0.07 | 0.10 | 0.725 | 0.09 | 0.15 | 0.04 | 0.06 | 0.020 |
| p__unclassified_d__Unclassified | 0.05 | 0.05 | 0.04 | 0.04 | 0.190 | 0.04 | 0.05 | 0.03 | 0.04 | 0.212 |
| p__Lentisphaerae | 0.04 | 0.11 | 0.02 | 0.05 | 0.325 | 0.03 | 0.10 | 0.02 | 0.06 | 0.336 |
| p__Chlamydiae | 0.02 | 0.05 | 0.02 | 0.04 | 0.758 | 0.01 | 0.09 | 0.02 | 0.08 | 0.971 |
| p__Elusimicrobia | 0.02 | 0.10 | 0.01 | 0.07 | 0.325 | 0.02 | 0.05 | 0.01 | 0.02 | 0.076 |
| p__TM7 | <0.01 | <0.01 | <0.01 | <0.01 | 0.325 | <0.01 | <0.01 | <0.01 | 0.01 | 0.578 |
| p__[Thermi] | <0.01 | <0.01 | <0.01 | <0.01 | 0.292 | <0.01 | <0.01 | <0.01 | 0.01 | 0.073 |
| p__Fusobacteria | <0.01 | <0.01 | <0.01 | <0.01 | 0.838 | <0.01 | <0.01 | <0.01 | <0.01 | 0.440 |
| p__Chloroflexi | <0.01 | <0.01 | <0.01 | <0.01 | 0.292 | <0.01 | <0.01 | <0.01 | <0.01 | 0.034 |
| p__Acidobacteria | <0.01 | <0.01 | <0.01 | <0.01 | 0.886 | <0.01 | <0.01 | <0.01 | <0.01 | 0.157 |
| p__Synergistetes | <0.01 | <0.01 | <0.01 | <0.01 | 0.292 | <0.01 | <0.01 | <0.01 | <0.01 | 0.282 |
| p__OD1 | <0.01 | <0.01 | <0.01 | <0.01 | 0.838 | <0.01 | <0.01 | <0.01 | <0.01 | 0.578 |
| p__norank_d__BacteriaBacteroidetes | <0.01 | <0.01 | <0.01 | <0.01 | 0.935 | <0.01 | <0.01 | <0.01 | <0.01 | 0.392 |
| p__WS6 | <0.01 | <0.01 | <0.01 | <0.01 | 0.697 | <0.01 | <0.01 | <0.01 | <0.01 | 0.578 |
| p__GN02 | <0.01 | <0.01 | <0.01 | <0.01 | 0.578 | |||||
| p__Planctomycetes | <0.01 | <0.01 | <0.01 | <0.01 | 0.578 |
TABLE A2.
Differences in the relative abundance of gut microbiota (at the genus level) in female and male rhesus macaques
| Species name | Female | Male | Corrected p value | ||
|---|---|---|---|---|---|
| Mean (%) | SD (%) | Mean (%) | SD (%) | ||
| g__Prevotella | 25.140 | 13.510 | 28.970 | 18.420 | 0.674 |
| g__norank_f__Ruminococcaceae | 9.835 | 4.475 | 8.848 | 4.339 | 0.596 |
| g__Lactobacillus | 4.472 | 9.130 | 5.329 | 9.095 | 0.564 |
| g__norank_f__Clostridiaceae | 3.254 | 4.975 | 3.833 | 5.298 | 0.724 |
| g__Oscillospira | 3.507 | 2.547 | 3.441 | 2.580 | 0.964 |
| g__norank_f__Lachnospiraceae | 3.541 | 2.093 | 2.822 | 1.645 | 0.341 |
| g__unclassified_o__Clostridiales | 3.289 | 1.296 | 2.543 | 1.280 | 0.064 |
| g__Succinivibrio | 3.669 | 4.758 | 2.055 | 4.864 | 0.064 |
| g__norank_o__Clostridiales | 2.754 | 2.408 | 2.178 | 1.788 | 0.341 |
| g__Blautia | 2.488 | 1.337 | 2.256 | 1.659 | 0.469 |
| g__norank_f__S24‐7 | 2.337 | 1.601 | 2.338 | 2.678 | 0.469 |
| g__Faecalibacterium | 2.375 | 1.922 | 2.291 | 2.600 | 0.720 |
| g__Coprococcus | 2.586 | 1.543 | 1.820 | 1.289 | 0.064 |
| g__unclassified_f__Ruminococcaceae | 2.143 | 1.113 | 1.728 | 0.890 | 0.393 |
| g__Treponema | 1.366 | 1.875 | 2.278 | 3.167 | 0.821 |
| g__Ruminococcus | 1.859 | 0.991 | 1.617 | 1.052 | 0.462 |
| g__Sarcina | 1.806 | 3.431 | 1.663 | 3.772 | 0.724 |
| g__Roseburia | 1.624 | 1.247 | 1.806 | 1.812 | 0.816 |
| g__unclassified_f__Lachnospiraceae | 1.845 | 1.068 | 1.516 | 0.991 | 0.341 |
| g__Enterococcus | 0.709 | 3.033 | 2.502 | 8.246 | 0.365 |
| g__norank_f__Peptostreptococcaceae | 1.308 | 1.659 | 1.308 | 1.550 | 0.720 |
| g__norank_o__Bacteroidales | 1.153 | 1.214 | 1.379 | 1.610 | 0.964 |
| g__Phascolarctobacterium | 1.341 | 1.818 | 1.149 | 1.623 | 0.604 |
| g__[Prevotella] | 0.948 | 0.934 | 0.945 | 1.231 | 0.720 |
| g__norank_f__Christensenellaceae | 1.048 | 0.925 | 0.724 | 0.586 | 0.427 |
| g__norank_f__Coriobacteriaceae | 0.818 | 0.705 | 0.904 | 1.162 | 0.720 |
| g__norank_o__RF39 | 0.866 | 0.563 | 0.524 | 0.401 | 0.047 |
| g__unclassified_o__Bacteroidales_c__Bacteroidia | 0.507 | 0.595 | 0.757 | 1.270 | 0.638 |
| g__Bifidobacterium | 0.230 | 0.803 | 1.003 | 2.392 | 0.644 |
| g__norank_f__[Mogibacteriaceae] | 0.662 | 0.365 | 0.488 | 0.307 | 0.145 |
| g__Dialister | 0.503 | 0.649 | 0.558 | 0.644 | 0.804 |
| g__[Ruminococcus] | 0.466 | 0.271 | 0.473 | 0.377 | 0.724 |
| g__Catenibacterium | 0.347 | 0.588 | 0.497 | 1.503 | 0.720 |
| g__Clostridium | 0.413 | 0.287 | 0.350 | 0.300 | 0.471 |
| g__Lachnospira | 0.348 | 0.392 | 0.332 | 0.474 | 0.736 |
| g__Bulleidia | 0.402 | 0.383 | 0.272 | 0.318 | 0.226 |
| g__norank_o__Streptophyta | 0.418 | 0.762 | 0.254 | 0.535 | 0.287 |
| g__CF231 | 0.352 | 0.409 | 0.311 | 0.438 | 0.634 |
| g__SMB53 | 0.354 | 0.654 | 0.293 | 0.406 | 0.644 |
| g__YRC22 | 0.340 | 0.467 | 0.287 | 0.522 | 0.473 |
| g__norank_o__YS2 | 0.355 | 0.328 | 0.264 | 0.396 | 0.194 |
| g__norank_o__GMD14H09 | 0.255 | 0.921 | 0.355 | 0.856 | 0.720 |
| g__norank_f__RF16 | 0.311 | 0.610 | 0.247 | 0.560 | 0.720 |
| g__Dorea | 0.264 | 0.121 | 0.280 | 0.211 | 0.564 |
| g__Pediococcus | 0.420 | 2.471 | 0.066 | 0.321 | 0.846 |
| g__Anaerovibrio | 0.271 | 0.457 | 0.200 | 0.317 | 0.638 |
| g__Lactococcus | 0.253 | 2.104 | 0.202 | 1.222 | 0.720 |
| g__unclassified_k__norank_d__Bacteria | 0.283 | 0.405 | 0.153 | 0.228 | 0.145 |
| g__norank_f__[Paraprevotellaceae] | 0.198 | 0.265 | 0.233 | 0.256 | 0.724 |
| g__norank_p__WPS‐2 | 0.289 | 0.570 | 0.136 | 0.179 | 0.852 |
| g__Megasphaera | 0.185 | 0.413 | 0.209 | 0.491 | 0.757 |
| g__Butyrivibrio | 0.148 | 0.170 | 0.219 | 0.331 | 0.638 |
| g__norank_f__RFP12 | 0.243 | 0.450 | 0.116 | 0.225 | 0.157 |
| g__norank_f__Erysipelotrichaceae | 0.178 | 0.219 | 0.124 | 0.172 | 0.287 |
| g__norank_f__Veillonellaceae | 0.160 | 0.259 | 0.136 | 0.317 | 0.564 |
| g__norank_f__Rikenellaceae | 0.130 | 0.154 | 0.154 | 0.262 | 0.720 |
| g__norank_f__Enterobacteriaceae | 0.096 | 0.687 | 0.170 | 0.734 | 0.761 |
| g__unclassified_f__Clostridiaceae | 0.122 | 0.165 | 0.123 | 0.171 | 0.871 |
| g__Flexispira | 0.103 | 0.142 | 0.142 | 0.330 | 0.837 |
| g__unclassified_o__Lactobacillales | 0.107 | 0.436 | 0.130 | 0.473 | 0.287 |
| g__Bacillus | 0.210 | 1.888 | 0.012 | 0.037 | 0.804 |
| g__norank_f__Lactobacillaceae | 0.155 | 0.754 | 0.038 | 0.258 | 0.469 |
| g__norank_o__RF32 | 0.092 | 0.130 | 0.075 | 0.130 | 0.474 |
| g__Fibrobacter | 0.093 | 0.159 | 0.074 | 0.100 | 0.737 |
| g__Desulfovibrio | 0.104 | 0.175 | 0.058 | 0.065 | 0.474 |
| g__Sphaerochaeta | 0.083 | 0.090 | 0.058 | 0.065 | 0.478 |
| g__Rummeliibacillus | 0.035 | 0.283 | 0.105 | 0.628 | 0.812 |
| g__unclassified_p__Firmicutes | 0.050 | 0.164 | 0.087 | 0.207 | 0.393 |
| g__Collinsella | 0.057 | 0.073 | 0.067 | 0.109 | 0.898 |
| g__norank_f__p‐2534‐18B5 | 0.060 | 0.094 | 0.050 | 0.085 | 0.564 |
| g__Sutterella | 0.055 | 0.109 | 0.050 | 0.064 | 0.964 |
| g__Slackia | 0.050 | 0.051 | 0.043 | 0.043 | 0.724 |
| g__Parabacteroides | 0.047 | 0.062 | 0.043 | 0.060 | 0.757 |
| g__unclassified_f__Coriobacteriaceae | 0.029 | 0.037 | 0.054 | 0.162 | 0.950 |
| g__unclassified_d__Unclassified | 0.048 | 0.046 | 0.035 | 0.041 | 0.385 |
| g__unclassified_p__Proteobacteria | 0.036 | 0.054 | 0.046 | 0.064 | 0.724 |
| g__unclassified_c__Bacilli | 0.024 | 0.102 | 0.058 | 0.194 | 0.845 |
| g__Streptococcus | 0.027 | 0.034 | 0.053 | 0.130 | 0.720 |
| g__unclassified_f__Veillonellaceae | 0.033 | 0.049 | 0.046 | 0.132 | 0.742 |
| g__Ruminobacter | 0.057 | 0.181 | 0.017 | 0.058 | 0.564 |
| g__Leuconostoc | 0.029 | 0.196 | 0.043 | 0.180 | 0.945 |
| g__norank_o__Rickettsiales | 0.040 | 0.047 | 0.030 | 0.035 | 0.504 |
| g__Acinetobacter | 0.069 | 0.636 | <0.001 | 0.001 | 0.724 |
| g__unclassified_f__Erysipelotrichaceae | 0.023 | 0.030 | 0.041 | 0.068 | 0.852 |
| g__RFN20 | 0.036 | 0.089 | 0.025 | 0.040 | 0.341 |
| g__[Eubacterium] | 0.027 | 0.025 | 0.032 | 0.042 | 0.821 |
| g__norank_f__Leuconostocaceae | 0.004 | 0.018 | 0.055 | 0.290 | 0.975 |
| g__norank_o__ML615J‐28 | 0.039 | 0.253 | 0.009 | 0.021 | 0.939 |
| g__p‐75‐a5 | 0.026 | 0.040 | 0.021 | 0.035 | 0.469 |
| g__Brachyspira | 0.019 | 0.028 | 0.022 | 0.038 | 0.996 |
| g__unclassified_c__Betaproteobacteria | 0.030 | 0.061 | 0.010 | 0.035 | 0.469 |
| g__norank_f__R4‐45B | 0.027 | 0.070 | 0.013 | 0.036 | 0.597 |
| g__norank_o__M2PT2‐76 | 0.020 | 0.043 | 0.018 | 0.067 | 0.575 |
| g__Oxalobacter | 0.018 | 0.020 | 0.016 | 0.024 | 0.720 |
| g__Mogibacterium | 0.014 | 0.023 | 0.019 | 0.038 | 0.928 |
| g__norank_f__Elusimicrobiaceae | 0.017 | 0.045 | 0.016 | 0.037 | 0.762 |
| g__unclassified_o__Bacillales | 0.031 | 0.277 | 0.001 | 0.002 | 0.720 |
| g__Candidatus_Rhabdochlamydia | 0.017 | 0.103 | 0.013 | 0.075 | 0.564 |
| g__unclassified_f__[Mogibacteriaceae] | 0.015 | 0.028 | 0.014 | 0.031 | 0.767 |
| g__Pseudomonas | 0.020 | 0.115 | 0.008 | 0.025 | 0.975 |
| g__L7A_E11 | 0.015 | 0.042 | 0.012 | 0.026 | 0.767 |
| g__rc4‐4 | 0.015 | 0.019 | 0.010 | 0.013 | 0.473 |
| g__Anaeroplasma | 0.012 | 0.017 | 0.011 | 0.026 | 0.341 |
| g__unclassified_c__Alphaproteobacteria | 0.015 | 0.045 | 0.008 | 0.017 | 0.891 |
| g__norank_f__Anaeroplasmataceae | 0.014 | 0.020 | 0.008 | 0.012 | 0.390 |
| g__Solibacillus | 0.022 | 0.204 | <0.001 | <0.001 | 0.469 |
| g__unclassified_c__Clostridia | 0.014 | 0.016 | 0.008 | 0.012 | 0.303 |
| g__norank_f__Victivallaceae | 0.015 | 0.048 | 0.007 | 0.016 | 0.742 |
| g__Anaerofustis | 0.012 | 0.015 | 0.009 | 0.019 | 0.064 |
| g__Dehalobacterium | 0.010 | 0.014 | 0.009 | 0.012 | 0.856 |
| g__Actinobacillus | 0.012 | 0.032 | 0.007 | 0.015 | 0.638 |
| g__Coprobacillus | 0.009 | 0.015 | 0.010 | 0.019 | 0.804 |
| g__Anaerostipes | 0.012 | 0.028 | 0.007 | 0.011 | 0.794 |
| g__norank_f__Streptococcaceae | <0.001 | 0.002 | 0.017 | 0.083 | 0.278 |
| g__Butyricimonas | 0.011 | 0.022 | 0.005 | 0.006 | 0.564 |
| g__Tetragenococcus | 0.001 | 0.007 | 0.014 | 0.047 | 0.469 |
| g__unclassified_f__Prevotellaceae | 0.007 | 0.007 | 0.007 | 0.008 | 0.841 |
| g__norank_f__Bacillaceae | 0.014 | 0.058 | <0.001 | 0.001 | 0.564 |
| g__unclassified_f__Planococcaceae | 0.014 | 0.116 | <0.001 | 0.001 | 0.720 |
| g__Veillonella | 0.008 | 0.018 | 0.006 | 0.010 | 0.720 |
| g__norank_f__Dehalobacteriaceae | 0.008 | 0.013 | 0.004 | 0.008 | 0.341 |
| g__unclassified_f__Enterobacteriaceae | 0.008 | 0.028 | 0.004 | 0.014 | 0.278 |
| g__norank_c__Alphaproteobacteria | 0.008 | 0.031 | 0.003 | 0.011 | 0.841 |
| g__norank_f__Peptococcaceae | 0.008 | 0.010 | 0.003 | 0.004 | 0.064 |
| g__norank_o__Acholeplasmatales | 0.009 | 0.039 | 0.001 | 0.008 | 0.478 |
| g__unclassified_f__Pasteurellaceae | 0.007 | 0.023 | 0.003 | 0.006 | 0.564 |
| g__unclassified_p__Tenericutes | 0.007 | 0.013 | 0.003 | 0.006 | 0.232 |
| g__norank_f__mitochondria | 0.004 | 0.009 | 0.006 | 0.016 | 0.720 |
| g__unclassified_o__Rickettsiales | 0.002 | 0.006 | 0.004 | 0.011 | 0.720 |
| g__norank_f__Desulfovibrionaceae | 0.003 | 0.006 | 0.002 | 0.004 | 0.804 |
| g__Alloscardovia | 0.001 | 0.005 | 0.003 | 0.011 | 0.638 |
| g__Bacteroides_f__Bacteroidaceae | 0.002 | 0.009 | 0.001 | 0.003 | 0.720 |
| g__Weissella | <0.001 | 0.004 | 0.003 | 0.022 | 0.757 |
| g__norank_f__Xenococcaceae | 0.001 | 0.003 | 0.002 | 0.010 | 0.720 |
| g__Actinomyces | 0.002 | 0.008 | 0.001 | 0.002 | 0.564 |
| g__unclassified_f__Bifidobacteriaceae | <0.001 | 0.002 | 0.002 | 0.009 | 0.139 |
| g__unclassified_f__[Paraprevotellaceae] | 0.002 | 0.003 | 0.001 | 0.002 | 0.341 |
| g__Staphylococcus | 0.001 | 0.004 | 0.002 | 0.004 | 0.347 |
| g__norank_f__Flavobacteriaceae | 0.002 | 0.023 | 0 | 0 | 0.720 |
| g__unclassified_c__Gammaproteobacteria | 0.002 | 0.004 | 0.001 | 0.002 | 0.462 |
| g__Wautersiella | 0.002 | 0.020 | 0 | 0 | 0.720 |
| g__Bilophila | 0.001 | 0.003 | 0.001 | 0.007 | 0.846 |
| g__norank_c__TM7‐3 | 0.001 | 0.003 | 0.001 | 0.003 | 0.663 |
| g__unclassified_p__Spirochaetes | 0.001 | 0.004 | 0.001 | 0.002 | 0.794 |
| g__Epulopiscium | 0.001 | 0.002 | 0.001 | 0.005 | 0.841 |
| g__unclassified_f__Rhizobiaceae | 0.001 | 0.002 | 0.001 | 0.002 | 0.720 |
| g__Rickettsiella | 0.002 | 0.010 | 0 | 0 | 0.411 |
| g__norank_f__Prevotellaceae | 0.001 | 0.002 | 0.001 | 0.002 | 0.723 |
| g__norank_f__[Cerasicoccaceae] | 0.001 | 0.003 | 0.001 | 0.002 | 0.928 |
| g__Rubellimicrobium | <0.001 | 0.001 | 0.001 | 0.005 | 0.637 |
| g__norank_f__Gaiellaceae | 0.001 | 0.002 | <0.001 | 0.002 | 0.755 |
| g__Rhodoplanes | 0.001 | 0.003 | <0.001 | 0.001 | 0.564 |
| g__Carnobacterium | <0.001 | 0.002 | 0.001 | 0.004 | 0.760 |
| g__unclassified_f__Gemellaceae | 0.001 | 0.002 | <0.001 | 0.002 | 0.898 |
| g__norank_o__HA64 | <0.001 | 0.001 | 0.001 | 0.002 | 0.852 |
| g__Rhodococcus | 0.001 | 0.003 | <0.001 | 0.001 | 0.570 |
| g__Leptotrichia | <0.001 | 0.002 | <0.001 | 0.002 | 0.943 |
| g__Halomonas | <0.001 | 0.001 | 0.001 | 0.003 | 0.720 |
| g__Paracoccus | <0.001 | 0.001 | <0.001 | 0.002 | 0.943 |
| g__Sphingomonas | <0.001 | 0.001 | 0.001 | 0.002 | 0.587 |
| g__Pseudonocardia | <0.001 | 0.002 | <0.001 | 0.001 | 0.898 |
| g__unclassified_c__Spirochaetes | <0.001 | 0.002 | <0.001 | 0.003 | 0.723 |
| g__unclassified_f__Comamonadaceae | 0.001 | 0.008 | 0 | 0 | 0.720 |
| g__Burkholderia | 0.001 | 0.002 | <0.001 | 0.001 | 0.393 |
| g__Granulicatella | 0.001 | 0.002 | <0.001 | 0.001 | 0.638 |
| g__Methyloversatilis | 0.001 | 0.006 | 0 | 0 | 0.638 |
| g__Neisseria | 0.001 | 0.004 | 0 | 0 | 0.365 |
| g__norank_f__AKIW874 | <0.001 | 0.002 | <0.001 | 0.001 | 0.930 |
| g__Rhodobacter | <0.001 | 0.002 | <0.001 | 0.002 | 0.762 |
| g__Mitsuokella | 0.001 | 0.005 | 0 | 0 | 0.570 |
| g__Porphyromonas | 0.001 | 0.002 | <0.001 | 0.001 | 0.564 |
| g__Methylobacterium | <0.001 | 0.001 | <0.001 | 0.001 | 0.756 |
| g__Rubrobacter | <0.001 | 0.001 | <0.001 | 0.002 | 0.478 |
| g__Salinivibrio | <0.001 | 0.003 | <0.001 | 0.001 | 0.767 |
| g__norank_o__Stramenopiles | <0.001 | 0.001 | <0.001 | 0.001 | 0.736 |
| g__Arthrobacter | <0.001 | 0.001 | <0.001 | 0.002 | 0.638 |
| g__unclassified_o__Burkholderiales | <0.001 | 0.001 | <0.001 | 0.001 | 0.623 |
| g__norank_o__JG30‐KF‐CM45 | <0.001 | 0.001 | <0.001 | 0.002 | 0.341 |
| g__norank_o__Rhizobiales | <0.001 | 0.002 | <0.001 | 0.001 | 0.604 |
| g__unclassified_f__Oxalobacteraceae | <0.001 | 0.002 | <0.001 | 0.001 | 0.720 |
| g__norank_f__Propionibacteriaceae | <0.001 | 0.002 | <0.001 | 0.001 | 0.943 |
| g__Rothia | <0.001 | 0.001 | <0.001 | 0.001 | 0.723 |
| g__Haemophilus | <0.001 | 0.003 | <0.001 | 0.001 | 0.812 |
| g__norank_f__Mycoplasmataceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.943 |
| g__Leucobacter | <0.001 | 0.001 | <0.001 | 0.002 | 0.821 |
| g__Truepera | <0.001 | 0.001 | <0.001 | 0.001 | 0.341 |
| g__unclassified_p__Bacteroidetes | <0.001 | 0.003 | 0 | 0 | 0.638 |
| g__norank_f__EB1017 | <0.001 | 0.001 | <0.001 | 0.001 | 0.945 |
| g__unclassified_o__Sphingomonadales | <0.001 | 0.001 | <0.001 | 0.001 | 0.929 |
| g__Balneimonas | <0.001 | 0.001 | <0.001 | 0.001 | 0.757 |
| g__norank_f__Acetobacteraceae | 0 | 0 | <0.001 | 0.001 | 0.168 |
| g__Bradyrhizobium | <0.001 | 0.001 | <0.001 | 0.001 | 0.674 |
| g__1‐68 | <0.001 | 0.002 | <0.001 | 0.001 | 0.812 |
| g__Corynebacterium | <0.001 | 0.001 | <0.001 | 0.001 | 0.812 |
| g__Anaerococcus | <0.001 | 0.001 | <0.001 | 0.001 | 0.928 |
| g__norank_f__Rhodospirillaceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.762 |
| g__Serratia | <0.001 | 0.001 | <0.001 | 0.001 | 0.928 |
| g__Kaistobacter | <0.001 | 0.001 | <0.001 | 0.001 | 0.928 |
| g__Brachybacterium | <0.001 | 0.001 | <0.001 | 0.002 | 0.821 |
| g__norank_o__SHA‐98 | <0.001 | 0.003 | 0 | 0 | 0.720 |
| g__Arcobacter | <0.001 | 0.002 | 0 | 0 | 0.638 |
| g__unclassified_f__Synergistaceae | <0.001 | 0.001 | 0 | 0 | 0.469 |
| g__Arcanobacterium | <0.001 | 0.003 | 0 | 0 | 0.638 |
| g__unclassified_f__Phyllobacteriaceae | <0.001 | 0.003 | 0 | 0 | 0.720 |
| g__unclassified_f__Streptomycetaceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.720 |
| g__norank_f__Rhizobiaceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.812 |
| g__Cetobacterium | <0.001 | 0.001 | <0.001 | 0.001 | 0.964 |
| g__Thermomonas | 0 | 0 | <0.001 | 0.001 | 0.393 |
| g__Campylobacter | <0.001 | 0.001 | 0 | 0 | 0.563 |
| g__norank_f__Xanthomonadaceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.638 |
| g__unclassified_o__Actinomycetales | <0.001 | 0.001 | <0.001 | 0.001 | 0.638 |
| g__unclassified_f__Xanthomonadaceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.638 |
| g__Megamonas | <0.001 | 0.001 | <0.001 | 0.001 | 0.638 |
| g__norank_f__Ellin6075 | <0.001 | 0.001 | <0.001 | 0.001 | 0.638 |
| g__unclassified_f__Fusobacteriaceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.638 |
| g__Phormidium | <0.001 | 0.001 | <0.001 | 0.001 | 0.638 |
| g__Chryseobacterium | <0.001 | 0.002 | 0 | 0 | 0.720 |
| g__Rhizobium | <0.001 | 0.001 | 0 | 0 | 0.563 |
| g__Peptoniphilus | <0.001 | 0.001 | <0.001 | 0.001 | 0.960 |
| g__norank_f__Caulobacteraceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.960 |
| g__Pseudoxanthomonas | <0.001 | 0.001 | <0.001 | 0.001 | 0.821 |
| g__Comamonas | <0.001 | 0.001 | <0.001 | 0.001 | 0.821 |
| g__Bacteroides_f__Bacteroidaceae_o__Bacteroidales | <0.001 | 0.001 | <0.001 | 0.001 | 0.960 |
| g__norank_f__Hyphomicrobiaceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.960 |
| g__Brevibacterium | 0 | 0 | <0.001 | 0.001 | 0.393 |
| g__Spirosoma | 0 | 0 | <0.001 | 0.001 | 0.564 |
| g__Flavisolibacter | 0 | 0 | <0.001 | 0.001 | 0.564 |
| g__Kocuria | 0 | 0 | <0.001 | 0.001 | 0.564 |
| g__unclassified_f__Pseudanabaenaceae | 0 | 0 | <0.001 | 0.001 | 0.564 |
| g__Pedomicrobium | 0 | 0 | <0.001 | 0.001 | 0.393 |
| g__norank_c__OPB41 | <0.001 | 0.001 | 0 | 0 | 0.570 |
| g__norank_o__CW040 | <0.001 | 0.001 | 0 | 0 | 0.638 |
| g__norank_f__Oxalobacteraceae | <0.001 | 0.002 | 0 | 0 | 0.720 |
| g__norank_c__ABY1 | <0.001 | 0.002 | 0 | 0 | 0.720 |
| g__Cupriavidus | <0.001 | 0.001 | 0 | 0 | 0.570 |
| g__Rathayibacter | <0.001 | 0.002 | 0 | 0 | 0.720 |
| g__norank_o__MBNT15 | <0.001 | 0.001 | <0.001 | 0.001 | 0.821 |
| g__Akkermansia | <0.001 | 0.001 | <0.001 | 0.001 | 0.821 |
| g__Mycobacterium | <0.001 | 0.001 | <0.001 | 0.001 | 0.821 |
| g__norank_f__Beijerinckiaceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.821 |
| g__Ochrobactrum | <0.001 | 0.001 | <0.001 | 0.001 | 0.821 |
| g__unclassified_f__Intrasporangiaceae | <0.001 | 0.001 | 0 | 0 | 0.638 |
| g__norank_f__Halobacteroidaceae | <0.001 | 0.001 | 0 | 0 | 0.720 |
| g__Allobaculum | <0.001 | 0.001 | 0 | 0 | 0.720 |
| g__Arenimonas | <0.001 | 0.001 | 0 | 0 | 0.720 |
| g__norank_o__PK29 | <0.001 | <0.001 | 0 | 0 | 0.638 |
| g__Exiguobacterium | <0.001 | <0.001 | 0 | 0 | 0.638 |
| g__norank_c__SC72 | <0.001 | <0.001 | 0 | 0 | 0.720 |
| g__DA101 | 0 | 0 | <0.001 | 0.001 | 0.564 |
| g__norank_o__Sphingomonadales | 0 | 0 | <0.001 | 0.001 | 0.564 |
| g__norank_c__ZB2 | 0 | 0 | <0.001 | 0.001 | 0.564 |
| g__norank_f__Cytophagaceae | 0 | 0 | <0.001 | 0.001 | 0.564 |
| g__Leptospira | 0 | 0 | <0.001 | 0.001 | 0.564 |
| g__Nannocystis | 0 | 0 | <0.001 | 0.001 | 0.564 |
| g__Paenibacillus | 0 | 0 | <0.001 | 0.001 | 0.564 |
| g__Odoribacter | 0 | 0 | <0.001 | 0.001 | 0.564 |
| g__Trichococcus | 0 | 0 | <0.001 | 0.001 | 0.564 |
| g__Deinococcus | <0.001 | 0.001 | 0 | 0 | 0.720 |
| g__norank_f__Actinomycetaceae | <0.001 | 0.001 | 0 | 0 | 0.720 |
| g__Wohlfahrtiimonas | <0.001 | 0.001 | 0 | 0 | 0.720 |
| g__norank_f__Methylobacteriaceae | <0.001 | 0.001 | 0 | 0 | 0.720 |
| g__Sanguibacter | <0.001 | 0.001 | 0 | 0 | 0.720 |
| g__norank_f__Frankiaceae | <0.001 | 0.001 | 0 | 0 | 0.720 |
| g__Pedobacter | <0.001 | 0.001 | 0 | 0 | 0.720 |
| g__norank_f__Anaerolinaceae | <0.001 | 0.001 | 0 | 0 | 0.720 |
| g__norank_f__Nocardioidaceae | <0.001 | 0.001 | 0 | 0 | 0.720 |
| g__Aeromicrobium | <0.001 | 0.001 | 0 | 0 | 0.720 |
TABLE A3.
Differences in the relative abundance of gut microbiota (at the genus level) in adult and immature rhesus macaques
| Species name | Adult | Immature | Corrected p value | ||
|---|---|---|---|---|---|
| Mean (%) | SD (%) | Mean (%) | SD (%) | ||
| g__Prevotella | 25.050 | 15.120 | 24.790 | 18.120 | 0.887 |
| g__norank_f__Ruminococcaceae | 9.543 | 4.686 | 7.094 | 4.114 | 0.028 |
| g__Lactobacillus | 4.226 | 8.527 | 6.031 | 9.846 | 0.237 |
| g__norank_f__Clostridiaceae | 3.430 | 5.158 | 6.061 | 5.933 | 0.102 |
| g__Enterococcus | 1.464 | 6.107 | 6.697 | 13.850 | <0.001 |
| g__Oscillospira | 3.509 | 2.619 | 2.461 | 2.227 | 0.081 |
| g__Sarcina | 2.443 | 5.712 | 3.330 | 5.166 | 0.231 |
| g__norank_f__Lachnospiraceae | 3.391 | 1.988 | 2.318 | 1.707 | 0.005 |
| g__unclassified_o__Clostridiales | 3.076 | 1.376 | 2.473 | 1.161 | 0.062 |
| g__Blautia | 2.299 | 1.430 | 2.618 | 2.165 | 0.973 |
| g__Faecalibacterium | 2.092 | 1.678 | 2.460 | 3.117 | 0.646 |
| g__norank_o__Clostridiales | 2.750 | 2.417 | 1.748 | 1.610 | 0.010 |
| g__norank_f__S24‐7 | 2.262 | 1.851 | 2.134 | 2.481 | 0.218 |
| g__Succinivibrio | 3.571 | 5.154 | 0.768 | 1.806 | <0.001 |
| g__norank_f__Peptostreptococcaceae | 1.380 | 1.692 | 2.883 | 4.069 | 0.180 |
| g__Coprococcus | 2.364 | 1.502 | 1.835 | 1.528 | 0.047 |
| g__Ruminococcus | 1.924 | 1.146 | 1.790 | 3.168 | 0.033 |
| g__unclassified_f__Ruminococcaceae | 2.035 | 1.093 | 1.305 | 0.970 | 0.004 |
| g__Roseburia | 1.616 | 1.491 | 1.620 | 1.700 | 0.588 |
| g__unclassified_f__Lachnospiraceae | 1.815 | 1.160 | 1.332 | 0.967 | 0.039 |
| g__Treponema | 1.727 | 2.573 | 1.057 | 1.553 | 0.076 |
| g__norank_o__Bacteroidales | 1.200 | 1.392 | 1.070 | 1.118 | 0.546 |
| g__Phascolarctobacterium | 1.332 | 1.841 | 0.613 | 1.050 | 0.047 |
| g__norank_f__Coriobacteriaceae | 0.801 | 0.681 | 1.028 | 1.356 | 0.811 |
| g__[Prevotella] | 0.880 | 1.003 | 0.766 | 1.059 | 0.195 |
| g__norank_f__Christensenellaceae | 0.994 | 0.878 | 0.518 | 0.493 | 0.007 |
| g__norank_o__Streptophyta | 0.629 | 1.878 | 0.745 | 2.291 | 0.650 |
| g__norank_o__RF39 | 0.826 | 0.586 | 0.492 | 0.388 | 0.008 |
| g__Bifidobacterium | 0.300 | 0.818 | 1.009 | 2.498 | 0.650 |
| g__Clostridium | 0.499 | 0.626 | 0.647 | 1.445 | 0.229 |
| g__norank_f__[Mogibacteriaceae] | 0.614 | 0.357 | 0.524 | 0.386 | 0.155 |
| g__unclassified_o__Bacteroidales_c__Bacteroidia | 0.645 | 0.964 | 0.402 | 0.951 | 0.008 |
| g__Dialister | 0.458 | 0.618 | 0.516 | 0.644 | 0.960 |
| g__[Ruminococcus] | 0.461 | 0.385 | 0.419 | 0.350 | 0.646 |
| g__SMB53 | 0.360 | 0.580 | 0.484 | 0.651 | 0.479 |
| g__Catenibacterium | 0.330 | 0.548 | 0.461 | 1.575 | 0.151 |
| g__Lachnospira | 0.340 | 0.411 | 0.327 | 0.482 | 0.565 |
| g__Bulleidia | 0.382 | 0.393 | 0.275 | 0.252 | 0.412 |
| g__norank_o__YS2 | 0.348 | 0.353 | 0.215 | 0.338 | 0.016 |
| g__Dorea | 0.260 | 0.161 | 0.299 | 0.213 | 0.747 |
| g__norank_o__GMD14H09 | 0.247 | 0.891 | 0.283 | 0.719 | 0.646 |
| g__CF231 | 0.345 | 0.440 | 0.184 | 0.272 | 0.025 |
| g__YRC22 | 0.331 | 0.493 | 0.189 | 0.365 | 0.047 |
| g__Pediococcus | 0.296 | 2.168 | 0.183 | 0.699 | 0.447 |
| g__norank_f__Enterobacteriaceae | 0.071 | 0.608 | 0.395 | 1.210 | 0.024 |
| g__Lactococcus | 0.334 | 2.053 | 0.114 | 0.436 | 0.104 |
| g__norank_f__RF16 | 0.309 | 0.644 | 0.130 | 0.203 | 0.097 |
| g__norank_f__[Paraprevotellaceae] | 0.212 | 0.279 | 0.223 | 0.556 | 0.447 |
| g__unclassified_o__Lactobacillales | 0.064 | 0.236 | 0.369 | 0.752 | 0.001 |
| g__unclassified_k__norank_d__Bacteria | 0.249 | 0.378 | 0.133 | 0.196 | 0.030 |
| g__Anaerovibrio | 0.258 | 0.443 | 0.113 | 0.201 | 0.039 |
| g__norank_p__WPS‐2 | 0.240 | 0.480 | 0.124 | 0.354 | 0.218 |
| g__Butyrivibrio | 0.166 | 0.207 | 0.174 | 0.292 | 0.878 |
| g__unclassified_f__Clostridiaceae | 0.142 | 0.215 | 0.185 | 0.223 | 0.465 |
| g__norank_f__Erysipelotrichaceae | 0.162 | 0.193 | 0.147 | 0.231 | 0.437 |
| g__norank_f__RFP12 | 0.210 | 0.401 | 0.092 | 0.260 | 0.001 |
| g__Megasphaera | 0.202 | 0.478 | 0.096 | 0.178 | 0.175 |
| g__Bacillus | 0.167 | 1.677 | 0.127 | 0.770 | 0.832 |
| g__unclassified_p__Firmicutes | 0.076 | 0.243 | 0.189 | 0.284 | 0.006 |
| g__norank_f__Rikenellaceae | 0.135 | 0.206 | 0.111 | 0.141 | 0.859 |
| g__Flexispira | 0.114 | 0.160 | 0.122 | 0.350 | 0.072 |
| g__norank_f__Lactobacillaceae | 0.092 | 0.619 | 0.143 | 0.522 | 0.650 |
| g__norank_f__Veillonellaceae | 0.168 | 0.302 | 0.062 | 0.119 | 0.084 |
| g__Rummeliibacillus | 0.027 | 0.251 | 0.200 | 0.801 | 0.102 |
| g__unclassified_c__Bacilli | 0.056 | 0.240 | 0.153 | 0.272 | 0.001 |
| g__Carnobacterium | 0.001 | 0.009 | 0.204 | 1.331 | 0.039 |
| g__Leuconostoc | 0.015 | 0.156 | 0.173 | 0.628 | 0.024 |
| g__Collinsella | 0.058 | 0.073 | 0.089 | 0.119 | 0.115 |
| g__norank_o__RF32 | 0.086 | 0.127 | 0.057 | 0.117 | 0.081 |
| g__Fibrobacter | 0.092 | 0.153 | 0.039 | 0.056 | 0.033 |
| g__Desulfovibrio | 0.095 | 0.159 | 0.035 | 0.058 | 0.001 |
| g__Sphaerochaeta | 0.080 | 0.087 | 0.036 | 0.050 | 0.007 |
| g__norank_f__Leuconostocaceae | 0.023 | 0.211 | 0.091 | 0.337 | 0.499 |
| g__Acinetobacter | 0.055 | 0.565 | 0.055 | 0.262 | 0.811 |
| g__Streptococcus | 0.031 | 0.060 | 0.077 | 0.190 | 0.878 |
| g__Pseudomonas | 0.020 | 0.104 | 0.084 | 0.274 | 0.201 |
| g__unclassified_f__Coriobacteriaceae | 0.033 | 0.038 | 0.068 | 0.176 | 0.918 |
| g__Slackia | 0.049 | 0.051 | 0.045 | 0.039 | 0.980 |
| g__norank_f__p‐2534‐18B5 | 0.059 | 0.096 | 0.034 | 0.063 | 0.084 |
| g__unclassified_f__Planococcaceae | 0.022 | 0.137 | 0.070 | 0.457 | 0.629 |
| g__Sutterella | 0.051 | 0.099 | 0.037 | 0.066 | 0.254 |
| g__Parabacteroides | 0.042 | 0.058 | 0.045 | 0.062 | 0.791 |
| g__unclassified_d__Unclassified | 0.042 | 0.045 | 0.033 | 0.037 | 0.254 |
| g__unclassified_f__Erysipelotrichaceae | 0.023 | 0.032 | 0.044 | 0.070 | 0.565 |
| g__unclassified_p__Proteobacteria | 0.038 | 0.058 | 0.028 | 0.047 | 0.274 |
| g__norank_o__Rickettsiales | 0.037 | 0.044 | 0.029 | 0.038 | 0.261 |
| g__Serratia | <0.001 | 0.001 | 0.063 | 0.420 | 0.432 |
| g__Mogibacterium | 0.017 | 0.027 | 0.042 | 0.096 | 0.372 |
| g__Ruminobacter | 0.048 | 0.164 | 0.011 | 0.037 | 0.039 |
| g__p‐75‐a5 | 0.024 | 0.033 | 0.034 | 0.069 | 0.878 |
| g__[Eubacterium] | 0.026 | 0.025 | 0.030 | 0.043 | 0.878 |
| g__unclassified_f__Veillonellaceae | 0.040 | 0.096 | 0.015 | 0.029 | 0.058 |
| g__RFN20 | 0.033 | 0.081 | 0.019 | 0.033 | 0.102 |
| g__Solibacillus | 0.037 | 0.258 | 0.015 | 0.087 | 0.948 |
| g__Comamonas | <0.001 | 0.001 | 0.052 | 0.345 | 0.546 |
| g__unclassified_o__Bacillales | 0.025 | 0.246 | 0.017 | 0.111 | 0.929 |
| g__Chryseobacterium | 0.001 | 0.005 | 0.041 | 0.269 | 0.256 |
| g__norank_o__ML615J‐28 | 0.032 | 0.225 | 0.006 | 0.012 | 0.666 |
| g__norank_f__R4‐45B | 0.021 | 0.057 | 0.015 | 0.058 | 0.412 |
| g__Brachyspira | 0.021 | 0.029 | 0.013 | 0.033 | 0.047 |
| g__unclassified_c__Betaproteobacteria | 0.028 | 0.059 | 0.004 | 0.012 | 0.020 |
| g__Candidatus_Rhabdochlamydia | 0.014 | 0.092 | 0.017 | 0.079 | 0.978 |
| g__norank_o__M2PT2‐76 | 0.025 | 0.062 | 0.004 | 0.013 | 0.010 |
| g__Trichococcus | <0.001 | <0.001 | 0.028 | 0.184 | 0.102 |
| g__unclassified_f__Enterobacteriaceae | 0.015 | 0.089 | 0.013 | 0.047 | 0.271 |
| g__Oxalobacter | 0.018 | 0.023 | 0.009 | 0.013 | 0.139 |
| g__norank_f__Elusimicrobiaceae | 0.018 | 0.046 | 0.007 | 0.016 | 0.102 |
| g__L7A_E11 | 0.015 | 0.041 | 0.009 | 0.013 | 0.565 |
| g__Coprobacillus | 0.009 | 0.017 | 0.013 | 0.021 | 0.586 |
| g__norank_f__Anaeroplasmataceae | 0.011 | 0.015 | 0.010 | 0.020 | 0.217 |
| g__unclassified_f__[Mogibacteriaceae] | 0.017 | 0.033 | 0.003 | 0.005 | 0.013 |
| g__Actinobacillus | 0.010 | 0.028 | 0.011 | 0.020 | 0.565 |
| g__rc4‐4 | 0.014 | 0.018 | 0.006 | 0.011 | 0.055 |
| g__Anaeroplasma | 0.013 | 0.020 | 0.007 | 0.022 | 0.007 |
| g__unclassified_c__Clostridia | 0.012 | 0.015 | 0.007 | 0.011 | 0.067 |
| g__Anaerostipes | 0.013 | 0.032 | 0.006 | 0.010 | 0.218 |
| g__Anaerofustis | 0.011 | 0.015 | 0.007 | 0.018 | 0.034 |
| g__unclassified_c__Alphaproteobacteria | 0.014 | 0.041 | 0.004 | 0.008 | 0.141 |
| g__norank_f__Streptococcaceae | 0.005 | 0.054 | 0.012 | 0.041 | 0.013 |
| g__Anaerovorax | 0.005 | 0.050 | 0.012 | 0.058 | 0.296 |
| g__norank_f__Victivallaceae | 0.013 | 0.044 | 0.004 | 0.007 | 0.650 |
| g__Dehalobacterium | 0.010 | 0.013 | 0.007 | 0.012 | 0.097 |
| g__Kocuria | 0.001 | 0.005 | 0.015 | 0.094 | 0.061 |
| g__Butyricimonas | 0.010 | 0.020 | 0.005 | 0.007 | 0.267 |
| g__unclassified_f__Prevotellaceae | 0.007 | 0.008 | 0.006 | 0.007 | 0.384 |
| g__norank_f__mitochondria | 0.005 | 0.011 | 0.008 | 0.020 | 0.650 |
| g__Tetragenococcus | 0.004 | 0.023 | 0.009 | 0.036 | 0.666 |
| g__Veillonella | 0.006 | 0.016 | 0.006 | 0.011 | 0.859 |
| g__Proteiniclasticum | 0 | 0 | 0.012 | 0.079 | 0.254 |
| g__norank_f__Bacillaceae | 0.011 | 0.052 | <0.001 | 0.002 | 0.650 |
| g__unclassified_f__Pasteurellaceae | 0.005 | 0.019 | 0.006 | 0.015 | 0.650 |
| g__norank_f__Dehalobacteriaceae | 0.007 | 0.012 | 0.003 | 0.005 | 0.026 |
| g__norank_f__Peptococcaceae | 0.007 | 0.010 | 0.002 | 0.004 | 0.024 |
| g__norank_c__Alphaproteobacteria | 0.006 | 0.028 | 0.003 | 0.010 | 0.254 |
| g__norank_f__Xanthomonadaceae | <0.001 | 0.001 | 0.008 | 0.054 | 0.546 |
| g__unclassified_p__Tenericutes | 0.007 | 0.012 | 0.001 | 0.003 | 0.010 |
| g__Sphingobacterium | 0 | 0 | 0.008 | 0.052 | 0.254 |
| g__norank_o__Acholeplasmatales | 0.008 | 0.036 | <0.001 | 0.001 | 0.180 |
| g__Weissella | 0.002 | 0.025 | 0.005 | 0.024 | 0.139 |
| g__Facklamia | 0 | 0 | 0.007 | 0.043 | 0.254 |
| g__norank_f__Xenococcaceae | 0.001 | 0.003 | 0.005 | 0.014 | 0.231 |
| g__Alloscardovia | 0.001 | 0.004 | 0.004 | 0.012 | 0.056 |
| g__unclassified_o__Rickettsiales | 0.002 | 0.006 | 0.003 | 0.010 | 0.668 |
| g__Arthrobacter | 0.003 | 0.023 | 0.002 | 0.011 | 0.133 |
| g__Bacteroides_f__Bacteroidaceae | 0.002 | 0.007 | 0.003 | 0.008 | 0.700 |
| g__Paracoccus | <0.001 | <0.011 | 0.004 | 0.013 | 0.039 |
| g__Actinomyces | 0.001 | 0.003 | 0.003 | 0.010 | 0.980 |
| g__Leucobacter | <0.001 | 0.001 | 0.004 | 0.022 | 0.859 |
| g__Anaerotruncus | 0 | 0 | 0.004 | 0.023 | 0.102 |
| g__norank_f__Desulfovibrionaceae | 0.002 | 0.006 | 0.001 | 0.002 | 0.689 |
| g__Rubellimicrobium | <0.001 | 0.001 | 0.003 | 0.010 | 0.006 |
| g__unclassified_f__Bifidobacteriaceae | <0.001 | 0.002 | 0.003 | 0.009 | 0.034 |
| g__norank_c__TM7‐3 | 0.001 | 0.002 | 0.002 | 0.005 | 0.546 |
| g__norank_f__Flavobacteriaceae | 0.002 | 0.020 | 0.001 | 0.006 | 0.650 |
| g__Wautersiella | 0.002 | 0.017 | 0.001 | 0.006 | 0.650 |
| g__unclassified_f__Rhizobiaceae | 0.001 | 0.002 | 0.002 | 0.004 | 0.139 |
| g__Staphylococcus | 0.001 | 0.004 | 0.001 | 0.004 | 0.356 |
| g__Luteimonas | 0.002 | 0.020 | <0.001 | 0.002 | 0.296 |
| g__unclassified_f__[Paraprevotellaceae] | 0.002 | 0.003 | 0.001 | 0.002 | 0.141 |
| g__Kaistobacter | <0.001 | 0.001 | 0.002 | 0.008 | 0.254 |
| g__Planomicrobium | 0 | 0 | 0.002 | 0.015 | 0.254 |
| g__Rhodoplanes | <0.001 | 0.001 | 0.002 | 0.004 | 0.086 |
| g__norank_f__Gaiellaceae | 0.001 | 0.002 | 0.001 | 0.003 | 0.254 |
| g__Sanguibacter | <0.001 | 0.001 | 0.002 | 0.010 | 0.918 |
| g__unclassified_c__Gammaproteobacteria | 0.001 | 0.003 | <0.001 | 0.001 | 0.026 |
| g__Deinococcus | <0.001 | 0.001 | 0.002 | 0.010 | 0.546 |
| g__Brachybacterium | <0.001 | 0.001 | 0.001 | 0.010 | 0.906 |
| g__Sphingomonas | <0.001 | 0.001 | 0.001 | 0.004 | 0.039 |
| g__unclassified_p__Spirochaetes | 0.01 | 0.004 | <0.001 | 0.002 | 0.544 |
| g__Epulopiscium | 0.001 | 0.003 | 0.001 | 0.004 | 0.928 |
| g__Sedimentibacter | 0.001 | 0.009 | <0.001 | 0.001 | 0.917 |
| g__Bilophila | 0.001 | 0.005 | <0.001 | 0.002 | 0.650 |
| g__norank_f__Prevotellaceae | 0.001 | 0.002 | 0.001 | 0.002 | 0.666 |
| g__Paenibacillus | <0.001 | 0.001 | 0.001 | 0.007 | 0.544 |
| g__Rickettsiella | 0.001 | 0.009 | <0.001 | 0.001 | 0.565 |
| g__Balneimonas | <0.001 | 0.001 | 0.001 | 0.004 | 0.057 |
| g__norank_f__[Cerasicoccaceae] | 0.001 | 0.002 | 0.001 | 0.003 | 0.878 |
| g__Rhodococcus | 0.001 | 0.002 | 0.001 | 0.002 | 0.906 |
| g__Pseudonocardia | <0.001 | 0.002 | 0.001 | 0.002 | 0.531 |
| g__unclassified_o__Actinomycetales | <0.001 | 0.001 | 0.001 | <0.006 | 0.254 |
| g__Rubrobacter | <0.001 | 0.001 | 0.001 | 0.003 | 0.012 |
| g__unclassified_f__Gemellaceae | <0.001 | 0.002 | 0.001 | 0.002 | 0.803 |
| g__norank_f__Caulobacteraceae | <0.001 | 0.003 | 0.001 | 0.003 | 0.442 |
| g__norank_f__AKIW874 | <0.001 | 0.002 | 0.001 | 0.002 | 0.386 |
| g__Methylobacterium | 0.001 | 0.002 | <0.001 | 0.002 | 0.859 |
| g__norank_o__JG30‐KF‐CM45 | <0.001 | 0.001 | 0.001 | 0.003 | 0.057 |
| g__unclassified_f__Intrasporangiaceae | <0.001 | 0.001 | 0.001 | 0.005 | 0.918 |
| g__norank_o__Rhizobiales | <0.001 | 0.002 | <0.001 | 0.002 | 0.940 |
| g__norank_f__Sanguibacteraceae | 0 | 0 | 0.001 | 0.006 | 0.254 |
| g__Tissierella_Soehngenia | 0 | 0 | 0.001 | 0.006 | 0.254 |
| g__Truepera | <0.001 | 0.001 | 0.001 | 0.002 | 0.028 |
| g__Halomonas | <0.001 | 0.001 | 0.001 | 0.003 | 0.878 |
| g__unclassified_c__Spirochaetes | <0.001 | 0.002 | <0.001 | 0.003 | 0.786 |
| g__norank_o__HA64 | <0.001 | 0.002 | <0.001 | 0.002 | 0.663 |
| g__Leptotrichia | <0.001 | 0.002 | <0.001 | 0.002 | 0.774 |
| g__Stenotrophomonas | 0 | 0 | 0.001 | 0.005 | 0.254 |
| g__Neisseria | 0.001 | 0.004 | <0.001 | 0.001 | 0.499 |
| g__Porphyromonas | <0.001 | 0.002 | <0.001 | 0.001 | 0.859 |
| g__norank_f__EB1017 | <0.001 | 0.001 | 0.001 | 0.001 | 0.214 |
| g__Granulicatella | <0.001 | 0.002 | <0.001 | 0.001 | 0.650 |
| g__Bradyrhizobium | <0.001 | 0.001 | <0.001 | 0.002 | 0.832 |
| g__norank_f__Acetobacteraceae | <0.001 | <0.001 | 0.001 | 0.002 | 0.026 |
| g__Burkholderia | 0.001 | 0.002 | <0.001 | 0.001 | 0.276 |
| g__norank_f__Propionibacteriaceae | <0.001 | 0.002 | <0.001 | 0.001 | 0.703 |
| g__unclassified_o__Sphingomonadales | <0.001 | 0.001 | 0.001 | 0.002 | 0.139 |
| g__unclassified_f__Comamonadaceae | 0.001 | 0.007 | 0 | 0 | 0.650 |
| g__Rhodobacter | <0.001 | 0.002 | <0.001 | 0.002 | 0.700 |
| g__Anaerococcus | <0.001 | 0.001 | 0.001 | 0.001 | 0.058 |
| g__Methyloversatilis | 0.001 | 0.005 | <0.001 | <0.001 | 0.553 |
| g__unclassified_f__Fusobacteriaceae | <0.001 | 0.001 | 0 | 0 | 0.139 |
| g__unclassified_f__Paenibacillaceae | <0.001 | 0.002 | <0.001 | 0.003 | 0.650 |
| g__Corynebacterium | <0.001 | 0.001 | <0.001 | 0.002 | 0.440 |
| g__Rothia | <0.001 | 0.001 | <0.001 | 0.002 | 0.432 |
| g__norank_o__Sphingomonadales | 0 | 0 | 0.001 | 0.002 | 0.039 |
| g__Dietzia | 0 | 0 | 0.001 | 0.003 | 0.254 |
| g__norank_f__Cytophagaceae | 0 | 0 | <0.001 | 0.002 | 0.020 |
| g__Mitsuokella | 0.001 | 0.004 | 0 | 0 | 0.465 |
| g__unclassified_f__Phyllobacteriaceae | <0.001 | 0.003 | <0.001 | 0.001 | 0.550 |
| g__Haemophilus | <0.001 | 0.003 | 0 | 0 | 0.296 |
| g__Salinivibrio | <0.001 | 0.002 | <0.001 | 0.001 | 0.906 |
| g__norank_o__Stramenopiles | <0.001 | 0.001 | <0.001 | 0.001 | 0.499 |
| g__norank_f__Rhizobiaceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.878 |
| g__norank_f__Mycoplasmataceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.878 |
| g__Thermomonas | 0 | 0 | <0.001 | 0.002 | 0.039 |
| g__norank_f__Jonesiaceae | 0 | 0 | <0.001 | 0.003 | 0.254 |
| g__norank_f__Dermatophilaceae | 0 | 0 | <0.001 | 0.003 | 0.254 |
| g__Phormidium | <0.001 | 0.001 | <0.001 | 0.002 | 0.546 |
| g__norank_f__Beijerinckiaceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.254 |
| g__Arcobacter | <0.001 | 0.002 | <0.001 | 0.001 | 0.650 |
| g__unclassified_o__Burkholderiales | <0.001 | 0.001 | <0.001 | 0.001 | 0.565 |
| g__norank_f__Ellin6075 | <0.001 | <0.001 | <0.001 | 0.001 | 0.139 |
| g__norank_c__BD1‐5 | <0.001 | <0.001 | <0.001 | 0.002 | 0.650 |
| g__norank_f__Nocardioidaceae | <0.001 | <0.001 | <0.001 | 0.002 | 0.650 |
| g__unclassified_f__Streptomycetaceae | <0.001 | 0.002 | 0 | 0 | 0.254 |
| g__unclassified_p__Bacteroidetes | <0.001 | 0.003 | 0 | 0 | 0.553 |
| g__unclassified_f__Oxalobacteraceae | <0.001 | 0.002 | <0.001 | <0.001 | 0.296 |
| g__norank_f__Rhodospirillaceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.550 |
| g__norank_o__PK29 | <0.001 | 0.001 | <0.001 | 0.001 | 0.686 |
| g__norank_f__Oxalobacteraceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.296 |
| g__norank_f__Sphingobacteriaceae | 0 | 0 | <0.001 | 0.002 | 0.254 |
| g__norank_o__Chlorophyta | 0 | 0 | <0.001 | 0.002 | 0.102 |
| g__Spirosoma | 0 | 0 | <0.001 | 0.002 | 0.102 |
| g__Janthinobacterium | 0 | 0 | <0.001 | 0.002 | 0.254 |
| g__Devosia | 0 | 0 | <0.001 | 0.001 | 0.039 |
| g__Rathayibacter | 0 | 0 | <0.001 | 0.002 | 0.254 |
| g__norank_f__Hyphomicrobiaceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.546 |
| g__unclassified_f__Xanthomonadaceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.546 |
| g__Cetobacterium | <0.001 | 0.001 | <0.001 | 0.001 | 0.906 |
| g__norank_o__SHA‐98 | <0.001 | 0.003 | 0 | 0 | 0.650 |
| g__1‐68 | <0.001 | 0.002 | 0 | 0 | 0.381 |
| g__unclassified_f__Synergistaceae | <0.001 | 0.001 | 0 | 0 | 0.296 |
| g__Arcanobacterium | <0.001 | 0.002 | 0 | 0 | 0.553 |
| g__Megamonas | <0.001 | <0.001 | <0.001 | 0.001 | 0.296 |
| g__Pedobacter | <0.001 | <0.001 | <0.001 | 0.001 | 0.650 |
| g__Campylobacter | <0.001 | 0.001 | 0 | 0 | 0.381 |
| g__Flavisolibacter | 0 | 0 | <0.001 | 0.001 | 0.254 |
| g__unclassified_f__Pseudanabaenaceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.254 |
| g__Peptoniphilus | <0.001 | 0.001 | <0.001 | 0.001 | 0.906 |
| g__Pseudoxanthomonas | <0.001 | 0.001 | <0.001 | 0.001 | 0.650 |
| g__norank_f__Methylobacteriaceae | <0.001 | 0.001 | <0.001 | 0.001 | 0.906 |
| g__Pedomicrobium | <0.001 | 0.001 | <0.001 | 0.001 | 0.906 |
| g__Rhizobium | <0.001 | 0.001 | 0 | 0 | 0.381 |
| g__norank_o__MBNT15 | <0.001 | <0.001 | <0.001 | 0.001 | 0.650 |
| g__Wohlfahrtiimonas | <0.001 | <0.001 | <0.001 | 0.001 | 0.650 |
| g__norank_f__Gemmataceae | <0.001 | <0.001 | <0.001 | 0.001 | 0.650 |
| g__Aeromicrobium | <0.001 | <0.001 | <0.001 | 0.001 | 0.650 |
| g__Ochrobactrum | <0.001 | <0.001 | <0.001 | 0.001 | 0.650 |
| g__Akkermansia | <0.001 | 0.001 | 0 | 0 | 0.465 |
| g__Bacteroides_f__Bacteroidaceae_o__Bacteroidales | <0.001 | 0.001 | 0 | 0 | 0.465 |
| g__norank_c__OPB41 | <0.001 | 0.001 | 0 | 0 | 0.465 |
| g__norank_o__CW040 | <0.001 | 0.001 | 0 | 0 | 0.553 |
| g__norank_c__ABY1 | <0.001 | 0.001 | 0 | 0 | 0.650 |
| g__Cupriavidus | <0.001 | 0.001 | 0 | 0 | 0.465 |
| g__norank_f__Erythrobacteraceae | 0 | 0 | <0.001 | 0.001 | 0.254 |
| g__norank_o__I025 | 0 | 0 | <0.001 | 0.001 | 0.254 |
| g__Aerococcus | 0 | 0 | <0.001 | 0.001 | 0.254 |
| g__norank_c__ZB2 | 0 | 0 | <0.001 | 0.001 | 0.254 |
| g__norank_f__Actinomycetaceae | 0 | 0 | <0.001 | 0.001 | 0.254 |
| g__norank_f__Methylocystaceae | 0 | 0 | <0.001 | 0.001 | 0.254 |
| g__norank_o__Burkholderiales | 0 | 0 | <0.001 | 0.001 | 0.254 |
| g__norank_f__Thermoactinomycetaceae | 0 | 0 | <0.001 | 0.001 | 0.254 |
| g__Leptospira | 0 | 0 | <0.001 | 0.001 | 0.254 |
| g__Nannocystis | 0 | 0 | <0.001 | 0.001 | 0.254 |
| g__Odoribacter | 0 | 0 | <0.001 | 0.001 | 0.254 |
| g__unclassified_p__Cyanobacteria | 0 | 0 | <0.001 | 0.001 | 0.254 |
| g__unclassified_p__Chloroflexi | 0 | 0 | <0.001 | 0.001 | 0.254 |
| g__DA101 | <0.001 | 0.001 | 0 | 0 | 0.553 |
| g__Helicobacter | <0.001 | 0.001 | 0 | 0 | 0.650 |
| g__norank_c__SC72 | <0.001 | 0.001 | 0 | 0 | 0.650 |
| g__Mycobacterium | <0.001 | 0.001 | 0 | 0 | 0.553 |
| g__norank_f__Halobacteroidaceae | <0.001 | 0.001 | 0 | 0 | 0.650 |
| g__Allobaculum | <0.001 | 0.001 | 0 | 0 | 0.650 |
| g__Arenimonas | <0.001 | 0.001 | 0 | 0 | 0.650 |
| g__Brevibacterium | <0.001 | 0.001 | 0 | 0 | 0.553 |
| g__norank_f__Frankiaceae | <0.001 | 0.001 | 0 | 0 | 0.553 |
| g__Exiguobacterium | <0.001 | 0.001 | 0 | 0 | 0.553 |
| g__norank_f__Anaerolinaceae | <0.001 | <0.001 | 0 | 0 | 0.650 |
| g__Cardiobacterium | <0.001 | <0.001 | 0 | 0 | 0.650 |
Li Y, Chen T, Li Y, Tang Y, Huang Z. Gut microbiota are associated with sex and age of host: Evidence from semi‐provisioned rhesus macaques in southwest Guangxi, China. Ecol Evol. 2021;11:8096–8122. 10.1002/ece3.7643
Contributor Information
Yin Tang, Email: 46543573@qq.com.
Zhonghao Huang, Email: hzh773@126.com.
DATA AVAILABILITY STATEMENT
All data are available in the open Figshare repository, and the link to the data is https://doi.org/10.6084/m9.figshare.14453421.v1
REFERENCES
- Amato, K. R. , Leigh, S. R. , Kent, A. , Mackie, R. I. , Yeoman, C. J. , Stumpf, R. M. , Wilson, B. A. , Nelson, K. E. , White, B. A. , & Garber, P. A. (2014). The role of gut microbes in satisfying the nutritional demands of adult and juvenile wild, black howler monkeys (Alouatta pigra). American Journal of Physical Anthropology, 155(4), 652–664. 10.1002/ajpa.22621 [DOI] [PubMed] [Google Scholar]
- Baker, J. M. , Al‐Nakkash, L. , & Herbst‐Kralovetz, M. M. (2017). Estrogen‐gut microbiome axis: Physiological and clinical implications. Maturitas, 103, 45–53. 10.1016/j.maturitas.2017.06.025 [DOI] [PubMed] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67(1), 1–48. http://hdl.handle.net/10.18637/jss.v067.i01 [Google Scholar]
- Bokulich, N. A. , Subramanian, S. , Faith, J. J. , Gevers, D. , Gordon, J. I. , Knight, R. , Mills, D. A. , & Caporaso, J. G. (2013). Quality‐filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nature Methods, 10(1), 57–59. 10.1038/nmeth.2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd, A. L. , Liu, M. , Fujimura, K. E. , Lyalina, S. , Nagarkar, D. R. , Charbit, B. , Bergstedt, J. , Patin, E. , Harrison, O. J. , Quintana‐Murci, L. , Mellman, I. , Duffy, D. , Albert, M. L. , & Milieu Interieur, C. (2020). Gut microbiome stability and dynamics in healthy donors and patients with non‐gastrointestinal cancers. Journal of Experimental Medicine, 218(1), e20200606. 10.1084/jem.20200606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso, J. G. , Kuczynski, J. , Stombaugh, J. , Bittinger, K. , Bushman, F. D. , Costello, E. K. , Fierer, N. , Pena, A. G. , Goodrich, J. K. , Gordon, J. I. , Huttley, G. A. , Kelley, S. T. , Knights, D. , Koenig, J. E. , Ley, R. E. , Lozupone, C. A. , McDonald, D. , Muegge, B. D. , Pirrung, M. , … Knight, R. (2010). QIIME allows analysis of high‐throughput community sequencing data. Nature Methods, 7(5), 335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T. , Li, Y. , Liang, J. , Li, Y. , & Huang, Z. (2020a). Gut microbiota of provisioned and wild rhesus macaques (Macaca mulatta) living in a limestone forest in southwest Guangxi, China. MicrobiologyOpen, 9(3), e981. 10.1002/mbo3.981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T. , Li, Y. , Liang, J. , Li, Y. , & Huang, Z. (2020b). Variations in the gut microbiota of sympatric François' langurs and rhesus macaques living in limestone forests in southwest Guangxi, China. Global Ecology and Conservation, 22, e00929. 10.1016/j.gecco.2020.e00929 [DOI] [Google Scholar]
- Cui, Y.‐F. , Wang, F.‐J. , Yu, L. , Ye, H.‐H. , & Yang, G.‐B. (2019). Metagenomic comparison of the rectal microbiota between rhesus macaques (Macaca mulatta) and cynomolgus macaques (Macaca fascicularis). Zoological Research, 40(2), 89–93. 10.24272/j.issn.2095-8137.2018.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David, L. A. , Maurice, C. F. , Carmody, R. N. , Gootenberg, D. B. , Button, J. E. , Wolfe, B. E. , Ling, A. V. , Devlin, A. S. , Varma, Y. , Fischbach, M. A. , Biddinger, S. B. , Dutton, R. J. , & Turnbaugh, P. J. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature, 505(7484), 559–563. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo, C. , Cavalieri, D. , Di Paola, M. , Ramazzotti, M. , Poullet, J. B. , Massart, S. , Collini, S. , Pieraccini, G. , & Lionetti, P. (2010). Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences of the United States of America, 107(33), 14691–14696. 10.1073/pnas.1005963107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cuesta‐Zuluaga, J. , Kelley, S. T. , Chen, Y. , Escobar, J. S. , Mueller, N. T. , Ley, R. E. , McDonald, D. , Huang, S. , Swafford, A. D. , Knight, R. , & Thackray, V. G. (2019). Age‐ and sex‐dependent patterns of gut microbial diversity in human adults. mSystems., 4(4), e00261‐19. 10.1128/mSystems.00261-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien, M. , Alvarez, A. S. , & de Vos, W. M. (2019). The gut microbiota in the first decade of life. Trends in Microbiology, 27(12), 997–1010. 10.1016/j.tim.2019.08.001 [DOI] [PubMed] [Google Scholar]
- Douglas, C. W. , Heath, J. , Hampton, K. K. , & Preston, F. E. (1993). Identity of viridans streptococci isolated from cases of infective endocarditis. Journal of Medical Microbiology, 39(3), 179–182. 10.1099/00222615-39-3-179 [DOI] [PubMed] [Google Scholar]
- Dunbar, R. I. , Korstjens, A. H. , & Lehmann, J. (2009). Time as an ecological constraint. Biological Reviews of the Cambridge Philosophical Society, 84(3), 413–429. 10.1111/j.1469-185X.2009.00080.x [DOI] [PubMed] [Google Scholar]
- Edgar, R. C. (2013). UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nature Methods, 10(10), 996–998. 10.1038/nmeth.2604 [DOI] [PubMed] [Google Scholar]
- Edgar, R. C. , Haas, B. J. , Clemente, J. C. , Quince, C. , & Knight, R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics, 27(16), 2194–2200. 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa, Y. , Curtis, B. , Yutuc, V. , Rulien, M. , Morrisroe, K. , Watkins, K. , Ferrier, C. , English, C. , Hewitson, L. , & Slupsky, C. M. (2018). Microbial structure and function in infant and juvenile rhesus macaques are primarily affected by age, not vaccination status. Scientific Reports, 8(1), 15867. 10.1038/s41598-018-34019-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashtanova, D. A. , Popenko, A. S. , Tkacheva, O. N. , Tyakht, A. B. , Alexeev, D. G. , & Boytsov, S. A. (2016). Association between the gut microbiota and diet: Fetal life, early childhood, and further life. Nutrition, 32(6), 620–627. 10.1016/j.nut.2015.12.037 [DOI] [PubMed] [Google Scholar]
- Koch, F. , Ganzhorn, J. U. , Rothman, J. M. , Chapman, C. A. , & Fichtel, C. (2017). Sex and seasonal differences in diet and nutrient intake in Verreaux's sifakas (Propithecus verreauxi). American Journal of Primatology, 79(4), e22595. 10.1002/ajp.22595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeck, D. E. , Pechtl, A. , Zverlov, V. V. , & Schwarz, W. H. (2014). Genomics of cellulolytic bacteria. Current Opinion in Biotechnology, 29, 171–183. 10.1016/j.copbio.2014.07.002 [DOI] [PubMed] [Google Scholar]
- Koren, O. , Goodrich, J. K. , Cullender, T. C. , Spor, A. , Laitinen, K. , Backhed, H. K. , Gonzalez, A. , Werner, J. J. , Angenent, L. T. , Knight, R. , Backhed, F. , Isolauri, E. , Salminen, S. , & Ley, R. E. (2012). Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell, 150(3), 470–480. 10.1016/j.cell.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Reau, A. J. , & Suen, G. (2018). The Ruminococci: Key symbionts of the gut ecosystem. Journal of Microbiology, 56(3), 199–208. 10.1007/s12275-018-8024-4 [DOI] [PubMed] [Google Scholar]
- Langille, M. G. , Zaneveld, J. , Caporaso, J. G. , McDonald, D. , Knights, D. , Reyes, J. A. , Clemente, J. C. , Burkepile, D. E. , Vega Thurber, R. L. , Knight, R. , Beiko, R. G. , & Huttenhower, C. (2013). Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature Biotechnology, 31(9), 814–821. 10.1038/nbt.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley, R. E. , Hamady, M. , Lozupone, C. , Turnbaugh, P. J. , Ramey, R. R. , Bircher, J. S. , Schlegel, M. L. , Tucker, T. A. , Schrenzel, M. D. , & Knight, R. (2008). Evolution of mammals and their gut microbes. Science, 320(5883), 1647–1651. 10.1126/science.1155725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Li, D. , Ren, B. , Hu, J. , Li, B. , Krzton, A. , & Li, M. (2014). Differences in the activity budgets of Yunnan snub‐nosed monkeys (Rhinopithecus bieti) by age‐sex class at Xiangguqing in Baimaxueshan Nature Reserve, China. Folia Primatologica, 85(6), 335–342. 10.1159/000368831 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Ma, G. , Zhou, Q. , Li, Y. , & Huang, Z. (2020). Nutrient contents predict the bamboo‐leaf‐based diet of Assamese macaques living in limestone forests of southwest Guangxi, China. Ecology and Evolution, 10(12), 5570–5581. 10.1002/ece3.6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone, C. A. , Stombaugh, J. I. , Gordon, J. I. , Jansson, J. K. , & Knight, R. (2012). Diversity, stability and resilience of the human gut microbiota. Nature, 489(7415), 220–230. 10.1038/nature11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle, J. G. , Frank, D. N. , Mortin‐Toth, S. , Robertson, C. E. , Feazel, L. M. , Rolle‐Kampczyk, U. , von Bergen, M. , McCoy, K. D. , Macpherson, A. J. , & Danska, J. S. (2013). Sex differences in the gut microbiome drive hormone‐dependent regulation of autoimmunity. Science, 339(6123), 1084–1088. 10.1126/science.1233521 [DOI] [PubMed] [Google Scholar]
- Moya, A. , & Ferrer, M. (2016). Functional redundancy‐induced stability of gut microbiota subjected to disturbance. Trends in Microbiology, 24(5), 402–413. 10.1016/j.tim.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Mukhopadhya, I. , Hansen, R. , El‐Omar, E. M. , & Hold, G. L. (2012). IBD‐what role do Proteobacteria play? Nature Reviews Gastroenterology & Hepatology, 9(4), 219–230. 10.1038/nrgastro.2012.14 [DOI] [PubMed] [Google Scholar]
- O'Mara, M. T. , & Hickey, C. M. (2014). The development of sex differences in ring‐tailed lemur feeding ecology. Behavioral Ecology and Sociobiology, 68(8), 1273–1286. 10.1007/s00265-014-1738-3 [DOI] [Google Scholar]
- Pafčo, B. , Sharma, A. K. , Petrželková, K. J. , Vlčková, K. , Todd, A. , Yeoman, C. J. , Wilson, B. A. , Stumpf, R. , White, B. A. , Nelson, K. E. , Leigh, S. , & Gomez, A. (2019). Gut microbiome composition of wild western lowland gorillas is associated with individual age and sex factors. American Journal of Physical Anthropology, 169(3), 575–585. 10.1002/ajpa.23842 [DOI] [PubMed] [Google Scholar]
- Peng, C. , Xu, X. , Li, Y. , Li, X. , Yang, X. , Chen, H. , Zhu, Y. , Lu, N. , & He, C. (2020). Sex‐specific association between the gut microbiome and high‐fat diet‐induced metabolic disorders in mice. Biology of Sex Differences, 11(1), 5. 10.1186/s13293-020-0281-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R‐project.org/ [Google Scholar]
- Reese, A. T. , Phillips, S. R. , Owens, L. A. , Venable, E. M. , Langergraber, K. E. , Machanda, Z. P. , Mitani, J. C. , Muller, M. N. , Watts, D. P. , Wrangham, R. W. , Goldberg, T. L. , Thompson, M. E. , & Carmody, R. N. (2021). Age patterning in wild chimpanzee gut microbiota diversity reveals differences from humans in early life. Current Biology, 31(3), 613–620. 10.1016/j.cub.2020.10.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reveles, K. R. , Patel, S. , Forney, L. , & Ross, C. N. (2019). Age‐related changes in the marmoset gut microbiome. American Journal of Primatology, 81(2), e22960. 10.1002/ajp.22960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiel, N. , Souto, A. , Huber, L. , & Bezerra, B. M. (2010). Hunting strategies in wild common marmosets are prey and age dependent. American Journal of Primatology, 72(12), 1039–1046. 10.1002/ajp.20860 [DOI] [PubMed] [Google Scholar]
- Schloss, P. D. , Westcott, S. L. , Ryabin, T. , Hall, J. R. , Hartmann, M. , Hollister, E. B. , Lesniewski, R. A. , Oakley, B. B. , Parks, D. H. , Robinson, C. J. , Sahl, J. W. , Stres, B. , Thallinger, G. G. , Van Horn, D. J. , & Weber, C. F. (2009). Introducing mothur: Open‐source, platform‐independent, community‐supported software for describing and comparing microbial communities. Applied and Environment Microbiology, 75(23), 7537–7541. 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, T. , Vich Vila, A. , Garmaeva, S. , Jankipersadsing, S. A. , Imhann, F. , Collij, V. , Bonder, M. J. , Jiang, X. , Gurry, T. , Alm, E. J. , D'Amato, M. , Weersma, R. K. , Scherjon, S. , Wijmenga, C. , Fu, J. , Kurilshikov, A. , & Zhernakova, A. (2019). Analysis of 1135 gut metagenomes identifies sex‐specific resistome profiles. Gut Microbes, 10(3), 358–366. 10.1080/19490976.2018.1528822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, B. , Gu, Z. , Wang, X. , Huffman, M. A. , Garber, P. A. , Sheeran, L. K. , Zhang, D. , Zhu, Y. , Xia, D. P. , & Li, J. H. (2018). Season, age, and sex affect the fecal mycobiota of free‐ranging Tibetan macaques (Macaca thibetana). American Journal of Primatology, 80(7), e22880. 10.1002/ajp.22880 [DOI] [PubMed] [Google Scholar]
- Tian, L. , Wang, X. W. , Wu, A. K. , Fan, Y. , Friedman, J. , Dahlin, A. , Waldor, M. K. , Weinstock, G. M. , Weiss, S. T. , & Liu, Y. Y. (2020). Deciphering functional redundancy in the human microbiome. Nature Communications, 11, 6217. 10.1038/s41467-020-19940-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg, L. N. , Colborn, T. , Hayes, T. B. , Heindel, J. J. , Jacobs, D. R. Jr , Lee, D. H. , Shioda, T. , Soto, A. M. , vom Saal, F. S. , Welshons, W. V. , Zoeller, R. T. , & Myers, J. P. (2012). Hormones and endocrine‐disrupting chemicals: Low‐dose effects and nonmonotonic dose responses. Endocrine Reviews, 33(3), 378–455. 10.1210/er.2011-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Steeg, L. G. , & Klein, S. L. (2017). Sex steroids mediate bidirectional interactions between hosts and microbes. Hormones and Behavior, 88, 45–51. 10.1016/j.yhbeh.2016.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujkovic‐Cvijin, I. , Sklar, J. , Jiang, L. , Natarajan, L. , Knight, R. , & Belkaid, Y. (2020). Host variables confound gut microbiota studies of human disease. Nature, 587(7834), 448–454. 10.1038/s41586-020-2881-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Feng, M. , & Li, Y. (1996). The study on population ecology of Macaca mulatta at Longhushan Nature Reserve, Guangxi. Acta Theriol Sinica, 16(4), 264–271. http://www.mammal.cn/CN/Y1996/V16/I4/264 [Google Scholar]
- Warton, D. I. , & Hui, F. K. C. (2011). The arcsine is asinine: The analysis of proportions in ecology. Ecology, 92(1), 3–10. 10.1890/10-0340.1 [DOI] [PubMed] [Google Scholar]
- Yurkovetskiy, L. , Burrows, M. , Khan, A. A. , Graham, L. , Volchkov, P. , Becker, L. , Antonopoulos, D. , Umesaki, Y. , & Chervonsky, A. V. (2013). Gender bias in autoimmunity is influenced by microbiota. Immunity, 39(2), 400–412. 10.1016/j.immuni.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Zhong, H. , Li, Y. , Shi, Z. , Ren, H. , Zhang, Z. , Zhou, X. , Tang, S. , Han, X. , Lin, Y. , Yang, F. , Wang, D. , Fang, C. , Fu, Z. , Wang, L. , Zhu, S. , Hou, Y. , Xu, X. , Yang, H. , … Ji, L. (2021). Sex‐ and age‐related trajectories of the adult human gut microbiota shared across populations of different ethnicities. Nature Aging, 1(1), 87–100. 10.1038/s43587-020-00014-2 [DOI] [PubMed] [Google Scholar]
- Zhao, J. , Yao, Y. , Li, D. , Xu, H. , Wu, J. , Wen, A. , Xie, M. , Ni, Q. , Zhang, M. , Peng, G. , & Xu, H. (2018). Characterization of the gut microbiota in six geographical populations of Chinese rhesus macaques (Macaca mulatta), implying an adaptation to high‐altitude environment. Microbial Ecology, 76(2), 565–577. 10.1007/s00248-018-1146-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the open Figshare repository, and the link to the data is https://doi.org/10.6084/m9.figshare.14453421.v1


